Abstract
Multivesicular bodies (MVBs) are defined by multiple internal vesicles enclosed within an outer, limiting membrane. MVBs have previously been quantified in neuronal cell bodies and in dendrites, but their frequencies and significance in axons are controversial. Despite lack of conclusive evidence, it is widely believed that MVBs are the primary organelle that carries neurotrophic factors in axons. Reliable information about axonal MVBs under physiological and pathological conditions is needed for a realistic assessment of their functional roles in neurons. We provide a quantitative ultrastructural analysis of MVBs in the normal postnatal rat hypoglossal nerve and under a variety of experimental conditions. MVBs were about 50 times less frequent in axons than in neuronal cell bodies or dendrites. Five distinct types of MVBs were distinguished in axons, based on MVB size, electron density and size of internal vesicles. While target manipulations did not significantly change MVBs in axons, dystrophic conditions such as delayed fixation substantially increased the number of axonal MVBs. Radiolabeled brain- and glial-cell derived neurotrophic factors (BDNF and GDNF) injected into the tongue did not accumulate during retrograde axonal transport in MVBs, as determined by quantitative ultrastructural autoradiography, and confirmed by analysis of quantum dot-labeled BDNF. We conclude that for axonal transport, neurotrophic factors utilize small vesicles or endosomes that can be inconspicuous at transmission electron microscopic resolution, rather than MVBs. Previous reports of axonal MVBs may be based, in part, on artificial generation of such organelles in axons due to dystrophic conditions.
Keywords: Axon, Node of Ranvier, Axonal Transport, Trophic Factor, Endosome, Ultrastructure, Organelle
Introduction
Multivesicular bodies (MVBs) are large, >250 nm diameter, single membrane delimited organelles that contain smaller, 50-80 nm diameter internal vesicles. They were first described in the 1950s and subsequently shown to be involved in the endocytotic pathway (Haigler et al., 1979; Russell et al., 2006; Piper and Katzmann, 2007). MVBs participate in intracellular degradation pathways, but also have roles as protein sorting stations. They are established organelles in neuronal cell bodies and dendrites, but the presence and functions of MVBs in axons have been a matter of controversy for many years. The published record of MVBs in axons is conflicting, with some researchers reporting MVBs as being “very rare” or not present in normal axons (Kapeller and Mayor, 1969; Castel et al., 1992a; Delcroix et al., 2003), while others consider MVBs to be the primary organelle for retrograde transport in axons (LaVail and LaVail, 1974; Chu-Wang and Oppenheim, 1980; Tsukita and Ishikawa, 1980; Parton et al., 1992; Weible and Hendry, 2004).
The role of MVBs in neurons has recently gained considerable attention due to several developments. MVBs were shown to deliver internal vesicles for extracellular release as exosomes in non-neuronal cells and possibly also in neurons (Faure et al., 2006; Smalheiser, 2007). Recent studies have re-emphasized older work that postulated MVBs to be the main organelles that retrogradely transport trophic molecules (ligand-receptor complexes) from targets to the cell bodies (Parton et al., 1992; Weible and Hendry, 2004; Bronfman et al., 2007; Valdez et al., 2007; Kaasinen et al., 2008). MVBs have been implicated in the pathogenesis of degenerative neuronal diseases such as Alzheimer's and frontotemporal lobar degeneration, where malfunction of ESCRT proteins, which are MVB-associated proteins, appears to be critical in the disease process (Filimonenko et al., 2007; Rusten et al., 2007).
Unlike other cells, neurons have large, specialized compartments (axons and dendrites) in addition to the cell body, and such expanded compartments require more complex transport and intra- and intercellular communication, some of which may be carried out by MVBs. Although various immunocytochemical markers have been suggested (Saito et al., 1997; Kobayashi et al., 1998; White et al., 2006), there is currently no single, unique marker for MVBs (Maxfield and McGraw, 2004), and therefore MVBs are most reliably identified at the ultrastructural level. MVBs are equally common in neuronal cell bodies (Schwab and Thoenen, 1976; Claude et al., 1982; Rind et al., 2005) and dendrites (Rind et al., 2005) with fractional areas (FAs) of 0.5-1.5% in the somatic or dendritic compartment. However, information about MVBs in axons is scarce and neither their frequency nor their functions have been systematically analyzed. This is a major impediment for progress in understanding the role of MVBs in neurons under normal and pathological conditions.
We have therefore embarked on a first comprehensive and quantitative study of MVBs in axons. In particular, we sought to answer five major questions: 1. Are MVBs present in axons? 2. How frequent are axonal MVBs and what is their fractional area? 3. Are MVBs distributed throughout the axon, or are they preferentially localized to nodal regions? 4. Does the frequency, fractional area, or distribution of MVBs change with manipulations of the target (injury or trophic factor injection), or with cooling conditions (that are known to slow axonal transport), or with stress-inducing conditions as evoked by hypoxia-induced dystrophy? 5. Are retrogradely transported neurotrophic factors (NTFs) contained within or associated with MVBs in axons?
To answer these questions, we measured the fractional area of MVBs in axons. We compared the location of these organelles between axon shaft and paranode/node region. By tracking the fate of radiolabeled BDNF and GDNF and quantum dot-conjugated BDNF, we tested the hypothesis that neurotrophic factors travel in MVBs during retrograde axonal transport. The peripheral axons of the postnatal rat hypoglossal nerve are ideal for this task. The hypoglossal nerve is a largely homogeneous population of motor axons with no significant contribution of sensory or intrafusal axons intermingled (O'Reilly and FitzGerald, 1990). The cell bodies and dendrites of the rat hypoglossal motor neurons have already been quantified for MVB content (Rind et al., 2005). Quantitative assessment of MVBs in axons will help to elucidate functions of these organelles in physiological and pathological conditions. Preliminary data of this work has been presented in abstract form (Altick et al., 2008).
Materials and Methods
Materials
Pregnant Sprague-Dawley rats were obtained from Simonsen Labs, (Gilroy, CA). Rat pups were used at the ages of postnatal days (P) 6.5-9.5. The animal studies were conducted in accordance with the Policies on the Use of Animals and Humans in Neuroscience Research (1995), and animal protocols were approved by the local animal care committee. A total of 25 rat pups were used. Glial cell line-derived neurotrophic factor (GDNF) was purchased from Peprotech (Rocky Hill, NJ) and brain-derived neurotrophic factor (BDNF) was a kind gift from Regeneron (Tarrytown, NY). Quantum dots were obtained from Invitrogen (streptavidin-QD585, Eugene, OR). All electron microscopy (EM) tissue processing reagents were purchased from EM Sciences (Gibbstown, NJ), with the exception of lead citrate (Sigma, St. Louis, MO).
Tissue processing for electron microscopic analyses
For tissue collection, rat pups were deeply anesthetized with an overdose of Euthasol and immediately perfused with 2% paraformaldehyde in 0.1M cacodylate buffer, pH 7.4, containing 0.5% EM grade glutaraldehyde. The brainstem with the hypoglossal nucleus and nerve was identified and dissected. Retaining hypoglossal nerve rootlets, the right and left sides of the brainstem were separated by cutting along the center midline. These hemi-medullas, approximately 2 mm in diameter, were then post-fixed in fixative with 1.5% EM grade glutaraldehyde overnight at room temperature, and processed for EM analyses as previously described (von Bartheld, 2001). Briefly, after perfusion and fixation, tissue was post-fixed in 1% osmium tetroxide in 0.1M sodium cacodylate buffer for 1 hour, dehydrated in a graded ethanol series, followed by final dehydration in 100% propylene oxide. Tissues were infiltrated in 1 part Spurr's resin 2 parts propylene oxide, then 1:1 Spurr's: propylene oxide, one hour each, followed by overnight 2:1 Spurr's to propylene. Infiltration and embedding was completed in fresh 100% Spurr's overnight at 37°C. Hemi-medullas were oriented for cutting longitudinal sections along the nerve rootlets, perpendicular to the rostrocaudal axis of the medulla. Flat-embedded samples were baked at 59-65°C until blocks hardened, overnight to 36 hours.
Initially, semi-thin (1 μm) sections were prepared and stained with 1% toluidine blue to confirm tissue orientation and adequate morphology. After identification of hypoglossal nerve rootlets and their entry into the brainstem in semi-thin sections, ultra-thin sections (70–80 nm) were cut on a Leica (Nussloch, Germany) ultramicrotome and stained with 2.5% aqueous lead citrate and uranyl acetate. For collection and analysis of serial thin sections, 2 mm × 1 mm slot grids (Agar Aids, UK) were coated with 0.3% Formvar (Hayat, 1981). Adjacent sections were collected onto a single grid, thus allowing the same axon to be more easily identified in a series of adjacent sections. Sections were examined in a Philips CM10 transmission electron microscope at magnifications ranging from ×490 to ×92,000. Magnification of ×25,000 was used for analysis of MVBs. Images were captured on a Gatan 792 BioScan digital imaging system (Software: Digital Micrograph 3.5.2) and were optimized exclusively for brightness and contrast by using Adobe Photoshop 7.0.
Classification, measurement and computation of fractional area (FA) of MVBs and MVB-like organelles
For each nerve, we scanned the entire proximal nerve rootlet (= sample area) at a magnification of ×4,600. Each organelle that possibly represented an MVB was then examined at high magnification, ×25,000, and images were taken and used for identification of MVB type, size measurement, and location in either axon shaft or paranode/node. We analyzed 334 images obtained from 37 different thin sections, representing 14 total nerves taken from 12 different animals. Within these 334 images, we found 507 profiles of multivesicular organelles (Table 1). Categorizing by type was done by evaluating each multivesicular organelle according to a list of parameters, fully described in results. Briefly, Type-1: 50-200 nm diameter, single limiting membrane, small (∼30 nm diameter) internal vesicles. Type-2: 70-200 nm diameter, single limiting membrane enclosing small internal vesicles, central empty space. Type-3: classic MVB, generally >250 nm diameter, single limiting membrane, multiple internal vesicles (∼50-80 nm diameter). Type-4: similar to type-3, but noticeably more electron dense. Type-5: generally >250 nm diameter, outer limiting membrane less distinct, very electron dense, internal vesicles less distinct. To measure the area and volume occupied by MVBs and MVB-like organelles, we used the point-counting method as described by Howard and Reed, 1998. Briefly, we overlaid a grid, 125 nm × 125 nm (15,625 nm2) squares, randomly on each image. All center points overlying an organelle were tallied, multiplied by the area of the grid-square, thereby estimating the area occupied by the organelle. The shape of MVBs was confirmed by collecting serial sections, 70-80 nm thick, and visually aligning images of identified MVBs and other landmarks. When a miniature MVB (type 1 or 2) did not appear in more than one section, we concluded that its largest diameter was <150 nm, and that the structure was relatively spherical or oval, but not tubular.
Table 1. Sample Numbers in Hypoglossal Nerve Analyzed in Normal and Experimental Conditions.
| Condition | Nerve Area (μm2) | Grids | Images | Axons | MVB Profiles |
|---|---|---|---|---|---|
| Normal | 66,719 | 10 | 60 | 381 | 97 |
| Buffer-Injected | 79,062 | 5 | 34 | 507 | 38 |
| + GDNF | 116,250 | 6 | 66 | 863 | 121 |
| + BDNF | 140,006 | 6 | 41 | 3,837 | 50 |
| Cold | 75,938 | 4 | 50 | 1,137 | 82 |
| Delayed Fixation | 26,875 | 6 | 83 | 895 | 119 |
| GDNF-I125 | 93,281 | 9 | 248 | - | - |
| BDNF I125 | 126,094 | 7 | 223 | - | - |
| QD-BDNF | - | 12 | 41 | - | - |
| Acid Phosphatase | - | 3 | 45 | - | - |
| Total | 645,243 | 69 | 891 | 7,620 | 507 |
Nerve rootlet area was calculated in the same way, using a grid-square of 156.25 μm2 overlaid on the entire nerve scanned for these images. Grid-squares were counted when they were covered at least ½ by rootlet area. The total area of the nerve rootlet equaled the total number of grid-squares × 156.25 μm2. Total axoplasm, shaft axoplasm, and node axoplasm were estimated from random samples of the nerve rootlet observed at higher magnification. When the MVB was located within the paranode or node region, we counted it as “node.” When it was located in the internode region we counted it as “shaft.” We estimated the percentage of nerve that is axoplasm by measuring the percent axoplasm in random samples and applying that percentage to the entire nerve rootlet area previously measured. Axoplasm did not include myelin but did include axoplasmic paranode/node region. Axoplasmic paranode/node region (“node”) was measured separately and subtracted from total axoplasm region. The fractional area (FA) for each organelle equals total area occupied by the organelle divided by the total area of axoplasm (=100%). We repeated these calculations to determine the FA of MVBs for node and shaft regions separately. The FA was indicated as % of total.
We measured the size of MVB profiles in the soma of hypoglossal neurons by taking random sample images from four different hypoglossal nuclei and measuring the diameter of all MVBs in the images. From the diameter measurements, area was calculated for all MVBs counted. A total of 36 somatic MVB profiles were quantified.
Estimation of the number of MVBs in axons
Precise numerical estimates of MVBs in the hypoglossal axons are beyond the scope of this study, because the entire reference space (including the branches into the tongue) would have to be identified and sampled. This task is also problematic because other (autonomic) nerves are known to join the hypoglossal nerve in the periphery (O'Reilly and FitzGerald, 1990). Nevertheless, we were able to estimate MVB numbers using the following strategy and with the following assumptions. In our sample volume, we calculated the total volume occupied by MVBs. We extrapolated for the total volume of an average hypoglossal axon. We then derived the number of MVBs, for each of the five types separately, by using the percentages of FA for each type. Because it is difficult to precisely measure the average size of organelles in sections (Hedreen, 1998; Howard and Reed, 1998), we used the entire range (minimum and maximum) for the sizes of MVB types and therefore report lower and higher estimates for each MVB type. For these estimates, the following assumptions were made: 1. The MVB volume in the sampled proximal segments is representative of both proximal and distal segments of the axons. 2. MVBs are considered to be spherical (this is a reasonable estimation, since elongated tubules in longitudinal or cross sections were extremely rare). 3. The actual average MVB size relevant for calculating MVB numbers is between the minimal and the maximal sizes used in the calculation. This approach provides a useful approximation of MVB numbers in hypoglossal axons and gives minimal and maximal estimates. We used the same strategy to estimate the total number of MVBs in an average hypoglossal motoneuron soma. The average hypoglossal neuron diameter was 25.2 μm and the average hypoglossal neuron soma volume was estimated at 8,180 μm3.
Acid phosphatase assay
The method used was adapted from published protocols (Gomori, 1956; Krizbai et al., 1997). Rat pups were deeply anesthetized with an overdose of Euthasol and immediately perfused with 0.1M cacodylate buffer, pH 7.4, containing 2.0% EM grade glutaraldehyde and 5% sucrose. Tissue was dissected into cold buffer and then post-fixed in perfusion buffer for 45 minutes at 4°C. After 3 washes in 0.1M cacodylate buffer, acid phosphatase reaction was carried out for 90 minutes at 37°C with gentle rocking, in 100× volume of 0.1M acetate buffer (pH 5.0), with 1mM β-glycerophosphate (Sigma, St. Louis, MO) and 3mM lead nitrate (Sigma, St. Louis, MO). Three washes in 0.1M acetate (pH 5.0) buffer were followed by 2 washes in 0.1M cacodylate buffer (pH 7.2) with 5% sucrose. Tissue was re-fixed in 0.1M cacodylate buffer (pH 7.2) with 3% glutaraldehyde for 1 hour and then washed overnight at 4°C in fresh buffer. The tissue was then processed for EM analysis as described above.
Experimental conditions
In addition to normal hypoglossal nerves, several experimental conditions were tested for effects on MVB parameters. Rat pups were anesthetized by hypothermia immediately prior to tongue injections. Approximately 100-200 ng of neurotrophic factors (NTFs), recombinant human BDNF or GDNF, or PBS as a control in a volume of 2 μl was injected into the base of the tongue, targeting both sides, thus labeling both right and left hypoglossal nerves. To verify injections, proteins were spiked with radio-iodinated protein as described previously (von Bartheld, 2001). After injections, pups were placed under a heat lamp to generate an ambient temperature of 30-31°C for 7.5 hours until tissue was collected as described above. For the cooling condition, rat pups were placed at 4°C for 30 min prior to tissue collection. For delayed fixation, rat pups were treated as described above for the “NTF injected” group, with the exception that after the overdose of anesthetic, intracardial perfusion was omitted. The dissected hindbrain and nerve were bathed in fixative during dissection instead of being immediately fixed by intracardial perfusion of fixative. Fixation was thereby delayed until after dissection, allowing for a period of dystrophic (ischemic/hypoxic) conditions.
Variability and statistical analysis
Because of the heterogeneity of MVBs encountered in axons, differential parameters for categorizing MVBs were developed, with the primary criteria being a visible single outer membrane, presence of internal vesicles, and size of internal vesicles. Two independent observers compared classification of specific types of MVBs in randomly selected images to confirm the reliability of the definitions for each MVB type. After preliminary analyses, the variability between the two observers was <5%, thus ensuring consistency of classification into MVB types. All counting and measuring was repeated three times, with a variability of approximately 5%. Data were analyzed by one-way and two-way ANOVA, and Student's T-test to determine statistical significance between different experimental conditions. We considered a value of p<0.05 as significant.
Protein iodination and method for in vivo injections
Proteins were radio-iodinated to specific activities of 80-90% as described previously (von Bartheld, 2001), using lactoperoxidase from CalBiochem (Gibbstown, NJ). Rat pups were anesthetized by hypothermia immediately prior to tongue injections. Approximately 50-200 ng of either 125I-BDNF or 125I-GDNF in 2 μl was injected into the base of the tongue, targeting both sides, thus labeling both right and left hypoglossal nerves. After injections, rats were placed under a heat lamp generating an ambient temperature of 30-31°C. Tissue samples were collected for analyses approximately 7.5 hours after injections.
Autoradiography
For autoradiographic electron microscopy, copper mesh 300 EM grids with ultra-thin sections were placed on glass slides and coated with a monolayer of Ilford L4 emulsion (Polysciences Inc., Warrington, PA) diluted to 40% in distilled water (Caro et al., 1962). After brief air drying, grid-containing slides were packaged in dessicated slide boxes and stored in the dark at 4°C for 1.5-3 months before developing, fixing, and staining with lead citrate (von Bartheld, 2001). Sections were examined in a Philips CM10 transmission electron microscope at magnifications ranging from ×490 to ×25,000. Magnification of ×25,000 was used for analysis of silver grains and axon structure. Images were captured on a Gatan 792 BioScan digital imaging system. As mentioned above, images were stored and processed exclusively for optimal brightness and contrast using Adobe Photoshop 7.0.
Analysis of EM autoradiographs
For each sample we scanned the entire proximal nerve rootlet at a magnification of ×4,900. All silver grains that were inside an axon were imaged at high magnification, ×25,000. On 16 grids, representing 7 nerves, from 7 different animals, we found 150-160 silver grains in axons. We counted silver grains by probability circle analysis (Salpeter, 1973), a method that results in partial silver grains being counted. We measured the maximal possible background by taking the grid with the lowest numbers of silver grains and considering them as 100% background. We calculated the silver grain/area on this grid and applied it as maximal background to all other grids. In this study, maximal background ranged from 5-10%, meaning that at least 90-95% of all silver grains represented radiolabeled protein. This is consistent with silver grains being extremely rare over emulsion-coated resin without tissue.
Analysis of quantum dot-BDNF axonal transport
Quantum dot-conjugated BDNF (QD-BDNF) was prepared using a biotin-streptavidin linking scheme. This synthesis scheme produces discrete QD bioconjugates with several advantages: 1) retained ligand bioactivity, 2) minimal non-specific binding (<10%), 3) stable complexes of QD-ligand after endocytosis (Vu et al., 2005; Liu and Vu, 2007; Sundara Rajan et al., 2008). BDNF was selectively biotinylated through primary amine groups by reaction with sulfo-NHS-PEO4-biotin (Pierce, Rockford, IL) at a ×20-fold molar excess of sulfo-NHS-PEO4-biotin, and unreacted biotin product was removed by dialysis. QD-BDNF complexes were formed at a molar ratio of 10 BDNF: 1 QD by incubation of the biotinylated BDNF with streptavidin-conjugated quantum dots.
Quantum dot-conjugated BDNF (QD-BDNF) was injected into the tongues of five P10 rat pups, as described above. Three pups were injected with 1-2 μg QD-BDNF only, in 2-4 μl buffer. To verify microtubule-mediated axonal transport, two pups were first injected with colchicine (Sigma), 200 ng in 2 μl buffer, followed 1 hour later with an injection of QD-BDNF combined with colchicine, resulting in a total injection volume of 4-5 μl. Dissection, fixation, and tissue processing were carried out as described above for experimental conditions. Quantum dots were visualized and identified by their intense dark electron density, size and shape at the ultrastructural level. We analyzed 12 grids representing 6 nerves from 3 animals. Each nerve section was scanned at low magnification, ×7,900. After quantum dots were located, they were analyzed at higher magnification (×25,000, ×46,000, and ×92,000) to identify any associated organelles.
Results
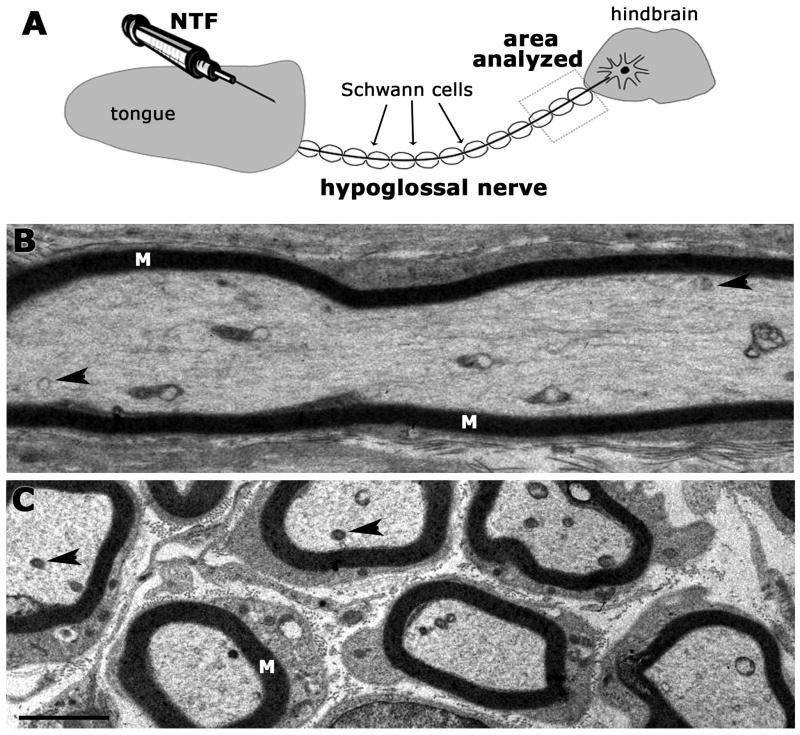
We focused on hypoglossal nerves of P6-9 rat pups within 2 mm of the nerve exit from the medulla (Fig. 1A) in order to quantify MVBs in axons and to determine the relationship between MVBs and retrograde axonal transport of neurotrophic factors. The hypoglossal nerve is myelinated, made up of several rootlets and is anatomically distinct from other cranial nerves such as the adjacent accessory nerve. The nerve area examined for each nerve rootlet ranged from 4,800 μm2 - 38,750 μm2. This area varied with the length of the dissected rootlets, their trajectory, and the plane of sectioning after the embedding process. Typical images of thin sections through the hypoglossal nerve are shown in Fig. 1B, C. Cross-sections of axons captured up to 37 myelinated fibers with an average diameter of 1.5 μm, while images taken along the longitudinal axis occasionally contained as few as one myelinated axon, but more often contained 5-8 axons (Fig. 1B, C).
Fig. 1A-C. Schematic of the hypoglossal nerve and representative images at low magnification.
A. Hypoglossal nerve showing the target (tongue), myelinated hypoglossal nerve with area used for analysis, and hindbrain. All sampling and analysis was done within a 2 mm segment of the nerve proximal to the hindbrain. In some cases, the target was injected with buffer or neurotrophic factor (NTF). B. Example of a longitudinal section through the hypoglossal nerve showing a myelinated (M) axon shaft. Organelles (arrowheads) are visible in the axoplasm at this magnification. All organelles of interest were subsequently examined at higher magnification (examples shown in Figs. 2, 5). C. Example of a cross-section through the hypoglossal nerve. Some of the organelles that are potential MVBs are marked with arrowheads. Organelles of interest were examined at higher magnification. Scale bar for B, C (shown in C) = 2 μm.
MVB types in axons
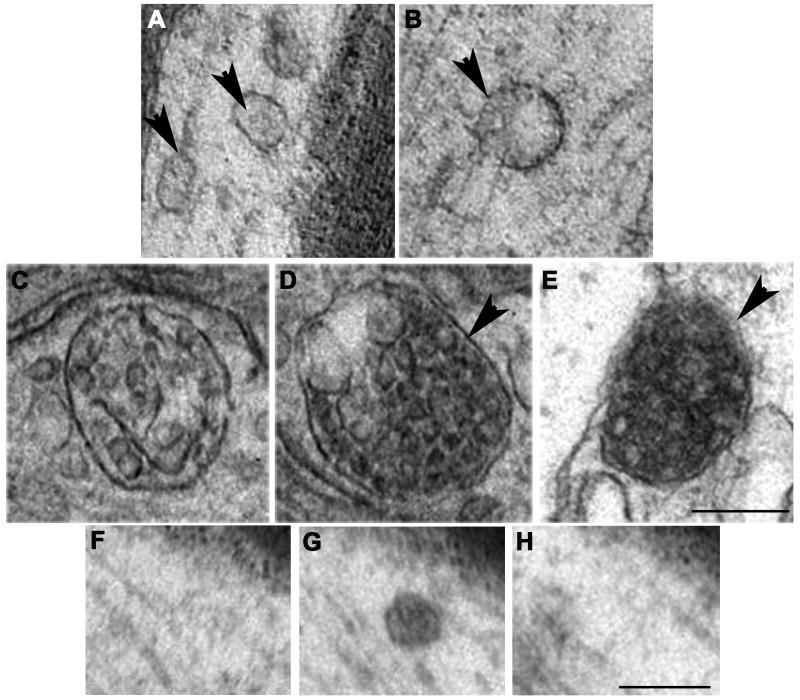
MVBs are classically described as spherical organelles of 250-1000 nm in diameter, containing multiple small (50-80 nm) vesicles, enclosed by a single outer limiting membrane (Roizin et al., 1967; Peters et al., 1991). Such large MVBs were rare in axons, but they were consistently observed. Unexpectedly, we found a range of MVB phenotypes in axons (Fig. 2A-E). We categorized five different types of MVBs and MVB-like organelles. The first two types were considered MVB-like, because they were smaller than typical MVBs as described and illustrated for cell bodies and dendrites in the literature. These “miniature” MVBs were barely above the resolution threshold, and the size of internal vesicles was smaller (20-40 nm) than typically described (50-80 nm). Serial section analysis confirmed that many of these small MVBs were not part of larger tubular structures, nor small fragments of larger MVBs (Fig. 2 F-H). We included these two “MVB-like” types in our analysis, to provide a complete account of MVB-like organelles that may be relevant for transport functions.
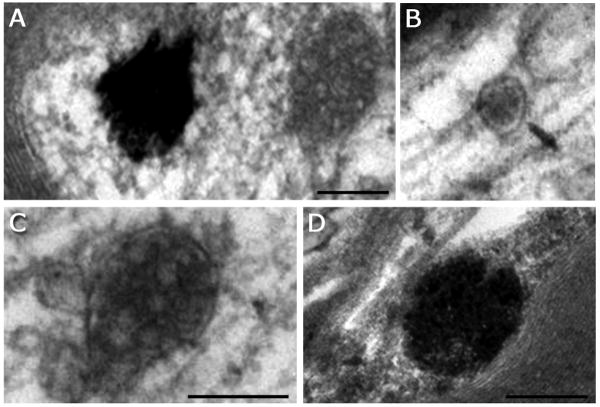
Fig. 2A-H. Images showing five types of MVBs and MVB-like organelles in hypoglossal axons.
A. Type-1: Two MVB-like organelles (arrowheads) containing small-sized internal vesicles scattered throughout the matrix. The left MVB-like organelle appears to be located in close vicinity of a microtubule. B. Type-2: Small MVB-like organelle with small-to-medium-sized internal vesicles, some of them adjacent to the outer membrane (arrowhead). C. Type-3: Classic, large MVB with large, distinct internal vesicles. D. Type-4: Large MVB with large internal vesicles that are less distinct (arrowhead), with internal vesicles showing increased electron density. E. Type-5: Large MVB-like organelle with a single, external membrane that lacks distinct demarcations in some areas (arrowhead). The internal vesicles appear partially fused, and the MVB begins to resemble a transitional state between an MVB and a late endosome. F-H. Serial sections through a type 1 MVB. The small MVB in panel G is visible in only one section (70-80 nm), proving that it is spherical rather than tubular. Scale bar for A-E = 250 nm (shown in E). Scale bar for F-H = 250 nm (shown in G).
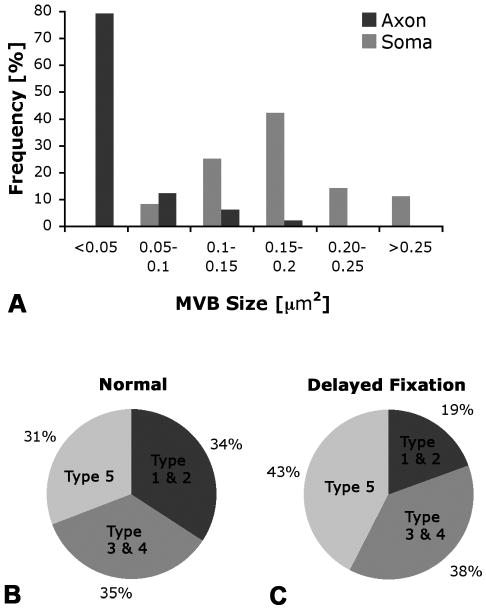
Type-1 is ≤150 nm in diameter, usually oval in shape, has no distinct vacuole or empty spaces, and contains small (30 nm) internal vesicles (Fig. 2A). Type-2 is similar to early endosomes, between 100-250 nm in diameter, with a central vacuole or “empty” space, a single membrane enclosing small (30 nm) internal vesicles, which are usually near the outer membrane (Fig. 2B). Type-3 is large, at least 250 nm in diameter, with a distinct single outer membrane, containing multiple internal vesicles of about 50 nm diameter (Fig. 2C). This type is the classic MVB as described for cell bodies in the literature (Roizin et al., 1967; Peters et al., 1991; Waxman et al., 1995). Type-4 is very similar to type-3 with the noticeable difference of being more electron-dense (compare Fig. 2C with 2D), making the internal vesicles more difficult to discern. Type-5 is similar to late endosomes described by others (Sachse et al., 2002). They are large, ≥300 nm in diameter, electron-dense, with an outer membrane that can have the appearance of a double membrane in some areas and is difficult to discern in other areas, internal vesicles are less distinct (Fig. 2E). We considered type-3 and type-4 as MVBs, type-1 and type-2 as small MVB-like organelles, and type-5 as a structure that has features similar to both MVBs and late endosomes. MVB-like organelles (types 1 and 2) are distinct from smooth endoplasmic reticulum (Lindsey and Ellisman, 1985; Peters et al., 1991) by the round shape and the presence of small internal vesicles. The variety of multivesicular organelles identified in axons made the term “multivesicular body” ambiguous, therefore we developed this classification system as a way to distinguish between the commonly described “classical” MVBs and the MVB-like organelles we found in hypoglossal axons. The range of MVB phenotypes appears more heterogeneous than what has been reported for MVBs in neuronal somata and dendrites. Comparison of MVB diameters measured in hypoglossal neuron soma and axon revealed that in the soma, MVBs were larger than in the axon (Fig. 3A).
Fig. 3A-C. Quantification of MVB size and fractional area (FA) of five MVB types in hypoglossal neurons.
A. In axons, most MVBs are small, < 0.05 μm2, while most MVBs in the soma are 3-4 times larger, 0.1-0.2 μm2. B. In normal axons, small MVBs (types 1 and 2), classic (types 3 and 4), and late-endosomal type MVBs (type 5) make up a similar fraction (about one third) of the total fractional area (FA) of MVBs. C. With delayed fixation, the FA of small MVBs, types 1 and 2, decreases, while the FA of large MVBs, especially the late endosomal type 5, increases.
Quantification of MVB types
To further assess the potential significance of MVBs and their different types (1-5) in axons, we quantified these five MVB types. We measured fractional area (FA), frequency (estimate of numbers per axon), and distribution within the axon for each type and also for all types together. Calculation of FA normalizes the data among various samples. For all MVBs and MVB-like organelles combined, we found an FA of 0.026% +/-0.013% (SEM) in the normal hypoglossal nerve – substantially less than in the hypoglossal motor neuron soma, where MVBs have an FA of about 1.5% (Rind et al., 2005). Comparing the FA of different types of MVBs, we found that about 34% of the total MVB FA was composed of small MVB-like organelles (types 1 and 2), while classic MVBs (types 3 and 4) accounted for 35% of the FA, and the late endosomal type, type 5, made up the remaining 31% (Fig. 3B). The complete data set, showing the number of each type and fractional area (FA) in the normal hypoglossal nerve, is listed in Table 2.
Table 2. Number of MVB types per axon, and fractional area (FA) +/- SEM in postnatal rat hypoglossal nerves.
| Condition | Axoplasm Area sampled [μm2] | Type 1 number/axon | FA [%] | Type 2 number/axon | FA [%] | Type 3 number/axon | FA [%] | Type 4 number/axon | FA [%] | Type 5 number/axon | FA [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 12,118 | 919 | 0.003 | 187 | 0.006 | 30 | 0.002 | 35 | 0.007 | 48 | 0.008 |
| ± SEM | ±0.0014 | ±0.0016 | ±0.0026 | ±0.0048 | ±0.0029 | ||||||
| (Range) | (195–5,667) | (34–538) | (4–274) | (11–87) | (7–853) | ||||||
| Cold | 17,281 | 203 | 0.001 | 80 | 0.001 | 85 | 0.007 | 15 | 0.002 | 69 | 0.012 |
| ± SEM | ±0.0007 | ±0.0005 | ±0.0078 | ±0.0016 | ±0.0054 | ||||||
| (Range) | (43–1,250) | (15–231) | (12–784) | (4–37) | (10–1,216) | ||||||
| Delayed Fix | 5,036 | 2,027 | 0.014 | 313 | 0.004 | 159 | 0.018 | 119 | 0.023 | 239 | 0.044 |
| ± SEM | ±0.0028 | ±0.0003 | ±0.014 | ±0.0012 | ±0.0114 | ||||||
| (Range) | (431–12,500) | (57–904) | (23–1,470) | (37–293) | (37–4,216) | ||||||
Based on the fractional areas for the five different types of MVBs and MVB-like organelles in the sample area, we extrapolated and estimated the volume of MVBs in an average hypoglossal axon, assuming an axon length of 7 mm (medulla to the base of the tongue) and an average diameter of 1.5 μm. According to these parameters, we estimated a total axoplasmic volume of ∼10,800 μm3 for a hypoglossal axon at this age. Based on the area sampled, we extrapolated to the total axon area, and then calculated fractional area (FA) of MVBs and estimated the total number of MVBs in an average hypoglossal axon.
Using the frequencies of MVB types 1-5 (Table 2), we then calculated the expected numbers of all types of MVBs, based on minimum and maximum organelle diameters, as described in the methods. An average hypoglossal axon contained a total of 65 (range: 15 – 361) large MVBs (types 3 and 4) and 1,106 (range: 230 – 6,205) smaller, type 1 and 2, MVB-like organelles. The smaller MVB-like organelles (types 1 and 2) outnumbered the larger MVBs (types 3-5) by a 10:1 ratio (Table 2). Assuming an evenly spaced distribution along the length of the axon, these numbers predict one large MVB for every 108 μm (range: 20 – 460 μm) of axon length and a small MVB-like organelle every 6 μm (range: 1 – 30 μm). Applying an average transport speed of 5 mm/hour (LaVail and LaVail, 1974; Viancour and Kreiter, 1993; Miller and Kaplan, 2001; Delcroix et al., 2003), these frequencies of MVBs translate into one large MVB (types 3-4) arriving at the soma approximately once every 1.3 minutes (range: 0.2 – 5.6 minutes). When we include the smaller types, 1 and 2, and the large late-endosomal type (type 5), the frequency of arrival increases to one multivesicular organelle every 4 seconds (range: 0.6 – 20 seconds). Using either calculation, organelles/axon length or frequency × transport rate, it is clear that MVBs of the classic type are relatively uncommon (one per 108 μm or one every 1.3 minutes at a chosen position) in myelinated axons of the hypoglossal nerve. Random samples from hypoglossal motoneuron somata from four different nuclei predict that an average soma contains 1,249 MVBs, types 3 and 4 (data not shown). Thus, the number of MVBs in the axon is similar to the number of MVBs in the soma, but in the axon the largest fraction consists of the smaller types 1 and 2, which were not observed in the cell soma. We did not attempt to estimate MVB number in dendrites because dendrite volume is difficult to measure or calculate. Considering exclusively types 3 and 4, the soma contains nearly 20 times more MVBs than the axon (1,249 vs. 65).
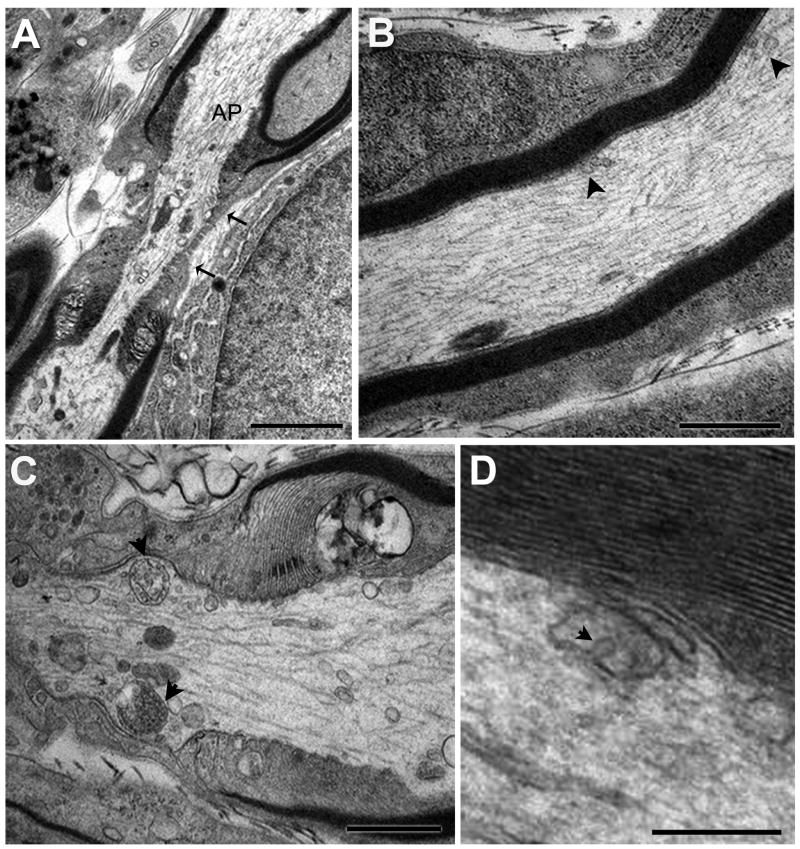
Previous work described differences in organelle distribution between axon shaft and node/paranode region and suggested that the distribution pattern had functional implications (Gatzinsky et al., 1988; Berthold et al., 1993; Waxman et al., 1995; Gatzinsky, 1996). To address this possibility, we identified and calculated the relative percentages of shaft and paranode/node (“node”) in the total nerve area sampled and then calculated FA of each MVB type at node and shaft (Fig. 4A-C). The node axoplasm occupied approximately 2.2% ±0.4% (SEM) of the total nerve area in our samples. In normal hypoglossal axons, the total FA of all types of multivesicular organelles at the node was 11-fold higher than the FA at the shaft. Our data confirms the notion that these organelles are located preferentially in the node region of the axon (Gatzinsky and Berthold, 1990; Berthold et al., 1993; Waxman et al., 1995).
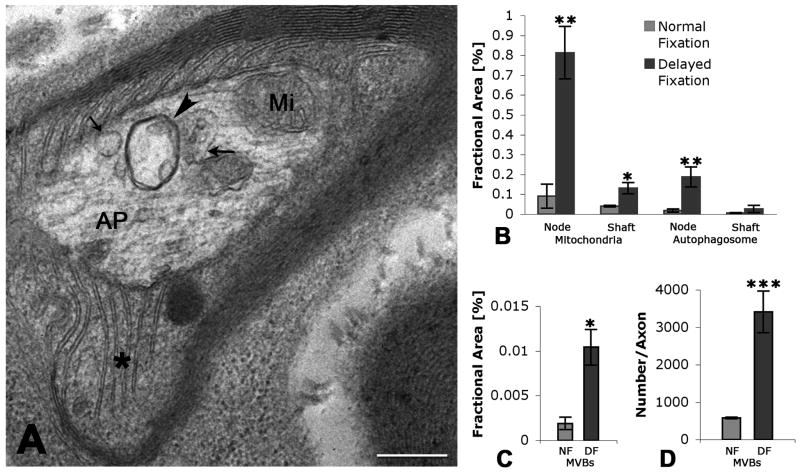
Fig. 4A-D. Morphological features of MVBs at the node of Ranvier and in the axon shaft of the hypoglossal nerve.
A. Representative section showing the axon at the node of Ranvier. The node is characterized by a lack of myelin and significantly restricted area of axoplasm (AP) in the “bottleneck” region (between arrows). B. High magnification of the axon shaft, characterized by dense myelin layers. Organelles (arrowheads) in the axon shaft are less numerous than in the paranode/node region. C. High magnification of the node of Ranvier. Note two MVBs (arrowheads), type 3 and type 4, in near symmetric positions, as well as numerous other organelles. D. High magnification of multivesicular-like organelle in an axon shaft apparently caught during membrane invagination (arrowhead) and formation of an internal vesicle. Scale bars: A = 2 μm, B and C =1 μm, D = 250 nm.
In conclusion, the quantification and frequency of all MVBs and MVB-like sub-types in normal axons, when considered as a fraction of total axoplasm area (FA), reveals that MVBs in axons are rare relative to those found in neuron cell bodies and dendrites, and they are smaller than their counterparts in the soma. Additionally, the MVBs are preferentially located within the paranode/node region of the axoplasm.
MVBs and acid phosphatase analysis
MVBs in other cell types are thought to be part of the sorting/degradation pathway (Piper and Katzmann, 2007) and can contain the degradative enzyme, acid phosphatase (Roizin et al., 1967; Holtzman et al., 1973). Therefore, we asked if MVBs in axons had properties associated with either transport or degradation. We collected tissue from animals immediately after perfusion and examined axons for acid phosphatase activity. We found that of the five MVB types identified in axons, only a fraction of the large types, 4 and 5, showed acid phosphatase activity with less than 10% of these organelles labeled (Fig. 5A, C). Types 1 and 2, the small MVB-like organelles, did not show reaction product (Fig. 5B), nor did the large classic MVBs, type 3. As a positive control, lysosomes in brain stem neurons and Schwann cell bodies showed acid phosphatase precipitates (Fig. 5D). These data support the notion that most MVBs in axons serve transport functions, although a smaller number of larger MVBs (that resemble late endosomes) become increasingly acidic and may have degradative functions (Gatzinsky, 1996; Overly and Hollenbeck, 1996).
Fig. 5A-D. Acid phosphatase activity is rare in axonal MVBs.
Immediately perfused and fixed hypoglossal nerves were processed for acid phosphatase activity (A-D). A. Acid phosphatase-positive large MVBs were found in axons, but they were rare. B. Small MVBs, types 1and 2, did not show acid phosphatase activity. C. MVB type 4 lacks acid phosphatase activity. D. Lysosome in Schwann cell, very close to myelin, is acid phosphatase positive (positive control). Scale bars (same for A and B) = 250 nm.
Target manipulations
Studies reporting MVBs in axons have often used injection of traceable exogenous protein to track endocytosis or transport within axons (LaVail and LaVail, 1974; Chu-Wang and Oppenheim, 1980; Buchner et al., 1987). Furthermore, treatment of the tongue with the trophic factors BDNF or GDNF increased the number of MVBs in vicinity of hypoglossal motor nucleus synapses (Rind et al., 2005). Since we observed very few MVBs in axons, we asked if target manipulation, specifically any injury inflicted during PBS injection into the muscle or increased levels of trophic factors in the muscle target, may cause an increase in MVBs or MVB-like organelles in axons. Comparing uninjected normal with buffer-injected control animals, we found an MVB FA of 0.026% +/- 0.013% (SEM) for normal vs. FA of 0.017% +/- 0.013% (SEM) for buffer-injected, when considering all types of MVBs and MVB-like organelles. There was no statistically significant difference in overall MVB area (p=0.6) between these conditions (normal vs. target injury). Thus, the injection procedure itself does not cause an increase in MVB FA in axons.
To test if increasing the amount of axonal cargo in the form of neurotrophic factors (NTFs) might affect the frequency of multivesicular organelles in these axons, we injected BDNF or GDNF in the tongue. These proteins are normally available to and transported by the hypoglossal nerve (Chiu et al., 1994; Russell et al., 2000). There was no significant difference between total MVB FA of hypoglossal axons in BDNF- or GDNF-injected animals, therefore we combined these data in one group (n=7). In the NTF- injected condition, we found an overall FA of 0.019% +/-0.006% (SEM), when considering all MVB types together. This reflects a slight increase in FA over buffer-injected animals (0.019% vs 0.017%), but there were no statistically significant differences between any of these groups. However, there was a change in the types of organelles, especially for the large MVBs, in the axons after NTF injection. NTF injection increased the FA of small MVBs (types 1, 2) by 1.4× (p=0.7), but increased the FA of large MVBs (types 3, 4, 5) by a statistically significant 3× (p<0.03) over buffer-injected axons.
Distribution of multivesicular organelles in axon shaft vs node may change in response to target manipulation. In buffer-injected animals, MVBs had a node FA that was four times that of the axon shaft, and NTF-injected animals had a node FA that was 2.4-fold that of shaft FA. When these differences were compared with normal (=11-fold higher node than shaft distribution), they were not statistically significant (p>0.05). These findings indicate that NTF injection shows a trend in the distribution of the MVBs, toward an increased presence in the shaft, but overall the “preferred” nodal distribution was retained.
Cooling
Several previous studies that identified MVBs in axons or axon terminals applied cold temperature during tissue fixation and/or analysis (Chu-Wang and Oppenheim, 1980; Tsukita and Ishikawa, 1980). Because axonal MVBs were relatively rare in our analysis, we tested if cooling increased MVBs in axons of the hypoglossal nerve. We subjected the animals to cold temperature for 30 – 60 minutes immediately prior to tissue collection. This cooling procedure resulted in an MVB FA of 0.024% +/- 0.0005% (SEM), which is not significantly different from either normal (room) temperature condition (p=0.08) or the normally treated, NTF injected condition (p= 0.3). When measured by fractional area, the distribution of MVBs and MVB-like organelles in the cooling experiments was also nodal more than shaft (a 7-fold difference), as found in buffer-injected and NTF-injected animals. This shows that MVB distribution is not significantly affected by cooling the animal. One of the images from the axon shaft of a cold-treated animals showed an MVB-like organelle with a membrane invagination – apparently an internal vesicle was captured during its formation or, conversely, during fusion with the organelle membrane (Fig. 4D). There was only one such image among the 507 MVB profiles we analyzed. While this single event does not allow for any statistical correlations or analysis, it nevertheless suggests that MVBs are dynamic structures within the axon.
Dystrophic condition
To test the idea that dystrophic, stress-related parameters may increase MVB formation, and specifically may cause an increase of axonal MVBs, we generated a “dystrophic condition” by delaying fixation after death of the animal (Kristensson et al., 1971). Delayed fixation was achieved by omitting immediate perfusion fixation, and instead, using immersion fixation 20-30 minutes after administering a lethal dose of Euthasol, thus avoiding any mechanical injury of axons. Delaying fixation caused a dramatic, 3-5-fold increase in FA of MVBs, MVB-like organelles, mitochondria, and autophagosomes in axons (Fig. 6A-D). The total FA, for all MVB types combined, in the delayed fixation condition was 0.09% +/- 0.02% (SEM) compared with a FA of 0.019% +/- 0.006% (SEM) for immediate fixation, an approximately 5-fold increase (p=0.0008) (Fig. 6C). This increase was not limited to just one type of MVB organelle, nor was it equally distributed among the types of MVBs. Type-1and 2 MVB-like organelles together increased by 2-fold, whereas the FA of type-5 MVBs increased by 7-fold. The distribution of the organelles remained predominately nodal, as was found in all conditions with target manipulations. From this data, we conclude that delaying fixation, essentially stressing the neuron with a period of hypoxia, resulted in a substantial increase in MVBs and MVB-like organelles in the axon.
Fig. 6A-D. Delayed fixation significantly increases total fractional area of MVBs and total number of MVBs/axon.
A. Cross-section of an axon with delayed fixation is shown at high magnification. Section shows node of Ranvier, as defined by lack of myelin and Schwann cell cytoplasm between myelin layers (asterisk). Organelles that accumulated within bottlenecked axoplasm (AP) include an autophagosome (arrowhead), mitochondria (Mi), classic MVB (large arrow) and MVB-like organelle (small arrow). B. Fractional area of mitochondria and autophagosomes was significantly increased in the node of Ranvier in axons that were treated with delayed fixation. C. Fractional area (FA) of MVBs (all types combined) was significantly increased with delayed fixation (DF) when compared to perfused, immediately fixed axons (Normal fixation, NF). D. Estimated number of MVBs (all types combined) per axon was significantly increased when axons were treated with delayed fixation (DF), indicating that new MVBs have formed. Error bars = SEM; *p<0.05; **p<0.025; ***p<0.005; NF: Normal (immediate) fixation; DF: delayed fixation; Scale bar for A = 500 nm.
Under normal conditions, the total FA of MVB organelles was distributed evenly (about one third each) among small MVB-like organelles, MVBs, and late-endosome-like MVBs (Fig. 3B). Under the experimental condition with delayed fixation, small MVB-like organelles (types 1 and 2) accounted for only 19% of the now increased total FA, larger MVBs (type 3 and 4) made up 38%, while 43% of the total MVB FA was now composed of late endosomal type MVBs (type 5) (Fig. 3C). Thus, in axons subjected to delayed fixation, we found a decrease in small, endosomal-like MVBs, while the other two groups, classic MVBs and the late endosomal type, both increased in terms of percentage of total FA (Fig. 3B-C). These findings suggest a highly dynamic relationship between the types of multivesicular organelles and the physiological state of the axon.
Radiolabeled neurotrophic factors do not associate with MVBs
NTFs accumulate in MVBs in the neuronal cell body after retrograde axonal transport (Stoeckel et al., 1975; Claude et al., 1982; Rind et al., 2005), but it has remained unclear whether NTFs use MVBs as retrograde carriers already during axonal transport (Parton et al., 1992; Weible and Hendry, 2004; Valdez et al., 2007), or whether they accumulate in MVBs only after arrival in the soma. To determine whether these NTFs travel in MVBs during axonal transport, we analyzed axons at the ultrastructural level, after tongue injection of radiolabeled GDNF or BDNF. Using this method, the location of the transported neurotrophin within the axon as well as other axonal structures such as MVBs, mitochondria, and microtubules can be visualized. Since radiolabeled trophins injected in the tongue of rat pups already accumulate in the hypoglossal nucleus in the brainstem eight hours after injection (Rind et al., 2005), we allowed 7.5 hours for internalization and axonal transport to visualize neurotrophins en route in proximal nerve segments.
Silver grains representing radiolabeled BDNF or GDNF were visible in both axon shafts and node regions. We found 152.2 silver grains in the axoplasm, 5% were associated with mitochondria, 11.2% were associated with unidentifiable structures, and only one silver grain (<1%) was in the vicinity of a large MVB (type 4), leaving the majority 83.6% unassociated with any visible structure in the axon excluding microtubules (Fig. 7A-D). Due to the relatively large size of silver grains, we were unable to absolutely rule out small MVBs, types 1 and 2, as the NTF carrier. To determine whether radiolabeled NTFs may accumulate in MVBs when the number of MVBs in axons was increased by dystrophic conditions (delayed fixation), one rat pup was injected with radiolabeled GDNF, and the tissue was processed for autoradiography after delayed fixation. In this case, we did not find any silver grains associated with large MVBs (types 3-5). Therefore, we conclude that even when the number and FA of large MVBs were artificially increased, radiolabeled GDNF still failed to associate with MVBs. These results strongly support the hypothesis that the retrograde transport vesicle is not a large MVB (types 3, 4 or 5).
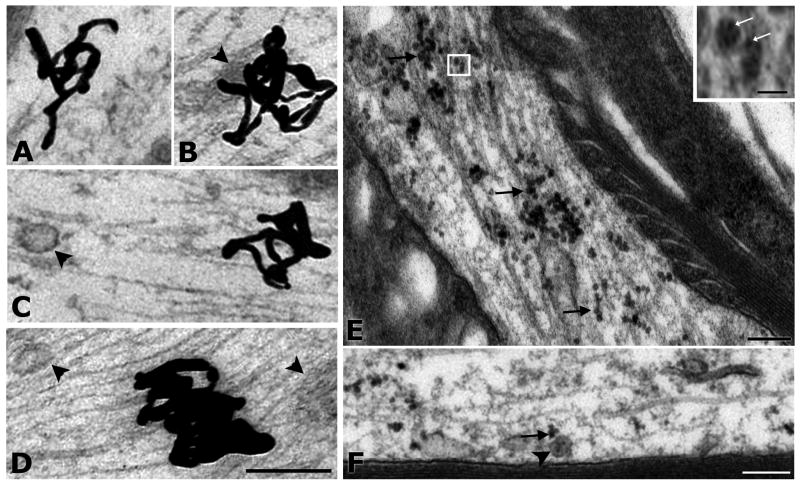
Fig. 7A-E. Retrogradely transported neurotrophic factors are not associated with MVBs in hypoglossal axons.
I125-GDNF and I125-BDNF were visualized by autoradiography of thin sections coated with a monolayer emulsion (A-D). A. Silver grain (SG) representing I125-GDNF, in axoplasm is not associated with any organelle. B. SG representing I125-GDNF in axoplasm next to an undefined structure (arrowhead). C. SG, representing I125-BDNF, is not associated with MVB type 1 (arrowhead). D. Dense SG in axoplasm is near, but not associated with MVB-like organelles (arrowheads). Quantum dot-conjugated BDNF (QD-BDNF) was evident by intense electron density of QDs in thin sections (E-F). E. Example of QD-BDNF in the node of a heavily-labeled axon. Many QDs appear to be associated with microtubules (arrows). At higher magnification (inset) it is apparent that the uniformly sized QD-BDNF is composed of multiple QDs (white arrows), possibly within a small endosomal organelle. Scale bar for inset = 50 nm. F. QD-conjugated BDNF (arrow) is located in close vicinity, but not within a “miniature” (type 1) MVB (arrowhead). Scale bars (same for panels A-D) = 200 nm.
We also analyzed the distribution of silver grains within the axon. We found that 68.6% of the silver grains were located in the axon shaft. However, because the axon shaft makes up 70-95% of the measured area in our samples, the labeling density of silver grains in the axon shaft was 0.8. The LD of silver grains at the node was 3.5. The silver grain accumulation (LD) at the node was significantly different when compared to the labeling in the shaft, p=0.01 for BDNF and GDNF combined. Therefore, consistent with the differential distribution of organelles such as MVBs among node and shaft, transported BDNF and GDNF were also found more frequently at the node than in the shaft of axons, suggesting that the node/paranode has specific cytoskeletal arrangements which may affect transport of multiple types of organelles.
Quantum dot (QD) analysis of BDNF transport
The relationship between radiolabled BDNF and larger (types 3-5) MVBs can be readily assessed by autoradiography as described above. However, silver grains are relatively large and may obscure the small type (“miniature”) MVBs (types 1-2), making it difficult to assess the potential association of these organelles with NTFs. We therefore used quantum-dot conjugated BDNF (QD-BDNF) to determine whether any types of axonal MVBs may carry BDNF retrogradely along hypoglossal motor axons. QDs are intensely electron dense and are thus distinguishable from other electron dense structures normally seen in small endosomes and MVBs. Following injection of QD-BDNF in the tongue, QDs were found in the axons of the hypoglossal nerve. When colchicine was co-injected with the QD-BDNF, no QDs were observed in the nerve, showing that QD-BDNF arrived in the nerve by axonal transport that was microtubule-dependent. In QD-labeled axons, the QDs were not associated with MVBs or MVB-like organelles, but rather appeared along microtubules, possibly in small endosomal organelles (Fig. 7E-F). These data confirm the conclusions reached for larger type MVBs. Thus, none of the five MVB types in axons function as the carrier organelle for retrograde transport of BDNF or GDNF, and we conclude that these NTFs accumulate in somatic MVBs only after their arrival in the soma.
Discussion
Our study is the first that quantifies MVBs in axons and tests the hypothesis that these organelles carry neurotrophic factors from axon terminals to the soma. MVBs in axons are relatively rare when compared with other neuronal compartments such as cell bodies and dendrites, but axons contain a surprising variety of MVB-like organelles. By tracking radiolabeled and quantum-dot conjugated neurotrophic factors, our study refutes the commonly held belief that MVBs are the primary carriers of neurotrophic factors during axonal transport from the axon terminals to the cell body (Weible and Hendry, 2004). Furthermore, we show that MVBs in axons can be artificially generated de novo by dystrophic conditions. Thus, our study adds several new perspectives to the understanding of functions of MVBs in axons.
Technical considerations
MVBs have previously been quantified in other neuronal compartments, but not in axons. Quantitative studies are always potentially biased (Guillery, 2002), each study has its limitations, and it is important to state such limitations clearly. We have spelled out any assumptions that were made with extrapolation or estimation of average MVB sizes in the methods. The type 1 and 2 MVB-like organelles (“miniature” MVBs) were near the threshold of resolution, and it is likely that some were missed because they were not recognized. Thus, the number of these “miniature” MVB-like organelles may be underestimated due to their small size. We believe that they can be differentiated from other organelles such as smooth endoplasmic reticulum (Lindsey and Ellisman, 1985; Peters et al., 1991). To render an account as complete as possible, we included and classified them as MVB-like organelles, based on the fact that they are single membrane-bound organelles containing multiple vesicles.
For the estimation of numbers of MVBs, we assumed that these organelles were spherical, because we rarely saw elongated tubular organelles with MVB-like characteristics in longitudinal or cross sections, and type 1 and 2 MVBs were shown by serial section analysis to be spherical. We sampled about 16% (Table 1) of all the hypoglossal axons, which number about 3,500 on each side in postnatal rats (Paxinos, 2004). Our sample area included the proximal segments of the peripheral nerve, adjacent to the medulla. Since the distal hypoglossal nerve is joined by additional nerve fibers (O'Reilly and FitzGerald, 1990), the peripheral course of the nerve is virtually impossible to sample systematically and with the same precision as the proximal segment.
Since we used radiolabeled and quantum-dot conjugated neurotrophic factors for our localization analysis, the question arises whether these modifications may alter the internalization, axonal transport or pathways of these proteins as compared to native trophins. Radiolabeling is considered the most insignificant protein modification, less disruptive than an epitope tag or reporter sequence, although receptor-binding and signaling can be affected in a small fraction of molecules, depending on the combination of altered moieties (tyrosine residues, von Bartheld, 2001). Quantum dot-conjugated neurotrophins have been shown to retain their biological activity and co-localize with Trk receptors, Rab and EEA1 early vesicle endosome markers, and the activated ERK1/2 during downstream signaling, thus supporting the notion that quantum dot probes serve as valid reporters of intracellular neurotrophin trafficking (Vu et al., 2005; Cui et al., 2007; Sundara Rajan et al., 2008). The work presented here is the first report of successful uptake of quantum dot-conjugated neurotrophin probes by myelinated axons in an in vivo preparation. Further details of the internalization and trafficking kinetics of quantum dot-conjugated neurotrophins at the ultrastructural level remain to be determined.
Types of axonal MVBs: progression through morphological types during maturation?
MVBs are defined in the literature as >250 nm diameter organelles being delimited by a single (outer) membrane, and containing multiple internal vesicles (Roizin et al., 1967). While MVBs in neuronal cell bodies and dendrites are of a more uniform, classical type, our analysis revealed a variety of multivesicular, MVB-like organelles in axons. The presence of a transitional appearance of MVB-like organelles suggests a relationship between the different types, and possibly a functional transition. In non-neuronal cells and in the neuronal cell soma, MVBs are thought to be part of the sorting mechanism for endocytosed proteins. Endocytosed proteins are sorted in MVBs and recycled back to the plasma membrane, or transferred to late endosomes (Gruenberg and Stenmark, 2004; Bache et al., 2006; Myromslien et al., 2006; Piper and Katzmann, 2007). Similarly, multivesicular organelles progress from an endocytic vesicle morphology to a tubular complex to either form a large MVB or may release small vesicles in dendrites of hippocampal neurons and cultured cortical neurons (Cooney et al., 2002; Faure et al., 2006). The morphological progression described by Cooney et al. and tracked via molecular techniques by Faure et al. is similar to the morphological progression of MVB formation described in non-neuronal cells (Piper and Katzmann, 2007).
The biogenesis of MVBs in axons is not known. The possibilities include de novo formation in axons, anterograde transport from the cell soma, transfer from neighboring Schwann cells, and formation in axon terminals followed by retrograde transport into axons. There is no direct evidence to favor any one particular possibility, except that MVBs minimally accumulate at the proximal side of a ligature (Smith, 1980; Tsukita and Ishikawa, 1980; Hirokawa et al., 1990), making anterograde axonal transport from the soma unlikely. MVBs can be present in axon terminals and they are always found in the soma (Schwab, 1977; Claude et al., 1982; Waxman et al., 1995; Rind et al., 2005; Valdez et al., 2005), but it is not known whether these are the same MVBs that can be observed in axons. Labeled proteins internalized via receptor-mediated endocytosis are found in endosomes in the axon (Delcroix et al., 2003; Bronfman et al., 2007; Cui et al., 2007) and eventually pass through MVBs in the neuronal soma (Rind et al., 2005). These findings are consistent with endosomes progressing to become MVBs by the time their transport along the axon is completed, but also with the notion that internalized proteins use a different type of transport organelle to reach the cell body and only subsequently become incorporated into somal MVBs (Pioro et al., 1991).
Endosomes are a heterogeneous population of vesicles, thus allowing for differential endocytosis according to cargo and/or ultimate destination: recycling or lysosomal degradation. There are differences in the molecular composition and reagent sensitivity among endosomes, which are not necessarily evident on the basis of morphology alone (Bronfman et al., 2003; Hibbert et al., 2006; Wu et al., 2009). The hypothesis that retrograde transport in the axon is accompanied by a similar progression of organelle maturation, possibly following a gradient towards more acidic pH (Overly and Hollenbeck, 1996), is still untested. Our finding of lack of degradative markers such as acid phosphatase in all “miniature” MVBs and in most larger MVBs is consistent with the idea that MVBs function primarily to traffick and sort proteins rather than to degrade them. Axonal MVBs did not contain any of the NTFs we tested (see below), but this does not negate trafficking functions for other proteins such as the cannabinoid receptor that can reside in axonal MVBs (Vitalis et al., 2008).
Lack of neurotrophic factors in axonal MVBs
MVB-mediated retrograde transport of internalized proteins, from target terminals to the cell body, has been suggested by numerous investigators and is widely believed to be the primary function of axonal MVBs (LaVail and LaVail, 1974; Smith, 1980; Schmied and Holtzman, 1987; Sandow et al., 2000; Weible and Hendry, 2004). However, none of these studies provided direct evidence that MVBs in normal axons actually transport neurotrophic factors retrogradely. Therefore, questions remained, especially as MVBs are rare in normal axons and it is unclear how activated receptors within MVBs would initiate signal transduction (Kapeller and Mayor, 1969; Castel et al., 1992b; Delcroix et al., 2003; Wu et al., 2009).
Based on our quantitative analysis of MVBs in hypoglossal axons, we first concluded that theoretically, there was a sufficient number of MVBs in these axons to serve a long-distance trophic signaling function. The MVBs observed in previous studies on retrograde transport are large, >250 nm diameter, single membrane limited, multivesicular organelles, types 3 and 4 in our categorization. We estimated the frequency of types 3 and 4 MVBs to be, on average, 65 MVBs per axon. For any organelle, to travel the distance of 7 mm from the axon terminal to the soma requires 1.4 hours, according to known rates of retrograde axonal transport. In the case of large MVBs, subsequent MVBs will arrive on average at intervals of 1.3 minutes. Previous studies have investigated temporal aspects of nerve growth factor (NGF) rescue after NGF deprivation of cultured neurons (Deckwerth and Johnson, 1993), appearance of phosphorylation of CREB after NGF application to neurons grown in compartmentalized cell culture dishes (Riccio et al., 1997), and rescue of neuron cell bodies by application of NTFs after axon transection in vivo (Oliveira et al., 2002). In all of these studies, the time course was not resolved to seconds or minutes, but rather hours. Therefore, the first set of data from our study is consistent with the hypothesis that MVBs may be carriers for retrograde transport of NTFs.
However, when we tested this hypothesis directly by tracking radiolabeled BDNF and GDNF that are known to accumulate in hypoglossal cell bodies in rat pups after tongue injections (Rind et al., 2005), we found that these NTFs did not associate with any of the five types of MVBs during retrograde axonal transport. Since the resolution of silver grains is not entirely conclusive for the smaller type MVBs (“miniature” MVBs types 1 and 2), we confirmed the lack of association with high-resolution QD-BDNF. Thus, contrary to widely held expectations, our experimental data conclusively demonstrate that these NTFs do not use axonal MVBs as a carrier organelle. Rather, NTFs appear to travel in the axon in very small endosomes. These endosomes accumulate several QD-BDNF molecules (Fig. 7E), unlike those described for QD-NGF in vitro which typically accumulates only 1 or 2 QDs in small vesicles (Cui et al., 2007). This may reflect differences between studies in the QD conjugation or tissue processing, differences between in vitro and in vivo preparations, or differences between the unmyelinated dendritic processes of sensory neurons versus myelinated axons. Whether native BDNF resides in such signaling endosomes during axonal transport (Oppenheim and von Bartheld, 2008) or whether they form in response to QDs remains to be determined. It should be noted that the lack of association of two different NTFs with MVBs does not allow one to conclude that MVBs do not traffick any transmembrane receptors and their ligands in axons. In fact, a recent study showed that cannabinoid receptors can reside within axonal MVBs (Vitalis et al., 2008), making it likely that axonal MVBs serve trafficking functions for these G-protein coupled receptors.
Other possible physiological functions of MVBs in axons
Besides axonal transport of internalized proteins, three other functions of axonal MVBs have been proposed. One is communication and membrane exchange between the axon and its myelinating Schwann cell (Gatzinsky and Berthold, 1990; Reles and Friede, 1991; Gatzinsky et al., 1997). Another is a function of “detoxification” and removal of harmful substances before they reach the CNS (Gatzinsky, 1996). In addition, a mechanical phenomenon has been suggested that occurs with slowing of vesicle axonal transport, in the area of the node “bottleneck” (Armstrong et al., 1987). The data presented in our study suggest a fourth function: a response of the axon to dystrophic conditions induced by ischemia/hypoxia, as discussed in the next section.
Our data are consistent with the hypothesis that the node of Ranvier interacts with the surrounding Schwann cells via MVBs. As previously proposed (Gatzinsky, 1996; Gatzinsky et al., 1997), the nodes of Ranvier may act to sequester, degrade, and export substances or to facilitate exchange of essential factors with surrounding Schwann cells before the axon enters the CNS. In all experimental conditions tested in this study, we found a nodal distribution of MVBs. This finding is consistent with the model that MVBs have a specific function at the node of Ranvier.
On the other hand, increased FA of MVBs in the node region could simply be the result of slowed transport through the node. It is well established that axonal transport is affected by nodal-specific cytoskeletal arrangements (Reles and Friede, 1991; Price et al., 1993; Nakazawa and Ishikawa, 1995). These lead to narrowing of axoplasm at the node, influence rheological parameters, increase organelle concentration at the node, and may cause “stop and go” saltatory movement of organelles during retrograde transport (Rebhun, 1972; Tsukita and Ishikawa, 1980; Armstrong et al., 1987; Viancour and Kreiter, 1993; Waxman et al., 1995; Cui et al., 2007).
MVBs can be generated by delayed fixation (dystrophic conditions)
Ischemia and hypoxia lead to accumulation of organelles, including vesiculotubular profiles, in myelinated axons (Korthals and Wisniewski, 1975; Radius and Anderson, 1981; Nukada and Dyck, 1987). Additionally, it has been shown that myelinated axons are more vulnerable to hypoxic stress than unmyelinated axons (Waxman et al., 1991). Studies employing immersion fixation or delaying fixation of tissue until lengthy dissections are completed seem more likely to encounter MVBs in axons (Chu-Wang and Oppenheim, 1980; Omata and Schatzle, 1980; Schmied and Holtzman, 1987; Sandow et al., 2000). Additionally, many of the studies that report MVBs in myelinated axons introduced exogenous proteins, i.e. horseradish peroxidase or wheat germ agglutinin, to the axons under investigation. These substances are known to have dystrophic effects on axons (Stoeckel and Thoenen, 1975; Bunt and Haschke, 1978; Chu-Wang and Oppenheim, 1980; Buchner et al., 1987). On the other hand, MVBs are only rarely observed in axons when endogenous proteins are used as markers, for example radiolabeled or QD-labeled nerve growth factor (Buchner et al., 1987; Castel et al., 1992a; Cui et al., 2007). Taken together, these findings suggest that MVBs appear in axons with an increased frequency when the axons are in a dystrophic or hypoxic state, or when axons or their terminals are loaded with an exogenous protein.
Dystrophic axons have a number of defining features that include axonal swelling, and organelle accumulation becomes manifest within 1-2 hours of insult. Additional biomarkers for neurons subjected to dystrophic or degenerative pathology are an increase in mitochondria size and/or number as well as the initiation of autophagy as determined by the presence of autophagosomes (Dixon, 1967; Matthews and Raisman, 1972; Marty and Peschanski, 1994; Einheber et al., 2006; Wang et al., 2006). The significant increase of both mitochondria and autophagosome FA, particularly in the node region (Fig. 6A, B), confirms that hypoglossal axons challenged by “delayed fixation” were indeed subjected to dystrophic conditions. Our finding that dystrophy increased MVBs in both number and FA suggests that formation of new MVBs is part of an axonal response to hypoxia, injury or pathology (Pioro et al., 1991). Thus, previous reports of MVBs in axons may be based, by up to 80%, on MVBs that are not normally present in healthy axons. The recent suggestion that MVBs are part of the autophagosome system (Kaasinen et al., 2008) supports this notion.
The conclusions from our study have importance in light of the accumulating evidence that axonal transport and MVB biology are altered in several neuronal pathologies, including Alzheimer's, Huntington's, Down syndrome, and amyotrophic lateral sclerosis (Zweifel et al., 2005; Castren and Tanila, 2006; Bronfman et al., 2007).
Acknowledgments
The trophic factor BDNF was kindly provided by Regeneron (Tarrytown, NY). Our work was supported by NIH grants NS 35931, EY 12841, DOD W81XWH-07-2-0107, and INBRE grant P20 RR 016464 from the NCRR. We gratefully acknowledge help with initial stages of this work by Dr. Hanna Damke and help with statistical analysis by Dr. Karen Schlauch, Director of the Center for Bioinformatics at the University of Nevada.
Grant Support: NIH grants NS 35931, EY 12841, DOD W81XWH-07-2-0107, and INBRE grant P20 RR 016464 from the NCRR.
Literature Cited
- Altick AL, Baryshnikova LM, Damke H, von Bartheld CS. Retrograde axonal transport of neurotrophic factors in vivo. Int J Dev Neurosci. 2008;26:870–871. [Google Scholar]
- Armstrong R, Toews AD, Morell P. Axonal transport through nodes of Ranvier. Brain Res. 1987;412:196–199. doi: 10.1016/0006-8993(87)91461-2. [DOI] [PubMed] [Google Scholar]
- Bache KG, Stuffers S, Malerod L, Slagsvold T, Raiborg C, Lechardeur D, Walchli S, Lukacs GL, Brech A, Stenmark H. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol Biol Cell. 2006;17:2513–2523. doi: 10.1091/mbc.E05-10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold CH, Fabricius C, Rydmark M, Andersen B. Axoplasmic organelles at nodes of Ranvier. I. Occurrence and distribution in large myelinated spinal root axons of the adult cat. J Neurocytol. 1993;22:925–940. doi: 10.1007/BF01218351. [DOI] [PubMed] [Google Scholar]
- Bronfman FC, Escudero CA, Weis J, Kruttgen A. Endosomal transport of neurotrophins: roles in signaling and neurodegenerative diseases. Dev Neurobiol. 2007;67:1183–1203. doi: 10.1002/dneu.20513. [DOI] [PubMed] [Google Scholar]
- Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M. Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J Neurosci. 2003;23:3209–3220. doi: 10.1523/JNEUROSCI.23-08-03209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner K, Seitz-Tutter D, Schonitzer K, Weiss DG. A quantitative study of anterograde and retrograde axonal transport of exogenous proteins in olfactory nerve C-fibers. Neuroscience. 1987;22:697–707. doi: 10.1016/0306-4522(87)90366-6. [DOI] [PubMed] [Google Scholar]
- Bunt AH, Haschke RH. Features of foreign proteins affecting their retrograde transport in axons of the visual system. J Neurocytol. 1978;7:665–678. doi: 10.1007/BF01205143. [DOI] [PubMed] [Google Scholar]
- Caro LG, Van Tubergen RP, Kolb JA. High-resolution autoradiography. I. Methods. J Cell Biol. 1962;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel MN, Woulfe J, Wang X, Laduron PM, Beaudet A. Autoradiographic localization of retrogradely transported neurotensin in nigrostriatal neurons. Ann N Y Acad Sci. 1992a;668:323–325. doi: 10.1111/j.1749-6632.1992.tb27364.x. [DOI] [PubMed] [Google Scholar]
- Castel MN, Woulfe J, Wang X, Laduron PM, Beaudet A. Light and electron microscopic localization of retrogradely transported neurotensin in rat nigrostriatal dopaminergic neurons. Neuroscience. 1992b;50:269–282. doi: 10.1016/0306-4522(92)90422-x. [DOI] [PubMed] [Google Scholar]
- Castren E, Tanila H. Neurotrophins and dementia--keeping in touch. Neuron. 2006;51:1–3. doi: 10.1016/j.neuron.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Chiu AY, Chen EW, Loera S. Distinct neurotrophic responses of axotomized motor neurons to BDNF and CNTF in adult rats. Neuroreport. 1994;5:693–696. doi: 10.1097/00001756-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Chu-Wang IW, Oppenheim RW. Uptake, intra-axonal transport and fate of horseradish peroxidase in embryonic spinal neurons of the chick. J Comp Neurol. 1980;193:753–776. doi: 10.1002/cne.901930312. [DOI] [PubMed] [Google Scholar]
- Claude P, Hawrot E, Parada I. Ultrastructural studies on the intracellular fate of 125I-nerve growth factor in cultured rat sympathetic neurons. J Cell Biochem. 1982;20:1–13. doi: 10.1002/jcb.240200102. [DOI] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci. 2002;22:2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci U S A. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckwerth TL, Johnson EM., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- Dixon JS. “Phagocytic” lysosomes in chromatolytic neurones. Nature. 1967;215:657–658. doi: 10.1038/215657a0. [DOI] [PubMed] [Google Scholar]
- Einheber S, Bhat MA, Salzer JL. Disrupted axo-glial junctions result in accumulation of abnormal mitochondria at nodes of Ranvier. Neuron Glia Biol. 2006;2:165–174. doi: 10.1017/S1740925X06000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerod L, Fisher EM, Isaacs A, Brech A, Stenmark H, Simonsen A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007;179:485–500. doi: 10.1083/jcb.200702115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzinsky KP. Node-paranode regions as local degradative centres in alpha-motor axons. Microsc Res Tech. 1996;34:492–506. doi: 10.1002/(SICI)1097-0029(19960815)34:6<492::AID-JEMT2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gatzinsky KP, Berthold CH. Lysosomal activity at nodes of Ranvier during retrograde axonal transport of horseradish peroxidase in alpha-motor neurons of the cat. J Neurocytol. 1990;19:989–1002. doi: 10.1007/BF01186826. [DOI] [PubMed] [Google Scholar]
- Gatzinsky KP, Berthold CH, Corneliuson O. Acid phosphatase activity at nodes of Ranvier in alpha-motor and dorsal root ganglion neurons of the cat. J Neurocytol. 1988;17:531–544. doi: 10.1007/BF01189808. [DOI] [PubMed] [Google Scholar]
- Gatzinsky KP, Persson GH, Berthold CH. Removal of retrogradely transported material from rat lumbosacral alpha-motor axons by paranodal axon-Schwann cell networks. Glia. 1997;20:115–126. doi: 10.1002/(sici)1098-1136(199706)20:2<115::aid-glia3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Gomori G. Histochemical methods for acid phosphatase. J Histochem Cytochem. 1956;4:453–461. doi: 10.1177/4.5.453. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Haigler HT, McKanna JA, Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979;81:382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat MA. Biological Applications. Baltimore: University Park Press; 1981. Principles and Techniques of Electron Microscopy; p. 522. [Google Scholar]
- Hedreen JC. What was wrong with the Abercrombie and empirical cell counting methods? A review. Anat Rec. 1998;250:373–380. doi: 10.1002/(SICI)1097-0185(199803)250:3<373::AID-AR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hibbert AP, Kramer BM, Miller FD, Kaplan DR. The localization, trafficking and retrograde transport of BDNF bound to p75NTR in sympathetic neurons. Mol Cell Neurosci. 2006;32:387–402. doi: 10.1016/j.mcn.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman E, Teichberg S, Abrahams SJ, Citkowitz E, Crain SM, Kawai N, Peterson ER. Notes on synaptic vesicles and related structures, endoplasmic reticulum, lysosomes and peroxisomes in nervous tissue and the adrenal medulla. J Histochem Cytochem. 1973;21:349–385. doi: 10.1177/21.4.349. [DOI] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology: Three-dimensional Measurement in Microscopy. New York: Springer-Verlag New York, Inc.; 1998. p. 246. [Google Scholar]
- Kaasinen SK, Harvey L, Reynolds AJ, Hendry IA. Autophagy generates retrogradely transported organelles: a hypothesis. Int J Dev Neurosci. 2008;26:625–634. doi: 10.1016/j.ijdevneu.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Kapeller K, Mayor D. An electron microscopic study of the early changes distal to a constriction in sympathetic nerves. Proc R Soc Lond B Biol Sci. 1969;172:53–63. doi: 10.1098/rspb.1969.0011. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Korthals JK, Wisniewski HM. Peripheral nerve ischemia. Part 1. Experimental model. J Neurol Sci. 1975;24:65–76. doi: 10.1016/0022-510x(75)90009-x. [DOI] [PubMed] [Google Scholar]
- Kristensson K, Olsson Y, Sjostrand J. Axonal uptake and retrograde transport of exogenous proteins in the hypoglossal nerve. Brain Res. 1971;32:399–406. doi: 10.1016/0006-8993(71)90332-5. [DOI] [PubMed] [Google Scholar]
- Krizbai I, Joo F, Pestean A, Preil J, Botcher H, Wolff JR. Localization and biochemical characterization of acid phosphatase isoforms in the olfactory system of adult rats. Neuroscience. 1997;76:799–807. doi: 10.1016/s0306-4522(96)00397-1. [DOI] [PubMed] [Google Scholar]
- LaVail JH, LaVail MM. The retrograde intraaxonal transport of horseradish peroxidase in the chick visual system: a light and electron microscopic study. J Comp Neurol. 1974;157:303–357. doi: 10.1002/cne.901570304. [DOI] [PubMed] [Google Scholar]
- Lindsey JD, Ellisman MH. The neuronal endomembrane system. III. The origins of the axoplasmic reticulum and discrete axonal cisternae at the axon hillock. J Neurosci. 1985;5:3135–3144. doi: 10.1523/JNEUROSCI.05-12-03135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Vu TQ. Identification of quantum dot bioconjugates and cellular protein co-localization by hybrid gel blotting. Nano Lett. 2007;7:1044–1049. doi: 10.1021/nl070239e. [DOI] [PubMed] [Google Scholar]
- Marty S, Peschanski M. Fine structural alteration in target-deprived axonal terminals in the rat thalamus. Neuroscience. 1994;62:1121–1132. doi: 10.1016/0306-4522(94)90348-4. [DOI] [PubMed] [Google Scholar]
- Matthews MR, Raisman G. A light and electron microscopic study of the cellular response to axonal injury in the superior cervical ganglion of the rat. Proc R Soc Lond B Biol Sci. 1972;181:43–79. doi: 10.1098/rspb.1972.0040. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR. On Trk for retrograde signaling. Neuron. 2001;32:767–770. doi: 10.1016/s0896-6273(01)00529-3. [DOI] [PubMed] [Google Scholar]
- Myromslien FD, Grovdal LM, Raiborg C, Stenmark H, Madshus IH, Stang E. Both clathrin-positive and -negative coats are involved in endosomal sorting of the EGF receptor. Exp Cell Res. 2006;312:3036–3048. doi: 10.1016/j.yexcr.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Nakazawa E, Ishikawa H. Occurrence of fasciculated microtubules at nodes of Ranvier in rat spinal roots. J Neurocytol. 1995;24:399–407. doi: 10.1007/BF01189066. [DOI] [PubMed] [Google Scholar]
- Nukada H, Dyck PJ. Acute ischemia causes axonal stasis, swelling, attenuation, and secondary demyelination. Ann Neurol. 1987;22:311–318. doi: 10.1002/ana.410220306. [DOI] [PubMed] [Google Scholar]
- O'Reilly PMR, FitzGerald MJT. Fibre composition of the hypoglossal nerve in the rat. J Anat. 1990;172:227–243. [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Risling M, Negro A, Langone F, Cullheim S. Apoptosis of spinal interneurons induced by sciatic nerve axotomy in the neonatal rat is counteracted by nerve growth factor and ciliary neurotrophic factor. J Comp Neurol. 2002;447:381–393. doi: 10.1002/cne.10248. [DOI] [PubMed] [Google Scholar]
- Omata T, Schatzle W. Afferent and efferent nerve endings of the outer hair cells in the rabbit. Arch Otorhinolaryngol. 1980;229:175–182. doi: 10.1007/BF02565519. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, von Bartheld CS. Programmed cell death and neurotrophic factors. In: Squire L, editor. Fundamental Neuroscience. 3rd. San Diego: Elsevier; 2008. pp. 437–467. [Google Scholar]
- Overly CC, Hollenbeck PJ. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. J Neurosci. 1996;16:6056–6064. doi: 10.1523/JNEUROSCI.16-19-06056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. Boston: Elsevier Academic Press; 2004. p. 1309. [Google Scholar]
- Peters A, Palay SL, Webster Hd. The Fine Structure of the Nervous System. New York: Oxford University Press; 1991. p. 494. [Google Scholar]
- Pioro EP, Ribeiro-Da-Silva A, Cuello AC. Similarities in the ultrastructural distribution of nerve growth factor receptor-like immunoreactivity in cerebellar Purkinje cells of the neonatal and colchicine-treated adult rat. J Comp Neurol. 1991;305:189–200. doi: 10.1002/cne.903050203. [DOI] [PubMed] [Google Scholar]
- Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007 doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RL, Lasek RJ, Katz MJ. Neurofilaments assume a less random architecture at nodes and in other regions of axonal compression. Brain Res. 1993;607:125–133. doi: 10.1016/0006-8993(93)91497-g. [DOI] [PubMed] [Google Scholar]
- Radius RL, Anderson DR. Morphology of axonal transport abnormalities in primate eyes. Br J Ophthalmol. 1981;65:767–777. doi: 10.1136/bjo.65.11.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebhun LI. Polarized intracellular particle transport: saltatory movements and cytoplasmic streaming. Int Rev Cytol. 1972;32:93–137. doi: 10.1016/s0074-7696(08)60339-3. [DOI] [PubMed] [Google Scholar]
- Reles A, Friede RL. Axonal cytoskeleton at the nodes of Ranvier. J Neurocytol. 1991;20:450–458. doi: 10.1007/BF01252273. [DOI] [PubMed] [Google Scholar]
- Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- Rind HB, Butowt R, von Bartheld CS. Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin. J Neurosci. 2005;25:539–549. doi: 10.1523/JNEUROSCI.4322-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizin L, Nishikawa K, Koizumi J, Keoseian S. The fine structure of the multivesicular body and their relationship to the ultracellular constituents of the central nervous system. J Neuropathol Exp Neurol. 1967;26:223–249. doi: 10.1097/00005072-196704000-00004. [DOI] [PubMed] [Google Scholar]
- Russell FD, Koishi K, Jiang Y, McLennan IS. Anterograde axonal transport of glial cell line-derived neurotrophic factor and its receptors in rat hypoglossal nerve. Neuroscience. 2000;97:575–580. doi: 10.1016/s0306-4522(00)00079-8. [DOI] [PubMed] [Google Scholar]
- Russell MR, Nickerson DP, Odorizzi G. Molecular mechanisms of late endosome morphology, identity and sorting. Curr Opin Cell Biol. 2006;18:422–428. doi: 10.1016/j.ceb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Rusten TE, Filimonenko M, Rodahl LM, Stenmark H, Simonsen A. ESCRTing autophagic clearance of aggregating proteins. Autophagy. 2007;4 [Google Scholar]
- Sachse M, Ramm G, Strous G, Klumperman J. Endosomes: multipurpose designs for integrating housekeeping and specialized tasks. Histochem Cell Biol. 2002;117:91–104. doi: 10.1007/s00418-001-0348-0. [DOI] [PubMed] [Google Scholar]
- Saito N, Okada Y, Noda Y, Kinoshita Y, Kondo S, Hirokawa N. KIFC2 is a novel neuron-specific C-terminal type kinesin superfamily motor for dendritic transport of multivesicular body-like organelles. Neuron. 1997;18:425–438. doi: 10.1016/s0896-6273(00)81243-x. [DOI] [PubMed] [Google Scholar]
- Salpeter M, McHenry FA. Electron microsocope autoradiography Analysis of autoradiograms. In: Kohler J, editor. Advanced Techniques in Biological Electron Microscopy. New York: Springer-Verlag; 1973. pp. 113–152. [Google Scholar]
- Sandow SL, Heydon K, Weible MW, 2nd, Reynolds AJ, Bartlett SE, Hendry IA. Signalling organelle for retrograde axonal transport of internalized neurotrophins from the nerve terminal. Immunol Cell Biol. 2000;78:430–435. doi: 10.1046/j.1440-1711.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- Schmied R, Holtzman E. A phosphatase activity and a synaptic vesicle antigen in multivesicular bodies of frog retinal photoreceptor terminals. J Neurocytol. 1987;16:627–637. doi: 10.1007/BF01637655. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Ultrastructural localization of a nerve growth factor-horseradish peroxidase (NGF-HRP) coupling product after retrograde axonal transport in adrenergic neurons. Brain Res. 1977;130:190–196. doi: 10.1016/0006-8993(77)90857-5. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Electron microscopic evidence for a transsynaptic migration of tetanus toxin in spinal cord motoneurons: an autoradiographic and morphometric study. Brain Res. 1976;105:213–227. doi: 10.1016/0006-8993(76)90422-4. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct. 2007;2:35. doi: 10.1186/1745-6150-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The short term accumulation of axonally transported organelles in the region of localized lesions of single myelinated axons. J Neurocytol. 1980;9:39–65. doi: 10.1007/BF01205226. [DOI] [PubMed] [Google Scholar]
- Stoeckel K, Schwab M, Thoenen H. Specificity of retrograde transport of nerve growth factor (NGF) in sensory neurons: a biochemical and morphological study. Brain Res. 1975;89:1–14. doi: 10.1016/0006-8993(75)90129-8. [DOI] [PubMed] [Google Scholar]
- Stoeckel K, Thoenen H. Retrograde axonal transport of nerve growth factor: specificity and biological importance. Brain Res. 1975;85:337–341. doi: 10.1016/0006-8993(75)90092-x. [DOI] [PubMed] [Google Scholar]
- Sundara Rajan S, Liu HY, Vu TQ. Ligand-bound quantum dot probes for studying the molecular scale dynamics of receptor endocytic trafficking in live cells. ACS Nano. 2008;2:1153–1166. doi: 10.1021/nn700399e. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980;84:513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Akmentin W, Philippidou P, Kuruvilla R, Ginty DD, Halegoua S. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J Neurosci. 2005;25:5236–5247. doi: 10.1523/JNEUROSCI.5104-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Philippidou P, Rosenbaum J, Akmentin W, Shao Y, Halegoua S. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proc Natl Acad Sci U S A. 2007;104:12270–12275. doi: 10.1073/pnas.0702819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viancour TA, Kreiter NA. Vesicular fast axonal transport rates in young and old rat axons. Brain Res. 1993;628:209–217. doi: 10.1016/0006-8993(93)90957-o. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Laine J, Simon A, Roland A, Leterrier C, Lenkei Z. The type 1 cannabinoid receptor is highly expressed in embryonic cortical projection neurons and negatively regulates neurite growth in vitro. Eur J Neurosci. 2008;28:1705–1718. doi: 10.1111/j.1460-9568.2008.06484.x. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS. Tracing with radiolabeled neurotrophins. Methods Mol Biol. 2001;169:195–216. doi: 10.1385/1-59259-060-8:195. [DOI] [PubMed] [Google Scholar]
- Vu TQ, Maddipati R, Blute TA, Nehilla BJ, Nusblat L, Desai TA. Peptide-conjugated quantum dots activate neuronal receptors and initiate downstream signaling of neurite growth. Nano Lett. 2005;5:603–607. doi: 10.1021/nl047977c. [DOI] [PubMed] [Google Scholar]
- Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Stys PK, editors. The Axon Structure, Function and Pathophysiology. 3. New York: Oxford University Press; 1995. p. 692. [Google Scholar]
- Waxman SG, Ransom BR, Stys PK. Non-synaptic mechanisms of Ca(2+)-mediated injury in CNS white matter. Trends Neurosci. 1991;14:461–468. doi: 10.1016/0166-2236(91)90046-w. [DOI] [PubMed] [Google Scholar]
- Weible MW, 2nd, Hendry IA. What is the importance of multivesicular bodies in retrograde axonal transport in vivo? J Neurobiol. 2004;58:230–243. doi: 10.1002/neu.10318. [DOI] [PubMed] [Google Scholar]
- White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. Embo J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Cui B, He L, Chen L, Mobley WC. The coming of age of axonal neurotrophin signaling endosomes. J Proteomics. 2009 doi: 10.1016/j.jprot.2008.10.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]