Abstract
Background
While adults who drink low to moderate amounts of alcohol have lower rates of cardiovascular disease (CVD) than other adults, the impact of alcohol on the brain is less clear. There is evidence that drinking large amounts of alcohol is tied to brain atrophy. It is uncertain whether consumption of smaller amounts of alcohol also negatively affects brain volume or may be protective in reducing the well-documented age-related decline in brain volume.
Methods
Participants were 1839 subjects from the Framingham Offspring Study who had a brain MRI between 1999-2001. Total cerebral brain volume (TCBV) was computed correcting for head size. Multivariate linear regression models were used to evaluate the association between five categories of alcohol consumption (abstainers, former drinkers, low, moderate, high) and TCBV, adjusting for age, sex, education, height, body-mass index (BMI) and the Framingham Stroke Risk Profile (FSRP). Pair-wise comparisons were also conducted between the alcohol consumption groups.
Results
Most participants reported low alcohol consumption and males were more likely than females to be moderate or heavy drinkers. There was a significant negative linear relationship between alcohol consumption and TCBV (r = -0.25, p < 0.001). This relationship was modified by sex, with alcohol consumption having a stronger association with TCBV in females than males (r=-0.29 vs. -0.20).
Conclusion
In contrast to studies on CVD, this study found that moderate alcohol consumption was not protective against normal age-related differences in total brain volume. Rather, the more alcohol consumed, the smaller the total brain volume.
Introduction
Many studies have considered the costs and benefits of alcohol consumption in diverse populations. Moderate alcohol consumption has frequently been reported to have a beneficial effect on cardiovascular disease (CVD) 1,2,3. Since the brain is perfused by the cardiovascular system, moderate alcohol consumption may attenuate age-related decline of brain volume.
It is recognized that brain volume declines with age4 at an estimated rate of 1.9% per decade5 while white matter lesions (WMH) increase with age6. In addition, decline in brain volume and increase in WMH accompany the progression of dementia7, 8 and cognitive deficits9. Lower brain volumes and larger WMH are also observed in persons with higher cardiovascular risk5, 10.
Excess alcohol consumption has often been correlated with decline in cognition and can lead to Korsakoff's syndrome11. Yet moderate alcohol consumption has been associated with improved cognitive functioning 12,13,14, a lower risk of Alzheimer's disease (AD)15 and less severe WMH16,17, although one study found no protective effect of moderate alcohol intake on cerebral infarction18.
The impact of alcohol on brain volume and WMH, however, has not been examined in a community-based sample that is free of clinically evident neurological disease. This cross-sectional study tested the hypothesis that low or moderate alcohol consumption was associated with larger brain volume and less white WMH when compared with no drinking or high levels of alcohol consumption in a sample of community-dwelling adults.
Methods
Study Subjects
The Framingham Offspring cohort, begun in 1971, included children of the original Framingham Heart Study cohort, and their spouses (n = 5124) who were aged 33-88 during the study. Study participants have had seven health exams every 4-8 years over the past 30 years. Participants at exam 7 (n=3539; 1999-2001) were invited to have an MRI of the brain; 1886 participants were imaged through August 2001. Major reasons for not being imaged were residence out of state, medical contraindications for MRI such as pacemakers, and claustrophobia. An additional 47 participants were excluded for prevalent stroke, dementia or other neurological disorder, resulting in a sample of 1839 stroke and dementia-free respondents. Unpublished data finds that those who did not have a brain MRI are older and show higher risk for cardiovascular disease, suggesting that our findings are a conservative estimate of the relationship between alcohol consumption and brain volume.
Measurement of total brain volume
The total (parenchymal) brain volume (TBV) and WMH volumes were corrected for total cranial volume (TCV); the resulting measures are TBV/TCV*100 (TCBV) and WMH/TCV*100 (WMHV) as described by DeCarli et al4.
Measurement of alcohol consumption
Participants reported at exam 7 the number of alcoholic drinks/week (beer, white wine, red wine or liquor), they consumed over the past month. Alcohol consumption was recorded as a continuous variable and participants were classified into one of five categories that have been used in other studies14, 17, 18: abstainers, former (drinkers based on their drinking status at earlier exams), low (1-7 drinks/week), moderate (8-14) and high (>15 drinks/week).
Covariates
Covariates obtained at exam 7 included age (in years), sex, education (categorized as 4th grade or less, 5th through 7th grade, high school or some college, and college graduate), height, body mass index (BMI) and Framingham Stroke Risk Profile (FSRP, an estimate of the 10-year risk of stroke, on a scale of 0 to 1, based on age, sex, systolic blood pressure, antihypertensive therapy, diabetes, smoking status, history of CVD and the presence of atrial fibrillation and left ventricular hypertrophy19).
Statistical Analysis
The mean TCBV and WMH were calculated for each of the five alcohol consumption groups and the means compared using an ANCOVA with pair wise comparisons (level of significance = 0.0125 for each pair wise comparison). Means were tested for a linear trend across alcohol consumption groups at a 0.05 level of significance. The initial model included all covariates; those covariates that did not contribute significantly to the model were eliminated one at a time, provided that their exclusion did not alter the beta coefficient for the alcohol consumption term and that they were not clinically important. Sex was assessed as an effect modifier to determine whether the association between amount of alcohol consumption and TCBV and WMH differed between males and females. Analyses were performed using SAS version 8.2 statistical software.
Results
The sample included 1839 participants who ranged in age from 33 to 88 years (mean age = 60.64, SD±9.42). There were 861 males and 978 females. As shown in Table 1, the majority of participants reported consuming 1-7 drinks/week, followed by former drinkers. While females were more likely than males to be abstainers, former drinkers, or drink 1-7 drinks/week, males were more likely to report moderate or high alcohol consumption.
Table 1.
Distribution of alcohol consumption among the total sample, males, and females, 1839 participants of the Framingham Offspring Cohort
| Total | Male | Female | |||
|---|---|---|---|---|---|
| n | n | % | n | % | |
| Participants | 1839 | 861 | 46.81 | 978 | 53.18 |
| Alcohol consumption | |||||
| Abstainers | 71 | 23 | 2.67 | 48 | 4.91 |
| Former drinkers | 545 | 217 | 25.20 | 328 | 33.54 |
| Low (1-7) | 762 | 326 | 37.86 | 436 | 44.58 |
| Moderate (8-14) | 276 | 163 | 18.93 | 113 | 11.55 |
| High (>14) | 185 | 132 | 15.33 | 53 | 5.42 |
For the total sample, the mean TCBV was 77.40± 3.41 for males (range 64.89-85.52) and 78.35±2.94 (range 67.84-85.85) for females.
The covariates for each group were evaluated separately for females and males (see Table 2a and 2b). Among females, amount of alcohol consumption was significantly associated with each of the covariates (age, sex, BMI and FSRP), but among males, alcohol consumption was associated only with FSRP. Notably, females who consumed moderate amounts of alcohol had the lowest BMI, most education and lowest FSRP, while males who drank low amounts had the lowest FSRP.
Table 2.
Comparison of means (±SD) of covariates within categories of alcohol consumption in 1839 males and females in the Framingham Offspring Cohort.
| Category of alcohol consumption | ||||||
|---|---|---|---|---|---|---|
| covariates | Abstainers | Former drinkers | Low | Moderate | High | p-value |
| A. Females | ||||||
| Age | 62.9±10.1 | 62.2±9.4 | 59.5±9.6 | 59.4±8.6 | 58.9±8.2 | < 0.01 |
| BMI | 28.3±7.3 | 28.1±6.3 | 27.1±5.4 | 25.8±4.4 | 26.9±5.6 | < 0.01 |
| Height | 63±2.3 | 63.2±2.5 | 63.6±2.5 | 63.8±2.2 | 64.2±2.1 | 0.01 |
| Education | 2.5±0.9 | 2.7±0.9 | 3.0 ±0.9 | 3.1±0.9 | 3.0±0.9 | <0.01 |
| FSRP | 0.05±0.06 | 0.05±0.06 | 0.04±0.05 | 0.04±0.04 | 0.05±0.05 | <0.01 |
| B. Males | ||||||
| Age | 63.2±9.5 | 61.6±10.1 | 60.1±9.1 | 60.8±9.8 | 60.5±8.5 | 0.29 |
| BMI | 27.9±3.4 | 28.9±4.9 | 28.4±4.1 | 28.2±3.6 | 28.8±4.7 | 0.47 |
| Height | 68.4±2.0 | 68.8±2.5 | 68.8±2.7 | 69.0±2.4 | 68.8±2.7 | 0.86 |
| Education | 3±1. | 2.9±1.0 | 3.2±0.9 | 3.1±1.0 | 3.1±0.9 | 0.04 |
| FSRP | 0.10±0.07 | 0.11±0.08 | 0.08±0.06 | 0.09±0.08 | 0.10±0.08 | 0.04 |
Association between alcohol consumption and TCBV and WMH in the total sample
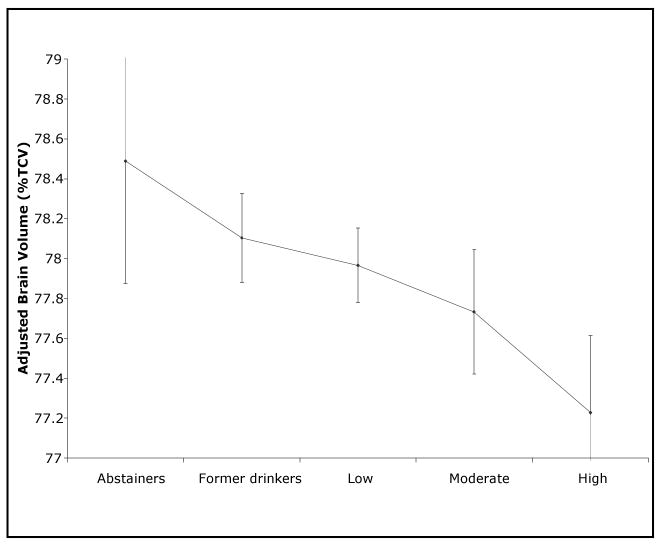
In unadjusted analyses, participants who had low alcohol consumption had slightly larger TCBV in comparison to all the other groups. However, when adjusted for covariates (age, sex, education, BMI and FSRP), there was a significant negative linear association between amount of alcohol consumed and TCBV (beta coefficient = -0.25, p < 0.01) (See Fig. 1). This slope of -0.25 is slightly larger than the average decline in brain volume/year with aging. From the pair-wise comparisons between groups, moderate drinkers had significantly smaller TCBV than former drinkers (p = 0.03); and participants who consumed more than 14 drinks/week had significantly smaller TCBV than all the other groups (high drinkers vs. abstainers p < 0.01; vs. former p < 0.01; vs. low p <0.01; vs. moderate p = 0.045). By contrast, there was no significant association between alcohol consumption and WMH.
Figure 1.
Mean TCBV, including confidence intervals, illustrated as error bars, adjusted for age, sex, BMI and FSRP (slope = -0.25, p < 0.01, R2 = 0.96).
Association between alcohol consumption and TCBV by sex
In sex-specific analyses, adjusted mean TCBV was higher for females than males in every category of alcohol consumption (see Figure 2). Moreover, the linear regression curve for the association between alcohol consumption and TCBV was steeper for females than for males. The interaction between drinking and sex on TCBV was significant (p <0.01).
Figure 2.
Mean values for TCBV, including confidence intervals, illustrated by error bars, for males and females, adjusted for age, sex, BMI and FSRP.
Among females, greater amount of alcohol consumed was significantly associated with smaller TCBV in multivariable analyses (p = 0.02). From the pair-wise comparisons, adjusted mean TCBV only differed significantly between females who were abstainers and moderate drinkers (p = 0.01), former and low drinkers (p = 0.01) and former and moderate drinkers (p < 0.01). Among males, alcohol consumption was also significantly associated with smaller TCBV (p = 0.02), but while the trend was in the same direction as for females, the overall association was somewhat weaker (beta coefficient = -0.20 for males versus -0.29 for females). Adjusted mean TCBV only differed significantly between males who were high drinkers and each of the other alcohol consumption categories: abstainers (p = 0.03), former (p = 0.02), low (p < 0.01), moderate drinkers (p < 0.01) (see Figure 2).
To verify that there was no protective effect of alcohol on TCBV, the low consumption group was contrasted with all the other alcohol consumption groups for both females and males and no significant difference was found for either sex (p = 0.07 and 0.39 for females and males, respectively). In addition, there was no quadratic relationship between alcohol consumption and TCBV for males (p = 0.31).
Discussion
This study found no protective effects of alcohol in reducing the normal age-related differences in brain volume in the Framingham Offspring Cohort. Instead, higher levels of alcohol consumption were consistently associated with a smaller brain volume, after adjusting for covariates. This association was modified by the participant's sex, with females showing larger TCBV than males at every level of alcohol consumption, and a steeper negative slope in the line relating alcohol consumption and TCBV. However, the direction of this relationship was the same for both sexes and the magnitude of the differences between genders was small. We also found no significant correlation between alcohol and WMH.
Our results are consistent with two recent studies in smaller samples. In one study of 405 Japanese males, both global and regional gray matter volumes were negatively correlated with lifetime alcohol intake20. In another sample of 385 adults aged 60 to 64 years, greater alcohol consumption was associated with more brain atrophy21. Neither of these studies found beneficial effects of low to moderate alcohol consumption.
Two other studies related alcohol consumption to ventricular and sulcal size17, 18. It is noteworthy that both studies found increasing ventricular size with increasing amounts of alcohol consumed. These findings are consistent with our results since ventricular size is an inverse measure of brain atrophy.
Our finding that sex modified the association between alcohol consumption and brain volume could be related to biological factors. Alcohol is absorbed more rapidly in females than in males, and in general females are more vulnerable to the effects of alcohol than males. Females on average are smaller than males and have less blood to dilute the alcohol. Results from this study suggest that alcohol also has a greater negative impact on brain volume in females than in males. In our sample, twice as many males as females reported high alcohol consumption and more males also reported moderate alcohol consumption, which may suggest a behavioral rather than a biological explanation for these findings.
Our hypothesis was based on many observations of a J-shaped association between amount of alcohol consumption and CVD. However, our results indicate that there is no neuroprotective effect of alcohol on neurons.
This study had several limitations. It was restricted to total brain volume while regional brain areas have not yet been considered independently. Because some of the alcohol consumption groups contained few participants, statistical power was low for some analyses. Additionally, the Framingham Offspring cohort is predominantly of European origin and the mean educational level is high, thus these results may not be generalizable to other racial and economic groups. Finally, only about 50% of the sample had MRI scans, however this participation rate is found for all the FHS reports and is consistent with most other MRI studies17,22,23.
Another potential limitation is the difficulty of disentangling effects of alcohol consumption from those of age on TCBV. Age is a normally accompanied by smaller brain volume4, and is inextricably linked to many of the other covariates especially BMI and FSRP.
Use of self-report data to determine the quantity of alcohol consumed is also a concern since participants are likely to under-report their true drinking pattern24. However, under-reporting of alcohol consumption would most likely have resulted in an underestimate of the association25, suggesting that the true association between alcohol consumption and brain volume would be even larger than the one observed.
Finally, these results were observed in cross sectional data. Thus, we cannot relate alcohol consumption to decline in brain volume. Prospective analyses of this association would be important to establish the temporal relationship between alcohol consumption and brain volume.
Nonetheless, this study has many strengths. It included MRIs performed on over 1800 Framingham Study participants without clinical dementia or stroke. These volumetric MRI data were examined after adjusting for a wealth of baseline data on cardiovascular risk. Finally, the MRI readers were blinded to participants' demographic and clinical data.
The public health impact of this study gives a clear message about the possible dangers of drinking alcohol. While there are many reports of the beneficial effects of moderate alcohol consumption, these reports are often accompanied by concerns that the data not be interpreted as encouraging drinking. Prospective longitudinal studies are needed to confirm these results as well as determine whether there are any functional consequences associated with increasing alcohol consumption. This study suggests that unlike the associations with CVD, alcohol consumption does not have any protective effect on brain volume.
Acknowledgments
Carol Ann Paul had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by: National Heart, Lung, and Blood Institute's Framingham Heart Study, National Institutes of Health (NIH/NHLBI Contract N01-HC-25195) and Grants: NIA # 5R01-AG08122, NIA # 2R01-AG16495 and NINDS # 2R01-NS017950.
References
- 1.Di Castelnuovo A, Costanzo S, Bagnardii V, et al. Alcohol dosing and total mortality in men and women. An updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 2.Ellison RC, Martinic M. The Harms and Benefits of Moderate Drinking: Summary of Findings of an International Symposium. Annals of Epidemiology. 2007:1–12. [Google Scholar]
- 3.Mukamal KJ, Majken KJ, Grønbaek M, et al. Drinking Frequency, Mediating Biomarkers, and Risk of Myocardial Infarction in Women and Men. Circulation. 2005;112(10):1379–81. doi: 10.1161/CIRCULATIONAHA.105.537704. [DOI] [PubMed] [Google Scholar]
- 4.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiology of Aging. 2005;4:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Seshadri D, Wolf P, Beiser A, et al. Stroke risk profile, brain volume and cognitive function. The Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 6.Bigler ED, Kerr B, Victoroff J, Tate DF, Breitner JC. White matter lesions, quantitative magnetic resonance imaging, and dementia. Alzheimer Disease and Associated Disorders. 2002;16(3):161–70. doi: 10.1097/00002093-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology. 2005;64(9):1548–52. doi: 10.1212/01.WNL.0000160115.55756.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128(9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 9.Au R, Massaro J, Wolf P, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Archives of Neurology. 2006;63(2):246–50. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 10.Jeerakathil T, Wolf P, Beiser A, et al. Stroke Risk Profile Predicts White Matter Hyperintensity Volume. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 11.Shimamura A, Squire LR. Korsakoff's syndrome: a study of the relation between anterograde amnesia and remote memory impairment. Behav Neurosci. 1986;100(2):165–70. doi: 10.1037//0735-7044.100.2.165. [DOI] [PubMed] [Google Scholar]
- 12.Espeland MA, Gu L, Masaki KH, et al. Association between reported alcohol intake and cognition: results from the Women's Health Initiative Memory Study. American Journal of Epidemiology. 2005;161:228–238. doi: 10.1093/aje/kwi043. [DOI] [PubMed] [Google Scholar]
- 13.Galanis DJ, Joseph C, Masaki KH, Petrovitch H, Ross GW, White L. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: the Honolulu-Asia Aging Study. American Journal of Public Health. 2000;90(8):1254–9. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA: The Journal of the American Medical Association. 2003;289(11):1405–13. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- 15.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. Journal of the American Geriatric Society. 2004;52(4):540–6. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 16.den Heijer T, Vermeer SE, van Dijk EJ, et al. Alcohol intake in relation to brain magnetic resonance imaging findings in older persons without dementia. American Journal of Clinical Nutrition. 2004;80(4):992–7. doi: 10.1093/ajcn/80.4.992. [DOI] [PubMed] [Google Scholar]
- 17.Mukamal KJ, Longstreth WT, Jr, Mittleman MA, Crum RM, Siscovick DS. Alcohol consumption and subclinical findings on magnetic resonance imaging of the brain in older adults: The Cardiovascular Health Study. Stroke. 2001;32(9):1939–46. doi: 10.1161/hs0901.095723. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Eigenbrodt M, Mosley T, et al. Alcohol Intake and Cerebral Abnormalities on Magnetic Resonance Imaging in a Community-Based Population of Middle-Aged Adults. Stroke. 2004;35:16–21. doi: 10.1161/01.STR.0000105929.88691.8E. [DOI] [PubMed] [Google Scholar]
- 19.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25(1):40–3. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 20.Taki Y, Kinomura S, Sato K, et al. Both Global Gray Matter Volume and Regional Gray Matter Volume Negatively Correlate with Lifetime Alcohol Intake in Non–Alcohol-Dependent Japanese Men: A Volumetric Analysis and a Voxel-Based Morphometry. Alcoholism, Clinical and Exper Research. 2006;30(6):1045–1050. doi: 10.1111/j.1530-0277.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 21.Anstey K, Jorm A, Reglade-Mesin R, et al. Weekly Alcohol Consumption, Brain atrophy and White Matter Hyperintensities in a Community-Based Sample Aged 60 to 64 years. Psychosomatic Medicine. 2006;68:778–785. doi: 10.1097/01.psy.0000237779.56500.af. [DOI] [PubMed] [Google Scholar]
- 22.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Carmelli D. Biobehavioral characteristics of nondemented older adults with subclinical brain atrophy. Neurology. 2000;54(11):2108–2114. doi: 10.1212/wnl.54.11.2108. [DOI] [PubMed] [Google Scholar]
- 23.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BEK, Liao DP, Hubbard LD, Mosley TH. Cerebral white matter lesions, retinopathy, and incident clinical stroke. Journal of the American Medical Association. 2002;288(1):67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 24.Stockwell T, Donath S, Cooper-Stanbury M, Chikritzhs T, Catalano P, Mateo C. Under-reporting of alcohol consumption in household surveys: a comparison of quantity-frequency, graduated-frequency and recent recall. Addiction. 2004;99(8):1024–33. doi: 10.1111/j.1360-0443.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S. Modern Epidemiology. 2nd. Lippincott Williams and Wilkins; Philadelphia: 1998. [Google Scholar]