Abstract
Objective
To compare validity of a parsimonious frailty index (components: weight loss, inability to rise from a chair, and poor energy [SOF index]) with that of the more complex CHS index (components: unintentional weight loss, low grip strength, poor energy, slowness, and low physical activity) for prediction of adverse outcomes in older men.
Design
Prospective cohort study
Setting
Six U.S. centers
Participants
3132 men ≥67 years
Measurements
Men classified as robust, intermediate stage or frail using SOF index and criteria similar to those used in CHS index. Falls reported tri-annually for 1 year. Disability (≥1 new impairment in performing IADL) ascertained at 1 year. Fractures and deaths ascertained during 3 years of follow-up. Area under the curve (AUC) statistics from receiver operating characteristic curve analysis compared for models containing SOF index versus CHS index.
Results
Greater evidence of frailty as defined by either index was associated with increased risks of adverse outcomes. Frail men had a higher age-adjusted risk of recurrent falls (odds ratio [OR] 3.0–3.6), disability (OR 5.3–7.5), nonspine fracture (hazards ratio [HR] 2.2–2.3), and death (HR 2.5–3.5) (P<0.001 for all models). AUC comparisons revealed no differences between models with SOF index versus models with CHS index in discriminating falls (AUC=0.63, P= 0.97), disability (AUC=0.68, P=0.86), nonspine fracture (AUC=0.63, P=0.90), or death (AUC=0.71 for model with SOF index and 0.72 for model with CHS index, P=0.19).
Conclusion
The simple SOF index predicts risk of falls, disability, fracture and mortality in men as well as the more complex CHS index.
Keywords: frailty, older men, hip fracture, mortality
INTRODUCTION
Frailty, a term in clinical geriatric medicine that typically describes the presence of multisystem impairment and expanding vulnerability, has not yet emerged as a discrete clinical syndrome with a consensus definition.1 In an attempt to standardize and operationalize the definition of frailty, Fried and colleagues systematically surveyed geriatricians and concluded that a critical mass of impairments added up to the phenotype of frailty.2 Using data from the Cardiovascular Health Study (CHS), they proposed a phenotype (CHS index) in which 3 or more of the following 5 components were present: unintentional weight loss, self-reported poor energy, weakness (reduced grip strength), slow gait speed and low physical activity. Frailty as defined by the CHS index was associated with an increased risk of falls, hospitalization, disability and mortality in older people.3 Subsequently, the predictive validity of the CHS index has been confirmed in other cohorts.4–8 It has been proposed that the CHS index be used to screen older persons for frailty.3
However, assessment of frailty using the CHS index is unrealistic in the clinical setting for several reasons. Identification of three of its components (grip strength, walking speed, physical activity) requires knowledge of the underlying distribution of the measure in a given population and also depends on gender and/or body size. Criteria including physical activity, timed walks, and grip strength are often not feasible to evaluate in the clinic. Unintentional weight loss is a component of the CHS frailty phenotype, but reporting of intention to lose weight is not straightforward as knowing the direction of weight change may bias patient responses to a question about intention. In addition, both intentional and unintentional weight loss have been associated with an increased risk of disease in older people.9,10
Based on the physiologic domains most frequently cited in the frailty literature,11,12 results from previous studies evaluating the predictive validity of individual components,9,10,13–17 and suitability of assessment of components in a clinical practice setting, we proposed a simple frailty index using 3 components (weight loss, inability to rise from a chair five times without using the arms, and poor energy [SOF index]).18 We previously reported that this parsimonious SOF index predicted risk of adverse outcomes as well as the more complex CHS index in older women.18 To test the hypothesis that the SOF and CHS indexes have similar predictive validity in older men, we used data collected in the Osteoporotic Fractures in Men (MrOS) Study to compare the value of the SOF index with that of the CHS index for prediction of falls, disability, fracture, and death in a cohort of 3132 community-dwelling men 67 years and older.
METHODS
Participants
From March 2000 through April 2002, 5,995 men who were at least 65 years of age were recruited for participation in the baseline examination of MrOS.19 Men were recruited from population based listings in six regions of the United States.20 Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded from the study.
From December 2003 through March 2005, MrOS participants were invited to participate in an ancillary study to identify outcomes of sleep disorders in older men (MrOS Sleep study). Of the 5995 men enrolled in the overall study, 3135 (>100% of goal of 3000) completed the MrOS Sleep (2nd) examination. Of these, a total of 3132 men provided data for frailty components and are the subject of this analysis. The Institutional Review Board (IRB) at each center approved the study protocol and written informed consent was obtained from all subjects.
Measurements
Participants completed a questionnaire and were interviewed at the 2nd examination. A selected medical history was obtained. Participants were asked to bring all current medications with them to the 2nd examination for verification of use. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).21 Depressive symptoms including the question, “Do you feel full of energy?”, were evaluated using the 15-item Geriatric Depression Scale. Cognitive function was assessed with the Teng Modified Mini-Mental State Exam (3MS).22 To assess functional disability, men were asked whether they had any difficulty performing any of five instrumental activities of daily living (IADL). Tests of physical function included grip strength (using a hand-held Jamar dynamometer), walk speed (time in seconds to walk 6 meters at usual pace expressed as m/sec), and inability to rise from a chair (without using his arms) five times. Body weight and height measurements were used to calculate a standard body mass index (BMI). Bone mineral density of the hip was measured using dual energy x-ray absorptiometry (QDR 4500, Hologic, Waltham, MA).
SOF Frailty Index
Frailty defined by the SOF index18 was identified by the presence of ≥2 of the following 3 components at the 2nd examination:
1. Weight loss (irrespective of intention to lose weight) of ≥5% between the baseline and 2nd examination (mean years between examinations 3.4 (± 0.5)),
2. Inability to rise from a chair five times without using the arms, and
3. Poor energy as identified by an answer of “no” to the question “Do you feel full of energy?” on the Geriatric Depression Scale.
Men with none of the above components were considered to be robust and those with 1 component were considered to be in an intermediate stage.
CHS Frailty Index
Frailty defined by the CHS index as proposed by Fried and colleagues3 was identified by the presence of ≥3 of the following 5 components at the 2nd examination:
1. Shrinking as identified by an unintentional weight loss of ≥5% between the baseline and 2nd examination (mean years between examinations 3.4 (± 0.5)),
2. Weakness as identified by a maximal grip strength in the lowest quintile stratified by body mass index quartile,
3. Poor energy as identified by an answer of “no” to the question “Do you feel full of energy?” from the Geriatric Depression Scale (GDS),
4. Slowness as identified by an average walk speed in the lowest quintile stratified by median standing height, and
5. Low physical activity level as identified by a PASE score in the lowest quintile. Measurements used to define the components of shrinking, poor energy and low physical activity were similar, but not identical, to those used in the original phenotype proposed by Fried and colleagues.3 Men with none of the above components were considered to be robust and those with 1 or 2 components were considered to be in an intermediate stage.
Ascertainment of Falls, Disability, Fractures and Mortality
After the 2nd examination, participants were contacted about about falls and fractures every 4 months. Over 99% of these follow-up contacts were completed. All falls reported on the first 3 postcards (covering approximately 1 year) returned after the 2nd examination were included in the falls analyses (average follow-up 9.9 (± 1.3) months). Functional status was assessed at the 2nd examination and an average of 1.2 years later at the 3rd examination; incident disability was defined as ≥1 new IADL impairment. Fractures were confirmed by review of radiographic reports. Average follow-up for fracture was 3.1 (± 0.7) years. Deaths were confirmed with death certificates. Follow-up for vital status was 99% complete. Average follow-up for death was 3.2 (± 0.5) years.
Statistical Analysis
We used logistic regression to analyze the association between frailty status as defined by SOF and CHS indexes and the odds of recurrent falls (≥2 falls vs. ≤1 fall) in the subsequent year and odds of disability after an average of 1.2 years of follow-up. Cox proportional hazards models were used to analyze the associations between frailty status as defined by SOF and CHS indices and subsequent outcomes including any incident nonspine fracture and death. The relative risk (approximated as hazard ratios or odds ratios) of each outcome with 95% confidence intervals was estimated for men categorized as intermediate and those categorized as frail using men categorized as robust as the referent group. We also utilized logistic regression to examine receiver operating characteristic (ROC) curves for each model and calculated area under the curve (AUC). All primary analyses were adjusted for age alone. Secondary analyses were adjusted for additional factors previously identified in the cohort to predict the outcomes.
A bootstrap procedure23 was used to compare area under the curve (AUC) statistics from logistic regression ROC curve analysis and to compare −2 log likelihood (−2LL) model fit statistics for logistic regression or proportional hazards models. The full study population was sampled (with replacement) 1000 times. Each bootstrap sample was fit to the two models being compared and the difference between the −2LL statistics and between AUC statistics was calculated. The distribution of these differences was used to make statistical inference about the likelihood that the statistics from the two models were significantly different (P<0.05). Findings regarding the comparison of observed AUC statistics between models were cross-validated using the 10-fold cross validation procedure (results not shown).
RESULTS
Characteristics of the Study Population
Characteristics of the cohort of 3132 men (average age 76.4 years) are shown in Table 1. Using the CHS index, 14% were classified as frail (≥3 components), 54% as intermediate (1–2 components) and 32% as robust (no components). Using the SOF index, 13% of men were classified as frail (≥2 components), 43% as intermediate (1 component) and 44% as robust (no components). Classification of frailty status using the indices was concordant in 2221 (71%) men. The Kappa statistic was 0.59. The Spearman correlation between indices was 0.70 (P<0.001).
Table 1.
Characteristics of 3132 Participants
| Variable | |
|---|---|
| Age, years, mean (± SD) | 76.4 (± 5.6) |
| Frailty status by CHS index, n (%) | |
| Robust | 1007 (32) |
| Intermediate | 1688 (54) |
| Frail | 437 (14) |
| Frailty status by SOF index, n (%) | |
| Robust | 1379 (44) |
| Intermediate | 1336 (43) |
| Frail | 417 (13) |
| Individual CHS index components | |
| Unintentional weight loss*, n (%) | 363 (12) |
| Weakness, n (%) | 754 (24) |
| Poor energy, n (%) | 1401 (45) |
| Slowness, n (%) | 624 (20) |
| Low physical activity level, n (%) | 625 (20) |
| Individual SOF index components | |
| Weight loss†, n (%) | 613 (20) |
| Inability to rise from chair‡, n (%) | 206 (7) |
| Poor energy, n (%) | 1401 (45) |
| ≥2 falls during 1st year, n (%) | 441 (14) |
| ≥1 new IADL impairment§, n (%) | 359 (12) |
| ≥1 incident nonspine fracture, n (%) | 169 (5) |
| Deaths, n (%) | 204 (7) |
Unintentional weight loss of 5% or more during the 3.4 years prior the exam
Weight loss of 5% or more during the 3.4 years prior to the exam
Inability to rise from a chair five times without using the arms
A total of 2891 men completed initial and follow-up IADL assessment
Abbreviations: CHS, Cardiovascular Health Study; SOF, Study of Osteoporotic Fractures; IADL, instrumental activities of daily living
During an average follow-up of 9.9 months, 441 (14%) men experienced ≥2 (recurrent) falls. An average of 1.2 years after the frailty assessment, 359(12%) men reported ≥1 new IADL impairment among the 2891 men with IADL data at both examinations. During an average follow-up of 3.1 (fracture) to 3.2 (death) years, 169 (5.4%) men experienced ≥1 nonspine fracture and 204 (7%) died.
SOF Index vs. CHS Index for Prediction of Falls, Disability, Fractures and Mortality
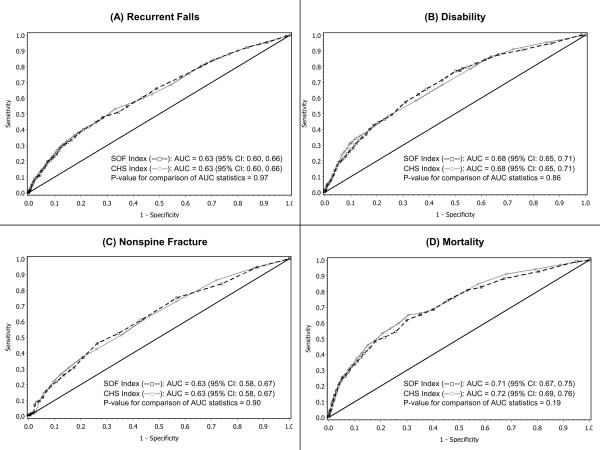
Greater evidence of frailty as identified using the SOF or CHS index was similarly associated with a higher odds of ≥2 (recurrent) falls in the subsequent year (Table 2). Compared with robust men, men in the intermediate group had a 1.6 -fold age-adjusted increase in risk (P<0.001 for both models) and frail men had a 3.0 to 3.6-fold increase in risk (P<0.001 for both models). ROC curves were essentially superimposed for the model containing the SOF index vs. the model containing the CHS index across a range of sensitivities and specificities, (Figure 1A). Using either index, AUC statistics were essentially identical (P=0.97 for comparison of AUC statistics).
Table 2.
Association between Frailty Indexes and Risk of Adverse Outcomes*
| Index of Frailty | Recurrent Falls† |
|
|---|---|---|
| No. with ≥2 falls (%) | Odds Ratio (95% CI) | |
| CHS | ||
| Robust (n=1006) | 86 (9) | 1.0 (referent) |
| Intermediate (n=1680) | 233 (14) | 1.62 (1.24–2.11) |
| Frail (n=432) | 122 (28) | 3.56 (2.58–4.93) |
| SOF | ||
| Robust (n=1378) | 133 (10) | 1.0 (referent) |
| Intermediate (n=1329) | 197 (15) | 1.56 (1.23–1.97) |
| Frail (n=411) | 111 (27) | 3.03 (2.27–4.05) |
| Disability‡ |
||
|---|---|---|
| No. with ≥1 new IADL impairment (%) | Odds Ratio (95% CI) | |
| CHS | ||
| Robust (n=968) | 49 (5) | 1.0 (referent) |
| Intermediate (n=1562) | 198 (13) | 2.61 (1.89–3.62) |
| Frail (n=361) | 112 (31) | 7.52 (5.14–11.02) |
| SOF | ||
| Robust (n=1314) | 82 (6) | 1.0 (referent) |
| Intermediate (n=1230) | 179 (15) | 2.47 (1.87–3.25) |
| Frail (n=347) | 98 (28) | 5.28 (3.80–7.33) |
| Nonspine Fracture§ |
|||
|---|---|---|---|
| No. with ≥ 1 fracture (%) | Age-adjusted rate per 1000 person-years | Hazard Ratio (95% CI)† | |
| CHS | |||
| Robust (n=1005) | 36 (4) | 12.4 | 1.0 (referent) |
| Intermediate (n=1674) | 90 (5) | 19.2 | 1.39 (0.94–2.06) |
| Frail (n=431) | 43 (10) | 31.7 | 2.30 (1.43–3.71) |
| SOF | |||
| Robust (n=1373) | 56 (4) | 14.5 | 1.0 (referent) |
| Intermediate (n=1327) | 73 (6) | 17.9 | 1.30 (0.91–1.84) |
| Frail (n=410) | 40 (10) | 33.1 | 2.15 (1.41–3.26) |
| Mortality∥ |
|||
|---|---|---|---|
| No. of deaths (%) | Age-adjusted rate per 1000 person-years | Hazard Ratio (95% CI)† | |
| CHS | |||
| Robust (n=1001) | 29 (3) | 10.4 | 1.0 (referent) |
| Intermediate (n=1674) | 104 (6) | 21.0 | 1.77 (1.17–2.68) |
| Frail (n=429) | 71 (17) | 40.0 | 3.51 (2.21–5.57) |
| SOF | |||
| Robust (n=1368) | 59 (4) | 14.8 | 1.0 (referent) |
| Intermediate (n=1324) | 84 (6) | 19.6 | 1.31 (0.94–1.83) |
| Frail (n=412) | 61 (15) | 37.8 | 2.53 (1.75–3.66) |
Adjusted for age
Note: Among the 3132 men with data for both frailty indices
14 who did not provide fall information at follow-up contacts during the subsequent year were excluded from this analysis
241 who did not provide IADL information at 1.2 years of follow-up were excluded from this analysis, including 56 men who had died (n=52) or terminated study participation (n=4) in the interim period, 173 who did not provide IADL data at the follow-up exam, and 12 who were unable to attend the follow-up exam
22 men with incident clinical vertebral fracture were excluded from this analysis
28 men whose reported deaths were in the adjudication process were excluded from this analysis
Abbreviations: CHS, Cardiovascular Health Study; SOF, Study of Osteoporotic Fractures; No., number; IADL, instrumental activities of daily living
Figure 1. Age-Adjusted Receiver Operating Characteristic (ROC) Curves for Prediction of (A) Recurrent Falls, (B) Disability, (C) Nonspine Fracture, and (D) Mortality with SOF and CHS Frailty Indices*.
*The black diagonal line indicates a reference AUC of 0.50 (no better than chance alone)
The odds of incident disability (≥1 new IADL impairment) were higher with increasing evidence of frailty using either the SOF or CHS index (Table 2). Compared with robust men, men in the intermediate group had an age-adjusted 2.5 to 2.6-fold increase in risk (P<0.001 for both models) and frail men had a 5.3 to 7.5-fold increase in risk (P<0.001 for both models). While the point estimate of the association between frailty and odds of new disability was higher in magnitude for the model with the CHS index, there was no difference between AUC statistics (P=0.86) (Figure 1B).
Frailty as identified using either the SOF or CHS index was also similarly related to an increased risk of incident nonspine fracture (Table 2). After adjustment for age, men in the intermediate stage appeared to have a 1.3 to 1.4-fold increase in risk that did not reach the level of significance (P=0.15 for model with SOF index and 0.10 for model with CHS index) and frail men had a 2.2 to 2.3-fold increase in risk (P<0.001 for both models). Models containing the SOF index performed similarly to those containing the CHS index in ROC analyses (Figure 1C, P=0.90 for comparison of AUC statistics).
All-cause mortality rates were higher with increasing evidence of frailty as identified using either the SOF or CHS index (Table 2). Using the model with the SOF index, men in the intermediate group compared with robust men had an age-adjusted 1.3-fold increase in risk of death that did not reach significance (P=0.11), while frail men had a significant 2.5-fold increase in risk (P<0.001). Point estimates of the magnitude of these associations were higher for the model with the CHS index. Compared with robust men, men in the intermediate stage had a 1.8-fold increase in risk of death (P=0.007) and frail men had a 3.5-fold increase in risk (P<0.001). However, there was no difference between AUC statistics (P=0.19) for the models (Figure 1D).
Results were similar when −2LL statistics were compared between models with the SOF index and those with the CHS index; p-values for these comparisons were 0.72 (falls), 0.42 (disability), 0.99 (non-spine fractures), and 0.43 (mortality).
For each outcome, the addition of multiple covariates (including race; living situation; health status; smoking; education; statin use; number of selected medical conditions including prior fracture, arthritis, hypo/hyperthyroidism, temporary ischemic attack or stroke, diabetes mellitus, hypertension, coronary or myocardial infarction, angina, congestive heart failure, chronic obstructive lung disease, chronic kidney disease or kidney failure, intermittent claudication, liver disease, parkinsonism, and non-skin cancer; fall history [models for fracture and mortality]; depressive symptoms; cognitive function; functional disability; body mass index; and femoral neck bone mineral density) to the age-adjusted model resulted in a small to moderate improvement in the predictive validity of the model. However, there was no difference in multivariable models with the SOF index compared to those with the CHS index in discriminating falls (AUC=0.68 for both models, P=0.84), disability (AUC=0.74 [SOF index] vs. 0.75 [CHS index], P=0.36), nonspine fracture (AUC=0.69 [SOF index] vs. 0.68 [CHS index], P=0.79), or death (AUC=0.75 for both models, P=0.65).
DISCUSSION
Measurement of frailty status using the simple SOF index based on three components (weight loss, inability to rise from a chair and poor energy) and evaluation of frailty using the more complex CHS index performed similarly in predicting falls, disability, fracture and mortality in this cohort of community-dwelling older men. In context of previously reported results in older women,18 these findings suggest that the parsimonious SOF index provides a useful operationalization of frailty to use in clinical practice to identify high-risk older people.
There is a widely held view that clinicians can easily determine whether an older patient is “frail” or not. However, there is no consensus definition or official International Classification of Diseases (ICD) diagnosis for frailty. While several instruments have been developed to operationalize the construct of frailty,11 including indexes based on clinical judgment,24 deficit accumulation,25,26 and comprehensive geriatric assessment,27 the CHS index3 has been most extensively studied. The validity of the CHS index in predicting risk of adverse outcomes has been confirmed in several cohorts of older adults.4–8 The CHS index has also been linked to specific alterations in physiologic and biological pathways that may lead to multisystem impairment underlying the development of the clinical frailty syndrome.28 It has been suggested that the CHS index might serve as the basis for screening older persons for frailty and risk of frailty3 and be integrated into the comprehensive care for older women.29
Classification of frailty status using the CHS and SOF indexes was concordant in 71% of participants. Thus, the SOF and CHS indexes similarly discriminate between groups of older men in the categorization of frailty status. In addition, our findings confirm the value of the CHS index in screening older men for risk of falls, disability, fractures and mortality, with an AUC as high as 0.72 for death in age-adjusted models. However, similar to our previously published results in older women,18 we did not find evidence that the prediction of these events was improved using the more complicated CHS index as compared with using the straightforward SOF index that was parsimonious without significant losses in its discriminative ability. Like the CHS index, the three criteria of the SOF index reflect impairment in one or more physiological domains most frequently cited in frailty literature.11,12 However, unlike the CHS index, the SOF criteria are not dependent on gender, body size, underlying distribution of the components in a population, or ability to determine intention to lose weight. In addition, SOF index components are easily evaluated and inexpensively obtained in a few minutes in the clinical setting.
This study has several strengths, including its prospective design, comprehensive set of measurements, and completeness of follow-up. However, this study has a number of limitations. Participants were older men living in the community and our findings may not be applicable to other population groups. The measures used to define three components of the CHS index in this analysis were similar, but not identical to those used in the original definition proposed by Fried and colleagues.3 While prediction of all-cause mortality is of essential prognostic importance in aged populations and disability, falls and fractures are a common cause of morbidity in older adults, future research should evaluate the ability of the SOF index to predict other outcomes, including hospitalization and institutionalization. These results indicate that both the CHS and SOF indexes are limited in their ability to discriminate falls and fracture. However, it should be noted that the validity of these indexes in predicting nonspine fracture was similar to that reported for other tools based on the use of clinical risk factors alone.30 Ascertainment of outcomes in this study was limited to one to three years, but our prior study in older women18 suggested that the findings regarding the predictive validity of the SOF versus CHS index persisted with longer follow-up. The objective of this analysis was to compare the ability of the SOF index to that of the CHS index in predicting adverse outcomes. Future investigations are needed to determine whether the SOF index is useful in the longitudinal characterization of change in frailty status over time. Finally, while we compared the predictive ability of the SOF index with that of the widely referenced CHS index, several indexes of frailty have been proposed and our findings are not generalizable across different frailty instruments.
The parsimonious SOF index provides an operational definition of frailty that predicts falls, disability, fracture and mortality as well as the more complicated CHS index. Thus, the SOF index provides a useful phenotype of frailty to identify high-risk older people in clinical practice.
ACKNOWLEDGMENTS
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Cancer Institute (NCI), the National Center for Research Resources (NCRR) and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140.
Conflict of Interest Disclosures:
| Elements of Financial/Personal Conflicts | *Author 1 KE Ensrud | Author 2 SK Ewing | Author 3 PM Cawthon | Author 4 HA Fink | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
For “yes” x mark(s): give brief explanation below:
Dr. Ensrud has received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page
| Elements of Financial/Personal Conflicts | *Author 5 BC Taylor | Author 6 JA Cauley | Author 7 T Dam | Author 8 LM Marshall | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
For “yes” x mark(s): give brief explanation below:
Dr. Cauley has received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page
| Elements of Financial/Personal Conflicts | Author 9 ES Orwoll | Author 10 SR Cummings | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | ||||||
| Grants/Funds | X | X | ||||||
| Honoraria | X | X | ||||||
| Speaker Forum | X | X | ||||||
| Consultant | X | X | ||||||
| Stocks | X | X | ||||||
| Royalties | X | X | ||||||
| Expert Testimony | X | X | ||||||
| Board Member | X | X | ||||||
| Patents | X | X | ||||||
| Personal Relationship | X | X | ||||||
For “yes” x mark(s): give brief explanation below:
Dr. Orwoll has received grant support from the NIH (and supporting agencies) grant as listed under Funding Sources on the title page
Other Contributions: We would like to thank Mr. Kyle A. Moen for his assistance with the manuscript and preparation and formatting of the tables and figure.
Footnotes
Statistical Analysis: Ms. Susan Ewing performed the statistical analyses and is independent of any commercial funder. She had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
Sponsor's Role: The funding agencies had no direct role in the conduct of the study; the collection, management, analyses and interpretation of the data; or preparation or approval of the manuscript.
REFERENCES
- 1.Rockwood K. Frailty and its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 5.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 6.Boyd CM, Xue QL, Simpson CF, Guralnik JM, Fried LP. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 7.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon PM, Marshall LM, Michael Y, et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 9.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–1747. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 10.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr., Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 12.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 13.Mannen Cawthon P, Fullman RL, Marshall L, et al. Physical Performance and Risk of Hip Fractures in Older Men. J Bone Miner Res. 2008 doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 15.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1997;157:857–863. [PubMed] [Google Scholar]
- 16.Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1998;158:2129–2135. doi: 10.1001/archinte.158.19.2129. [DOI] [PubMed] [Google Scholar]
- 17.Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS. Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:484–490. doi: 10.1001/archinte.159.5.484. [DOI] [PubMed] [Google Scholar]
- 18.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 19.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Washburn RA, Ficker JL. Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 23.Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall; New York, NY: 1993. [Google Scholar]
- 24.Studenski S, Hayes RP, Leibowitz RQ, et al. Clinical Global Impression of Change in Physical Frailty: development of a measure based on clinical judgment. J Am Geriatr Soc. 2004;52:1560–1566. doi: 10.1111/j.1532-5415.2004.52423.x. [DOI] [PubMed] [Google Scholar]
- 25.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52:1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 28.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 29.Correa-de-Araujo R. An operational definition of frailty predicted death, hip fracture, and hospitalization in older women. ACP J Club. 2006;144:23. [PubMed] [Google Scholar]
- 30.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]