Abstract
Purpose
To investigate the expression of CK2 subunits, and CK2 effects on NF-κB and TP53 mediated signal activation and gene expression, the malignant phenotype, and chemosensitivity in head and neck squamous cell carcinoma (HNSCC) in vitro and in vivo.
Experimental Design
Protein expression of CK2 subunits was investigated by Western blot and immunohistochemistry. CK2 subunits were knocked down by siRNA, and NF-κB activation was examined using DNA binding, Western blot, and luciferase reporter assays. Gene expression was measured by quantitative RT-PCR. Cell growth, survival, motility, and sensitivity to cisplatin were measured by MTT, flow cytometry and migration assays. In vivo targeting of CK2α/α′ in HNSCC xenograft models was achieved using anti-CK2α/α′ oligodeoxynucleotide (ODN) encapsulated in sub-50 nm tenfibgen nanocapsules.
Results
CK2 subunit proteins were overexpressed in HNSCC lines and tissues. Knockdown of CK2 subunits differentially inhibited IκBα degradation, NF-κB nuclear localization, phosphorylation, DNA binding, and reporter activity. CK2 subunits modulated gene expression and the malignant phenotype involved in cell cycle and migration, while CK2α is critical to promote proliferation, anti-apoptosis and cisplatin resistance in vitro. Further, in vivo delivery of anti-CK2α/α′ ODN nanocapsules significantly suppressed tumor growth in HNSCC xenograft models, in association with modulation of CK2 and NF-κB regulated molecules, TP53 family proteins, and induction of apoptosis.
Conclusions
Our study reveals a novel role of CK2 in co-regulating NF-κB activation, and TP53/p63 expression, and downstream gene expression. Downregulation of CK2 in HNSCC models in vitro and in vivo demonstrates antitumor effects as well as sensitization to cisplatin.
Keywords: CK2, nanocapsules, NF-κB, p53, head and neck cancer
Introduction
Protein kinase CK2 (formerly Casein Kinase 2 or II) is a highly conserved and ubiquitous protein serine/threonine kinase. It consists of catalytic subunits (42 kDa α, 38 kDa α′) and a regulatory subunit (28 kDa β), producing holoenzyme structures linking the catalytic subunits through the β subunit into α2β2, αα′β2, or α′2β2 tetramers (1–4). Because CK2 has a large number of potential substrates, it plays an important functional role in numerous disease processes, including cancer, where it has been found to be dysregulated in all cancers examined (2, 3, 5, 6). CK2 is diffusely localized to both the nuclear and cytoplasmic compartments in normal cells, whereas, in cancer cells the kinase localization is more intense in the nuclear compartment (3, 6). Further, the various subunits in cancer cells can be differentially distributed (2, 7). CK2 has long been implicated in cell proliferation and differentiation; however, subsequent studies have demonstrated that CK2 is also a potent suppressor of apoptosis (5, 8), thus providing an important link of the kinase to the critical phenotypic hallmarks of malignancy (9).
Previous work has shown that CK2 is highly elevated in head and neck squamous cell carcinoma (HNSCC) and its elevation is linked to disease status and prognosis in patients (3, 6, 10, 11). Elevated levels of CK2 in tumor specimens have been suggested to serve as a marker of the disease severity in different cancers including HNSCC (3, 10, 11). Indeed, immunohistochemistry analysis of CK2 signal compared with that of Ki-67 in HNSCC suggested that CK2 was not only elevated in the proliferating edge of the tumor but also throughout the tissue section reflecting a state of dysregulation (3). Further, the relative increase in nuclear distribution and association with chromatin by CK2α observed in HNSCC suggests these subunits may have differential functional roles in the cytoplasm and nucleus in cancer. In this regard, it is noteworthy that the most prominent signaling of CK2 in response to altered growth is observed in the nuclear matrix compartment of the cell (5, 6, 8).
CK2 is known to have a large number of potential substrates (12), and it is one of several kinases involved in the activation of Nuclear Factor-kappaB (NF-κB), a transcription factor that controls many genes associated with proliferation, anti-apoptosis, angiogenesis, invasion, and resistance to therapies in cancer (13). NF-κB is comprised of hetero- or homodimers from 5 different subunits, RELA (p65), NF-κB1 (p50), NF-κB2, cREL, and RELB. The p65/p50 complex is involved in the canonical NF-κB pathway activated by cytokines like Tumor Necrosis Factor (TNF)-α. Under the stimulation of upstream signaling, a trimeric Inhibitor-kappaB Kinase (IKK) phosphorylates IκBs, the inhibitor of NF-κB, leading to IκB proteasomal degradation and releasing IκB bound NF-κB p65 and p50 complex to nuclear translocation and DNA binding (14). Previously, we established that NF-κB is aberrantly activated in cell lines and tumor specimens from patients with HNSCC (15, 16). We demonstrated that CK2 contributes to aberrant NF-κB activation through a novel mechanism involving IKKβ (17). In addition, CK2 has been shown to directly phosphorylate the COOH-terminal PEST domain of IκBα (18), as well as p65 (19, 20). Phosphorylation of the IκBα PEST domain enhances proteasome degradation, while direct phosphorylation of the p65 subunit is necessary to activate and alter its transcriptional activity (14).
NF-κB is an important regulator of a broad program of genes that promote cell proliferation, survival, migration, inflammation and angiogenesis, critical in the malignant phenotype (13, 15, 16, 21). Activation of the NF-κB subunit RELA/p65 and a related gene cluster is linked with repressed tumor suppressor TP53 mRNA and protein expression in a subset of HNSCC (21, 22). In addition, CK2 has been identified as an upstream kinase involved in post-translational modification of TP53 phosphorylation and function (23, 24). These observations lead us to hypothesize that CK2 could be a critical regulatory node affecting both NF-κB and TP53 pathways, and promoting the malignant phenotype and progression.
CK2 has been proposed as a target for cancer therapy (5, 6, 25–27) and downregulation of CK2 results in induction of apoptosis in HNSCC cultured cells (5, 6, 28). However, because it is ubiquitous and essential for cell survival, it is desirable to enhance targeting of cancer cells while sparing normal cells in vivo. This has become possible with the development of novel sub-50 nanometer size nanocapsules containing tenfibgen (fibrinogen binding domain of tenascin), which have been shown to enhance tumor delivery of anti-CK2α/α′ ODN against both CK2α and α′ subunits (25, 26). These nanocapsules enter the tumor cells via the caveolar pathway promoting enhanced intracellular delivery of antisense or siRNA cargo to tumor sites in prostate and HNSCC xenograft models, detectable by histology as well as functional imaging (6, 28). Furthermore, delivery of anti-CK2α/α′ ODN in the prostate xenograft tumor model has shown enhanced delivery of anti-CK2α/α′ ODN to the nucleus and significant anti-tumor effects in vivo (27, 28). These observations lead us to explore the efficacy of CK2 targeted therapy in HNSCC xenograft animal models, and examine the effects on NF-κB and TP53 as molecular targets.
In the present work, we demonstrate differential functions of the CK2 subunits in NF-κB activation, repression of pro-apoptotic TP53 family transcription factors TP53 and TAp63, and expression of key genes implicated in controlling cell cycle, survival, and adhesion in the malignant phenotype of HNSCC. These mechanisms identified in vitro are consistent with anti-tumor responses observed using in vivo models where anti-CK2α/α′ ODN nanocapsules significantly suppressed HNSCC tumor growth and altered expression of multiple proteins involved in NF-κB, TP53, and apoptotic pathways.
Methods
Cell lines
A panel of 9 HNSCC cell lines from the University of Michigan squamous cell carcinoma (UM-SCC) series was obtained from Dr. T.E. Carey (University of Michigan, Ann Arbor, MI). These UM-SCC cell lines were extensively characterized in previous studies in our laboratory and found to reflect many of the molecular and phenotypic alterations important in pathogenicity of HNSCC. The Fadu tumor line was purchased from American Type Culture Collection (ATCC, Manassas, VA). Normal human epidermal keratinocytes (HEKA, Invitrogen, Carlsbad, CA) were isolated from skin of different individual adults, established as primary cell cultures under low calcium conditions, and used as a non-malignant control within 5 passages. The UM-SCC cell lines and HEKA cells were cultured as previously described (21).
Real time RT-PCR
Western blot
Whole cell, nuclear, and cytoplasmic lysates were obtained using a Nuclear Extraction Kit from Active Motif (Carlsbad, CA). Western blot analysis was performed as described previously (22), using the following antibodies: goat anti-CK2α 1:500 (sc-6479), goat anti-CK2α′ 1:500 (sc-6481), rabbit anti-CK2β (sc-2071) 1:500, and rabbit anti-NF-κBp65 1:500 (sc-109) from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Additional antibodies included: mouse anti-CK2 α & α′ 1:500 (MA-5004, Affinity Bioreagents, Golden, CO), rabbit anti-phospho-NF-κBp65-ser536 1:1000 (3031, Cell Signaling, Danvers, MA), rabbit anti-phospho NF-κBp65-ser529 1:500 (ab47395, Abcam, Cambridge, MA); donkey anti-goat IgG-HRP 1:4000 (sc-2020, Santa Cruz), goat anti-rabbit IgG-HRP 1:2000 (AP132P, Chemicon, Billerica, MA). Each blot was incubated with Pierce Super Signal West Pico substrate (Pierce Biotechnology Inc., Rockford, IL) and exposed to Kodak X-OMAT film.
Immunohistochemistry
CK2 small interfering RNA
Cultured cells were transfected with 50nM siRNAs from Dharmacon (Chicago, IL): ON-TARGETplus Non-targeting Pool (001810), CK2α (003475), CK2α′ (004752), CK2β (007679), Cyclin D1 (003210) using Lipofectamine 2000 (Invitrogen) for 24, 48, and 72 hours. Knockdown efficiency was assessed by RT-PCR and by Western blot.
NF-κB DNA binding assays
Reporter gene assay
MTT cell proliferation assay
Analysis of cell cycle and apoptosis by flow cytometry
Wound migration assay
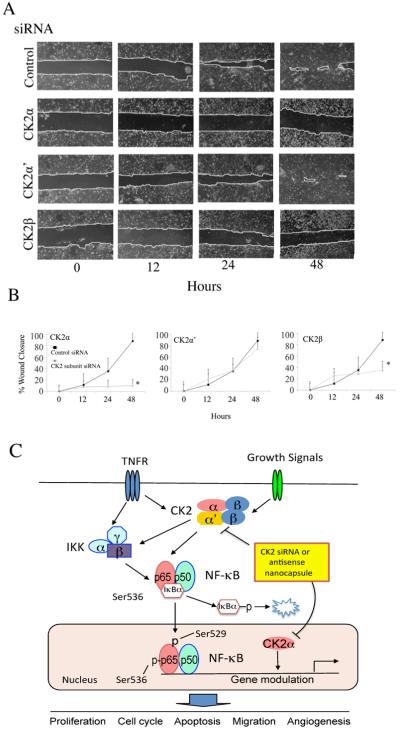
Cells were transfected with siRNA for 48 hours to allow for sufficient protein knockdown. Wounds were made through the confluent cell sheets using a 200 μL pipette tip. Scratches were monitored for percentage of wound closure over the next 48 hours. 12 measurements at preset distances on the wound were made and averaged. The wound healing was quantified and the statistical analysis relative to the control siRNA was performed (t-test, * p<0.05).
Preparation of tenfibgen nanocapsules containing anti-CK2α/α′ ODN against CK2
The sequence for the chimeric oligonucleotide directed against and CK2α′ (AS-CK2αα′) was 5′-ATACAACCCAAACT-2′-o-methyl-(ccacat)-propyl-3′ which targets the conserved region of both molecules. A published chimeric sequence directed against GAPDH was used as a control sequence (29).
A dispersion atomization method was used to package 20-mer chimeric oligonucleotides into sub-50 nm nanocapsules comprised of tenfibgen, the recombinant fibrinogen fragment of tenascin (27, 28, 30). Incorporation of oligos at greater than 90% and average particle size of less than 50 nm was confirmed by published methods (31).
HNSCC xenograft tumor model
Four to six week old male BALB/c SCID (severe combined immunodeficient) mice were obtained from Frederick Cancer Research and Development Center (NCI), and housed in a pathogen-free animal facility. UM-SCC 11A cells were cultured in DMEM medium with 10% FCS. Mice were injected subcutaneously in flanks with 1.5 × 107 UM-SCC 11A cells (32) and treated 23 days later when tumors were 6–8 mm in diameter. Mice received three i.p. injections every three days. Tumors were collected by snap freezing 24 hours after the third dose. For FaDu model, weanling BALB/c athymic nude females (Harlan, Indianapolis, IN.) were inoculated intradermally with 4 × 106 cells and treated twice 48 hours apart by tail vein injection when tumors were palpable. In both models, mice were treated at 10 μg/kg of body weight. Tumor diameters were followed by caliper measurements and volumes were estimated using the formula V = 0.5(L*W*W) where L equals the longer of the two tumor measurements.
TUNEL assay
Results
Aberrant CK2α, α′ and β subunit expression and cellular distribution in HNSCC cell lines and tissues
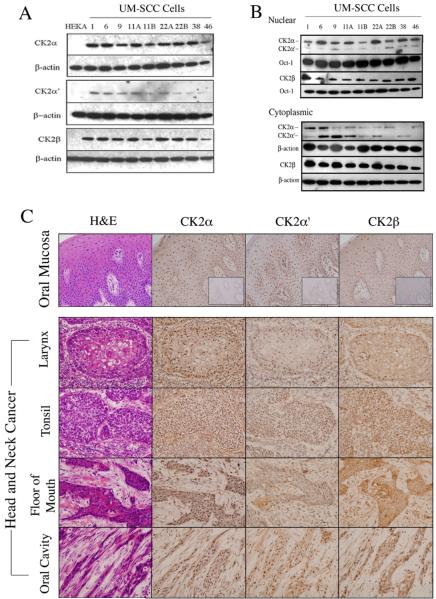
We previously observed aberrant CK2 activity in HNSCC (10, 17), but the expression and cellular distribution of the CK2 subunits were not well defined. Here, we examined the relative expression levels and cellular localization of individual CK2 subunits in a panel of HNSCC (UM-SCC) cell lines and tumors. We detected increased expression of CK2 subunit proteins α, α′ and β by Western blot in 9/9 UM-SCC lines, relative to that detected in non-malignant HEKA cells (Fig. 1A and supplemental Fig. 1A). CK2 proteins in HEKA were detectable with prolonged exposure (data not shown). Previous reports indicated that aberrant CK2 kinase activity was associated with the nuclear chromatin compartment in HNSCC tissues (10, 17). Therefore, we compared the relative abundance of individual CK2 subunits in nuclear and cytoplasmic compartments (Fig. 1B). Expression of CK2α relative to CK2α was distinctly enriched in the nuclear versus cytoplasmic extracts using the antibody detecting both subunits. Conversely, CK2α was relatively enriched in the cytoplasmic extracts in several lines, and CK2β was abundant in all UM-SCC lines (Fig. 1B).
Figure 1. Aberrant expression of CK2 subunits in HNSCC.
(A) The protein expression of CK2 subunits in whole cell lysates isolated from HNSCC and HEKA cells were detected by Western blot. Representative data from one of three independent experiments are shown. β-actin was used as a loading control. (B) The protein expression of CK2 in the nucleus and cytoplasmic lysates were detected by Western blot using antibody against both α and α′ or β. Oct-1 was used as loading control for nuclear fraction, and β-actin was used as a loading control for cytoplasmic fraction. (C) IHC of individual CK2 subunits was performed and showed the nuclear and cytoplasm localization in normal oral mucosa and four tumor specimens from different anatomic sites. The negative control with isotype antibodies were shown in the inner boxes of normal mucosa staining images. The photomicrographs were taken at 400× magnification.
To investigate the expression levels of different CK2 subunits in normal oral cavity, we performed IHC in the gingival mucosa from several healthy nonsmokers and images from a representative specimen are shown (Fig. 1C). All CK2 subunits exhibited variable light cytoplasmic but denser staining in nuclei of cells in the basal layer of the mucosa, with lesser staining in the parabasal layer. We further compared the presence and localization of CK2 expression in four HNSCC specimens originating from different anatomic sites (Fig. 1C). Tumor tissues demonstrated strong expression of CK2α, α′ and β subunits, with variable staining pattern detectable in both nucleus and cytoplasm. Similar to the differential distribution observed in UMSCC lines, a relatively strong nuclear immunostaining for CK2α is observed in 4/4 tumors shown, while predominantly cytoplasmic or nuclear staining for CK2α′ and β was observed in 2 cases. In addition, in two cases (floor of mouth and oral cavity) where a distinct stroma is shown, the intensity of CK2 staining was higher in tumor compartment than in adjacent connective tissue.
Knockdown of CK2 subunits suppress aberrant NF-κB activity
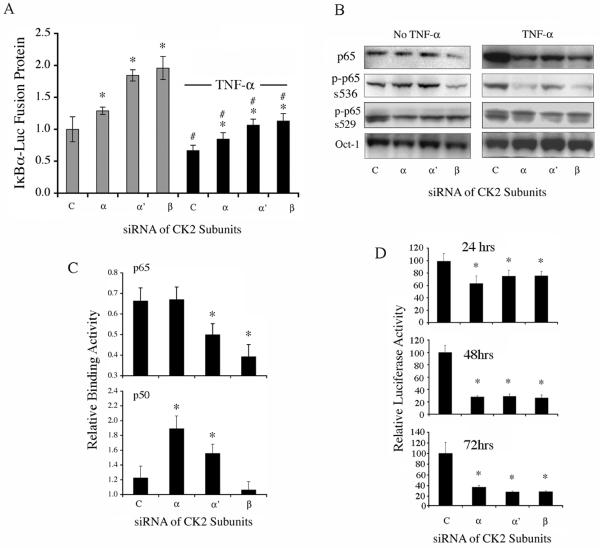
To delineate functions of specific CK2 subunits, we knocked down CK2 subunits by siRNA in two lines (UM-SCC 6 and 11A) from the UM-SCC subset with elevated NF-κB activation and low wild type (wt) TP53 expression (22, 32). A highly specific and efficient knockdown for individual subunit mRNA and protein levels was obtained in UM-SCC 11A (supplemental Fig. 2) and UM-SCC 6 cells (data not shown). To delineate the contribution of CK2 subunits to the IκBα kinase (IKK)-dependent activation of NF-κB, we tested degradation of an IκBα-luciferase fusion protein (33). In this assay, the inhibition of CK2 dependent IKK kinase activity is indicated by an increase of IκBα-luciferase signal, which reflects increased concentration of this inhibitor of NF-κB activation. After knockdown of individual CK2 subunits, a significant increase in IκBα-luciferase protein was observed (Fig. 2A, left columns). Knockdown of CK2β and α′, the predominant subunits detected in the cytoplasm, had more potent effects. TNF-α, a classical inducer of IKK activity, IκBα degradation, and NF-κB activation, further promoted IκBα degradation in all conditions, with knockdown of CK2 subunits attenuating TNF-α-induced IκBα degradation in a fashion similar to that observed without TNF-α (Fig. 2A, right columns).
Figure 2. Knockdown of CK2 subunits by siRNA inhibits aberrant NF-κB activation.
(A) Knockdown of individual CK2 subunits for 48 hours significantly blocked IκBα-Luc protein degradation, which increased luciferase activity without or with TNF-α treatment (concentration and time). * indicates significant differences when comparing the conditions after CK2 knockdown with control siRNA and # indicates the difference after TNF-α treatment (t-test, p<0.05). [siRNAs for control (c); CK2α (α); CK2α′ (α′ CK2β (β)]. (B) Knockdown of individual CK2 subunit for 48 hours modulated NF-κB p65 nuclear translocation, phospho-serine536, and phospho-serine529 detected in nuclear fractions without TNF-α (left panel), or after 30 min of TNF-α treatment (right panel). In UM-SCC 11A cells, the basal level of phosphorylated p65 was relatively low as compared to that under stimulation by classical inducer of NF-κB, TNF-α (supplemental Fig. 3). To detect and show it, a more sensitive chemiluminesence reagent and a longer exposure time were used and shown for the left panel, reducing the apparent differences between the No TNF-α and TNF-α conditions (Fig 2B, left and right panels). (C) Knockdown of CK2 α′ or β subunit significantly reduced NF-κB p65 binding activity in nuclear fractions by 48 hours (upper panel), while knockdown of CK2 α or α′ subunit significantly increased NF-κB p50 binding activity (lower panel). (D) Knockdown of individual CK2 subunits reduced aberrant NF-κB activity by reporter gene assay at different time points. * indicates statistical significance (t-test, p<0.05) when comparing CK2 knockdown with control siRNA.
We next examined the effects of knockdown of the CK2 subunits on constitutive and TNF-α induced nuclear translocation of the NF-κB transactivating subunit p65 (RELA), as well as phosphorylation of p65 serine536, which is dependent on IKKβ activation (14), and serine529, dependent on CK2 activity (20) (Fig. 2B, supplemental Fig. 2). TNF-α stimulated an increase in total nuclear p65, and phosphorylation of p65 at serine536 and serine529 (Fig. 2B, supplemental Fig. 3). Knockdown of CK2β, and to a minimal extent CK2α, reduced total p65 in nuclear extracts without TNF-α (Fig 2B, upper left panel). Knockdown of all 3 subunits strongly reduced TNF-α-induced translocation of nuclear p65, with CK2α and β demonstrating a greater effect (Fig 2B, upper right panel). The effects of CK2 subunit knockdown on IKKβ-phosphorylation of serine536 demonstrated a similar pattern (Fig. 2B), suggesting the CK2α and β subunits have similar effects on IKK function in promoting both translocation and serine536 phosphorylation of p65. In cultured UM-SCC 11A cells, the basal level of p65 phosphorylation was relatively lower as compared to that under TNF-α stimulation (supplemental Fig. 3).
NF-κB family members are transcription factors, which activate gene expression though binding to the regulatory sequences of gene promoters. Next, NF-κB binding activity of transcriptionally functional p65 or inactive p50 to an NF-κB DNA consensus sequence was determined. Knockdown of CK2β, and to a lesser extent, the α' subunit, significantly lowered constitutive NF-κB p65 binding activity (Fig. 2C, upper panel). In contrast, knockdown of and to a lesser extent the α′ subunit, significantly increased NF-κB binding activity of p50 (Fig 2C, lower panel), which lacks the transactivation domain and serves as a negative regulator (14). The binding activities of other NF-κB family members, NF-κB2, cREL, or RELB, regulated by the alternative pathway, were not affected by CK2 knockdown (data not shown). Consistent with the combined effects of knockdown of CK2 subunits on NF-κB nuclear translocation, phosphorylation, or binding activity, knockdown of each CK2 subunit significantly suppressed constitutive NF-κB reporter activity, which is a direct measurement of NF-κB function (Fig. 2D).
Knockdown of CK2 subunits differentially regulates gene expression
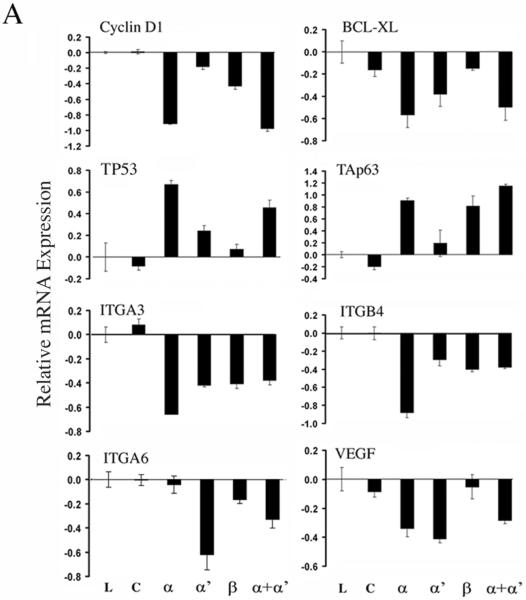
We next examined if CK2 subunits affect expression of genes previously shown to individually modulate the malignant phenotype of HNSCC (22, 32, 34, 35). The most profound effects were observed with knockdown of the subunit, including decreased expression of NF-κB target genes involved in cell survival (BCL-XL) and cell cycle progression (CCND1), while increasing expression of TP53 family genes known to promote growth arrest and apoptosis (p53 and TAp63, Fig. 3A, upper two panels). Integrin genes ITGA3 and ITGB4 were also significantly modulated after CK2α knockdown (Fig. 3A, third panels). Additionally, integrin ITGA6 was significantly decreased after depletion of CK2α′, and angiogenic factor VEGF was significantly down regulated by both CK2 and CK2α′ knockdown (Fig. 3A, lower panels).
Figure 3. Knockdown of different CK2 subunits differentially modulated gene expression.
Gene expression was assayed by real-time RT-PCR 48 hrs after transfection with CK2 siRNA in UM-SCC 11A cells. Lipofectamine alone (L); siRNAs for control (c); CK2α (α); CK2α′ (α′); CK2β (β); CK2α plus α′ (α+α′).
The roles of the aforementioned genes modulated by CK2 in the malignant phenotype have been established in HNSCC lines previously (21, 32, 34-39). To confirm a direct link between anti-CK2 mediated down regulation of NF-κB target genes and functional inhibition of tumor cell proliferation, CCND1 was knocked down and its effect on cell proliferation was measured (supplemental Fig. 4). CCND1 mRNA was significantly decreased by siRNA, and cell growth was suppressed through day 6.
Knockdown of the CK2α subunit alters cell proliferation, survival and chemosensitivity
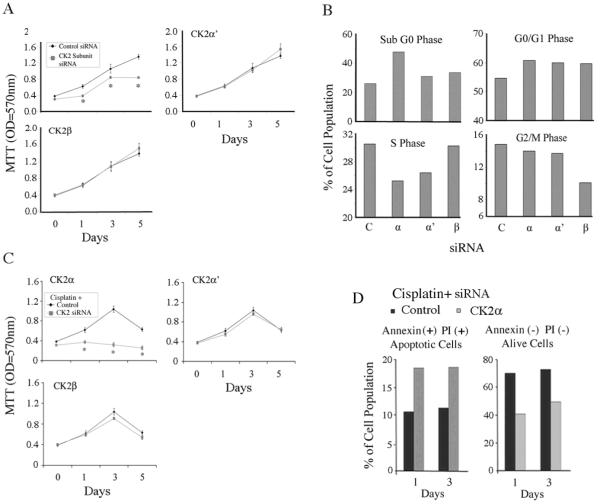
To examine the biological effects of knockdown of individual CK2 subunits, we measured the effects on cell proliferation of UM-SCC 11A (Fig. 4A) and UM-SCC 6 cells (Supplemental Fig. 5A) by MTT assay. CK2α knockdown specifically inhibited proliferation of both cell lines to an extent that was not seen with either α' or β knockdown. We further analyzed whether knockdown of individual subunits would affect cell cycle and death by DNA flow cytometry (Fig. 4B). Cell death, measured by sub-G0 DNA fragmentation, was specifically increased after CK2α, but not by α′ or β subunit knockdown (Fig. 4B). Knockdown of each CK2 subunit increased cells arrested in the G0/G1 phase, while knockdown of CK2α and α′ significantly decreased cells in S phase and CK2β decreased cells in G2/M phase (Fig. 4B).
Figure 4. Knockdown of CK2 subunits differentially modulates cell survival and chemosensitivity.
(A) UM-SCC 11A cells were transfected with siRNA targeting of CK2 subunits and cell proliferation was measured by MTT. * indicates statistical difference using t-test (p<0.05). (B) Cell cycle and death were measured 48 hrs after transfection by staining DNA with propidium iodide (PI) using the Cycletest Plus DNA reagent kit. (C) Cells were transfected with siRNA overnight and then replated in 96-well plates in quadruplicates. The next day, the cells were treated with the sub-optimal dose of cisplatin (2.5μM), and the proliferation was measured by MTT assay. (D) Cells were subjected to knockdown of CK2 subunits plus cisplatin treatment following which cell apoptosis was analyzed with an Annexin V/FITC (x axis) and PI/PE (y axis) apoptotic assay by flowcytometry. The experiments were repeated twice.
Next, we tested whether knockdown of CK2 subunits would sensitize cells to cisplatin, a chemotherapy drug used for HNSCC. Knockdown of CK2α, but not CK2α′ or CK2β, strongly enhanced sensitization to a suboptimal dose of cisplatin, completely blocking the proliferation of UM-SCC 11A (Fig. 4C) and UM-SCC 6 cells (Supplemental Fig. 5B). To confirm if CK2α knockdown synergized with cisplatin by increasing apoptosis, we tested cells with annexin V and propidium iodide (PI) using flow cytometry. Knockdown of CK2α plus cisplatin showed greater increase in the quantity of late apoptotic cells (annexin positive/PI positive) than the control, accompanied by a decrease in the non-apoptotic cell population (Fig. 4D and Supplemental Fig 5C).
CK2 knockdown inhibits cell migration by wound healing assay
As we showed above that CK2 siRNA inhibited expression of integrin genes specifically involved in HNSCC adhesion and migration (38, 39), therefore, we examined the effects of CK2 subunit knockdown on UM-SCC cell migration after a wounding assay. Knockdown of CK2 and β had profound inhibitory effects on wound closure, when compared with control and CK2α′ knockdown (Fig. 5A, B), supporting their important roles in cell migration.
Figure 5. Knockdown of CK2 subunits inhibits cell migration by wound healing assay, and a model for role of CK2 in HNSCC.
(A) UM-SCC 11A cells were transfected with CK2 subunit specific siRNAs for 48 hrs. The cell monolayers were wounded by scraping and the wound closure was followed at 12, 24, and 48 hrs. (B) The distance of the wound was measured, and * indicates significant differences by t-test (p<0.05). (C) Proposed model for CK2 modulation of NF-κB and TP53 pathways. Increased external signaling, such TNF-α, or other factors induce aberrant CK2 and NF-κB activation. CK2 subunits modulate IKK mediated IκBα phosphorylation and degradation, as well as IKK mediated phosphorylation of p65 at serine536, and CK2 phosphorylation of p65 at serine529. NF-κB subunits translocated into nucleus, bound to the NF-kB target gene promoters, and modulated NF-κB transcriptional activity. In addition, CK2α co-regulates NF-κB, TP53/ TAp63 family gene expression. CK2 subunits regulate broad cellular functions affecting proliferation, cell cycle, apoptosis, migration and angiogenesis, which promote HNSCC tumorigenesis. TNFR: Tumor necrosis factor-α receptor, IKK: Inhibitor kappaB kinase, IκBα: Inhibitor kappaBα
Proposed model for CK2 modulated NF-κB and other signaling pathways that enhance malignant progression of HNSCC
Based on the evidence presented above, we propose a model illustrating the key mechanisms by which CK2 may contribute to the malignant phenotype of HNSCC (Fig. 5C). This includes roles for the different CK2 subunits in mediating signals by TNF-α and other factors to modulate IKKβ mediated IκBα degradation, NF-κB nuclear translocation, and p65 serine536 and serine529 phosphorylation. In addition, we revealed a critical function of CK2α in modulating gene expression that is associated with its predominant localization in the nucleus. These genes promote the malignant phenotype of HNSCC, including proliferation, cell cycle, anti-apoptosis, cell migration and angiogenesis. Based on this model, we next examined the effects of molecular downregulation of CK2 by employing sub-50 nm tenfibgen nanocapsules designed for systemic delivery of anti-CK2α/α′ ODN targeting both and α′ in HNSCC xenograft models in vivo.
Knockdown of CK2α/α′ by anti-CK2α/α′ ODN in tenfibgen nanocapsules suppresses tumor growth and modulates NF-κB and TP53 in HNSCC xenograft models
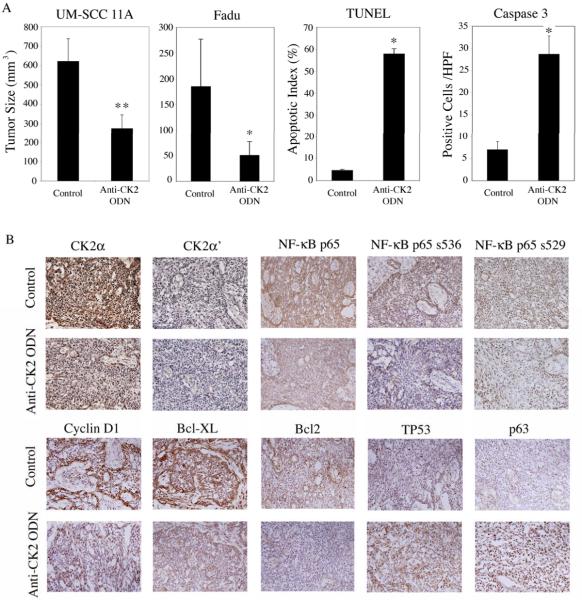
In order to test antitumor activity of knockdown CK2 in vivo, we administered tenfibgen nanocapsules containing anti-CK2α/α′ ODN for targeting both and α′ catalytic subunits in mice bearing human laryngeal (UM-SCC 11A) or hypopharyngeal tumor xenografts (FaDu). After three injections of the nanocapsules, tumor size was significantly reduced in both tumor models (Fig 6A, left two panels). Quantitation of cell apoptosis by both TUNEL and cleaved caspase 3 methods showed anti-CK2α/α′ ODN treatment significantly increased cell death in UM-SCC 11A tumor specimens (Fig 6A, right two panels). Consistent with above observations, protein expression detected by IHC in anti-CK2α/α′ ODN treated UM-SCC 11A tumors exhibited decreased and α′ expression, total NF-κB p65, and p65 serine536 and serine529 phosphorylation (Fig 6B, upper panels). In addition, anti-CK2α/α′ ODN decreased Cyclin D1, BCL-XL and BCL2 expression, and increased expression of TP53 and its family member p63 (Fig 6B, lower panels).
Figure 6. Targeting CK2 by nanocapsule delivered anti-CK2α/α′ ODN inhibits tumor growth in xenograft animal models.
HNSCC cells were inoculated either subcutaneously or intradermally and were randomized either after tumors were well-established (23 days, 6–8 mm, UMSCC-11A) or after palpable tumors were observed (5days, 3–4 mm, FaDu). Nanocapsules containing anti-CK2α/α′ ODN were administered at 10 μ/kg every three days. (A) Left panel: UM-SCC 11A tumor bearing mice were treated i.p. with three doses of anti-CK2α/α′ ODN (n=4), while control animals (n=5) received equivalent dosing with diluent or nanoencapsulated anti-GAPDH ODN. Tumor dimensions were measured by caliper and reported at day 7. The second left panel: FaDu tumor bearing mice were treated i.v. with two doses of anti-CK2α/α′ ODN (n=6) 48 hours apart; tumor size was reported at day 7 compared with diluent controls (n=8). * indicates statistical significance p<0.05, and ** indicates p<0.01. (B) IHC was performed using UM-SCC 11A tumors harvested on day 7 after anti-CK2α/α′ ODN treatment and compared with controls. Photomicrographs were taken by light microscopy at 400× magnification.
Discussion
The present findings represent an important advance in understanding the roles of CK2 in dysregulation of NF-κB and TP53, and the translational potential of targeting CK2 for therapy in HNSCC. Increased NF-κB activation was recently linked with decreased TP53 expression and activation in a subset of HNSCC with wt TP53 genotype, suggesting that a common mechanism may dysregulate the balance between these important prosurvival and proapoptotic transcription factors in this HNSCC subset (21, 35). As illustrated in Fig. 5C, our data support a model for the role of the three CK2 subunits in different steps of IKKβ-IκBα-NF-κB activation, and p65 serine536 or serine529 phosphorylation (Fig. 2). Strikingly, CK2α exhibited a prominent function in enhancing transactivation of NF-κB prosurvival genes while inhibiting that of proapoptotic genes TP53 and TAp63, associated with its enhanced localization in the nucleus (Fig. 1, 3, 4). Further, CK2α played a key role in modulating growth, apoptosis, migration and chemoresistance, with varying contribution of other CK2 subunits to regulation of genes involved in cell cycle, migration and angiogenesis (Fig. 3 and 4). Consistent with this model, anti-CK2α/α′ ODN nanocapsules demonstrated the translational potential for CK2 targeted therapy to concurrently inhibit NF-κB and induce TP53, and promote cytotoxic antitumor activity in human HNSCC xenografts in vivo (Fig. 6).
Targeting any of the three CK2 subunits reduced transcriptional activation of an NF-κB reporter, but through different mechanisms (Fig. 2), and lead to different consequences for expression of specific genes and features of the malignant phenotype (Fig. 3–5). Canonical signal activation of NF-κB takes place in the cytoplasm through KKα/β/γ complex-mediated IκBα phosphorylation, ubiquitination, and proteasome degradation, which release p65/p50 for nuclear translocation (14, 15) (Fig. 5C). Consistent with this, CK2α′ and β were predominantly expressed in the cytoplasm and played a greater role in degradation of IκBα (Fig. 2A) and enhancement of p65 DNA binding (Fig. 2C). The effects of CK2α′ and β knockdown on expression of an N-terminal IκBα-Luc fusion protein containing phosphorylation sites for IKKβ supported our previous findings demonstrating that CK2 is a key upstream kinase promoting activation of IKKβ, as well as phosphorylation and degradation of IκBα (17, 18). Furthermore, when IκBα expression was increased upon knockdown of CK2α′ or β, we observed a corresponding decrease in p65 DNA binding, consistent with the reported role of IκBα in promoting turnover and nuclear export of DNA bound p65 (14). Although CK2α was detected at lower levels in the cytoplasm and played a lesser role in IκBα degradation, CK2α or β knockdown demonstrated a greater inhibitory effect on the TNF-α induced increase in total and serine536 phosphorylated p65 in the nucleus (Fig. 2B). These results suggest that the interaction of different CK2α subunits with CK2β may differentially contribute to functions of IKKβ in promoting degradation of IκBα, and nuclear translocation and phosphorylation of p65 at serine536. As expected, knockdown of all three subunits attenuated phosphorylation of p65 at serine529 (Fig. 2B), previously reported to be a direct target of the CK2 holoenzyme (20).
Remarkably, CK2α knockdown revealed that this subunit has an essential role in promoting expression of multiple NF-κB regulated proliferative (CCND1), anti-apoptotic (BCL2 and BCL-XL) and integrin (ITGA3 and ITGB4) genes, while inhibiting expression of growth arrest and proapoptotic genes TP53 and TAp63 (Fig. 3). By contrast CK2α′ knockdown strongly modulated expression of ITGA6 and VEGF without significantly affecting TP53 or TAp63. Knockdown of CK2α but not α′ had the greatest effect on proliferation, apoptosis, chemosensitivity, and migration, consistent with their effects on expression and the important role of these genes in the malignant phenotype (21, 22, 32, 34-39). These differences were linked with the predominant nuclear distribution and apparent role of CK2α in repressing DNA binding of the non-transactivating p50 subunit, without attenuating binding of the transactivating p65 subunit (Fig. 2C). These results, and previous demonstration that CK2α is associated with nuclear chromatin (10), suggest that it is involved in novel nuclear and transcriptional mechanisms that orchestrate inactivation of p53 and activation of NF-κB and its target genes in HNSCC.
Although both and β were previously implicated in the regulation of G1 and G2/M phases of cell cycle in lower eukaryotes and other cell types (4), here we showed distinct cellular functions of CK2 subunits in modulation of cell cycle. Knockdown of CK2α showed the most profound inhibition of proliferation, blocking cells in the G1 phase (Fig. 4A–C), and promoting cell death with increased Sub G0 DNA fragmentation in vitro (Fig. 4B), consistent with CK2α regulated TP53 and Cyclin D1 expression and activities (Fig. 3 and 6). However, the role of both CK2α′ and CK2β in G1 arrest is less clear, although CK2β could modulate p21 and p27 activities as previously suggested (40, 41). CK2β is the major regulator for G2/M transition, consistent with its holoenzyme-independent interaction with molecules controlling G2/M, such as the Chks (40, 41). A recent report also supports our observation, that downregulated CK2β results in delayed cycle progression at the onset of mitosis, through stabilization of Wee1 and increased phosphorylation of CDK1 (42).
Knockdown of either β alone significantly blocked cell migration (Fig. 5A, B), consistent with their effects on the expression of multiple cell adhesion molecules and TAp63 (Fig. 3), which was previously shown to affect cell adhesion and migration (43). We previously showed that α3β1, α6β1, and integrins are overexpressed and involved in cell adhesion to laminins and collagens, is a marker for decreased survival in patients with HNSCC (38, 39). CK2α, but not CK2α′, promoted migration, which could be due to its regulation of β4 gene expression (Fig. 3). Restricted expression of integrin β4, together with α6, is found near the basal membrane zone associated with the desmosomes in normal stratified and transitional epithelia. Integrin α6β4 has been shown to play a pivotal role in promoting carcinoma invasion, and an aggressive clinical course in HNSCC (38, 39). Although CK2α′ modulated integrin α6, it had no apparent effect on cell migration in vitro. However, we could not exclude the role of CK2α′ in vivo, as previous studies suggested (25–27, 44). CK2α and α′ also regulated angiogenesis factor VEGF gene expression (Fig. 4A). VEGF is an important angiogenesis factor overexpressed by HNSCC, detected in cell culture supernatants, patient serum, and tumor specimens (34), and its effects are appreciated more in the interaction between tumor cell and stroma in the tumor microenvironment. Consistent with this, overexpression of mRNA was identified in metastatic colon cancer specimens (44), and is elevated in the paired UM-SCC 22A and 22B cell lines from a patient with lymph node metastasis (Fig. 1). Further, as prior studies have shown that knockdown of CK2α alone could not effectively block tumor growth in vivo, blocking both α′ is an essential strategy for targeted therapy (26).
These findings regarding function of CK2 are potentially important in developing therapeutics for other cancers as well as HNSCC. Overexpression of CK2 has been observed in many types of solid tumors including prostate, breast, kidney, and lung, as well as in hematopoietic malignancies, such as acute myeloid leukemia (2, 3, 6, 40). In transgenic mouse models, overexpression of CK2 subunits promotes transformation and development of mammary and lymphoid malignancies (45, 46). Additionally, increases in oncogenic activity and progression of tumorigenesis have been identified when CK2 overexpression has been combined with deficient tumor suppressor gene function, such as in TP53(−/−) deficient mice (47), or with aberrant activation of oncogenes, such as c-Myc (48), H-Ras (49), and Her-2/neu (19). Increased CK2 activity has also been implicated in a variety of tumor processes including invasion and metastasis. Recently, in a rat breast cancer model, ectopic co-expression of the NF-κB transcription factor family member c-Rel and CK2α in untransformed mammary epithelial cells was sufficient to transform tumors and induce an epithelial to mesenchymal transition, which lead to a more invasive phenotype (50). Our data suggest the role of CK2 in regulation of NF-κB and TP53 and key genes and phenotypic features merits investigation in other cancers.
Unger and Ahmed previously developed an anti-CK2α/α′ ODN that evoked a strong apoptotic response, with ODN against CK2α/α′ being more potent than targeting CK2β, in a variety of prostate carcinoma models (26, 27). Recent novel approaches, such as the delivery of the anti-CK2α/α′ ODN encapsulated in sub-50 nm (s-50 nm) tenascin or tenfibgen nanocapsules, have become available for more specific targeting of CK2 in vivo. Here, we present the data from two HNSCC xenograft models, where significant reduction of primary tumors were observed with increased TUNEL and caspase 3 staining in tumor specimens after the anti-CK2α/α′ ODN treatment (Fig. 6A). In support of the effects of CK2 modulation on NF-κB signaling, gene expression and malignant phenotype observed in vitro, immunohistochemical staining revealed decreased α′, total and phospho-NF-κB, and expression of proteins Cyclin D1, BCL-XL, and BCL2, while TP53 and p63 protein expression were increased after treatment. Our in vivo investigation confirmed that CK2 activates NF-κB and target genes, while decreasing expression of pro-apoptotic TP53 family members, thereby regulating a broad gene program that promotes proliferation, survival, and malignant progression of HNSCC. Since overexpression of CK2 subunits has been documented in a variety of other types of cancers, including prostate, breast, lung and kidney (5–8, 19), and the delivery of the anti-CK2α/α′ ODN in nanocapsules also successfully suppressed prostate tumor growth in the mouse model (25–28), knockdown of CK2 using nanocapsules technology could greatly enhance the tissue specific targeting and therapeutic efficacy in a broad range of cancers. Our study suggests that CK2 subunits warrant further investigation as the critical indicator of malignant phenotype and aggressiveness of HNSCC and other solid tumors in future clinical trials. The molecular mechanisms and in vivo effects of targeting CK2α/α′ in the inhibition of HNSCC progression and enhancement of the efficacy of cisplatin-based chemotherapy are currently under investigation in our laboratories.
Translational Relevance.
We previously reported that protein kinase CK2 contributes to aberrant NF-κB activation, a critical transcription factor promoting head and neck squamous cell carcinoma (HNSCC) survival and chemoresistance. Here, we demonstrate that CK2 subunits are overexpressed and CK2α exhibits a predominantly nuclear distribution in HNSCC cell lines and tissue specimens. CK2 subunit knockdown, especially CK2α, differentially modulated NF-κB signal activation and downstream molecules, expression of proapoptotic genes TP53 and TAp63, the malignant phenotype, and cisplatin sensitivity in HNSCC cells in vitro. Using sub-50 nm tenfibgen nanocapsules to deliver anti-CK2α/α′, oligodeoxynucleotide, we demonstrate significant suppression of tumor growth, down-modulation of NF-κB, and up-modulation of TP53 family members, as well as their regulated proteins in vivo. These observations support the notion that CK2 contributes to the malignant phenotype and aggressiveness of HNSCC, and that targeting CK2α/α′ effectively inhibits tumor proliferation, survival, and migration, as well as enhances the efficacy of cisplatin-based chemotherapy.
Supplementary Material
Acknowledgements
The authors thank Drs. Liesl Nottingham, Jay Friedman, Bin Yan, Ning T Yeh, Mr. Jonah Cohen, and Chris Silvin (NIH) for their technical assistance and scientific suggestions. The authors are very grateful for Nancy E. McDermott, DMD, MD, Staff Oral & Maxillofacial Surgeon, and her team at NIDCR/NIH, who provided gingival specimens for the immunohistochemical staining. We also express appreciation to Justin Ricker (Abbott Labs, Abbott Park, IL), and Ms. Cindy Clark (NIH Library) for providing helpful and critical comments on the manuscript.
This work was supported by NIH/NIDCD intramural research projects ZIA-DC-000016, ZIA-DC-000073-01, R43CA-9936601 to GMU, and by VA Merit Review Funds and UO1-CA 15062 to KA.
Research funded by Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc.
Footnotes
Disclosure of Potential Conflict of Interest Gretchen Unger is the CSO of GeneSegues, Inc., Chaska, MN. Drs. Ahmed and Unger are authors on the patent application for anti-CK2 ODN nanocapsules filed by University of Minnesota.
References
- 1.Pinna LA. Protein kinase CK2: a challenge to canons. J Cell Sci. 2002;115:3873–8. doi: 10.1242/jcs.00074. [DOI] [PubMed] [Google Scholar]
- 2.Guerra B, Issinger OG. Protein kinase CK2 in human diseases. Curr Med Chem. 2008;15:1870–86. doi: 10.2174/092986708785132933. [DOI] [PubMed] [Google Scholar]
- 3.Tawfic S, Yu S, Wang H, Faust R, Davis A, Ahmed K. Protein kinase CK2 signal in neoplasia. Histol Histopathol. 2001;16:573–82. doi: 10.14670/HH-16.573. [DOI] [PubMed] [Google Scholar]
- 4.Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad KA, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2--a key suppressor of apoptosis. Adv Enzyme Regul. 2008;48:179–87. doi: 10.1016/j.advenzreg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trembley JH, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell Mol Life Sci. 2009;66:1858–67. doi: 10.1007/s00018-009-9154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra B, Boldyreff B, Sarno S, Cesaro L, Issinger OG, Pinna LA. CK2: a protein kinase in need of control. Pharmacol Ther. 1999;82:303–13. doi: 10.1016/s0163-7258(98)00064-3. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–30. doi: 10.1016/s0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 10.Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: association with malignant transformation. Cancer Lett. 1996;101:31–5. doi: 10.1016/0304-3835(96)04110-9. [DOI] [PubMed] [Google Scholar]
- 11.Gapany M, Faust RA, Tawfic S, Davis A, Adams GL, Ahmed K. Association of elevated protein kinase CK2 activity with aggressive behavior of squamous cell carcinoma of the head and neck. Mol Med. 1995;1:659–66. [PMC free article] [PubMed] [Google Scholar]
- 12.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–68. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 13.Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076–82. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 14.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Allen CT, Ricker JL, Chen Z, Van Waes C. Role of activated nuclear factor-kappaB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck. 2007;29:959–71. doi: 10.1002/hed.20615. [DOI] [PubMed] [Google Scholar]
- 16.Brown M, Cohen J, Arun P, Chen Z, Van Waes C. NF-kappaB in carcinoma therapy and prevention. Expert Opin Ther Targets. 2008;12:1109–22. doi: 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M, Yeh J, Van Waes C. Protein kinase casein kinase 2 mediates inhibitor-kappaB kinase and aberrant nuclear factor-kappaB activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res. 2006;66:6722–31. doi: 10.1158/0008-5472.CAN-05-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, Channavajhala P, Seldin DC, Sonenshein GE. Phosphorylation by the protein kinase CK2 promotes calpain-mediated degradation of IkappaBalpha. J Immunol. 2001;167:4919–25. doi: 10.4049/jimmunol.167.9.4919. [DOI] [PubMed] [Google Scholar]
- 19.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Protein kinase CK2 promotes aberrant activation of nuclear factor-kappaB, transformed phenotype, and survival of breast cancer cells. Cancer Res. 2002;62:6770–8. [PubMed] [Google Scholar]
- 20.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr. Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–7. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 21.Lee TL, Yang XP, Yan B, et al. A novel nuclear factor-kappaB gene signature is differentially expressed in head and neck squamous cell carcinomas in association with TP53 status. Clin Cancer Res. 2007;13:5680–91. doi: 10.1158/1078-0432.CCR-07-0670. [DOI] [PubMed] [Google Scholar]
- 22.Friedman J, Nottingham L, Duggal P, et al. Deficient TP53 expression, function, and cisplatin sensitivity are restored by quinacrine in head and neck cancer. Clin Cancer Res. 2007;13:6568–78. doi: 10.1158/1078-0432.CCR-07-1591. [DOI] [PubMed] [Google Scholar]
- 23.Keller DM, Zeng X, Wang Y, et al. A DNA damage-induced p53 serine 392 kinase complex contains CK2, hSpt16, and SSRP1. Mol Cell. 2001;7:283–92. doi: 10.1016/s1097-2765(01)00176-9. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Keller DM, Scott JD, Lu H. CK2 phosphorylates SSRP1 and inhibits its DNA-binding activity. J Biol Chem. 2005;280:11869–75. doi: 10.1074/jbc.M413944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slaton JW, Unger GM, Sloper DT, Davis AT, Ahmed K. Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model. Mol Cancer Res. 2004;2:712–21. [PubMed] [Google Scholar]
- 26.Unger GM, Davis AT, Slaton JW, Ahmed K. Protein kinase CK2 as regulator of cell survival: implications for cancer therapy. Curr Cancer Drug Targets. 2004;4:77–84. doi: 10.2174/1568009043481687. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad KA, Wang G, Slaton J, Unger G, Ahmed K. Targeting CK2 for cancer therapy. Anticancer Drugs. 2005;16:1037–43. doi: 10.1097/00001813-200511000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Unger G, Ahmad KA, Slaton JW, Ahmed K. Downregulation of CK2 induces apoptosis in cancer cells--a potential approach to cancer therapy. Mol Cell Biochem. 2005;274:77–84. doi: 10.1007/s11010-005-3077-1. [DOI] [PubMed] [Google Scholar]
- 29.Fukuhara Y, Takeshima T, Kashiwaya Y, Shimoda K, Ishitani R, Nakashima K. GAPDH knockdown rescues mesencephalic dopaminergic neurons from MPP+ -induced apoptosis. Neuroreport. 2001;12:2049–52. doi: 10.1097/00001756-200107030-00051. [DOI] [PubMed] [Google Scholar]
- 30.Unger GM. Metal ion-treated biocompatible polymers useful for nanoparticles. Jun 14, 2007. PCT/US08/67158. [Google Scholar]
- 31.Kren BT, Unger GM, Sjeklocha L, et al. Nanocapsule-delivered Sleeping Beauty mediates therapeutic Factor VIII expression in liver sinusoidal endothelial cells of hemophilia A mice. J Clin Invest. 2009;119:2086–99. doi: 10.1172/JCI34332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Ricker JL, Malhotra PS, et al. Differential bortezomib sensitivity in head and neck cancer lines corresponds to proteasome, nuclear factor-kappaB and activator protein-1 related mechanisms. Mol Cancer Ther. 2008;7:1949–60. doi: 10.1158/1535-7163.MCT-07-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam LT, Davis RE, Pierce J, et al. Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res. 2005;11:28–40. [PubMed] [Google Scholar]
- 34.Chen Z, Malhotra PS, Thomas GR, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5:1369–79. [PubMed] [Google Scholar]
- 35.Lee TL, Yeh J, Friedman J, et al. A signal network involving coactivated NF-kappaB and STAT3 and altered p53 modulates BAX/BCL-XL expression and promotes cell survival of head and neck squamous cell carcinomas. Int J Cancer. 2008;122:1987–98. doi: 10.1002/ijc.23324. [DOI] [PubMed] [Google Scholar]
- 36.Deyoung MP, Ellisen LW. p63 and p73 in human cancer: defining the network. Oncogene. 2007;26:5169–83. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 37.Pena JC, Thompson CB, Recant W, Vokes EE, Rudin CM. Bcl-xL and Bcl-2 expression in squamous cell carcinoma of the head and neck. Cancer. 1999;85:164–70. doi: 10.1002/(sici)1097-0142(19990101)85:1<164::aid-cncr23>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.Van Waes C, Kozarsky KF, Warren AB, et al. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin alpha 6 beta 4. Cancer Res. 1991;51:2395–402. [PubMed] [Google Scholar]
- 39.Van Waes C. Cell adhesion and regulatory molecules involved in tumor formation, hemostasis, and wound healing. Head Neck. 1995;17:140–7. doi: 10.1002/hed.2880170212. [DOI] [PubMed] [Google Scholar]
- 40.Duncan JS, Litchfield DW. Too much of a good thing: the role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta. 2008;1784:33–47. doi: 10.1016/j.bbapap.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 41.St-Denis NA, Litchfield DW. Protein kinase CK2 in health and disease: From birth to death: the role of protein kinase CK2 in the regulation of cell proliferation and survival. Cell Mol Life Sci. 2009;66:1817–29. doi: 10.1007/s00018-009-9150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yde CW, Olsen BB, Meek D, Watanabe N, Guerra B. The regulatory beta-subunit of protein kinase CK2 regulates cell-cycle progression at the onset of mitosis. Oncogene. 2008;27:4986–97. doi: 10.1038/onc.2008.146. [DOI] [PubMed] [Google Scholar]
- 43.Carroll DK, Carroll JS, Leong CO, et al. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551–61. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- 44.Saha S, Bardelli A, Buckhaults P, et al. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294:1343–6. doi: 10.1126/science.1065817. [DOI] [PubMed] [Google Scholar]
- 45.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–57. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- 46.Seldin DC, Leder P. Casein kinase II alpha transgene-induced murine lymphoma: relation to theileriosis in cattle. Science. 1995;267:894–7. doi: 10.1126/science.7846532. [DOI] [PubMed] [Google Scholar]
- 47.Landesman-Bollag E, Channavajhala PL, Cardiff RD, Seldin DC. p53 deficiency and misexpression of protein kinase CK2alpha collaborate in the development of thymic lymphomas in mice. Oncogene. 1998;16:2965–74. doi: 10.1038/sj.onc.1201854. [DOI] [PubMed] [Google Scholar]
- 48.Channavajhala P, Seldin DC. Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 2002;21:5280–8. doi: 10.1038/sj.onc.1205640. [DOI] [PubMed] [Google Scholar]
- 49.Orlandini M, Semplici F, Ferruzzi R, Meggio F, Pinna LA, Oliviero S. Protein kinase CK2alpha' is induced by serum as a delayed early gene and cooperates with Haras in fibroblast transformation. J Biol Chem. 1998;273:21291–7. doi: 10.1074/jbc.273.33.21291. [DOI] [PubMed] [Google Scholar]
- 50.Belguise K, Guo S, Yang S, et al. Green tea polyphenols reverse cooperation between c-Rel and CK2 that induces the aryl hydrocarbon receptor, slug, and an invasive phenotype. Cancer Res. 2007;67:11742–50. doi: 10.1158/0008-5472.CAN-07-2730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.