Abstract
Accumulation of mutations in dihydrofolate reductase (dhfr) and dihydropteroate synthetase (dhps) is strongly associated with sulfadoxine-pyrimethamine (SP) treatment failure. Routine surveillance for these resistance markers was conducted annually at 26 sentinel sites in Maputo Province, Mozambique, before and after the phased deployment of artesunate plus SP (AS-SP), with 15,758 children sampled between 2004 and 2008. Mean asexual parasite prevalence, polymerase chain reaction (PCR) corrected, decreased from 44.2% in 2004 to 3.8% in 2008 (P < 0.0001). Among the 2,012 PCR-confirmed falciparum samples, the dhfr triple mutation remained close to fixation, whereas both dhps double and dhfr/dhps “quintuple” mutations increased from 11.0% in 2004, to 75.0% by 2008 (P < 0.0001). Adding artesunate to SP did not retard the spread of SP-resistant parasites. The high “quintuple” mutation prevalence suggests a limited useful therapeutic lifespan of AS-SP for treating uncomplicated malaria, and may curb efficacy of SP-monotherapy for intermittent preventive treatment in Mozambique.
Introduction
A major factor contributing to the continued public health burden of malaria is the spread of drug-resistant Plasmodium parasites.1,2 In response to the threat posed by antimalarial drug resistance the World Health Organization (WHO) has recommended a shift from antimalarial monotherapy, particularly chloroquine (CQ) and sulfadoxine-pyrimethamine (SP) to combination therapy.3 It is expected that combining antimalarials with differing modes of action would reduce the probability of a resistant (mutant) parasite surviving treatment.4 Artemisinin-based combination therapies (ACTs) are preferred to other combination therapies or monotherapies, as they have higher cure rates, more rapid parasite clearance times, and the potential to reduce malaria transmission caused by their gametocidal effect, further limiting the spread of antimalarial resistance.5–7
By 2008, 77 countries had adopted an ACT as their first line antimalarial treatment policy.8 Artesunate plus SP (AS-SP), one of the WHO recommended ACTs,7 has the operational advantages of lower cost and the full dose of the partner drug being administered as a single dose, but unfortunately is not suitable for manufacture as a fixed dose combination. Separate tablets, even if blister packed, allow patients to choose which drug to take, potentially negating the purported benefits of combination therapy. The widespread use of SP monotherapy has resulted in a high prevalence of SP-resistant Plasmodium falciparum isolates in many southern African countries.9–13 There may be further selection for these resistant parasites through the current large-scale use of SP-monotherapy for intermittent preventive treatment (IPT) of high-risk groups, particularly pregnant women.14
Resistance to SP develops because of an accumulation of single nucleotide polymorphisms in the dihydrofolate reductase (dhfr) and dihydropteroate synthetase (dhps) genes. The presence of three mutations in the dhfr gene (at codons 51, 59, and 108, known as the dhfr triple) and two mutations in the dhps gene (at codons 437 and 540, known as the dhps double), together referred to as the “quintuple mutation,” is strongly associated with in vivo and in vitro SP resistance in East and Southern Africa.10,11
Within the case management component of the Lubombo Spatial Development Initiative malaria control program,15 large-scale deployment of AS-SP as first line treatment of definitely diagnosed uncomplicated malaria commenced in Maputo Province, southern Mozambique, in 2004. This ACT was selected to replace chloroquine following in vivo efficacy trials, conducted from 2003 until 2005, which showed AS-SP to be highly effective with an adequate clinical and parasitological response of 98% at 42 days.16 This study, conducted on uncomplicated malaria patients 1 to 65 years of age, found an over 3-fold increased risk of recrudescence among patients infected with parasites carrying the “quintuple” mutation. Phased implementation began in the Namaacha District in April 2004 and included all districts in Maputo Province by May 2006 (Figure 1). Adequate supplies of AS-SP have been sustained in all public sector facilities since implementation of this policy, with ACT availability extended to the community health post level in 2006. This has ensured high ACT coverage following the deployment of this treatment policy.17
Figure 1.

Sentinel sites and AS-SP implementation date in Maputo Province, Mozambique.
Limiting the spread of antimalarial resistance is one of the key rationales motivating ACT deployment. However, surprisingly little research has been conducted on the spread of antimalarial resistance following large-scale ACT deployment, with almost all the research in this area being conducted in Asia.18,19 Antimalarial resistance is usually monitored through in vivo therapeutic efficacy studies, which are costly and may not be the most sensitive tool for detecting resistance given the contribution of partial immunity to clinical and parasitological treatment response.20 With highly effective malaria control programs, the marked reductions in malaria incidence decrease the feasibility of conducting adequately powered in vivo therapeutic efficacy studies. This study reports on the large-scale surveillance of dhfr and dhps mutations over a 5-year period before and after the wide-scale deployment of AS-SP as first line treatment of uncomplicated malaria in Maputo Province, southern Mozambique.
Materials and Methods
Study population and blood sample collection.
Finger-prick blood from a random sample of children (2 to 15 years of age) were collected during annual cross-sectional surveys of asexual parasite prevalence in 26 sentinel sites in Maputo Province, Mozambique (Figure 1) from 2004 to 2008.15,21 Areas within each district were identified as sentinel sites based on population size, geographical structure, and proximity to health facilities. Blood samples were collected on filter paper strips (Whatman filter paper no 1; Merck Laboratory Suppliers (Pty) Ltd., Durban, South Africa) from 120 children per sentinel site. The air dried filter paper blood spots were stored individually in zip-lock bags containing desiccant at room temperature until assayed.
Sample preparation and analysis.
Parasite DNA, from filter paper blood samples of rapid test (ICT; Global Diagnostics, Cape Town, South Africa) positive individuals, was extracted using the Chelex method.22 Once a sample was confirmed as P. falciparum positive by nested PCR,23 it was subjected to dhfr (codons 51, 59, 108, and 164) and dhps (codons 436, 437, 540, and 518) mutational analysis.24 Digestion products separated on a 2% agarose gel using electrophoresis were visualized and photographed using the MiniBIS documentation system (BioSystematica, Ceredigion Wales, UK). Codons were classified as either pure wild type, pure mutant, or mixed (both wild type and mutant haplotypes present in an individual sample). All genotyping analyses were run in duplicate with a third trial conducted on discordant results. When calculating overall prevalence of P. falciparum isolates with mutant codons, codons with mixed genotypes were analyzed together with pure mutant codons.
Statistical analysis.
Statistical analysis was performed using Stata 10 (Stata Corp., College Station, TX). The prevalence of dhfr and dhps mutations were calculated for individual years and odds ratios (ORs), relative to 2004, and the association between “quintuple” mutation prevalence and prospectively defined explanatory variables (time since the introduction of AS-SP, time since deployment of SP-IPTp, age, and parasite prevalence) was assessed using a multilevel mixed effects logistic regression model.25 Spearman's correlation coefficients were used to quantify the level of association between year and these more biologically plausible explanatory variables. “Quintuple mutation” prevalence and parasite prevalence were assumed constant within each site for each study year. Sentinel sites were nested within districts, within which time since AS-SP and SP-IPT deployment remained constant. Within site and within district correlations of responses, and the number of children surveyed, were taken into account in the estimation of 95% confidence intervals (CIs).
Ethical considerations.
Ethics approval for this study was obtained from the South African Medical Research Council and the Ministry of Health in Mozambique. Blood samples were only taken if full informed consent from a parent/guardian had been obtained. Children testing positive for malaria were referred to the closest health facility for appropriate treatment.
Results
A total of 15,758 samples were collected over the 5-year study period, of which 2,361 (15.0%) were rapid test positive for P. falciparum. DNA could be extracted from 2,329 (98.6%) rapid test positive samples of which 2,012 (86.4%) were confirmed as P. falciparum positive by PCR. This discrepancy could partially be explained by the rapid test detection of the histidine-rich protein 2 malaria antigens in children in whom all parasites had been cleared 2 to 4 weeks previously.26 Mean asexual parasite prevalence, based on PCR results, decreased from 44.2% in 2004 to 3.8% in 2008 (rate ratio [RR] 0.09; 95% CI: 0.07–0.11; P < 0.0001, Table 1). Mixed genotypes were detected in 1030 (51%) samples.
Table 1.
Parasite prevalence (%) based on polymerase chain reaction (PCR) results by district and year
| District | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|
| Namaacha | 4.6% (11/239) | 3.9% (10/256) | 3.8% (10/263) | 1.7% (2/118) | 1.0% (2/200) |
| Matutuine | 8.5% (45/529) | 6.3% (18/286) | 3.3% (19/576) | 2.4% (9/375) | 1.5% (7/467) |
| Boane | 28.0% (87/311) | 15.3% (46/301) | 12.8% (58/453) | 1.4% (4/286) | 4.0% (14/350) |
| Marracuene | 29.5% (115/390) | 34.4% (97/282) | 21.4% (68/318) | 4.0% (12/300) | 2.9% (10/345) |
| Magude | 62.0% (337/544) | 40.7% (190/467) | 24.2% (123/508) | 11.1% (54/486) | 5.2% (18/346) |
| Moamba | 42.5% (16 /393) | 18.3% (81/443) | 19.3% (59/306) | 6.2% (9/145) | 2.1% (9/429) |
| Matola | 36.8% (79/215) | 47.9% (104/217) | 34.8% (71/204) | 37.1% (51/137) | 5.1% (16/314) |
| Total | 44.2% (841/1903) | 33.7% (546/1620) | 21.8% (408/1872) | 18.6% (141/758) | 3.8% (76/2000) |
No mutant alleles were detected in any of the samples tested at dhfr codon 164 and dhps codon 581. The dhps 436 mutation was rare, with a prevalence of 0.003%.
Before the introduction of AS-SP, dhfr triple mutation prevalence in Maputo Province was at 91.7%, increasing to 96.3% by 2005, and remaining close to fixation (> 98%) for the remainder of the study period (Figure 2A).
Figure 2.
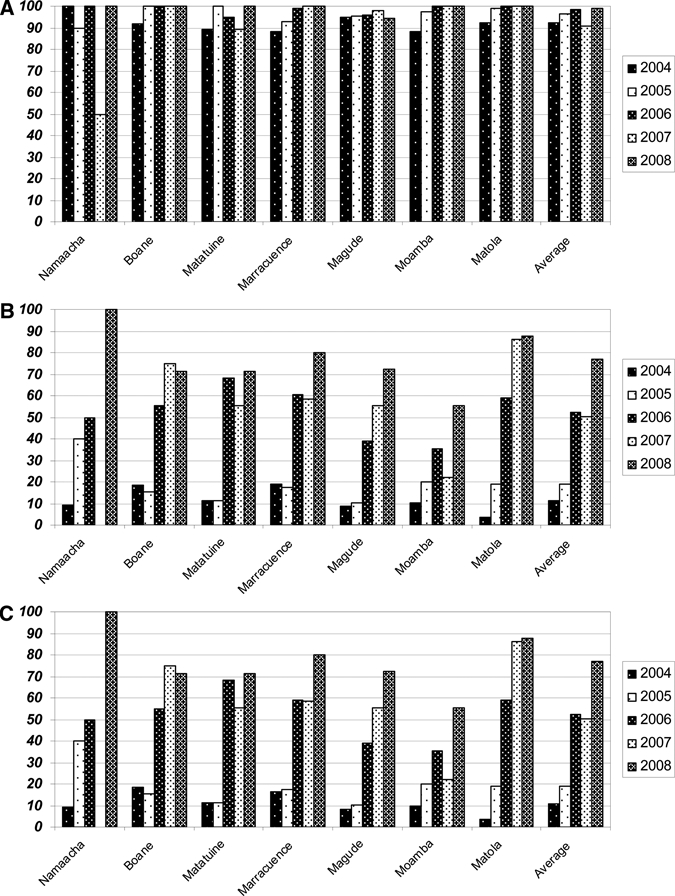
Prevalence of the (A) dhfr triple mutation, (B) dhps double mutation, and (C) “quintuple” mutation by district and year in Maputo Province, Mozambique.
Prevalence of the dhps double was below 20% throughout all districts in Maputo Province before the introduction of AS-SP, with a mean prevalence of 11% (Figure 2B). However, as AS-SP implementation progressed through Maputo Province, the dhps double mutation prevalence began increasing, reaching high levels (64.5%) in 2007, a year after AS-SP and IPT with SP monotherapy had been deployed nationally (OR = 21.3; 95% CI: 12.6–36.3; P < 0.0001, Figure 2B). This increase in prevalence continued, reaching 75.0% by 2008 (OR = 40.4; 95% CI: 20.4–80.3; P < 0.0001, Figure 2B).
Because the dhfr triple mutation was already at fixation in 2004, the prevalence of parasites carrying the “quintuple” mutation tracked the dhps double mutation prevalence. “Quintuple” mutation prevalence had increased markedly by 2006 (OR = 9.4; 95% CI: 6.9–13.0; P < 0.0001) and continued to increase in 2007 (OR = 22.1; 95% CI: 13.0–37.6; P < 0.0001), reaching 75.0% by 2008 (OR = 42.2; 95% CI: 21.3–83.7; P < 0.0001; Figure 2C).
Univariate and multiple logistic regression analysis were used to explore whether prospectively defined variables could explain the observed increase in “quintuple” mutation prevalence over time (Table 2). Univariate analysis showed a positive relationship between “quintuple” mutation prevalence and months since artesunate plus SP deployment (OR = 1.11; 95% CI: 1.10–1.12; P < 0.0001), years since deployment of SP IPTp policy (OR = 4.40; 95% CI: 3.43–5.65; P < 0.0001), and fever (OR = 2.26; 95% CI: 1.32–3.87; P = 0.003). There was a negative association with sentinel site parasite prevalence (OR = 0.95; 95% CI: 0.94–0.95; P < 0.0001) and with age (OR = 0.95; 95% CI: 0.93–0.98; P = 0.003). There was strong co-linearity between calendar year and duration of AS-SP use (R = 0.83); a weak negative correlation with parasite prevalence (R = 0.49) but none with age (R = 0.04) (Figure 3). As SP-IPTp was implemented nationally (and thus in all study sites) in mid 2006 there was perfect correlation of this explanatory variable with the year after 2005. Probably as a result of these correlations with time, only age and fever remained significantly associated with “quintuple” mutation prevalence after adjusting for confounding with study year (Table 2). No association between “quintuple” mutation prevalence and gender (P = 0.31) was found.
Table 2.
Factors associated with “quintuple” mutation prevalence in Maputo Province between 2004 and 2008 (within district and site correlations are taken into account in the estimation of confidence intervals)
| Unadjusted OR | 95% CI* | P value | Adjusted OR | 95% CI* | P value | |
|---|---|---|---|---|---|---|
| 2004 | 1.0 | – | – | 1.0 | – | – |
| 2005 | 1.56 | 1.12–2.18 | 0.009 | 1.50 | 1.05–2.14 | 0.026 |
| 2006 | 9.44 | 6.86–13.00 | < 0.0001 | 9.30 | 5.48–15.80 | < 0.0001 |
| 2007 | 22.14 | 13.03–37.60 | < 0.0001 | 21.47 | 8.48–54.34 | < 0.0001 |
| 2008 | 42.24 | 21.32–83.67 | < 0.0001 | 37.04 | 9.45–145.19 | < 0.0001 |
| Duration AS-SP use (months) | 1.11 | 1.10–1.12 | < 0.0001 | 0.99 | 0.95–1.02 | 0.416 |
| Duration SP-IPTp use (years) | 4.40 | 3.43–5.65 | < 0.0001 | † | † | † |
| Age (years) | 0.95 | 0.93–0.99 | 0.003 | 0.92 | 0.89–0.96 | < 0.0001 |
| Parasite prevalence (%) | 0.95 | 0.94–0.95 | < 0.0001 | 0.99 | 0.98–1.00 | 0.290 |
| Gender | 0.90 | 0.74–1.11 | 0.311 | 0.84 | 0.66–1.07 | 0.152 |
| Fever | 2.26 | 1.32–3.87 | 0.003 | 2.35 | 1.29–4.29 | 0.005 |
Within site and within district correlations of mutation frequency were taken into account in the estimation of 95% confidence intervals (CI).
The adjusted odds ratio (OR) for the effect of intermittent preventive treatment in pregnancy (IPTp) could not be evaluated because of perfect correlation with the year after 2005.
Figure 3.
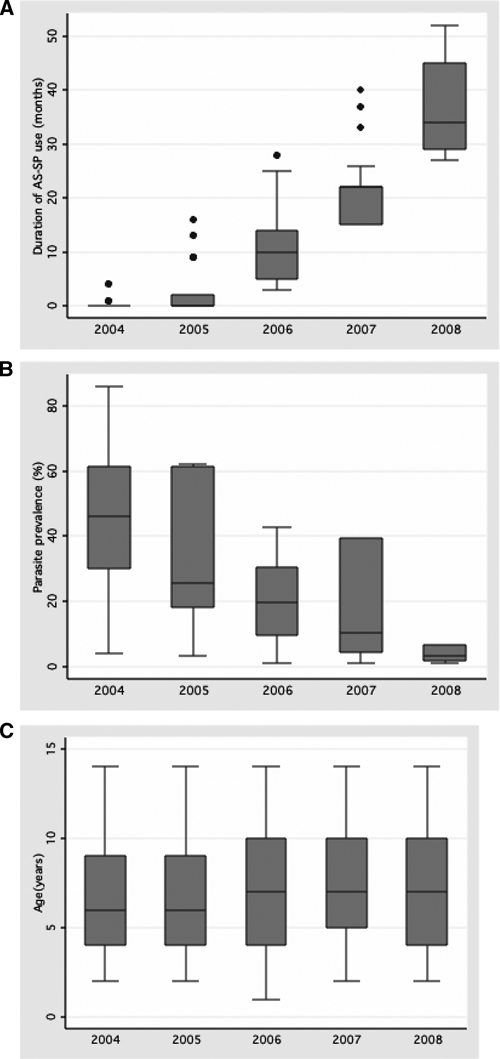
Correlation between study year and (A) duration of AS-SP use (R = 0.84), (B) parasite prevalence (R = −0.49), and (C) age (R = 0.04).
Discussion
We report the first data documenting the routine surveillance of temporal changes in resistance after large-scale systematic deployment of an ACT in Africa. Following the phased deployment of AS-SP in Maputo Province, southern Mozambique, dhfr triple mutation prevalence remained at fixation. More importantly, the prevalence of parasites with the dhps double, associated with sulfadoxine resistance, and “quintuple” mutations, associated with SP treatment failure, increased rapidly, both reaching an overall prevalence of 75% by 2008. Our findings suggest that the systematic, large-scale deployment of artesunate plus SP, as first line treatment of uncomplicated malaria since 2004, has not delayed the spread of SP resistance markers and may be contributing to the selection of parasites carrying these resistance markers.
These findings contrast sharply with the observed decrease in mefloquine resistance after the systematic deployment of the artesunate-mefloquine combination on the north-west border of Thailand,27,28 although falciparum malaria transmission reduced significantly over the study periods in both locations.15,27 Both mefloquine and SP have a long elimination half-life, which provides a selective filter for resistant parasites acquired elsewhere.29–32 A plausible explanation for these contrasting results is that on the north-west border of Thailand there is minimal local transmission, with most malarial infections originating in neighboring Burma, where the limited availability of mefloquine has resulted in most falciparum isolates being mefloquine sensitive. This is not the case in Mozambique where most neighboring countries show a high prevalence of parasites carrying the dhfr and dhps mutations.11–13,21
There are a number of local factors other than the region wide increase in dhfr and dhps mutations that may have contributed to the alarmingly rapid spread of SP-resistant parasites in Maputo Province after the systematic large-scale deployment of AS-SP. These include the fixation of the dhfr triple mutation before the commencement of AS-SP deployment in 2004,21 and the use of SP monotherapy for IPT of pregnant women since 2006. People with resistant parasites tend to have enhanced gametocyte carriage and are more infectious to mosquitoes than individuals with wild parasites, even during the primary infection.33,34 Although gametocyte carriage is substantially reduced after the addition of AS to SP,35 gametocyte transmission to mosquitoes is not completely halted36 and data are needed to determine whether this ACT alters the ratio of resistant to sensitive infections seen after SP monotherapy.
In addition to the factors listed previously that could have contributed to the rapid selection and spread of SP-resistant falciparum parasites in southern Mozambique, pharmacokinetic studies have shown that children less than 5 years of age have been systematically under-dosed with the currently recommended SP dose.37 Sub-therapeutic drug concentrations together with lack of acquired immunity could explain the significantly increased risk of mutations found in parasites infecting young children in Maputo Province.16
For the benefits of ACTs to be realized, it is essential that the individual component drugs are effective in their own right. This is most critical now that artemisinin resistance has been confirmed in South East Asia38,39 In infections with concomitant resistance to the longer-acting partner drug, ACTs provide selective pressure for artemisinin resistance. It is imperative that resistance in partner drugs is closely monitored, to ensure that national treatment policies remain effective. This is of particular importance in countries where malaria control interventions have been successful, markedly reducing malaria incidence and thereby limiting the feasibility of in vivo therapeutic efficacy studies.
Although the negative association between parasite prevalence and “quintuple” mutation prevalence was not confirmed in our multivariable analysis, the primary sources of antimalarial drug resistance historically have been low intensity transmission areas. Because of the lack of immunity in these populations, most infections are symptomatic, resulting in increased treatment seeking behavior and drug pressure. This is becoming increasingly pertinent in Africa in light of the recent successes in drastically reducing the malaria burden in countries with previously high transmission intensities, including Kenya, Rwanda, Tanzania, Zanzibar, The Gambia, Eritrea, Equatorial Guinea, and southern Mozambique.40
The role of SP monotherapy for IPT may need to be reconsidered in light of our data showing that the “quintuple” mutation is nearing fixation in southern Mozambique, and the potential contribution of SP-IPTp to the spread of SP resistance.14 Although a relationship between SP resistance and the benefits provided by SP-IPTp must exist, the SP resistance threshold at which IPTp ceases to be effective has not yet been determined.41
African countries with high pre-existing levels of SP resistance have achieved poor cure rates using AS-SP.42,43 Although AS-SP was shown to be highly efficacious at the start of ACT roll out,16 the high pre-existing dhfr triple mutation prevalence at the time of AS-SP implementation, together with the rather sharp increase in both the dhps double and “quintuple” mutations following ACT implementation, is cause for concern. Allen and colleagues16 showed the presence of the “quintuple” mutation resulted in a 3-fold increased risk of treatment failure in Maputo Province, after adjusting for treatment arm, age, and temperature. Despite the marked increase in the “quintuple” mutation over the study period, the dhfr 164 mutation, associated with highly pyrimethamine-resistant parasites was not detected.
The dramatic reduction in asexual parasite prevalence in Maputo Province can be attributed to a combination of intensive indoor residual spraying15 and effective case management following the AS-SP deployment. For this impressive malaria control program to be sustained it is essential that effective insecticides and antimalarials continue to be used. The steep rise in “quintuple” mutations found in this study points to a reduced useful therapeutic lifespan of AS-SP. The low asexual parasite prevalence and high “quintuple” mutation prevalence found in our study, combined with the ongoing use of SP monotherapy for IPTp, could provide favorable conditions for artesunate resistance emergence. Additional concerns were that SP dosing is probably sub-optimal in young children and that AS-SP could not be provided as a fixed dose combination. On these grounds a change in antimalarial treatment policy to artemether-lumefantrine is currently being implemented by the Mozambican Ministry of Health.
Supplementary Material
Acknowledgments
We thank the Global Fund to fight AIDS, Tuberculosis and Malaria and the South African Medical Research Council for financial support without which this study would not have been possible, the Lubombo Spatial Development Initiative staff involved in the annual cross sectional surveys, V. Kelly for assistance with mutational assays, Natashia Morris for GIS support, and members of the Database section of the Malaria Research Programme for assistance with database management. The contents of this manuscript were greatly enhanced following discussions at the Drug Strategies meeting hosted by Resources for the Future, funded by the Bill & Melinda Gates Foundation in Mpumalanga, South Africa (30 March–4 April 2008) and by the comments of the anonymous reviewers.
Disclaimer: The authors have no conflict of interest to declare.
Note: Supplemental appendix appears online at www.ajtmh.org.
Footnotes
Financial support: The Global Fund to fight AIDS, Tuberculosis and Malaria (grants MAF-202-GO1-M-00 and MAF-202-GO2-M-00).
Authors' addresses: Jaishree Raman and Rajendra Maharaj, Malaria Research Programme, Medical Research Council, KwaZulu-Natal, South Africa, E-mails: jaishree.raman@mrc.ac.za and rajendra.maharaj@mrc.ac.za. Francesca Little, Department of Statistical Sciences, University of Cape Town, Cape Town, South Africa, E-mail: francesca.little@uct.ac.za. Cally Roper, Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, England, E-mail: cally.roper@lshtm.ac.uk. Immo Kleinschmidt, Tropical Epidemiology Group, Department of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London, England, E-mail: immo.kleinschmidt@lshtm.ac.uk. Yasmin Cassam, Direccao Provincial de Saude de Maputo, Cidade Da Matola, Maputo, Mozambique, E-mail: yascassam@gmail.com. Karen I. Barnes, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Groote Schuur Hospital, Cape Town, Western Cape, South Africa, E-mail: karen.barnes@uct.ac.za.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2004;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olliaro P. Drug resistance hampers our capacity to roll back malaria. Clin Infect Dis. 2005;41((Suppl 4)):S247–S257. doi: 10.1086/430785. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. 2003. http://www.who.int/malaria/docs/ProtocolWHO.pdf Available at.
- 4.White NJ. Antimalarial drug resistance and chemotherapy. Philos Trans R Soc Lond B Biol Sci. 1999;354:739–749. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate combinations for the treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GAT. Reduction of malaria transmission to Anopheles mosquitoes with a six dose regimen of co-artmether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Guidelines for the treatment of malaria. 2006. http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf Available at.
- 8.World Health Organization World Malaria Report. 2008. http://www.who.int/malaria/wmr2008 Available at.
- 9.Enosse S, Magnussen P, Abacassamo F, Gomez-Olive X, Ronn AM, Thompson R, Alifrangis M. Rapid increase of Plasmodium falciparum dhfr/dhps resistant haplotypes after the adoption of sulphadoxine-pyrimethamine as first line treatment in 2002, in southern Mozambique. Malar J. 2008;7:115. doi: 10.1186/1475-2875-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bwijo B, Kaneko A, Takechi M, Zungu IL, Moriyama Y, Lum JK, Tsukahara T, Mita T, Takahashi N, Bergqvist Y, Bjorkman A, Kobayakawa T. High prevalence of quintuple mutant dhps/dhfr genes in Plasmodium falicaparum infections seven years after introduction of sulfadoxine and pyrimethamine as first line treatment in Malawi. Acta Trop. 2003;85:363–373. doi: 10.1016/s0001-706x(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 11.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, Chandramohan D, Sharp B. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 12.Nkhoma S, Molyneux M, Ward S. Molecular surveillance for drug-resistant Plasmodium falciparum malaria in Malawi. Acta Trop. 2007;102:138–142. doi: 10.1016/j.actatropica.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Mlambo G, Sullivan D, Mutambu SL, Soko W, Mbedzi J, Chivenga J, Gemperli A, Kumar N. High prevalence of molecular markers for resistance to choloroquine and pyrimethamine in Plasmodium falciparum from Zimbabwe. Parasitol Res. 2007;101:1147–1151. doi: 10.1007/s00436-007-0597-5. [DOI] [PubMed] [Google Scholar]
- 14.O'Meara WP, Smith DL, McKenzie FE. Potential impact of intermittent preventive treatment on the spread of drug resistant malaria. PLoS Med. 2006;3:e141. doi: 10.1371/journal.pmed.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp BL, Kleinschmidt I, Streat E, Maharaj R, Barnes KI, Durrheim DN, Ridl FC, Morris N, Seocharan I, Kunene S, La Granga JJP, Mthembu JD, Maartens F, Martin CL, Barreto A. Seven years of regional malaria control collaboration–Mozambique, South Africa and Swaziland. Am J Trop Med Hyg. 2007;76:42–72. [PMC free article] [PubMed] [Google Scholar]
- 16.Allen EN, Little F, Camba T, Cassam Y, Raman J, Boulle A, Barnes KI. Efficacy of sulphadoxine-pyrimethamine with or without artesunate for the treatment of uncomplicated Plasmodium falciparum malaria in southern Mozambique: a randomized controlled trial. Malar J. 2009;8:141. doi: 10.1186/1475-2875-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The LSDI Annual Report Lubombo Spatial Development Initiataive, Mapato Province, Annual Report, 2009. 2009. http://www.malaria.org.za/lsdi/Reports/2009/LSDIMaputoAnnualReport2009.pdf Available at.
- 18.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Laing K, Looareesuwan S, White NJ, Nosten F, Krishna S. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah NK, Alker AP, Sem R, Susanti AI, Muth S, Maguire JD, Duong S, Areiy F, Meshnick SR, Wongsrichanalai C. Molecular surveillance for multidrug-resistant Plasmodium falciparum, Cambodia. Emerg Infect Dis. 2008;14:1637–1640. doi: 10.3201/eid1410.080080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talisuna AO, Nalunkuma-Kazibwe A, Langi P, Mutabingwa TK, Watkins WW, Van Marck E, Egwang TG, D'Alsessandro U. Two mutations in dihydrofolate reductase combined with one in the dihydropteroate sunthase gene predict sulphadoxine-pryimethamine parasitological failure in Ugandan children with uncomplicated falciparum malaria. Infect Genet Evol. 2004;4:321–327. doi: 10.1016/j.meegid.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Raman J, Sharp B, Kleinschmidt I, Roper C, Streat E, Kelly V, Barnes KI. Differential effect of regional drug pressure on dihydrofolate reductase and dihydropteroate synthetase mutations in southern Mozambique. Am J Trop Med Hyg. 2008;78:256–261. [PMC free article] [PubMed] [Google Scholar]
- 22.Wooden J, Keyes S, Sibley CH. PCR and strain identification in Plasmodium falciparum. Parasitol Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-x. [DOI] [PubMed] [Google Scholar]
- 23.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity to detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biol Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 24.Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA, Estrada-France JG, Mollinedo R, Avila JC, Cespedes JL, Carter D, Doumbo OK. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- 25.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modelling Using Stata. College Station, TX; StataCorp LP: 2005. [Google Scholar]
- 26.Hopkins H, Kambale W, Kamya MR, Staedke SG, Dorsey G, Rosenthal PJ. Comparison HRP2- and pLDH-based rapid diagnostic tests for malaria with longitudinal follow-up in Kampala, Uganda. Am J Trop Med Hyg. 2007;78:256–261. [PubMed] [Google Scholar]
- 27.Nosten F, van Vugt M, Price R, Luxemburger C, Thway KL, Broackman A, McGready R, ter Kuile F, Looareesuwan S, White NJ. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand. Lancet. 2000;356:297–302. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 28.Brockman A, Price RN, van Vugt M, Heppner DG, Walsh D, Sookto P, Wimonwattrawatee T, Looareesuwan S, White NJ, Nosten F. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans R Soc Trop Med Hyg. 2000;94:537–544. doi: 10.1016/s0035-9203(00)90080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins WM, Mosobo M. Treatment of Plasmodium falciparum malaria with pyrimethamine-sulfadoxine: selective pressure for resistance is a function of long elimination half live. Trans R Soc Trop Med Hyg. 1993;87:75–78. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- 30.Zongo I, Dorsey G, Rouamba N, Tinto H, Dokomajilar C, Guiguemde RT, Rosenthal PJ, Ouedraogo JB. Artemether-lumefantrine versus amodiaquine plus sufaldoxine-pyrimethamine for uncomplicated malaria in Burkina Faso: a randomised non-inferiority trial. Lancet. 2007;369:491–498. doi: 10.1016/S0140-6736(07)60236-0. [DOI] [PubMed] [Google Scholar]
- 31.Bell DJ, Nyirongo SK, Mukaka M, Zijlstra EE, Plowe CV, Molyneux ME, Ward SA, Winstanley PA. Sulfadoxine-pyrimethamine-based combinations for malaria: a randomised blinded trial to compare efficacy, safety and selection of resistance in Malawi. PLoS One. 2008;3:e1578. doi: 10.1371/journal.pone.0001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlemann A-C, McGready R, Ashley EA, Brockman A, Singhasivanon P, Krishna S, White NJ, Nosten F, Price RN. Intrahost selection of Plasmodium falciparum pfmdr1 alleles after antimalarial treatment on the northwestern border of Thailand. J Infect Dis. 2007;195:134–141. doi: 10.1086/509809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes KI, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, Roper C, Watkins B, White NJ. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J Infect Dis. 2008;197:1605–1613. doi: 10.1086/587645. [DOI] [PubMed] [Google Scholar]
- 34.Mabuza A, Govere J, La Grange K, Mngomezulu N, Allen E, Zitha A, Mbokazi F, Durrheim D, Barnes K. Therapeutic efficacy of sulfadoxine-pyrimethamine for Plasmodium falciparum malaria. S Afr Med J. 2005;95:346–349. [PubMed] [Google Scholar]
- 35.Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate combinations for treatment of malaria: meta-analysis. International Artemisinin Study Group. Lancet. 2004;363:18–22. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 36.Targett G, Drakeley C, Jawara M, von Seidlein L, Coleman R, Deen J, Pinder M, Doherty T, Sutherland C, Walraven G, Milligan P. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis. 2001;183:1254–1259. doi: 10.1086/319689. [DOI] [PubMed] [Google Scholar]
- 37.Barnes KI, Little F, Smith PJ, Evans A, Watkins WM, White NJ. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin Pharmacol Ther. 2006;80:582–596. doi: 10.1016/j.clpt.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Artemisinin resistance in Cambodia 1 (ARC1) study consortium. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 39.Dondorp AM, Nosten F, Poravuth P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization World Malaria Report. 2008. http://www.who.int/malaria/wmr2008 Available at.
- 41.World Health Organization Technical expert group meeting on intermittent preventive treatment in pregnancy (IPTp) 2008. http://apps.who.int/malaria/docs/IPTp/TechnicalExpertMtgIPTpReport.pdf Available at.
- 42.Dorsey G, Njama D, Kamya MR, Cattamanchi A, Kyabayinze D, Staedke SG, Gasasira A, Rosenthal PJ. Sulfadoxine/pyrimethamine alone or with amodiaquine or artesunate for treatment of uncomplicated malaria: a longitudinal randomised trial. Lancet. 2002;360:2031–2038. doi: 10.1016/S0140-6736(02)12021-6. [DOI] [PubMed] [Google Scholar]
- 43.Rwagacondo CE, Niyitegeka F, Sarushi J, Karema C, Mugisha V, Dujardin JC, Van Overmeir C, van den Ende J, D'Alessandro U. Efficacy of amodiaquine alone and combined with sulfadoxine-pyrimethamine and of sulfadoxine-pyrimethamine combined with artesunate. Am J Trop Med Hyg. 2003;68:743–747. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


