Abstract
The basoapical organization of monolayered epithelia is defined by the presence of hemidesmosomes at the basal cellular pole, where the cell makes contacts with the basement membrane, and tight junctions at the opposite apical pole. In the mammary gland, tight junctions seal cell–cell contacts against the lumen and separate the apical and basolateral cell membranes. This separation is critical to organize intracellular signaling pathways and the cytoskeleton. The study of the impact of the highly organized apical pole, and notably apical polarity regulators (Crb complex, Par complex, and Scrib, Dlg, Lgl proteins) and tight junction proteins on cell phenotype and gene expression has revealed an intricate relationship between apical polarity and the cell nucleus. The goal of this review is to highlight the role of the apical pole of the tissue polarity axis in the epigenetic control of tissue phenotype. The organization of the apical pole and its importance in mammary homeostasis and tumorigenesis will be emphasized before presenting how apical polarity proteins impact gene expression indirectly, by influencing signal transduction and the location of transcription regulators, and directly, by participating in chromatin-associated complexes. The relationship between apical polarity and cell nucleus organizations might explain how apical polarity proteins could switch from nuclear repressors to nuclear promoters of cancerous behavior following alterations in the apical pole. The impact of apical polarity proteins on epigenetic mechanisms of gene expression will be discussed in light of increased evidence supporting a role for apical polarity in the fate of breast neoplasms.
Keywords: Apical polarity, Tight junction, Chromatin, Cell nucleus, Proliferation, Cancer progression
Introduction
Epigenetics is an evolving concept [1] that can be roughly described as the changes in chromatin structure that control gene expression. However, chromatin structure and gene expression are under the influence of factors, like transcription factors and other coregulators of transcription, housed in the cytoplasm. Therefore epigenetics can also be broadened to include all factors that directly or indirectly exert an effect on chromatin, resulting in a modification of gene transcription. For simplicity we will refer to these factors as epigenetic factors.
Many epigenetic factors have multiple engagements; they can be found in an extranuclear compartment in parallel to their nuclear location, and either shuttle between cytoplasm and nucleus or become free from their cytoplasmic location to travel to the cell nucleus upon specific signals [2]. Signal transducers trapped within the cell cortex may be released upon alteration of cell adhesion complexes and then, trigger pathways that influence gene transcription; however, an increasing body of literature is reporting that scaffold proteins that participate in the making of cell adhesion complexes might also directly impact gene transcription by traveling to the cell nucleus. Cell adhesion complexes are part of a higher order cellular organization referred to as the polarity axis (i.e., a geometric feature of cells originating from the existence of a basal pole—against the extracellular matrix- and an apical pole—generally against a lumen—that contain different sets of proteins and protein complexes) in phenotypically normal epithelial tissue; therefore the epigenetic relationship in not simply between cytoplasmic epigenetic factors and gene transcription complexes, it is between the polarity axis and the cell nucleus, another compartment governed by the higher order organization of its elements [3].
Polarity
Adhesion Complexes and Beyond Polarity is a fascinating feature of all epithelia. It results from the asymmetrical distribution of cell adhesion complexes, culminating with the formation of tight junctions at the tip of the apical pole of cells. This pole is located against the lumen in glandular and ductal structures of the mammary gland. The basal pole, opposite to the apical pole, corresponds to the contact between the cell membrane and extracellular basement membrane molecules. Understandably, this organization is critical for the directional secretion of milk. As described initially by Farquhar and Palade, typically, the lateral cell–cell contacts or junctional complex of the polarity axis include, from apical to basal locations, the tight junctions (or zonula occludens), the adherens junctions (or zonula adhaerens), and the desmosomes (or macula adhaerens; not always present) [4, 5]. Tight junctions separate the apical membrane, characterized by the presence of ion channels and nucleotide receptors [6, 7] from the basolateral portion of the cell membrane, and the combination of tight junctions and adherens junctions forms the apical junctional complex (AJC) (Fig. 1) [8]. Tight junctions are the definite markers of apical polarity (i.e., a structural feature of cells that corresponds to the cellular pole containing specific adhesion complexes formed close to a lumen). They prevent diffusion of cell membrane components, including proteins and lipids, between apical and basolateral plasma membranes [9, 10]. In the mammary gland, apical polarity is organized according to the usual schematic with tight junctions located at the top third of cell–cell contacts toward the lumen. Tight junctions play a critical role in changes in intercellular sealing, culminating with very tight junctions in lactation [11]. The high plasticity of these complexes in the different physiological stages (resting, pregnancy, lactation, involution) is well illustrated by changes in the subtype and/or quantity of apical polarity proteins, notably the claudins [12].
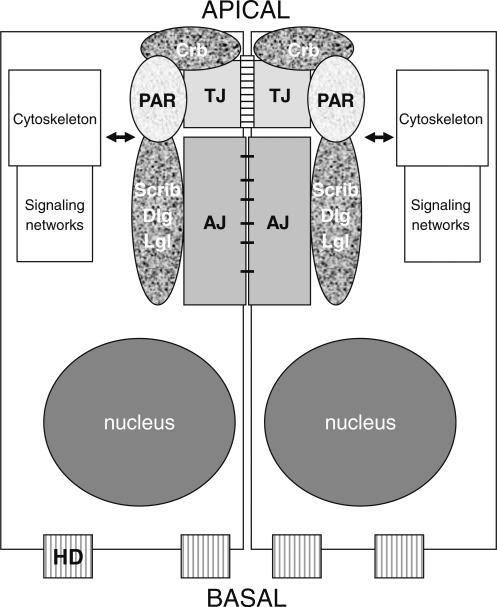
Figure 1.
Organization of the polarity complexes. At the apical side interactions among apical polarity regulatory proteins of the Crb, PAR and Scrib groups and tight junction (TJ) and adherens junction (AJ) proteins stabilize the apical junctional complex (AJC). Interactions of apical polarity proteins with elements of the cytoskeleton and signal transduction networks connect the AJC to other compartments in the cell. HD: hemidesomsomes that make contact with extracellular basement membrane proteins. Although a number of integrins make contact with extracellular matrix molecules, only α6-β4 integrin dimers forming the hemidesmosomes have been linked to basal polarity in breast epithelial cells.
Asymmetry in the organization of the cell membranes in polarized epithelia is reflected in the organization of the cytoskeleton [13–15]. For instance, in polarized breast epithelial cells, actin is associated with the plasma membrane with higher density at the apical pole giving the impression of an apical ring. The cytoskeleton cooperates with apical junctions to maintain epithelial structure; the actin network is dynamic and connected to adhesion complexes via adaptor proteins (e.g., ZO-1 for tight junctions) (for a review refer to [8, 14]).
Nuclear Localization of Proteins of the Apical Pole
Expanding Functional Latitude Tight junctions and adherens junctions consist of transmembrane proteins connected to cytoplasmic plaque or adaptor proteins that control the junction assembly and function [5, 16] (Fig. 1). Surprisingly, most nontransmembrane apical polarity proteins involved in the formation of the AJC have also been observed in the cell nucleus, thus raising the possibility of their potential direct involvement in gene expression control.
The nuclear location of AJC proteins was initially observed with adherens junction representative molecule β-catenin, before progressively including apical polarity regulatory molecules (PAR, Crb, and Scrib groups) and tight junction ZO proteins. Protein trafficking between cytoplasm and nucleus is a highly regulated mechanism of information exchange. As expected, shuttling apical polarity proteins, like for instance ZO proteins, contain nuclear localization and export signals [17]. As described later in this review, apical polarity proteins found in the cell nucleus have been associated with nuclear functions such as gene transcription/repression and DNA repair. The diverse apical polarity proteins represent an emerging family of powerful epigenetic factors that bring unprecedented means to better understand how tissue organization (i.e. the tissue structure that results from the arrangement of cell–cell and cell-extracellular matrix contacts) controls gene expression. An important question to answer is whether the apical polarity-related epigenetic factors act redundantly or provide specific epigenetic signals resulting in different modifications of cell behavior.
How Could Apical Polarity Influence Gene Expression? In addition to binding to cytoskeletal elements, molecules of the AJC make contact with signal transducers like G proteins, kinases and phosphatases, themselves connected to signal transduction networks involved in the regulation of cell proliferation, differentiation and gene expression [16, 18] (Fig. 1). Cytoskeletal molecules and signal transducers influence the organization and function of the gene transcription machinery (for a review refer to [3]), therefore the formation or disruption of the apical pole of epithelial cells that impact signal transduction, should also affect gene expression.
A powerful means for epigenetic control of gene expression is via chromatin remodeling [3]. A wide variety of chromatin-associated complexes regulate chromatin compaction necessary for gene expression control via transcription factors, histone tail modifiers, DNA methylation and ATP-dependent remodeling activities (for a detailed review refer to [19]). Thus, any cytoplasmic factor, including apical polarity proteins or other proteins trapped in junction complexes, that reaches the cell nucleus could influence gene expression control via a breadth of epigenetic mechanisms. Although this aspect is still a largely uncharted territory when dealing with adhesion complexes, certain apical polarity proteins have been found associated with transcription factors at gene promoters, thus giving them the possibility to directly influence chromatin organization.
The importance of apical polarity for epithelial homeostasis necessary for proper organ function is supported by the highly conserved structure of the apical pole and the underlying regulatory mechanisms, and by the demonstration that several AJC proteins can act as tumor suppressors. Here epithelial homeostasis is referring to the expected behavior of epithelial cells, including their arrangement within a tissue, associated with their physiologically normal function in a particular organ. Such behavior is ultimately controlled by gene expression. The important role of apical polarity in epithelial homeostasis is no exception in the mammary gland where tissue polarity has been found to be altered early during cancer development, as shown notably by changes in the position of the cell nucleus and formation of multilayers of epithelial cells [20]. Moreover, as detailed later in this review, we and others have reported findings linking tight junction organization and/or specific apical polarity regulators and tight junction proteins to the control of mammary cell proliferation.
In this review, we discuss how proteins located specifically within the apical polarity complex (i.e., the complex formed by apical polarity regulators and tight junction-related proteins) could influence steps critical for breast cancer development by acting at the chromatin level, and whether cell fate and thus, breast cancer prognosis might be differently impacted depending on the type of apical polarity complex protein that travels to the cell nucleus. The influence of apical polarity on epithelial homeostasis is presented in a first part in order to comprehend how alterations in the organization of the apical pole could contribute to breast cancer development. In a second part, the relationship between apical polarity and gene expression control is scrutinized. In particular, the indirect and direct contributions of proteins that organize the apical pole are examined in order to unravel the mechanisms underlying the influence of the apical cellular pole on nuclear epigenetic functions.
The Place of Apical Polarity in Breast Cancer
Developmental Aspects of Apical Polarity: a Critical Role for Epithelial Homeostasis
The formation of apical polarity is hierarchical and interactive. Three major and highly conserved groups of regulatory proteins have been identified in Drosophila and Caenorhabditis Elegans to contribute to apical polarity formation by controlling the location of tight junctions at the apical pole of cells (refer to [21–23]). These groups of proteins called stardust (std), Bazooka (Baz) and Scribble (Scrib) in Drosophila, have also been identified in the epithelia of vertebrates and are made of Crumbs3/PALS-1/PATJ (std or Crb complex), Par-3/Par-6/aPKC (Baz or Par complex), and proteins Scrib, Discs-large (Dlg) and Lethal giant larvae (Lgl) (refer to [21]).
The Interactive Nature of Apical Polarity Building
The current view, based on work mainly performed in nonmammals, is that apical polarity is formed upon the recruitment of the Par complex via adherens junctions. The Par complex recruits the Crb complex to the apical cell membrane, which, in turn, forces tight junctions to remain lateroapical (right underneath the Crb complex) by maintaining the Par complex to lateroapical cell–cell contacts. The basolateral location of Scrib, Dlg and Lgl proteins prevents the propagation of tight junctions toward the basal side of cells due to Par complex activity and contributes to the maintenance of apical polarity by preserving the basolateral side of cells. Finally, the Crb complex antagonizes the activity of Scrib, DLg and Lgl, thus preserving the apical cell membrane.
Interactions among members of apical polarity complexes play a critical role in the development of apical polarity in mammalian cells. Crumbs3 (Crb3) is thought to recruit and stabilize PALS1 [24]. In turn, PALS1 mediates the association between Crumbs3 and PATJ and binds Par-6 [25]. The interaction between Par-6 and PALS1 is regulated by GTPase Cdc42, a member of the Par complex that binds Par-6 [25, 26]. Reports from work performed in Drosophila also indicate that Cdc42 activates the recruitment of kinase aPKC by Par-6, which is a step critical for the formation of tight junctions, and that aPKC controls apical polarity formation by phosphorylating Lgl and Crb [27, 28]. Both Par-3 and Par-6 interact with aPKC in mammalian cells [26].
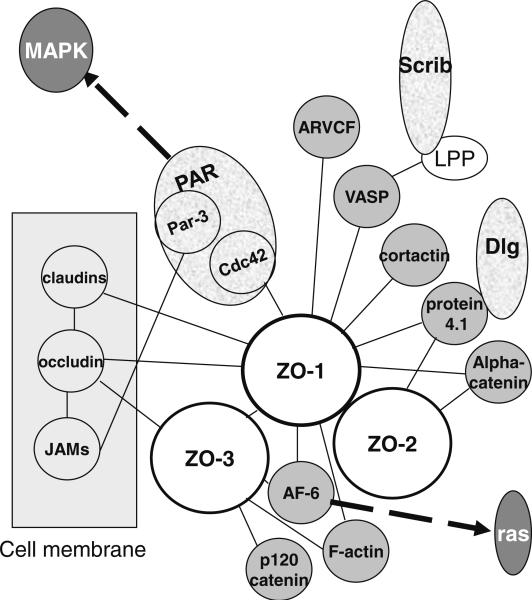
The series of interaction continues with tight junction molecules. Transmembrane JAMs (junctional adhesion molecules) appear essential for tight junction assembly [29, 30]. They associate with Par-3 and membrane-associated guanylate kinase (MAGUK) ZO subfamily of proteins. Other transmembrane tight junction proteins, claudins and occludin, also bind to ZO proteins. ZO proteins are considered scaffolding proteins because they bind to a plethora of molecules including cytoskeletal elements and thus, play a central role in tight junction maintenance [14–16, 18] (Fig. 2).
Figure 2.
Diagram of some of the possible ligands of ZO proteins within the apical junctional complex. ZO-1 can heterodimerize either with ZO-2 or with ZO-3. Proteins indicated in black in a dark gray circle are involved in cytoskeleton organization. Links to specific pathways (labeled in white in the darkest gray circles) important for breast cancer development are shown by discontinuous arrows. Of note: ZO-1 interacts with Cdc42 which is part of the PAR complex and LPP interacts with VASP and apical polarity regulatory protein Scrib.
In light of these numerous interactions, it is understandable that any change in the presence of these proteins at the apical pole might impact the interaction network and trigger signaling. Even, too much or too little of these proteins can alter the basoapical polarity axis by perturbing the proper placement of cell–cell complexes and membrane domains and/or altering the functionality and stability of cell–cell junctions. Such tight regulation of apical polarity is an indication of how sensitive these structures might be to extracellular conditions as well as internal changes.
Apical Polarity-Based Control of Mammary Function
It will come to no surprise that apically localized tight junctions play a critical role in the function of the powerful glandular activity of the mammary epithelium. Most of the known apical polarity proteins have been observed in breast epithelial cells in culture and in vivo [23, 24, 31, 32]. A direct impact of Par-3 on mammary morphogenesis was recently demonstrated in mice. Indeed the proper formation of ductal structures was found to depend on the binding of Par-3 to aPKC. Par-3 also affected progenitor cell renewal [33].
Interestingly, tight junction structure is highly dynamic in the breast epithelium; it loosens in nonlactating gland, as shown by fewer interconnections seen in electron microscopy, compared to the very tight organization necessary to prevent leakiness during lactation [11, 34]. The composition of transmembrane proteins of tight junctions, notably claudins, varies with the physiological stage, which accounts for changes in sealing capacity [12]. Tight junction permeability increases with milk stasis suggesting that environmental changes, such as pressure, rapidly affect apical organization. Tight junction permeability is also under the control of hormones and growth factors like glucocorticoids and TGF-β, respectively. An increase in the amount of ZO-1 was observed upon treatment with dexamethasone that induced closure of tight junctions and synthesis of milk components. In contrast, TGF-β prevented the closure of tight junctions induced by dexamethasone; this effect was accompanied with a change in the distribution of ZO-1 and cell cycling (for a review refer to [11]). More recent findings implicated the Rho pathway in the control of tight junction sealing response to glucocorticoids; however, the inhibitory effect of Rho effector kinase PKN1 on glucocorticoid stimulation was not accompanied with striking distribution changes for ZO-1 or its binding partner occludin [35]. Knowing whether modifications in the composition of the whole apical polarity complex accompany these effects would greatly clarify the molecular mechanisms associated with tight junction leakiness and possibly unravel changes in gene expression that might underlie tight-junction related cell cycling control.
Apical Polarity as a Tumor Suppressor
Interest in the possibility that apical polarity acts as a barrier to cancer development spurred originally from work done in Drosophila [36, 37]. In particular, it was elegantly shown that loss of expression of each of the three proteins involved in protecting the organization of the basolateral membrane, Dlg, Scrib, and Lgl, prevented the establishment of polarity in the drosophila embryonic epithelium and triggered tumor development [38]. All three series of proteins necessary for the formation of apical polarity, including Human Crb, and Par complexes and the Scrib group, and a number of tight junction proteins have also been involved in human malignancies [39–47]. Interestingly, apical polarity complex proteins do not seem to act all in a similar fashion as tumor suppressors. Nevertheless, their action has mainly been correlated with two major aspects of cancer: promotion of a proliferative phenotype and invasion.
Involvement of Apical Polarity Regulators
In Drosophila, Scrib has been involved in neoplastic growth in the eye in collaboration with Ras or Notch [48]. A demonstration that human Scrib plays a role in protecting tissue homeostasis was brought by experiments in which it rescues flies from tumorigenesis [49]. The tumor suppressive potential of Scrib was proposed to rely on the stabilization of α and β-catenins that bind E-cadherin at the apical cortex of cells and on its effect on E-cadherin function in mammalian systems. Such mechanism was identified when studying Scrib's role in the regulation of cell adhesion and migration in the model of Mardin-Darby Canine kidney (MDCK) epithelial cells [45]. This is an important aspect of the potential contribution of apical polarity to tumor development and progression since Scrib influences adherens junction proteins known to play a role in the aggressive behavior of breast cancer cells. In another study Scrib was found to bind to LPP, a zyxin-related protein which has been described as a partner in fusion proteins associated with different types of cancers [50]. A possible way to decrease Scrib expression and prevent its tumor suppression effect is via ubiquitination. Indeed human Scrib, as well as Dlg, were targeted for degradation via ubiquitination upon expression of human papillomavirus protein E6 in cervical carcinoma cells [51]. E6 expression also affected tight junction organization as shown by the change in localization of ZO-1 [41].
The impact of Scrib on the development of breast neoplasia has been investigated but these studies generated different opinions. One report corroborates results obtained with MDCK cells that suggest a role for Scrib in cell migration. Non-neoplastic mammary epithelial MCF10A cells depleted from Scrib failed to regulate Cdc42 and to migrate due to the lack of polarization at the leading edge; there was no effect on basal polarity, as shown by α6-integrin staining, and on the apical location of Golgi marker GP130. No effect on proliferation was observed as well [52]. One of the conclusions was that in contrast to Drosophila, Scrib is not critical for basoapical polarity formation in human mammary epithelial cells. Such conclusion might be premature since it is well established that apical polarity is already compromised in MCF10A cells. Indeed most of the MCF10A cell lines notoriously lack tight junctions upon forming acini in 3D culture [24, 53].
Another report demonstrated that loss of Scrib expression in MCF10A cells led to loss of apically polarized distribution of Golgi marker, and inhibition of acinus-like morphogenesis in 3D culture. Epithelial outgrowth and tumor formation was also induced by murine epithelial cells depleted from Scrib in mice [54]. It was further shown that Scrib loss associated with myc overexpression (a common phenomenon in breast cancer) leads to tumor formation in 3D culture. Furthermore, the effects of Scrib loss were mimicked by Scrib release from the cell membrane indicating that alteration in Scrib compartmentalization was sufficient to induce phenotypic changes. It was also reported that Scrib is absent from cell–cell location already in preinvasive tumors (ductal carcinoma in situ) supporting a potentially important role for Scrib in breast cancer development [54]. Thus far, the role of Scrib on cell migration was only shown in non-neoplastic cells and awaits further demonstration in a tumor context.
Changes in the equilibrium of molecules located in the AJC might play a critical role in tumor development. Hence, tumor promoter effects could occur via the targeted disruption of interactions within the Par complex. For instance PKCξII (a truncated form of apical polarity regulator aPKCξ that lacks catalytic activity) can disrupt tight junctions, but not adherens junctions, by interacting with Par-6. It is thought that such disruption is linked to prevention of aPKC accumulation and activation at tight junction. The resulting effect was cell proliferation in murine mammary epithelial cells, leading to the production of multiple cell layers [31]. The PKCξII form has been detected in MCF10A and MCF7 human breast epithelial cells, suggesting that the influence of this pathway on breast cancer development might be important to study. It was also reported that disruption of the Par3-Par6-aPKC-Cdc42 complex upon activation of ErbB2 in canine kidney MDCK and human mammary MCF10A cells leads to the formation of mutilayered epithelial structures resulting from the association of ErbB2 with Par6-aPKC [55].
Involvement of Tight Junctions
Tight junction proteins have also been implicated in the control of tumor development when their location at cell membranes is prevented. A form of ZO-1 that cannot bind the cell membrane was shown to trigger tumorigenicity in MDCK cells thus, suggesting that the membrane localization of this protein could act as a tumor suppressor [56]. Interestingly, in mammary epithelial cells, a form of ZO-1 that cannot bind the cell membrane was associated with the activation of proteinase MT-MMP1 [46]. The possibility that loss of membrane localization of ZO-1 triggers invasive potentials is supported by the observation that ZO-1 is found at the plasma membrane in noninvasive tumor cells but it is located in the cytoplasm of invasive cells [46]. In addition, ZO-1 was observed to be lost in a high percentage of breast cancer cell lines and primary tumors [40, 57]. Finally ZO-1 expression could be positively correlated to glandular differentiation in tumors and also within different regions of the tumor. Thus, progressive alterations in ZO-1 distribution might participate in breast tumor progression.
Another tight-junction scaffolding protein, ZO-2, was also associated with cancer development, as suggested by the inhibition of transformation activity of several viral proteins in CREF fibroblasts upon the increase of ZO-2 expression [44]. Interestingly, ZO-2 is targeted by the oncogenic adenoviral E4-ORF1 protein that only leads to mammary cancer in animals. In support for a critical role of ZO-2 in breast cancer development, its expression was found to be lost or greatly decreased in breast cancer samples and cell lines. ZO-2 loss (especially the A-isoform) accompanied ductal types of carcinoma and was already obvious in DCIS, suggesting a link between an alteration of ZO-2 function and the deregulation of ductal homeostasis [42].
The loss of apical polarity proteins from the cell membrane appears to trigger key aspects of breast cancer cell behavior like transformation, proliferation and invasive potential. However, a modification of the breast acinar phenotype could already be unraveled upon loss of the strict apical location of ZO-1 without its absence from the cell membrane. Our findings using non-neoplastic breast epithelial S1 HMT-3522 cells capable of forming basoapically polarized acini with lateroapical tight junctions in three-dimensional (3D) culture support the idea that the loss of apical polarity, and notably proper tight junction protein location, is paramount for very early stages of breast tumor development. Indeed, only acini in which tight junction marker ZO-1 was relocated away from the apical domain of the cell membrane could be pushed into the cell cycle upon induction of chromatin alterations [58] or stimulation with defined growth factors (Lelièvre laboratory unpublished data). Whether apical polarity proteins other than ZO-1 need to leave the cell membrane area to trigger such an effect remains to be clarified.
The findings linked to the role of apical polarity proteins in breast cancer development so far indicate that depending on the type of apical polarity protein and the degree of its alteration, the resulting phenotypes might be different. Surprisingly, alterations in some of the apical polarity proteins can be linked to specific types of breast cancer as discussed in the next section.
A Role for Apical Polarity Proteins in Aggressive Types of Cancer
From Synergism with Oncogenes to Progressive Alterations in Expression
One striking observation is the association between alterations in proteins of the Scrib group and aggressive forms of cancer. For instance, the loss of hDlg has been associated with a more undifferentiated phenotype of cervical cancer cells [59]. Similarly loss of Scrib function leads to an increase in tumor aggressiveness in Drosophila [60]. The latter observation is transposable to human cancers since the absence of Scrib1 correlates with progression of uterine cervical carcinoma from precursor lesions to invasive tumors [61].
The importance of the alteration of Scrib in the development of an aggressive form of tumors is beautifully shown by experiments revealing that mutated Scrib associated with ras activation in Drosophila leads to metastatic behavior of eye disc cells because it suppresses E-cadherin expression. If there is only ras activation, it leads to overproliferation [60]. A Similar effect was observed when mutating Lgl, Dlg, Cdc42, baz (Par3) or std (PALS1), suggesting that apical polarity protects against the activation of yet-to-be-known pathways necessary for the invasive phenotype. In another study, still in Drosophila, it was observed that mutated Scrib cooperates with Ras or with Notch, two oncogenes associated with breast cancer, leading to synergistic overgrowth of larval tissue [48]. In the sole Scrib mutants overgrowth remained minimal due to the proapoptotic activity of JNK. Thus, the alteration of apical polarity works in synergy with other proteins and pathways to foster tumor development.
Loss of expression of the tight junction proteins has been associated with specific breast cancer phenotypes. For instance, ZO-1 has been correlated with poorly differentiated breast invasive ductal carcinoma (IDC). Specifically, loss of ZO-1 coincided with the lack of glandular differentiation in IDC rather than with other differentiation characteristics (i.e., nuclear grade and mitotic index) [40]. The decrease of ZO-1 expression seemed to parallel that of E-cadherin hence confirming an association with more aggressive types of breast cancer. However, it is important to note that ZO-1 did not leave the cell membrane until cells became invasive (it was then found in the cytoplasm when still expressed). These findings suggest that there is a hierarchy in ZO-1 alterations associated with breast cancer progression. Several reports and our unpublished observations attest of the lack of significant alterations in ZO-1 distribution in early stages of breast cancer, emphasizing the potentially important contribution of the late delocalization of this protein from the cell membrane for the acquisition of invasive potential.
The Impact of Apical Polarity on Progenitor Cells
Reports indicating that certain oncogenes have specific ties with progenitor cells have shed a new light on the complex mechanisms leading to the development of different forms of breast cancer. Notch has been associated with aggressive, basal-like breast cancers [62], themselves proposed to derive from the progenitor/stem cell compartment [63]. It was further proposed that Notch participates in early events necessary for breast cancer progression [64]. Thus, the link between apical polarity proteins and the Notch pathway (presented in a previous section) warrants further investigation of the effects of early changes in apical polarity on the progenitor cell compartment, in order to better understand the formation of aggressive forms of breast cancer.
The recent demonstration that Par-3 influences the progenitor cell compartment [33] strengthens the urgency to investigate the relationship between apical polarity and progenitor/stem cells. Silencing of Par-3 led to the formation of multilayered mammary epithelium in mice cleared fatpad, with a higher level of proliferation compared to control epithelia based on staining for cell cycle marker Ki67. The accompanying increase in bipotential progenitor K8+/K14+ cells led to the hypothesis that the binding of Par-3 to aPKC occurring at the apical pole of cells would normally limit the expansion of this type of cells. Therefore, one could imagine that the loss of Par-3 from the apical cell membrane and thus, disruption of Par3-aPKC interaction without necessarily a global change in Par-3 level, might be sufficient to affect progenitor cells, which could have consequences on tumor development. The type of breast cancer corresponding to alterations in Par-3 remains to be unraveled.
An emerging concept is that events that occur early on during breast cancer development will determine the fate of the neoplasm (i.e., more or less aggressive). Based on the findings linking certain alterations in apical polarity with more aggressive types of breast cancer and the fact that apical polarity alterations occur early during breast cancer development, I would like to propose that the type of apical polarity protein involved in early alterations in breast tissue architecture plays a critical role in the fate of the breast neoplasm. In particular, it might determine whether additional changes in the AJC necessary for tumor progression will occur (e.g., changes in ZO-1 distribution).
Using 3D culture of breast glandular structures, we have observed that apical polarity is highly labile compared to basal polarity and remarkably, is very sensitive to changes in the extracellular matrix [32]. If changes in apical polarity occur early during breast cancer development, possibly even before a tumor develops, it is important to understand how these early changes could favor later phenotypic changes, like the acquisition of the invasive phenotype or the formation of tumors of adverse prognosis. One of the possible reasons for apical polarity changes affecting the long-term fate of breast cancer development might be found at the level of the cell nucleus and notably in epigenetic changes.
Relationship Between Apical Polarity and Nuclear Mechanisms of Gene Expression Control
A powerful means for apical polarity proteins to influence proliferation and other phenotypic changes necessary for tumor development is via their effect on gene expression control. For instance Crb influences p53 that targets genes important for tissue homeostasis (e.g., cell cycle regulation, proliferation, apoptosis, DNA repair) as shown in Drosophila. More particularly, decreasing levels of Crb reinforced p53-induced apoptosis-linked rough eye phenotype. These findings led to the hypothesis that p53 senses changes at the level of cell–cell junctions [65]. One possibility to explain how the cell nucleus senses changes at cell–cell junctions is the control of apical polarity over the location of signal transducers and transcription regulators.
Impact of Apical Polarity on Phenotypes Via the Control of the Location of Transcription Regulators
Effects Linked to Apical Polarity Regulators
A relationship between apical polarity and proliferation control was beautifully demonstrated by Bilder and colleagues in Drosophila for all three members of the Scrib group (scribble, Dlg, Lgl). Mutation of these proteins led to overproliferation of larval imaginal discs. These data were correlated to the lack of apical polarity formation as shown by altered distribution of Crb and disruption of adherens junctions [38].
Further investigation of Scrib revealed an action on transcription regulators. In particular, Scrib has been reported to affect the function of LPP, a zyxin-related protein that shuttles between plasma membrane and nucleus and has transcriptional capacity, as shown in reporter gene assays [50, 66]. LPP has been directly involved in lung and soft tissue tumors and in leukemia; it is considered to act as a protooncogene [67]. In the cell nucleus, LPP is recruited to PEA3-dependent promoter regions and functions as a coregulatory protein in gene transactivation [68]. Interestingly, a number of PEA3 regulated genes are proteases involved in invasive phenotype and have been linked to breast cancer metastasis and HER2/neu-mediated mammary oncogenesis (for a review refer to [66]. Since LPP is acting as a scaffolding protein influencing signal transduction from cell–cell contacts to the cell nucleus, it could mediate some of the Scrib-related cellular effects. Scrib binds LPP via its DPZ domains but it does not seem to be involved in LPP targeting to cell–cell contacts [50]. LPP also interacts with VASP and α-actinin at cell–cell junctions. Whether an alteration of Scrib at the cell membrane might trigger LPP action on PEA3 regulatory regions remains to be known.
As presented in a previous section, in mammalian cells evidence of the involvement of apical polarity in cell proliferation control is found in several studies. For instance, Dlg was proposed to participate in progression block from G0/G1 to S phase by forming a complex with tumor suppressor adenomatous polyposis coli (APC) thus, helping convey APC blocking signal [69]. Although APC has been found to associate with euchromatin, its role in this compartment needs to be further deciphered [70].
The possible mechanism underlying Par-6 involvement in proliferation control is more detailed. Overexpression of Par-6 in MCF10A non-neoplastic mammary epithelial cells led to the formation of a multilayered epithelium in 3D culture. Par-6 acted via binding to aPKC and Cdc42 and triggering the sustained activation of the mitogen-activated protein kinase (MAPK) pathway, notably the extracellular signal-regulated kinase (ERK) [71] previously associated with breast cancer development [72].
The effect of Par-6 on MAPK is a highway to chromatin regulation since ras-MAPK pathway activation is known to induce modifications in histone 3 and architectural transcription factor high mobility group (HMG)N1 (for a review refer to [73]). Increase in H3 phosphorylation could affect the transcription of immediate-early genes (e.g., c-myc, c-fos, c-jun) important for cancer development. From an architectural standpoint, modifications in histone 3 and HMGN1 have been associated with alterations in higher chromatin structure. For instance phosphorylation of HMGN1 by mitogen- and stress-activated kinase 1 (MSK1) perturbed its interaction with DNA, thus allowing MSK1 access to the long N-terminal tail of histone 3 [74]. The genes that could be affected by upregulation of this pathway in the breast are mostly unknown. However, chromatin immunoprecipitation experiments have shown that histone 3 phosphorylated on ser10 is associated with HER2 promoter in cells overexpressing this gene, suggesting that the ras-MAPK pathway could stimulate the expression of HER2 observed during breast cancer development [75]. Interestingly, a general pattern of chromatin decondensation has been associated with the activation of the ras-MAPK pathway [76–78], suggesting that a new organization of the cell nucleus is induced, which could, in turn, affect the response to subsequent incoming signals at the level of chromatin [3] and influence cancer progression.
Effects Linked to Tight Junction Proteins
Tight junctions were proposed to participate in proliferation control more than a decade ago [79]. A powerful epigenetic control of proliferation in mammalian cells was associated with the sequestration of y-box protein ZO-1 nucleic acid binding protein (ZONAB or human DbpA) to apical junctions via binding to ZO-1 since this resulted in reduced proliferation of MDCK cells. Overexpression of ZO-1 also contributed to cell cycle arrest, confirming that this tight junction protein was involved in proliferation control [80].
The y-box transcription factors are generally thought to promote cell cycle entry [81]. Thus, a change in ZONAB sequestration upon apical polarity loss could be initiating cell cycle entry at the early stages of tumor development. Indeed the control of ZO proteins on the localization of transcription factors (ZONAB and AP1) and other molecules is considered a strong way to alter gene expression [5]. ZONAB (or its human ortholog DbpA), which is overexpressed in carcinomas, controls the expression of cyclin D1 and PCNA involved in G1 to S transition. Specifically, ZONAB works with RNA-processing factor symplekin to control cyclin D1 expression and thus, proliferation in colon carcinoma cells [82]. It also binds the promoter of PCNA and cyclin D1 genes [83]. Interestingly, ZONAB upregulation was found to stimulate transcription of histone 4 and architectural transcription factor HMG-I [83], indicating a more general role in epigenetic control of gene expression. Recent work in wallaby has reported a significant upregulation of ZONAB in mammary tumors mimicking basal-like breast cancer [84], suggesting a link between this ZO-1 binding partner and breast cancer aggressiveness.
Thus far ZO-1 is thought to inhibit cell proliferation by sequestering ZONAB at tight junctions hence, preventing the nuclear function of ZONAB. It has been proposed that this interaction might explain how ZO-1 is involved in differentiation control and that the ZO-1-ZONAB pathway can be deregulated in cancer [5]. A similar trapping role of gene transcription regulators could be envisioned for ZO-2 that interacts with AP1 (Jun & Fos) and C/EBP, two transcription factors involved in breast cancer [85, 86] and associated with tight junctions [87].
Direct Impact of Apical Polarity Proteins on Gene Expression
The role of apical polarity proteins in tissue homeostasis and as tumor suppressors is complicated to grasp. Indeed, in addition to controlling the nuclear compartmentalization of regulators of gene expression by trapping them at cell–cell junctions, most apical polarity proteins are also found in the cell nucleus, suggesting that they might directly interfere with gene expression control in that compartment.
ZO Proteins in the Cell Nucleus: the Potential to Affect Chromatin Organization at First Hand
ZO-1 has been observed in the cell nucleus of epithelial cells in culture as well as in vivo [88, 89]. It is known to influences gene expression, including tumor promoting genes, once delocalized from the plasma membrane [46]. However, to this date the possibility for ZO-1 to directly control gene expression in the cell nucleus has not been demonstrated. In contrast, the involvement of ZO-2 in the cell nucleus is being progressively clarified.
ZO-2 was observed in vivo in the nucleus of epithelial cells throughout the nephron of rabbit kidney [89] and was also reported to partially colocalize with SC-35 [17]. Like the other ZO proteins, ZO-2 possesses nuclear import and export signals indicating that it is capable of shuttling between nucleus and cytoplasm. Interactions between ZO-2 and several proteins involved in gene expression control have been demonstrated. Of particular interest is the binding of ZO-2 to the scaffold attachment factor, SAF-B that interacts with matrix attachment regions in the DNA and controls chromatin organization at gene boundaries and thus, influences gene expression [90]. Interestingly, Like ZO-2, SAF-B colocalizes with SC-35 in regions permissive for gene expression; it also interacts with RNA polymerase II [91]. SAF-B might also be involved in repression of gene transcription; it has been reported to act as a corepressor of estrogen receptor α transactivation and proliferation inhibition in breast tissue [92]. More generally, it acts as a corepressor for nuclear receptors by modifying chromatin (for a review refer to [93, 94]. Indeed SAF-B1 activity was altered upon treatment with histone deacetylase (HDAC) inhibitors, suggesting that it is accompanied with histone tail modifying action.
Other investigations suggest that ZO-2 is also involved in the repression of gene transcription. It interacts with c-myc to recruit HDAC1 at the promoter of cyclin D1 leading to inhibition of cyclin D1 transcription as shown in MDCK cells [95]. This repressive action on proliferation is proposed to occur upon ZO-2 binding to the E box region in the cyclin D1 gene promoter, probably via c-myc.
An Emerging Role for Nuclear Apical Polarity Proteins in Tumor Repression
The involvement of ZO-2 in inhibiting cyclin D1 transcription as presented above goes against the idea that it is upon apical polarity loss that certain tight junction proteins become free to travel to the cell nucleus and, once there, stimulate proliferation. The latter idea would suggest that the tumor suppressor activity of apical polarity is primarily linked to the sequestration of AJC proteins at cell–cell junction. In agreement with this idea, it was reported recently that accumulation of ZO-2 in the nuclei of MDCK cells led to increased proliferation and stimulated the expression of M2 type of pyruvate kinase [96]. This was accompanied with a reduction of intercellular junction stability. How can we reconcile the presence of apical polarity proteins in the cell nucleus in both quiescence and proliferation, and the possibility of an opposite effect for a given apical polarity protein on cell phenotype (i.e. repression of proliferation and stimulation of proliferation)?
The fact that apical polarity proteins are found in the cell nucleus also when apical polarity is intact (see above) argues for an involvement of tight junction proteins in nuclear functions, and particularly gene expression control, in the normal phenotype. This hypothesis is demonstrated by the findings reported in the precedent section for ZO-2. A similar situation exists for apical polarity protein Dlg1 that interacts with RhoA-specific Guanine nucleotide exchange factor protein Net 1 (a protein that can transform cells once in the cytoplasm). Especially, in nontransformed cells Net1 leads to Dlg1 relocalization to nuclear PML bodies [97] that are known to contribute to differentiation and suppression of cell transformation [98], suggesting that the tumor suppressor activity of Dlg1 occurs in the cell nucleus.
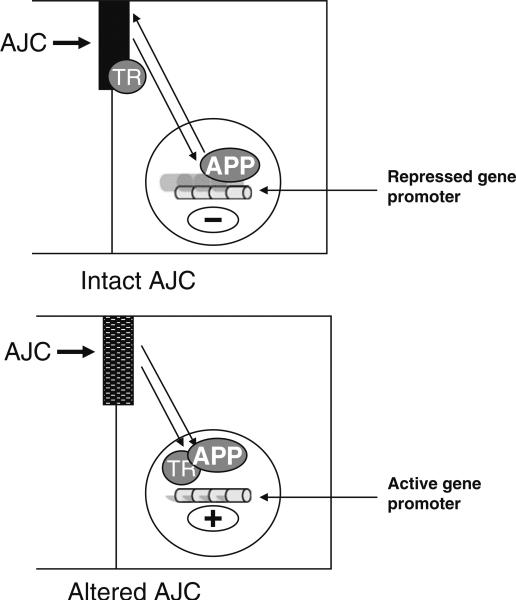
There might be two different situations: On one hand if cell–cell junctions are intact, the effect of apical polarity proteins in the cell nucleus is preventing tumor development. On the other hand, if cell–cell junctions are altered, the actions of apical polarity proteins in the cell nucleus favor proliferation and invasion (Fig. 3). This hypothesis is supported by the observation that the amount of ZO-2 can increase in the cell nucleus in nontransformed cells with decrease in cytoplasm but no change at the cell membrane and no change in occludin localization [90]. Moreover, ZO-1 localizes armadillo-like protein ARVCF to the plasma membrane, whereas upon alteration of cell–cell contacts ZO-2 localizes ARCVF to the cell nucleus [99]. Thus, the switch from a tumor suppressor to a tumor promoter action of polarity proteins in the cell nucleus might depend on the concomitant release of epigenetic factors, like ZONAB and potentially ARVCF (its function is still largely unknown) from cell–cell junction that would change local chromatin organization and the interaction of apical polarity proteins with their ligands.
Figure 3.
The action of apical polarity proteins in the cell nucleus depends on the integrity of the apical junctional complex (AJC). If the AJC is intact, certain apical polarity proteins (APP) shuttle between nucleus and cytoplasm and participate in a repressive action on the transcription of genes that have to be silenced in order to maintain a differentiated, quiescent stage. Transcription regulators (TR) remain trapped within the AJC (top panel). If the AJC is disrupted (e.g., disturbed tight junctions) the release of a transcription regulator (TR) from the AJC might modify the local environment of certain genes. Apical polarity proteins can stay in the cell nucleus and their action on the new promoter environment now helps to promote gene transcription.
Linking Early Alterations in Apical Polarity Proteins to Breast Cancer Progression Via an Epigenetic Action
Proliferation and invasion are two major aspects of tumor development that have been linked to the alteration of apical polarity. It is not surprising that apical polarity is observed to be altered early if it is involved in the control of cell proliferation. We have shown that unless apical polarity is perturbed, as illustrated by the relocation of tight junction marker ZO-1 away from the apical pole, mammary epithelial HMT-3522 S1 cells that form polarized glandular structures in 3D culture cannot be pushed into the cell cycle, suggesting that in tissue, apical polarity acts as a safety brake to prevent loss of quiescence [58].
Changes in apical polarity linked to regulatory proteins (e.g., Scrib, Par complex) and tight junction ZO proteins impact proliferation control and in some cases induce a multilayering of cells that mimics preinvasive breast lesions. This suggests that the alteration of the integrity of the AJC in general is conducive to proliferation. However, as presented in an earlier section, several publications also associate apical polarity proteins with the acquisition of aggressive forms of cancer (e.g. progression to invasion, metastatic behavior, lesions of adverse prognosis) although these phenotypes are not present already at the onset of tumor formation.
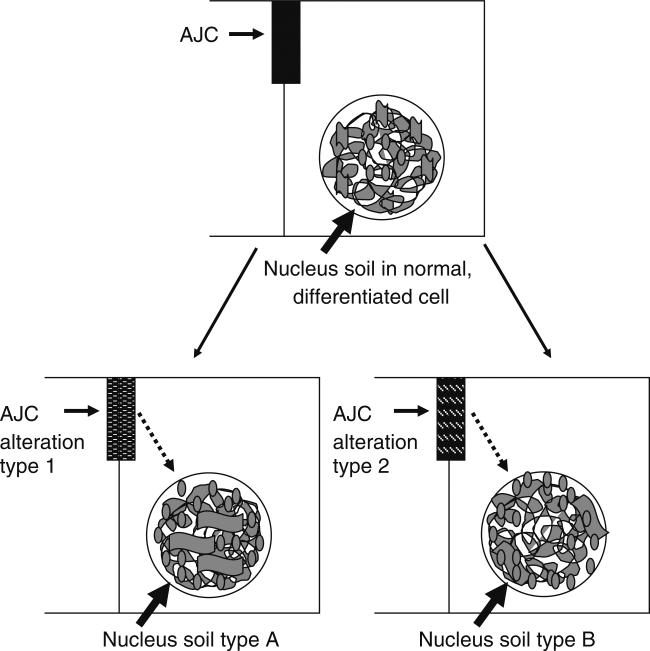
The involvement of apical polarity in the development of aggressive forms of cancer might be related to the impact of apical polarity proteins on epigenetic mechanisms in the cell nucleus. For instance, the initial alteration occurring within the AJC could be critical for the future of the breast neoplasm by altering chromatin organization, and subsequently gene expression and the signaling network (Fig. 4). In a recent publication I have discussed how changes in the cell nucleus leading to a new chromatin signature might, in turn, affect future responses to incoming signals [3]. Some of the effectors (e.g., ZONAB, MAPK pathway) of apical polarity proteins have the potential to profoundly alter chromatin organization by influencing the expression of histones and HMG proteins, thus potentially producing a new ‘nucleus soil’ in the targeted cells for the action of incoming signals.
Figure 4.
Effect of apical polarity alterations on the nucleus soil. The hypothesis is that the organization of the cell nucleus characteristic of normal, differentiated cells can be altered by modifications occurring at the level of the apical junctional complex (AJC). A specific modification leading to the release of a transcription regulator or the activation of a signal transduction pathway (e.g., type 1 or 2 alteration) might trigger changes in chromatin structure (e.g., via changes in DNA methylation and histone tail modifications) that affect different chromatin regions (e.g., via HMG proteins) thus, leading to a new nuclear organization (nucleus soil type A or B) (i.e., the new organization might correspond to changes in heterochromatin regions in the genome; this is represented by thick gray, convoluted ribbons in the cell nuclei in the drawing). The cell with nucleus soil type A might respond differently to certain incoming signals compared to a cell with nucleus soil type B. Therefore, future alterations and progression to cancer might be influenced by the initial modification(s) of the AJC.
We have only presented the link with the cell nucleus of apical polarity proteins for which there is already a good understanding of this particular relationship. However, most of the apical polarity proteins have been linked to the cell nucleus. This suggests that, depending on the apical polarity protein altered, a number of different actions might occur in the cell nucleus, which will shape the progression and possibly the outcome of cancer. Nok a homolog of PALS1 in zebrafish can localize to the cell nucleus [100]. Par-6 has also been observed in the cell nucleus in association with splicing factor speckles and it interacts with transcriptional activator Tax [101]. There seems to be a link between Par-3 and Ku to control double-strand break repair [102], thus potentially revealing another critical aspect of apical polarity for the maintenance of tissue homeostasis.
Another possibility to explain the involvement of apical polarity proteins in breast cancer progression is that a particular modification of apical polarity will affect a specific category of progenitor cells because of their different chromatin organization (and thus, ‘nucleus soil’) compared to nonprogenitor cells and other categories of progenitor cells [103]. The different chromatin organization among certain cells that make the mammary glandular structures is suggested by our experiment with DNA hypomethylation in mammary acini in 3D culture. Upon treatment with a DNA hypomethylating drug only certain cells in the acini expressed the progenitor cell marker CK19, indicating that these cells had a chromatin compaction status that made them responsive to DNA hypomethylation for this particular gene [104]. The specific type of progenitor cells affected would then lead the way to progression to a specific subtype of breast cancer, like for instance the basal type. This possibility is reinforced by the increasing number of reports that link progenitor mammary epithelial cells to more aggressive types of breast cancer and, as illustrated in this review, associate apical polarity proteins to progenitor cell behavior.
Concluding Remarks
We have outlined possible ways for apical polarity to control epigenetic mechanisms taking place in the cell nucleus, either by indirect effect (upon release of epigenetic factors or triggering signaling pathways) or by direct effect in the cell nucleus. However, there are additional possibilities to link apical polarity to gene expression. As suggested by others, tight junction disruption could allow contacts between proteins that were previously physically separated by the membrane seal, hence activating certain signal transduction pathways. Changes in ion channels linked to polarity could also alter gene expression [105].
The remarkable involvement of apical polarity proteins in the cell nucleus and gene expression control does not seem to only rely on the presence of these proteins in the cell nucleus upon disruption of apical polarity. Indeed, the presence of certain apical polarity proteins in the nucleus of cells with intact cell–cell junctions indicates that the shuttling of these proteins between their AJC location and the nucleus might be critical to maintain differentiation. This fundamental aspect of apical polarity needs to be further explored in the context of breast epithelial homeostasis.
The concept of barriology proposed by Shoichiro Tsukita, in which apical polarity functions as a physical barrier in multicellular organisms by regulating the epithelial microenvironment [106], can be extended to include a functional barrier linked to the cell nucleus. By participating in the epigenetic control of gene expression and chromatin organization, polarity regulates breast tissue differentiation and notably, prevents the expression of genes involved in proliferation and invasion.
Acknowledgments
Support provided via the National Institutes of Health (NIH) (CA112017 and CA112613 to SAL) and the Susan G. Komen Breast Cancer Foundation (BCTR-0707641 to SAL).
Abbreviations
- 3D
Three-dimensional
- AJC
Apical junctional complex
- APC
Adenomatous polyposis coli
- Baz
Bazooka
- Dlg
Discs-large
- HDAC
Histone deacetylase
- HMG
High mobility group
- IDC
Invasive ductal carcinoma
- JAM
Junctional adhesion molecule
- Lgl
Lethal giant larvae
- MAGUK
Membrane-associated guanylate kinase
- MAPK
Mitogen-activated protein kinase
- MDCK
Mardin-Darby Canine Kidney
- SAF
Scaffold-attachment factor
- Scrib
Scribble
- std
Stardust
- ZO
Zonula Occludens
- ZONAB
ZO-1 nucleic acid binding protein
References
- 1.Morange M. The relations between genetics and epigenetics: a historical point of view. Ann N Y Acad Sci. 2002;981:50–60. doi: 10.1111/j.1749-6632.2002.tb04911.x. [DOI] [PubMed] [Google Scholar]
- 2.Lelievre SA, Bissell MJ. Communication between the cell membrane and the nucleus: role of protein compartmentalization. J Cell Biochem Suppl. 1998;30–31:250–63. [PMC free article] [PubMed] [Google Scholar]
- 3.Lelièvre SA. Contributions of extracellular matrix signaling and tissue architecture to nuclear mechanisms and spatial organization of gene expression control. Biochim Biophys Acta. 2009;1790(9):925–35. doi: 10.1016/j.bbagen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matter K, Balda MS. Epithelial tight junctions, gene expression and nucleo-junctional interplay. J Cell Sci. 2007;120(Pt 9):1505–11. doi: 10.1242/jcs.005975. [DOI] [PubMed] [Google Scholar]
- 6.Shennan DB, Peaker M. Transport of milk constituents by the mammary gland. Physiol Rev. 2000;80(3):925–51. doi: 10.1152/physrev.2000.80.3.925. [DOI] [PubMed] [Google Scholar]
- 7.Blaug S, Rymer J, Jalickee S, Miller SS. P2 purinoceptors regulate calcium-activated chloride and fluid transport in 31EG4 mammary epithelia. Am J Physiol Cell Physiol. 2003;284(4):C897–909. doi: 10.1152/ajpcell.00238.2002. [DOI] [PubMed] [Google Scholar]
- 8.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–20. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 10.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286(6):C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen DA, Neville MC. Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia. 1998;3(3):233–46. doi: 10.1023/a:1018707309361. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard AA, Watson PH, Shiu RP, Leygue E, Nistor A, Wong P, et al. Differential expression of claudin 1, 3, and 4 during normal mammary gland development in the mouse. DNA Cell Biol. 2006;25(2):79–86. doi: 10.1089/dna.2006.25.79. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16(6):2636–50. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyoshi J, Takai Y. Structural and functional associations of apical junctions with cytoskeleton. Biochim Biophys Acta. 2008;1778(3):670–91. doi: 10.1016/j.bbamem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778(3):660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778(3):729–56. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Islas S, Vega J, Ponce L, Gonzalez-Mariscal L. Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp Cell Res. 2002;274(1):138–48. doi: 10.1006/excr.2001.5457. [DOI] [PubMed] [Google Scholar]
- 18.Paris L, Tonutti L, Vannini C, Bazzoni G. Structural organization of the tight junctions. Biochim Biophys Acta. 2008;1778((3):646–59. doi: 10.1016/j.bbamem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Abad PC, Yagci B, Lelièvre SA. Nanoprocesses in gene expression. In: Nalwa HS, editor. Encyclopedia of Nanoscience and Nanotechnology, Vols 11–20. American Scientific Publishers; in press. [Google Scholar]
- 20.Konska G, Guillot J, De Latour M, Fonck Y. Expression of Tn antigen and N-acetyllactosamine residues in malignant and benign human breast tumors detected by lectins and monoclonal antibody 83D4. Int J Oncol. 1998;12(2):361–7. doi: 10.3892/ijo.12.2.361. [DOI] [PubMed] [Google Scholar]
- 21.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5(1):53–8. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 22.Macara IG. Par proteins: partners in polarization. Curr Biol. 2004;14(4):R160–2. [PubMed] [Google Scholar]
- 23.Stucke VM, Timmerman E, Vandekerckhove J, Gevaert K, Hall A. The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol Biol Cell. 2007;18(5):1744–55. doi: 10.1091/mbc.E06-11-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogg VC, Liu CJ, Margolis B. Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J Cell Sci. 2005;118(Pt 13):2859–69. doi: 10.1242/jcs.02412. [DOI] [PubMed] [Google Scholar]
- 25.Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5(2):137–42. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- 26.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2(8):540–7. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 27.Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6(6):845–54. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Sotillos S, Diaz-Meco MT, Caminero E, Moscat J, Campuzano S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol. 2004;166(4):549–57. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(Pt 13):2363–74. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 30.Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J. 2001;20(14):3738–48. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkinson SJ, Le Good JA, Whelan RD, Whitehead P, Parker PJ. Identification of PKCzetaII: an endogenous inhibitor of cell polarity. EMBO J. 2004;23(1):77–88. doi: 10.1038/sj.emboj.7600023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plachot C, Chaboub LS, Adissu HA, Wang L, Urazaev A, Sturgis J, et al. Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: example of the breast epithelium. BMC Biol. 2009;7:77. doi: 10.1186/1741-7007-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaffrey LM, Macara IG. The Par3/aPKC interaction is essential for end bud remodeling and progenitor differentiation during mammary gland morphogenesis. Genes Dev. 2009;23(12):1450–60. doi: 10.1101/gad.1795909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK. Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J Cell Biol. 1973;56(3):797–818. doi: 10.1083/jcb.56.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer A, Stuckas H, Gluth M, Russell TD, Rudolph MC, Beeman NE, et al. Impaired tight junction sealing and precocious involution in mammary glands of PKN1 transgenic mice. J Cell Sci. 2007;120(Pt 13):2272–83. doi: 10.1242/jcs.03467. [DOI] [PubMed] [Google Scholar]
- 36.Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200(4349):1448–59. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- 37.Jacob L, Opper M, Metzroth B, Phannavong B, Mechler BM. Structure of the l(2)gl gene of Drosophila and delimitation of its tumor suppressor domain. Cell. 1987;50(2):215–25. doi: 10.1016/0092-8674(87)90217-0. [DOI] [PubMed] [Google Scholar]
- 38.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289(5476):113–6. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 39.Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci U S A. 1997;94(13):6670–5. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoover KB, Liao SY, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153(6):1767–73. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papilloma-virus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20(21):8244–53. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chlenski A, Ketels KV, Korovaitseva GI, Talamonti MS, Oyasu R, Scarpelli DG. Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta. 2000;1493(3):319–24. doi: 10.1016/s0167-4781(00)00185-8. [DOI] [PubMed] [Google Scholar]
- 43.Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol. 2000;10(12):697–707. doi: 10.1016/s0960-9822(00)00535-2. [DOI] [PubMed] [Google Scholar]
- 44.Glaunsinger BA, Weiss RS, Lee SS, Javier R. Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2. EMBO J. 2001;20(20):5578–86. doi: 10.1093/emboj/20.20.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171(6):1061–71. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polette M, Gilles C, Nawrocki-Raby B, Lohi J, Hunziker W, Foidart JM, et al. Membrane-type 1 matrix metalloproteinase expression is regulated by zonula occludens-1 in human breast cancer cells. Cancer Res. 2005;65(17):7691–8. doi: 10.1158/0008-5472.CAN-04-4230. [DOI] [PubMed] [Google Scholar]
- 47.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27(55):6970–80. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 48.Brumby AM, Richardson HE. Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22(21):5769–79. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dow LE, Brumby AM, Muratore R, Coombe ML, Sedelies KA, Trapani JA, et al. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene. 2003;22(58):9225–30. doi: 10.1038/sj.onc.1207154. [DOI] [PubMed] [Google Scholar]
- 50.Petit MM, Meulemans SM, Alen P, Ayoubi TA, Jansen E, Van de Ven WJ. The tumor suppressor Scrib interacts with the zyxin-related protein LPP, which shuttles between cell adhesion sites and the nucleus. BMC Cell Biol. 2005;6(1):1. doi: 10.1186/1471-2121-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18(40):5487–96. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- 52.Dow LE, Kauffman JS, Caddy J, Zarbalis K, Peterson AS, Jane SM, et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26(16):2272–82. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 53.Underwood JM, Imbalzano KM, Weaver VM, Fischer AH, Imbalzano AN, Nickerson JA. The ultrastructure of MCF-10A acini. J Cell Physiol. 2006;208(1):141–8. doi: 10.1002/jcp.20639. [DOI] [PubMed] [Google Scholar]
- 54.Zhan L, Rosenberg A, Bergami KC, Yu M, Xuan Z, Jaffe AB, et al. Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell. 2008;135(5):865–78. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8(11):1235–45. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- 56.Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem. 2000;275(13):9492–500. doi: 10.1074/jbc.275.13.9492. [DOI] [PubMed] [Google Scholar]
- 57.Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat. 1994;31(2–3):325–35. doi: 10.1007/BF00666165. [DOI] [PubMed] [Google Scholar]
- 58.Chandramouly G, Abad PC, Knowles DW, Lelievre SA. The control of tissue architecture over nuclear organization is crucial for epithelial cell fate. J Cell Sci. 2007;120(Pt 9):1596–606. doi: 10.1242/jcs.03439. [DOI] [PubMed] [Google Scholar]
- 59.Mantovani F, Massimi P, Banks L. Proteasome-mediated regulation of the hDlg tumour suppressor protein. J Cell Sci. 2001;114(Pt 23):4285–92. doi: 10.1242/jcs.114.23.4285. [DOI] [PubMed] [Google Scholar]
- 60.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302(5648):1227–31. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- 61.Nakagawa S, Yano T, Nakagawa K, Takizawa S, Suzuki Y, Yasugi T, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer. 2004;90(1):194–9. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A, et al. A functional Notch-survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10(6):R97. doi: 10.1186/bcr2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15(5):193–7. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99(8):616–27. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 65.Yamaguchi M, Hirose F, Inoue YH, Ohno K, Yoshida H, Hayashi Y, et al. Genetic link between p53 and genes required for formation of the zonula adherens junction. Cancer Sci. 2004;95(5):436–41. doi: 10.1111/j.1349-7006.2004.tb03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petit MM, Fradelizi J, Golsteyn RM, Ayoubi TA, Menichi B, Louvard D, et al. LPP, an actin cytoskeleton protein related to zyxin, harbors a nuclear export signal and transcriptional activation capacity. Mol Biol Cell. 2000;11(1):117–29. doi: 10.1091/mbc.11.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grunewald TG, Pasedag SM, Butt E. Cell adhesion and transcriptional activity—defining the role of the novel protooncogene LPP. Transl Oncol. 2009;2(3):107–16. doi: 10.1593/tlo.09112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo B, Sallis RE, Greenall A, Petit MM, Jansen E, Young L, et al. The LIM domain protein LPP is a coactivator for the ETS domain transcription factor PEA3. Mol Cell Biol. 2006;26(12):4529–38. doi: 10.1128/MCB.01667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene. 2000;19(3):365–72. doi: 10.1038/sj.onc.1203309. [DOI] [PubMed] [Google Scholar]
- 70.Kouzmenko AP, Takeyama K, Kawasaki Y, Akiyama T, Kato S. Truncation mutations abolish chromatin-associated activities of adenomatous polyposis coli. Oncogene. 2008;27(36):4888–99. doi: 10.1038/onc.2008.127. [DOI] [PubMed] [Google Scholar]
- 71.Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred DC, et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008;68(20):8201–9. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milde-Langosch K, Bamberger AM, Rieck G, Grund D, Hemminger G, Muller V, et al. Expression and prognostic relevance of activated extracellular-regulated kinases (ERK1/2) in breast cancer. Br J Cancer. 2005;92(12):2206–15. doi: 10.1038/sj.bjc.6602655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83(1):1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 74.Lim JH, Catez F, Birger Y, West KL, Prymakowska-Bosak M, Postnikov YV, et al. Chromosomal protein HMGN1 modulates histone H3 phosphorylation. Mol Cell. 2004;15(4):573–84. doi: 10.1016/j.molcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Mishra SK, Mandal M, Mazumdar A, Kumar R. Dynamic chromatin remodeling on the HER2 promoter in human breast cancer cells. FEBS Lett. 2001;507(1):88–94. doi: 10.1016/s0014-5793(01)02951-9. [DOI] [PubMed] [Google Scholar]
- 76.Chadee DN, Taylor WR, Hurta RA, Allis CD, Wright JA, Davie JR. Increased phosphorylation of histone H1 in mouse fibroblasts transformed with oncogenes or constitutively active mitogen-activated protein kinase kinase. J Biol Chem. 1995;270(34):20098–105. doi: 10.1074/jbc.270.34.20098. [DOI] [PubMed] [Google Scholar]
- 77.Taylor WR, Chadee DN, Allis CD, Wright JA, Davie JR. Fibroblasts transformed by combinations of ras, myc and mutant p53 exhibit increased phosphorylation of histone H1 that is independent of metastatic potential. FEBS Lett. 1995;377(1):51–3. doi: 10.1016/0014-5793(95)01314-8. [DOI] [PubMed] [Google Scholar]
- 78.Chadee DN, Hendzel MJ, Tylipski CP, Allis CD, Bazett-Jones DP, Wright JA, et al. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblasts. J Biol Chem. 1999;274(35):24914–20. doi: 10.1074/jbc.274.35.24914. [DOI] [PubMed] [Google Scholar]
- 79.Stevenson BR, Keon BH. The tight junction: morphology to molecules. Annu Rev Cell Dev Biol. 1998;14:89–109. doi: 10.1146/annurev.cellbio.14.1.89. [DOI] [PubMed] [Google Scholar]
- 80.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160(3):423–32. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ladomery M, Sommerville J. A role for Y-box proteins in cell proliferation. Bioessays. 1995;17(1):9–11. doi: 10.1002/bies.950170104. [DOI] [PubMed] [Google Scholar]
- 82.Kavanagh E, Buchert M, Tsapara A, Choquet A, Balda MS, Hollande F, et al. Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J Cell Sci. 2006;119(Pt 24):5098–105. doi: 10.1242/jcs.03297. [DOI] [PubMed] [Google Scholar]
- 83.Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, et al. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26(6):2387–98. doi: 10.1128/MCB.26.6.2387-2398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharp JA, Mailer SL, Thomson PC, Lefevre C, Nicholas KR. Identification and transcript analysis of a novel wallaby (Macropus eugenii) basal-like breast cancer cell line. Mol Cancer. 2008;7:1. doi: 10.1186/1476-4598-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41(16):2449–61. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 86.Zahnow CA. CCAAT/enhancer-binding protein beta: its role in breast cancer and associations with receptor tyrosine kinases. Expert Rev Mol Med. 2009;11:e12. doi: 10.1017/S1462399409001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res. 2004;292(1):51–66. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci U S A. 1996;93(20):10779–84. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, et al. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000;57(6):2386–402. doi: 10.1046/j.1523-1755.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 90.Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem. 2003;278(4):2692–700. doi: 10.1074/jbc.M206821200. [DOI] [PubMed] [Google Scholar]
- 91.Nayler O, Stratling W, Bourquin JP, Stagljar I, Lindemann L, Jasper H, et al. SAF-B protein couples transcription and pre-mRNA splicing to SAR/MAR elements. Nucleic Acids Res. 1998;26(15):3542–9. doi: 10.1093/nar/26.15.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Townson SM, Sullivan T, Zhang Q, Clark GM, Osborne CK, Lee AV, et al. HET/SAF-B overexpression causes growth arrest and multinuclearity and is associated with aneuploidy in human breast cancer. Clin Cancer Res. 2000;6(9):3788–96. [PubMed] [Google Scholar]
- 93.Oesterreich S. Scaffold attachment factors SAFB1 and SAFB2: Innocent bystanders or critical players in breast tumorigenesis? J Cell Biochem. 2003;90(4):653–61. doi: 10.1002/jcb.10685. [DOI] [PubMed] [Google Scholar]
- 94.Debril MB, Dubuquoy L, Feige JN, Wahli W, Desvergne B, Auwerx J, et al. Scaffold attachment factor B1 directly interacts with nuclear receptors in living cells and represses transcriptional activity. J Mol Endocrinol. 2005;35(3):503–17. doi: 10.1677/jme.1.01856. [DOI] [PubMed] [Google Scholar]
- 95.Huerta M, Munoz R, Tapia R, Soto-Reyes E, Ramirez L, Recillas-Targa F, et al. Cyclin D1 is transcriptionally down-regulated by ZO-2 via an E box and the transcription factor c-Myc. Mol Biol Cell. 2007;18(12):4826–36. doi: 10.1091/mbc.E07-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Traweger A, Lehner C, Farkas A, Krizbai IA, Tempfer H, Klement E, et al. Nuclear Zonula occludens-2 alters gene expression and junctional stability in epithelial and endothelial cells. Differentiation. 2008;76(1):99–106. doi: 10.1111/j.1432-0436.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Mata R, Dubash AD, Sharek L, Carr HS, Frost JA, Burridge K. The nuclear RhoA exchange factor Net1 interacts with proteins of the Dlg family, affects their localization, and influences their tumor suppressor activity. Mol Cell Biol. 2007;27(24):8683–97. doi: 10.1128/MCB.00157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borden KL. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol Cell Biol. 2002;22(15):5259–69. doi: 10.1128/MCB.22.15.5259-5269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kausalya PJ, Phua DC, Hunziker W. Association of ARVCF with zonula occludens (ZO)-1 and ZO-2: binding to PDZ-domain proteins and cell-cell adhesion regulate plasma membrane and nuclear localization of ARVCF. Mol Biol Cell. 2004;15(12):5503–15. doi: 10.1091/mbc.E04-04-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bit-Avragim N, Rohr S, Rudolph F, Van der Ven P, Furst D, Eichhorst J, et al. Nuclear localization of the zebrafish tight junction protein nagie oko. Dev Dyn. 2008;237(1):83–90. doi: 10.1002/dvdy.21389. [DOI] [PubMed] [Google Scholar]
- 101.Cline EG, Nelson WJ. Characterization of mammalian Par 6 as a dual-location protein. Mol Cell Biol. 2007;27(12):4431–43. doi: 10.1128/MCB.02235-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang L, Wang Y, Du D, Yang G, Tak Kwok T, Kai Kong S, et al. Cell polarity protein Par3 complexes with DNA-PK via Ku70 and regulates DNA double-strand break repair. Cell Res. 2007;17(2):100–16. doi: 10.1038/sj.cr.7310145. [DOI] [PubMed] [Google Scholar]
- 103.Boheler KR. Stem cell pluripotency: a cellular trait that depends on transcription factors, chromatin state and a checkpoint deficient cell cycle. J Cell Physiol. 2009;221(1):10–7. doi: 10.1002/jcp.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298(1):122–32. doi: 10.1016/j.yexcr.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 105.Isfort RJ, Cody DB, Stuard SB, Ridder GM, LeBoeuf RA. Calcium functions as a transcriptional and mitogenic repressor in Syrian hamster embryo cells: roles of intracellular pH and calcium in controlling embryonic cell differentiation and proliferation. Exp Cell Res. 1996;226(2):363–71. doi: 10.1006/excr.1996.0237. [DOI] [PubMed] [Google Scholar]
- 106.Tsukita S, Yamazaki Y, Katsuno T, Tamura A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27(55):6930–8. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]