Gibberellins (GAs) are a class of phytohormones that impact various aspects of plant growth and development (reviewed in Fleet and Sun, 2005). For more than 50 years, GAs have been known for their dramatic impact on plant stature. Inhibition of GA biosynthesis results in dwarfism (Ninnemann et al., 1964), whereas exogenously applied gibberellic acid promotes internodal stem growth (Brian et al., 1954). Recent evidence suggests that GAs also play an important role in lateral root development. Mutants defective in GA biosynthesis (Berova and Zlatev, 2000) or signaling (Busov et al., 2006) were found to have enhanced lateral root formation. Prompted by these later findings, Gou et al. (pages 623–639) set out to decipher the role of GAs in lateral root formation using GA-deficient and GA-insensitive transgenic Populus lines.
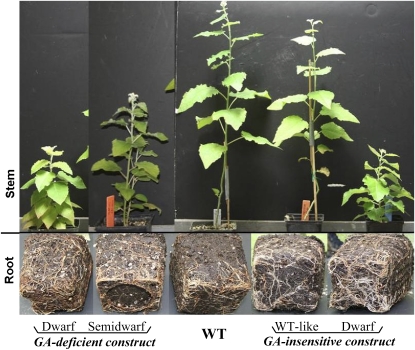
The authors first analyzed lateral root density in the transgenic poplar lines. As expected, both lines had reduced stature and increased lateral root density. Treatment with exogenous GAs rapidly inhibited lateral root formation, and to a lesser extent elongation, in the GA-deficient plants. Quantitative analysis revealed that lateral root density in these transgenic lines was positively correlated with the degree of dwarfism (see figure), providing further evidence that the changes in lateral root density were due to reductions in GAs. In planta levels of GAs were bolstered using RNA interference technology. Two genes encoding root-localized GA2ox enzymes, which degrade bioactive GAs, were simultaneously silenced, and the resulting transgenic lines were analyzed. Increased levels of GA1 and GA4 in the roots were accompanied by reduced lateral root density. These findings confirmed that GAs inhibit lateral root formation.
Lateral root density is greatest in the shortest transgenic plants. Photos on bottom show roots corresponding to the same 2-month-old plant shown in the photo directly above. (Figure adapted from Figure 1B of Gou et al. [2010].)
Whole-genome microarray analysis of the GA-deficient and GA-insensitive lines identified 2069 genes that were differentially expressed in both transgenic lines relative to the wild type. The expression of many genes involved in cell proliferation, growth, and cell wall loosening correlated with the severity of the lateral root phenotype. Interestingly, genes involved in hormone responses were found to be the most highly enriched category in both transgenic lines. The authors presented several lines of evidence that GAs interact with auxin to regulate lateral root formation. First, auxin levels were slightly elevated in the roots of the GA-deficient and GA-insensitive lines. Second, the gene encoding PIN9, an auxin efflux carrier, was strongly upregulated in both transgenic plants, and RT-PCR analysis showed that this gene was expressed mainly in the roots. Third, transgenic poplar plants overexpressing PIN9 had significantly more lateral root primordia than wild-type controls, and lateral root development in these plants was inhibited by GA application. Finally, exogenous GA treatment rapidly repressed PIN9 expression.
This study provides important insights into how plant hormones regulate lateral root development.
References
- Berova M., Zlatev Z. (2000). Physiological response and yield of paclobutrazol treated tomato plants (Lycopersicon esculentum Mill.). Plant Growth Regul. 30: 117–123 [Google Scholar]
- Brian P.W., Elson G.W., Hemming H.G., Radley M. (1954). The plant-growth-promoting properties of gibberellic acid, a metabolic product of the fungus gibberella fujikuroi. J. Sci. Food Agric. 5: 602–612 [Google Scholar]
- Busov V., Meilan R., Pearce D.W., Rood S.B., Ma C., Tschaplinski T.J., Strauss S.H. (2006). Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta. 224: 288–299 [DOI] [PubMed] [Google Scholar]
- Fleet C.M., Sun T.P. (2005). A DELLAcate balance: The role of gibberellin in plant morphogenesis. Curr. Opin. Plant Biol. 8: 77–85 [DOI] [PubMed] [Google Scholar]
- Gou J., Strauss S.H., Tsai C.J., Kai F., Chen Y., Jiang X., Busov V.B. (2010). Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell. 22: 623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann H., Zeevaart J.A.D, Kende H., Lang A. (1964). The plant growth retardant CCC as inhibitor of gibberellin biosynthesis inFusarium moniliforme. Planta. 61: 229–235 [Google Scholar]