Abstract
A prospective cohort design was used to measure the association between daily cotrimoxazole-prophylaxis and infection with Plasmodium falciparum containing mutations associated with antifolate resistance among persons infected with human immunodeficiency virus (HIV) in Tororo and Busia District, in eastern Uganda. Of 149 cases of P. falciparum parasitemia diagnosed, 147 (99%) (smears from participants taking prophylaxis = 91 and smears from those not taking cotrimoxazole prophylaxis = 56) were successfully assessed for mutations in the dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutations associated with antifolate resistance. Prevalences of the dhfr pure triple mutant (74% and 70%; P = 0.71), the dhps pure double mutant (95% and 88%; P = 0.21), and the dhfr/dhps pure quintuple mutant (73% and 64%; P = 0.36), were not significantly different between those taking and those not taking cotrimoxazole-prophylaxis, respectively. The overall prevalence of the pure quintuple mutant in this study was 69%, which is among the highest in Africa. Although resistance rates of P. falciparum to antifolate drugs are high, cotrimoxazole-prophylaxis in HIV-infected persons was not associated with a higher prevalence of mutations associated with antifolate resistance.
Introduction
Because of the high morbidity and mortality caused by human immunodeficiency virus (HIV) and malaria, the overlap of these two diseases in sub-Saharan Africa produces interactions of tremendous public health importance. In Uganda, the prevalence of HIV is estimated to be 6.4% in adults and 0.7% in children,1 The transmission intensity of malaria in Tororo, Uganda, the site of the study, is very high, with a parasite prevalence of 91% among children 2–9 years of age2 and an entomologic inoculation rate of 562 infectious bites per person per year.3 Past evidence has demonstrated that clinical malaria is more likely to develop in HIV-infected patients than in those who are uninfected,4–6 with an estimated 10% of clinical malaria in Africa attributable to concurrent HIV infection.7 Furthermore, HIV-infected women are at greater risk of acquiring placental malaria during pregnancy than HIV-uninfected women.8 Those HIV-infected patients who contract malaria are also more likely than HIV-uninfected patients to acquire severe malaria in low or unstable transmission areas, and the risk of clinical treatment failure in patients with Plasmodium falciparum malaria increases with HIV infection and decreasing CD4 cell count.9,10
The incidence of malaria is decreased substantially in HIV-infected patients taking prophylactic trimethoprim-sulfamethoxazole (cotrimoxazole).11 The motivation for the current escalation in cotrimoxazole prophylaxis results from several recent studies documenting clinical benefit, including a reduction in opportunistic infections and mortality among HIV-infected adults and children taking this medication.12–15 Currently, the World Health Organization (WHO) recommends cotrimoxazole prophylaxis in all HIV-infected patients with mild to advanced disease (WHO Stages 2, 3, and 4) and in Stage 1 HIV-infected children with a low CD4 lymphocyte percentage.16 In spite of the well-documented benefits of cotrimoxazole prophylaxis, there is concern that its widespread use will lead to the selection of P. falciparum resistance to the antifolate class of antimalarial drugs, including sulfadoxine-pyrimethamine (SP).17,18 In Uganda, SP, in combination with chloroquine, was used as a provisional first-line therapy from 2000 until 2007, when artemether-lumefantrine became the first-line option.19 In addition, chloroquine/SP is still being used by the Ugandan Ministry of Health for home-based management of fever, and SP remains the only recommended drug for intermittent preventive treatment of malaria in pregnancy.20
Resistance to SP in sub-Saharan Africa is accrued in a step-wise fashion with three mutations in the dihydrofolate reductase (dhfr) gene (triple mutant 108N + 51I + 59R) reducing the efficacy of pyrimethamine and two mutations in the dihydropteroate synthase (dhps) gene (double mutant 437G + 540Q) decreasing the efficacy of sulfadoxine.21,22 Acquisition of a quintuple mutant parasite with all five mutations is associated with an increased risk of failure after treatment with SP.21,23 Previous studies have not demonstrated an increase in the prevalence of antifolate resistance mutations among the uninfected household members of HIV-infected patients on long-term cotrimoxazole prophylaxis in Tororo.24 However, there is concern that HIV-infected patients who take cotrimoxazole prophylaxis may select for antifolate-resistant parasites, especially in areas in which malaria is highly endemic.25
To investigate the impact of daily cotrimoxazole prophylaxis on the selection of SP-related resistance mutations in P. falciparum, we compared the prevalence of antifolate resistance mutations between HIV-infected patients who were taking and not taking cotrimoxazole prophylaxis in the Tororo District in eastern Uganda.
Methods
Study participants and clinical study.
The cohort and study methods have been described.11 Briefly, in April and May 2001, HIV-infected patients were recruited sequentially after coming to the AIDS Support Organization in Tororo, Uganda. Written, informed consent was provided by all participants. In 2003, Uganda Ministry of Health policy changes mandated cotrimoxazole use in HIV-infected patients, and beginning in July 2003 study participants were provided with weekly supplies of cotrimoxazole prophylaxis (160 mg of trimethoprim and 800 mg of sulfamethoxazole daily in adults and corresponding dosing in children based on weight). Doses were provided weekly in pre-packaged pill boxes for adults or in liquid concentrate form for children. However, some of the HIV-infected participants were not taking cotrimoxazole prophylaxis at the time of certain episodes of clinical malaria because of allergies to drugs, severity of illness that precluded taking the drug, or delay in initiation of prophylaxis after enrollment. During the study period from July 2003 through April 2006, a total of 3,601 blood smears were obtained from study participants, 2,154 smears were obtained from HIV-infected participants taking cotrimoxazole prophylaxis, of which 58 (2.7%) were positive, and 1,447 smears were obtained from HIV-infected participants not taking cotrimoxazole, of which 94 (6.5%) were positive. Of the 152 positive smears, there were 3 smears in which the accompanying filter paper samples had been used in previous studies, leaving 149 episodes of parasitemia available for analysis.
Each participant was visited weekly by study staff and was administered a standardized questionnaire regarding fever or illness in the preceding seven days. At the same visit, weekly blood smears and filter paper samples were collected. Slides were evaluated for Plasmodium species at the study clinic and antimalarial treatment was provided to clients at their homes. Home-based treatment consisted of SP with or without chloroquine, per Uganda Ministry of Health guidelines at the time of the study.
Laboratory methods.
Thick blood smears for malaria parasites were stained with Leishman's stain and parasite density was estimated by counting the number of asexual parasites per 200 leukocytes and calculating parasites per microliter, assuming a leukocyte count of 8,000 cells/µL. Thin blood smears were used to identify Plasmodium species. Symptomatic malaria was defined as a parasitemia with either reported fever in the two days before the home visit or an axillary temperature ≥ 38.0°C at the time of the home visit.
We selected all available filter paper specimens from positive blood smears diagnosed in HIV-infected persons to test for molecular markers associated with antifolate resistance. We assessed for the presence of three mutations in the dhfr gene (108N, 51I, and 59R) and two mutations in the dhps gene (437G and 540Q) commonly found in eastern Africa. Additionally, we tested for one dhfr mutation (164L) and three dhps mutations (436S, 581G, and 613S) rarely found in Africa, but also associated with antifolate resistance.22 Parasite DNA was isolated from filter paper using the Chelex extraction method,26 and genotypes were determined by using nested polymerase chain reaction amplification, digestion with restriction endonucleases, and visualization after gel electrophoresis as described.23,27 Specimens were classified as wild-type, pure mutant, or mixed (both mutant and wild-type alleles detected in the same specimen).
Data analysis.
Data were double-entered using Epi Info (Centers for Disease Control and Prevention, Atlanta, GA), and analyzed using STATA 10.0 (STATA Corp., College Station, TX). The chi-square test was used to compare binary characteristics between those patients with parasitemia during cotrimoxazole prophylaxis and those with parasitemia who were not taking prophylaxis. Median ages were compared by using the Wilcoxon rank-sum test. A t-test was used to evaluate geometric mean parasite density between the two groups. Prevalence estimates of assessed genotype mutations were compared between groups by using Fisher's exact test. To account for the possibility of repeated testing of parasite strains after episodes of asymptomatic parasitemia or recrudescent malaria, we also tested only the first episode from each participant for an association between cotrimoxazole use and prevalence of antifolate resistance–conferring mutations. Multivariate analysis was used to test the association of different independent variables, including age, sex, presence of fever, and time of specimen collection, with the presence of the dhfr/dhps pure quintuple mutant compared with the presence of the mixed mutant or wild-type genotypes. Generalized estimating equation methods with exchangeable correlation structure were used to account for repeated measures among the same persons in comparing the association of independent variables with the presence of the dhfr/dhps quintuple mutant. To investigate changes in prevalence of the dhfr triple pure mutant and dhps double pure mutant over time, we analyzed results of the genotyping episodes of P. falciparum malaria obtained from HIV-infected participants from July 2003 through April 2006 by using a non-parametric extension of the rank-sum test for trend.28
The study was reviewed and approved by the Science and Ethics Committee of the Uganda Virus Research Institute, the Uganda National Council of Science and Technology, and the Institutional Review Boards of the Centers for Disease Control and Prevention, the University of Washington, and the University of California, San Francisco.
Results
Characteristics of study population.
During July 2003–April 2006, we identified 149 episodes of parasitemia for analysis among the HIV-infected population. Of the 149 episodes, we were able to establish genotypes for 147 episodes (98.7%). Of these 147 episodes, 91 occurred in 60 participants taking cotrimoxazole prophylaxis and 56 occurred in 37 participants not taking prophylaxis. The reasons why 37 HIV-infected individuals were not taking cotrimoxazole prophylaxis when parasitemia was diagnosed are as follows: 8 were discontinued because of an adverse reaction to cotrimoxazole, with an average of 324 days to the first incident case of malaria; 6 were discontinued because of being too ill to take the medication, with an average of 545 days to the first incident case of malaria; and 23 acquired malaria infection after enrollment but before being started on cotrimoxazole prophylaxis, with an average of 267 days to the first incident case of malaria.
The characteristics of both groups are shown in Table 1. There were no differences in age, sex, mean parasite density, or percent of parasitemic cases resulting in symptomatic malaria between the two groups.
Table 1.
Characteristics of Plasmodia-positive blood smears diagnosed in persons infected with human immunodeficiency virus, Tororo and Busia Districts, Uganda*
| Characteristic | Taking cotrimoxazole (n = 91) | Not taking cotrimoxazole (n = 56) | P |
|---|---|---|---|
| Female sex (%) | 59 (65) | 38 (68) | 0.71 |
| Median age, years (IQR) | 12 (3–30) | 9 (3–34.5) | 0.74 |
| Symptomatic malaria (%) | 55 (60) | 29 (52) | 0.30 |
| Parasite density/μL† (range) | 3,830 (120–80,000) | 2,821 (80–80,000) | 0.40 |
| Date sample collected (%) | |||
| August 2003–July 2004 | 33 (36) | 28 (50) | 0.25 |
| August 2004–July 2005 | 33 (36) | 15 (27) | |
| August 2005–April 2006 | 25 (27) | 13 (23) | |
IQR = interquartile range.
Geometric mean.
Prevalence of molecular markers of folate resistance.
All of the samples contained the dhfr 51I and dhfr 108N (Table 2). The dhfr 59R mutation (either mixed or pure mutant) was found in 94% of the samples from patients taking cotrimoxazole prophylaxis and in 88% of the samples from patients not taking prophylaxis (P = 0.24). All positive blood smears from patients taking cotrimoxazole contained the pure dhps 437G mutation compared with 93% pure mutants and 7% mixed mutant/wild-type from patients not taking cotrimoxazole (P = 1.00), and the dhps 540Q mutation was found in 99% of the samples from those taking cotrimoxazole and in 98% of samples from those not taking cotrimoxazole (P = 1.00). No significant differences were found between either the mixed or pure mutant dhfr triple mutant (P = 0.24 and P = 0.71, respectively) or the mixed or pure mutant dhps double mutant (P = 1.00 and P = 0.21, respectively) in the two prophylaxis groups. The pure quintuple mutant parasite was found in 73% of the samples from patients taking cotrimoxazole and in 64% of samples from patients not taking the drug (P = 0.36). When only first episodes of each participant were tested (n = 97), the prevalence of the dhfr pure triple mutant in samples from those participants taking and not taking cotrimoxazole was 70% and 67%, respectively (P = 0.48); the prevalence of the dhps pure double mutant in samples from those participants taking and not taking cotrimoxazole was 91% and 91%, respectively (P = 0.63); and the prevalence of the dhfr/dhps pure quintuple mutant in samples from those participants taking and not taking cotrimoxazole was 67% and 65%, respectively (P = 0.52).
Table 2.
Prevalence of key dhfr/dhps alleles, Tororo and Busia Districts, Uganda, stratified by cotrimoxazole use*
| Allele | Taking cotrimoxazole, no. (%) (n = 91) | Not taking cotrimoxazole,† no. (%) (n = 56) | P‡ |
|---|---|---|---|
| dhfr 51 mixed or pure mutant | 91 (100) | 56 (100) | 1.00 |
| dhfr 59 mixed or pure mutant | 85 (94) | 49 (88) | 0.24 |
| dhfr 108 mixed or pure mutant | 91 (100) | 56 (100) | 1.00 |
| dhps 437 mixed or pure mutant | 91 (100) | 56 (100) | 1.00 |
| dhps 540 mixed or pure mutant | 90 (99) | 55 (98) | 1.00 |
| dhfr 51 triple pure mutant only | 67 (74) | 39 (70) | 1.00 |
| dhfr triple mixed or pure mutant | 85 (93) | 49 (88) | 0.24 |
| dhps double pure mutant only | 86 (95) | 49 (88) | 0.21 |
| dhps double mixed or pure mutant | 90 (99) | 55 (98) | 1.0 |
| dhfr/dhps quintuple pure mutant only | 66 (73) | 36 (64) | 0.36 |
| dhfr/dhps quintuple mixed or pure mutant | 84 (92) | 48 (86) | 0.26 |
dhfr = dihydrofolate reductase; dhps = dihyropteroate synthase.
Two samples with no molecular results were not included.
By Fisher's exact test.
All other tested mutations were either not detected or rarely found. The dhfr 164L mutation and the dhps 613S were not found in any samples. The dhps 436S mutation was present in only 6% of cases from patients taking cotrimoxazole and in 2% of cases from patients not taking cotrimoxazole (P = 0.70), and the dhps 581G mutation was found in 4% and 0% of cases from those same groups, respectively (P = 0.41).
Use of cotrimoxazole prophylaxis was not associated with an increased prevalence of the dhfr/dhps quintuple mutant even after adjusting for age, sex, presence of symptomatic malaria, and time of sample collection (odds ratio = 1.16, 95% confidence interval = 0.91–1.46, P = 0.23).
Prevalence of the double and triple pure mutants over time.
During July 2003–April 2006, enrolled HIV-infected patients who had weekly sample collection for parasitemia provided 147 cases to investigate the changes in prevalence of the dhps double pure mutant and dhfr triple pure mutant over time. The data from all genotyped episodes of P. falciparum malaria in this study are shown in Figure 1 and demonstrate a statistically significant increase in prevalence of the dhfr triple pure mutant over time (P = 0.04). The prevalence of the dhps double pure mutant was very high (87%) at the beginning of the study and showed a slight increase over time that was not statistically significant (P = 0.52). Although the prevalence of dhfr triple mutant increased significantly over the three years of the study, the lack of association between the triple mutant and cotrimoxazole use did not vary over time.
Figure 1.
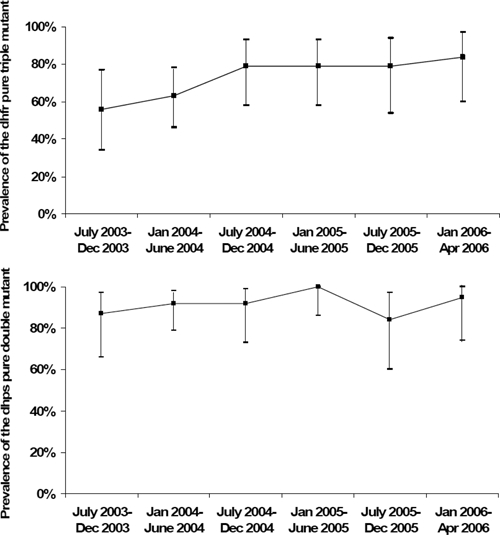
Prevalence of the dihydrofolate reductase pure triple mutants and dihydropteroate synthase pure double mutants among participants infected with human immunodeficiency virus over time (P = 0.04 and P = 0.52, respectively), Uganda.
Discussion
In this prospective cohort study, we found no difference in the proportion of parasitemic episodes caused by antifolate resistant genotypes between HIV-infected people taking and not taking cotrimoxazole prophylaxis. However, three of the common antifolate resistance-conferring mutations (dhfr-51I, 108N and dhps 437G) were already saturated among our participants, limiting our ability to detect a difference among these genotypes between groups. The two other common antifolate resistance–conferring mutations in our population (dhfr 59R and dhps 540Q) had prevalences greater than 80%. Age or diagnosis of symptomatic malaria were not associated with the presence of markers of antifolate resistance. Previously, a five-fold reduction in the incidence of malaria has been demonstrated among HIV-infected persons in Tororo taking cotrimoxazole prophylaxis.11 Our findings suggest that the use of cotrimoxazole prophylaxis for HIV-infected patients in areas with a baseline of high prevalence of P. falciparum dhfr and dhps mutations associated with antifolate resistance may not lead to an increase in these same mutations. This conclusion is further supported by the fact that HIV-uninfected participants and HIV-unknown participants of other studies being conducted concurrently in Tororo had nearly identical prevalences of the dhfr and dhps mutations compared with our study of HIV-infected participants.24,29,30 The prevalence of mutations associated with antifolate resistance appears to be increasing over time in Tororo, reaching extremely high levels in our patient population and achieving saturation in some alleles.
Cross-resistance between trimethoprim and pyrimethamine31 and between sulfamethoxazole and sulfadoxine32 has been documented in vitro with mutations in the dhfr and dhps genes, respectively. However, the in vivo effect of cotrimoxazole use on the acquisition of antifolate-resistant malaria parasites has not yet been established. At this point, cumulative studies in sub-Saharan Africa indicate that cotrimoxazole prophylaxis does not contribute to increased prevalence of antifolate-resistant markers,24,33,34 although the efficacy of cotrimoxazole prophylaxis in decreasing the incidence of malaria in some of these studies limits the power to detect a difference in these markers between those samples from participants taking cotrimoxazole prophylaxis and those samples from participants not taking this prophylaxis.33,34
We have directly observed the increasing prevalence of mutations associated with antifolate resistance in P. falciparum over time in Tororo (Figure 1). One of several theories addressing the cause of increasing antifolate resistance hypothesizes that weak selection of antifolate-resistant parasites may be catalyzed through cotrimoxazole prophylaxis.25 Although we cannot prove that cotrimoxazole is not contributing to the increasing prevalence of P. falciparum antifolate resistance, we believe that the high levels of antifolate resistance in Uganda before widespread use of cotrimoxazole prophylaxis and the uniform increase in the prevalences of antifolate-resistant parasites in different patient populations taking and not taking cotrimoxazole prophylaxis in Tororo provide evidence against this theory. An alternate hypothesis attributes the etiology of the increasing prevalence of antifolate-resistant parasites to the common use of SP in the region, either alone or in combination with other drugs, as a primary therapy for clinical malaria and for intermittent preventive therapy for pregnant women. A previous study has shown that after the introduction of dhfr and dhps mutations associated with antifolate resistance into eastern Africa from southeast Asia in the 1980s and 1990s, these mutations have spread across the continent after the increased use of SP as a commonly used anti-malarial therapy in southern and eastern Africa.35 Furthermore, some of these antifolate mutations provide a transmission advantage to infect anopheline mosquitoes after therapy of patients that includes SP.36 Causal factors associated with the increasing prevalence of antifolate resistance–conferring mutations in P. falciparum remains controversial, and may be multifactorial.
To our knowledge, the prevalence of the pure quintuple mutant (69%) and the pure and mixed quintuple mutant (90%) in Tororo are among the highest recorded in Africa. The prevalence of dhfr and dhps mutations in other studies of HIV-uninfected and HIV-unknown patients concurrently being conducted in Tororo were almost identical to those in our study.24,29,30 Although these other studies are not directly comparable, they provide an indication that there was little difference in the prevalence of antifolate resistance–conferring mutations between HIV-infected and uninfected populations in Tororo. Despite this high prevalence of antifolate-resistant mutations, we have previously demonstrated that daily cotrimoxazole prophylaxis reduces the incidence of malaria and improves mortality in HIV-infected patients in this region.11,15 The mechanism by which cotrimoxazole can prevent malaria caused by parasites containing mutations associated with antifolate resistance has not been determined. It is possible these mutations do not diminish the protective efficacy of cotrimoxazole or perhaps that drug levels required for malaria prevention are lower than those needed for treatment.
Cotrimoxazole or other sulfa-drug use was not assessed biochemically. Therefore, we can not rule out the possibility of misclassification bias if some cotrimoxazole use was over reported in the HIV-infected patients taking cotrimoxazole and/or if use of other sulfa-drugs such as SP was under-reported by malaria-infected persons not taking cotrimoxazole prophylaxis. Second, the near saturation of mutant alleles in the HIV-infected overall population at the time of study may have limited us from observing selection of P. falciparum dhfr and dhps mutations.
In summary, cotrimoxazole prophylaxis was not associated with a higher prevalence of P. falciparum dhfr and dhps mutations below saturation in our population. There was also no difference in the prevalences of the dhfr/dhps quintuple mutant between those taking and not taking cotrimoxazole prophylaxis. The efficacy of cotrimoxazole prophylaxis in reducing the incidence of malaria and preventing morbidity and mortality in HIV-infected patients has been well-established, even in the setting of high population levels of antifolate resistance–conferring mutations. Our present data add to the growing collection of evidence suggesting that chronic cotrimoxazole prophylaxis may not select for antifolate-resistant P. falciparum malaria in HIV-infected persons in an area of high transmission intensity and with high levels of dhfr and dhps mutations associated with resistance to antifolate drugs. However, the near or total saturation of mutant alleles in the HIV-infected overall population at the time of study may have limited us from observing selection of some P. falciparum dhfr and dhps mutations. More studies are needed to determine the effect of cotrimoxazole on HIV-infected persons in areas with differing malaria transmission intensities and prevalence of antifolate resistance–conferring mutations.
Footnotes
Financial support: This study was supported by CDC and the United States Agency for International Development through the Emergency Plan for AIDS Relief. Samuel Malamba was supported by the Fogarty AIDS International Training and Research Program (1-D43-TW00003) at the University of California, Berkeley. Testing for molecular markers was supported by the Fogarty International Center/National Institutes of Health (TW00007).
Authors' addresses: Samuel Malamba and John Lule, Global AIDS Program, National Center for HIV, STD and TB Prevention, Centers for Disease Control and Prevention–Uganda, Entebbe, Uganda. Taylor Sandison, Department of Medicine, University of Washington School of Medicine, Seattle, WA. Arthur Reingold, Division of Epidemiology, School of Public Health, University of California, Berkeley, CA. Jordan Walker and Grant Dorsey, Department of Medicine, San Francisco General Hospital, University of California, San Francisco, CA. Jonathan Mermin, Coordinating Office for Global Health, Centers for Disease Control and Prevention–Kenya, Nairobi, Kenya.
References
- 1.World Health Organization Uganda HIV/AIDS Sero-Behavioral Survey (UHSBS) 2004–2005. www.who.int/3by5/support/june2005_uga.pdf Availabe at.
- 2.Talisuna AO, Erhart A, Samarasinghe S, Van Overmeir C, Speybroeck N, D'Alessandro U. Malaria transmission intensity and the rate of spread of chloroquine resistant Plasmodium falciparum: why have theoretical models generated conflicting results. Infect Genet Evol. 2006;6:241–248. doi: 10.1016/j.meegid.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 4.French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS. 2001;15:899–906. doi: 10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS. 2004;18:547–554. doi: 10.1097/00002030-200402200-00023. [DOI] [PubMed] [Google Scholar]
- 6.Korenromp EL. Malaria attributable to the HIV-1 epidemic, sub-Saharan Africa. Emerg Infect Dis. 2005;11:1410–1419. doi: 10.3201/eid1109.050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 8.ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, van Eijk AM, Rogerson SJ, Steketee RW. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am J Trop Med Hyg. 2004;71:41–54. [PubMed] [Google Scholar]
- 9.Van Geertruyden JP, Mulenga M, Mwananyanda L, Chalwe V, Moerman F, Chilengi R, Kasongo W, Van Overmeir C, Dujardin JC, Colebunders R, Kestens L, D'Alessandro U. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J Infect Dis. 2006;194:917–925. doi: 10.1086/507310. [DOI] [PubMed] [Google Scholar]
- 10.Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobya SL, Francis D, Rosenthal PJ, Dorsey G, Havlir D. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 11.Mermin J, Ekwaru JP, Liechty CA, Were W, Downing R, Ransom R, Weidle P, Lule J, Coutinho A, Solberg P. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–1261. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 12.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, Manlan K, N'Dri-Yoman T, Salamon R. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 13.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, Djomand G, Ackah A, Domoua K, Kadio A, Yapi A, Combe P, Tossou O, Roels TH, Lackritz EM, Coulibaly D, De Cock KM, Coulibaly IM, Greenberg AE. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d'Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 14.Chintu C, Bhat GJ, Walker AS, Mulenga V, Sinyinza F, Lishimpi K, Farrelly L, Kaganson N, Zumla A, Gillespie SH, Nunn AJ, Gibb DM. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 15.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, Kaharuza F, Culver D, Kizito F, Bunnell R, Kigozi A, Nakanjako D, Wafula W, Quick R. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–1434. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 16.Eggena MP, Barugahare B, Okello M, Mutyala S, Jones N, Ma Y, Kityo C, Mugyenyi P, Cao H. T cell activation in HIV-seropositive Ugandans: differential associations with viral load, CD4+ T cell depletion, and coinfection. J Infect Dis. 2005;191:694–701. doi: 10.1086/427516. [DOI] [PubMed] [Google Scholar]
- 17.Gill CJ, Sabin LL, Tham J, Hamer DH. Reconsidering empirical cotrimoxazole prophylaxis for infants exposed to HIV infection. Bull World Health Organ. 2004;82:290–297. [PMC free article] [PubMed] [Google Scholar]
- 18.Whitty CJ, Jaffar S. Plasmodium falciparum cross resistance. Lancet. 2002;359:80. doi: 10.1016/S0140-6736(02)07300-2. [DOI] [PubMed] [Google Scholar]
- 19.Kamya MR, Bakyaita NN, Talisuna AO, Were WM, Staedke SG. Increasing antimalarial drug resistance in Uganda and revision of the national drug policy. Trop Med Int Health. 2002;7:1031–1041. doi: 10.1046/j.1365-3156.2002.00974.x. [DOI] [PubMed] [Google Scholar]
- 20.Greenwood B. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70:1–7. [PubMed] [Google Scholar]
- 21.Dorsey G, Dokomajilar C, Kiggundu M, Staedke SG, Kamya MR, Rosenthal PJ. Principal role of dihydropteroate synthase mutations in mediating resistance to sulfadoxine-pyrimethamine in single-drug and combination therapy of uncomplicated malaria in Uganda. Am J Trop Med Hyg. 2004;71:758–763. [PubMed] [Google Scholar]
- 22.Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, Mberu EK, Cowman AF, Winstanley PA, Watkins WM, Nzila AM. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next. Trends Parasitol. 2001;17:582–588. doi: 10.1016/s1471-4922(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 23.Kyabayinze D, Cattamanchi A, Kamya MR, Rosenthal PJ, Dorsey G. Validation of a simplified method for using molecular markers to predict sulfadoxine-pyrimethamine treatment failure in African children with falciparum malaria. Am J Trop Med Hyg. 2003;69:247–252. [PubMed] [Google Scholar]
- 24.Malamba SS, Mermin J, Reingold A, Lule JR, Downing R, Ransom R, Kigozi A, Hunt BM, Hubbard A, Rosenthal PJ, Dorsey G. Effect of cotrimoxazole prophylaxis taken by human immunodeficiency virus (HIV)-infected persons on the selection of sulfadoxine-pyrimethamine-resistant malaria parasites among HIV-uninfected household members. Am J Trop Med Hyg. 2006;75:375–380. [PubMed] [Google Scholar]
- 25.Laufer MK, van Oosterhout JJ, Thesing PC, Thumba F, Zijlstra EE, Graham SM, Taylor TE, Plowe CV. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 26.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 27.Duraisingh MT, Curtis J, Warhurst DC. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes by PCR and restriction digestion. Exp Parasitol. 1998;89:1–8. doi: 10.1006/expr.1998.4274. [DOI] [PubMed] [Google Scholar]
- 28.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 29.Bukirwa H, Yeka A, Kamya MR, Talisuna A, Banek K, Bakyaita N, Rwakimari JB, Rosenthal PJ, Wabwire-Mangen F, Dorsey G, Staedke SG. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clin Trials. 2006;1:e7. doi: 10.1371/journal.pctr.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis D, Nsobya SL, Talisuna A, Yeka A, Kamya MR, Machekano R, Dokomajilar C, Rosenthal PJ, Dorsey G. Geographic differences in antimalarial drug efficacy in Uganda are explained by differences in endemicity and not by known molecular markers of drug resistance. J Infect Dis. 2006;193:978–986. doi: 10.1086/500951. [DOI] [PubMed] [Google Scholar]
- 31.Iyer JK, Milhous WK, Cortese JF, Kublin JG, Plowe CV. Plasmodium falciparum cross-resistance between trimethoprim and pyrimethamine. Lancet. 2001;358:1066–1067. doi: 10.1016/S0140-6736(01)06201-8. [DOI] [PubMed] [Google Scholar]
- 32.Triglia T, Menting JG, Wilson C, Cowman AF. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94:13944–13949. doi: 10.1073/pnas.94.25.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamya MR, Gasasira AF, Achan J, Mebrahtu T, Ruel T, Kekitiinwa A, Charlebois ED, Rosenthal PJ, Havlir D, Dorsey G. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;21:2059–2066. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 34.Thera MA, Sehdev PS, Coulibaly D, Traore K, Garba MN, Cissoko Y, Kone A, Guindo A, Dicko A, Beavogui AH, Djimde AA, Lyke KE, Diallo DA, Doumbo OK, Plowe CV. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192:1823–1829. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, Chandramohan D, Sharp B. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 36.Dunyo S, Milligan P, Edwards T, Sutherland C, Targett G, Pinder M. Gametocytaemia after drug treatment of asymptomatic Plasmodium falciparum. PLoS Clin Trials. 2006;1:e20. doi: 10.1371/journal.pctr.0010020. [DOI] [PMC free article] [PubMed] [Google Scholar]


