Abstract
Dengue infection can be challenging to diagnose early in the course of infection before severe manifestations develop, but early diagnosis can improve patient outcomes and promote timely public health interventions. We developed age-based predictive models generated from 2 years of data from an enhanced dengue surveillance system in Puerto Rico. These models were internally validated and were able to differentiate dengue infection from other acute febrile illnesses with moderate accuracy. The accuracy of the models was greater than either the current World Health Organization case definition for dengue fever or a proposed modification to this definition, while requiring the collection of fewer data. In young children, thrombocytopenia and the absence of cough were associated with dengue infection; for adults, rash, leucopenia, and the absence of sore throat were associated with dengue infection; in all age groups, retro-orbital pain was associated with dengue infection.
Introduction
Dengue is the world's most common mosquito-borne viral infection and a leading cause of morbidity throughout the tropics and subtropics.1 Although at least 50–100 million infections are known to occur worldwide each year, the true burden of disease is unknown.2 Illness caused by one of the four dengue virus serotypes can range from nonspecific febrile illness to classic dengue fever (DF), which may then progress to severe disease (e.g., dengue hemorrhagic fever or dengue shock syndrome in the 1997 dengue classification system). Countries often report only the most severe cases, many cases of DF are not diagnosed or reported and case-fatality rates between countries are not currently comparable.
The diagnosis of dengue infection early in the course of illness, before development of severe manifestations of the disease, can be challenging. Serologic tests, the mainstay of laboratory diagnosis, are unreliable early in infection (during the first 3 days after symptom onset) and usually require collection of paired acute- and convalescent-phase samples. Polymerase chain reaction (PCR) testing, which is more sensitive early in dengue infection, is usually unavailable in the countries with the highest burden of disease, and no rapid diagnostic test is in widespread clinical use. Diagnosis, therefore, typically relies on identification of clinical features consistent with the World Health Organization (WHO) case definitions.3 Unlike dengue hemorrhagic fever, which is solely a clinical diagnosis, the diagnosis of DF relies on recognition of clinical features along with serologic confirmation or an epidemiologic link to a confirmed case. For practical purposes, in dengue-endemic regions, the latter two caveats are often ignored and a diagnosis of DF made solely on the basis of clinical features consistent with the WHO case definition. However, the sensitivity and specificity of this definition have been questioned and a proposed revised case definition is under consideration.4–8
Although early laboratory diagnosis is often not feasible, it has critical implications for the care of individual patients and improving public health. Early diagnosis can improve patient outcomes by enabling more timely assessment and initiation of supportive clinical management of patients with warning signs of severe disease.3,9 Identification of patients with dengue infection early, while the patient is still febrile (and viremic), can additionally help limit further transmission of dengue virus within households and communities. For these reasons, the WHO recently identified among its global research priorities the need for “clinical and laboratory indicators of early dengue.”10 A recent systematic literature review identified multiple clinical and laboratory features that could potentially differentiate dengue from other febrile illnesses, but concluded that published studies to date have been hampered by methodological limitations.11 In addition, clinical features of dengue were noted to vary significantly between age groups and stages of illness.11–16
An analysis performed by our group using data from the first year of our enhanced dengue surveillance system, published subsequent to the systematic review,11 identified several early clinical features that were independently associated with laboratory-positive dengue infections in children and adults.17 These features varied somewhat between children to adults, and while a combination of clinical features predictive of dengue in children was identified; no similar combination of clinical features was found in adults. Recent improvements in our enhanced dengue surveillance system now enable us to systematically collect laboratory results in addition to clinical data on patients reported to the system. We therefore decided to undertake an analysis of data from the second and third years of the system to assess whether the incorporation of these laboratory results could enable us to identify a constellation of early clinical and laboratory features predictive of dengue infection in an endemic-disease area, including those that may vary by patient age. We collected data from the time of initial physician contact at an outpatient facility, rather than at hospitalization, thereby allowing identification of features present early in the disease course. We validated our model internally using bootstrap re-sampling techniques, a step not previously performed in any previous dengue prediction model to our knowledge.11 We additionally sought to compare the discriminatory ability of our model with the current (1997)3 and proposed (November 2009) WHO case definitions for DF.8
Materials and Methods
Study area and population.
Puerto Rico has a population of 3.8 million (2000 U.S. Census data) and is divided into 78 administrative municipalities. Passive, laboratory-based dengue surveillance has been conducted island-wide in Puerto Rico for more than 3 decades. Through this system, it was determined that rates of suspected and confirmed dengue in the rural, southeastern coastal municipality of Patillas have historically been among the highest municipality-specific rates in Puerto Rico (CDC, unpublished data). Since 2005, the Patillas Enhanced Dengue Surveillance System (P-EDSS) has been operational at the sole health center in Patillas, the Centro de Servicios Primarios de Salud de Patillas, which serves the majority of the municipality's 20,152 inhabitants (2000 U.S. Census data). This health center consists of an emergency room and an extended-hours clinic for children and adults but does not provide any inpatient care services. Details of this enhanced surveillance system have been described previously.18 Briefly, on-site Centers for Disease Control and Prevention (CDC) staff at the health center work with health-care providers to optimize identification and reporting of dengue cases and to ensure the completeness and accuracy of the dengue case identification form (DCIF), which accompanies every serum specimen submitted for testing to CDC's Dengue Branch in San Juan, Puerto Rico.
Collection of patient data.
An analysis was performed using data from the P-EDSS from January 1, 2007 through December 31, 2008. All Centro de Servicios Primarios de Salud de Patillas patients suspected of having a dengue infection, as defined in the case definitions described below, were to be reported to the P-EDSS. Only patients for whom a definitive laboratory positive or negative diagnosis of dengue infection was made were included in the study. The DCIF records data on patient age, sex, days from symptom onset to specimen collection, and the provider's clinical diagnosis. Clinical data collected on the DCIF include signs and symptoms included in the 1997 WHO case definition of probable DF: headache, retro-orbital pain, myalgia, arthralgia, rash, and hemorrhage. Other signs and symptoms recorded are cough, nasal congestion, sore throat, abdominal pain, nausea, vomiting, and diarrhea. Laboratory data are collected if available, including white blood cell count, platelet count, hematocrit, albumin, and tourniquet test results. A random sample of 35 DCIFs from the study period was compared with medical records at the Centro de Servicios Primarios de Salud de Patillas to assess the accuracy and completeness of captured data. Data reported on the DCIF for this sample were 96% accurate and 96% complete compared with information available in the medical record (CDC, unpublished data).
Case definitions.
Suspected dengue case.
Documented fever of ≥ 38°C or history of fever lasting 2–7 days and two or more of the following symptoms or signs: headache, rash, retro-orbital pain, myalgia, arthralgia, hemorrhage, hypotension, hemoconcentration (hematocrit elevated ≥ 20% of population mean),19 thrombocytopenia (platelet count < 100,000 platelets/mm3), or attending physician suspicion of clinical dengue infection in a patient who does not meet the above criteria. Patients with a clinical diagnosis of infection by another pathogen (e.g., chickenpox, measles) were excluded.
Laboratory-positive dengue case.
A case of suspected dengue with anti-dengue IgM seroconversion or single anti-dengue IgM positivity or dengue virus identification through serotype-specific reverse transcriptase-polymerase chain reaction (RT-PCR).
Laboratory-negative dengue case.
A suspected dengue case that is negative for anti-dengue IgM antibodies in a convalescent-phase specimen and with neither dengue virus nor anti-dengue IgM detected in the acute specimen.
Laboratory-indeterminate dengue case.
A suspected dengue case negative for anti-dengue IgM antibodies and RT-PCR for dengue in an acute-phase specimen and for which a convalescent-phase specimen is not available.
Laboratory testing.
Blood specimens were collected from all suspected dengue cases and initially centrifuged and serum separated in Patillas. Extensive efforts were undertaken to collect both acute and convalescent specimens when possible (i.e., patient education about the importance of collecting the convalescent sample, reminder flyers, and follow-up reminder phone calls). Serum samples were stored at 4°C until transported once a week by CDC staff to the CDC Dengue Branch laboratory. Serum samples collected within 5 days of onset of symptoms (acute-phase samples) were tested by serotype-specific RT-PCR for dengue virus20,21 and by dengue-specific IgM antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA).22 Serum samples collected 6 or more days after symptom onset (convalescent-phase samples) were tested by MAC-ELISA. All samples with positive PCR results or seroconversion of IgM between acute- and convalescent-phase samples were tested by quantitative IgG ELISA to differentiate between primary and secondary dengue infections.
Statistical analysis.
Persons with laboratory-indeterminate dengue cases and infants (i.e., children < 365 days old) were excluded from our study analysis. Using laboratory-positive and laboratory-negative dengue cases, we performed univariate analysis to identify clinical and laboratory features associated with dengue positivity. Categorical variables were compared using the χ2 test or Fisher's exact test as appropriate, and continuous variables were compared using Student's t test and the Mann-Whitney U test when applicable.
Variables with a P value of < 0.1 on univariate analysis, or those features that are part of the 1997 WHO clinical case definition of probable DF,3 were entered into a multivariate logistic regression analysis using backward elimination with 95% confidence intervals calculated using Bonferroni correction to account for multiple comparisons. Age and sex were forced into the model as potential confounders. Stratified analyses were additionally performed for young children aged 1–9 years, adolescents aged 10–19 years, and adults aged ≥ 20 years. Receiver-operator characteristic (ROC) curves were generated for the overall logistic regression model and for the age-stratified models.
We dichotomized the continuous variable platelet count into a dichotomous variable, using a threshold of < 240,000 platelets/mm3 as “low platelet count.” This threshold was determined by ROC curve analysis as the level that best discriminated between the presence and absence of laboratory-positive dengue infection (data not shown). The ROC curve analysis of white blood cell count did not show a threshold level that clearly discriminated between the presence and absence of dengue, and therefore the commonly used definition of leukopenia (i.e., a total white blood cell count of < 5,000/mm3) was used.
We additionally created prediction models using the current WHO case definition for probable DF and a revised probable dengue case definition proposed by an international working group of dengue experts and included in the third edition of the WHO's “Dengue guidelines for diagnosis, treatment, prevention and control” (Table 1).3,8 For the purpose of this analysis, the non-specific term “aches and pains” was considered to refer only to generalized musculoskeletal complaints (i.e., arthralgia and myalgia as collected on the DCIF) and not to localized pain like headache or retro-orbital pain. The ROC curves were generated from these models according to the methods described previously. The accuracy of these models as measured by the area under the ROC curve (AUC) was compared with the AUC of our prediction model. Diagnostic accuracy was considered to be high if the AUC was > 0.9, moderate if in the range of 0.7 to 0.9, and low if between 0.5 and 0.7.23,24 We subsequently used bootstrap resampling methods to assess the significance of the difference in predictive ability between the models.25 One thousand bootstrap samples equal in size to the original dataset of 1,734 individuals were drawn (with replacement) and the various models fit to these bootstrap samples. Differences in AUCs (Δ AUC) between the models were compared using the percentile confidence intervals method to evaluate if Δ AUC was significantly different than zero. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Table 1.
Current and proposed World Health Organization (WHO) dengue case definitions
| Current (1997) WHO case definition3 | Proposed (2009) WHO case definition8 |
|---|---|
| An acute febrile illness with two or more of the following: | Live in or travel to endemic area Plus |
| • Headache | Fever and two or more of the following: |
| • Retro-orbital pain | • Nausea/vomiting |
| • Myalgia | • Rash |
| • Arthralgia | • Aches and pains |
| • Rash | • Tourniquet test positive |
| • Leukopenia | • Leukopenia |
| • Hemorrhagic manifestations | • Any warning sign |
| (includes the tourniquet test) | - Abdominal pain or tenderness |
| And | - Persistent vomiting |
| • Supportive serology | - Clinical fluid accumulation |
| • Occurrence at the same location and time as other confirmed cases of dengue fever | - Mucosal bleed |
| - Lethargy; restlessness | |
| - Liver enlargement > 2 cm | |
| - Increase in hematocrit concurrent with rapid decrease in platelets |
Ethical review.
The study protocol was reviewed and approved by the Human Subjects Institutional Review Board of the Centers for Disease Control and Prevention.
Results
Data were collected for 3,270 patients with suspected dengue infections from January 1, 2007 through December 31, 2008. One hundred seventy-eight infants and 1,137 persons with laboratory-indeterminate cases were excluded from analysis. Of the remaining 1,955 cases, 108 (5.5%) were dengue laboratory-positive and 1,847 (94.5%) were laboratory-negative. Of the laboratory-positive cases, 60 (55.5%) were confirmed by positive PCR results (2 DENV-1, 33 DENV-2, 24 DENV-3, 1 DENV-4), 15 (13.9%) by seroconversion from negative to positive IgM status, and 33 (30.5%) by a positive IgM result on a single specimen. Patients with laboratory-positive dengue infections were older (median 18.8 years, range 1–91 years) than patients who were laboratory-negative for dengue (median 11.5 years, range 1–95 years) (Table 2). Persons with laboratory-confirmed dengue were more likely to be male than those who were laboratory-negative for dengue, (52% versus 46%, respectively) but this difference did not reach statistical significance. Patients, regardless of diagnosis, presented to the health-care center a median of 1 day after fever onset (range, 0–17 days all patients; 0–8 days for laboratory-positives). There were 1,810 (92.6%) patients seen within 3 days of symptom onset. Only 11 (0.005%) of the 1,955 patients required hospitalization for this illness, and that included five laboratory-positive patients and six laboratory-negative patients.
Table 2.
Characteristics of dengue laboratory-positive and laboratory-negative patients, Enhanced Dengue Surveillance System, Patillas, Puerto Rico, January 1, 2007–December 31, 2008
| Dengue-positive (N = 108) | Dengue-negative (N = 1847) | P value* | |
|---|---|---|---|
| Age in years, median (range) | 18.8 (1–91) | 11.5 (1–95) | < 0.01 |
| Age 1 to 9, no. | 23 | 796 | |
| Age 10 to 19, no. | 35 | 397 | |
| Age 20 and over, no. | 50 | 654 | |
| Sex, no. (%) | |||
| Male | 56 (52) | 847 (46) | NS |
| Female | 52 (48) | 1000 (54) | NS |
| Fever, no. (%) | 101 (93) | 1719 (93) | NS |
| Headache, no. (%) | 77 (71) | 1201 (65) | NS |
| Retro-orbital pain, no. (%) | 38 (35) | 277 (15) | < 0.01 |
| Body aches, no. (%) | 71 (66) | 923 (50) | < 0.01 |
| Joint pain, no. (%) | 48 (44) | 591 (32) | 0.02 |
| Rash, no. (%) | 23 (21) | 74 (4) | < 0.01 |
| Nausea or vomiting, no. (%) | 32 (30) | 517 (28) | NS |
| Diarrhea, no. (%) | 11 (10) | 160 (9) | NS |
| Reported bleeding, no. (%) | 4 (4) | 15 (1) | NS |
| Sore throat, no. (%) | 41 (38) | 1,034 (56) | < 0.01 |
| Cough, no. (%) | 41 (38) | 1,145 (62) | < 0.01 |
| Nasal congestion, no. (%) | 38 (35) | 1,090 (59) | < 0.01 |
| Leukocyte count,† median (no. with data available) | 6.9 (N = 53) | 8.4 (N = 1102) | < 0.01 |
| Platelet count,† median (no. with data available) | 201 (N = 61) | 263 (N = 1,132) | < 0.01 |
| Hematocrit value (%), median | 41.3 (N = 47) | 39.6 (N = 824) | < 0.01 |
χ2 test for categorical variables, Mann-Whitney U test for continuous variables; NS = not significant.
×103 cells/mm3.
Primary or secondary infection status was determined for 51 (47%) of the 108 patients with laboratory-positive dengue; 17 had primary and 34 had secondary infections. Patients with primary infection were significantly younger than patients with secondary infection (12.1 versus 25.2 years; P = < 0.01) and were more likely to be male (76% versus 44%; P = 0.04). Those with primary and secondary infection did not differ significantly in time from symptom onset to presentation for care. On univariate analysis, patients with primary infections were more likely to have a rash and less likely to have symptoms of body ache, joint pains, or nausea and vomiting.
There were several differences in the frequency of presenting clinical features between patients with laboratory-positive dengue and patients that were dengue laboratory-negative (Table 2). Compared with dengue laboratory-negative patients, patients with laboratory-positive dengue infections were significantly more likely to have retro-orbital pain, rash, joint pain, and body aches. In contrast, patients with laboratory-positive dengue infections were significantly less likely than laboratory-negative patients to have symptoms of upper respiratory tract infection, such as sore throat, nasal congestion, or cough. There was no significant difference between groups in the proportion of patients reporting headache, nausea and vomiting, or hemorrhagic manifestations.
Laboratory and clinical data from 1,734 (88.6%) patients were sufficient to be included in the logistic regression model (97 laboratory-positive patients and 1,637 laboratory-negative patients). Five variables were found to be independently associated with a laboratory-positive dengue infection: retro-orbital pain, rash, low platelet count (platelets < 240,000 cells per mm3), absence of sore throat, and absence of cough (Table 3). The ROC curve (Figure 1) arising from our predictive model for all age groups incorporating these variables had an AUC of 0.76. For all age groups, the AUC of the logistic regression model from the current WHO case definition was 0.67 and for the logistic regression model generated from the proposed WHO case definition was 0.66. Similarly, when stratified by age group, AUCs for the predictive model exceeded AUCs from models generated from the current and proposed WHO case definitions (Figure 1). Performances of the models did not change significantly if only patients with definitive laboratory confirmation of dengue (PCR positivity or IgM seroconversion) were included (data not shown).
Table 3.
Multivariate predictors of laboratory-positive dengue infection by age group*
| Covariate | Adjusted odds ratio | 95% confidence interval |
|---|---|---|
| All Ages | ||
| Retro-orbital pain | 2.9 | (1.8–4.9) |
| Rash | 5.7 | (3.1–10.5) |
| Platelets < 240,000 cells/mm3 | 2.1 | (1.3–3.4) |
| No sore throat | 2.1 | (1.3–3.4) |
| No cough | 1.9 | (1.1–3.2) |
| Age 1–9 years† | ||
| Retro-orbital pain | 5.8 | (1.5–23.0) |
| No cough | 3.4 | (1.2–9.3) |
| Platelets < 240,000 cells/mm3 | 4.0 | (1.3–11.9) |
| Age 10–19 years‡ | ||
| Retro-orbital pain | 3.7 | (1.7–8.2) |
| Rash | 13.2 | (4.8–36.1) |
| No cough | 3.0 | (1.4–6.8) |
| Age ≥ 20 years§ | ||
| Retro-orbital pain | 2.4 | (1.2–4.7) |
| Rash | 7.0 | (2.0–24.9) |
| No sore throat | 8.1 | (3.1–18.4) |
| Leukopenia | 3.9 | (1.8–8.8) |
Data from 97 laboratory-positive cases and 1,637 laboratory-negative cases.
Data from 18 laboratory-positive cases and 667 laboratory-negative cases.
Data from 33 laboratory-positive cases and 371 laboratory-negative cases.
Data from 46 laboratory-positive cases and 599 laboratory-negative cases.
Figure 1.
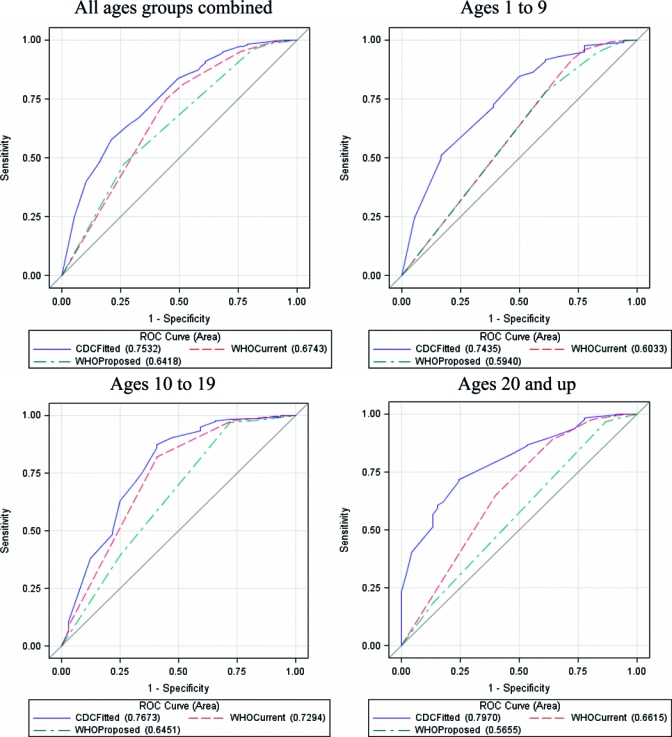
Receiver-operator characteristic (ROC) curves by age groups for Centers for Disease Control and Prevention (CDC) predictive model* and models generated from current† and proposed‡ World Health Organization (WHO) dengue case definitions. * Variables included in CDC predictive model: retro-orbital pain, rash, platelet count < 240,000 cells/mm3, no sore throat, no cough. † Variables included in current WHO case definition model: headache, retro-orbital-pain, myalgia, arthralgia, rash, leucopenia, hemorrhagic manifestation. ‡ Variables included in proposed WHO case definition model: nausea, vomiting, rash, aches and pains (myalgia or arthralgia), leucopenia, abdominal pain/tenderness, clinical fluid accumulation, mucosal bleed.
Clinical and laboratory features that were predictive of laboratory-positive dengue infections were found to vary by patient age (Table 3). For patients aged 1–9 years, retro-orbital pain, low platelet count, and absence of cough were independently associated with laboratory-positive dengue infection. In the 10–19-year age group, retro-orbital pain, rash, and absence of cough were predictive. For patients ≥ 20 years of age, retro-orbital pain, rash, absence of sore throat, and leukopenia were associated with laboratory-positive dengue infection.
As evaluated using bootstrap resampling, the ROC curves generated from the all-ages predictive model performed significantly better than either the model generated from the proposed WHO case definition (Table 4). When the performance of each model was examined by age group, the performance of the predictive model was statistically significantly better than models generated from either the current or proposed WHO case definitions for every age group, except in the 10–19 year age group as compared with the model generated from the current WHO case definition (data not shown).
Table 4.
Comparison of area under the ROC curves (AUCs) of different models by bootstrap resampling for all age groups
| Model | AUC or Δ AUC | Standard error | Lower percentile confidence boundary* | Upper percentile confidence boundary* |
|---|---|---|---|---|
| 1. Dengue Branch | 0.75 | 0.0011 | ||
| 2. Current WHO | 0.67 | 0.0012 | ||
| 3. Proposed WHO | 0.64 | 0.0012 | ||
| ΔAUC1–2 | 0.0830 | 0.0412 | 0.1754 | |
| ΔAUC1–3 | 0.1160 | 0.0543 | 0.1784 | |
| ΔAUC2–3† | 0.0330 | −0.0245 | 0.0876 | |
Non-parametric 95% confidence intervals with Bonferroni correction for multiple comparisons.
Negligible improvement of one model over the other.
Discussion
An accurate assessment of the burden of dengue in many parts of the world is hampered by inability to make a laboratory diagnosis and difficulties in identifying less severe forms of dengue by clinical presentation alone. A clinically applicable diagnostic algorithm could improve reporting in areas where laboratory resources are limited, while simultaneously benefiting busy clinicians by identifying dengue cases so that appropriately targeted prevention messages and management can be provided. This analysis using data from our enhanced dengue surveillance system shows that a prediction model can be constructed from readily available data collected early in the disease course. A combination of four clinical features and one routine laboratory test predicted dengue positivity with moderate accuracy, and more accurately than either the current or proposed WHO dengue case definition while requiring the collection of fewer data. Additionally, our study shows that for our population the proposed WHO dengue case definition is, at best, no better than the existing WHO case definition in predicting laboratory-positive dengue.
One important feature of our study, which has received relatively little attention previously, is the variation in clinical and laboratory features of dengue by patient age. Although these findings have also been reported by other groups,12,15 the WHO case definition makes no distinction between pediatric and adult patients. Moreover, among 19 previous studies of clinical features of dengue,5,12–17,26–37 only three have examined children and adults separately.12,17,36 It is not surprising, given the differences between children and adults in prior immunologic exposure to dengue and other viral infections, that pathophysiological responses to dengue infection12,38 and clinical and laboratory features may vary significantly. For example, subgroup analysis of our data shows that leukopenia, one of the manifestations of dengue listed in the current WHO case definition, is predictive of dengue early in the course of infection only among adults aged ≥ 20 years. Leukopenia is a common clinical finding in many viral childhood infections,39 and as children have an average of 6 to 8 viral infections annually,40,41 the finding that this is not a good early predictor for dengue among children could be anticipated. Although a previous study from Thailand did identify leukopenia as a good early predictor of dengue infection in children,14 another study from Nicaragua found, similar to our results, that leukopenia was significantly associated with early dengue infection in adults but not in children.12
Another key finding of our study was the importance of retro-orbital pain as a discriminator of dengue among all age groups in our population. This is especially important given that the proposed new WHO dengue case definition does not specifically mention this symptom in its list of clinical features.8 Omitting retro-orbital pain from the dengue case definition or grouping retro-orbital pain in with other possibly less specific pain syndromes in the definition may reduce clinical diagnostic accuracy. In our study when we performed an alternative analysis including headache and retro-orbital pain along with myalgia and arthralgia in the new WHO criteria of “aches and pains,” the model performed non-significantly worse than when we included only myalgia and arthralgia (data not shown). This is not surprising given that univariate analysis showed that body aches, joint pain, and retro-orbital pain were associated with dengue positivity, but headache was not (Table 1). Previous studies that have combined retro-orbital pain with headache showed no association between retro-orbital pain/headache and dengue positivity,11 whereas studies that have examined retro-orbital pain separately have seen this association;29,33,35 although sometimes only in certain age groups.17 Retro-orbital pain has been traditionally considered a relatively nonspecific manifestation of DF, but to our knowledge, no other common acute febrile illnesses have retro-orbital pain or eye pain listed in their case definitions. Retro-orbital pain has also been criticized for lacking sensitivity in children, given the presumed limited ability of young children to express location of pain. However, of 1,211 children aged 1–14 years with laboratory-confirmed dengue in Nicaragua, 59% were documented to have retro-orbital pain.12
Recent articles have highlighted a variety of ocular manifestations associated with dengue infection, including macular and retinal hemorrhages,42,43 maculopathy,44,45 optic neuritis and neuropathy,46 vasculitis,47 uveitis,48 retinal artery occlusion,49 venular occlusion,50 and retinal and macular edema.43,47 In a recent prospective study from Singapore, Seet and colleagues51 observed “clinically significant” ocular symptoms, defined as symptoms severe enough to warrant referral to an ophthalmologist, in 18% of hospitalized patients with serologically confirmed dengue. A 2006 study from India examined 134 consecutive hospitalized dengue patients and discovered ocular findings on funduscopic examination in over 40%.42 Similarly, maculopathy was documented in 10% of consecutively admitted dengue patients in Singapore.44 Based on the timing of the development of ocular signs relative to the course of dengue illness, and the association between low complement C3 levels and “dengue-associated maculopathy,”44 an immune-mediated process has been proposed for the ocular manifestations of dengue.44,45 These studies suggest that eye involvement with dengue might be more common than is appreciated and it is plausible that retro-orbital pain could be a more specific indicator of dengue infection than previously realized.
A recent systematic review identified 15 studies that have examined the differences in clinical and laboratory features between dengue and other febrile illnesses,11 and since that publication at least three additional studies have been published.16,27,34 Our study differs from most previous studies in that data were recorded at the time of the initial clinic or emergency department visit rather than at the time of hospitalization. Hence, we were able to identify features that were predictive of dengue positivity early in the disease course, rather than mid-to-late in the course. When we reanalyzed our data excluding patients who presented more than 3 days after symptom onset (7.4% of our total patients), the only significant change in the findings was that rash was no longer associated with dengue-positivity in patients ≥ 20 years of age (data not shown). This is not surprising as the association between rash and dengue in this age group may have been driven by patients who presented for medical care at the time of the appearance of the relatively classical convalescent rash of dengue. Several of the age-specific predictive features for dengue identified in our study have been previously noted, including the presence of rash and leukopenia in adults,12,26,33,52 reduced platelet counts in children,12,14 and absence of upper respiratory symptoms in children.5,17
Apart from the collection of data early in the disease course, our study has several other strengths. Unlike some previous studies, it includes only cases that could be laboratory confirmed as dengue infection or not dengue infection, thereby reducing the potential for misclassification bias. Data were collected over more than one dengue season and in a period when all four dengue virus serotypes were circulating; thereby giving a more accurate overall clinical picture than studies that took place in an outbreak setting. Finally, our study had a relatively large sample size compared with other similar studies11 and we stratified by age group to reduce potential confounding by age.
Nonetheless, the study has several limitations. We used enhanced surveillance data which, while it is extremely accurate and complete overall, was missing information for certain variables such as petechiae and the results of the tourniquet test. The presence of petechiae was seen almost exclusively among patients with dengue, but because of the small numbers of patients for whom this variable was recorded, it could not be incorporated into our multivariate model. The tourniquet test is not routinely performed in Puerto Rico. Because both current and proposed WHO case definitions for probable DF include this as one possible example of the hemorrhagic manifestations criteria, absence of information on this feature may have led to an underestimation of the sensitivity of both case definitions. A prospective study is underway in Puerto Rico to evaluate the effect of inclusion of the tourniquet test on the diagnostic accuracy of the current and proposed WHO case definitions and whether including tourniquet test results would improve the performance of our predictive model. Because of the retrospective nature of our study, we were unable to assess the variation in clinical and laboratory features predictive of dengue by day of illness. Patients were seen only once or twice in the course of their illnesses so a final determination of case severity was not possible in many cases. Therefore, this study is unable to identify early predictors that are specific to severe dengue, a pressing research need. Finally, the data are from only one region of Puerto Rico and may not be representative of other areas with different dengue transmission patterns, population demographics, underlying causes for acute febrile illness (e.g., typhoid, malaria), or where patients present for medical care later in the course of illness.
This study suggests that simple clinical and laboratory data can be used to identify early dengue infections in both adults and children. Further efforts should be made to validate these findings in other geographic settings and time periods. If the models continue to perform well over time in these settings, they could be used to develop clinical diagnostic algorithms. Future studies should also seek to detect early clinical and laboratory markers that can predict the development of severe dengue. Findings from our study and others11,12,15,17 suggest that separate clinical case definitions or diagnostic algorithms may be needed for children and adults. Additionally, changes to the DF case definition that deemphasize retro-orbital pain should be carefully evaluated, as this symptom was one of the strongest predictors of dengue infection in our study.
Acknowledgments
We thank Gladys Gonzalez-Zeno and E. Brian Irizarry-Perez for assistance in data collection and Brad Biggerstaff for guidance and advice on statistical methodology.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authors' addresses: Christopher J. Gregory, Luis Manuel Santiago, D. Fermin Argüello, Elizabeth Hunsperger, and Kay M. Tomashek, Dengue Branch, Division of Vector-Borne Infectious Diseases, National Center for Zoonotic, Vector-Borne and Enteric Diseases, Centers for Disease Control and Prevention, San Juan, Puerto Rico, E-mail: hgk4@cdc.gov.
References
- 1.Gubler DJ. The global pandemic of dengue/dengue haemorrhagic fever: current status and prospects for the future. Ann Acad Med Singapore. 1998;27:227–234. [PubMed] [Google Scholar]
- 2.Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ. 2002;324:1563–1566. doi: 10.1136/bmj.324.7353.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Second edition. Geneva: World Health Organization; 1997. [Google Scholar]
- 4.Rigau-Perez JG. Severe dengue: the need for new case definitions. Lancet Infect Dis. 2006;6:297–302. doi: 10.1016/S1473-3099(06)70465-0. [DOI] [PubMed] [Google Scholar]
- 5.Phuong CX, Nhan NT, Kneen R, Thuy PT, Thien CV, Nga NT, Thuy TT, Solomon T, Stepniewska K, Willis B, Dong Nai Study Group Clinical diagnosis and assessment of severity of confirmed dengue infections in Vietnamese children: is the world health organization classification system helpful? Am J Trop Med Hyg. 2004;70:172–179. [PubMed] [Google Scholar]
- 6.Bandyopadhyay S, Lum LC, Kroeger A. Classifying dengue: a review of the difficulties in using the WHO case classification for dengue haemorrhagic fever. Trop Med Int Health. 2006;11:1238–1255. doi: 10.1111/j.1365-3156.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 7.Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, Dung NM, Hung NT, Hien TT, Farrar J. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–173. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. Third edition. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 9.Wills BA, Nguyen MD, Ha TL, Dong TH, Tran TN, Le TT, Tran VD, Nguyen TH, Nguyen VC, Stepniewska K, White NJ, Farrar JJ. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877–889. doi: 10.1056/NEJMoa044057. [DOI] [PubMed] [Google Scholar]
- 10.Farrar J, Focks D, Gubler D, Barrera R, Guzman MG, Simmons C, Kalayanarooj S, Lum L, McCall PJ, Lloyd L, Horstick O, Dayal-Drager R, Nathan MB, Kroeger A. Towards a global dengue research agenda. Trop Med Int Health. 2007;12:695–699. doi: 10.1111/j.1365-3156.2007.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health. 2008;13:1328–1340. doi: 10.1111/j.1365-3156.2008.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond SN, Balmaseda A, Perez L, Tellez Y, Saborio SI, Mercado JC, Videa E, Rodriguez Y, Perez MA, Cuadra R, Solano S, Rocha J, Idiaquez W, Gonzalez A, Harris E. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–1070. [PubMed] [Google Scholar]
- 13.Deparis X, Murgue B, Roche C, Cassar O, Chungue E. Changing clinical and biological manifestations of dengue during the dengue-2 epidemic in French Polynesia in 1996/97–description and analysis in a prospective study. Trop Med Int Health. 1998;3:859–865. doi: 10.1046/j.1365-3156.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 14.Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 15.Kittigul L, Pitakarnjanakul P, Sujirarat D, Siripanichgon K. The differences of clinical manifestations and laboratory findings in children and adults with dengue virus infection. J Clin Virol. 2007;39:76–81. doi: 10.1016/j.jcv.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Premaratna R, Pathmeswaran A, Amarasekara ND, Motha MB, Perera KV, de Silva HJ. A clinical guide for early detection of dengue fever and timing of investigations to detect patients likely to develop complications. Trans R Soc Trop Med Hyg. 2009;103:127–131. doi: 10.1016/j.trstmh.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Ramos MM, Tomashek KM, Arguello DF, Luxemburger C, Quiñones L, Lang J, Muñoz-Jordan JL. Early clinical features of dengue infection in Puerto Rico. Trans R Soc Trop Med Hyg. 2008;103:878–884. doi: 10.1016/j.trstmh.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Ramos MM, Arguello DF, Luxemburger C, Quinoñes L, Muñoz JL, Beatty M, Lang J, Tomasek KM. Epidemiological and clinical observations on patients with dengue in Puerto Rico: results from the first year of enhanced surveillance—June 2005–May 2006. Am J Trop Med Hyg. 2008;79:123–127. [PubMed] [Google Scholar]
- 19.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C, Centers for Disease Control and Prevention, National Center for Health Statistics Hematological and iron-related analytes–reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat 11. 2005;247:1–156. [PubMed] [Google Scholar]
- 20.Chien LJ, Liao TL, Shu PY, Huang JH, Gubler DJ, Chang GJ. Development of real-time reverse transcriptase PCR assays to detect and serotype dengue viruses. J Clin Microbiol. 2006;44:1295–1304. doi: 10.1128/JCM.44.4.1295-1304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol. 2005;43:4977–4983. doi: 10.1128/JCM.43.10.4977-4983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Santayakorn S, Puttisri P, Hoke CH. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 23.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 24.Fischer JE, Bachmann LM, Jaeschke R. A readers' guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043–1051. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- 25.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 26.Chadwick D, Arch B, Wilder-Smith A, Paton N. Distinguishing dengue fever from other infections on the basis of simple clinical and laboratory features: application of logistic regression analysis. J Clin Virol. 2006;35:147–153. doi: 10.1016/j.jcv.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Dietz VJ, Gubler DJ, Rigau-Perez JG, Pinheiro F, Schatzmayr HG, Bailey R, Gunn RA. Epidemic dengue 1 in Brazil, 1986: evaluation of a clinically based dengue surveillance system. Am J Epidemiol. 1990;131:693–701. [PubMed] [Google Scholar]
- 28.Kumar R, Tripathi P, Tripathi S, Kanodia A, Pant S, Venkatesh V. Prevalence and clinical differentiation of dengue fever in children in northern India. Infection. 2008;36:444–449. doi: 10.1007/s15010-008-7172-6. [DOI] [PubMed] [Google Scholar]
- 29.Low JG, Ooi EE, Tolfvenstam T, Leo YS, Hibberd ML, Ng LC, Lai YL, Yap GS, Li CS, Vasudevan SG, Ong A. Early dengue infection and outcome study (EDEN)—study design and preliminary findings. Ann Acad Med Singapore. 2006;35:783–789. [PubMed] [Google Scholar]
- 30.Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, De A. Concurrent outbreak of leptospirosis and dengue in Mumbai, India, 2002. J Trop Pediatr. 2005;51:174–181. doi: 10.1093/tropej/fmh100. [DOI] [PubMed] [Google Scholar]
- 31.Bruce MG, Sanders EJ, Leake JA, Zaidel O, Bragg SL, Aye T, Shutt KA, Deseda CC, Rigau-Perez JG, Tappero JW, Perkins BA, Spiegel RA, Ashford DA. Leptospirosis among patients presenting with dengue-like illness in Puerto Rico. Acta Trop. 2005;96:36–46. doi: 10.1016/j.actatropica.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Buchy P, Vo VL, Bui KT, Trinh TX, Glaziou P, Le TT, Le VL, Bui TC. Secondary dengue virus type 4 infections in Vietnam. Southeast Asian J Trop Med Public Health. 2005;36:178–185. [PubMed] [Google Scholar]
- 33.McBride WJ, Mullner H, LaBrooy JT, Wronski I. The 1993 dengue 2 epidemic in Charters Towers, North Queensland: clinical features and public health impact. Epidemiol Infect. 1998;121:151–156. doi: 10.1017/s0950268898001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phuong HL, de Vries PJ, Nga TT, Giao PT, Hung LQ, Binh TQ, Nam NV, Nagelkerke N, Kager PA. Dengue as a cause of acute undifferentiated fever in Vietnam. BMC Infect Dis. 2006;6:123. doi: 10.1186/1471-2334-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes-Araujo FR, Ferreira MS, Nishioka SD. Dengue fever in Brazilian adults and children: assessment of clinical findings and their validity for diagnosis. Ann Trop Med Parasitol. 2003;97:415–419. doi: 10.1179/000349803235002263. [DOI] [PubMed] [Google Scholar]
- 36.Suwandono A, Kosasih H, Nurhayati, Kusriastuti R, Harun S, Ma'roef C, Wuryadi S, Herianto B, Yuwono D, Porter KR, Beckett CG, Blair PJ. Four dengue virus serotypes found circulating during an outbreak of dengue fever and dengue haemorrhagic fever in Jakarta, Indonesia, during 2004. Trans R Soc Trop Med Hyg. 2006;100:855–862. doi: 10.1016/j.trstmh.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Watt G, Jongsakul K, Chouriyagune C, Paris R. Differentiating dengue virus infection from scrub typhus in Thai adults with fever. Am J Trop Med Hyg. 2003;68:536–538. doi: 10.4269/ajtmh.2003.68.536. [DOI] [PubMed] [Google Scholar]
- 38.Gamble J, Bethell D, Day NP, Loc PP, Phu NH, Gertside IB, Farrar JF, White NJ. Age-related changes in microvascular permeability: a significant factor in the susceptibility of children to shock? Clin Sci (Lond) 2000;98:211–216. [PubMed] [Google Scholar]
- 39.Karavanaki K, Polychronopoulou S, Giannaki M, Haliotis F, Sider B, Brisimitzi M, Dimitriou C, Scordias G, Marangou F, Stamatiadou A, Avionitis S. Transient and chronic neutropenias detected in children with different viral and bacterial infections. Acta Paediatr. 2006;95:565–572. doi: 10.1080/08035250500477537. [DOI] [PubMed] [Google Scholar]
- 40.Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16:351–373. doi: 10.1093/oxfordjournals.epirev.a036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monto AS. Epidemiology of viral respiratory infections. Am J Med. 2002;112((Suppl 6A)):4S–12S. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- 42.Kapoor HK, Bhai S, John M, Xavier J. Ocular manifestations of dengue fever in an East Indian epidemic. Can J Ophthalmol. 2006;41:741–746. doi: 10.3129/i06-069. [DOI] [PubMed] [Google Scholar]
- 43.Pek DC, Teoh SC. Ocular manifestations in dengue fever. Can J Ophthalmol. 2007;42:755–756. doi: 10.3129/i07-129. [DOI] [PubMed] [Google Scholar]
- 44.Su DH, Bacsal K, Chee SP, Flores JV, Lim WK, Cheng BC, Jap AH. Prevalence of dengue maculopathy in patients hospitalized for dengue fever. Ophthalmology. 2007;114:1743–1747. doi: 10.1016/j.ophtha.2007.03.054. Dengue Maculopathy Study Group. [DOI] [PubMed] [Google Scholar]
- 45.Lim WK, Mathur R, Koh A, Yeoh R, Chee SP. Ocular manifestations of dengue fever. Ophthalmology. 2004;111:2057–2064. doi: 10.1016/j.ophtha.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 46.Sanjay S, Wagle AM, Au Eong KG. Dengue optic neuropathy. Ophthalmology. 2009;116:170. doi: 10.1016/j.ophtha.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Tan CS, Teoh SC, Chan DP, Wong IB, Lim TH. Dengue retinopathy manifesting with bilateral vasculitis and macular oedema. Eye. 2007;21:875–877. doi: 10.1038/sj.eye.6702748. [DOI] [PubMed] [Google Scholar]
- 48.Gupta A, Srinivasan R, Setia S, Soundravally R, Pandian DG. Uveitis following dengue fever. Eye. 2009;23:873–876. doi: 10.1038/eye.2008.124. [DOI] [PubMed] [Google Scholar]
- 49.Kanungo S, Shukla D, Kim R. Branch retinal artery occlusion secondary to dengue fever. Indian J Ophthalmol. 2008;56:73–74. doi: 10.4103/0301-4738.37606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bacsal KE, Chee SP, Cheng CL, Flores JV. Dengue-associated maculopathy. Arch Ophthalmol. 2007;125:501–510. doi: 10.1001/archopht.125.4.501. [DOI] [PubMed] [Google Scholar]
- 51.Seet RC, Quek AM, Lim EC. Symptoms and risk factors of ocular complications following dengue infection. J Clin Virol. 2007;38:101–105. doi: 10.1016/j.jcv.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Wilder-Smith A, Earnest A, Paton NI. Use of simple laboratory features to distinguish the early stage of severe acute respiratory syndrome from dengue fever. Clin Infect Dis. 2004;39:1818–1823. doi: 10.1086/426029. [DOI] [PMC free article] [PubMed] [Google Scholar]


