Abstract
To assess water contamination and the relative effectiveness of three options for point-of-use water treatment in South India, we conducted a 6-month randomized, controlled intervention trial using chlorine, Moringa oleifera seeds, a closed valved container, and controls. One hundred twenty-six families participated. Approximately 70% of public drinking water sources had thermotolerant coliform counts > 100/100 mL. Neither M. oleifera seeds nor containers reduced coliform counts in water samples from participants' homes. Chlorine reduced thermotolerant coliform counts to potable levels, but was less acceptable to participants. Laboratory testing of M. oleifera seeds in water from the village confirmed the lack of reduction in coliform counts, in contrast to the improvement seen with Escherichia coli seeded distilled water. This discrepancy merits further study, as M. oleifera was effective in reducing coliform counts in other studies and compliance with Moringa use in this study was high.
Introduction
In developing countries, 2.6 billion people lack access to basic sanitation and 1.1 billion do not have access to improved water sources. This combination leads to 1.6 million deaths each year from preventable diarrheal diseases, 90% of which are among children less than 5 years of age.1 The Millennium Development Goals' target for improvement is to reduce by half the proportion of people without access to safe drinking water by 2015.2 The optimal approach to preventing water-borne diseases includes the construction of water disinfection and delivery systems and sewage treatment facilities, which is expensive and time-consuming. In tropical developing countries, even if facilities are developed, their maintenance and monitoring may not be reliable because of a variety of factors.
Previous studies in India have shown that many of the public water sources that serve both the peri-urban and rural areas are often contaminated and the type of pathogens vary in each outbreak.3–6 Even when population-based methods of water purification, such as chlorination of tanks, are successful, practices in the home, such as dipping tumblers or cups into wide-mouthed containers to obtain water, can cause recontamination.7 Studies have shown that in the absence of recontamination, counts of thermotolerant coliforms will decrease significantly over hours to days in stored water samples.8 Water storage containers, which prevent in-home contamination, can lead to almost 70% reduction in coliform counts and 31% reduction in diarrhea in children.9 A combination of point-of-use disinfection methods and prevention of recontamination is likely optimal, but difficult to achieve. Boiling, one of the most common methods of disinfection used in settings with contaminated water sources, is expensive and time-consuming.10 Two promising alternative technologies for point-of-use decontamination are the use of M. oleifera seed preparations and in-home chlorination.
The use of natural materials of plant origin to clarify turbid surface waters can be found in many cultures. Sanskrit writings in India dating from several centuries BC make reference to seeds of the tree Strychnos potatorum as a clarifier. Moringa oleifera seeds were first used for domestic household water treatment by women in the Sudan, who placed powdered seeds in a small cloth bag that was then swirled in turbid water.11,12 Laboratory testing of the seeds of the M. oleifera show that the seeds flocculate bacteria by low molecular weight cationic water soluble proteins that attach themselves to suspended particles, including bacteria, and thereby assist in the removal of harmful pathogens from water.11,13–15 Safety tests have shown that doses typically used for water treatment posed no serious threat to human health;16,17 however, few interventions using Moringa have been tested against bacterial contamination in field trials.13,18 The Moringa tree grows wild over much of India, and most of the population recognizes it as an ingredient in many sauces, with the colloquial name, “drumstick.” Its effectiveness and acceptability in water decontamination, however, has not yet been assessed in the rural setting in India.
Chlorine is the cornerstone of most municipal water purification systems, and outbreaks have been noted to occur when chlorine has not been regularly administered.19 The Latin American cholera outbreak in 2001 led the Pan American Health Organization (PAHO) and the Centers for Disease Control and Prevention (CDC) to develop the Safe Water System,20 a chlorine-based intervention for in-home decontamination of water. Previous studies on chlorine use in disinfecting water for drinking have showed a marked decrease in coliform counts in beverages using disinfected water and in diarrheal episodes in homes using chlorine in conjunction with health education and designated water storage vessels.21,22 Despite concerns about safety and long-term effects,23 the positive impact of chlorine on preventing disease is undeniable and it remains the cornerstone of the movement for access to clean water around the world. Currently in India, chlorine-based bleaching powders are used for disinfection of most water supply systems on a regular basis.24
Unfortunately, one of the limiting factors in developing effective methods for water decontamination is the acceptability to the population. Chlorine in particular has been noted to be unacceptable to many participants in previous studies.9,25 Freeman and others26 showed that 30% of participants who had tried Klorin (a chlorine-based water treatment, Jet Chemicals Ltd., Kenya) disliked the smell or taste and only 50% of persons who had used chlorine at least once continued to use it. Other studies have shown even lower levels of continued use.27
Given the suboptimal quality of most water available to rural Indian communities, this 6-month randomized, controlled intervention trial aimed to assess the relative efficacy and acceptability of three specific interventions in the rural Indian context: closed valved containers, M. oleifera seeds, and chlorine.
Materials and Methods
Study area and participants.
The study took place in Adukamparai Kattupadi, a village with a population of 239 households located 15 km outside of Vellore, Tamil Nadu, a city with a population of 400,000 approximately 3 hours from Chennai (Madras). The study protocol was approved by the Research Committee of Christian Medical College. An initial evaluation of households in the village determined who was responsible for collecting water in the home, and whether they boiled the water before drinking it. In the event that more than one family inhabited a single structure, only one “household” (defined as an individual or group of people using a common cooking area) was chosen. Initially, those members of the community who reported boiling their water regularly were to be excluded from the study, but were later included because the water in most households was found to be microbially contaminated.
Drinking water is provided to the village from a nearby government-maintained deep bore well. Every night, the water is pumped from the well to a main overhead tank at the periphery of the village. This tank is cleaned, chlorinated, and maintained by the government. Each morning the water flows from this main tank through a series of public taps that run for approximately 2–3 hours each morning. Women in each household collect water each morning and store it in containers in the home for use throughout the day. The following morning, the previous day's water is discarded, containers are rinsed, and new water is collected.
After consent was obtained, the women in the participating households were interviewed to assess their knowledge, attitudes, and practices regarding water, hygiene, and water-borne disease. A baseline water sample was taken to determine the level of contamination in the household's drinking water. Additionally, four focus groups of approximately 10 women per group were held to determine the general consensus on the current beliefs and practices of the village. This information was compiled and considered to be the “baseline data” for the village, before the intervention.
After the baseline survey was completed, all women who had consented to participate in the baseline study were stratified by membership in one of the two predominant castes in the community (Harijan and Reddiar) and then randomized to one of four intervention arms. The stratification was done to ensure similar numbers of each caste in each intervention arm because socioeconomic status and education were significantly associated with caste. The randomization was done using a random number table.28 The study coordinator visited each participating household, informed them of the level of contamination in their drinking water, delivered a health education message, and then described the study.
Health education intervention.
Each woman participating in the study received a health education message at the first visit of the intervention study, and again at each subsequent visit. The topics covered in the health education included: the importance of using soap for hand washing, particularly after defecation; instructions on designating separate drinking water vessels, keeping them covered, and cleaning them with soap; and information on preventing contamination of drinking water by not allowing hands to touch the water.
Intervention.
The four arms were a closed valved container (20 L plastic container with tap purchased locally), M. oleifera seeds (Veg India exports, Erode, India), chlorine (sodium hypochlorite 5%), and control. Sample size was calculated to detect a 25% difference between the percentage of potable samples (< 1 thermotolerant coliform/100 mL) between the four intervention arms with 80% power and significance of 0.05. Each arm required at least 27 participants. A total of 127 women completed the initial questionnaire, and 126 participated in the intervention. After randomization, the participants were instructed in the method for implementing the assigned intervention.
For the group assigned to receive the container, specific instructions were given about the importance of keeping the container tightly closed and that all members of the household should drink water from that container exclusively. For the M. oleifera group, the participants were instructed to take 1 seed per 5 L (generally 2 seeds per container) and crush it into a powder using a clean piece of paper and a hard object; put the M. oleifera powder into the water container and allow it to sit. Initially, the duration was left to the participants' preference, as some preferred to have a second container in circulation rather than wait 1 hour for the water to be clean; however, after laboratory tests indicated that the maximum effect was achieved after 4 hours, all participants were instructed to allow the water to sit for at least 4 hours or overnight. The “Moringa water” was then poured through a plastic mesh filter into a second container and this water was used for drinking and cooking. For the group using chlorine, participants were given liquid sodium hypochlorite and instructed in the use of a small plastic pipette marked with the amount of chlorine necessary for a 12 L standard sized container, calculated to result in a chlorine concentration of 1 ppm (~1 mL liquid sodium hypochlorite per 5 L water). They were instructed to add the chlorine, shake the water container slightly to allow it to mix, and then allow it to sit for 30 minutes before drinking or using.
To assess compliance, study participants were instructed to show the remaining seeds of M. oleifera and level of chlorine in the bottles to the health worker at each visit. Residual chlorine in water samples was also tested in the laboratory.
Collection of water samples.
At baseline and at 7 days, 6 weeks, and 4 months during the intervention phase, 25–50 mL water samples were collected from the previous day's water and transported within 4 hours of collection to the laboratory to undergo testing for thermotolerant coliform count after 1 in 10 dilution.
Before the commencement of the intervention, and halfway through the intervention trial, samples were taken from identified drinking water sources in the community and tested for thermotolerant coliform counts. The Geographic Information Systems (GIS) map of bore wells and public taps in relation to the main tank and the public toilet (which was largely unused) is included as Figure 1.
Figure 1.
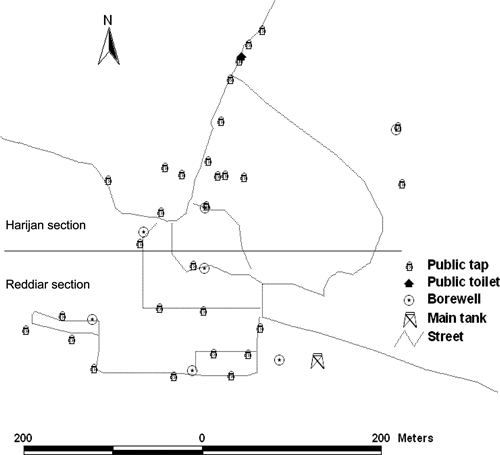
Study village: Adukampadai Kattupadi divided into Harijan and Reddiar sections
Laboratory analysis of samples.
All water samples were tested in the laboratory at Christian Medical College. All laboratory estimations of presumptive and confirmed thermotolerant coliform counts were carried out by the most probable number technique using McCready's tables and further identification of thermotolerant coliforms, as previously used in multiple studies on water contamination in this area.3,8,29 Testing was also done to establish the efficacy of M. oleifera. Distilled water samples were seeded with 104 to 107 Escherichia coli per 1 mL, then M. oleifera was added and samples were taken at different time intervals to assess reduction in coliform counts. At 1 hour with 1 seed per 5 L, there was a 3 log reduction in coliform counts and this increased to 4 logs at 4 hours.
Data analysis.
Questionnaire data were analyzed for frequencies and significant correlations using SPSS (version 17.0, SPSS, Inc., Chicago, IL). Analysis of variance was determined to assess for significant differences between the relative efficacies of each intervention in reducing coliform counts.
Results
Demographics and hygienic practices.
Table 1 shows the general demographics of the 126 households included in the study. There was a significant difference between the castes, as expected, in terms of housing, fuel, source of water, and educational level, but not age. There was a significant difference (P < 0.001) between castes in terms of where defecation occurred, as 42% of Reddiars had a toilet in the home, whereas 95% of Harijans reported defecating in the fields. After stratification by caste and randomization, there were no significant differences in demographics and practices in the four arms of the study.
Table 1.
Demographics of study participants enrolled in an interventional trial on household water treatment in Adukamparai Kattupadi village
| Total | Reddiar | Harijan | P value | |
|---|---|---|---|---|
| Number of participants | 126 | 66 | 60 | |
| Average age (years) | 44 | 47 | 43 | 0.087 |
| Education | ||||
| Secondary or higher | 23% | 36% | 9% | 0.001 |
| Middle school or less | 77% | 64% | 92% | |
| House Type | ||||
| Mansion (cement, > 5 rooms) | 13% | 21% | 3% | 0.001 |
| Pucca (cement, < 5 rooms) | 35% | 43% | 25% | |
| Hut | 28% | 22% | 35% | |
| Other (intermediate) | 24% | 14% | 37% | |
| Type of cooking fuel | ||||
| Wood/cow dung only | 42% | 13% | 73% | 0.001 |
| Gas/electric, etc. | 58% | 87% | 27% | |
| Source of water | ||||
| Domestic tap | 32% | 48% | 13% | 0.001 |
| Public tap | 65% | 45% | 87% | |
| Closed well | 4% | 7% | 0% |
Most participants reported that they used soap during bathing (56%), and not after defecating. In addition, most participants reported defecating primarily in the fields (72%). There was no significant difference in soap usage, place of defecation, or frequency of diarrhea between the intervention groups.
Intervention efficacy.
Initial water samples from homes and sources.
Of the 135 initial samples taken, 13 were taken from households who stated that they boiled their water regularly, to assess the relative effectiveness of this practice. The sources of water were 61% public tap, 29.5% domestic tap, and 9.5% from a domestic or public bore well. The materials of the containers used for drinking water storage were 47% steel, 30% brass, 20% plastic, 1.5% clay, and 1.5% copper. Of the 135 initial water samples tested, only one house that reported boiling water, had potable water by World Health Organization (WHO) standards. Three households (2.4%) that did not boil their water had thermotolerant coliform counts less than 100 organisms/100 mL, and 85% had thermotolerant coliform counts above 900 organisms/100 mL. Because households who reported boiling water also had high counts, we included seven households reporting regular water boiling in the intervention study.
The public taps had levels of contamination that ranged from 35 to 270 thermotolerant coliforms per 100 mL. Five of the seven public bore wells connected to hand pumps had potable water; however, these wells were generally used for drinking water only when an alternate source of water was needed during the day after the public taps had stopped running in the morning. Thus, there were situations in which microbiologically safe water was being used to wash cows and clothing, whereas water from contaminated sources was kept for drinking.
Testing of water sources.
Table 2 shows the results from testing the 11 wells during the baseline and 4-month sample collections. An additional 7 samples were collected from other public taps in April and May (dry season), all of which had > 180 thermotolerant coliforms per 100 mL. The level of contamination was so persistent that the main overhead tank was then checked and found to have thermotolerant coliform counts in excess of 150 per 100 mL. According to the government program, the tank is scheduled to be chlorinated at least once a month.
Table 2.
Contamination of public water system
| Public taps | Bore wells | ||
|---|---|---|---|
| Coliform count | Dec/Jan | Apr/May | Dec |
| < 1 per 100 mL | 0 | 0 | 5 (71.4%) |
| 1 < × < 180 | 3 (27.3%) | 2 (18.2%) | 1 (14.3%) |
| > 180 | 8 (72.7%) | 9 (81.8%) | 1 (14.3%) |
| Total | 11 | 11 | 7 |
| Average thermotolerant coliform count | 204 | 166 | 29.9 |
| Main tank thermotolerant coliform count | > 180 | 164 | |
Results of home water samples during intervention.
Results of the 1 week, 6 weeks, and 4 months testing for thermotolerant coliforms and residual chlorine are presented in Table 3. A separate column has been included to indicate samples that were significantly improved (thermotolerant coliform counts from 1 to 10 per 100 mL), but did not meet the standard of < 1/100 mL. Additional chlorination was effective. Eighteen of the 22 (82%) samples without thermotolerant coliforms were from homes using chlorine (P < 0.001), and 15 (68%) contained residual chlorine. However, the number assigned to chlorine that had potable samples declined at each time interval, indicating decreasing compliance with this intervention. Neither M. oleifera nor the closed valved container had any significant effect on thermotolerant coliform counts; the one home noted to have consistently potable water despite only using the container was boiling their water. However, there was no significant association between boiling and improved water quality among the 7 families who regularly boiled their water or the 45 families who reported boiling water occasionally.
Table 3.
Thermotolerant coliform testing in 126 households randomized to use chlorine, Moringa seeds, a closed container or follow prior practice over a 6-month period tested at baseline, 1 week, 6 weeks, and 4 months post-introduction of the intervention*
| Intervention | < 1 | > 1 to ≤ 10 | > 11 to ≤ 180 | > 180 | P value | Total no. samples | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||||
| Control | 0 | 0.00% | 1 | 3.20% | 1 | 3.20% | 29 | 93.50% | n/a | 31 |
| Chlorine | 0 | 0.00% | 0 | 0.00% | 1 | 3.20% | 30 | 96.80% | 0.601 | 31 |
| Moringa | 0 | 0.00% | 0 | 0.00% | 3 | 9.40% | 29 | 90.60% | 0.371 | 32 |
| Container | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 31 | 100.00% | 0.356 | 31 |
| Totals | 0 | 0.00% | 1 | 0.80% | 5 | 4.00% | 119 | 95.20% | 125 | |
| Sample at 1 week | ||||||||||
| Control | 1 | 3.30% | 0 | 0.00% | 0 | 0.00% | 29 | 96.70% | n/a | 30 |
| Chlorine | 10 | 32.30% | 0 | 0.00% | 2 | 6.50% | 19 | 61.30% | 0.003 | 31 |
| Moringa | 0 | 0.00% | 0 | 0.00% | 2 | 6.70% | 28 | 93.30% | 0.221 | 30 |
| Container | 1 | 3.20% | 0 | 0.00% | 1 | 3.20% | 29 | 93.50% | 0.611 | 31 |
| Totals | 12 | 9.80% | 0 | 0.00% | 5 | 4.10% | 105 | 86.10% | 122 | |
| Sample at 6 weeks | ||||||||||
| Control | 0 | 0.00% | 1 | 3.30% | 5 | 16.70% | 24 | 80.00% | n/a | 30 |
| Chlorine | 6 | 21.40% | 1 | 3.60% | 5 | 17.90% | 16 | 57.10% | 0.057 | 28 |
| Moringa | 0 | 0.00% | 0 | 0.00% | 5 | 17.20% | 24 | 82.80% | 0.612 | 29 |
| Container | 1 | 3.20% | 2 | 6.50% | 4 | 12.90% | 24 | 77.40% | 0.699 | 31 |
| Totals | 7 | 5.90% | 4 | 3.40% | 19 | 16.10% | 88 | 74.60% | 118 | |
| Sample at 4 months | ||||||||||
| Control | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 30 | 100.00% | n/a | 30 |
| Chlorine | 2 | 7.10% | 1 | 3.60% | 0 | 0.00% | 25 | 89.30% | 0.184 | 28 |
| Moringa | 0 | 0.00% | 0 | 0.00% | 1 | 3.30% | 29 | 96.70% | 0.313 | 30 |
| Container | 1 | 3.20% | 0 | 0.00% | 2 | 6.50% | 28 | 90.30% | 0.217 | 31 |
| Totals | 3 | 2.50% | 1 | 0.00% | 4 | 3.40% | 112 | 94.10% | 119 | |
At baseline, the samples were diluted 1 in 10, post-intervention they were tested undiluted. P values are for each intervention in comparison to control. n/a = not available.
Additional testing of Moringa.
Because of the continuing microbial contamination found in the samples from homes using Moringa, in contrast to the laboratory testing done before the intervention, additional tests were performed using crushed Moringa seeds in the laboratory at higher concentrations to gain more accurate assessment of thermotolerant coliform count reductions (Table 4). Families using this intervention were asked to use increased numbers of seeds; however, this did not result in decreased thermotolerant coliform counts. At this point, water from the village was brought to the laboratory and used in seeding experiments similar to those conducted earlier. The water from the village showed no decrease in thermotolerant coliform counts with increasing amounts of crushed Moringa seeds.
Table 4.
Laboratory testing of the efficacy of crushed Moringa oleifera seeds in reducing coliform counts of seeded laboratory and village water after 4-hour treatment
| Coliform count in distilled water* | Coliform count in village water* | ||
|---|---|---|---|
| 100,000 | 1 seed/5 L | 43 | NT |
| 10000 | 1 seed/5 L | 3 | NT |
| 1000 | 1 seed/5 L | < 1 | NT |
| 100,000 | 1 seed/1 L | 18 | NT |
| 10000 | 1 seed/1 L | < 1 | NT |
| 1000 | 1 seed/1 L | < 1 | NT |
| 100,000 | 3 seeds/1 L | < 1 | > 180 |
| 10000 | 3 seeds/1 L | < 1 | 161 |
| 1000 | 3 seeds/1 L | < 1 | 17 |
Measurements are thermotolerant coliforms per 100 mL water.
NT = not tested.
Intervention acceptability and sustainability.
This village was chosen because its inhabitants had been cooperative with previous studies, including questionnaires administered by medical students. The 126 women who participated were divided first by community, and then into the four intervention arms. There was significant resistance to the chlorine arm, with 26 (83%) of 32 women expressing dissatisfaction, and 11 (34%) initially refusing to use it, mostly because of the change in taste and smell. On the other hand, both the closed valved container and Moringa seeds were very popular and had very high levels of interest and compliance, with 100% of assigned women using the container and 90% of assigned women using Moringa as instructed. When asked which intervention they would have preferred to have been offered during the follow-up questionnaire, 46% said container, 11% said Moringa, and only three women (2.5%) said chlorine.
Discussion
Contamination of the public water system and disease transmission.
There was significant fecal coliform contamination in the public water system, similar to findings in prior studies in this area.3,8 Because the main overhead tank was found to have high thermotolerant coliform counts (Table 2), the contamination may have occurred before delivery to the main overhead tank and continued despite intermittent chlorination. We were unable to test the deep bore well source water directly to determine if it was also contaminated, as there is no current method of sampling the water directly from the well without it passing through pipes that may be cracked or otherwise compromised and thereby contaminated.
Efficacy of interventions.
Additional chlorination was the most effective water treatment method, with 82% of negative samples coming from households using chlorine. Neither M. oleifera nor the closed valved container had any significant effect on coliform counts. In the case of the closed container, this could be because the contamination level at the source was too great for recontamination in the home to make any difference in thermotolerant coliform counts, and water was not kept long enough for the usual time-related reduction in thermotolerant coliform counts seen in other studies.8 However, recontamination in the home was likely a significant factor in all groups, as most of the participants reported defecating in the fields, and not washing hands with soap after defecation.
For homes using Moringa, the reason for the lack of reduction in thermotolerant coliform counts is unclear. It is possible that there are dissolved solutes or particulates in the village water that interfered with the flocculating action of the Moringa seeds, which has been postulated to be caused by ionic charge on the particles attracting the negatively charged cell wall of bacteria.14 In discussion with experts, another possible reason for the lack of effect was postulated to be the absence of turbidity, which could result in less effective flocculation (Folkard GK, personal communication), but this does not account for the reduction in counts when testing was carried out using laboratory water, which is also non-turbid. Drinking water turbidity in these areas has been estimated several times and is consistently less than 5 NTU.
The lack of 100% potable water in the chlorine group, despite its clear efficacy, was likely caused by both refusal to use chlorine by participants, and in-home recontamination. In prior studies, in-home recontamination of samples treated with chlorine has been shown to occur less than 6 hours after treatment.9 Additionally, in-home chlorination at specified doses may be inadequate during certain times of the year when conditions cause increasing levels of contamination in the water supply, particularly in the rainy season. Previous studies in the area have shown that during an outbreak, Vibrio cholerae was grown from tap water containing 1 ppm of residual chlorine.3
The lack of association between reported boiling and improved water quality was likely due either to inadequate boiling time, recontamination after boiling, or a combination of both. Despite the generally held belief that boiling water is financially unfeasible in many developing world contexts,10 almost 35% of participants reported boiling water on occasion, generally when a family member was sick. According to the Indian Demographic and Health Survey conducted in 2005–2006, 10.6% of the Indian population reported boiling their water regularly.30 Additionally, a study in rural India proposed that boiling may actually be more feasible in India than other places, costing only US$0.88 per month for gas and US$0.69 for wood needed to boil 6 L of water per family per day.31 In our study, there was no association between regular or occasional boiling and education, caste, type of home, or fuel.
Health education and intervention acceptability.
Although this study was not designed to assess the effectiveness of the health education message, participants' answers to the initial and follow-up surveys indicate an increase in awareness of the necessity of washing hands with soap after defecating. Other studies have shown increased practice of hand washing with soap among children less than 15 years of age who have received health messages at school, but minimal dissemination of this practice among other family members.32 Further work on spreading this message is needed, as studies focused on hand washing with soap have shown reductions in acute respiratory infections, diarrhea, and impetigo.33
The closed valved container was the most popular intervention, most likely because additional containers for water are always welcome. The cost of each container was US$2. If cleaned and properly maintained they could last for several years, but eventually would need to be replaced. Moringa oleifera was the second most popular intervention in the study. No participants assigned to this group indicated any resistance to using the method, although some did not want to wait several hours before using the water, and it is unclear if they actually did so. Moringa for study purposes was purchased from a local grower for US$0.01 per seed (i.e., per 5 L treatment). As previously noted the plant grows locally; if the plant had been shown to be an effective water treatment, a “cottage-industry” of Moringa orchards could have been established to scale-up the intervention locally and provide income-generating projects from extracting oil and selling seeds. However, given the lack of effectiveness shown in this study, this is not currently being pursued.
The chlorine provided to study participants cost US$0.04 per 5 L treated water. There was significant resistance to the use of chlorine, based on perceived smell and taste. Women in the focus groups reported that the people in the village would notice when the bleaching powder was put into the overhead tanks and refuse to drink the water for 2 to 3 days to allow the chlorine to diffuse out of the water. As mentioned previously, chlorine's acceptability to communities has been an issue in many previous studies.9,25–27
Other than social marketing, which was not part of this project, another method to increase acceptability might have been to offer taste neutralizers; however, the cost of these would likely have been prohibitive for people of the lowest socioeconomic strata. To use an adequate number of neutralizing tablets for 5 L, would cost about 15 rupees,34 which is a significant amount where the average daily wage is 50 rupees (45 rupees approximately equal to US$1 at the time of this study). Another option for improving acceptability of effective methods would be to combine Moringa or closed containers with a more effective method, such as solar disinfection, or ceramic or biosand filters.35 If the reasons for ineffectiveness in this study could be determined and overcome, a combination approach using closed containers or Moringa would be a very attractive option given their high acceptability. In summary, it is important that members of the community be made aware of the levels of contamination in their water supply and be given effective means to disinfect water to protect themselves from water-borne diseases.
Acknowledgments
We thank the staff at the CMC-affiliated hospitals in Vellore and Bagayam and the field staff of the Community Health Department, especially Mr. Iyenar, Mr. Ranjit, Mrs. Manjula, and Mr. Singarayan for conducting interviews and counseling sessions. A special thank you to Mr. Chandresekhar for translating questionnaires and training the Onsite Coordinator to administer them, to Mr. Thyagarajan for transporting samples and people back and forth to the village early in the morning, and to Mr. Thirumani for obtaining the materials necessary for the implementation of the intervention. In addition, we thank the staff of the Department of Biostatistics at CMC for their assistance in compiling and analyzing the data.
Footnotes
Financial support: This study was supported by the Fluid Research Funds of Christian Medical College. Jacqueline Firth received financial and logistical support from the Fogarty-Ellison Fellowship administered by the U.S. National Institutes of Health for this research as a medical student.
Authors' addresses: Jacqueline Firth, Combined Internal Medicine/Pediatrics residency, Warren Alpert School of Medicine at Brown University, Providence, Rhode Island, E-mail: jacqfirt@gmail.com. Vinohar Balraj, Department of Community Health, Christian Medical College, Vellore, Tamil Nadu, India, E-mail: Vinohar@cmcvellore.ac.in. Jayaprakash Muliyil, Department of Community Health, Christian Medical College, Vellore, Tamil Nadu, India, E-mail: Jayaprakash@cmcvellore.ac.in. Sheela Roy, Department of Gastroenterology, Christian Medical College, Vellore, Tamil Nadu, India, E-mail: wellcome@cmcvellore.ac.in. Lilly Michael Rani, Department of Gastroenterology, Christian Medical College, Vellore, Tamil Nadu, India, E-mail: wellcome@cmcvellore.ac.in. R. Chandresekhar, Department of Gastroenterology, Christian Medical College, Vellore, Tamil Nadu, India, E-mail: wellcome@cmcvellore.ac.in. Gagandeep Kang, Department of Gastroenterology, Christian Medical College, Vellore, Tamil Nadu, India, E-mail: gkang@cmcvellore.ac.in.
References
- 1.World Health Organization Water, sanitation, and hygiene links to health: facts and figures. 2004. http://www.who.int/water_sanitation_health/publications/facts2004/en/ Available at. Accessed February 27, 2009.
- 2.United Nations Millennium Development Goals. 2000. http://www.un.org/millenniumgoals/ Available at. Accessed February 27, 2009.
- 3.Ramakrishna B, Kang G, Rajan D, Mathan M, Mathan V. Isolation of Vibrio cholerae 0139 from the drinking water supply during an epidemic of cholera. Trop Med Int Health. 1996;1:854–858. doi: 10.1111/j.1365-3156.1996.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 4.Pai M, Kang G, Ramakrishna B, Venkataraman A, Muliyil J. An epidemic of diarrhea in south India caused by enteroaggregative Escherichia coli. Indian J Med Res. 1997;106:7–12. [PubMed] [Google Scholar]
- 5.Kang G, Ramakrishna B, Daniel J, Mathan M, Mathan V. Epidemiological and laboratory investigation of outbreaks of diarrhoea in rural South India: implications for control of disease. Epidemiol Infect. 2001;127:107–112. doi: 10.1017/s0950268801005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarkar R, Prabhakar A, Manickam S, Selvapandian D, Raghava M, Kang G, Balraj V. Epidemiological investigation of an outbreak of acute diarrhoeal disease using geographic information systems. Trans R Soc Trop Med Hyg. 2007;101:587–593. doi: 10.1016/j.trstmh.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Jensen P, Ensink J, Jayasinghe G, Van der Hoek W, Cairncross S, Dalsgaard A. Domestic transmission routes of pathogens: the problem of in-house contamination of drinking water during storage in developing countries. Trop Med Int Health. 2002;7:604. doi: 10.1046/j.1365-3156.2002.00901.x. [DOI] [PubMed] [Google Scholar]
- 8.Brick T, Primrose B, Chandresekhar R, Roy S, Muliyil J, Kang G. Water contamination in urban south India: household storage practices and their implications for water safety and enteric infections. Int J Hyg Environ Health. 2004;207:1–8. doi: 10.1078/1438-4639-00318. [DOI] [PubMed] [Google Scholar]
- 9.Roberts L, Chartier Y, Chartier O, Malenga G, Toole M, Rodka H. Keeping clean water clean in a Malawi refugee camp: a randomized intervention trial. Bull World Health Organ. 2001;79:280–287. [PMC free article] [PubMed] [Google Scholar]
- 10.Tumwine JK. Clean drinking water for homes in Africa and other less developed countries. BMJ. 2005;331:468–469. doi: 10.1136/bmj.331.7515.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen M, Schlundt J, Omer EF. Effect of water coagulation by seeds of Moringa oleifera on bacterial concentrations. J Trop Med Hyg. 1987;90:101–109. [PubMed] [Google Scholar]
- 12.Sutherland J. Moringa oleifera in water treatment. 1996. http://www.le.ac.uk/engineering/staff/Sutherland/moringa/water/water.htm Available at. Accessed March 3, 2009.
- 13.Sutherland J, Folkard G, Mtawali M, Grant W. Moringa oleifera as a natural coagulant. Proceedings of the 20th Water, Engineering, and Development Center International Conference: Affordable Water Supply and Sanitation. Colombo, Sri Lanka: WEDC; 1994. [Google Scholar]
- 14.Broin M, Santaella C, Cuine S, Kokou K, Peltier G, Joet T. Flocculent activity of a recombinant protein from Moringa oleifera Lam. seeds. Appl Microbiol Biotechnol. 2002;60:114–119. doi: 10.1007/s00253-002-1106-5. [DOI] [PubMed] [Google Scholar]
- 15.Amagloh FK, Benang A. Effectiveness of Moringa oleifera seed as a coagulant for water purification. African Journal of Agricultural Research. 2009;4:119–123. [Google Scholar]
- 16.Ayotunde E, Fagbenro O, Adebayo O, Amoo A. Proceedings of the 6th International Symposium on Tilapia in Aquaculture. Manila, Phillipines: 2004. pp. 200–208. (Toxicity of Aqueous Extracts of Drumstick, Moringa oleifera, Seeds to Nile tilapia, Oreochromis niloticus, Fingerlings and Adults). [Google Scholar]
- 17.Grabow W, Slabert JL, Morgan W, Jahn S. Toxicity and mutagenicity evaluation of water coagulated with Moringa oleifera seed preparation using fish protozoan, bacterial coliphage, enzyme, and Salmonella assay. Water SA. 1985;11:9–14. [Google Scholar]
- 18.Ezeji J. Mor-sand filter. 2008. http://www.joachimezeji.com/mor-sand-filter/ Available at. Accessed March 3, 2009.
- 19.Swerdlow D, Mintz E, Rodriguez M, Tejada E, Ocampo C, Espejo L, Greene K, Saldana W, Seminario L, Tauxe R. Waterborne transmission of epidemic cholera in Trujillo, Peru: lessons for a continent at risk. Lancet. 1992;340:28–33. doi: 10.1016/0140-6736(92)92432-f. [DOI] [PubMed] [Google Scholar]
- 20.CDC/CARE Health Initiative Safe Water Systems for the Developing World: A Handbook for Implementing Household-Based Water Treatment and Safe Storage Projects. 2003. http://www.cdc.gov/safewater/manual/sws_manual.pdf Second printing. Available at. Accessed March 1, 2009.
- 21.Sobel JM, Mendoza CE, Passaro D, Cano F, Baier K, Racioppi F, Hutwagner L, Mintz E. Reduction of fecal contamination of street-vended beverages in Guatemala by a simple system for water purification and storage, handwashing, and beverage storage. Am J Trop Med Hyg. 1998;59:380–387. doi: 10.4269/ajtmh.1998.59.380. [DOI] [PubMed] [Google Scholar]
- 22.Quick R, Venczel L, Mintz E, Soleto L, Aparicio J, Gironaz M, Hutwagner L, Greene K, Bopp C, Maloney K, Chavez D, Sobsey M, Tauxe R. Diarrhoea prevention in Bolivia through point-of-use water treatment and safe storage: a promising new strategy. Epidemiol Infect. 1999;122:83–90. doi: 10.1017/s0950268898001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bing Fang H, Jaakkola J, Guo H. Water disinfection by-products and the risk of specific birth defects: a population-based cross-sectional study in Taiwan. Environ Health. 2008;7:23. doi: 10.1186/1476-069X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Government of India Ministry of Rural Development National Rural Drinking Water Programme: Movement Towards Ensuring People's Drinking Water Security in Rural India. 2009. http://ddws.nic.in/popups/FinalRWSGuideLines.pdf Available at. Accessed March 1, 2009.
- 25.Jacob A, Ramani S, Banerjee I, Kang G. Efficacy and acceptability of chlorine dioxide water. Indian J Med Microbiol. 2007;25:74–75. doi: 10.4103/0255-0857.31074. [DOI] [PubMed] [Google Scholar]
- 26.Freeman M, Quick R, Rheingans R, Abbott D. Removing barriers to point-of-use water treatment products through social marketing and entrepreneurship: a case study in Western Kenya. 2003. [DOI] [PubMed]
- 27.Makutsa P, Nzaku K, Ogutu P, Barasa P, Ombeki S, Mwaki A, Quick R. Challenges in implementing a point-of-use water quality intervention in rural Kenya. Am J Public Health. 2001;91:1571–1573. doi: 10.2105/ajph.91.10.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cochran W, Cox G. Experimental Designs. New York: Wiley; 1957. [Google Scholar]
- 29.Senior B. In: Practical Medical Microbiology. Collee J, Duguid J, Fraser A, Marmion B, editors. Edinburgh: Churchill Livingstone Press; 1989. pp. 204–213. (Examination of water, milk, food and air). [Google Scholar]
- 30.Government of India Demographic and Health Survey Demographic and Health Survey 2005–2006. 2006. http://www.measuredhs.com/pubs/pdf/FRIND3/02Chapter02.pdf Available at. Accessed March 3, 2009.
- 31.Clasen T, McLaughlin C, Nayaar N, Boisson S, Gupta R, Desai D, Shah N. Microbiological effectiveness and cost of disinfecting water by boiling in semi-urban India. J Trop Med Hyg. 2008;79:407–413. [PubMed] [Google Scholar]
- 32.Banda K, Sarkar R, Gopal S, Govindarajan J, Harijan B, Jeyakumar M, Mitta P, Sadanala M, Selwyn T, Suresh C. Water handling, sanitation and defecation practices in rural southern India: a knowledge, attitudes and practices study. Trans R Soc Trop Med Hyg. 2007;101:1124–1130. doi: 10.1016/j.trstmh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Luby S, Agboatwalla M, Feikin D, Painter J, Billhimer W, Altaf A, Hoekstra R. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366:225–233. doi: 10.1016/S0140-6736(05)66912-7. [DOI] [PubMed] [Google Scholar]
- 34.Stratford Pharmacy Cost of chlorine neutralizing tablets: US$0.07 each, 1 tablet to 1 liter. 2009. www.stratford-pharmacy.co.uk Available at. Accessed March 3, 2009.
- 35.Sobsey MD, Stauber CE, Casanova LM, Brown JM, Elliott MA. Point of use household drinking water filtration: a practical, effective solution for providing sustained access to safe drinking water in the developing world. Environ Sci Technol. 2008;42:4261–4267. doi: 10.1021/es702746n. [DOI] [PubMed] [Google Scholar]


