Abstract
Congenital transmission (CT) has acquired relevance in Chagas disease (CHD). A cohort of pregnant CHD women (4,355) and their babies were studied in the period 1994–2004. Children were excluded when they had received blood transfusions, or were born or had been in endemic areas; CT rate was 6.1%. Babies were diagnosed between months 1 and 5 in 68.9% of the cases and between months 6 and 12 in 31.1%. In the latter group, parasitemia was detected in 94% and serology in 74.7%. Between months 6 and 9, parasitemia diagnosed 36.2% (P = 0.000) more cases than serology. If serology had been the diagnosis method, those children would have been considered CT free. Taking the overall outcomes, 38.1% of babies were CT free, and 55.8% did not complete the follow-up. Establishing CT as a public health priority and improving first-line health service, congenital CHD coverage could be more efficient in endemic countries.
Introduction
In the early years, on the discovery of Chagas disease (CHD), the risk of congenital transmission (CT) was considered one of the potential transmission routes of Trypanosoma cruzi infection. However, as recently as the 1970s, substantial changes were introduced thanks to progress in the physiopathology and treatment of this particular T. cruzi transmission route. Since 1991, the Initiative for the Southern Cone, a regional control program for CHD, has encouraged elimination of the vector Triatoma infestans and control of T. cruzi transmission through blood banks.1
In the countries of Latin America, this initiative has enabled the implementation of active and sustainable actions in relevant areas for CHD vectorial control, allowing the present reinforcement of the CT strategy and the network of clinical diagnosis and treatment of CHD patients.2
Recently, two meetings organized in Bolivia and Uruguay have emphasized the relevance of congenital CHD in the panorama of the disease and the need to control it in endemic countries.3,4 However, in countries such as Spain, Switzerland, and the United States, congenital T. cruzi infection at present constitutes a public health issue caused by people migration from the endemic CHD areas.5–9
Between 1993 and 2000, the Argentinean Program for Pregnant Women Control studied 245,583 women from different endemic provinces, and the serological prevalence was estimated to be 11.8% during the period 1994–1995; 9.1% in 1996–1997, and 6.8% in 2000, whereas the estimated incidence of CT was 1.9% in the 1970s (range 0.1–3.5) and 2.5% (range 0.7–10.4) in the 1980s (Blanco S and others, Programa Nacional de Chagas. Coordinación de Vectores 2001, National Chagas Program, Vector Coordination, unpublished data).10,11
This study was developed in the Instituto Nacional de Parasitología “Dr Mario Fatala Chaben” (National Parasitology Institute of Argentina), an urban reference health care center located in the main non-endemic area of Argentina, that sees around 800 pregnant women who are referred for CHD diagnosis confirmation per year. The purpose of this work is to evaluate certain aspects relative to the efficacy in the application of a CT strategy in our institute during the 1994–2004 period. Strengths and weaknesses of the strategy related to the control of congenital T. cruzi infection were evaluated.
Material and Methods
Population.
A total of 4,355 CHD pregnant women and their newborns from different public and private centers of the City of Buenos Aires and Greater Buenos Aires were referred to the Instituto Nacional de Parasitología “Dr Mario Fatala Chaben,” to diagnose potential CT during the 1994–2004 period. The serological prevalence of CHD pregnant women was evaluated in a group of 6,204 pregnant women of the whole population referred to our institute in the sub-period between October 1997 and December 2004. Inclusion criteria for pregnant women in the congenital CHD protocol was serological reactivity for CHD in at least two serological tests, such as indirect immunofluorescence assay (IFA), enzyme-linked immunosorbent assay (ELISA), and indirect hemagglutination (IHA) standardized in our institute.12–15 Babies born to CHD mothers met the inclusion criterion in the protocol. Children were excluded when they had received blood transfusions, or were born or had been in transit in endemic areas.
Study design.
A follow-up strategy for CT was designed and implemented in the 1994–2004 period, where a cohort of CHD mothers and their children were prospectively enrolled. Initially, the data were recorded in especially designed manual forms, and from 1997 they were captured in a database. The variables used were age, gender, present location, blood transfusions, occupation, medical history of CHD mother, proportions of nulliparous/mulltiparous women in transmitting and non-transmitting mothers, other previous or present diseases, and number of children in the family. Recorded data about newborns included type of delivery, weight at birth, and whether the babies were symptomatic or asymptomatic at birth.
The diagnosis protocol of congenital CHD included three controls: in the period between birth and 30 days, at 6 and 12 months. The CT monitoring was considered complete when the diagnosis of the congenital T. cruzi infection was detected at any point in the first 12 months, or in those babies confirmed as non-infected at the end of follow-up, i.e., 12 months of age. The CT monitoring was considered incomplete when the second control before month 10 was negative by parasitological and serological methods, and the absence of a third control after month 10 did not allow the children's medical discharge from the protocol.
Case definition.
A child was considered to have congenital T. cruzi infection when parasitemia was positive at any time of the follow-up, or serology was reactive above the cut-off value in two serological tests in children between 6 and 12 months of age. A child was considered to be free of CT when the serology for at least two tests was non-reactive as from the 10th month on.
Methods.
The parasitological diagnosis of CT was performed through a “micromethod” (De Rissio AM and others, unpublished data) developed and validated in relation to the xenodiagnosis.16 Briefly, 0.5 mL of blood was collected by venopuncture in an eppendorf tube with a drop of heparin, centrifuged at 3,000 rpm during 1 minute. The buffy coat between the sera and the blood cells was used in at least four smears with coverslips of 22 × 22 mm in size, and a reading was obtained at 400×.
Serological diagnosis was performed with ELISA, IHA, and IFA, with “in-house” antigens, compliant with domestic and international rules. The parasite antigens used in those methods vary widely, from whole cells for IFA to crude cell extracts for IHA and ELISA. The sensitivity of the tests varies between 99.0% and 99.8%. When two or three tests were performed simultaneously, the sensitivity ranges from 99.7% to 100% and the specificity from 97.4% to 97.9%.12–15 Titers equal or higher than 0.200 of optical density at 490 nm for ELISA, and 1/32 for IHA and IFA were considered reactive for T. cruzi infection.
To determine persistence time of anti-T. cruzi antibodies transmitted by the CHD mothers, a subgroup of 1,052 CT-free children at the 12-month follow-up was selected using a systematic method. The evaluation point was at 6 months by IHA, ELISA, and IFA tests.
Statistical analysis.
Fisher's exact test was applied to evaluate differences between proportions. Confidence intervals (CI) were calculated, and P was considered significant at least at 0.05. Data were expressed as mean ± SD. To evaluate consistency between diagnosis methods, we calculated sensitivity and specificity, positive and negative predictive values, and the Kappa concordance index with its CI.
Bioethical criteria.
The CHD mothers were invited to be included in the protocol of congenital T. cruzi infection, allowing the follow-up of their children. Informed consent forms were signed by all mothers after detailed interviews. The infected mothers group was given descriptive and explanatory material on CHD, and the value of monitoring and the recovery of their potentially infected children by means of specific treatment were explained in detail. All children with congenital T. cruzi infection were treated with benznidazole. The Bioethical Committee of the ANLIS CG Malbran approved the study and the informed consent form.
Results
Within the framework of the longitudinal prospective study, the serological prevalence in the group of CHD mothers was evaluated in our institution between October 1997 and December 2004 (sub-period in which that data were recorded in a database). It was 49.5 ± 5.1% (3,068/6,204), as shown in Figure 1, ranging between 47.4% in 1998 and 57.2% in 2003.
Figure 1.
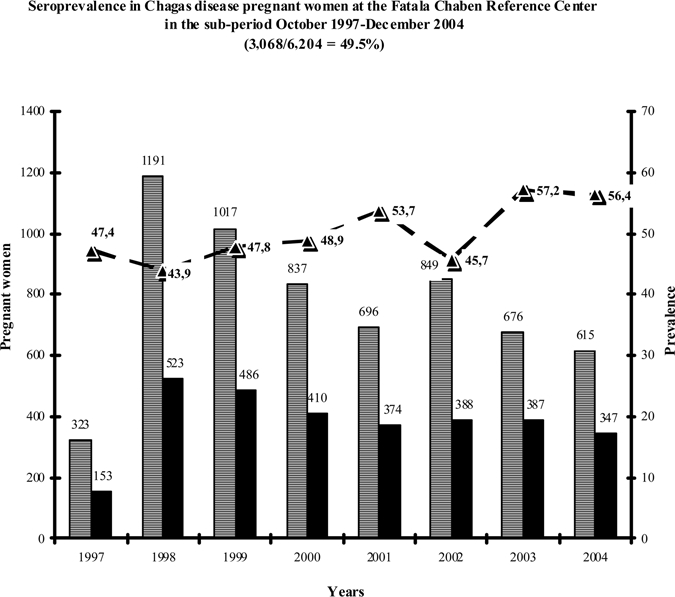
 Total number of mothers referred per year. ■ Chagas disease mothers per year. –▲– Rate of serological prevalence. The serological prevalence was 49.5 ± 5.1% (mean ± SD) in the sub-period 1997–2004.
Total number of mothers referred per year. ■ Chagas disease mothers per year. –▲– Rate of serological prevalence. The serological prevalence was 49.5 ± 5.1% (mean ± SD) in the sub-period 1997–2004.
Specifically regarding the CT, 4,355 mothers and their children were evaluated in the 1994–2004 period and 265 were CT transmitting mothers (two of them had twins).
The distribution of pregnant CHD women whose babies were diagnosed with congenital T. cruzi infection by place of origin is shown in Figure 2. Pregnant CHD women from different provinces and different endemicity areas in Argentina amounted to 41.8% (111/265). It is interesting to point out that 26% of the mothers were born in urban, non-endemic areas: 7.9% were from the City of Buenos Aires (21/265), and 18.1% (48/265) from Greater Buenos Aires. Chaco, a highly endemic Argentinian province, contributed 8%. The highest contributor to CT was Bolivia, a neighboring country, whose CHD mothers were 27.1% (72/265). At the time of this study, all pregnant CHD mothers were living in urban, non-endemic areas.
Figure 2.
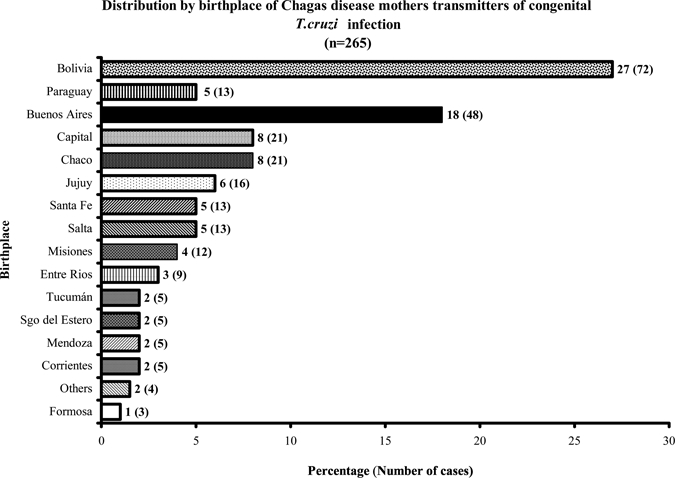
Twenty-seven percent of Chagas disease mothers were from Bolivia, a neighboring country; 26% of mothers were from the main urban areas of Argentina, City of Buenos Aires and Greater Buenos Aires. The rest were from the different endemic Argentinian provinces.
The age distribution of the 265 CHD mothers was 76.6% of the mothers were between the ages of 21 and 35 (203/265), whereas 13.2% (35/265) were young mothers, between the ages of 15 and 20, and 10.2% (27/265) were mature mothers, between 36 and 45 years of age. In connection with the age of transmitting and non-transmitting mothers, there were no significant differences between both populations. In relation to CT, 267 babies were diagnosed with congenital T. cruzi infection in the period studied. The overall CT rate was 6.1 ± 1.1%, with the lowest rate in 1996 (4.3%) and the highest one in 1999 (7.7%). The distribution of these values is shown in Figure 3. There was no difference between nulliparous/multiparous women in connection with transmitting and non-transmitting mothers.
Figure 3.
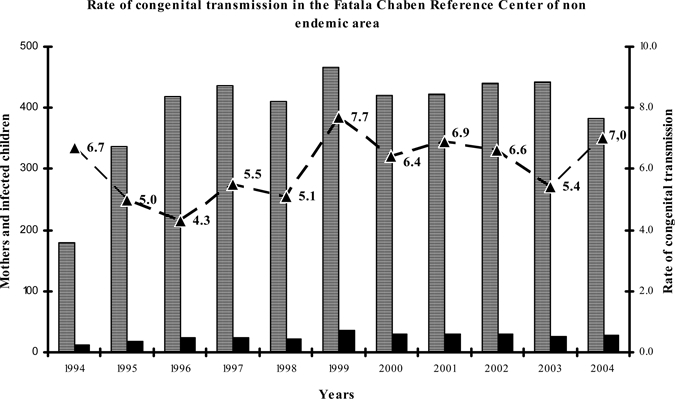
 Number of Chagas disease mothers. ■ Children with congenital Trypanosoma cruzi infection. –▲–Rate of congenital transmission. Overall congenital transmission was 6.1 ± 1.1% (mean ± SD).
Number of Chagas disease mothers. ■ Children with congenital Trypanosoma cruzi infection. –▲–Rate of congenital transmission. Overall congenital transmission was 6.1 ± 1.1% (mean ± SD).
The gender distribution of the 267 babies was 46.1% male (123/267), and 53.9% (144/267) female.
Regarding the weight of the congenitally infected T. cruzi newborns, it was normal in 87%; 9% of the children had low weight at birth, and 4% of them had very low weight. All babies were asymptomatic; no symptoms of congenital CHD were detected.
Table 1 shows the persistence of maternal anti-T. cruzi antibodies at 6 months of age in 1,052 children who were CT free after the complete follow-up. The data show that the pair ELISA/IHA was non-reactive; the pair ELISA/IFA was reactive in 0.3% of children; IHA/IFA in 0.3%, and 5.9% (62/1,052) of the babies were reactive for only one test, usually IFA. A total of 6.5% (68/1,052) of non-infected children were serologically reactive for serological pairs or for one test. In all positive cases, the values found were a maximum of two titers above cut-off level.
Table 1.
Behavior of maternal antibodies to the 6th month of age in children born to Chagas disease mothers without congenital transmission*
| Years | Number of babies | ELISA- IHA | ELISA- IFA | IHA- IFA | Discordant serology† | Total | % |
|---|---|---|---|---|---|---|---|
| 1999 | 240 | 0 | 1 | 0 | 10 | 11 | 4.6 |
| 2000 | 182 | 0 | 1 | 1 | 11 | 13 | 7.1 |
| 2001 | 200 | 0 | 0 | 0 | 12 | 12 | 2.0 |
| 2002 | 200 | 0 | 1 | 2 | 15 | 18 | 9.0 |
| 2003 | 230 | 0 | 0 | 0 | 14 | 14 | 6.0 |
| Total | 1,052 | 0 | 3 | 3 | 62 | 68 | 6.5 |
| % | 100 | 0 | 0.3 | 0.3 | 5.9 |
Diagnosis of maternal anti-Typanosoma cruzi antibodies up to 6 months of children's age. Numbers indicate serological reactivity by two tests or one test (discordant serology†), usually for indirect immunofluorescence assay (IFA). The 6.5% of children without congenital transmission had reactive or discordant serology up to the 6th month of age.
To determine the sensitivity and specificity of the micromethod, only children with complete follow-up, with or without positive CT diagnosis, were considered (N:1,924). The value of the micromethod in relation to xenodiagnosis is shown in Table 2. The sensitivity of the test was 0.938 (CI 95%, 0.925–0.940) and its specificity was 1 (0.998–1). The micromethod was able to detect at least 25 parasites/mL (De Rissio AM and others, unpublished data).
Table 2.
Contingency table: relationship between micromethod and xenodiagnosis*
| Micromethod | Xenodiagnosis | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 251 | 0 | 251 |
| Negative | 16 | 1657 | 1673 |
| Total | 267 | 1657 | 1924 |
The sensitivity of the micromethod vis-à-vis xenodiagnosis is 0.938 (confidence interval [CI] 95%, 0.925–0.940) and the specificity is 1 (CI 95%, 0.998–1). The positive predictive value was 0.998 (CI 95%, 0.984–1) and negative predictive value, 0.990 (CI 95%, 0.988–1). Kappa index: 96.4% (CI 95%, 0.9469–0.9817).
Furthermore, the positive predictive value was 0.998 (0.984–1) and the negative predictive value 0.990 (0.988–1), showing the good performance of the micromethod; the kappa index between both methods was 0.9643%, with very good concordance (CI 95%, 0.9469–0.9817).
The diagnosis age for the 267 babies is shown in Figure 4. During the first 5 months of life, diagnosis was performed in 68.9% of the children: 44.6% (119/267) of the newborns were diagnosed around the first month, and 24.3% (65/267) between months 2 and 5, whereas 31.1% (83/267) of the babies were diagnosed between the 6th and the 12th month. Before 6 months of age, the diagnosis was always performed by means of parasitemia using micromethod, and xenodiagnosis. After the 6th month, the diagnosis was based on parasitemia and serology. The diagnosis of babies with congenital T. cruzi infection between 6 to 12 months was compared by means of the relationship between the parasitemia and the serology against T. cruzi as shown in Table 3. Parasitemia was detected in 94% (78/83) of the babies, whereas in 6% (5/83) it was not detected. Out of 83 children, serology was reactive at least by two tests in 74.7% (62/83), negative in 20.5% (17/83), and discordant, usually by IFA, in 4.8% (4/83) of the babies. Between the 6th and 9th months, these 21 children with non-reactive and discordant serology had detectable T. cruzi parasitemia. The overall difference between the parasitemia and serology at 6 to 9 months was 36.2% (CI 95%, 23.8–48.6, P = 0.000). When the analysis was performed at specific months, for instance at 6 months, it was observed that parasitemia detected 38.7% (12 cases) more than serology (CI 95%, 21.6–55.9, P = 0.000); at 7 to 8 months, 25% (4 cases) more than serology (CI 95%, 3.8–46.2, P = 0.101); at 9 months, 45.5% (5 cases) more than serology (CI 95%, 16.0–74.9, P = 0.035). In this study, parasitemia usually showed more sensitivity than serology in babies who were congenitally infected with T. cruzi at 6 to 9 months of age. Parasitemia was able to detect 38.7%, 25%, and 45.5% more infected children than serology at months 6, 7–8, and 9, respectively. The relevance of these results is that if serology had been the only diagnosis criterion, those children would have been considered free of congenital T. cruzi infection in that critical period. At 10 to 12 months, serology always showed more sensitivity and was more significant in the diagnosis of CT than parasitemia.
Figure 4.
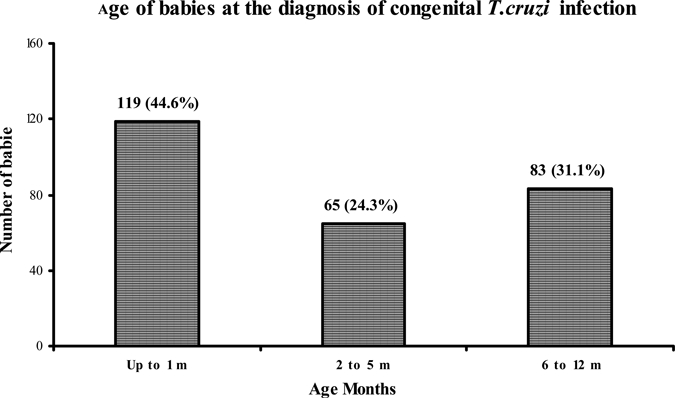
The 68.9% of babies were diagnosed with congenital Trypanosoma cruzi infection since the perinatal period up to the 5th month of life. The rest, 31.1%, were diagnosed during the second semester of life.
Table 3.
Relationship between parasitemia and serology in the period between 6 and 12 months of age congenitally infected babies by Trypanosoma cruzi*
| Age of babies (months) | Parasitemia | Serology | Total | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Reactive | No reactive | Discordant | ||
| 6 | 31 | 0 | 19 | 10 | 2 | 31† |
| 7 | 13 | 0 | 10 | 3 | 0 | 13‡ |
| 8 | 3 | 0 | 2 | 1 | 0 | 3‡ |
| 9 | 11 | 0 | 6 | 3 | 2 | 11§ |
| 10 | 1 | 2 | 3 | 0 | 0 | 3 |
| 11 | 4 | 0 | 4 | 0 | 0 | 4 |
| 12 | 15 | 3 | 18 | 0 | 0 | 18 |
| Total | 78 | 5 | 62 | 17 | 4 | 83 |
| % | 94 | 6 | 74.7 | 20.5 | 4.8 | 100 |
The overall difference between parasitemia and serology at 6 to 9 months was 36.2% (confidence interval [CI] 95%, 23.8–48.6, P = 0.000).
At 6 months, it was 38.7% (CI 95%, 21.6–55.9, P = 0.000).
At 7–8 months, 25% (CI 95%, 3.8–46.2, P = 0.101).
At 9 months, 45.5% (CI 95%, 16.0–74.9, P = 0.035).
When the overall outcome of the 4,355 babies born to CHD mothers was analyzed, as shown in Figure 5, the results observed were as follows: 267 cases (6.1%) were diagnosed as infected, 1,657 cases (38.1%) completed the monitoring and were considered to be free of congenital infection, and 2,431 (55.8%) were cases with incomplete monitoring and did not get an end diagnosis despite the fact that two controls had been performed during the year.
Figure 5.
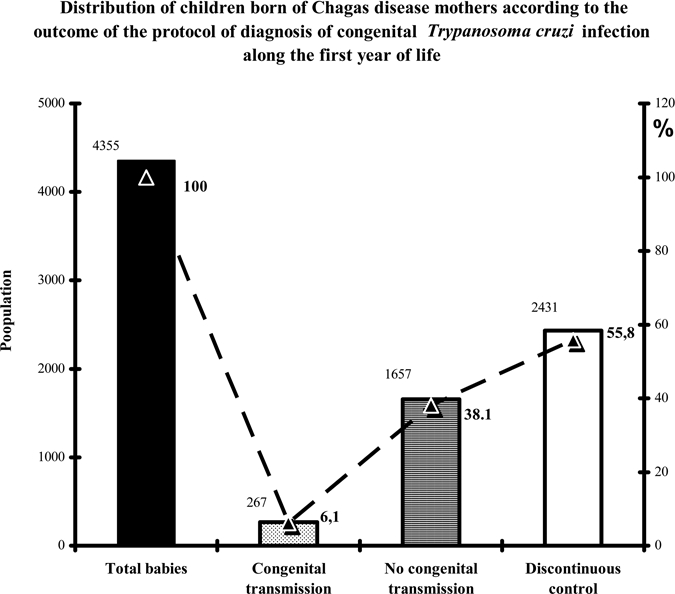
Outcome of 4,355 children born to Chagas disease mothers along the follow-up. Congenital transmission: positive diagnosis at any point of the first 12 months of life. No congenital transmission: confirmed as non-infected at the end of the follow-up, i.e., 12 months of age. Discontinuous control: two negative controls before the 10th month and absence of a third control.
Discussion
Control of congenital CHD involves four sequential stages: 1) determination of serological reactivity against T. cruzi in pregnant women; 2) monitoring and diagnosis of CT along the first year of life of babies born to CHD mothers; 3) treatment in children with congenital CHD or T. cruzi infection, and 4) follow-up of treatment to establish therapeutic cure or failure. Our study shows the different stages in the surveillance of CT, except for the effects of treatment, focusing on the strengths and weaknesses of control of such stages in an urban, non-endemic area of Argentina.
The study was performed at the Instituto Nacional de Parasitología Dr Mario Fatala Chaben, an institution that receives pregnant mothers referred from different health centers to confirm CHD serology, and follows up their babies to diagnose potential CT. The CHD mothers and their babies lived in the main urban, non-endemic, more densely populated areas of Argentina, the City of Buenos Aires, and Greater Buenos Aires, which host 31.6% of the population in the country. The overall serological prevalence of CHD mothers was 49.5%, a very high percentage. It is worth mentioning that the data are biased because we receive patients that are referred to our institution, in most cases to confirm CHD diagnosis.
In 2000, the serological prevalence of pregnant women in Argentina was 6.78%, (Blanco S and others, Programa Nacional de Chagas. Coordinación de Vectores 2001, National Chagas Program, Vector Coordination, unpublished data); 12.7% in Paraguay, in the departments of Paraguary and Cordillera, which are endemic areas17; in Brazil, 0.1%18; in Chile, between 1% and 8% in different endemic areas,19 and in Bolivia, 42.2% in Yacuiba, a highly endemic area,20 and 33.8% in Bermejo with control of vectorial transmission.21 Variations in the support of vectorial control activities in the different regions, countries, or areas of Latin America seem to determine the levels of serological prevalence of CHD in pregnant women.
Argentina receives external migration from neighboring countries such as Paraguay and Bolivia. The mean CT rate in our cohort was 6.1% along the 11-year period of the study. Bolivia's contribution to CT in our study was the highest (27%), and this fact was sustained because of Bolivian migration from the poorest and more endemic regions of that country. Different rates of CT were detected in different provinces of our country: 8.8% in Tucumán,22 4–8.8% in Salta,23,24 2.6% in Santa Fe,25 2.4% in Córdoba,26 and 2.5% in the City of Buenos Aires in a reference pediatric center, mainly caused by internal migration from endemic areas to the urban center.27 According to our data, it is not possible to determine if those children born in the City of Buenos Aires or Greater Buenos Aires, whose mothers are also native of non-endemic areas, constitute second generation congenital CHD children, a piece of information that is clearly reported in studies from Chile and the province of Salta, Argentina.28,29 In other countries of the Southern Cone, CT rate figures are as follows: 5.1% in Yacuiba and 5.2% in Bermejo, both from Bolivia,20,21 5.4% in Paraguay,17 0.7% in Brazil,30,31 and 21.2% in Chile.32 The variability expressed in CT rates in countries and areas of Latin America could be related to the degree of success or difficulty in the application of the strategy, recording of cases, the impact of vectorial control and policies on surveillance and poverty.33,34 In this sense, in Argentina, it has been estimated that the number of detected cases of infected children was smaller than expected,35 which emphasizes the fact that CT constitutes a serious Public Health issue.
Sustained vectorial surveillance actions in CHD have an impact on CT rate, as proven by Brazil, where vectorial transmission has been successfully interrupted. This relevant public health event has induced a marked reduction in the indicators of congenital T. cruzi infection, and has supported the influence of vector control in CHD.30,31 On the other hand, CT rate in Chile, another country where vectorial transmission has been successfully interrupted is still very high; there, congenital T. cruzi infection was diagnosed by means of polymerase chain reaction (PCR), which is a high sensitivity method, and has shown controversial results when used as a tool in congenital T. cruzi infection diagnosis (Russomando G and others, Neonatal diagnosis and follow-up treatment of congenital Chagas disease by polymerase chain reaction. J Microbiol Methods 30: 237, 1997).19,36–38
At present, direct parasitological methods seem to be the method of choice in the diagnosis of newborns because of ease of accessibility and adequate sensitivity, although the efficacy of these tests has always depended on the skill of the operator. Other methods, such as the micromethod, developed and validated in our institute (De Rissio and others, unpublished data), and the microhematocrit concentration method39 seem to be better suited for diagnosis in the field because they can be performed in laboratories with less complexity. Serological tests require moderate complexity laboratories, and molecular biology tests need high complexity laboratories.
In this study, out of 267 children with CT, 68.9% were diagnosed within the first 5 months of life (44.6% in the first month, and 24.3% until the 5th month). The rest, i.e., 31.1%, were diagnosed between the 6th and 12th months. Diagnosis for CT was more efficacious within the first 5 months than in the second semester. In most CT studies from endemic countries, the diagnosis is mostly carried out around birth or within the first semester, mainly because there are better recruitment possibilities for CHD mothers and their children around the perinatal period. Few reports emphasize follow-up until the end of the first year of life.
In our experience, the success of the follow-up in the first year was because serologically reactive mothers were informed through extensive individual interviews about the relevance of control of their asymptomatic and apparently healthy children. Follow-up during the first year is a crucial issue in the monitoring of congenital T. cruzi infection, and it is essential for whole coverage in the CT strategy. In that sense, a strong weakness was detected in our study in the application of the entire protocol because a significant number of children (55.8%) failed to complete the follow-up and therefore were not diagnosed in the first 12 months of their life, even when they had up to two controls within the year of follow-up. This large number of children lost during the follow-up could be distorting the true CT rate in our studied population.
The CT monitoring strategy has, in our view, a weak point that makes it vulnerable; in a lot of papers published about this issue in Latin America, there is no clear follow-up extending to the end of the first year of life of the babies born to CHD mothers. In our study, this vulnerability was expressed by the loss of subjects, which we recognize as amounting to 55.8%. We attribute this weakness, in our endemic countries, to lack of a strong network within health systems to allow for the application of an integrated strategy. Moreover, the difficulties associated to geographical distances and other relevant social emergent factors in diagnosis and follow-up are more marked in rural areas.17,20–22 Increasing the effectiveness of the first line of health services in direct contact with the community in our endemic countries and establishing congenital T. cruzi infection as a priority in Public Health Programs could contribute to achieve better surveillance coverage and to reduce potential undiagnosed and untreated children, facts that have failed to be clearly reported in different studies.20–23
In connection with the diagnosis protocol, in our experience parasitemia was a determining factor in the diagnosis of congenital T. cruzi infection within the first 5 months, by micromethod and xenodiagnosis, which is similar to reported findings in other studies.17,19,20,22,23 Between 6 and 12 months of age, serology confirmed congenital T. cruzi infection in 74.7% of the babies when the serological titers were clearly over the cut-off level. However, serology was not always conclusive in the diagnosis in this period, especially when it was non-reactive or discordant. The interplay between parasitemia and serology had a dynamic role in the diagnosis of children with CT. Between 6 and 9 months of age, parasitemia identified 36.2% more cases than serology. A non-reactive serology in this period was not always indicative of absence of congenital T. cruzi infection. In our experience, 10 months was the definitive cut-off age that unmistakably separated parasitemia from serology in the diagnosis; more specifically, between 6 and 9 months of age, a non-reactive serology diagnosis should be confirmed after 10 months of age to complete the CT protocol.40
This became more relevant because maternal anti-T. cruzi antibodies were persistent up to 6 months17 and 8 months after birth26 in children who were free of infection. In our study, a total of 6.5% of children without congenital T. cruzi infection had reactive serology for one or two tests at 6 months of age. The persistence of maternal antibodies until months 6 to 8 suggests that physicians should adopt a cautious position regarding the serological diagnosis of CT in these critical months of life in the cases of children born to CHD mothers.
In summary, overall CT surveillance requires a closer interrelation between the different Public Health players, defined health policies, and active involvement in the different levels of health services in contact with the community. Focusing on diagnosis by means of low and high complexity laboratories, both in the field and in reference health care centers, will potentially contribute to increasing and improving coverage of the congenital T. cruzi infection.
Acknowledgments
We thank the technical staff at the Diagnosis and Investigation Departments of this institution for the quality of patient management and their contributions in the diagnosis of the different tests performed.
Footnotes
Financial support: This study was supported by the ANLIS CG Malbran, Ministry of Health of Argentina.
Authors' addresses: Ana María De Rissio, Adelina Rosa Riarte, Miriam Martín García, Mónica Inés Esteva, and Andrés Mariano Ruiz, Instituto Nacional de Parasitologia Dr M Fatala Chaben, Buenos Aires, Argentina, E-mail: amderissio@yahoo.com.ar. Marta Quaglino, Universidad Nacional de Rosario, Santa Fe, Argentina.
Reprint request: Ana María De Rissio, Instituto Nacional de Parasitología Dr M Fatala Chaben, Av. Paseo Colón 568 (1063), Buenos Aires, Argentina, E-mail: amderissio@yahoo.com.ar.
References
- 1.Silveira CA, Rojas de Arias A, Segura E, Guillén G, Pinto Dias JC, Lorca M, Schenone H, Valdez Padilla J, Russomando G, Salvatella R. El control de la enfermedad de Chagas en los países del Cono Sur de América. Historia de una iniciativa internacional OPS, 1991–2001. 2002. http://www.paho.org/Spanish/ad/dpc/cd/dch-historia-incosur.pdf Available at. Accessed August 2009.
- 2.OPS/MSF Consulta técnica regional sobre Organización y Estructura de la atención médica del enfermo o infectado por Trypanosoma cruzi (enfermedad de Chagas) Montevideo, Uruguay. 13–14 Octubre OPS/DPC/CD/353/05. 2005. http://www.paho.org/Spanish/AD/DPC/CD/dch-consulta-ops-msf-2005.pdf Available at. Accessed August 2009.
- 3.Carlier Y, Torrico F. Congenital infection with Trypanosoma cruzi: from mechanism of transmission to strategies for diagnosis and control. Rev Soc Bras Med Trop. 2003;36:767–771. doi: 10.1590/s0037-86822003000600024. [DOI] [PubMed] [Google Scholar]
- 4.OPS Consulta sobre Enfermedad de Chagas congénita, su epidemiología y manejo. Montevideo, Uruguay. 24 y 25 Junio OPS/DPC/CD/301/04. 2004. http://www.mex.ops-oms.org/documentos/chagas/dch-chagas-congenita-2004.pdf Available at.
- 5.Riera C, Guarro A, El Kassab H, Jorba JM, Castro M, Angrill R, Gállego M, Fisa R, Martín C, Lobato A, Portús M. Congenital transmission of Trypanosoma cruzi in Europe (Spain): a case report. Am J Trop Med Hyg. 2006;75:1078–1081. [PubMed] [Google Scholar]
- 6.Muñoz J, Portús M, Corachan M, Fumadó V, Gascon J. Congenital Trypanosoma cruzi in a non-endemic area. Trans R Soc Trop Med Hyg. 2007;11:1161–1162. doi: 10.1016/j.trstmh.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Flores-Chávez M, Faez Y, Olalla JM, Cruz I, Gárate T, Rodríguez M, Blanc P, Cañavate C. Fatal congenital Chagas' disease in a non-endemic area: a case report. BioMed Central. 2008. http//www.casesjournal.com/content/I/I/302 Available at. Accessed January 2010. [DOI] [PMC free article] [PubMed]
- 8.Jackson I, Myers C, Diana A, Marti HP, Wolff H, Chappuis F, Loutan L, Gervaix A. Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis. 2009;5:601–603. doi: 10.3201/eid1504.080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadon ZE, Schmunis GA. Congenital Chagas disease: estimating the potential risk in the United States. Am J Trop Med Hyg. 2009;81:927–933. doi: 10.4269/ajtmh.2009.09-0257. [DOI] [PubMed] [Google Scholar]
- 10.Freilij H, Altcheh J, Storino R. In: Chagas Congénito en Enfermedad de Chagas. Storino R, Milei J, editors. Buenos Aires, Argentina: Mosby-Doyma; 1994. pp. 267–278. [Google Scholar]
- 11.Martín García M, De Rissio AM, Ruiz AM. Estudio epidemiológico y diagnóstico de la infección por Trypanosoma cruzi en mujeres embarazadas del Centro Nacional de Referencia Instituto Nacional de Parasitología “Dr. Mario Fatala Chaben.”. Archivos Argentinos de Epidemiología. 2007;10:45–51. [Google Scholar]
- 12.Cura EN, Segura EL. Quality assurance of the serologic diagnosis of Chagas disease. Pan American J Publ Health. 1998;3:242–248. doi: 10.1590/s1020-49891998000400004. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez M, Cerisola JA, Rohwedder RW. Inmunofluorescence test in the diagnosis of Chagas' diseases. Bol Chil Parasitol. 1968;23:4–8. [PubMed] [Google Scholar]
- 14.Cura EN, Ruiz AM, Velázquez E, Malagrino N, Örn A, Segura EL. 1993. Estandarización de un kit de confirmación (Fatala Kit) para el immunodiagnóstico para infección para Trypanosoma cruzi. Medicina (B Aires) 53((Suppl 1)):82. [Google Scholar]
- 15.Ruiz AM, Segura EL. Investigación biotecnológica en la enfermedad de Chagas y su aplicación al control de la transmisión. 1er Congreso Virtual de Cardiología. 2000. http//www.fac.org.ar/cvirtual/cvirtesp/cientesp/ecesp/ecc4520c/cruiz/cruiz.htm Available at. Accessed August 2009.
- 16.Cerisola JA, Rohwedder RW, Segura EL, Del Prado CE, de Martini GJW. El Xenodiagnóstico. Normalización. Utilidad. Premio Lab. Ciba-Geigy. Buenos Aires: Secretaría Estado Salud Pública; 1974. [Google Scholar]
- 17.Russomando G, Almiron M, Candia N, Franco L, Sanchez Z, de Guillen I. Implementation and evaluation of a locally sustainable system of prenatal diagnosis to detect cases of congenital Chagas disease in endemic areas of Paraguay. Rev Soc Bras Med Trop. 2005;38((Suppl 2)):49–54. [PubMed] [Google Scholar]
- 18.Figueiró Filo EA, de Almeida Senefonte FR, Antunes López AH, Oliveira de Morais A, Gonçalvez Souza Júnior V, Lemos Maia T, Duarte G. Frecuency of HIV-1, rubella, syphilis, toxoplasmosis, cytomegalovirus, simple herpes virus, hepatitis B, hepatitis C, Chagas disease and HTLV I/II infections in pregnant women in the State of Mato Grosso Do Sul. Rev Soc Bras Med Trop. 2007;40:181–187. doi: 10.1590/s0037-86822007000200007. [DOI] [PubMed] [Google Scholar]
- 19.Lorca MH, Bahamonde MI, García A, Tassara R, Urarte E, Contreras Mdel C, Salinas P, Núñez E. Trypanosoma cruzi transplacental infection in Chile: diagnosis, treatment and control. Rev Soc Bras Med Trop. 2005;38((Suppl 2)):46–48. [PubMed] [Google Scholar]
- 20.Salas NA, Cot M, Schneider D, Mendoza B, Santalla JA, Postigo J, Chippaux JP, Brutus L. Risk factors and consequences of congenital Chagas disease in Yacuiba, south Bolivia. Trop Med Int Health. 2007;12:1498–1505. doi: 10.1111/j.1365-3156.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 21.Brutus L, Schneider D, Postigo J, Romero M, Santalla J, Chippaux JP. Congenital Chagas disease: diagnostic and clinical aspects in an area without vectorial transmission, Bermejo, Bolivia. Acta Trop. 2008;106:195–199. doi: 10.1016/j.actatropica.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Blanco SB, Segura EL, Cura EN, Chuit R, Tulián L, Flores I, Garbarino G, Villalonga JF, Gürtler R. Congenital transmission of Trypanosoma cruzi: an operational outline for detecting and treating infected infants in north-western Argentina. Trop Med Int Health. 2000;5:293–301. doi: 10.1046/j.1365-3156.2000.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Contreras S, Fernandez MR, Agüero F, Desse Desse J, Orduna T, Martino O. Congenital Chagas-Mazza disease in Salta, Argentina. Rev Soc Bras Med Trop. 1999;32:633–636. doi: 10.1590/s0037-86821999000600004. [DOI] [PubMed] [Google Scholar]
- 24.Zaidenberg M. La enfermedad de Chagas congénita en la Provincia de Salta, Argentina, años 1980–1997. Rev Soc Bras Med Trop. 2005;32((Suppl 2)):689–695. doi: 10.1590/s0037-86821999000600012. [DOI] [PubMed] [Google Scholar]
- 25.Streiger M, Fabbro D, del Barco M, Beltramino R, Bovero N. Congenital Chagas disease in Santa Fe, diagnosis and treatment. Medicina (B Aires) 1995;55:125–132. [PubMed] [Google Scholar]
- 26.Moya P, Basso B, Moretti E. Congenital Chagas disease in Córdoba, Argentina: epidemiological, clinical, diagnostic, and therapeutic aspects. Experience of 30 years of follow up. Rev Soc Bras Med Trop. 2005;38((Suppl 2)):33–40. [PubMed] [Google Scholar]
- 27.Altcheh J, Biancard M, Lapeña A, Ballering G, Freilij H. Congenital Chagas disease: experience in the Hospital de Niños Ricardo Gutiérrez, Buenos Aires, Argentina. Rev Soc Bras Med Trop. 2005;38((Suppl 2)):41–45. [PubMed] [Google Scholar]
- 28.Schenone H, Gaggero M, Sapunar J, Contreras Md C, Rojas A. Congenital Chagas disease of second generation in Santiago, Chile. Report of two cases. Rev Inst Med Trop Sao Paulo. 2001;43:231–232. doi: 10.1590/s0036-46652001000400011. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez Negrete O, Mora MC, Basombrío MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics. 2005;115:668–672. doi: 10.1542/peds.2004-1732. [DOI] [PubMed] [Google Scholar]
- 30.Luquetti A, Ferreira AW, Oliveira RA, Tavares SBL, Rassi A, Pinto Dias JC, Prata A. Congenital transmission of Trypanosoma cruzi in Brasil: estimation of prevalence based on preliminary data of national serological surveys in children under 5 years old. Rev Soc Bras Med Trop. 2005;38((Suppl 2)):24–26. [PubMed] [Google Scholar]
- 31.Rassi A, Amado Neto V, Rassi GG, Sabagga Amato V, Rassi Júnior A, Luquetti A, Rassi SG. A retrospective search for maternal transmission of Chagas infection from patients in the chronic phase. Rev Soc Bras Med Trop. 2004;37:485–489. doi: 10.1590/s0037-86822004000600011. [DOI] [PubMed] [Google Scholar]
- 32.Lorca M, García A, Contreras M, Schenome H, Rojas A. Evaluation of a Triatoma infestans elimination program by the decrease of Trypanosoma cruzi infection frequency in children younger than 10 years, Chile, 1991–1998. Am J Trop Med Hyg. 2001;6:861–864. doi: 10.4269/ajtmh.2001.65.861. [DOI] [PubMed] [Google Scholar]
- 33.Torrico F, Alonso-Vega C, Suarez E, Rodriguez P, Torrico MC, Dramaix M, Truyens C, Carlier I. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201–209. [PubMed] [Google Scholar]
- 34.Sosa-Estani S, Dri L, Touris C, Abalde S, Dell'Arcipreste A, Braunstein J. Transmisión vectorial y congénita del Trypanosoma cruzi en Las Lomitas, Formosa. Medicina (B Aires) 2009;69:424–430. [PubMed] [Google Scholar]
- 35.Gürtler R, Segura EL, Cohen JL. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Infect Dis. 2003;9:29–32. doi: 10.3201/eid0901.020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, Carlier I, Svoboda M. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am J Trop Med Hyg. 2003;68:574–582. doi: 10.4269/ajtmh.2003.68.574. [DOI] [PubMed] [Google Scholar]
- 37.Diez CN, Manattini S, Zanuttini JC, Botasso JC, Marcipar I. The value of molecular studies for the diagnosis of congenital Chagas disease in northeastern Argentina. Am J Trop Med Hyg. 2008;78:624–627. [PubMed] [Google Scholar]
- 38.Russomando G, Figueredo A, Almiron M, Sakamoto M, Morita K. Polymerase chain reaction-based detection of Trypanosoma cruzi DNA in serum. J Clin Microbiol. 1992;30:2864–2868. doi: 10.1128/jcm.30.11.2864-2868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freilij H, Müller L, Gonzalez Cappa SM. Direct micromethod for diagnosis of acute and congenital Chagas disease. J Clin Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Rissio AM, Scollo K, Cardoni RL. La transmisión madre-hijo del Trypanosoma cruzi en la Argentina. Medicina (B Aires) 2009;69:529–535. [PubMed] [Google Scholar]


