Abstract
Nestling birds are rarely sampled in the field for most arboviruses, yet they may be important in arbovirus amplification cycles. We sampled both nestling and adult house sparrows (Passer domesticus) in western Nebraska for West Nile virus (WNV) or WNV-specific antibodies throughout the summer of 2008 and describe pathology in naturally infected nestlings. Across the summer, 4% of nestling house sparrows were WNV-positive; for the month of August alone, 12.3% were positive. Two WNV-positive nestlings exhibited encephalitis, splenomegaly, hepatic necrosis, nephrosis, and myocarditis. One nestling sparrow had large mural thrombi in the atria and ventricle and immunohistochemical staining of WNV antigen in multiple organs including the wall of the aorta and pulmonary artery; cardiac insufficiency thus may have been a cause of death. Adult house sparrows showed an overall seroprevalence of 13.8% that did not change significantly across the summer months. The WNV-positive nestlings and the majority of seropositive adults were detected within separate spatial clusters. Nestling birds, especially those reared late in the summer when WNV activity is typically greatest, may be important in virus amplification.
Introduction
Most field surveys of bird-associated arthropod-borne viruses (arboviruses) have sampled adult birds for the presence of virus or antibodies, perhaps because in many species adults can be sampled more easily and in greater numbers than can more dispersed and cryptic nestling birds. However, it is important to understand whether age influences birds' exposure to or relative involvement in arbovirus transmission, given potential differences in both the response to infection in adult versus nestling birds1 and their ability to behaviorally avoid and/or to attract mosquito vectors.2–4 Some general avian surveys reported that nestlings and immature birds were overrepresented in the virus-positive population,5,6 and in one study peaks in West Nile virus (WNV) transmission were correlated with high seroprevalence and infection rates in hatching-year birds.7 In contrast, other studies of WNV found relatively low seroprevalence in hatching-year birds in comparison to adults.8,9
Nestling birds may be more susceptible to viruses and more negatively affected by them than are adults of the same species. For example, WNV-infected young white leghorn chicks (Gallus gallus domesticus) exhibited greater viremias and more severe clinical response than older ones.10 Western equine encephalomyelitis virus (WEEV) caused low to no mortality in adult house sparrows (Passer domesticus) in experimental infection studies4,11 but is fatal to sparrow nestlings when they are infected naturally in the field12 or experimentally infected in the laboratory.4 Adult house sparrows inoculated with Buggy Creek virus (BCRV), an alphavirus in the WEEV antigenic complex,13,14 develop transient to no viremia15 and are not found viremic in field surveys (O'Brien V and Brown C, unpublished data), but field-sampled nestling house sparrows have high viremias and often die of virus infection.16
Although some information on WNV pathology is known for relatively young individuals of several raptor species,17,18 we know almost nothing about the clinical pathology of arboviruses in nestling passerine birds,16 possibly because nestlings or recently fledged passerines are rarely found dead or exhibiting clinical pathology attributable to arboviruses.6,7,19 One study reported WNV RNA in kidney tissue of field-collected blue jay (Cyanocitta cristata) nestling carcasses, but necropsy was not performed on these birds.20 Understanding WNV disease etiology in nestling birds may assist in explaining patterns of virus or antibody prevalence in field surveys and yield insight into ways that the virus may be transmitted.
A recent study of WNV in an urban area around Chicago, IL concluded that nestling passerine birds were not important in virus amplification: samples from 194 nestlings of 12 bird species yielded only one virus-positive and one antibody-positive individual.21 However, the sampling was done in early summer before mosquitoes were abundant and before virus was commonly detected in vectors,21 and thus not finding WNV in these nestlings is not surprising. A more complete understanding of the role of nestling birds in WNV amplification and transmission will require locating nests and sampling nestlings throughout a summer, including mid-to-late summer when WNV activity is most often detected,7,9 and when late nesting, which is likely more common than generally assumed in North American birds,22 is occurring.
The house sparrow historically has been heavily sampled for arboviruses, perhaps in part because of its abundance and peridomestic occurrence.23 House sparrows were implicated in the transmission cycles of St. Louis encephalitis virus (SLEV)24–26 and WEEV,27 and they may serve as useful sentinels for SLEV activity.28 House sparrows have previously been demonstrated to be competent hosts of WNV,29,30 and studies in New York City during the initial outbreak concluded that sparrows likely contributed importantly to WNV transmission in that area in 1999.31 A mid-summer roost of house sparrows in Colorado served as a prominent source of blood meals for Culex tarsalis Coquillett,32 showing both behavioral (late-season aggregations of juveniles) and mosquito host-preference mechanisms for the involvement of house sparrows in WNV transmission.
As part of a study of the role of nestling birds in the transmission of BCRV,16 we systematically sampled house sparrows of all ages in western Nebraska throughout the summer of 2008 and tested nestlings for WNV and adults for WNV-specific antibodies. This allowed us 1) to assess the potential role of this species and the different age classes in arbovirus transmission throughout the bird's breeding season, including in late summer at a time when some house sparrows are still nesting and when WNV should be most prevalent; 2) to describe pathology associated with WNV infection in nestlings; and 3) to study potential spatial and temporal differences in virus incidence. The northern Great Plains (Nebraska, South Dakota, North Dakota) have reported among the highest incidences of WNV cases of any region within the United States each year from 2003 through 2008,33 and thus better understanding of WNV transmission dynamics in these relatively rural prairie areas is desirable.
Materials and Methods
Study area and study species.
Our study area, in western Nebraska along the North and South Platte rivers, was centered at the Cedar Point Biological Station (41°13′N, 101°39′W) in Keith County, and included portions of Garden, Lincoln, Deuel, and Morrill counties.34,35 Because BCRV is associated exclusively with cliff swallow (Petrochelidon pyrrhonota) nesting colonies35–37 and WNV samples were taken in the course of our work on BCRV, sampling of house sparrows in our study was restricted to those nesting in abandoned swallow nests on bridges and highway culverts, often in close proximity to human habitations. Cliff swallows are highly colonial passerine birds that build gourd-shaped mud nests on cliff faces, in highway or railroad culverts, and under bridges. The mud nests sometimes persist for many years after initial construction, particularly on man-made substrates. House sparrows evict cliff swallows from nests or occupy abandoned nests, usually at colonies near human activity, and will perennially use them until the nests fall from the substrate. House sparrows are semi-colonial, with colony size ranging from 1 to 20 active nests, and are highly sedentary, using the same breeding colony year after year.38
Sampling.
House sparrow nestlings were sampled at 16 colony sites between May 29 and August 11, 2008. Each colony was visited from 1 to 4 times during the season to maximize coverage of first and subsequent broods produced at colony sites. Nestlings 4–17 days of age were removed from the nest, banded with U.S. Geological Survey numbered bands, and a 0.1 mL blood sample was taken by jugular venipuncture with a 29-gauge insulin syringe and placed in 0.4 mL of virus diluent.39 Nestlings were then returned to the nest. Nestling age was estimated based on our experience with known-age house sparrow nestlings. Between one and six nests were sampled at each visit to a site, and each nestling was sampled only once. At one colony, two nestlings were found on the ground near an active nest, one dead about 5 m from the nest, and the other at the base of the nest, alive but showing clinical symptoms of disease (gasping, ruffled feathers, unresponsiveness, and lethargy). A blood sample was collected from the moribund nestling, and it died in hand. Both nestling carcasses were stored on wet ice and then at 4°C until shipment within 24 h to the National Wildlife Health Center (NWHC) for necropsy and virus isolation. Blood samples from nestlings were stored on wet ice in the field, returned to the laboratory, clarified by centrifugation, supernatant removed, and stored at −70°C until taken to the University of Tulsa for RNA extraction.
Adult and juvenile house sparrows were captured in mist nets at 14 colony sites every 5 to 7 days between May 25 and August 10, 2008. Birds were banded, sexed, bled as previously described for nestlings, and released. Blood samples were handled as for nestlings and shipped to the Center for Vectorborne Diseases Laboratory at the University of California-Davis (CVEC) for serological testing. All birds recaptured in the course of the season were re-sampled.
Human case data for WNV were obtained from the Nebraska Department of Health and Human Services (http://www.hhs.state.ne.us) website.
Virus detection.
Viral RNA was extracted from house sparrow nestling sera by adding 25 µL sera in diluent to 100 µL of a guanidine thiocyanate-based lysis buffer. After the addition of 100 µL of 100% ethanol, RNA was extracted using the QIAmp Viral RNA Mini Kit (Qiagen, Valencia, CA) following the manufacturer's protocol. A positive control was included in each extraction, and negative controls were placed between every five samples. Extracted RNA samples were frozen at −20°C until shipped to CVEC for testing by real-time reverse transcription-polymerase chain reaction (RT-PCR) using primer and protocols described previously.40,41 Blood samples were insufficient to also determine virus titers by plaque assay. In 2008, our TaqMan critical threshold values (Ct values) decreased significantly as a linear function of increasing virus concentration over a dilution series ranging from 1 to 5 log10 plaque forming units (PFU) of WNV per mL (Ct = 35.07–2.77 log10 PFU/mL; R2 = 0.99, P < 0.0001). The Ct values of 13.1 and 36.9 would equate to virus titers of 7.9 to < 1.0 log10 PFU/mL.
To detect virus in tissue, portions of the spleen, brain, and liver were removed from the two nestling carcasses and virus isolation attempted. Each tissue was homogenized in a Stomacher 400 Circulator (Seward, Norfolk, UK) in 10 volumes of viral transport media.42 The suspensions were centrifuged at 800 × g for 30 min at 4°C, and 1 mL of the supernatant was inoculated onto Vero cell (ATCC CRL-1587) monolayers in 12 cm2 flasks. The flasks were incubated at 37°C and 5% CO2 and examined daily for cytopathic effects (CPE). Samples showing CPE were subjected to RT-PCR with WNV-specific primers to identify isolates as WNV.43 Tissues also were tested for avian influenza using RT-PCR.44
Gross and microscopic pathology.
At necropsy, body condition was scored, the carcasses were examined for external and internal pathology, and brain, spinal cord, liver, spleen, bursa, trachea, lung, heart, kidney, esophagus, proventriculus, ventriculus, pancreas, and intestine were collected for histopathology. Tissues were placed in 10% neutral buffered formalin, trimmed and embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin. Immunohistochemical (IHC) staining on tissues from the sparrow that died in hand (Bird P10, Table 1) was performed at the Histology Laboratory, Department of Pathology, College of Veterinary Medicine, University of Georgia, following their protocols previously described.45 Briefly, following deparaffinization, proteinase K was used for antigen retrieval and endogenous peroxidase was blocked using 3% hydrogen peroxide (H312-500, Fisher Scientific, Fair Lawn, NJ). Antigen retrieval was performed using Protease III, which is a mild enzymatic pretreatment (760-2020, Ventana Medical Systems, Inc., Tucson, AZ). Rabbit polyclonal anti-WNV antibody (80-015, BioReliance Corp., Rockville, MD) diluted 1:1000 using Dako Antibody Diluent (S0809, Dako, Carpinteria, CA) was applied to slides for 45 minutes, followed by biotinylated goat anti-rabbit IgG (BA-1000, Vector Laboratories, Burlingame, CA) and Streptavidin conjugated to horseradish peroxidase (Dako's LSAB 2; K1016, Dako). The substrate-chromogen system used was DAB (K3466, Dako) and slides were counterstained with Gills II hematoxylin and bluing. Positive tissue controls consisted of formalin fixed, paraffin-embedded heart from a WNV-infected raptor. As a negative control, the primary antibody was substituted with Universal Negative (N1699, Dako).
Table 1.
West Nile virus-positive house sparrow nestlings, Morrill County, Nebraska, tested/collected on August 10, 2008*
| Bird no. | Colony name | Location | Bird age (days) | Where found | Nest no. | Brood size | Virus identification (Ct score and/or tissue) |
|---|---|---|---|---|---|---|---|
| 316 | CP | 41° 30.869′ N 102° 38.971′ W | 14 | Fledged, hand caught | Likely 19 | 5 | 28.3 |
| 317 | CP | 41° 30.869′ N 102° 38.971′ W | 14 | In nest | 19 | 5 | 16.5 |
| 319 | CP | 41° 30.869′ N 102° 38.971′ W | 14 | In nest | 19 | 5 | 26.2 |
| P10 | CP | 41° 30.869′ N 102° 38.971′ W | 14 | On ground, clinically ill | Likely 19 | 5 | 13.1/brain, spleen, liver |
| P11 | CP | 41° 30.869′ N 102° 38.971′ W | 14 | On ground, dead | Likely 19 | 5 | Brain, spleen, liver |
| 322 | BC | 41° 31.743′ N 102° 41.016′ W | 12 | In nest | 14 | 4 | 36.9 |
| 333 | CH | 41° 34.106′ N 102° 46.576′ W | 17 | In nest | 28 | 5 | 35.7 |
Virus was identified by real-time reverse transcription-polymerase chain reaction (RT-PCR) (TaqMan Ct score) from sera, and virus isolation in tissue was confirmed with RT-PCR.
Liver was cultured for aerobic bacteria using 5% sheep blood and eosin-methylene-blue agars. Inoculated plates were incubated at 36°C for 48 h and bacterial isolates were identified using standard methods.
Serology.
Sera were screened for antibodies against flaviviruses with an enzyme immunoassay (EIA) using a crude antigen prepared from Vero cell cultures of St. Louis encephalitis virus.46 Positive EIAs had a ratio of the mean optical density of two antigen-positive wells divided by an antigen-negative well > 2.0. EIA positives were confirmed and identified with a 90% end-point plaque reduction neutralization test (PRNT90) using the NY99 strain of WNV and the KERN217 strain of SLEV (70–80 PFU) on Vero cell culture. Twenty-seven of 28 EIA positives (96.4%) confirmed using PRNT90 at a titer > 1:20 (3 EIA positives had too little sample for PRNT confirmation attempts). All positive samples were identified as WNV because the end-point titers were ≥ 4× the titer of the next most likely flavivirus (i.e., SLEV). Because greater than 95% of the samples EIA positive were confirmed by PRNT, and as others have reported that EIA is more sensitive than PRNT,46,47 all samples that were EIA positive were considered to represent birds with prior exposure to WNV.
Results
Virus detection.
We tested 173 nestling house sparrows aged 4–17 days old from 53 nests for WNV. Mean overall age of those tested was 9.7 days (±0.3 SE). Seven nestlings (4%) were positive for WNV by RT-PCR and/or plaque assay. Older nestlings (≥ 12 days old) were more likely to be virus positive than younger ones (Wilcoxon two-sample test; Z = 2.63, P = 0.009) with mean age for virus-positive nestlings 14.1 days (±0.6 SE) and for virus-negative nestlings 9.6 days (±0.3 SE).
There was a strong temporal and spatial focus of WNV infection. All WNV-positive house sparrow nestlings were from three colonies 3 to 12 km apart along U.S. Highway 26 in Morrill County in the extreme western portion of the study area (Cluster A, Figure 1), and all virus detection occurred on August 10, 2008. Five of the seven WNV-positive nestlings were from the same colony, perhaps from the same nest (Table 1). Of the total nestlings sampled in August across the study area (N = 57), 12.3% were WNV positive, and of those sampled in August in Morrill County alone (N = 21), 33.3% were WNV positive.
Figure 1.

Locations of house sparrow colonies sampled for West Nile virus (WNV) in nestlings and antibodies in adults, May 29–August 11, 2008, western Nebraska. (A) Clusters of WNV amplification and (B) highest seroprevalence are circled. The colony with the greatest number of WNV-positive nestlings (CP) is the southeasternmost site in Cluster A.
West Nile virus was isolated from all tissues cultured from the two nestling carcasses (brain, liver, and spleen; Table 1). Screening tests on tracheal and cloacal swabs from these two birds were negative for avian influenza. There was no evidence of bacterial infections grossly or microscopically, and only a few colonies of contaminant bacteria were isolated from the liver.
Gross and microscopic pathology.
The two necropsied WNV-positive nestlings were in good body condition. There was a moderate amount of fecal material on the feet and ventral feathers of both birds, suggesting that they had been recumbent and sitting in feces. The spleens were approximately four times normal size. The bursae were very small, and the lobes of thymus were too small to be identified. The kidneys were enlarged beyond the renal crypts and tan rather than the normal deep red color. Histopathology was similar in both birds, although the bird found already dead had confounding autolysis.
For the initially moribund nestling (Bird P10, Table 1), the heart had large mural thrombi in the left ventricular apex and at the base of the left atria (Figure 2A). These organized thrombi effaced the endothelium and contained fibrin, necrotic cell debris, and heterophils. A small accumulation of heterophils was also adhered to the left atrioventricular heart valve. The thrombi were IHC negative for WNV antigen, but adjacent endocardium was positive. Moderate necrotic foci in the atrea were IHC positive. Positive IHC-WNV staining was also present in cells in the intermyofiber spaces, epicardium, the endocardium of the atria, ventricles, and heart valves, as well as the vascular endothelium.
Figure 2.
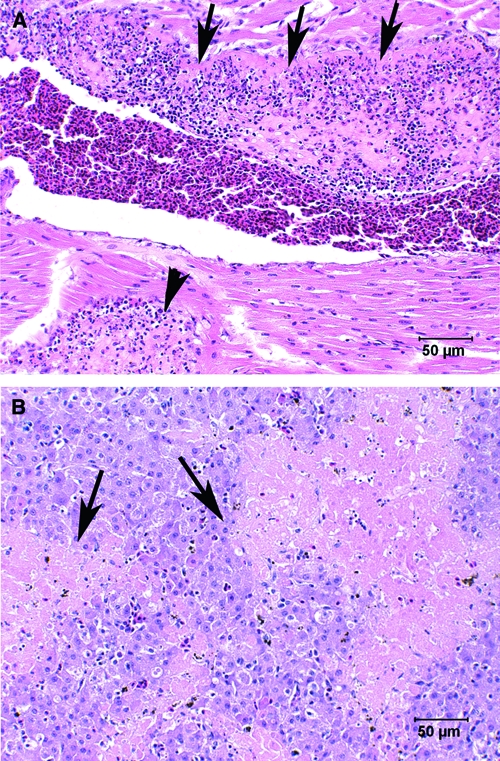
Hematoxylin and eosin stained heart and liver sections from a 14-day-old moribund house sparrow that died during handling as a result of natural infection with West Nile virus (WNV) (Bird P10; Table 1). (A) Heart with mural thrombus adhered to the left ventricular endocardium; loss of detectible endocardium associated with this mature thrombus (arrows). A subendocardial thrombus is also present (arrowhead). (B) Liver with acute multifocal to coalescing regions of necrosis (arrows) without inflammation.
The liver had severe, multifocal to coalescing acute hepatic necrosis without associated inflammation (Figure 2B). The distribution of necrosis was random without affinity for portal or central zones. No bacteria were seen in the liver, and none was isolated. Kupffer cells lining sinusoids stained more intensely for WNV antigen with IHC than hepatocytes in necrotic areas (Figure 3B). A small amount of hemosiderin pigment was present in hepatocytes and Kupffer cells.
Figure 3.
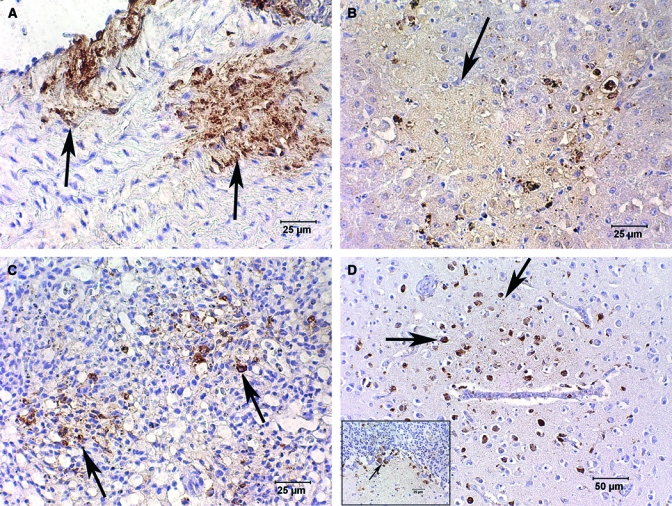
West Nile virus (WNV) immunohistochemical (IHC) staining of tissues from the same house sparrow as in Figure 2 using Dako K3466, Dako Corp. DAB brown chromogen and counterstained with Gills II hematoxylin and bluing. (A) Aorta with positive IHC for WNV antigen in the tunica intima and tunica media (arrows). Disruption of fibers can be seen in the regions of positive staining. (B) Liver with faint WNV antigen staining of necrotic areas (arrow) and more intense staining of Kupffer cells at the margin of necrotic regions. (C) Multifocal areas of lung have lost alveolar architecture (arrows) and stain positively for WNV antigen. (D) Cerebrum with focus of intensely stained WNV antigen-positive glial cells and neurons (arrows). (D) Inset: cerebellum with WNV antigen staining of necrotic Purkinje cells (arrow) and surrounding glial cells associated with necrosis in the molecular layer and granular layer.
The tunica intima and tunica media of both the aorta (Figure 3A) and the pulmonary artery had relatively large areas of multifocal IHC-positive WNV antigen. The areas of IHC-positive staining also defined the disruption of fibers in the tunica media of these arteries that was not obvious in the hematoxylin-eosin (HE)-stained sections. Multifocal areas of lung lost distinguishable alveolar architecture, and the interstitium was widened with edema, vacuolation, and a light infiltrate of heterophils and mononuclear cells. These regions of the lung subsequently stained intensely with WNV IHC (Figure 3C).
Vessels in the brain had multifocal swelling and vacuolation of endothelial cells with rare lymphocytes and plasma cells traversing vessel walls and forming loose accumulations in the perivascular space. There was regionally extensive IHC-WNV antigen staining of neurons and glial cells (Figure 3D) even in areas without detectible pathology or inflammation with HE stains. Clusters of Purkinje cells and scattered cells in the associated granular and molecular layers of the cerebellum were IHC positive (inset: Figure 3D). Inflammation could not be confirmed in the small sections of spinal cord; cut artifacts complicated diagnostic evaluation of spinal cord tissue and IHC staining was not performed.
Multifocal regions of kidney had acute tubular necrosis with casts of necrotic cells, protein, and urate material in the lumen; inflammation was minimal. Positive IHC staining was associated with necrotic tubules, multifocal clusters of renal interstitial cells, and occasional glomeruli. The bursa was markedly atrophied with only multifocal IHC-positive follicles. Staining in these bursal follicles was limited to the remnant medullary cells and epithelial cells. The cortical cells did not stain. The spleen was congested with a moderate accumulation of hemosiderin pigment. Spleen and skin were lost during sectioning, preventing IHC testing of these tissues. Trachea, esophagus, ventriculus, and proventriculus were negative for WNV IHC. The pancreas and intestine were too autolyzed for diagnostic evaluation.
Serology.
A total of 167 adult and juvenile house sparrows were captured, with 25 birds captured ≥ 2 times, resulting in 194 samples tested for antibodies to WNV. Of these, 31 samples were positive by EIA. No recaptured birds seroconverted between first and subsequent captures. Five birds were seropositive on both first and second capture. Overall, seroprevalence across all dates and all sites was 13.8% (N = 189; the birds seropositive on both captures were considered only once in this total). All hatching-year (fledged juvenile) house sparrows sampled (N = 12) were seronegative.
House sparrows with antibodies to WNV were found at eight colony sites across the study area (Figure 1). There appeared to be a spatial cluster of seropositive birds at three colonies between 0.22 and 4.98 km apart (Cluster B, Figure 1). Two colonies were located directly adjacent to a farmyard with large numbers of livestock, including domestic geese and chickens, and the other site was 0.3 km from an active agricultural operation. Seropositivity was higher in these three colonies (27.1%, N = 61) than in all others combined (11.3%, N = 128; χ21 = 4.33, P = 0.037).
When examining temporal seroprevalence, we excluded second capture data on birds that were EIA positive on both first and second capture, and used only the date of first capture for these individuals in our analysis. There was no increase in seropositivity in the study area as the season progressed, as demonstrated by no significant difference in seroprevalence between the three months of May, June, and July (χ22 = 0.12, P = 0.94). Prevalence of antibodies also remained the same in the clustered colonies (Cluster B, Figure 1) from May to July at 18.5%, 21.4%, and 23.8%, respectively (χ22 = 0.20, P = 0.90).
Discussion
Our systematic sampling of house sparrows throughout summer 2008 resulted in seven detections of WNV in nestling birds, all in mid-August near the end of the house sparrow's reproductive season. Across the summer, only 4% of nestlings sampled were virus positive, but when only the month of August was considered, 12.3% were positive. These results show that nestling house sparrows were relatively frequently infected with WNV in rural western Nebraska in late summer 2008. We found WNV in nestling birds only in August, a time when WNV activity in mosquitoes tends to peak at most locations.7,9 This suggests that surveys of nestlings earlier in the summer at times of low mosquito activity21 are not likely to yield virus positives, even in areas where the virus may be endemic. Surveys throughout the summer are necessary to determine the full role of young birds in WNV amplification and transmission.
To our knowledge, this is the first description of WNV pathology with virus isolation from tissues of a naturally infected nestling passerine bird in North America. The IHC staining of WNV antigen in the lung, aorta, pulmonary artery, heart valves, endocardium, liver, brain, and kidney, with minimal inflammation, suggests that the terminal stage of viremia was rapidly fatal. The tropism WNV had for the endocardium, demonstrated by the IHC-staining cells lining the heart, was the likely initiator of the large thrombi in the left ventricle and left atria. It is reasonable to assume that the thrombi resulted in dramatic cardiac insufficiency. Hepatic necrosis was seen in both house sparrow nestlings, but was severe only in the nestling with the cardiac thrombi. Although the pattern of necrosis in the liver did not have a peri-central distribution, which would be expected in congestive heart failure, the relatively light staining of hepatocytes with WNV IHC (when compared with Kupffer cells) suggests that the severe hepatic necrosis seen was not due solely to WNV and that cardiac insufficiency may have exacerbated the necrosis. West Nile virus infection of the aorta and pulmonary arteries has not been previously reported, and infection may have compromised circulatory function.
The isolation of WNV from brain, liver, and spleen in the two field-collected nestlings, along with additional organs staining positively with WNV IHC, indicated extensive viral replication in these birds. Our findings agree with virus isolation from these same tissues in experimentally infected adult house sparrows29 and other bird species.48 The house sparrow nestlings in our study had pathology in multiple organs. Interestingly, although there is typically little pathology in corvids infected with WNV, virus is frequently isolated from multiple tissues.48,49 This may reflect species differences in susceptibility to the virus and a rapid course of disease leading to death in crows and jays. The tissue pathology shown in the two field-collected house sparrows indicates that these nestlings likely maintained high viremic titers in blood, perhaps for several days before death, and presumably lived longer after infection than do corvids.
The lack of an increase in antibody prevalence across the 2008 season both for the study area as a whole and within a cluster of colonies with 27% seroprevalence (Cluster B, Figure 1), together with the relatively high percentage of house sparrows positive for antibodies to WNV, indicates that the area may have been involved in intensive WNV amplification in a preceding year (perhaps 2007) but not in early season 2008. High adult seroprevalence in May might signal prior-year exposure,50 with WNV antibodies known to persist in house sparrows for up to 3 years.51
In contrast, our finding of WNV-positive house sparrow nestlings at colonies in Morrill County showed a potential zone of WNV amplification in 2008 that was farther to the west than in earlier years. Morrill County is sparsely populated, but still reported two human cases of WNV in 2008. Seroprevalence among adult house sparrows in the same Morrill County colonies in 2008 was only 4.5%. Although the sample size for adults there was small (N = 22), the low seroprevalence in 2008 is consistent with that area having minimal WNV activity in preceding years. House sparrows are relatively sedentary during and between nesting seasons,38 and consequently serology data from one year to the next may reflect localization of virus transmission. A caveat, however, is that adult house sparrows are known to succumb to WNV infection with variable fatality rates when experimentally infected,29,30,51 so adult mortality cannot be ruled out as a cause of low seroprevalence in an area (e.g., Morrill County in 2008).
Our data showing different spatial clusters of WNV transmission between years match those from other regions that showed transmission occurring in discrete temporal and spatial foci, with some areas maintaining high infection rates between years9 and others varying widely in both spatial and temporal parameters.21,52 Highly focal WNV transmission was documented in Florida, with 78% of virus isolations from mosquitoes coming from 1 of 5 sites monitored in an area where WNV transmission had previously occurred.53
The house sparrows we studied were in nesting aggregations brought about by their use of cliff swallow nesting colonies, and thus they were perhaps more likely to be exposed to mosquitoes (and therefore to WNV) than sparrows nesting in a more dispersed distribution. Groups of birds can be highly attractive to mosquitoes: Brown and Sethi54 found a positive relationship between cliff swallow nesting colony size and local mosquito abundance, with the more ornithophilic species recruited to the vicinity of the birds' nests. In a study of California birds, the house finch (Carpodacus mexicanus) was frequently found seropositive and at high risk for WNV infection,55 perhaps because this species often nests in low-lying vegetation in loose aggregations that may increase its exposure to mosquitoes. Clusters of American crows (Corvus brachyrhynchus) have been linked to increased infection rates in Culex pipiens quinquefasciatus Say mosquitoes in California,56 and crow roosting habits may also promote bird-to-bird transmission of WNV.57
In one colony (CP; Figure 1), all five WNV-positive nestlings may have been from the same nest (Table 1). All appeared to be the same age (14 days old) and about ready to fledge, whether found in the nest or on the ground. There were two known active nests in the colony at the time, and the other nest contained 7-day-old nestlings that were virus negative. The presence of five, near-adult size birds occupying a single nest may be highly attractive to host-seeking ornithophilic mosquitoes: as brooding decreases and nestlings become larger and thus more exposed to mosquito attack, feeding on nestlings by mosquitoes may increase.4,58 Furthermore, the crowding experienced by older and larger nestlings before fledging, the high titer and duration of viremias documented in nestlings infected with arboviruses,1,10 and the decreased nest sanitization by house sparrows in the days immediately before fledging of young23,59 could lead to an increased likelihood of bird-to-bird transmission of WNV.60 In one study, contact transmission rates of WNV through oral and cloacal shedding were as high as mosquito-induced transmission in some corvids.29 House sparrow nestlings testing positive for WEEV in a Texas survey tended to be clustered by nest, with virus concurrently detected in oral sampling, which indicates possible contact transmission of WEEV between birds in nests.12 Direct transmission of BCRV was demonstrated in an experimental inoculation study involving house sparrows,15 perhaps through fecal contamination of water or food sources. The two nestlings examined by necropsy had feces on the ventral body surface, indicating they may have become unable to preen or perhaps to properly defecate out of the nest entrance because of morbidity from virus infection, which could further expose nest mates to WNV.
The finding of WNV-positive house sparrow nestlings in late summer illustrates the importance of continued research into the potential contribution of nestling birds to arbovirus transmission cycles, especially among late nesting individuals. The clustering of WNV in these birds also suggests that virus infection may occur in spatially discrete foci even in areas where it is generally common and means that widespread sampling across a landscape may sometimes be necessary to detect focal hotspots.
Acknowledgments
The Cedar Point Biological Station of the University of Nebraska-Lincoln provided logistical support. Immunohistochemical staining was performed by Abbie Butler, Histology Laboratory, Department of Pathology, College of Veterinary Medicine, University of Georgia. Eric Nelson produced the map of the Nebraska study area. We thank Ananda Ellis, Ying Fang, Sandra Garcia, Kristen Lear, Renee Long, and Amy Moore for field and laboratory assistance, and Carol Fassbinder-Orth and several anonymous reviewers for comments on the manuscript.
Disclaimer: Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. government.
Footnotes
Financial support: This work was funded by NIH grant AI057569 and NSF grant DEB-0514824 (to CRB) and NIH grant AI55607 (to WKR).
Authors' addresses: Valerie A. O'Brien, Department of Entomology and Plant Pathology, Oklahoma State University, Stillwater, OK, E-mail: valerie.obrien@okstate.edu. Charles R. Brown, Department of Biological Sciences, University of Tulsa, Tulsa, OK, E-mail: charles-brown@utulsa.edu. Carol U. Meteyer and Hon S. Ip, U.S. Geological Survey, National Wildlife Health Center, Madison, WI, E-mails: cmeteyer@usgs.gov and hip@usgs.gov. William K. Reisen, Center for Vectorborne Diseases, School of Veterinary Medicine, University of California, Davis, CA, E-mail: arbo123@pacbell.net.
References
- 1.Mahmood F, Chiles RE, Fang Y, Barker CM, Reisen WK. Role of nestling mourning doves and house finches as amplifying hosts of St. Louis encephalitis virus. J Med Entomol. 2004;41:965–972. doi: 10.1603/0022-2585-41.5.965. [DOI] [PubMed] [Google Scholar]
- 2.Blackmore JS, Dow RP. Differential feeding of Culex tarsalis on nestling and adult birds. Mosq News. 1958;18:15–17. [Google Scholar]
- 3.Scott TW, Edman JD, Lorenz JH, Hubbard JL. Effects of disease on vertebrates' ability behaviorally to repel host-seeking mosquitoes. Misc Publ Entomol Soc Am. 1988;68:9–17. [Google Scholar]
- 4.Scott TW, Lorenz LH, Edman JD. Effects of house sparrow age and arbovirus infection on attraction of mosquitos. J Med Entomol. 1990;27:856–863. doi: 10.1093/jmedent/27.5.856. [DOI] [PubMed] [Google Scholar]
- 5.Cockburn TA, Sooter CA, Langmuir AD. Ecology of western equine and St. Louis encephalitis viruses: a summary of field investigations in Weld County, Colorado, 1949 to 1953. Am J Hyg. 1957;65:130–146. doi: 10.1093/oxfordjournals.aje.a119861. [DOI] [PubMed] [Google Scholar]
- 6.Milby MM, Reeves WC. In: Epidemiology and Control of Mosquito-Borne Arboviruses in California, 1943–1987. Reeves WC, editor. Sacramento, CA: California Mosquito and Vector Control Association, Inc.; 1990. pp. 26–65. (Natural infection in vertebrate hosts other than man). [Google Scholar]
- 7.Hamer GL, Walker ED, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, Schotthoefer AM, Brown WM, Wheeler E, Kitron UD. Rapid amplification of West Nile virus: the role of hatch-year birds. Vector Borne Zoonotic Dis. 2008;8:57–68. doi: 10.1089/vbz.2007.0123. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs SE, Allison AB, Yabsley MJ, Mead DG, Wilcox BR, Stallknecht DE. West Nile virus antibodies in avian species of Georgia, USA: 2000–2004. Vector Borne Zoonotic Dis. 2006;6:57–72. doi: 10.1089/vbz.2006.6.57. [DOI] [PubMed] [Google Scholar]
- 9.Reisen WK, Carroll BD, Takahashi R, Fang Y, Garcia S, Martinez VM, Quiring RO. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J Med Entomol. 2009;46:139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth NM, Bowen RA. Dynamics of passive immunity to West Nile virus in domestic chickens (Gallus gallus domesticus) Am J Trop Med Hyg. 2007;76:310–317. [PubMed] [Google Scholar]
- 11.Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Experimental infection of California birds with western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 2003;40:968–982. doi: 10.1603/0022-2585-40.6.968. [DOI] [PubMed] [Google Scholar]
- 12.Holden P, Hayes RO, Mitchell CJ, Francy DB, Lazuick JS, Hughes TB. House sparrows, Passer domesticus (L.), as hosts of arboviruses in Hale County, Texas. I. Field studies, 1965–1969. Am J Trop Med Hyg. 1973;22:244–253. doi: 10.4269/ajtmh.1973.22.244. [DOI] [PubMed] [Google Scholar]
- 13.Hopla CE, Francy DB, Calisher CH, Lazuick JS. Relationship of cliff swallows, ectoparasites, and an alphavirus in west-central Oklahoma. J Med Entomol. 1993;30:267–272. doi: 10.1093/jmedent/30.1.267. [DOI] [PubMed] [Google Scholar]
- 14.Padhi A, Moore AT, Brown MB, Foster JE, Pfeffer M, Gaines KP, O'Brien VA, Strickler SA, Johnson AE, Brown CR. Phylogeographical structure and evolutionary history of two Buggy Creek virus lineages in the western Great Plains of North America. J Gen Virol. 2008;89:2122–2131. doi: 10.1099/vir.0.2008/001719-0. [DOI] [PubMed] [Google Scholar]
- 15.Huyvaert KP, Moore AT, Panella NA, Edwards EA, Brown MB, Komar N, Brown CR. Experimental inoculation of house sparrows (Passer domesticus) with Buggy Creek virus. J Wildl Dis. 2008;44:331–340. doi: 10.7589/0090-3558-44.2.331. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien VA, Meteyer CU, Ip HS, Long RR, Brown CR. Pathology and virus detection in tissues of nestling house sparrows naturally infected with Buggy Creek virus. J Wildl Dis. 2010;46:23–32. doi: 10.7589/0090-3558-46.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Nemeth NM, Hahn DC, Gould DH, Bowen RA. Experimental West Nile virus infection in eastern screech owls (Megascops asio) Avian Dis. 2006;50:252–258. doi: 10.1637/7466-110105R1.1. [DOI] [PubMed] [Google Scholar]
- 18.Ellis AE, Mead DG, Allison AB, Stallknecht DE, Howerth EW. Pathology and epidemiology of natural West Nile viral infection of raptors in Georgia. J Wildl Dis. 2007;43:214–223. doi: 10.7589/0090-3558-43.2.214. [DOI] [PubMed] [Google Scholar]
- 19.Wobeser G, Wobeser AG. Carcass disappearance and estimation of mortality in a simulated die-off of small birds. J Wildl Dis. 1992;28:548–554. doi: 10.7589/0090-3558-28.4.548. [DOI] [PubMed] [Google Scholar]
- 20.Garvin MC, Tarvin KA, Smith J, Ohajuruka OA, Grimes SD. Patterns of West Nile virus infection in Ohio blue jays: implications for initiation of the annual cycle. Am J Trop Med Hyg. 2004;70:566–570. [PubMed] [Google Scholar]
- 21.Loss SR, Hamer GL, Goldberg TL, Ruiz MO, Kitron UD, Walker ED, Brawn JD. Nestling passerines are not important hosts for amplification of West Nile virus in Chicago, Illinois. Vector Borne Zoonotic Dis. 2009;9:13–17. doi: 10.1089/vbz.2008.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig WD, Stahl JT. Late summer and fall nesting in the acorn woodpecker and other North American terrestrial birds. Condor. 2007;109:334–350. [Google Scholar]
- 23.Lowther PE, Cink CL. In: The Birds of North America Online. Poole A, editor. Ithaca, NY: Cornell Lab of Ornithology; 2006. http://bna.birds.cornell.edu.bnaproxy.birds.cornell.edu/bna/species/012doi:10.2173/bna.12 (House sparrow (Passer domesticus)). Available at. Accessed July 14, 2009. [Google Scholar]
- 24.Gruwell JA, Fogarty CL, Bennett SG, Challet GL, Vanderpool KS, Jozan M, Webb JP., Jr Role of peridomestic birds in the transmission of St. Louis encephalitis virus in southern California. J Wildl Dis. 2000;36:13–34. doi: 10.7589/0090-3558-36.1.13. [DOI] [PubMed] [Google Scholar]
- 25.McLean RG, Kirk LJ, Shriner RB, Townsend M. Avian hosts of St. Louis encephalitis virus in Pine Bluff, Arkansas, 1991. Am J Trop Med Hyg. 1993;49:46–52. doi: 10.4269/ajtmh.1993.49.46. [DOI] [PubMed] [Google Scholar]
- 26.Reisen WK. In: The Flaviviruses: Detection, Diagnosis and Vaccine Development. Monath TP, Chambers TJ, editors. San Diego, CA: Elsevier Academic Press; 2003. pp. 139–183. (Epidemiology of St. Louis encephalitis virus). [Google Scholar]
- 27.Holden P, Francy DB, Mitchell CJ, Hayes RO, Lazuick JS, Hughes TB. House sparrows, Passer domesticus (L.), as hosts of arboviruses in Hale County, Texas. II. Laboratory studies with western equine encephalitis virus. Am J Trop Med Hyg. 1973;22:254–262. doi: 10.4269/ajtmh.1973.22.254. [DOI] [PubMed] [Google Scholar]
- 28.McLean RG, Mullenix J, Kerschner J, Hamm J. The house sparrow (Passer domesticus) as a sentinel for St. Louis encephalitis virus. Am J Trop Med Hyg. 1983;32:1120–1129. doi: 10.4269/ajtmh.1983.32.1120. [DOI] [PubMed] [Google Scholar]
- 29.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- 31.Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001;7:621–625. doi: 10.3201/eid0704.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent R, Juliusson L, Weissmann M, Evans S, Komar N. Seasonal blood-feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- 33.United States Geological Survey West Nile virus maps: historical. 2009. http://diseasemaps.usgs.gov/wnv_historical.html Available at. Accessed July 20, 2009.
- 34.Brown CR, Brown MB. Coloniality in the Cliff Swallow: The Effect of Group Size on Social Behavior. Chicago, IL: University of Chicago Press; 1996. [Google Scholar]
- 35.Brown CR, Brown MB, Padhi A, Foster JE, Moore AT, Pfeffer M, Komar N. Host and vector movement affects genetic diversity and spatial structure of Buggy Creek virus (Togaviridae) Mol Ecol. 2008;17:2164–2173. doi: 10.1111/j.1365-294X.2008.03747.x. [DOI] [PubMed] [Google Scholar]
- 36.Brown CR, Komar N, Quick SB, Sethi RA, Panella NA, Brown MB, Pfeffer M. Arbovirus infection increases with group size. Proc R Soc Lond B. 2001;268:1833–1840. doi: 10.1098/rspb.2001.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown CR, Padhi A, Moore AT, Brown MB, Foster JE, Pfeffer M, O'Brien VA, Komar N. Ecological divergence of two sympatric lineages of Buggy Creek virus, an arbovirus associated with birds. Ecology. 2009;90:3168–3179. doi: 10.1890/08-1731.1. [DOI] [PubMed] [Google Scholar]
- 38.Anderson TR. Biology of the Ubiquitous House Sparrow. New York: Oxford University Press; 2006. [Google Scholar]
- 39.Moore AT, Edwards EA, Brown MB, Komar N, Brown CR. Ecological correlates of Buggy Creek virus infection in Oeciacus vicarius, southwestern Nebraska, 2004. J Med Entomol. 2007;44:42–49. doi: 10.1603/0022-2585(2007)44[42:ecobcv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi PY, Kauffman EB, Ren P, Felton A, Tai JH, Dupuis Ii AP, Jones SA, Ngo KA, Nicholas DC, Maffei J, Ebel GD, Bernard KA, Kramer LD. High-throughput detection of West Nile virus RNA. J Clin Microbiol. 2001;39:1264–1271. doi: 10.1128/JCM.39.4.1264-1271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Docherty DE, Slota PG. Use of Muscovy duck embryo fibroblasts for the isolation of viruses from wild birds. J Tissue Cult Methods. 1988;11:165–170. [Google Scholar]
- 43.Docherty DE, Long RR, Griffin KM, Saito EK. Corvidae feather pulp and West Nile virus detection. Emerg Infect Dis. 2004;10:907–909. doi: 10.3201/eid1005.030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ip H, Flint P, Franson JC, Dusek R, Derksen D, Gill R, Ely C, Pearce J, Lanctot R, Matsuoka S, Irons D, Fischer J, Oates R, Petersen M, Fondell T, Rocque D, Pedersen J, Rothe T. Prevalence of Influenza A viruses in wild migratory birds in Alaska: patterns of variation in detection at a crossroads of intercontinental flyways. Virol J. 2008;5:71. doi: 10.1186/1743-422X-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellis AE, Mead DG, Allison AB, Gibbs SE, Gottdenker NL, Stallknecht DE, Howerth EW. Comparison of immunohistochemistry and virus isolation for diagnosis of West Nile virus. J Clin Microbiol. 2005;43:2904–2908. doi: 10.1128/JCM.43.6.2904-2908.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiles RE, Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J Vector Ecol. 1998;23:123–135. [PubMed] [Google Scholar]
- 47.Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Encephalitis virus persistence in California birds: experimental infections in mourning doves (Zenaidura macroura) J Med Entomol. 2004;41:462–466. doi: 10.1603/0022-2585-41.3.462. [DOI] [PubMed] [Google Scholar]
- 48.Steele KE, Linn MJ, Schoepp RJ, Komar N, Geisbert TW, Manduca RM, Calle PP, Raphael BL, Clippinger TL, Larsen T, Smith J, Lanciotti RS, Panella NA, McNamara TS. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City, New York. Vet Pathol. 2000;37:208–224. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- 49.Gibbs SE, Ellis AE, Mead DG, Allison AB, Moulton JK, Howerth EW, Stallknecht DE. West Nile virus detection in the organs of naturally infected blue jays (Cyanocitta cristata) J Wildl Dis. 2005;41:354–362. doi: 10.7589/0090-3558-41.2.354. [DOI] [PubMed] [Google Scholar]
- 50.Marshall JS, Zuwerink AZ, Restifo RA, Grubb TC. West Nile virus in the permanent-resident bird community of a fragmented Ohio landscape. Ornithol Monogr. 2006;60:79–85. [Google Scholar]
- 51.Nemeth NM, Oesterle PT, Bowen RA. Humoral immunity to West Nile virus is long-lasting and protective in the house sparrow (Passer domesticus) Am J Trop Med Hyg. 2009;80:864–869. [PMC free article] [PubMed] [Google Scholar]
- 52.Gu W, Lampman R, Krasavin N, Berry R, Novak R. Spatio-temporal analyses of West Nile virus transmission in Culex mosquitoes in northern Illinois USA, 2004. Vector Borne Zoonotic Dis. 2006;6:91–98. doi: 10.1089/vbz.2006.6.91. [DOI] [PubMed] [Google Scholar]
- 53.Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- 54.Brown CR, Sethi RA. Mosquito abundance is correlated with cliff swallow (Petrochelidon pyrrhonota) colony size. J Med Entomol. 2002;39:115–120. doi: 10.1603/0022-2585-39.1.115. [DOI] [PubMed] [Google Scholar]
- 55.Wheeler SS, Barker CM, Fang Y, Armijos MV, Carroll BD, Husted S, Johnson WO, Reisen WK. Differential impact of West Nile virus on California birds. Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reisen WK, Barker CM, Carney R, Lothrop HD, Wheeler SS, Wilson JL, Madon MB, Takahashi R, Carroll B, Garcia S, Fang Y, Shafii M, Kahl N, Ashtari S, Kramer V, Glaser C, Jean C. Role of corvids in epidemiology of West Nile virus in southern California. J Med Entomol. 2006;43:356–367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 57.Dawson JR, Stone WB, Ebel GD, Young DS, Galinski DS, Pensabene JP, Franke MA, Eidson M, Kramer LD. Crow deaths caused by West Nile virus during winter. Emerg Infect Dis. 2007;13:1912–1914. doi: 10.3201/eid1312.070413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Griffing SM, Kilpatrick AM, Clark L, Marra PP. Mosquito landing rates on nesting American robins (Turdus migratorius) Vector Borne Zoonotic Dis. 2007;7:437–443. doi: 10.1089/vbz.2006.0560. [DOI] [PubMed] [Google Scholar]
- 59.Weaver RL. Growth and development of English sparrows. Wilson Bull. 1942;54:183–191. [Google Scholar]
- 60.Hartemink NA, Davis SA, Reiter P, Hubálek Z, Heesterbeek JAP. Importance of bird-to-bird transmission for the establishment of West Nile virus. Vector Borne Zoonotic Dis. 2007;7:575–584. doi: 10.1089/vbz.2006.0613. [DOI] [PubMed] [Google Scholar]


