Abstract
We sought to test the association of polymorphisms in Plasmodium falciparum nhe-1 (Pfnhe-1, gene PF13_0019) with in vitro susceptibility to quinine, which was previously reported in a limited number of reference strains or culture-adapted isolates. Determination of in vitro susceptibility to quinine, genotyping of Pfnhe-1 ms4760 microsatellite and polymorphism in codon 76 of Pfcrt were performed for 83 isolates obtained from symptomatic malaria-infected travelers returning from various African countries to France or from subjects living in Madagascar. Nineteen different ms4760 microsatellite profiles of Pfnhe-1 were found including 14 not previously described. Multivariate analysis showed no significant association between the in vitro susceptibility to quinine with particular ms4760 profiles. Contrary to previous reports, we only observed that the number of NHNDNHNNDDD repeats was positively associated with the increased IC50 of QN (P = 0.01). We concluded that the studied polymorphisms in Pfnhe-1 did not appear as valid molecular markers of in vitro susceptibility to quinine in P. falciparum isolates from Africa. Because we did not include any isolate of Asian origin in our series, these results did not exclude the possibility of regional associations, for example in South-East Asia.
Introduction
Quinine (QN), a natural compound found in Cinchona bark, used for four centuries in malaria endemic regions, is still a major antimalarial drug, especially to treat severe malaria cases or malaria in pregnant women. Although the first cases of quinine resistance were reported nearly 100 years ago in Brazil1,2 and sporadic observations of clinical failures have been successively reported since the 1960s from Asia (Thai-Cambodian border),3 Western Oceania, or South America,4 the emergence and spreading of resistance to QN remained particularly low in comparison to other antimalarials as chloroquine or antifolates.
Contrary to those more recent and synthetic drugs, the mechanisms of parasite resistance to QN are not well known. Some polymorphisms in multiple transporters and in the Plasmodium falciparum chloroquine resistance transporter gene (Pfcrt) have been associated with QN resistance.4 However, the degree of implication or linkage of those genes in QN resistance remains uncertain. Another candidate gene involved in in vitro QN resistance, the P. falciparum Na+/H+ exchanger (Pfnhe-1) gene (PF13_0019), was identified by Ferdig and others in 2004.5 The physiological contribution of PfNHE-1 in QN resistance is still evaluated and debated, and could be strain-dependent.6 Concomitantly, the evaluation of the association of polymorphisms in PF13_0019 with QN susceptibility is under progress. The princeps study by Ferdig and others described three point polymorphisms at three separate codons (790, 894, 950) and microsatellite variations in three different repeat sequences (msR1, ms3580, ms4760). Only variations in ms4760 showed significant association with in vitro QN response in 71 P. falciparum lines. Among the eight profiles of ms4760 described, one profile, the ms4760-1, was relatively frequent in less quinine-susceptible lines but was also present in fully susceptible parasites. More interestingly, the number of a DNNND repeat motif, shared by the different ms4760 profiles, was reported as associated with QN response. Since then, another study investigated the association of polymorphisms in PF13_0019 with in vitro QN susceptibility.7 In that series of 23 culture-adapted isolates or reference strains, the relationship between the number of DNNND repeats and the inhibitory concentration 50% values (IC50) to QN was confirmed and the increased number of another repeat motif, NHNDNHNNDDD, was associated with decreased IC50s to QN. A limitation of those previous studies was that the in vitro QN susceptibility and polymorphisms determinations were performed on culture-adapted cloned isolates or reference strains, which could lead to biased results caused by accumulated mutations selected by in vitro conditions.
In this study, we have tried to test, in isolates originating from various African countries, the association of the previously reported polymorphisms of PF13_0019 with in vitro QN response. Fresh isolates obtained from symptomatic, malaria-infected travelers returning from Africa to France or from subjects living in Madagascar were used.
Materials and Methods
Reference culture-adapted strains.
Three culture-adapted and cloned strains of P. falciparum (chloroquine-resistant and chloroquine-susceptible strains) were used for quality control (3D7Africa and W2 Indochina in Paris; 3D7Africa and FCM29 Cameroon in Madagascar). These clones were obtained from MR4-ATCC (Manassas, VA). Reference strains were cryopreserved and thawed before each measurement. They were cultivated in erythrocytes resuspended in RPMI 1640 medium supplemented with 10% human serum (Abcys Biowest, Paris, France) at 37°C in a 5% CO2, 5% O2, 90% N2 atmosphere until a minimum parasite density of 1% was achieved. The in vitro susceptibilities to chloroquine (CQ) and QN were determined after suspension in the same medium and addition of uninfected erythrocytes to obtain 0.3% parasitemia and 1.5% hematocrit. The culture obtained within three asexual cycles after thawing contained a minimum of 90% ring forms and did not require synchronization.
Patients and clinical samples.
Clinical isolates of P. falciparum from symptomatic malaria-infected travelers returning to France from various African countries in 1997–2007 were obtained at the National Reference Center for Malaria (NRCM), Paris, France. Clinical samples from Madagascar were collected, as part of the surveillance of antimalarial drug resistance, in 2006/2007 from symptomatic patients before treatment in six health centers. The health centers were located in areas exhibiting three of the four epidemiological patterns of malaria transmission: Ihosy in South Madagascar (sub-desert stratum, epidemic prone), Maevatanana and Miandrivazo in West Madagascar (tropical stratum, seasonal and endemic area), and Tsiroanomandidy, Saharevo, and Moramanga in the foothills of the Central Highlands of Madagascar (highlands stratum, low-endemic area). Venous blood samples (10 mL) were collected in tubes coated with EDTA (Vacutainer tubes, Becton Dickinson, Rutherford, NJ), from malaria-positive patients (> 2 years of age). Parents or guardians gave their consent for participation in the study (No. 007/SANPF/2007, registration number from the Ethics Committee of the Ministry of Health of Madagascar). Malaria positivity was evaluated by using a rapid diagnostic test based on the detection of Plasmodium-specific lactate dehydrogenase (pLDH) (OptiMAL-IT, DiaMed AG, Cressier sur Morat, Switzerland). Positive patients were treated with artesunate-amodiaquine combination, according to the National Malaria Control Program (NMCP) in Madagascar.
Determination of QN and CQ in vitro IC50s.
At the NMRC, the determinations of IC50s were routinely done on fresh isolates as part of the surveillance of antimalarial susceptibility of P. falciparum isolates obtained in travelers returning to France. In vitro assays performed on isolates obtained at the NRMC, Paris, France and at the Malaria Research Unit (MRU), Institut Pasteur de Madagascar, Antananarivo, Madagascar were processed using the isotopic microtest method.8,9 Antimalarial drugs in the appropriate solvent were distributed on 96-well tissue culture plates and dried: chloroquine disulfate (Sigma Aldrich, Saint-Quentin Fallavier, France), 12 to 3,200 nM (NCRM, Paris) or 12.5 to 1,600 nM (MRU, Antananarivo) and quinine (Sigma Aldrich), 25 to 3,200 nM (NCRM, Paris and MRU, Antananarivo). For each drug tested, three control wells were drug free, and each concentration was studied in duplicate or triplicate. Clinical isolates with at least a 0.1% parasite density were included in the study and were maintained at +4°C (for up to 48 h after collection) before a culture was started. The blood samples were washed three times with a solution of RPMI 1640 (Gibco, Invitrogen Life Technologies, Cergy-Pontoise, France) plus 25 mM HEPES (Sigma) and 25 mM NaHCO3 (Sigma). The blood samples were then resuspended in the same culture medium supplemented with 10% human serum (Abcys Biowest, Paris, France). If necessary, a dilution was performed by adding uninfected O-positive-group erythrocytes (from Etablissement Français du Sang, Rungis, France, or from Blood Transfusion Center, Antananarivo, Madagascar) to obtain a 0.3% parasite density and a 1.5% hematocrit. For in vitro isotopic microtest, 200 μL/well of the suspension of parasitized erythrocytes were distributed in 96-well plates predosed with antimalarial agents. Plates were incubated for 42 h at 37°C in an atmosphere of 5% O2, 5% CO2, and 90% N2, and a relative humidity of 95%. Plates were then frozen and kept at −20°C.
Isotopic measurement.
For the isotopic assay, 1 mL RPMI 1640 with [8-3H] hypoxanthine (40 mCi/L; Amersham Biosciences, Orsay, France) was added to 20 mL of the homogeneous parasite suspension (~0.4 µCi [8-3H]hypoxanthine/well) at the beginning of the in vitro assay. After the plates were thawed, the content of each well was harvested onto fiber filter disks (FilterMAT, Wallac, Turku, Finland). The fiber filter papers were washed and dried and mixed with 2 mL of scintillation fluid (OptiScint, Perkin-Elmer; NBCS 204, Amersham Biosciences, Orsay, France), and the level of parasite incorporation of radioactivity (in counts per minute) was measured with a liquid scintillation counter (Wallac 1410; Perkin-Elmer). The IC50s were determined by nonlinear regression analysis with an inhibitory sigmoid maximum-effect model.8,9 The cut-off values, defined as > 2 SD above the mean and/or after correlation with clinical failures, for in vitro resistance or reduced susceptibility to CQ and QN, were 100 and 800 nM, respectively.
Genotyping of Pfnhe ms4760 microsatellite polymorphisms and of Pfcrt codon76.
Parasites DNA from 100 μL infected blood were extracted using the phenol chloroform method as described elsewhere.10 The polymerase chain reaction (PCR) genotyping of highly polymorphic genetic markers was performed to select monoclonal isolates. The polymorphic regions of msp-1 (block2) and msp-2 (block3) were amplified by nested PCR. Second-round appropriate PCR primers were used to amplify the K1, MAD20, and RO33 allelic families of msp-1 and the 3D7 and FC27 allelic families of msp-2. The primers and conditions used for amplifications were those previously reported by Ranford-Cartwright and others.11 For each sample, 25 µL reactions mixtures were prepared containing 1X PCR buffer, 150 µM of each dNTPs, 100 nM of each primer, and 1.25 U of Amplitaq Gold (Applied Biosystems, Roche). Five microliters (5 µL) of sample DNA were used for outer amplification and 2 µL of the outer PCR product was transferred as template for the nested amplification. Eight microliters (8 µL) of nested PCR products were analyzed by electrophoresis using 2% agarose gels. Isolates showing only one band in each marker were processed for further genotyping studies including genotyping of Pfnhe-1 ms4760 microsatellite polymorphisms and of Pfcrt codon76.
For Pfnhe-1, the inner amplification reaction mixture contained 1X PCR buffer, 2.5 mM MgCl2, 1 mM of dNTPs, and 0.25 µM of primers (NHE-1: 5′-TCCTGATAGTAGCGAAGAAGAA-3′ and NHE-2: 5′-CAGTGCATGGACCAAAATTA-3′). Reactions were carried out in 25 μL and 1.25 U DNA polymerases (ExTaq, TaKaRa Bio Inc, Japan). The outer PCR was carried out in 55 μL with identical mixture with nested primers (NHE-3: 5′-AGTCGAAGGCGAATCAGATG-3′ and NHE-4: 5′-CCTGAACAAGCACTGCAAGA-3′). The PCR conditions used to genotype codon 76 of Pfcrt were as previously described.12 After purification by filtration using Macherey-Nagel plate (NucleoFast 96 PCR, Macherey-Nagel, Düren, Germany), sequencing reactions were carried out with the ABI PRISM BigDye Terminator cycle sequencing ready reaction kit run on a 3730xl Genetic Analyser (Applied Biosystems, Courtaboeuf, France). Electrophoregrams were visualized and analyzed with CEQ2000 Genetic Analysis System software (Beckman Coulter, Sykesville, MD). Amino-acid sequences were compared with wild-type sequence (GenBank accession no. XM_001349726 for NHE-1 gene). Pfnhe-1 ms4760 microsatellite genotypes were constructed from full sequence presenting an unambiguous single allele signal at all positions.
Statistical analysis.
Epi Info software (version 6.04, CDC, Atlanta, GA) and MedCalc software (version 9.1.0.1, Mariakerke, Belgium) were used for data analysis. Continuous variables were compared using an independent-sample analysis of variance (ANOVA) or Mann-Whitney test. The CQ and QN geometric means IC50s were analyzed with respect to location of the isolates collection, polymorphism in Pfcrt gene, Pfnhe-1 ms4760 profiles (only the four most numerous profiles were included in the analysis), number of DNNND or NHNDNHNNDDD repeats or repeats ratio. A P value < 0.05 was considered statistically significant. For the multivariate analysis, variables with a P value < 0.25 were initially introduced into the model and removed following a backward-stepwise selection procedure to leave only those with a P value < 0.05 in the final model.
Nucleotide sequence accession numbers.
The exact sequence of each new ms4760 genotype has been submitted to GenBank (accession no. FJ947067 to FJ947073).
Results
A total of 83 isolates from Madagascar (N = 40) and from 13 other African countries (36 from West Africa, 6 from Central Africa, 1 from East Africa), collected between 1997 and 2007, appearing monoclonal on the basis of msp-1/msp-2 genotyping and with Pfnhe-1 full sequences presenting only one single allele at all positions, were included in the study (Table 1). The Pfcrt 76T mutant-type allele was present in 30 isolates (36.1%) and its prevalence distribution was significantly heterogeneous: from 66.7% to 100% in mainland Africa and absent in Madagascar (P < 0.0001).
Table 1.
Characteristics (country and year of collection, in vitro susceptibility, and Pfcrt codon 76 and Pfnhe-1 polymorphisms) of the 83 Plasmodium falciparum isolates from African countries collected in 1997–2007
| Isolates | Country | Year of collection | Pfcrt codon76† | In vitro susceptibility, CI50 (nM)* | Pfnhe-1 ms4760 microsatellite | |||
|---|---|---|---|---|---|---|---|---|
| QN | CQ | No. DNNND repeat | No. NHNDNHNNDDD repeat | No. genotype profile‡ | ||||
| 565 | Mozambique | 1997 | 76T | 1600 | ND | 2 | 2 | ms-1 |
| 1785 | Togo | 1999 | 76T | 1267 | ND | 3 | 1 | ms-7 |
| 1648 | Benin | 1999 | 76T | 1191 | 111 | 1 | 2 | ms-20 |
| 1563 | Ivory Coast | 1999 | 76T | 1179 | 64 | 2 | 2 | ms-1 |
| 5805 | Cameroon | 2005 | 76T | 855 | 498 | 2 | 2 | ms-1 |
| 1553 | DRCongo | 1999 | 76T | 821 | 67 | 2 | 1 | ms-28 |
| TDD01 | Madagascar | 2006 | 76K | 612 | 138 | 1 | 2 | ms-3 |
| 4274 | Guinea | 2003 | 76T | 611 | ND | 2 | 2 | ms-1 |
| TDD02 | Madagascar | 2007 | 76K | 435 | 50 | 2 | 2 | ms-1 |
| 6268 | Mali | 2006 | 76K | 413 | 30 | 1 | 2 | ms-3 |
| IHO01 | Madagascar | 2007 | 76K | 393 | 47 | 2 | 2 | ms-1 |
| MOR01 | Madagascar | 2006 | 76K | 385 | ND | 2 | 2 | ms-1 |
| 6569 | Mali | 2006 | 76T | 371 | 77 | 2 | 2 | ms-26 |
| MIA01 | Madagascar | 2006 | 76K | 363 | 104 | 2 | 2 | ms-1 |
| TDD03 | Madagascar | 2006 | 76K | 353 | 30 | 1 | 2 | ms-3 |
| 5813 | DRCongo | 2005 | 76T | 330 | 237 | 3 | 2 | ms-23 |
| TDD04 | Madagascar | 2006 | 76K | 327 | 38 | 3 | 1 | ms-7 |
| 6643 | Mali | 2006 | 76T | 310 | 216 | 1 | 2 | ms-3 |
| 6562 | Mali | 2006 | 76T | 299 | 167 | 1 | 2 | ms-3 |
| 6685 | Mali | 2006 | 76K | 285 | 55 | 1 | 2 | ms-3 |
| TDD05 | Madagascar | 2006 | 76K | 275 | ND | 1 | 2 | ms-3 |
| 6514 | Mauritania | 2006 | 76K | 266 | 43 | 2 | 1 | ms-6 |
| 3406 | DRCongo | 2002 | 76T | 260 | 63 | 1 | 2 | ms-3 |
| MIA02 | Madagascar | 2006 | 76K | 247 | 63 | 2 | 2 | ms-1 |
| MAE01 | Madagascar | 2006 | 76K | 240 | 47 | 2 | 1 | ms-6 |
| MOR02 | Madagascar | 2006 | 76K | 222 | 27 | 2 | 2 | ms-1 |
| MIA03 | Madagascar | 2006 | 76K | 222 | 86 | 0 | 2 | ms-33 |
| MIA04 | Madagascar | 2006 | 76K | 221 | 14 | 2 | 2 | ms-1 |
| 6339 | Burkina Faso | 2006 | 76T | 196 | 163 | 2 | 2 | ms-1 |
| 4627 | Ivory Coast | 2004 | 76T | 187 | ND | 1 | 2 | ms-24 |
| 4275 | Cameroon | 2003 | 76T | 186 | ND | 1 | 2 | ms-3 |
| MIA05 | Madagascar | 2006 | 76K | 185 | 42 | 1 | 2 | ms-31 |
| 6534 | Niger | 2006 | 76T | 177 | 187 | 2 | 2 | ms-1 |
| 5864 | Senegal | 2005 | 76T | 176 | 52 | 2 | 2 | ms-1 |
| MIA06 | Madagascar | 2006 | 76K | 166 | 51 | 1 | 2 | ms-3 |
| 6410 | Mali | 2006 | 76T | 163 | 139 | 1 | 2 | ms-3 |
| 6588 | Mali | 2006 | 76K | 159 | 40 | 3 | 1 | ms-7 |
| 6529 | Mali | 2006 | 76T | 150 | 114 | 2 | 2 | ms-1 |
| 5773 | Mali | 2005 | 76T | 145 | 154 | 2 | 1 | ms-25 |
| 5832 | Ivory Coast | 2005 | 76T | 144 | 294 | 2 | 2 | ms-1 |
| 5774 | Senegal | 2005 | 76K | 142 | 40 | 3 | 1 | ms-7 |
| 5833 | Benin | 2005 | 76T | 137 | 231 | 2 | 1 | ms-21 |
| 5769 | Burkina Faso | 2005 | 76K | 135 | 37 | 2 | 3 | ms-22 |
| MIA07 | Madagascar | 2006 | 76K | 125 | 57 | 3 | 1 | ms-7 |
| MIA08 | Madagascar | 2006 | 76K | 125 | 127 | 3 | 1 | ms-7 |
| MIA09 | Madagascar | 2006 | 76K | 123 | 140 | 2 | 2 | ms-1 |
| 5693 | Ivory Coast | 2005 | 76K | 123 | 74 | 2 | 1 | ms-21 |
| 6506 | Burkina Faso | 2006 | 76T | 115 | 173 | 2 | 1 | ms-6 |
| MIA10 | Madagascar | 2007 | 76K | 112 | 48 | 1 | 2 | ms-30 |
| SHV01 | Madagascar | 2006 | 76K | 110 | 84 | 3 | 1 | ms-7 |
| 6340 | Mali | 2006 | 76T | 109 | ND | 2 | 2 | ms-1 |
| 6481 | Mali | 2006 | 76T | 107 | 188 | 2 | 2 | ms-1 |
| MIA11 | Madagascar | 2006 | 76K | 106 | 74 | 1 | 2 | ms-3 |
| IHO02 | Madagascar | 2007 | 76K | 105 | 67 | 1 | 2 | ms-3 |
| 6539 | Mali | 2006 | 76T | 105 | 120 | 3 | 2 | ms-27 |
| MIA12 | Madagascar | 2006 | 76K | 105 | 79 | 2 | 1 | ms-32 |
| 5322 | Benin | 2005 | 76T | 104 | 138 | 2 | 2 | ms-1 |
| 6612 | Mali | 2006 | 76K | 104 | 35 | 1 | 3 | ms-12 |
| 6126 | Niger | 2006 | 76T | 103 | 212 | 2 | 2 | ms-1 |
| MIA13 | Madagascar | 2006 | 76K | 102 | 85 | 2 | 2 | ms-1 |
| 6659 | Mali | 2006 | 76K | 97 | 22 | 1 | 2 | ms-3 |
| MIA14 | Madagascar | 2006 | 76K | 96 | 63 | 2 | 1 | ms-29 |
| 6532 | Mali | 2006 | 76K | 94 | 39 | 1 | 3 | ms-12 |
| MIA15 | Madagascar | 2007 | 76K | 92 | 25 | 3 | 1 | ms-7 |
| IHO03 | Madagascar | 2007 | 76K | 90 | 33 | 2 | 1 | ms-6 |
| 6430 | Chad | 2006 | 76K | 88 | 29 | 1 | 2 | ms-3 |
| SHV02 | Madagascar | 2006 | 76K | 87 | 126 | 2 | 1 | ms-6 |
| MIA16 | Madagascar | 2007 | 76K | 76 | 13 | 2 | 2 | ms-1 |
| MIA07 | Madagascar | 2006 | 76K | 74 | 88 | 1 | 2 | ms-3 |
| 5866 | Mali | 2005 | 76T | 71 | 34 | 2 | 1 | ms-6 |
| MIA18 | Madagascar | 2007 | 76K | 70 | 3 | 3 | 1 | ms-7 |
| MOR03 | Madagascar | 2006 | 76K | 70 | 37 | 3 | 1 | ms-7 |
| MIA19 | Madagascar | 2006 | 76K | 64 | 94 | 1 | 3 | ms-12 |
| SHV03 | Madagascar | 2006 | 76K | 63 | 142 | 1 | 2 | ms-31 |
| 5655 | Ivory Coast | 2005 | 76T | 57 | 223 | 2 | 2 | ms-1 |
| SHV04 | Madagascar | 2006 | 76K | 57 | 17 | 3 | 1 | ms-7 |
| IHO04 | Madagascar | 2007 | 76K | 51 | 88 | 3 | 1 | ms-7 |
| 6299 | Burkina Faso | 2006 | 76K | 49 | 21 | 1 | 3 | ms-12 |
| MIA20 | Madagascar | 2006 | 76K | 37 | 99 | 3 | 1 | ms-7 |
| 6374 | Mali | 2006 | 76K | 27 | 30 | 2 | 2 | ms-1 |
| TDD06 | Madagascar | 2006 | 76K | 23 | 87 | 2 | 2 | ms-1 |
| MOR04 | Madagascar | 2007 | 76K | 23 | 142 | 3 | 1 | ms-7 |
| MOR05 | Madagascar | 2006 | 76K | 22 | 126 | 2 | 1 | ms-6 |
QN = quinine, CQ = chloroquine.
Pfcrt 76K (wild-type allele) and Pfcrt 76T (mutant-type allele).
Pfnhe-1 ms4760 microsatellite genotype profiles are detailed in Figure 1.
ND = not done.
The quality controls of batches of plates using reference lines were as follow: (IC50s geometric mean ± SD): for CQ, 3D7 Africa, 28 nM ± 10 nM (N = 116, Paris) and 13 nM ± 3 nM (N = 5, Madagascar), W2 Indochina, 389 nM ± 106 nM (N = 154, Paris) and FCM29 Cameroon, 216 nM ± 19 nM (N = 5, Madagascar); for QN, 3D7 Africa, 130 nM ± 52 nM (N = 43, Paris) and 138 nM ± 30 nM (N = 5, Madagascar), W2 Indochina, 455 nM ± 177 nM (N = 68, Paris) and FCM29 Cameroon, 372 nM ± 86 nM (N = 5, Madagascar).
The IC50 median in vitro activities of drugs were 137 nM for QN (confidence interval [CI] 95%: 107–183 nM, range: 22–1,600 nM) and 74 nM for CQ (CI 95%: 51–89 nM, range: 3–498 nM), respectively. Contrary to CQ (P = 0.07), the QN IC50 geometric means displayed significant differences (P = 0.002) ranging from 121 nM from Madagascar (N = 40) to 171 nM from West Africa (N = 36), 315 nM from Central Africa (N = 6), and 1600 nM from East Africa (N = 1).
Nineteen different ms4760 microsatellite profiles of Pfnhe-1 were found including 14 not previously described (Figure 1). The profiles ms-1 (31.3%), ms-3 (19.3%), ms-7 (16.9%), and ms-6 (8.4%) were the most common. No significant associations were noticed between the Pfnhe-1 profiles and the geographical origin of the isolate collection (P = 0.4). The number of DNNND repeats ranged from 0 to 3 (median = 2; 48.2% of the isolates), whereas the number of NHNDNHNNDDD repeats ranged from 1 to 3 (median = 2; 61.5% of the isolates) (Table 1). The median of the DNNND: NHNDNHNNDDD repeats ratio was 1 (range: 0–3).
Figure 1.
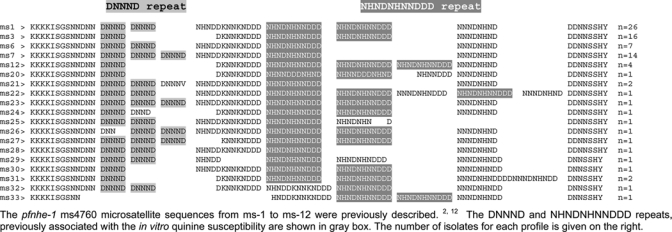
Multiple amino acid sequence alignment of the 19 pfnhe-1 ms4760 microsatellite observed in 83 Plasmodium falciparum isolates collected in African countries between 1997 and 2007.The pfnhe-1 ms4760 microsatellite sequences from ms-1 to ms-12 were previously described.2,12 The DNNND and NHNDNHNNDDD repeats, previously associated with the in vitro quinine susceptibility are shown in the gray box. The number of isolates for each profile is given on the right.
In univariate analysis, the CQ IC50 geometric mean was significantly associated with the Pfcrt polymorphism (P < 0.0001, 50 nM for Pfcrt K76 versus 138 nM for Pfcrt 76T), and the QN IC50 geometric mean was significantly associated with the Pfcrt polymorphism (P = 0.001, 121 nM for Pfcrt K76 versus 242 nM for Pfcrt 76T), the number of NHNDNHNNDDD repeats (P = 0.02, 117 nM for one repeat and 192 nM for two repeats) and the location of the samples collection (as previously shown). There was no significant association between in vitro QN susceptibility and the number of DNNND repeats (P = 0.4).
In the multivariate analysis performed to control confounding factors, five variables (Pfcrt polymorphism, number of NHNDNHNNDDD repeats, DNNND/NHNDNHNNDDD repeats ratio, ms4760 microsatellite profiles, and location of the sample collection) were initially introduced into the model. Backward stepwise selection procedure to leave only variables with a P value < 0.05 in the final model showed that the remaining significant associations were the in vitro susceptibility to CQ with the Pfcrt polymorphism (P < 0.001) and the in vitro susceptibility to QN with the number of NHNDNHNNDDD repeats (P = 0.01).
Discussion
Because no valid molecular marker of QN resistance is currently available, the identification of genes potentially involved in QN resistance is of paramount importance to develop new tools for the surveillance of emergence and spreading of P. falciparum-resistant strains. The finding of association of polymorphisms in putative genes with clinical failures and/or in vitro susceptibility constitutes a pivotal step in this process. Such associations must be verified on numerous isolates originating from various geographical areas and further molecular studies are required to assess the involvement of the candidate genes in drug resistance. Recent genetic and physiological studies reinforced the observation that QN resistance is a complex trait requiring multiple factors;6 those studies did not exclude a potential role for PfNHE in QN resistance, but in a strain-dependent manner. The recent study of microsatellite markers flanking Pfnhe-1 gene reported an absence of selective sweep in 108 Indian P. falciparum isolates and also a lack of association of microsatellite markers with DNNND repeats,13 possibly indicating that there is no strong selection pressure on this target gene. In this context, the validity and reliability of candidate polymorphisms in Pfnhe-1 gene as molecular markers of QN resistance has to be carefully evaluated.
In this study, we have compiled in vitro susceptibility data from France and from Madagascar to perform statistical tests for association. As shown by the IC50s values of reference lines used as quality controls, both drug testing sites had comparable results and reproducibility, which enabled us to merge the two sets of data for analysis. We noticed that isolates having the highest values of QN IC50s (> 600 nM) had also a mutant allele in codon 76 of Pfcrt (associated with CQ resistance). The significant relationship found in univariate analysis between in vitro QN response and the polymorphism in codon 76 of Pfcrt was consistent with that found by Ferdig and others.5 Henry and others7 did not report such an association though all strains of their series having QN IC50s > 400 nM, except one, had also a mutant allele in codon 76 of Pfcrt. Although some CQ IC50s were not performed in our series, results of genotyping of codon 76 of Pfcrt were consistent with the measured CQ IC50s in French isolates.12 A particularity of Malagasy isolates at this point is that Pfcrt mutant alleles are almost totally absent from Madagascar14,15 and thus in vitro CQR Malagasy isolates did not present this type of mutations, but they frequently have single nucleotide polymorphisms (SNPs) in the Pfmdr1 gene.15,16
The discovery of the different ms4760 microsatellite profiles of Pfnhe-1 appeared as a burgeoning process as we reported 14 not previously described, which added to the many already known polymorphisms in this locus.5,7,17 The only association revealed by the multivariate analysis of our study, except the already known Pfcrt 76/in vitro CQ response association, was that of the number of NHNDNHNNDDD repeats with increased QN IC50s geometric mean. These results were conflicting with those of Henry and others,7 who reported an association of a greater number of NHNDNHNNDDD repeats with decreased QN IC50s. We did not confirm the previously reported associations of particular ms4760 profiles with in vitro QN response. Indeed, the ms4760-1 that was found by Ferdig and others5 as associated with higher QN IC50s was not confirmed by Henry and others who claimed that strains having another particular profile (ms4760-7) had reduced susceptibility to QN. In our series, isolates having either ms4760-1 or ms4760-7 had not particularly reduced susceptibility to QN. We did not confirm either the previously reported associations of the number of DNNND repeats with in vitro QN response.
Some hypotheses may explain our data. First, the associations previously reported could be geographically restricted for a part. Thus, among the 15 less susceptible strains of the series of Ferdig and others,5 11 strains originated from South-East Asia (mainly Thailand, Vietnam, and Cambodia) as only four strains among the 56 other more susceptible ones originated from South-East Asia. In the same way, five out the seven strains having the highest QN IC50 values in the work by Henry and others originated from South-East Asia as none of the 16 other more susceptible strains came from this region. Contrary to those previous studies, we did not include any isolate of Asian origin in our series. It may be underlined that at present P. falciparum QN resistance is much less frequently found in Africa than in Asia.4,18 Second, the likely multigenic nature of QN resistance and the probable strain-specific relative role of each molecular actor may hamper the accurate detection of reliable association of genetic polymorphisms with in vitro QN response. The contrasted results found by different teams about the associations of Pfnhe-1 polymorphisms with QN resistance were not without recalling us about the discussed status of the association of Pfmdr-1 N86Y mutation with CQ resistance, which fluctuated largely according to studies.19
In conclusion, the studied polymorphisms in PF13_0019 did not appear as valid molecular markers of in vitro QN response in our series of isolates originating from various African countries. At present, the study of these polymorphisms did not appear useful to monitor QN resistance in Africa and help policy markers.
Acknowledgments
We thank the patients and healthcare workers involved in the national network for the surveillance of malaria resistance in Madagascar (Réseau d'Etude de la Résistance, RER) from which the Malagasy samples were obtained, and the staff of the Ministry of Health of Madagascar.
Footnotes
Financial support: This study was supported by grants from Natixis/Impact Malaria through the Observatoire de la Résistance aux Antipaludiques Project and the Genomics Platform, Pasteur Génopôle, Pasteur Institute, France. Samples collection in France is part of surveillance of imported malaria, funded by Institut de Veille Sanitaire, Ministry of health. Samples collection in Madagascar was funded by the Global Fund project for Madagascar round 3 (Community Action to Roll Back Malaria grant no. MDG-304-G05-M). Valérie Andriantsoanirina is a graduate PhD student funded by the Institut Pasteur de Madagascar (Bourse « Girard ») and the Direction des Affaires Internationales (Institut Pasteur).
Authors' addresses: Valérie Andriantsoanirina and Stéphane Rabearimanana, Institut Pasteur de Madagascar, Unité de Recherche sur le Paludisme, Antananarivo, Madagascar. Didier Ménard, Institut Pasteur de Madagascar, Unité de Recherche sur le Paludisme, Antananarivo, Madagascar and Institut Pasteur, Unité d'Immunologie Moléculaire des Parasites, CNRS URA 2581, Paris, France. Véronique Hubert and Jacques Le Bras, Centre National de Référence du Paludisme, AP-HP, Hôpital Bichat-Claude Bernard, Université Paris Descartes, Paris, France. Christiane Bouchier and Magali Tichit, Institut Pasteur, Plate-forme Genomique, Paris, France. Rémy Durand, Laboratoire de Parasitologie-Mycologie, Hôpital Avicenne, AP-HP, Bobigny, France, E-mail: remy.durand@avc.aphp.fr.
References
- 1.Neiva A. Ueber die Bildung einur chininresistenten Rasse des Malariaparasiten. Mem Inst Oswaldo Cruz. 1910;2:131–140. [Google Scholar]
- 2.Nocht B, Werner H. Beobachtungen uber eine relative Chininresistenz bei malaria aus Brasilien. Dtsch Med Wochenschr. 1910;36:1557–1560. [Google Scholar]
- 3.Peters W. Chemotherapy and Drug Resistance in Malaria. Second edition. London: Academic Press; 1987. pp. 543–568. (Resistance of human malaria I, III, and IV). 593–658, 659–786. [Google Scholar]
- 4.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 5.Ferdig MT, Cooper RA, Mu J, Deng B, Joy DA, Su XZ, Wellems TE. Dissecting the loci of low-level quinine resistance in malaria parasites. Mol Microbiol. 2004;52:985–997. doi: 10.1111/j.1365-2958.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 6.Nkrumah LJ, Riegelphaupt PM, Moura P, Johnson DJ, Patel J, Hayton K, Ferdig MT. Probing the multifactorial basis of Plasmodium falciparum quinine resistance: evidence for a strain-specific contribution of the sodium-proton exchanger PfNHE. Mol Biochem Parasitol. 2009;165:122–131. doi: 10.1016/j.molbiopara.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry M, Briolant S, Zettor A, Pelleau S, Baragatti M, Baret E, Mosnier J, Amalvict R, Fusai T, Rogier C, Pradines B. Plasmodium falciparum Na+/H+ exchanger 1 transporter is involved in reduced susceptibility to quinine. Antimicrob Agents Chemother. 2009;53:1926–1930. doi: 10.1128/AAC.01243-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaddouri H, Nakache S, Houzé S, Mentré F, Le Bras J. Assessment of the drug susceptibility of Plasmodium falciparum clinical isolates from Africa by using a Plasmodium lactate dehydrogenase immunodetection assay and an inhibitory maximum effect model for precise measurement of the 50-percent inhibitory concentration. Antimicrob Agents Chemother. 2006;50:3343–3349. doi: 10.1128/AAC.00367-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rason MA, Randriantsoa T, Andrianantenaina H, Ratsimbasoa A, Menard D. Performance and reliability of the SYBR Green I-based assay for the routine monitoring of susceptibility of Plasmodium falciparum clinical isolates. Trans R Soc Trop Med Hyg. 2008;102:346–351. doi: 10.1016/j.trstmh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Rakotonirina H, Barnadas C, Raherijafy R, Andrianantenaina H, Ratsimbasoa A, Randrianasolo L, Jahevitra M, Andriantsoanirina V, Menard D. Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria-endemic countries. Am J Trop Med Hyg. 2008;78:217–221. [PubMed] [Google Scholar]
- 11.Ranford-Cartwright LC, Taylor J, Umasunthar T, Taylor LH, Babiker HA, Lell B, Schmidt-Ott JR, Lehman LG, Walliker D, Kremsner PG. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans R Soc Trop Med Hyg. 1997;91:719–724. doi: 10.1016/s0035-9203(97)90539-3. [DOI] [PubMed] [Google Scholar]
- 12.Durand R, Jafari S, Vauzelle J, Delabre JF, Jesic Z, Le Bras J. Analysis of pfcrt mutations and chloroquine susceptibility in isolates of Plasmodium falciparum. Mol Biochem Parasitol. 2001;114:95–102. doi: 10.1016/s0166-6851(01)00247-x. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary V, Sharma YD. Extensive heterozygosity in flanking microsatellites of Plasmodium falciparum Na+/H+ exchanger (pfnhe-1) gene among Indian isolates. Acta Trop. 2009;109:241–244. doi: 10.1016/j.actatropica.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Randrianarivelojosia M, Fidock DA, Belmonte O, Valderramos SG, Mercereau-Puijalon O, Ariey F. First evidence of pfcrt mutant Plasmodium falciparum in Madagascar. Trans R Soc Trop Med Hyg. 2006;100:826–830. doi: 10.1016/j.trstmh.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriantsoanirina V, Andrianaranjaka V, Ratsimbasoa A, Bouchier C, Jahevitra M, Rabearimanana S, Radrianjafy R, Randriantsoa T, Rason MA, Tichit M, Rabarijaona LP, Mercereau-Puijalon O, Durand R, Ménard D. Plasmodium falciparum drug resistance in Madagascar: facing the spread of unusual pfdhfr and pfmdr-1 haplotypes and the decrease of the dihydroartemisinin susceptibility. Antimicrob Agents Chemother. 2009;53:4588–4597. doi: 10.1128/AAC.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rason MA, Andrianantenaina HB, Ariey F, Raveloson A, Domarle O, Randrianarivelojosia M. Prevalent Pfmdr-1 N86Y mutant Plasmodium falciparum in Madagascar despite absence of Pfcrt mutant strains. Am J Trop Med Hyg. 2007;76:1079–1083. [PubMed] [Google Scholar]
- 17.Vinayak S, Alam MT, Upadhyay M, Das MK, Dev V, Singh N, Dash AP, Sharma YD. Extensive genetic diversity in the Plasmodium falciparum Na+/H+ exchanger 1 transporter protein implicated in quinine resistance. Antimicrob Agents Chemother. 2007;51:4508–4511. doi: 10.1128/AAC.00317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jelinek T, Grobusch MP, Loscher T. Patterns of Plasmodium falciparum drug resistance in nonimmune travellers to Africa. Eur J Clin Microbiol Infect Dis. 2001;20:284–286. doi: 10.1007/pl00011266. [DOI] [PubMed] [Google Scholar]
- 19.Picot S, Olliaro P, de Monbrison F, Bienvenu AL, Price RN, Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]


