Summary
Activated platelets release large lipid-protein complexes termed microparticles. These platelet microparticles (PMP) are composed of vesicular fragments of the plasma membrane and α-granules. PMP facilitate coagulation, promote platelet and leukocyte adhesion to the subendothelial matrix, support angiogenesis and stimulate vascular smooth muscle proliferation.
Objectives
PMP were separated into 4 size classes to facilitate identification of active protein and lipid components. PMP were obtained from activated human platelets and separated into 4 size classes by gel filtration chromatography. Proteins were identified using 2-dimensional, liquid chromatography tandem mass spectrometry. Functional effects on platelets were determined using the PFA-100® and on endothelial cells by measuring transendothelial cell electrical resistance. PMP size classes differed significantly in their contents of plasma membrane receptors and adhesion molecules, chemokines, growth factors and protease inhibitors. The two smallest size classes (3 and 4) inhibited collagen/adenosine-diphosphate-mediated platelet thrombus formation, while fractions 2 and 4 stimulated barrier formation by endothelial cells. Heat denaturation blocked the effect of fraction 4 on endothelial cell function, but not fraction 2 implying that the active component in fraction 4 is a protein and in fraction 2 is a heat-stable protein or lipid but not sphingosine-1-phosphate. Proteomic and functional analysis of PMP size fractions has shown that PMP can be separated into different size classes that differ in protein components, protein/lipid ratio, and functional effects on platelets and endothelial cells. This analysis will facilitate identification of active components in the PMP and clarify their involvement in diseases such as atherosclerosis and cancer.
Keywords: Blood platelets, microparticles, proteomics, platelet activation, transendothelial cell electrical resistance, sphingosine-1-phosphate
Introduction
The existence of platelet microparticles (PMP) was established in the late 1960s as a subcellular pro-coagulant released from platelets that was initially termed “platelet dust” (1). PMP are estimated to vary in size from 0.1–1.0 μm with the smaller particles originating from α-granules and the larger particles from the plasma membrane (2). Sheer stress on platelets adhering to endothelium has been proposed as a contributing factor to PMP formation (3). Circulating microparticles in normal individuals are largely derived from platelets and megakaryocytes (4–7). Key protein components of PMP have been identified using immunoelectron microscopy, flow cytometry and mass spectrometry (8–10). Proteins in the large, plasma membrane-derived PMP include integrin α2bβ3, glycoprotein 1b, PECAM, P-selectin and other platelet plasma membrane proteins (4). Subcellular origins of other PMP proteins identified by mass spectrometry include α-granules, mitochondria, the nucleus and the cytosol (8–10).
The function of PMP first identified was enhancement of coagulation. PMP exhibit exposed aminophospholipids and are therefore binding sites for activated factor V, factor VIIIa and factor IXa, and provide a surface for thrombin formation (11, 12). Removal of PMP from plasma drastically inhibits coagulation in vitro which is not mitigated by addition of phosphatidylserine vesicles thus indicating that participation of PMP in coagulation is more extensive than just providing a surface for activated factor binding (13). Isolated PMP have been reported to utilise both the extrinsic and intrinsic coagulation cascades (14). Procoagulent activity of microparticles is 50–100 fold higher than activated platelets (15). PMP also participate in platelet adhesion to the subendothelium, likely due to the presence of collagen-binding receptors in PMP (16). In a genetic condition termed Scott Syndrome, platelets fail to produce microparticles which results in a severe bleeding disorder (13, 17, 18).
In addition to their role in coagulation, the involvement of PMP in angiogenesis and proliferation of vascular smooth muscle has recently been demonstrated. Kim et al. (19) reported that treatment of endothelial cells with PMP in vitro promoted cell survival, stimulated migration and elicited formation of tube-like structures. PMP have also been reported to promote proliferation of coronary artery smooth muscle cells by a PDGF-independent (platelet-derived growth factor) mechanism (20). In addition, the concentration of PMP in blood has been correlated with the presence of atherosclerosis (21), hypertension (22), peripheral artery disease (23) and aortic valve stenosis (24) indicating that measurement of PMP levels may be an important indicator of disease.
One mechanism for the observed effects of PMP on endothelial cells is the delivery of the thromboxane precursor, arachidonic acid, to these cells (25). In addition, Pfister (26) demonstrated that PMP can convert arachidonic acid originating from endothelial cells to thromboxane A2 via endogenous cyclooxygenase. It has also been shown that PMP can deliver arachidonic acid to other tissues for conversion to prostaglandins and other bioactive lipids (27).
Another bioactive lipid component released by platelets that affects endothelial cell function is sphingosine-1-phosphate (S1P) (28–30). However, this lipid appears to be secreted directly into the blood where it binds to albumin and lipoproteins (31).
The studies described in this paper show that different size classes of PMP contain different protein components and exhibit different functional activities. Furthermore, S1P was not detected in PMP. These data will aid in the identification of the functional significance of specific proteins in PMP.
Methods
Platelet and PMP isolation
Platelets were isolated by differential centrifugation from 80–400 ml of freshly drawn, citrate anti-coagulated blood obtained from healthy volunteers (32). The final platelet pellets were resuspended in calcium-free Tyrodes buffer (5–8 × 108 platelets/ml) and activated in the presence of 1 mM CaCl2, 0.2 U/ml thrombin and 10 μg/ml collagen (11). After incubation for 30 min at 30°C, the platelets were removed by centrifugation at 5,000 × G and the supernatants centrifuged at 130,000 × G to collect PMP which were resuspended in 0.01 M Tris containing 0.1 M NaCl at approximately 1 mg/ml protein. PMP were frozen in liquid nitrogen and stored at −80°C. Platelets used for functional tests were obtained from 20–40 ml of freshly drawn blood of healthy volunteers. Platelet rich plasma was isolated by centrifugation of the citrate anti-coagulated blood at 140 × G for 15 min and was used within 2 hours of blood draw.
Gel filtration of PMP
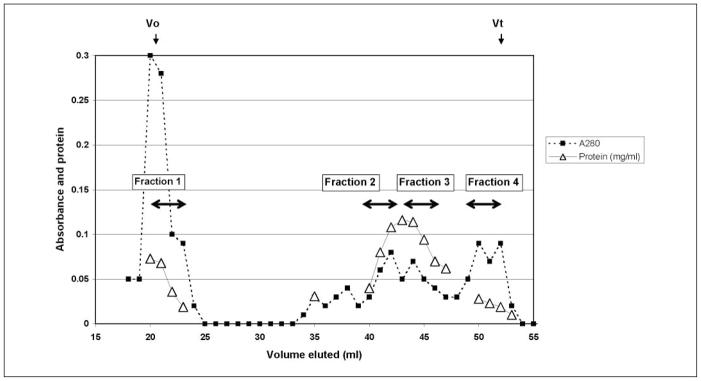
The PMP from 7–10 individuals were pooled and loaded onto a 1 cm × 30 cm Sepharose CL-4B column equilibrated with 0.01 M Tris, 0.1 M NaCl at pH 7.4. The flow rate was adjusted to 1 ml/min and the absorbance at 280 nm was continuously monitored. The void volume was determined with blue dextran while the total volume was indicated by β-mercaptoethanol. The eluate was divided into 4 fractions based on the absorbance at 280 nm as shown in Figure 1. Protein was determined by the BCA method (Pierce, Rockford, IL). Fractions were frozen in liquid nitrogen and stored at −80°C.
Figure 1. Chromatography of PMP on Sepharose CL-4B. PMP from 10 individuals were pooled and were separated according to size.
The void volume (Vo) and total volume (Vt) are indicated. Double headed arrows indicate eluted material that was pooled to give the indicated fractions.
Measurement of vesicle size by dynamic light scattering
Immediately after fractionation on Sepharose CS-4B, pooled fractions were analysed on a Precision Detector PD2000DLS (Precision Detectors, Leeds, UK) in the Stokes Radius mode. Values represent weighted averages of vesicle distributions.
Measurement of lipid phosphorous
Frozen fractions were thawed and extracted with 2:1 (v:v) chloroform:methanol at a ratio of 5:1 organic solvent to column fraction volume. The organic phase was dried with a stream of nitrogen and the organic phosphorous content was determined by the method of Bartlett (33).
Mass spectrometric analysis of proteins
Briefly, the PMP fractions were thawed and resuspended in 60% methanol and 0.1 M ammonium bicarbonate. The pH was adjusted to pH 8.0 with 1 M Tris and proteins were reduced by incubation with dithiothreitol, followed by cysteine alkylation with iodoacetamide. Proteins were digested directly in solution with trypsin (Promega, Madison, WI, USA) overnight at 37°C. The resulting tryptic peptides were desalted using a reversed phase cartridge by washing with 0.5% acetic acid and then eluting with 95% acetonitrile, 0.5% acetic acid. The eluted peptides were lyophilised and resuspended in 0.5% acetic acid and directly analysed using a previously described 2-dimensional, liquid chromatography tandem mass spectrometry (2D-LC-MS/MS) approach (34). Briefly, the samples were loaded onto a microcapillary strong cation exchange column and fractions were collected using an increasing salt step elution gradient. Subsequently, each fraction was analysed by microcapillary RP-LC coupled with electrospray ionisation-tandem mass spectrometry using an LTQ Plus ion trap mass spectrometer (Thermo-Fisher Scientific, Waltham, MA). The acquired MS/MS spectra were searched against a human protein database (Human Ref-Seq) using the Sequest algorithm (Sequest Sorcerer, Sage-N Research, Inc., San Jose, CA). Data processing of the SEQUEST output files into a list of proteins has been previously described (35). The complete data set is supplied as supplemental data in an Excel spreadsheet (Ingenuity_F1–F4.xls) where F1–F4 refers to fractions 1–4.
HPLC assay for S1P
S1P was extracted with acidified chloroform:methanol (2:1; v/v) (30). The acidic chloroform:methanol extract was dried under a stream of nitrogen and dissolved in ethanol at a concentration of 50 μM lipid phosphate. The lipids were then treated with ophthalaldehyde (36). The labeled lipids were then applied to a C18 column (Shimadzu Premier; 25 cm × 4.6 mm; Shimadzu VP series HPLC system, Kyoto, Japan), eluted with a mobile phase of methanol:10 mM potassium phosphate (pH 7.2):1 M tetra-butylammonium dihydrogen phosphate (83:16:1, v/v/v) and detected with an in-line fluorescence detector (Shimadzu, RF-10A XL) (37).
Platelet function analysis
Platelet thrombus formation in citrated whole blood was determined using a Dade-Behring Platelet Function Analyser PFA-100® (Dade Behring, Inc., Atlanta, GA, USA)with collagen-ADP cartridges. The output of this instrument is time for capillary occlusion (38, 39).
Transendothelial cell electrical resistance
An ECIS Model 1600R (Applied BioPhysics, New York, USA) electric cell-substrate impedance sensing apparatus was used to measure the transendothelial electrical resistance (TEER) in confluent human umbilical cord vein endothelial cells (HUVEC) (Clonetics; Cc-2517, passage 4–12). Briefly, 200 μl of HUVEC cell suspensions (5 × 105 cells per ml) were seeded onto each well of a 8W1E array (Applied BioPhysics), which was pre-equilibrated with 200 μl of medium at 37°C for 30 min. Two days later, endothelial cells were serum-starved in plain M199 medium for 2 h at 37°C. After treatment with PMP or S1P (500 nM) as a positive control, the TEER was measured in real-time as described earlier (40,41).
Results
We reasoned that separation of vesicle size classes of PMP by gel filtration might also separate PMP into different functional classes which could facilitate studying their mechanism of action. Platelet microparticles were obtained from washed human platelets activated with thrombin and collagen which has been shown to produce the greatest release of microparticles (11). Activated and aggregated platelets were removed from the solution by centrifugation at low speed and the microparticles were then isolated by ultracentrifugation at 130,000 × G. PMP preparations from 7–10 individuals were pooled for chromatography on Sepharose CL-4B. Figure 1 shows a typical elution profile that has been observed repeatedly with PMP from more than 40 volunteers. There is a large peak of protein at the void volume of the column followed by a broad peak that elutes before the total volume. The eluted material was divided into 4 fractions as shown on the chromatography profile (Fig. 1). General properties of the 4 fractions are indicated in Table 1. The phospholipid/protein ratios of fractions 2 and 3 are typical of plasma membranes, while the composition of fractions 1 and 4 indicate a greater proportion of lipid (42). The diameters of microparticles determined by light scattering are in the range of sizes reported using electron microscopy and flow cytometry (2, 4). Thus, although it is possible that freezing and thawing of the microparticles prior to chromatography could potentially change the size and protein distribution of the microparticles, the range of vesicle sizes is similar to earlier reports (2, 4), suggesting that no major changes have occurred.
Table 1.
Properties of PMP size fractions.
| Fraction | Protein (mg/ml) | Phospholipid (μmol/ml) | Lipid/Protein (μmol/mg) | Average diameter (nm) |
|---|---|---|---|---|
| 1 | 0.06 | 0.71 | 11.8 | 550 |
| 2 | 0.40 | 0.57 | 1.4 | 330 |
| 3 | 0.31 | 0.71 | 2.3 | 260 |
| 4 | 0.08 | 0.50 | 6.2 | 130 |
In order to determine the subcellular source of the PMP and identify potential functionally relevant protein components, 2D-LC-MS/MS analysis was carried out on the fractions. The complete data set is supplied as supplemental data in an Excel spreadsheet (Ingenuity_F1–F4.xls) where F1–F4 refers to fractions 1–4. Table 2 indicates the number of proteins identified in each fraction and their subcellular origin. The decrease of proteins of mitochondrial origin with decreasing fraction size is striking. It appears that mitochondrial fragments are being ejected from platelets in the largest PMP. Furthermore, plasma membrane and cytoskeleton-associated proteins are enriched in fractions 3 and 4 indicating increased contributions of plasma membrane and its associated cytoskeleton in the smaller size fractions. Finally, as would be expected based on the proposed α-granule origin of the smaller exosomes (2, 4), α-granule proteins are absent in the larger fractions while highly enriched in the smallest fraction. Table 3 shows the components in the PMP fractions that may be associated with functional activity of the PMP, including plasma membrane adhesion receptors, growth factors, chemokines, and metalloproteinase inhibitors. Fractions 1 and 2 are essentially devoid of obvious functional proteins. Fraction 3 has the richest assortment of possibly functional proteins including fibrinogen binding proteins, von Willebrand factor binding proteins, chemokines and metalloproteinase inhibitors. Fraction 4 protein content overlaps with many of the fraction 3 proteins, but in addition has 2 growth factors.
Table 2.
Subcellular origin of proteins in PMP fractions.
| Fraction | Total Proteins | Plasma membrane (%) | Cytoskeletal (%) | Cytosol/ER (%) | Mitochondrial (%) | Nuclear (%) | α-granule (%) |
|---|---|---|---|---|---|---|---|
| 1 | 54 | 11 | 15 | 13 | 23 | 15 | 0 |
| 2 | 49 | 8 | 13 | 15 | 17 | 42 | 0 |
| 3 | 293 | 20 | 18 | 17 | 7 | 33 | 4 |
| 4 | 150 | 16 | 19 | 22 | 3 | 11 | 8 |
Table 3.
Proteins involved in signal transduction.
| Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 |
|---|---|---|---|
| 0 proteins |
Plasma membrane receptors/adhesion intergrin αX |
Chemokines CCL23 CCL5 CXCL4 CXCL7 Plasma membrane receptors/adhesions integrin α2β integrin β3 GPIX GP4 PECAM1 tetraspanin 29 thrombospondin-1 fibrinogen αβγ Protease inhibitors TIMP3 α2-macroglobulin |
Chemokines CXCL4 CXCL7 Growth factors fibroblast growth factor 23 TGF β-1 Plasma membrane receptors/adhesion integrin α2β integrin β3 GPIX tetraspanin 29 thrombospondin-1 fibrinogen βγ cadherin-4 |
The purity of the fractions is indicated by the complete absence in any fractions of neutrophil or erythrocyte signature membrane proteins. Neutrophils make up 40–60% of blood leukocytes in normal individuals and contamination by leukocytes would be easily detected by the presence of major neutrophil membrane proteins. Major neutrophil membrane proteins include β2 integrin (CD18), leukocyte common antigen (CD45), glycoprotein MAC-1 α-subunit (CD11b), IL-8 receptor (CXCR-2), leukotriene B4 receptor and ICAM3. None of these proteins were detected in any fraction. Furthermore, the signature erythrocyte membrane proteins glycophorin and band 3 (anion exchanger 1) were not detected. However, haemoglobin subunits were detected in fractions 3 and 4 likely originating from the haemolysis of erythrocytes.
S1P, a sphingolipid metabolite secreted by platelets during activation, has been shown to promote endothelial cell growth (28–31); consequently, we assayed for its presence in the PMP fractions. No S1P was detected in any of the fractions indicating that the functional effects of PMP fractions cannot be attributed to this bioactive lipid.
Since there are substantial differences in many of the physical properties of the PMP fractions including size, phospholipids/protein ratio, and protein components, we determined the effects of these fractions on platelets and endothelial cells. Standard aggregometry using both thrombin and collagen as platelet activators did not reveal significant effects of any of the fractions on the rate and extent of platelet activation, although fractions 3 and 4 did enhance the extent of the shape change phase of activation (data not shown). However, use of the platelet function PFA-100® (38) with ADP-collagen cartridges revealed a significant amount of delay of capillary closure time for both fractions 3 and 4 as shown in Table 4. This instrument measures the time for whole citrated blood to form a thrombus that blocks blood flow in capillaries coated with collagen and ADP. It is a technique that measures platelet activity in an environment resembling damaged blood vessels. It is not surprising that fractions 3 and 4 affected platelet function since they contain more bioactive proteins than fractions 1 and 2 (Table 3).
Table 4.
Effects of PMP fractions on PFA-100 closure time.
| Sample | Closure time (seconds, mean ± SE, n=3) |
|---|---|
| Whole blood | 74 ± 0 |
| + 1 μg fraction 1 | 79 ± 12 (P<0.46) |
| + 1 μg fraction 2 | 73 ± 4 (P<0.81) |
| + 1 μg fraction 3 | 88 ± 8 (P<0.03) |
| + 0.5 μg fraction 4 | 92 ± 10 (P<0.04) |
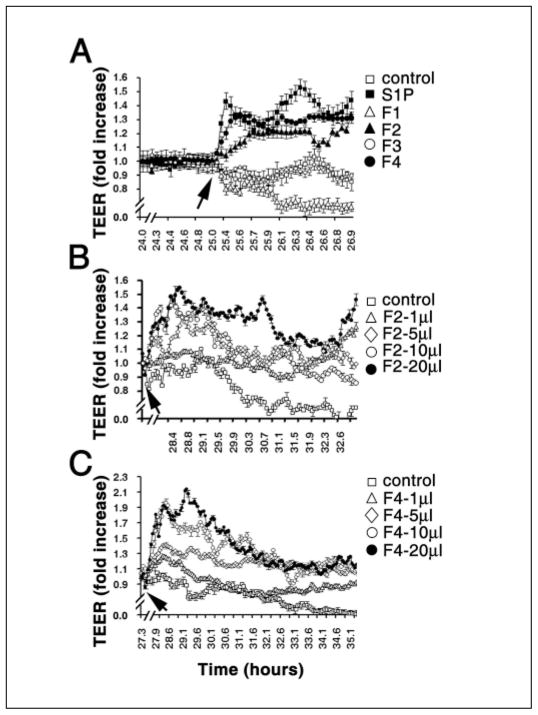
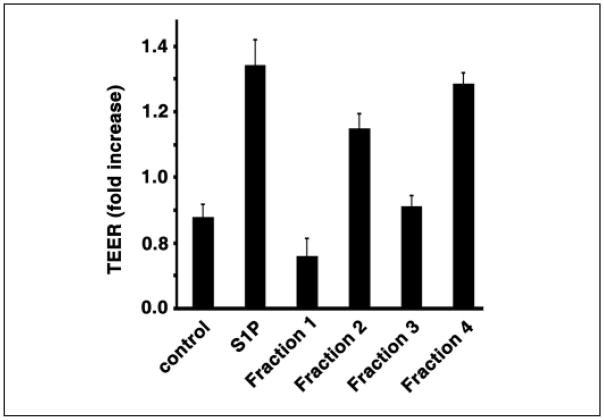
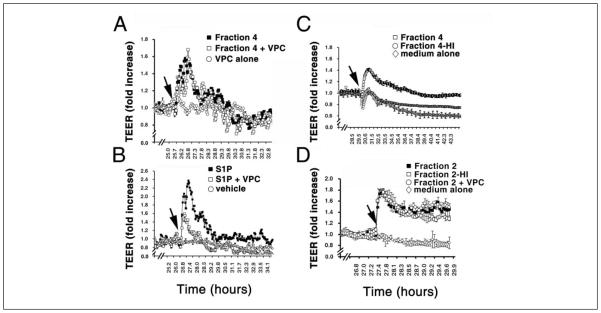
PMP have been shown to affect endothelial cell function (16). Therefore we determined the effects of PMP fractions on TEER using HUVEC (40, 41). In this assay, cells are grown on a grid with electrodes on either side of the cellular monolayer. As tight junctions form between the cells, the electrical resistance increases. Agents such as S1P that enhance tight junction formation cause a greater increase in electrical resistance compared with controls without S1P (41). The results of treating HUVEC cells with PMP fractions are shown in Figures 2 and 3. S1P (500 nM) caused an increase in resistance relative to the control with no additions as observed earlier (Fig. 2A and 3)(41). Fractions 2 and 4 also robustly enhanced the rise in TEER which is similar to the effect of S1P. In contrast, fraction 1 inhibited TEER and fraction 3 had no effect (Fig. 2A and 3). Figures 2B and 2C show the concentration dependence of fractions 2 and 4. Clearly, fractions 2 and 4 cause concentration-dependent increases in electrical resistance. Furthermore this effect is abrogated by heat denaturation of the most active fraction, fraction 4, indicating that the active component(s) in this fraction are proteins and not lipids (Fig. 4C). In addition, fraction 4 does not appear to operate through the S1P1 receptor, which is abundantly expressed in endothelial cells (41, 43). Addition of VPC23109, a specific antagonist of S1P1 and S1P3 receptors (43), had no effect on the increase in TEER induced by fraction 4 (Fig. 4A), but markedly reduced the effect of added S1P on TEER by nearly 50% (Fig. 4B). This agrees with the observation that S1P was not detected in any of the fractions. Thus a protein or proteins in PMP fraction 4 stimulates barrier integrity in HUVECs. In contrast to fraction 4, the induction of a rise in TEER by fraction 2 was not affected by heat treatment (Fig. 4D), suggesting that a lipid and not a protein component induces this effect. However, VPC23109 did not abrogate the effect of fraction 2 showing that the lipid component is not S1P as would be predicted from the absence of detectable S1P in this fraction. Thus a heat insensitive component in fraction 2, likely a lipid, enhances TEER, while it is a protein component in fraction 4 promoting TEER barrier integrity.
Figure 2. Effects of PMP fractions on TEER of endothelial cells.
HUVECs were cultured in ECIS arrays. A) After the addition of PMP fractions (5 μl) (arrow), TEER (transendothelial electric resistance) was measured in real-time as described in the Methods section. Note that fractions 2 and 4 significantly induce TEER rises in endothelial cells. Fractions 2 (B) and 4 (C) dose-dependently induce TEER rises in endothelial cells. PMP fractions were added to the culture medium (arrow) and the electrical resistance was measured in real time. Volumes of fractions added (0.5 mg/ml protein) are indicated. Data represent mean ± SD of triplicate determinations.
Figure 3. Effect of PMP fractions on TEER of endothelial cells.
HUVECs were cultured in ECIS arrays. After the addition of PMP fractions (5 μl), TEER was measured in real-time as described in Figure 2. Measured resistance relative to the control resistance 2 hours after additions are shown. Data represent mean ± SD of triplicate determinations.
Figure 4. Heat denaturation abrogates PMP fraction 4 but not fraction 2-induced endothelial TEER rise.
A) Treatment of VPC23019, a specific antagonist for S1P1 and S1P3 receptors, has no effects on PMP fraction 4-induced TEER rise. Cultures were washed and pretreated with or without VPC23019 (0.5 μM) for 15 min. Subsequently, cells were added in the presence or absence of PMP fraction 4 (10 μl, 0.5 mg/ml, arrow). The TEER rises were measured in a real-time manner. Note that functional blockage of S1P receptors has no effect on PMP fraction 4-mediated TEER rise. B) Endothelial cells were washed, pretreated with or without VPC23019 (0.5 μM) for 15 min. After treatment with or without S1P (0.5 μM, arrow), endothelial TEER rises were measured in real-time. Note that VPC23019 treatment significantly diminishes the S1P-induced TEER rise. C) PMP fraction 4 was incubated at 95°C for 10 min. Subsequently, endothelial cells were treated with PMP fraction 4 (10 μl, 0.5 mg/ml, arrow) or heat-denatured PMP fraction 4 (Fraction 4-HI) (arrow), and the TEER rises were measured in real-time as described. Note that heat denaturation completely abrogates PMP fraction 4-induced TEER rises. D) PMP fraction 2 was incubated at 95°C for 10 min. Subsequently, endothelial cells were treated with PMP fraction 2 (20 μl, 0.2 mg/ml, arrow) or heat-denatured PMP fraction 2 (Fraction 2-HI) (arrow), and the TEER rises were measured in real-time. In addition the effect of VPC23019 on the TEER rise induced by fraction 2 was also determined as in A and B. VPC23019 had no effect on the TEER increase induced by fraction 2. Data in panels A-D represent mean ± SD. of triplicate determinations.
Discussion
In this study we have separated the total PMP into 4 size classes ranging from greater than 500 nm to approximately 100 nm using gel filtration chromatography. This size range is the same that has been reported earlier for PMP (2, 11). These size classes have different lipid/protein ratios, different protein components and different functional effects on platelets and endothelial cells. Protein components in each fraction were identified using 2D-LC-MS/MS. The largest size fraction, fraction 1, is enriched in mitochondrial proteins suggesting that mitochondria are ejected from platelets during platelet activation. The relative contribution of platelet membrane proteins to PMP increases as the size of the vesicles decreases. This was also true for α-granule proteins since proteins of known α-granule origin such as growth factors and chemokines were only found in fractions 3 and 4 (Table 3). Thus the observation that smaller PMP termed exosomes originate from platelet α-granules (2, 4, 11) is supported by the present work. The smaller fractions (3 and 4) also contain a significant number of proteins that could affect vascular function by interacting with the subendothelium, platelet protein ligands such as fibrinogen and von Willebrand factor or by acting as chemokines and growth factors (Table 3).
Comparison of the results of this study with earlier studies describing proteomic analysis of platelet microparticles by Garcia et al. (9) and platelet membrane proteins by Moebius et al. (44) reveals that the present work has identified many proteins not reported in the earlier studies. For fraction 1, 26% of the reported proteins are also reported in the earlier studies (9, 44). An additional 11% of the proteins reported herein are isoforms of proteins reported earlier. Fraction 2 has the least number of proteins in common with the earlier studies, 14% matches and 8% isoforms. Fractions 3 and 4 are similar in that 22% of the proteins are exact matches and 14% are isoforms. In addition, 65% of the proteins in the Garcia et al. (9) data set are not found in the present data set. Garcia et al. (9) reported the presence of proteins of mitochondrial origin such as the ADP/ATP exchanger and F1 AT-Pase subunits indicating agreement that mitochondria or mitochondrial fragments are clearly released by activated platelets. Comparison between the platelet membrane proteome (44) and the present work is shown in Table 5. Two factors contribute to the differences in the data sets. First, Garcia et al. (9) used very mild conditions to activate platelets to produce microparticles, ADP activation. In contrast we used very strong conditions consisting of simultaneous activation with two strong platelet agonists, thrombin and collagen, as had been used by others (11). This could certainly explain the presence of additional proteins in our data set. A second contributing factor to differences in data sets is the method used to produce peptides. Both Garcia et al. (9) and Moebius et al. (44) used in-gel digestion after separation of proteins on one dimensional SDS polyacrylamide gels (9) or on 2-dimensional SDS/benzyldimethyl-n-hexadecyl ammonium chloride gels (44). In contrast, in the current work we used direct digestion of isolated membrane vesicles in 60% methanol after chromatographic separation of microparticles into size classes. Although our technique yields excellent results for both soluble and membrane-associated proteins, it is quite possible that proteins are lost during the purification of microparticles and some may be inefficiently digested in 60% methanol.
Table 5.
Comparison with platelet membrane proteome (44).
| Cellular location* | Cytoplasmic | Endoplasmic reticulum | Mitochondrial | Plasma membrane | Secreted | Unknown |
|---|---|---|---|---|---|---|
| Matches (%) | 63 | 7 | 41 | 23 | 50 | 12 |
No matches were observed for golgi, lysosomal, peroxisome or vesicle proteins.
The effects of PMP fractions on platelet function were demonstrated using the platelet function analyser PFA-100®. Only fractions 3 and 4 affected platelet function. Inhibition of capillary closure time by fractions 3 and 4 could result from competition between platelets and PMP for the collagen-coated surface of the PFA-100 capillary, although many other mechanisms are possible based on the abundance of surface receptors and chemokines in the PMP.
The effects of PMP fractions on endothelial cell barrier integrity were determined using an electric cell-substrate impedance sensing apparatus (Fig. 2–4). This technique indicates whether added agents enhance or inhibit endothelial cell barrier integrity. Fractions 2 and 4 significantly enhanced barrier integrity, while fraction 1 inhibited it (Fig. 2 and 3). The mechanism for the functional effects of fractions 2 and 4 was shown not to involve the presence of S1P or other lipids that interact with S1P receptors using the receptor antagonist, VPC23109 (43). This agrees with our inability to detect S1P in any of the fractions. However, while heat treatment abrogated the TEER rise induced by fraction 4 (Fig. 4C), it had no effect on fraction 2 (Fig. 4D). Thus the active component in fraction 2 is either a bioactive lipid other than S1P or a heat-stable protein.
In conclusion, PMP size classes exhibit different functional effects on platelets and endothelial cells. The proteomic analysis of these size classes presented here will facilitate identification of active proteins in the PMP by correlation of protein content with activity. Since the levels of PMP are increased in disease states including cancer and atherosclerosis (5, 19–24), these studies provide new avenues of research into the role of PMP in disease and development of therapeutic strategies. Furthermore, since PMP of different size and composition may be cleared from the circulation at different rates, the functional effects of specific size classes may predominate in vivo.
What is known about this topic?
– Activated platelets release vesicular membrane fragments in the size range of 0.1–1.0 μm termed microparticles.
– Large microparticles originate from the platelet plasma membrane while smaller particles originate from internal storage vesicles such as α granules.
– Microparticles have several functions including promotion of coagulation, angiogenesis, and proliferation of smooth muscle.
– Proteomic analysis of the total microparticle population has been reported confirming their membrane origin and showing the presence of proteins of other origins including mitochondrial, nuclear and cytosolic.
What does this paper add?
– Platelet microparticle size classes contain proteins of different subcellular origin with mitochondrial proteins highly represented in the largest microparticles and α granule proteins predominating in the smallest particles.
– Different size classes of platelet microparticles have different functional effects on platelets and endothelial cells.
– The active component in the smallest microparticle fraction is a protein while the active component in an intermediate sized microparticle fraction is likely a lipid.
Supplementary Material
References
- 1.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 2.Heijnen HF, Schiel AE, Fijnheer R, et al. Activated platelets release two types of membrane vesicles; microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and a-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 3.Reininger AJ, Heijnen HF, Schumann H, et al. Mechanism of platelet adhesion to vonWillebrand factor and microparticle formation under high shear stress. Blood. 2006;107:3537–3545. doi: 10.1182/blood-2005-02-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denzer K, Kleijmeer MJ, Heijnen HFG, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 5.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Flaumenhaft R, James R, Dilks JR, et al. Mega-karyocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–1121. doi: 10.1182/blood-2008-06-163832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smalley DM, Root KE, Cho HJ, et al. Proteomic discovery of 21 proteins expressed in human plasma-derived but not platelet-derived microparticles. Thromb Haemost. 2007;97:67–80. [PubMed] [Google Scholar]
- 8.Jin M, Drwal G, Bourgeois T, et al. Distinct proteome features of plasma microparticles. Proteomics. 2005;5:1940–1952. doi: 10.1002/pmic.200401057. [DOI] [PubMed] [Google Scholar]
- 9.Gracia BA, Smalley DM, Cho H, et al. The platelet microparticle proteome. J Proteome Res. 2005;4:1516–1521. doi: 10.1021/pr0500760. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Pujol S, Marker PH, Key NS. Platelet micro-particles are heterogeneous and highly dependent on the activation mechanism: studies using a new digital flow cytometer. Cytometry A. 2007;71:38–45. doi: 10.1002/cyto.a.20354. [DOI] [PubMed] [Google Scholar]
- 11.Biro E, Akkerman JW, Hoek FJ, et al. The phospholipid composition and cholesterol content of platelet derived microparticles: a comparison with platelet membrane fractions. Thromb Haemost. 2005;3:2754–2763. doi: 10.1111/j.1538-7836.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 12.Sims PJ, Wiedmer T, Esmon CT, et al. Assembly of the platelet prothrombinase complex is linked to vesiculation of the platelet plasma membrane: studies in Scott syndrome, an isolated defect in platelet procoagulent actvitity. J Biol Chem. 1989;264:17049–17057. [PubMed] [Google Scholar]
- 13.Berckmans RJ, Nieuwland R, Tak PP, et al. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002;46:2857–2866. doi: 10.1002/art.10587. [DOI] [PubMed] [Google Scholar]
- 14.Nieuwland R, Berckmans RJ, Rotteveel-Eijkman RC, et al. Cell-derived microparticles generated in patients during cardiopulmonary bypass are highly pro-coagulent. Circulation. 1997;96:3534–3541. doi: 10.1161/01.cir.96.10.3534. [DOI] [PubMed] [Google Scholar]
- 15.Sinauridze EI, Kireev DA, Popenko NY, et al. Platelet microparticle membranes have 50– to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97:425–434. [PubMed] [Google Scholar]
- 16.Merten M, Pakala R, Thiagarajan P, Benedict CR. Platelet microparticles promote platelet interaction with subendothelial matrix in a glycoprotein IIb-IIIa-dependent mechanism. Circulation. 1999;99:2577–2582. doi: 10.1161/01.cir.99.19.2577. [DOI] [PubMed] [Google Scholar]
- 17.Dachary-Prigent J, Pasquet JM, Fressinaud E, Toti F, Freyssinet JM, Nurden AT. Aminophospholipid exposure, microvesicularization and abnormal protein tyrosine phosphorylation in the platelets of a patient with Scott syndrome: a study using physiologic agonists and local anaesthetics. Br J Haematol. 1997;99:959–67. doi: 10.1046/j.1365-2141.1997.5003302.x. [DOI] [PubMed] [Google Scholar]
- 18.Toti F, Satta N, Fressinaud E, et al. Scott syndrome, characterized by impaired transmembrane migration of procoagulent phosphatidylserine and hemorrhagic complications, is an inherited disorder. Blood. 1996;87:1409–15. [PubMed] [Google Scholar]
- 19.Kim HK, Song KS, Chung JH, et al. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124:376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 20.Weber A, Koppen HO, Schror K. Platelet-derived microparticles stimuilate coronary artery smooth muscle cell mitogenesis by a PDGF-independent mechanism. Thromb Res. 2000;98:461–466. doi: 10.1016/s0049-3848(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 21.Boulanger CM, Amabile N, Tedgui A. Circulating Microparticles: a potential prognosis marker for atherosclerotic vascular disease. Hypertension. 2006;48:180–186. doi: 10.1161/01.HYP.0000231507.00962.b5. [DOI] [PubMed] [Google Scholar]
- 22.Preston RA, Jy W, Jimenez JJ, et al. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 23.van der Zee PM, Biró E, Ko Y, et al. P-Selectin and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin Chem. 2006;52:657–664. doi: 10.1373/clinchem.2005.057414. [DOI] [PubMed] [Google Scholar]
- 24.Diehl P, Nagy F, Sossong V, et al. Increased levels of circulating microparticles in patients with severe aortic valve stenosis. Thromb Haemost. 2008;99:711–719. doi: 10.1160/TH07-05-0334. [DOI] [PubMed] [Google Scholar]
- 25.Barry OP, Pratico D, Lawson JA, et al. Transcellular activiation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99:2118–2127. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfister SL. Role of Platelet Microparticles in the production of thromboxane by rabbit pulmonary artery. Hypertension. 2004;43:428–433. doi: 10.1161/01.HYP.0000110906.77479.91. [DOI] [PubMed] [Google Scholar]
- 27.Barry OP, Kazanietz MG, Praticò D, et al. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinaseC/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–7556. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- 28.Alvarex SE, Milstien S, Spiegel S. Acutocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Sano T, Baker D, Virag T, et al. Multiple Mechanisms linked to platelet activation results in lysophosphatidic acid and sphingosine-1-phosphate generaion in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 30.Yatomi Y, Ohmori T, Rile G, et al. Sphingosine-1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- 31.Kobayashi N, Nishi T, Hirata T, et al. Sphingosine-1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Bozulic LD, Malik MT, Powell DW, et al. Plasma membrane Ca2+-ATPase associates with the actin cytoskeleton via CLP36 in human platelets. Thromb Haemost. 2007;87:587–597. [PubMed] [Google Scholar]
- 33.Bartlett GR. Phosphorus Assay in Column Chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 34.Powell DW, Merchant ML, Link AJ. Discovery of regulatory molecular events and biomarkers using 2D capillary chromatography and mass spectrometry. Expert Rev Proteomics. 2006;3:63–74. doi: 10.1586/14789450.3.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Powell DW, Weaver CM, Jennings JL, et al. Cluster analysis of mass spectrometry data reveals a novel component of SAGA. Mol Cell Biol. 2004;24:7249–7259. doi: 10.1128/MCB.24.16.7249-7259.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon HT, Yoo HS, Shin BK, et al. Improved fluorescent determination method of cellular sphingoid bases in high-performance liquid chromatography. Arch Pharm Res. 1999;22:294–299. doi: 10.1007/BF02976365. [DOI] [PubMed] [Google Scholar]
- 37.Caligan TB, Peters K, Ou J, et al. A high-performance liquid chromatographic method to measure sphingosine-1-phosphate and related compounds from sphingosine kinase assays and other biological samples. Anal Biochem. 2000;281:36–44. doi: 10.1006/abio.2000.4555. [DOI] [PubMed] [Google Scholar]
- 38.Kundu SK, Heilmann EJ, Sio R, et al. Description of an in vitro platelet function analyzer, PFA-100. Semin Thromb Hemost. 1995;21:106–112. doi: 10.1055/s-0032-1313612. [DOI] [PubMed] [Google Scholar]
- 39.Heilmann EJ, Kundu SK, Sio R, et al. Comparison of four commercial citrate blood collection systems for platelet function analysis by the PFA-100 system. Thromb Res. 1997;87:159–164. doi: 10.1016/s0049-3848(97)00115-1. [DOI] [PubMed] [Google Scholar]
- 40.Keese CR, Wegener J, Walker SR, et al. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci USA. 2001;101:1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JF, Zeng Q, Ozaki H, et al. Dual roles of tight junction-associated protein, zonula occuledens-1, in sphingosine 1 phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281:29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- 42.Guidotti G. Membrane Proteins. Annu Re Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- 43.Davis MD, Clemens JJ, Macdonald TL, et al. Sphingosine-1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 44.Moebius J, Zahedi RP, Lewandrowski U, et al. The human platelet membrane proteome reveals several new potential membrane proteins. Mol Cell Prot. 2005;4:1754–1761. doi: 10.1074/mcp.M500209-MCP200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.