This work provides evidence that both bZIP28 and NF-Y subunits are recruited under stress to form a transcriptional complex that upregulates the expression of endoplasmic reticulum stress-induced genes. The bZIP28/NF-Y complex specifically binds to an element common to the promoters of the targeted stress genes.
Abstract
Stress agents known to elicit the unfolded protein response in Arabidopsis thaliana upregulate the expression of a constellation of genes dependent on the membrane-associated basic domain/leucine zipper (bZIP) transcription factor, bZIP28. Among the stress-activated genes, a consensus promoter sequence corresponding to the endoplasmic reticulum (ER) stress-responsive element I (ERSE-I), CCAAT-N10-CACG, was identified. Disruption of either the CCAAT or CACG subelement in ERSE-I resulted in reduction of the transcriptional response to ER stress. bZIP28 forms homo- and heterodimers with other bZIP TF family members (in subgroup D) and interacts with CCAAT box binding factors, heterotrimeric factors composed of NF-Y subunits. Arabidopsis encodes 36 NF-Y subunits, and it was found that subunits NF-YB3 and -YC2 interact with bZIP28 and NF-YA4, respectively, in a yeast three-hybrid system. A transcriptional complex containing bZIP28 and the above-mentioned three NF-Y subunits was assembled in vitro on DNA containing ERSE-I. bZIP28, on its own, binds to the CACG subelement in ERSE-I to form a smaller complex I, and in combination with the NF-Y subunits above, bZIP28 assembles into a larger transcriptional complex (complex II). bZIP28 was shown to interact with NF-Y subunits in vivo in bimolecular fluorescence complementation analyses and in coimmunoprecipitation assays. Treatment of seedlings with ER stress agents led to the upregulation of NF-YC2 and the relocation of NF-YB3 from the cytoplasm to the nucleus. Thus, in response to ER stress, bZIP28 is mobilized by proteolysis and recruits NF-Y subunits to form a transcriptional complex that upregulates the expression of ER stress-induced genes.
INTRODUCTION
The unfolded protein response (UPR) is a stress mitigation mechanism that monitors the folding of proteins in the cell's secretory pathway (for example, see Schroder and Kaufman, 2005a, 2005b; Bernales et al., 2006). When unfolded or misfolded proteins accumulate in the endoplasmic reticulum (ER), UPR is activated to produce protein folding factors and other factors to aid in protein folding. UPR transcriptional regulation is quite extensive in yeast and in mammalian cells. Genome expression profiling has shown that >5% of the yeast genome is regulated by UPR (Travers et al., 2000).
In plants, adverse environmental conditions can produce ER stress and UPR. Two membrane-associated, basic domain/leucine zipper (bZIP) transcription factors (TFs) play key roles in transducing ER stress signals in Arabidopsis thaliana (Iwata and Koizumi, 2005; Liu et al., 2007a). One of the bZIP TFs involved in UPR in Arabidopsis is bZIP28, which is similar in structure and mode of action to the ER stress-activated TF, Activating Transcription Factor 6 (ATF6), in mammalian cells (Schroder and Kaufman, 2005b). ATF6 is a type II transmembrane protein normally retained in the ER by its association with binding protein, BiP/GRP78 (Chen et al., 2002; Shen et al., 2002). In response to stress, ATF6 dissociates from BiP/GRP78 and is transported to the Golgi where it is subjected to proteolytic processing (Chen et al., 2002; Shen et al., 2002), first by a Ser protease, S1P, and then by a metalloprotease, S2P (Ye et al., 2000; Feng et al., 2007). The released N-terminal domain of ATF6 contains a bZIP DNA binding domain and a transcriptional activation domain and is translocated to the nucleus where it activates the transcription of ER stress response genes, such as Grp78/BiP (Wang et al., 2000).
In mammalian cells, UPR genes are upregulated through the action of TFs acting on promoter cis-elements called ER stress response elements (ERSEs; Yoshida et al., 1998). There are three ERSE types in mammalian cells: ERSE-I (CCAAT-N9-CCACG), ERSE-II (ATTGG-NCCACG), and XBP1-BS [GA-TGACGT-G(T/G)] (Yoshida et al., 1998, 2000, 2001; Wang et al., 2000; Kokame et al., 2001; Shen et al., 2001; Yamamoto et al., 2004). RNA profiling studies showed that ERSEs were also found in promoters of several genes induced by ER stress in Arabidopsis (Martinez and Chrispeels, 2003; Noh et al., 2003; Kamauchi et al., 2005), but information is lacking on the role of ERSEs in the regulation of ER stress-induced genes in plants. The consensus ERSE-I sequence is composed of two subelements: CCAAT-N9-CCACG. Yoshida et al. (2000) showed that the stress-activated ATF6 binds as a dimer to the 3′ CCACG subelement, and the constitutively active NF-Y, a CCAAT box binding factor, binds to the 5′ CCAAT subelement.
In mammals, the NF-Y TF complex is composed of three unique subunits: NF-YA, NF-YB, and NF-YC, with one copy of each subunit. Arabidopsis, unlike yeast and mammals, has numerous NF-Y subunit genes, also known as heme activator proteins (HAPs), 10 of which encode NF-YA (HAP2), 13 encode NF-YB (HAP3), and 13 encode NF-YC (HAP5) (Edwards et al., 1998; Gusmaroli et al., 2001; Siefers et al., 2009). The NF-Y subunits can theoretically combine to form 1690 unique trimers. To date, no complete NF-Y complex has been reported in plants, although individual NF-Y subunits are known to be important for embryo development (West et al., 1994; Lotan et al., 1998; Lee et al., 2003), plastid biogenesis (Miyoshi et al., 2003), flowering time regulation (Ben-Naim et al., 2006; Wenkel et al., 2006; Cai et al., 2007), and abiotic stress tolerance (Nelson et al., 2007; Li et al., 2008). Several groups have demonstrated that each of the three plant NF-Ys can substitute for their yeast counterparts in gene expression assays (Masiero et al., 2002; Ben-Naim et al., 2006; Kumimoto et al., 2008), implying the existence of NF-Y trimers in plants. Nonconventional NF-Y trimeric complexes have also been reported in plants. A rice (Oryza sativa) MADS box protein, MADS18, either with or without the natural partner, interacts with Os NF-YB1. Os NF-YB1 is capable of heterodimerizing with Os NF-YC, but not Os NF-YA, the CCAAT box binding subunit (Masiero et al., 2002). In Arabidopsis and tomato (Solanum lycopersicum), CONSTANS (CO) and CONSTANS-like (COL) proteins form complexes with their NF-YB and NF-YC subunits through their plant-specific CCT domains (for CO, COL, and TOC1) (Ben-Naim et al., 2006; Wenkel et al., 2006). Recently, an Arabidopsis seed-specific bZIP TF bZIP67 was found to interact with either of two NF-YBs, LEAFY COTYLEDON1 or LEC1-LIKE, by its association with NF-YC2 or other NF-YCs to activate the CRUCIFERIN C promoter and sucrose synthase 2, independent of its CCAAT box binding factor, NF-YA. Coexpression of any of four tested NF-YAs, including NF-YA4, strongly inhibited the transcriptional activity (Yamamoto et al., 2009).
This study focuses on transcriptional complexes involving bZIP28 in Arabidopsis, a TF activated by typical ER stress agents that elicit UPR, such as tunicamycin (TM) and DTT (Liu et al., 2007a), and by heat stress (Gao et al., 2008). Overexpression of an activated form of bZIP28 upregulates the expression of UPR genes in the absence of stress (Liu et al., 2007a). bZIP28 is in the same bZIP family subgroup B (Jakoby et al., 2002) with bZIP17, a factor involved in salt stress responses (Liu et al., 2007b). An additional member of the ER stress response group is bZIP60, which was uncategorized in the grouping of bZIP TFs by Schutze et al. (2008). Similar to bZIP28, overexpression of an activated form of bZIP60 upregulates UPR genes in the absence of stress (Iwata and Koizumi, 2005), and bZIP60 is reportedly processed in response to treatment by ER stress agents (Iwata et al., 2008). In this article, we identify genes that are upregulated by UPR and dependent on bZIP28 for their upregulation. One of the highly upregulated, bZIP28-dependent genes is BINDING PROTEIN3 (BiP3), which contains tandem ERSE-I elements in its promoter. We used the BiP3 ERSE-I as a model for understanding the transcriptional role of bZIP28 in UPR. We find that bZIP28 has the capacity to homo- or heterodimerize with members in the small bZIP TF family subgroup that are membrane associated (Jakoby et al., 2002; Deppmann et al., 2004), including bZIP60. In addition, we found that bZIP28 forms a transcriptional complex with a NF-YA4/NF-YB3/NF-YC2 trimer. The proteolytic activation of the bZIP28 protein, the upregulation of the NF-YC2 gene, and the translocation of the NF-YB3 protein from cytosol to nucleus are all involved in orchestrating the formation of a transcriptional complex that activates UPR-associated genes.
RESULTS
bZIP28-Regulated Genes
We previously showed that bZIP28 is a transducer of ER stress responses elicited by TM and DTT, stress agents known to induce UPR (Liu et al., 2007a). Other laboratories have reported on genes in Arabidopsis that are upregulated by treatment with these stress agents (Martinez and Chrispeels, 2003; Noh et al., 2003; Iwata et al., 2008). We wanted to know which genes among those that are upregulated by TM require bZIP28 for expression; therefore, we compared the RNA profiles of wild-type and bZIP28 mutant plants (zip28-2) subjected to TM treatment and ranked genes with significant expression changes (P < 0.1) by the differences of differences (the difference in TM-treated versus untreated wild type less the difference in TM-treated versus untreated zip28-2 mutant) (Table 1). In a previous study, we demonstrated that the T-DNA insertion mutant zip28-2 with an insertion in the first exon of bZIP28 was a null mutant (Liu et al., 2007a). The comparison clearly demonstrated that a broad range of genes involved in ER protein folding and secretion require bZIP28 for full induction. They include genes encoding BiP3, HSP90-like protein, calnexin, DNA J domain-containing proteins, protein disulfide isomerase, signal peptide peptidase, and a coated vesicle component. Among the genes on the top of the list, six of them (BiP3, SHD, CNX1, SDF2, HSP70, and PDIL1-1) were tested by quantitative RT-PCR and found to be significantly dependent on bZIP28 for expression (Figure 1).
Table 1.
UPR Upregulated Genes Dependent on bZIP28 Function for Expression
| −TM Wild Typea | +TM Wild Typea | Diff. Wild Typea | −TM Mutanta | +TM Mutanta | Diff. Mutanta | Diff. of Diffs.b | P Value | UPR cis-Elementc | Locus | Description |
| 0.5 | 14.5 | 13.9 | 0.1 | 5.1 | 5.1 | 8.8 | 0.07 | ERSEx2 | At1g09080 | BiP3/GRP78 |
| 11.1 | 17.7 | 6.6 | 8.4 | 10.5 | 2.2 | 4.4 | 0.03 | ERSE, ERSE-L, UPRE | At4g24190 | SHD (shepherd)/GRP94/HSP90-like protein |
| 9.1 | 15.7 | 6.6 | 6.1 | 9.1 | 3.0 | 3.3 | 0.03 | ERSE, UPRE | At5g61790 | CNX1 (calnexin 1) |
| 4.8 | 8.7 | 3.9 | 3.2 | 4.5 | 1.2 | 2.7 | 0.06 | ERSE-Lx2 | At2g25110 | SDF2 (stromal cell-derived factor 2) |
| 5.1 | 7.5 | 2.4 | 3.2 | 3.3 | 0.1 | 2.3 | 0.00 | ERSE | At3g62600 | DNAJ heat shock family protein |
| 2.5 | 5.5 | 3.0 | 1.2 | 2.2 | 1.0 | 2.0 | 0.05 | ERSE-L | At4g29520 | Unknown protein |
| 2.1 | 4.6 | 2.5 | 1.2 | 2.1 | 0.8 | 1.7 | 0.08 | ERSE, UPRE | At4g16660 | Heat shock protein 70 |
| 5.1 | 7.0 | 1.9 | 4.3 | 4.6 | 0.3 | 1.6 | 0.03 | ERSEx2, UPRE | At1g21750 | PDIL1-1 (protein disulfide isomerase like) |
| 1.4 | 3.7 | 2.3 | 0.8 | 1.5 | 0.7 | 1.6 | 0.09 | – | At5g07340 | Calnexin, putative |
| 3.1 | 4.9 | 1.8 | 2.7 | 3.0 | 0.2 | 1.6 | 0.08 | – | At2g03120 | Signal peptide peptidase |
| 5.5 | 7.2 | 1.7 | 4.3 | 4.7 | 0.4 | 1.3 | 0.01 | UPRE | At2g47470 | PDIL2-1 (protein disulfide isomerase like) |
| 2.7 | 4.0 | 1.3 | 2.3 | 2.5 | 0.1 | 1.2 | 0.06 | ERSE, ERSE-L, UPRE | At3g07680 | emp24/gp25L/p24 family protein-coated vesicle component |
| 2.2 | 3.8 | 1.6 | 1.7 | 2.2 | 0.5 | 1.1 | 0.10 | ERSE, ERSE-L | At1g61780 | Postsynaptic protein-related |
| 2.2 | 4.3 | 2.0 | 1.2 | 2.2 | 1.0 | 1.0 | 0.06 | ERSE | At1g04980 | PDIL2-2 (protein disulfide isomerase like) |
| 10.1 | 11.2 | 1.0 | 12.1 | 12.2 | 0.1 | 0.9 | 0.01 | – | At5g35630 | GS2 (Gln synthetase 2) |
Microarray data are mean values × 103, representing genes with P values < 0.1. Seedlings were treated with or without 5 μg/mL TM for 2 h.
Difference of differences between the wild type and the zip28-2 mutant.
ERSE, CCAAT-N10-CACG; ERSE-L, one ERSE mismatch; XBP1-BS, GA-TGACGT-GK; UPRE, TGACGTGR.
Figure 1.
Genes Dependent on bZIP28 for UPR Upregulation.
Wild-type and zip28-2 Arabidopsis seedlings were treated with 5 μg/mL TM or DMSO (mock treatment) for 2 h, and total RNA was extracted and analyzed by quantitative RT-PCR. Expression levels of BiP3 (At1g09080) (A), SHD (At4g24190) (B), CNX1 (At5g61790) (C), SDF2 (At2g25110) (D), SHP70 (At4g16660) (E), and PDIL1-1 (At1g21750) (F) were normalized to actin 2/8. The values in DMSO control were normalized to 1 (At3g18780/ At1g49240). Error bars represent se for three biological replicates. Differences between genotypes were statistically significant (P = 0.05) according to Tukey's range (honestly significant difference) test and two-way analysis of variance.
In an effort to understand the basis for the coregulation in expression of the group of genes dependent on bZIP28, we searched for common motifs in the promoters of the upregulated genes. Iwata et al. (2008) conducted a similar analysis to identify promoter motifs in the larger set of genes upregulated by TM treatment in Arabidopsis. From this they identified upstream consensus sequences ERSE-I (CCAAT-N10-CACG), ERSE-II (ATTGG-N2-CACG), XBP1-BS (GA-TGACGT-GK), and UPRE (TGACGT-GR), which were similar to those found in mammalian cell promoters. The consensus Arabidopsis ERSE-I is composed of two subelements: a CCAAT box and a bZIP factor binding subelement (CACG). Using PSCAN (Zambelli et al., 2009), we analyzed the upstream region of the top 15 genes rank ordered as described above and found a consensus sequence corresponding to the promoter element, bZIP911 (Steffens et al., 2005), which occurred more frequently in the upstream sequences of these genes than in a similar group of random sequences in the Arabidopsis genome (P value = 1.11e−09). The consensus sequence had a core of TGACGTGG (reverse complement = CCACGTCA) containing a canonical bZIP binding site (underlined) in the ERSE-I and ERSE-II elements (Figure 2A). In 11 of the 15 genes that are TM induced and highly bZIP28 dependent, an ERSE-I or ERSE-L (one mismatch to ERSE-I) was found at least one time in the 500-bp upsteam promoter region. The ERSE-II and XBP1-BS elements were not found, and UPRE was less well represented compared with ERSE-I (6 versus 11) in the top 15 genes (Table 1). For example, in the 500-bp upstream region of BiP3, which was most highly dependent on bZIP28 function and strongly induced by TM, there are two ERSE-I elements in tandem. Both ERSE-I elements are required for full function, in that mutations in one of the ERSE-I elements resulted in partial reduction of the transcriptional activity (Iwata et al., 2009).
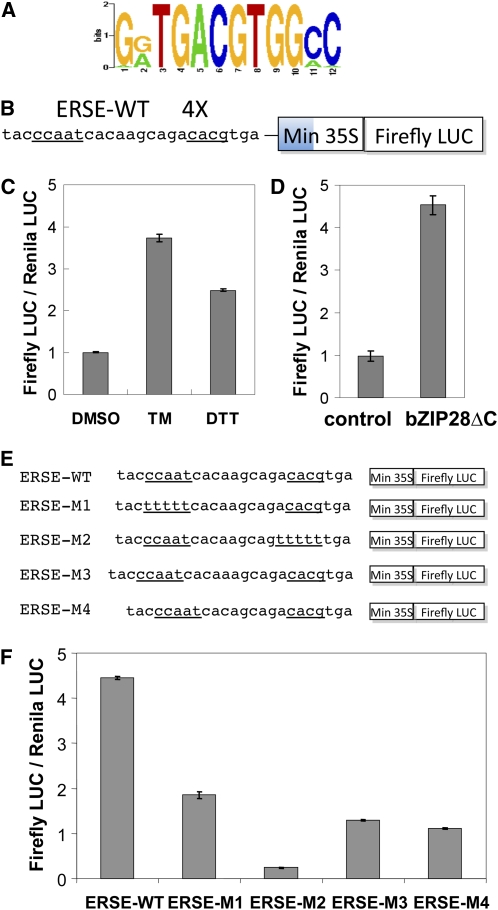
Figure 2.
The ERSE-I Promoter Element Confers Responsiveness to ER Stress Agents in UPR-Induced Genes.
(A) Consensus sequence for the common promoter element in bZIP28-dependent genes derived from PSCAN analysis.
(B) A construct was developed containing (as shown) a 4X multimer of an ERSE-I promoter element from the BiP3 gene linked to a minimal CaMV 35S promoter and firefly luciferase (LUC). The underlined sequences are two core subelements in ERSE-I.
(C) and (D) The construct was tested for induction in an Arabidopsis protoplast system treated with UPR stress agents, TM or DTT, or mock treated (DMSO) (C) or cotransfected with a constitutively active form of bZIP28ΔC or empty vector (control) (D). Test plasmids also encode a constitutively active Renilla LUC, and activity was expressed as a ratio of firefly LUC/Renilla LUC activity. According to a Student's t test, the treatment (C), bZIP28ΔC addition (D), and each ERSE-I mutation (F) were statistically significant (P = 0.05).
(E) Mutated forms of the ERSE-I (ERSE-M1 through -M4) in which the CCAAT box or the bZIP binding site was multiply mutated by base substitution.
(F) Activity of the mutated ERSE-I forms in Arabidopsis protoplasts was also expressed as a ratio of firefly LUC/Renilla LUC activity. Error bars represent se for three independent experiments.
To determine whether ERSE-I does, in fact, mediate UPR, a 4X multimer of the ERSE-I element (ERSE-WT) from the BiP3 gene was fused to a minimal 35S promoter (Figure 2B), which was then linked to a firefly luciferase reporter gene and tested for response to stress agents in an Arabidopsis protoplast transient expression system. It was found that the multimer construct did indeed support a significant response to treatment by TM (∼4-fold) and DTT (∼2.5-fold) (Figure 2C). Induction by these stress agents was comparable to the induction resulting from cotransformation of a constitutively active, truncated form of bZIP28ΔC without stress treatment (Figure 2D). Hence, the construct responds to both stress agent induction and the activity of a truncated form of bZIP28.
To learn whether each of the subelements, the CCAAT box and the putative bZIP binding site (CACG), contributes to the response, the separate elements were mutated by multiple base substitutions (Figure 2E). Base substitutions in the CCAAT box (ERSE-M1) reduced the luciferase expression levels induced by TM treatment by about half, and substitutions in the putative bZIP binding site (ERSE-M2) reduced the induced expression level much further (Figure 2F). The subelements are separated from each other by about one helical turn of DNA. To determine whether that spacing is important for activity, the spacing was altered between the subelements (Figure 2E). It was found that subtraction of a base (ERSE-M3) or addition of an extra base to the spacer (ERSE-M4) reduced TM induced expression significantly (Figure 2F).
Dimer Formation
bZIP factors are dimeric proteins; therefore, we asked whether bZIP28 could homo- or heterodimerize with other members of its class (class B according to Jakoby et al., 2002) and with bZIP60, which falls into the same family (called family D) according to Deppmann et al. (2004). There are some 75 genes in Arabidopsis encoding bZIP factors, and four of these (bZIP17, -28, -49, and -60) are in the D family. D family members are predicted to undergo intragroup dimerization because sequence characteristics within the repeating heptads of the bZIP domains are attractive for pairing (Deppmann et al., 2004). To test for homodimerization, the yeast two-hybrid system was used in which bZIP28T (a truncated form of bZIP28 missing its N terminus transcription activation domain, transmembrane, and C terminus luminal domain) served as both bait and prey. We observed in the yeast system that, indeed, bZIP28T homodimerizes (Figures 3A and 3B). To test for intragroup dimerization, all four bZIP factors under study (bZIP17, -28, -49, and -60) were used as bait and as prey in a similar way to bZIP28T. It was found that bZIP28T interacted with all the other bZIPs (Figures 3A and 3B). bZIP60T, on the other hand, showed little tendency to homodimerize and to heterodimerize with bZIP49T in the yeast two-hybrid system.
Figure 3.
Formation of Homo- and Heterodimers between bZIP TFs.
The N-terminal segments of members of the D family of bZIP TFs (bZIP17, -28, -49, and -60) were tested as bait and prey in a yeast two-hybrid system. In the N-terminal segments, the transcription activation domain, transmembrane domain, and C-terminal regions were removed.
(A) Growth of yeast on SD-Trp-Leu plates.
(B) Growth of yeast on SD-Trp-Leu-His plates. Activation of HIS gene (shown by ability to grow on –His plates) was used as the indication of interaction.
CCAAT Box Binding Factor
To determine whether NF-Y subunits interact with bZIP28 and, if so, with which subunits, bZIP28T was used as the bait and tested individually with eight of the major NF-YB and eight major NF-YC subunits as prey in a yeast two-hybrid system (Table 2). However, none of the subunits, on their own, interacted with bZIP28T.
Table 2.
Members of the NF-YB and -YC Gene Families Used as Prey in the Yeast Three-Hybrid System
| NF-YB Family |
NF-YC Family |
||
| Name | AGI No. | Name | AGI No. |
| NF-YB1 | At2g38880 | NF-YC1 | At3g48590 |
| NF-YB2 | At5g47640 | NF-YC2 | At1g56170 |
| NF-YB3 | At4g14540 | NF-YC3 | At1g54830 |
| NF-YB4 | At1g09030 | NF-YC4 | At5g63470 |
| NF-YB5 | At2g47810 | NF-YC5 | At5g50490 |
| NF-YB6 | At5g47670 | NF-YC6 | At5g50480 |
| NF-YB7 | At2g13570 | NF-YC7 | At5g50470 |
| NF-YB10 | At3g53340 | NF-YC9 | At1g08970 |
AGI, Arabidopsis Genome Initiative.
As a result, we tested whether bZIP28T could interact with a combination of NF-YB and -YC subunits in a yeast three-hybrid system (Figure 4A). Given the large number of combinations of potential interactors, NF-YB and -YC subunits were pooled as preys and screened with the bZIP28T bait. NF-YB and NF-YC constructs had different selection markers, and yeast colonies were selected that had been cotransformed with NF-YB and NF-YC as well as the bZIP28T bait. Two NF-YB plasmids (NF-YB3 and -YB5) and two NF-YC plasmids (NF-YC2 and -YC4) were identified as interesting candidates based on their ability to activate the HIS reporter gene and tested pairwise in a yeast three-hybrid system against bZIP28T. Also included in the test were the close relatives (by sequence similarity) of the two recovered NF-YB genes (NF-YB2 and -YB4) (Siefers et al., 2009). When tested in this manner, the interaction of bZIP28T with the selected NF-YB and -YC subunits was quite stringent in that only NF-YB3 and -YC2 interacted productively with bZIP28T in the yeast system (Figure 4B).
Figure 4.
Interaction of NF-Y Subunits with bZIP28.
(A) The N-terminal segment of bZIP28 (bZIP28T) was fused to the Gal4 UAS binding domain (BD) and used as bait in a yeast three-hybrid system against a pool of NF-YB and -YC subunits. The transcription activation domain, transmembrane domain, and C-terminal region were removed in bZIP28T. Prey plasmids harboring NF-YB (prey B, with URA selection marker) and -YC (prey C, with TRP selection marker) subunit genes were pooled and challenged against bZIP28T as bait (with LEU selection marker) and tested for their ability to activate the HIS interaction marker.
(B) The recovered plasmids from the screening were tested pairwise (one NF-YB and -YC subunit in each test) against bZIP28T as bait in the three-hybrid system.
(C) NF-YA4 was used as bait in the three-hybrid system in a similar pairwise challenge with NF-Y subunits (-Trp-Leu-Ura were noninteraction selective conditions, and -Trp-Leu-Ura-His were interaction selective conditions).
(D) NF-YA4 was used as bait in a two-hybrid system to test the interaction with prey NF-YB3 or NF-YC2.
(E) NF-YC2 was used as bait in a two-hybrid system to investigate the potential for dimer formation. Activation of the HIS gene was used as the indication of interaction in (D) and (E).
To identify the third subunit composing the NF-Y heterotrimer, we consulted the Arabidopsis Interactions Viewer (Geisler-Lee et al., 2007), a predicted Arabidopsis interactome database based on interacting orthologs in yeast, nematode worm, fruitfly, and human. Surprisingly, when NF-YC2 was used as the query protein, NF-YA4 had the highest interolog confidence value (value 40) followed by NF-YB3 (value 16). Subsequently, NF-YA was considered as the best potential candidate for the interaction with NF-YB3/NF-YC2 dimer. When NF-YA4 was used as bait in a three-hybrid system and challenged pairwise against the various NF-YB and -YC subunits described above, it was found that NF-YA4 was most interactive with NF-YC2 and less so with NF-YC4 (Figure 4C). However, unlike the interaction with bZIP28T, there was not much selectivity for the four different NF-YBs included in the pairwise test. NF-YA4 did not interact with individual NF-YB3 or NF-YC2 subunits in the yeast two-hybrid system (Figure 4D), which indicates that a NF-YB subunit is required for the interaction of NF-YA4 with NF-YC2. The likely interpretation of this is that NF-YC2 forms a heterodimer with NF-YB3, which is required for the interaction of NF-YA4 (Figure 4E). Nonetheless, based on the combined yeast two- and three-hybrid data, we concluded that the NF-YB3/NF-YC2 dimer interacts with NF-YA4 and with the bZIP28 dimer. However, we cannot exclude the possibility that other NF-YA subunits may associate with the NF-YB3/NF-YC2 dimer in the interaction with bZIP28.
In Vitro Assembly of the Transcriptional Complex
To determine whether bZIP28 and the NF-Y components described above form an ERSE-I binding complex, the various components expressed in Escherichia coli were tested for assembly in vitro in the presence and absence of a multimer of the ERSE-I DNA (ERSE-WT). Attempts to express full-length, His-tagged NF-YB3 in E. coli were not successful, so the C-terminal 35 amino acids of NF-YB3 were removed, giving rise to H6-NF-YB3ΔC, which was expressed. Complex assembly was monitored in pull-down assays testing for the interaction of glutathione S-transferase- and His-tagged GST-H6-bZIP28T with maltose binding protein- and His-tagged MBP-H6-NF-YA4 in the presence of H6-NF-YB3ΔC and His-tagged H6-NF-YC2 (Figure 5). When all three NF-Y subunits were incubated along with GST-H6-bZIP28T in the presence of the multimeric ERSE-I DNA, MBP-H6-NF-YA4 was readily pulled down by glutathione agarose beads with GST-H6-bZIP28T, as detected using an anti-MBP antibody (Figure 5, lane 6). When individual NF-YB3 and -YC2 subunits or both together were omitted from the assembly assay, MBP-H6-NF-YA4 was not pulled down (Figure 5, lanes 3 to 5). MBP-H6-NF-YA4 was not pulled down when the components were incubated in the absence of BiP3 ERSE-I DNA (Figure 5, lane 7 versus lane 6). Hence, an ERSE-I DNA binding complex can be assembled in vitro by the interaction of tagged forms of NF-YA4, -YB3, and -YC2 subunits with bZIP28 in the presence of DNA containing ERSE-I.
Figure 5.
Pull-Down Assay to Detect the in Vitro Assembly of a Transcriptional Complex on DNA Containing ERSE-I.
DNA was incubated with an epitope-tagged, truncated form of bZIP28 (GST-H6-28T) and various epitope-tagged forms of NF-Y subunits (MBP-H6-NF-YA4, H6-NF-YB3ΔC, and H6-NF-YC2). The proteins were synthesized in E. coli, and the amounts of tagged NF-Y subunits (lanes contain 30% input material) added to the assembly reactions were estimated by protein immunoblot using anti-His antibody (lanes 8 to 10). Protein complexes containing GST-H6-28T were pulled down using glutathione agarose beads. Transcriptional complexes containing MBP-H6-NF-YA4 were detected with anti-MBP.
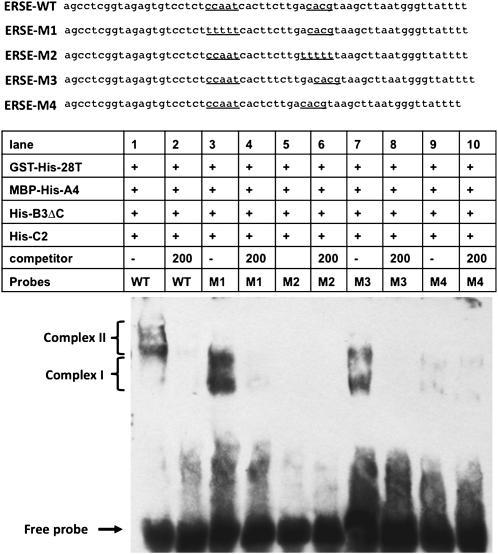
Comparable in vitro assembly reactions were performed using electrophoretic mobility shift assays, in this case with a biotin-labeled ERSE-I DNA (ERSE-WT, Figure 6). When GST-H6-bZIP28T alone was incubated with the ERSE-WT, a band shift was observed reflecting the formation of a complex (complex I) consisting of a major faster-moving and a minor slower-migrating band (Figure 6, lane 2). However, when GST-H6-bZIP28T was incubated with the ERSE-WT in the presence of the three NF-Y subunits (MBP-H6-NF-YA4, H6-NF-YB3ΔC, and H6-NF-YC2), a more slowly migrating complex was formed (complex II), which again consisted of two bands (Figure 6, lane 3). To show that the complex was specific for ERSE-I containing DNA, excess unlabeled ERSE-WT was added and shown to be an effective competitor of complex II formation (Figure 6, lanes 4 to 6). To demonstrate that complex II is made up of GST-H6-bZIP28T and/or the His-tagged NF-Y subunits, the assembly reaction was incubated along with anti-His antibody, resulting in a supershift of complex II (Figure 6, lane 7). Complex II formation was dependent on the presence of both NF-YB3 and -YC2 (in the form of H6-NF-YB3ΔC and H6-NF-YC2; Figure 6, lane 9) and on the presence of NF-YA4 (MBP-H6-NF-YA4; Figure 6, lane 10). In the absence of all three NF-Y subunits, only complex I was formed (Figure 6, lanes 2, 9, and 10). The origin of the doublet bands representing these complexes in native gels is unknown.
Figure 6.
Electrophoretic Mobility Shift Assays to Detect the in Vitro Assembly of a Transcriptional Complex on DNA Containing ERSE-I.
Biotinylated DNA containing ERSE-I was incubated with an epitope-tagged, truncated form of bZIP28 and various epitope-tagged forms of NF-Y subunits as described in Figure 5. To determine whether the assembly of the transcriptional complex was specific for DNA containing ERSE-I, 50- to 200-fold excess unlabeled competitor ERSE-I DNA was added to the reactions as indicated. The migration positions of complexes I and II are indicated. To demonstrate that the tagged proteins are involved in the assembly of complex II, the reactions were postincubated with anti-His antibody. Complex II* represents the resulting supershifted complex.
We looked into the properties of the ERSE-I DNA that might affect complex formation in the in vitro assembly reaction. A set of ERSE-I mutations were used similar to those that had been tested for ERSE-I activity in the transient expression system (described above) in which the CCAAT box and bZIP binding sites were altered by multiple base substitutions (Figure 7). When base substitutions were made in the CCAAT box (ERSE-M1), complex I was formed, but complex II was not (Figure 7, lane 3). This was interpreted to mean that GST-H6-bZIP28T can bind to ERSE-I DNA even in the absence of NF-Y. This is consistent with the finding in the transient expression system in which the ERSE-I multimer with the mutated CCAAT box supported reporter gene expression, although not at as high a level as the intact ERSE-I multimer (Figures 2E and 2F).
Figure 7.
Effect of Modifications of the ERSE-I DNA on the Assembly of a bZIP28-Containing Transcriptional Complex.
Various ERSE-I constructs (ERSE-M1 to -M2) were tested in electrophoretic mobility shift assays in which the CCAAT box or bZIP binding site was altered by multiple base substitutions or in which the length of the spacer was altered. Migration positions of complex I and II are indicated as described in Figure 6. The 200-fold excess unlabeled competitor DNA was added to the control reactions to determine whether binding is specific.
However, multiple base substitutions in the bZIP binding site (ERSE-M2) prevented detectable complex formation (Figure 7, lane 5), indicating that NF-Y does not stably bind to the ERSE-I containing DNA in the absence of bZIP28. This is consistent with the transient expression system finding on the activity of the construct in which the bZIP binding site was multiply mutated (Figures 2E and 2F). Almost no luciferase activity was observed with these constructs. In the ERSE-I constructs in which the spacing between the bZIP binding site and the CCAAT box subelements was altered, the addition of a base to the spacer allowed some complex I to be formed, but prevented complex II formation (Figure 7, lane 7), while subtraction of a base prevented formation of either complex (Figure 7, lane 9). That, too, is consistent with the transient expression system, in which addition or subtraction of a base reduced expression of the luciferase gene (Figures 2E and 2F). However, the complete absence of complex formation in the base subtracted spacer construct indicates that, whereas the construct has some activity in a transient expression system, the complex formed is too unstable to be detected by an electrophoretic mobility assay. In any case, the effects of altering the spacer indicate that bZIP28 and NF-Y interact with each other and that the spacing or the topological positioning of the binding sites is critical for promoting or stabilizing the interaction.
In Vivo Detection of the Interaction between bZIP28 and NF-Y Subunits
The functional association of bZIP28 with NF-Y subunits in vivo was investigated using bimolecular fluorescence complementation (BiFC). In BiFC, fluorescence is restored when the N-terminal (nYFP) and C-terminal (cYFP) parts of the yellow fluorescent protein (YFP) are brought together by either direct protein interaction or scaffolding attachment (Citovsky et al., 2006; Kerppola, 2008). The truncated soluble form of bZIP28 (bZIP28T) lacking the transcriptional activation domain, transmembrane domain, and C-terminal domain was fused with cYFP, and three NF-Y subunits (NF-YA4, -YB3, and -YC2) were fused individually with nYFP. Fluorescence was observed in the nucleus of the root cells of Arabidopsis seedlings stably expressing bZIP28T-cYFP and nYFP-NF-YA4 or nYFP-NF-YC2 constructs with or without TM treatment (nYFP-NF-YA4 in Figures 8A and 8D and nYFP-NF-YC2 in Figures 8C and 8F). The strong signal indicates a physical interaction/association between bZIP28T and NF-YA4 or -YC2 in vivo. Interaction between bZIP28T and NF-YB3 was observed in vivo only following TM treatment (compare Figures 8B and 8E).
Figure 8.
Detection the Interaction between bZIP28 and NF-Y Subunits in Vivo with BiFC and Pull-Down Assays.
The BiFC assay involves the interaction of proteins fused to N-terminal (nYFP) and C-terminal (cYFP) fragments of YFP to restore fluorescence of the split fluorophore.
(A) to (F) Individual NF-Y subunits were fused to nYFP, and the truncated form of bZIP28 (bZIP28T) was fused to cYFP. Each NF-Y subunit fusion protein was coexpressed with bZIP28T-cYFP in Arabidopsis roots. Roots were counterstained with 50 μg/mL propidium iodide (red). Bars = 20 μm.
(A) to (C) Untreated (DMSO).
(D) to (F) TM treated for 4 h.
(A) and (D) nYFP-NF-YA4 plus bZIP28T-cYFP.
(B) and (E) nYFP-NF-YB3 plus bZIP28T-cYFP.
(C) and (F) nYFP-NF-YC2 plus bZIP28T-cYFP.
(G) Pull-down assay with NF-YB3-YFP–overexpressing seedlings. Total proteins were extracted from nontreated and TM-treated (5 μg/mL) 2-week old seedlings and incubated with purified GST-His-bZIP28T (GST-His is a negative control). The complex was pulled down with glutathione beads and detected with anti-GFP antibody against YFP. Purified His-NF-YC2 was also added along with GST-His-bZIP28T in nontreated sample (lane 5). Two percent of the input material in the pull-down reactions was also loaded as a sample in lanes 6 and 7. The origin of doublet bands of NF-YB3-YFP is not known.
The association of bZIP28 with NF-Y subunits was also demonstrated by pull-down assays in transgenic plants overexpressing NF-YB3-YFP driven by the cauliflower mosaic virus (CaMV) 35S promoter. Only when seedlings were treated with TM could NF-YB3-YFP be pulled down with GST-His-bZIP28T (Figure 8G, lane 3 versus 4). The empty vector control (GST-His) was used as a negative control (Figure 8G, lanes 1 and 2). The amount of NF-YC2 appears to be a limiting factor under untreated (DMSO) conditions because when His-NF-YC2 protein was added to the DMSO-treated sample, NF-YB3-YFP was readily pulled down (Figure 8G, lane 5). This could explain why the physical interaction/association between NF-YB3 and bZIP28T was observed only under TM treatment in the BiFC analysis described above (Figures 8B and 8E).
Expression Patterns and Nuclear Localization of the NF-Y Subunits
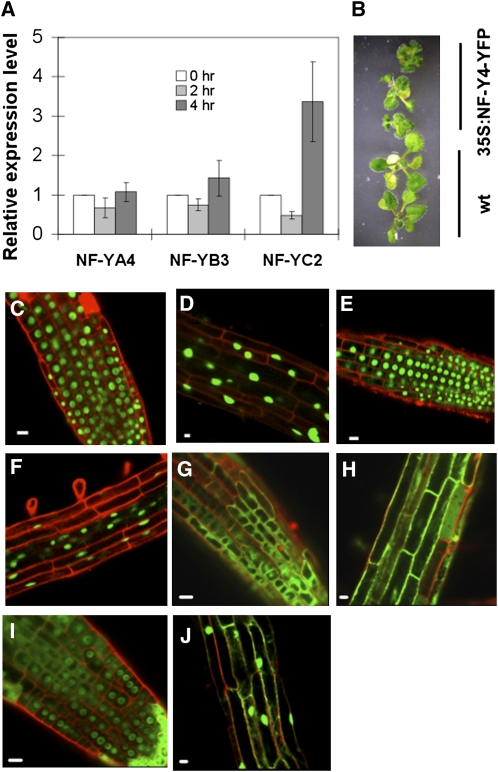
The expression patterns for NF-YA4 (probe 267315_at), -YB3 (probe 245592_at), and -YC2 (probe 262098_at) are available online at the Arabidopsis eFP browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi), and these data demonstrate that the NF-YA4 and -YB3 genes are constitutively expressed in seedlings and throughout most subsequent developmental stages. On the other hand, NF-YC2 is normally expressed only at low levels during seedling development but is upregulated during seed development, particularly during stages of active storage protein synthesis. In observations on promoter:β-glucuronidase reporter gene constructs, Siefers et al. (2009) also observed strong expression from the NF-YA4 and -YB3 promoters in unstressed Arabidopsis seedlings but almost no detectable expression from the NF-YC2 promoter under the same conditions.
However, our microarray expression data show that the expression of NF-YC2 in seedlings, along with bZIP60, is upregulated in response to ER stress and that a significant part of that upregulation is dependent on functional bZIP28 (see Supplemental Data Set 1A online). We confirmed that by quantitative RT-PCR analysis in which we found that NF-YA4 and -YB3 transcript levels were not significantly altered by TM treatment but that NF-YC2 expression was upregulated after 4 h of TM treatment. Unlike BiP3, NF-YC2 transcript levels did not rapidly rise after TM treatment and, in fact, were even somewhat lower 2 h after treatment (Figure 9A). To assess the effect of disruption of NF-YA4/YB3/YC2 on the expression of UPR target genes, homozygous T-DNA insertion mutants for NF-YA4 (SALK_003337 and SALK_144313) and NF-YC2 (SALK_111422) were obtained. Both T-DNA lines of NF-YA4 were verified as null mutants, while the T-DNA insertion line for NF-YC2 has similar NF-YC2 expression levels to the wild type. Knockout NF-YA4 did not affect the induction of BiP3 by TM (see Supplemental Figure 1 online), indicating possible functional redundancy in the NF-YA family. The upregulation of BiP3 in the NF-YA4 knockout line was, perhaps, not unexpected since bZIP28 on its own provides a certain level of transcriptional activity in the transient expression assay, and perhaps there are other UPR pathways in Arabidopsis. Unfortunately, we did not recover T-DNA homozygous plants for NF-YB3 (SALK_130295 and SALK_130296) after several attempts, although heterozygous plants for these two lines were obtained.
Figure 9.
Induction of NF-Y Gene Expression and Subcellular Relocation of NF-Y Subunits.
(A) Quantitative RT-PCR analysis of the expression of NF-Y subunits following TM treatment.
(B) NF-YA4-YFP overexpression plants were smaller than wild-type plants at 2 weeks.
(C) to (J) Merged confocal microscopy images of YFP-tagged NF-Y subunits in Arabidopsis root tips ([C], [E], [G], and [I]) or in elongated cells in the root ([D], [F], [H], and [J]). Samples were counterstained with propidium iodide (red).
(C) and (D) NF-YA4-YFP, untreated (DMSO).
(E) and (F) NF-YC2-YFP, untreated.
(G) and (H) NF-YB3-YFP, untreated.
(I) and (J) NF-YB3-YFP, TM treated for 4 h.
Bars = 10 mm in (B) and 10 μm in (C) to (J). Error bars in (A) represent se of three biological replicates. The relative expression level at 0 h was normalized to 1. Only the treatment for NF-YC2 is statistically significant (P = 0.05) according to Student's t test.
To determine the subcellular localization of the NF-Y subunits in Arabidopsis, we expressed C-terminal YFP fusions of NF-YA4, -YB3, and -YC2 in transgenic plants (under the control of the 35S CaMV promoter) and examined the location of the factors in the roots of seedlings under different conditions. Unlike NF-YB3 and NF-YC2 overexpression plants, NF-YA4 overexpression plants had a delayed flowering phenotype as reported (Wenkel et al., 2006), and the seedlings were smaller than the wild type (Figure 9B), which is similar to transgenic plants overexpressing active forms of bZIP28 (bZIP28ΔC, Liu et al., 2007a) or bZIP17 (bZIP17ΔC, Liu et al., 2008). We observed that NF-YA4-YFP and NF-YC2-YFP were located largely in nuclei of root and root tip cells under nontreated conditions (Figures 9C to 9F). However, NF-YB3-YFP was located mainly in the cytosol of root cells in untreated seedlings (Figures 9G and 9H) but largely relocated to the nucleus in TM-treated seedlings (Figures 9I and 9J). Note that the subcellular localization of NF-YB3 was quite different from that of a phylogenetically close relative, NF-YB2, which was reported to be constitutively nuclei localized in Arabidopsis and involved in regulating flowering time (Cai et al., 2007). Therefore, the expression of NF-YC2, but not the subcellular localization of the NF-YC2 protein, is affected by TM treatment, whereas, by contrast, the subcellular localization of NF-YB3, but not the expression of the NF-YB3 gene, is influenced by TM treatment.
DISCUSSION
A model that emerges from this study is one in which there are several control points in the assembly of transcriptional complexes on the ERSE-I elements of genes upregulated by the UPR in Arabidopsis (Figure 10). The transcriptional complex analyzed in this study is composed of an ER stress-specific TF, bZIP28, and possibly also bZIP60, and a general TF, NF-Y. It was interesting to find that both bZIP28 and NF-Y are regulated by ER stress. In a previous study, we showed that bZIP28 is posttranslationally activated in response to stress (Liu et al., 2007a). This involves the mobilization of ER membrane-bound bZIP28 (bZIP28p) and its proteolysis. The resulting N-terminal TF component (labeled in Figure 10 as bZIP28n) is released into the cytoplasm and then relocates into the nucleus where it upregulates the expression of target genes, such as BiP3 (Figure 10).
Figure 10.
Model for the Assembly of bZIP28 and NF-Y Containing Transcriptional Complexes and the Upregulation of UPR Genes.
In response to stress, bZIP28p is proteolytically activated by Golgi-localized S1P (and probably also by S2P) to release bZIP28n, which relocates to the nucleus to form transcriptional complexes on target genes (BiP3 and NF-YC2) bearing ERSEs in their promoters. Although the steps in nuclear translocation of the NF-Y subunits have not been studied in detail in plants, we infer from the mammalian cell literature (Kahle et al., 2005) that NF-YC2 dimerization with NF-YB3 allows the latter to be translocated to the nucleus and for the pair (NF-YC2 and NF-YB3) to interact with NF-YA4, which likely enters independently. Transcriptional complexes are composed of NF-Y trimers, which bind to CCAAT box subelements of the ERSEs, and bZIP28n dimers, which bind to bZIP binding subelements.
The TF component of bZIP28 (bZIP28n) interacts with NF-Y, a heterotrimeric protein complex composed of subunits NF-YA4, NF-YB3, and NF-YC2. Treatment with UPR stress agents upregulates the expression of the gene encoding NF-YC2, and the induction appears to be partially dependent on bZIP28, whereas genes encoding NF-YA4 and NF-YB3 are constitutively expressed. In mammalian cells, NF-Y subunits are thought to be assembled in a stepwise manner. Mammalian NF-YB and -YC are histone-fold containing protein partners and enter the nucleus as heterodimers through the importin-13 nuclear import system. The third subunit, NF-YA, which is imported by an importin-β mechanism, is recruited to generate the mature, heterotrimeric NF-Y TF (Frontini et al., 2004; Kahle et al., 2005). If Arabidopsis behaves as mammalian cells, one would expect NF-YA4 to be able to enter the nucleus on its own, since it has a SV-40-like nuclear targeting signal, RRPR (Jans et al., 2000). We showed that NF-YC2 heterodimerizes with NF-YB3, and since neither has a nuclear targeting signal, it is possible that the groove formed by dimerization at the histone fold constitutes a recognition signal for nuclear import, similar to the mechanism postulated for animal systems (Kahle et al., 2005). In the nucleus, the NF-YB3/NF-YC2 dimer would be expected to interact with NF-YA4 subunit to mediate the binding of the complex to the CCAAT box subelement of ERSE-I. One might expect that without the availability of its pairing partner (NF-YC2) in unstressed cells, NF-YB3 would be localized in the cytosol. Indeed, in nontreated Arabidopsis root cells, the YFP-tagged form of NF-YB3 is largely localized in the cytosol. In response to ER stress, tagged forms of NF-YB3 enter the nucleus. However, tagged forms of NF-YC2 expressed constitutively in transgenic plants do not follow such rules. NF-YC2-YFP is nuclear localized both before and after stress treatment. A possible explanation for this finding is that NF-YB3 cannot enter the nucleus unless it dimerizes with NF-YC2 but that NF-YC2 is not so constrained, perhaps because it can partner with other NF-YB subunits. Therefore, we tentatively conclude that the mechanisms that govern the subcellular localization of NF-YB3 may be influenced by the availability of NF-YC2 (Figure 10). However, we remain open to the possibility that nuclear relocalization for NF-YB3 may operate by a mechanism that is independent of the availability of NF-YC2 for dimerization but dependent on postmodification during UPR.
bZIP TFs are dimeric proteins, and the formation of homo- and heterodimers is thought to expand the combinatorial diversity of these TFs (Singh, 1998; Wolberger, 1998; Remenyi et al., 2004). The tendency of specific bZIP TFs to form homo- and heterodimers depends on the amino acid sequences in their Leu zippers. The zippers contain structural repeats, termed heptads, of two α-helical turns containing seven amino acid residues. The specificity for heterodimerization in plants appears to be largely determined by the placement of Asn residues in the first (a) position of the heptads (Deppmann et al., 2004). Asn residues in this position produce stable N–N interactions with partners that have Asn residues in the same position. bZIP28 is predicted to homodimerize and undergo intragroup dimerization with other members of the D family of bZIP factors (Deppmann et al., 2004). We demonstrated that experimentally with the yeast two-hybrid system, in which bZIP28 homodimerizes and heterodimerizes most efficiently with bZIP17 and bZIP60. We disregard the ability of bZIP28 to dimerize with bZIP17 in the nucleus during UPR because bZIP17 is activated by salt stress and not by UPR stress (Liu et al., 2007a; Tajima et al., 2008). However, the dimerization might be significant under conditions of combined stresses. The ability of bZIP28 to interact with bZIP60, however, may be an important transcriptional issue in UPR. Using size exclusion chromatography analysis, Iwata et al. (2009) showed that the nuclear form of bZIP60 (bZIP60n) exists as a protein complex of ∼260 kD, which is much larger than either the bZIP60n homodimer or the bZIP60n/bZIP28n heterodimer. It is quite possible that bZIP60n is also associated with the transcriptional complex identified in this study involving the bZIP60n/bZIP28n heterodimer.
The expression of the gene encoding bZIP60 is UPR regulated and partially depends on the function of bZIP28 (see Supplemental Data Set 1A online). bZIP60 was first identified by Iwata and Koizumi (2005) as being involved in UPR in Arabidopsis. bZIP60, like bZIP28, is a membrane-associated bZIP TF that is thought to undergo proteolysis and release from secretory pathway membranes in response to ER stress (Iwata et al., 2008). The difference between the two factors is that bZIP28 has a canonical S1P site, while bZIP60 does not, and bZIP28 is proteolytically processed in a manner similar to ATF6 in mammalian cells (Liu et al., 2007a). Iwata and Koizumi (2005) demonstrated that expression of a truncated, constitutively activated form of bZIP60 upregulates the expression of several genes reported here (e.g., genes encoding BiP3 and calnexin). In addition, Iwata et al. (2008) reported in a bZIP60 knockout mutant that a number of these genes responded less to TM treatment. Hence, bZIP28 and bZIP60 regulate an overlapping set of genes, except that bZIP28 also appears to be a regulator of bZIP60 gene expression, although once expressed, bZIP60 appears to be able to autoregulate its own expression (Iwata and Koizumi, 2005; Iwata et al., 2008).
In any case, bZIP28 appears to play a pivotal role in UPR in Arabidopsis in that it is mobilized by ER stress and is involved in upregulating the expression of an NF-Y subunit (NF-YC2), which by its induction may influence the entry of another NF-Y subunit (NF-YB3) into the nucleus (Figure 10). bZIP28 and -60 are both activated by the UPR, and they heterodimerize with each other in the yeast two-hybrid system. The apparent overlap may not represent redundancy in function. It may be that the factors are differently expressed or activated in different tissues or that the two TFs have different time courses for activation with bZIP28 initiating the response and bZIP60 sustaining it. More work is needed to uncover possible different functions for these two TFs.
Although a number of the TM-induced genes that depend on bZIP28 for expression have ERSEs in their promoters, others have only bZIP binding sites. If these genes are direct targets of bZIP28 action, then one might wonder what functionality NF-Ys add to the transcription complex. It is likely that bZIP28 is a first responder. It is activated (undergoes proteolysis) rapidly (within 30 min) after UPR stress agent treatment (Liu et al., 2007a). The upregulation of NF-YC2 is slower, taking several hours. Indeed, bZIP28 binds to DNA when only the CACG subelement is present. However, when both subelements are present and appropriately spaced, more transcriptional activity is observed. Thus, NF-Y support might be important in maintaining or adding to the magnitude of the response. The BiP3 gene, for example, is a robust responder to UPR stress agent treatment, and it has two tandem ERSEs, each complete with CCAAT boxes and bZIP binding sites. As for the role of NF-Ys in transcription, NF-YB and -YC in mammalian cells have been found to interact with the TFIID component of the TATA factor binding protein, a factor known to make contacts with many proteins involved in pol-II–mediated transcription (Bellorini et al., 1997).
METHODS
Plant Materials and Growth Conditions
The T-DNA insertion mutant lines for NF-YA4 (SALK_144313, insertion on the 4th exon; SALK_003337C, insertion on the 1st intron), NF-YB3 (SALK_130296, insertion on the 1st exon), and NF-YC2 (SALK_111422C, insertion on the 5′-untranslated region) were obtained from the ABRC, and SALK_130295 (for NF-YB3, insertion on the 1st exon) was provided by Kate Warpeha of the University of Illinois at Chicago. The T-DNA lines were genotyped by PCR using gene-specific primers and a left border T-DNA primer (see Supplemental Data Set 1B online). Stable transformed transgenic plants were generated by floral dip (Clough and Bent, 1998). Seeds were germinated on agar plates containing Linsmaier and Skoog salts (Linsmaier and Skoog, 1965) and 1% sucrose after being stratified at 4°C for 2 d. Seedlings were grown either on agar plates or in soil in an illuminated growth chamber at 23°C.
Microarray Analysis and Quantitative RT-PCR
For microarray analysis, wild-type (Arabidopsis thaliana ecotype Columbia) and mutant plants were grown vertically on agar plates for 1 week and then transferred to Linsmaier and Skoog liquid medium supplied with 5 μg/mL TM or DMSO (mock treatment) for 2 h. Whole seedlings were collected and total RNA was extracted from ground tissues using an RNeasy Kit according to the manufacturer's instructions (Qiagen). Affymetrix Arabidopsis gene chips (ATH1) were used to compare the gene expression profiles in the wild type and the bZIP28 T-DNA mutant in a randomized complete block design with two independent replications. P and Q values for the comparison of genotypes were calculated using EDGE software (http://www.genomine.org/edge/), and a P = 0.1 cutoff was used to select the genes that are differentially regulated by TM between the wild type and the mutant. Genes were rank ordered by the difference in the level of upregulation between the wild type and the mutant to give a measure of the TM-induced genes that were mostly dependent on bZIP28 for expression. PSCAN (http://www.beaconlab.it/pscan) was used to search for consensus sequences in the promoter region of the 15 genes on the top list. Promoter consensus sequences were compared with UPR cis-elements defined according to Iwata et al. (2008) as follows: CCAAT-N10-CACG for ERSE-I, ATTGG-N2-CACG for ERSE-II, GA-TGACGT-GK for XBP1-BS, TGACGTGR for UPRE, and one mismatch to ERSE-I as ERSE-L. Seven of the genes (BiP3, SHD, CNX1, SDF2, HSP70, PDIL1-1, and DNA J) at the top of the list (Table 1) were selected for quantitative RT-PCR with three biological replicates. Quantitative RT-PCR and statistical analysis were performed according to Liu et al. (2007a). Briefly, gene expression levels in three biological replicates were normalized to that of actin 2/8 measured in the same RNA sample with SYBR Green PCR master mix (Applied Biosystems) in a multiplex quantitative PCR system, Mx4000 (Stratagene). Expression levels were calculated with a ΔCt (threshold cycle) method. Two-way analysis of variance was performed and Tukey's range (honestly significant difference) test was used to determine significant differences among genotypes. A level of 0.05 was used for statistical significance. All primers used in this study are listed in Supplemental Data Set 1B online.
Luciferase Reporter Assay
A 4X multimer of the ERSE-I motif (5′-TCACGTGTCTGCTTGTGATTGGGTA-3′) derived from BiP3 promoter (−162 to −102 nucleotides) and various mutated forms (Figure 2) were synthesized and cloned into pGreenII 0800-LUC (Hellens et al., 2005) at HindIII and BamHI sites. A CaMV 35S minimal promoter was introduced into the construct at SpeI and SacII sites to make firefly luciferase reporter constructs, which also contain a full 35S promoter driving a Renilla luciferase expression cassette. Arabidopsis leaf protoplasts were isolated from 4-week-old soil-grown seedlings and transfected according to a protocol developed by Jen Sheen (http://genetics.mgh.harvard.edu/sheenweb/) with various reporter constructs or cotranstransfected with truncated forms of bZIP28 (bZIP28ΔC) in which the transmembrane domain and C terminus domain sequences were removed (Liu et al., 2007a). After transformation, the protoplasts were incubated either with DMSO or 5 μg/mL TM for 16 h. Firefly and Renilla luciferase were quantified with a dual-luciferase reporter assay according to the manufacturer's instructions (Promega) in a Centro Microplate Luminometer LB 960 (Berthold Technologies). Three independent transformation assays were used to calculate the mean and se for luciferase expression. Student's t test was used for statistical analysis.
Protein Interactions in Yeast
Yeast two-hybrid assays were performed using a PROQUEST system (Invitrogen) with yeast strain MaV203. Truncated forms of bZIP17, -28, -49, and -60 were developed by removing the N-terminal transcription activation domain, transmembrane domain, and C-terminal domain and then were cloned into pENTR/D-TOPO vectors and recombined into prey pDEST22 and bait pDEST32, respectively, using LR recombinase (Invitrogen). The screen was performed on SD medium lacking His, Leu, and Trp plus 30 mM 3-aminotriazole. NF-YB1, NF-YB2, NF-YB4, NF-YC5, and NF-YC7 were also cloned and recombined into pDEST22 using LR recombinase. Ten other NF-YB and NF-YC genes, which were gifts from George Coupland at the Max Planck Institute for Plant Breeding Research (Wenkel et al., 2006), were recombined in pDEST22. For yeast three-hybrid screening, TRP1 selection markers in eight NF-YB pDEST22 constructs (Table 2) were replaced by URA markers at SnaBI and AarI sites, and all the eight NF-YB and eight NF-YC plasmids were mixed with equal molar ratio to make the NF-Y library. Yeast strain PJ69-4A was transformed with the bZIP28 bait vector and subsequently transformed with the NF-Y library. Approximately 2000 yeast colonies were screened for -Trp-Leu-Ura-His resistance, and the first 100 colonies showing faster growth were streaked on fresh selection plates. Twenty yeast colonies were genotyped with gene-specific primers for bZIP28, NF-YB, or NF-YC genes, and the genotyping results were confirmed by direct sequencing of the PCR products. The interactions were also reconfirmed by pairwise testing of the interactions between specific NF-YBs and -YCs with bZIP28T in the yeast three-hybrid system. Closely related gene family members, NF-YB2 and -YB4, were also included in the reconfirmation tests. NF-YA4 and NF-YC2 were cloned into pENTR/D-TOPO vectors and recombined into bait vector pDEST32. The interaction of NF-YA4 with NF-YB and NF-YC was also tested similarly using NF-YA4 bait instead of bZIP28T bait. Interaction between individual NF-Y subunits (NF-YA4, NF-YB3, and NF-YC2) was also assayed with the yeast two-hybrid system as described above.
Protein Expression and Pull-Down Assays of in Vitro Assembled Complexes
The truncated form of bZIP28 (bZIP28T) was cloned into pETMALc-H (Merck) at EcoRI and NotI sites to produce a GST-H6-bZIP28T protein expression construct. NF-YA4 was cloned into pET42 at EcoRI and NotI sites to produce a MBP-H6-NF-YA4 protein expression construct. Full-length NF-YB3, NF-YC2, and a truncated form of NF-YB3 (NF-YB3ΔC, in which the C-terminal 35 amino acids were removed) were cloned into pET28 to produce the H6-NF-YB3, H6-NF-YC2, and H6-NF-YB3ΔC protein expression constructs, respectively. The constructs were introduced into Escherichia coli strain BL21, and the cells were induced with 300 μM IPTG overnight at 16°C. MBP-H6-NF-YA4 was purified with amylase resin (NEB), and the other His-tagged proteins were purified with Ni-NTA agarose beads (Qiagen). For in vitro GST pull-down assays, GST-H6-bZIP28T or GST-H6 (control) was bound to glutathione agarose beads (Sigma-Aldrich), and subsequently various recombinant proteins (0.5 μg) were added with or without ERSE-I multimeric DNA. The pull-down mixture contained 25 mM Tris-HCl, pH 7.4, 75 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 0.05% Nonidet P-40, and 1 mg/mL BSA (NEB). The mixture was rotated at 4°C for 3 h and washed four times with pull-down buffer. The beads were resuspended and boiled in 2× SDS buffer for 5 min, and the eluted material was subjected to protein blotting in which anti-MBP antibody (NEB) was used to detect the MBP-H6-NF-YA4 protein. The input material in the pull-down assay was analyzed with anti-His antibody (Amersham Bioscience).
Electrophoretic Mobility Shift Assay
A wild-type ERSE-I probe (60 bp; Figure 7) and various mutated forms (M1 to M4; Figure 7) were created by annealing together complementing biotinylated oligonucleotides. Electrophoretic mobility shift assays were performed using a LightShift Chemiluminescent EMSA kit (Pierce), according to the manufacturer's protocols. Each 20-μL binding reaction contained 20 mM HEPES buffer, pH 7.9, 100 mM KCl, 5% glycerol, 1 mM MgCl2, 1 mM DTT, 0.1% Tween 20, and 25 ng/μL poly(dI-dC). For electrophoretic mobility shift assay analysis of TF complexes assembled in vitro, 40 fmol biotin-labeled probe, 3.0 μg GST-H6-bZIP28T, 1.5 μg MBP-H6-NF-YA4, 1.5 μg H6-NF-YB3ΔC, and 1.5 μg H6-NF-YC2 were added to the binding reaction. Nonlabeled DNA was used as competitor, and 1 μL of anti-His antibody (Amersham Bioscience) was added after the incubation for test for supershifting. The binding reactions were allowed to incubate on ice for 1 h. A 5% polyacrylamide minigel (37.5:1 acrylamide-bisacrylamide in 0.5× Tris-borate-EDTA containing 3% glycerol) was prerun for 1 h at 90 V with 0.5× Tris-borate-EDTA, and then 5 μL of 5× loading buffer were added to the binding reaction, and subsequently 25 μL of the reaction were subjected to gel electrophoresis at 90 V for 1 h. The gel was then transferred to a charged nylon membrane (Amersham Bioscience) for 45 min at 90 V, and subsequently UV cross-linked to the membrane at 120 μJ/cm2 for 1 min. The membrane was treated with developing buffers according to the manufacturer's protocol and then exposed to film and developed.
BiFC Analysis
BiFC was performed as described by Li et al. (2008). NF-YA4, NF-YB3, and NF-YC2 were cloned into pXY106 (one of the pXY series of plasmids from Yu et al., 2008) at XbaI and SalI sites to produce nYFP-NF-Y fusion proteins. The truncated form of bZIP28 (bZIP28T) was cloned into pXY104 at BamHI and XbaI sites to make the bZIP28T-cYFP fusion protein. Arabidopsis was cotransformed with nYFP-NF-Y and bZIP28T-cYFP driven by the 35S CaMV promoter, and transgenic plants were selected for both gentamycin and kanamycin resistance. Seedling roots were observed and images were acquired with a NikonC1si confocal scanning system attached to a 90i microscope after treating with either DMSO (solvent control) or 5 μg/mL TM for 4 h. Roots were counterstained with 50 μg/mL propidium iodide (PI). For pull-down assays, YFP was fused to the C terminus of NF-YB3 to produce NF-YB3-YFP, and NF-YB3-YFP was overexpressed in Arabidopsis driven by the 35S CaMV promoter. Transgenic plants were treated with either DMSO (solvent control) or 5 μg/mL TM for 4 h. Total proteins were extracted with extraction buffer (50 mM Tris-HCl, pH 7.6, 100 mM NaCl, 0.05% Tween 20, 1 mM EDTA, 1 mM PMSF, and 1/100 protease inhibitor [Sigma-Aldrich]) and incubated with Ni-NTA agrose purified proteins (GST-His-bZIP28T, GST-His, or His-NF-YC2 as indicated in Figure 8G) for 1 h in incubation buffer (50 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.01% Tween 20, and 1 mM PMSF). Subsequently, glutathione beads were added and incubated overnight. The beads were washed four times with incubation buffer and resuspended in 2× SDS buffer. The samples were boiled for 10 min and analyzed by protein blots using anti-GFP antibody (Medical and Biological Laboratories).
Protein Subcellular Localization and Transcript Level Analysis
YFP C terminus tagged forms of NF-YA4, NF-YB3, and NF-YC2 were expressed through the action of the 35S CaMV promoter in Arabidopsis, and the subcellular localization of the fluorescent proteins was determined using a NikonC1si confocal scanning system attached to a 90i microscope. Roots were counterstained with 50 μg/mL PI. Emission signals for YFP and PI were acquired using a sequential scanning mode to eliminate emission signal bleed through. Seedlings were treated with either DMSO or 5 μg/mL TM for 4 h. Transcript level analysis for NF-YA4, NF-YB3, and NF-YC2 genes was performed by quantitative RT-PCR as described above. The relative expression level at 0 h was normalized to 1.
Accession Numbers
Microarray data from this article can be found in ArrayExpress under the accession number 072173. Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At3g10800 (bZIP28), At2g40950 (bZIP17), At3g56660 (bZIP49), At1g42990 (bZIP60), At3g18780 (ACT2), and At1g49240 (ACT8); other accession numbers are listed in Tables 1 and 2.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Quantitative RT-PCR Analysis of the Expression of the BiP3 Gene in the T-DNA Insertion Mutant nfy-a4 (SALK_003337C).
Supplemental Data Set 1A. UPR Upregulated Genes Dependent on bZIP28 Function or Expression.
Supplemental Data Set 1B. Primers Used in This Study.
Acknowledgments
This work is supported by the National Science Foundation 2010 program (IBN0420015) and the Iowa State University Plant Sciences Institute. We thank Yanhai Yin and Bing Yang (Iowa State University) and Daniel Voytas (University of Minnesota) for providing cloning vectors and bacterial or yeast strains. We thank George Coupland (Max Planck Institute for Plant Breeding Research) for sharing the recombined yeast prey vectors harboring some of the NF-YB and NF-YC genes. We also thank the ABRC and Kate Warpeha (University of Illinois at Chicago) for providing the T-DNA insertion lines and Renu Srivastava and Sabrina Humbert for their helpful comments.
References
- Bellorini M., Lee D.K., Dantonel J.C., Zemzoumi K., Roeder R.G., Tora L., Mantovani R. (1997). CCAAT binding NF-Y-TBP interactions: NF-YB and NF-YC require short domains adjacent to their histone fold motifs for association with TBP basic residues. Nucleic Acids Res. 25: 2174–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Naim O., Eshed R., Parnis A., Teper-Bamnolker P., Shalit A., Coupland G., Samach A., Lifschitz E. (2006). The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J. 46: 462–476 [DOI] [PubMed] [Google Scholar]
- Bernales S., Papa F.R., Walter P. (2006). Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 22: 487–508 [DOI] [PubMed] [Google Scholar]
- Cai X., Ballif J., Endo S., Davis E., Liang M., Chen D., DeWald D., Kreps J., Zhu T., Wu Y. (2007). A putative CCAAT-binding transcription factor is a regulator of flowering timing in Arabidopsis. Plant Physiol. 145: 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Shen J., Prywes R. (2002). The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 277: 13045–13052 [DOI] [PubMed] [Google Scholar]
- Citovsky V., Lee L.Y., Vyas S., Glick E., Chen M.H., Vainstein A., Gafni Y., Gelvin S.B., Tzfira T. (2006). Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J. Mol. Biol. 362: 1120–1131 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Deppmann C.D., Acharya A., Rishi V., Wobbes B., Smeekens S., Taparowsky E.J., Vinson C. (2004). Dimerization specificity of all 67 B-ZIP motifs in Arabidopsis thaliana: A comparison to Homo sapiens B-ZIP motifs. Nucleic Acids Res. 32: 3435–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D., Murray J.A., Smith A.G. (1998). Multiple genes encoding the conserved CCAAT-box transcription factor complex are expressed in Arabidopsis. Plant Physiol. 117: 1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Yan H., Wu Z., Yan N., Wang Z., Jeffrey P.D., Shi Y. (2007). Structure of a site-2 protease family intramembrane metalloprotease. Science 318: 1608–1612 [DOI] [PubMed] [Google Scholar]
- Frontini M., Imbriano C., Manni I., Mantovani R. (2004). Cell cycle regulation of NF-YC nuclear localization. Cell Cycle 3: 217–222 [PubMed] [Google Scholar]
- Gao H., Brandizzi F., Benning C., Larkin R.M. (2008). A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105: 16398–16403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler-Lee J., O'Toole N., Ammar R., Provart N.J., Millar A.H., Geisler M. (2007). A predicted interactome for Arabidopsis. Plant Physiol. 145: 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmaroli G., Tonelli C., Mantovani R. (2001). Regulation of the CCAAT-Binding NF-Y subunits in Arabidopsis thaliana. Gene 264: 173–185 [DOI] [PubMed] [Google Scholar]
- Hellens R.P., Allan A.C., Friel E.N., Bolitho K., Grafton K., Templeton M.D., Karunairetnam S., Gleave A.P., Laing W.A. (2005). Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y., Fedoroff N.V., Koizumi N. (2008). Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 20: 3107–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y., Koizumi N. (2005). An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc. Natl. Acad. Sci. USA 102: 5280–5285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y., Yoneda M., Yanagawa Y., Koizumi N. (2009). Characteristics of the nuclear form of the Arabidopsis transcription factor AtbZIP60 during the endoplasmic reticulum stress response. Biosci. Biotechnol. Biochem. 73: 865–869 [DOI] [PubMed] [Google Scholar]
- Jakoby M., Weisshaar B., Droge-Laser W., Vicente-Carbajosa J., Tiedemann J., Kroj T., Parcy F. (2002). bZIP transcription factors in Arabidopsis. Trends Plant Sci. 7: 106–111 [DOI] [PubMed] [Google Scholar]
- Jans D.A., Xiao C.Y., Lam M.H. (2000). Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays 22: 532–544 [DOI] [PubMed] [Google Scholar]
- Kahle J., Baake M., Doenecke D., Albig W. (2005). Subunits of the heterotrimeric transcription factor NF-Y are imported into the nucleus by distinct pathways involving importin beta and importin 13. Mol. Cell. Biol. 25: 5339–5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamauchi S., Nakatani H., Nakano C., Urade R. (2005). Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J. 272: 3461–3476 [DOI] [PubMed] [Google Scholar]
- Kerppola T.K. (2008). Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu. Rev. Biophys. 37: 465–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K., Kato H., Miyata T. (2001). Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J. Biol. Chem. 276: 9199–9205 [DOI] [PubMed] [Google Scholar]
- Kumimoto R.W., Adam L., Hymus G.J., Repetti P.P., Reuber T.L., Marion C.M., Hempel F.D., Ratcliffe O.J. (2008). The Nuclear Factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 228: 709–723 [DOI] [PubMed] [Google Scholar]
- Lee H., Fischer R.L., Goldberg R.B., Harada J.J. (2003). Arabidopsis LEAFY COTYLEDON1 represents a functionally specialized subunit of the CCAAT binding transcription factor. Proc. Natl. Acad. Sci. USA 100: 2152–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.X., Oono Y., Zhu J., He X.J., Wu J.M., Iida K., Lu X.Y., Cui X., Jin H., Zhu J.K. (2008). The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20: 2238–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsmaier E.M., Skoog F. (1965). Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 18: 100–127 [Google Scholar]
- Liu J.X., Srivastava R., Che P., Howell S.H. (2007a). An endoplasmic reticulum stress response in Arabidopsis is mediated by proteolytic processing and nuclear relocation of a membrane-associated transcription factor, bZIP28. Plant Cell 19: 4111–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.X., Srivastava R., Che P., Howell S.H. (2007b). Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic stress signaling. Plant J. 51: 897–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.X., Srivastava R., Howell S.H. (2008). Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis. Plant Cell Environ. 31: 1735–1743 [DOI] [PubMed] [Google Scholar]
- Lotan T., Ohto M., Yee K.M., West M.A., Lo R., Kwong R.W., Yamagishi K., Fischer R.L., Goldberg R.B., Harada J.J. (1998). Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93: 1195–1205 [DOI] [PubMed] [Google Scholar]
- Martinez I.M., Chrispeels M.J. (2003). Genomic analysis of the unfolded protein response in Arabidopsis shows its connection to important cellular processes. Plant Cell 15: 561–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero S., Imbriano C., Ravasio F., Favaro R., Pelucchi N., Gorla M.S., Mantovani R., Colombo L., Kater M.M. (2002). Ternary complex formation between MADS-box transcription factors and the histone fold protein NF-YB. J. Biol. Chem. 277: 26429–26435 [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Ito Y., Serizawa A., Kurata N. (2003). OsHAP3 genes regulate chloroplast biogenesis in rice. Plant J. 36: 532–540 [DOI] [PubMed] [Google Scholar]
- Nelson D.E., et al. (2007). Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. USA 104: 16450–16455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh S.J., Kwon C.S., Oh D.H., Moon J.S., Chung W.I. (2003). Expression of an evolutionarily distinct novel BiP gene during the unfolded protein response in Arabidopsis thaliana. Gene 311: 81–91 [DOI] [PubMed] [Google Scholar]
- Remenyi A., Scholer H.R., Wilmanns M. (2004). Combinatorial control of gene expression. Nat. Struct. Mol. Biol. 11: 812–815 [DOI] [PubMed] [Google Scholar]
- Schroder M., Kaufman R.J. (2005a). ER stress and the unfolded protein response. Mutat. Res. 569: 29–63 [DOI] [PubMed] [Google Scholar]
- Schroder M., Kaufman R.J. (2005b). The mammalian unfolded protein response. Annu. Rev. Biochem. 74: 739–789 [DOI] [PubMed] [Google Scholar]
- Schutze K., Harter K., Chaban C. (2008). Post-translational regulation of plant bZIP factors. Trends Plant Sci. 13: 247–255 [DOI] [PubMed] [Google Scholar]
- Shen J., Chen X., Hendershot L., Prywes R. (2002). ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3: 99–111 [DOI] [PubMed] [Google Scholar]
- Shen X., Ellis R.E., Lee K., Liu C.Y., Yang K., Solomon A., Yoshida H., Morimoto R., Kurnit D.M., Mori K., Kaufman R.J. (2001). Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107: 893–903 [DOI] [PubMed] [Google Scholar]
- Siefers N., Dang K.K., Kumimoto R.W., Bynum W.E., IV, Tayrose G., Holt B.F., III (2009). Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 149: 625–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.B. (1998). Transcriptional regulation in plants: The importance of combinatorial control. Plant Physiol. 118: 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens N.O., Galuschka C., Schindler M., Bulow L., Hehl R. (2005). AthaMap web tools for database-assisted identification of combinatorial cis-regulatory elements and the display of highly conserved transcription factor binding sites in Arabidopsis thaliana. Nucleic Acids Res. 33: W397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima H., Iwata Y., Iwano M., Takayama S., Koizumi N. (2008). Identification of an Arabidopsis transmembrane bZIP transcription factor involved in the endoplasmic reticulum stress response. Biochem. Biophys. Res. Commun. 374: 242–247 [DOI] [PubMed] [Google Scholar]
- Travers K.J., Patil C.K., Wodicka L., Lockhart D.J., Weissman J.S., Walter P. (2000). Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101: 249–258 [DOI] [PubMed] [Google Scholar]
- Wang Y., Shen J., Arenzana N., Tirasophon W., Kaufman R.J., Prywes R. (2000). Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 275: 27013–27020 [DOI] [PubMed] [Google Scholar]
- Wenkel S., Turck F., Singer K., Gissot L., Le Gourrierec J., Samach A., Coupland G. (2006). CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18: 2971–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M.A.L.W., Yee K.M., Danao J., Zimmerman J.L., Fischer R.L., Goldberg R.B., Harada J.J. (1994). LEAFY COTYLEDON1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. Plant Cell 6: 1731–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolberger C. (1998). Combinatorial transcription factors. Curr. Opin. Genet. Dev. 8: 552–559 [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Kagaya Y., Toyoshima R., Kagaya M., Takeda S., Hattori T. (2009). Arabidopsis NF-YB subunits LEC1 and LEC1-LIKE activate transcription by interacting with seed-specific ABRE-binding factors. Plant J. 58: 843–856 [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Yoshida H., Kokame K., Kaufman R.J., Mori K. (2004). Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J. Biochem. 136: 343–350 [DOI] [PubMed] [Google Scholar]
- Ye J., Rawson R.B., Komuro R., Chen X., Dave U.P., Prywes R., Brown M.S., Goldstein J.L. (2000). ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6: 1355–1364 [DOI] [PubMed] [Google Scholar]
- Yoshida H., Haze K., Yanagi H., Yura T., Mori K. (1998). Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 273: 33741–33749 [DOI] [PubMed] [Google Scholar]
- Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., Mori K. (2000). ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20: 6755–6767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M., Mori K. (2001). Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol. Cell. Biol. 21: 1239–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Li L., Li L., Guo M., Chory J., Yin Y. (2008). Modulation of brassinosteroid-regulated gene expression by Jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proc. Natl. Acad. Sci. USA 105: 7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambelli F., Pesole G., Pavesi G. (2009). Pscan: Finding over-represented transcription factor binding site motifs in sequences from co-regulated or co-expressed genes. Nucleic Acids Res. 37: W247–W252 [DOI] [PMC free article] [PubMed] [Google Scholar]