Abstract
Signaling by the Wnt family of secreted glycolipoproteins via the transcription co-activator β-catenin controls embryonic development and adult homeostasis. Here we review recent progresses in this so-called canonical Wnt signaling pathway. We discuss Wnt ligands, agonists and antagonists and their interactions with Wnt receptors. We also dissect critical events that regulate β-catenin stability from Wnt receptors to the cytoplasmic β-catenin destruction complex, and nuclear machinery that mediates β-catenin-dependent transcription. Finally we highlight some key aspects of Wnt/β-catenin signaling in human diseases including congenital malformations, cancer and osteoporosis and potential therapeutic implications.
Introduction
Signaling by the Wnt family of secreted glycolipoproteins is one of the fundamental mechanisms that direct cell proliferation, cell polarity and cell fate determination during embryonic development and tissue homeostasis (Logan and Nusse, 2004). As a result, mutations in the Wnt pathway are often linked to human birth defects, cancer and other diseases (Clevers, 2006). A critical and most studied Wnt pathway is canonical Wnt signaling, which functions by regulating the amount of the transcriptional co-activator β-catenin that controls key developmental gene expression programs. This review focuses on our current understanding of Wnt/β-catenin signaling, drawing mainly from genetic, developmental and biochemical analyses in Drosophila, Xenopus, mice and humans. For more comprehensive and historic perspective we refer readers to earlier reviews (Clevers, 2006; Logan and Nusse, 2004) and the Wnt homepage (www.stanford.edu/~rnusse/wntwindow.html). The nematode Caenorhabditis elegans exhibits similar but also divergent Wnt/β-catenin pathways, which are covered elsewhere (Mizumoto and Sawa, 2007) and in the accompanying review (Kimble 2009). Wnt also activates a number of non-canonical signaling pathways that are independent of β-catenin and have been recently reviewed (Seifert and Mlodzik, 2007; Wang and Nathans, 2007).
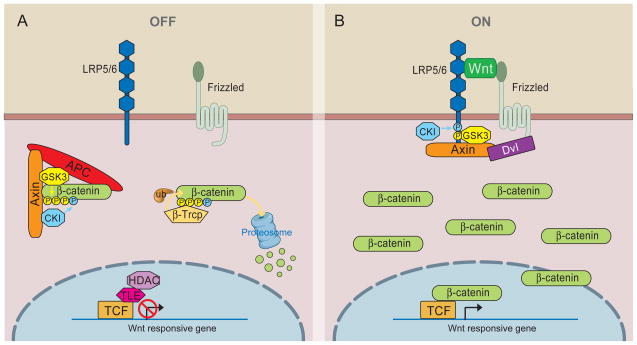
The central logic of Wnt/β-catenin signaling has emerged from two decades of studies (Figure 1). In the absence of Wnt, cytoplasmic β-catenin protein is constantly degraded by the action of the Axin complex, which is composed of the scaffolding protein Axin, the tumor suppressor adenomatous polyposis coli gene product (APC), casein kinase 1 (CK1), and glycogen synthase kinase 3 (GSK3). CK1 and GSK3 sequentially phosphorylate the amino terminal region of β-catenin, resulting in β-catenin recognition by β-Trcp, an E3 ubiquitin ligase subunit, and subsequent β-catenin ubiquitination and proteasomal degradation (He et al., 2004). This continual elimination of β-catenin prevents β-catenin from reaching the nucleus, and Wnt target genes are thereby repressed by the DNA-bound T cell factor/lymphoid enhancer factor (TCF/LEF) family of proteins (Figure 1a). The Wnt/β-catenin pathway is activated when a Wnt ligand binds to a seven-pass transmembrane Frizzled (Fz) receptor and its co-receptor, low-density lipoprotein receptor related protein 6 (LRP6) or its close relative LRP5. The formation of a likely Wnt-Fz-LRP6 complex together with the recruitment of the scaffolding protein Dishevelled (Dvl) results in LRP6 phosphorylation and activation and the recruitment of the Axin complex to the receptors. These events lead to inhibition of Axin-mediated β-catenin phosphorylation and thereby to the stabilization of β-catenin, which accumulates and travels to the nucleus to form complexes with TCF/LEF and activates Wnt target gene expression (Figure 1b).
Figure 1. Overview of Wnt/β-catenin signaling.
A) In the absence of Wnt, cytoplasmic β-catenin forms a complex with Axin, APC, GSK3 and CK1, and is phosphorylated by CK1 (blue) and subsequently by GSK3 (yellow). Phosphorylated β-catenin is recognized by the E3 ubiquitin ligase β-Trcp, which targets β-catenin for proteosomal degradation. Wnt target genes are repressed by TCF-TLE/Groucho and histone deacetylases (HDAC). B) In the presence of Wnt ligand, a receptor complex forms between Fz and LRP5/6. Dvl recruitment by Fz leads to LRP5/6 phosphorylation, and Axin recruitment. This disrupts Axin-mediated phosphorylation/degradation of β-catenin, allowing β-catenin to accumulate in the nucleus where it serves as a co-activator for TCF to activate Wnt responsive genes.
Wnt ligands and biogenesis
Wnts are conserved in all metazoan animals. In mammals, complexity and specificity in Wnt signaling are in part achieved through 19 Wnt ligands, which are cysteine rich proteins of approxiamately 350-400 amino acids that contain an N-terminal signal peptide for secretion. Murine Wnt3a represents the first purified and biochemically characterized Wnt protein (Willert et al., 2003) owing to its relatively efficient secretion (in contrast to most other Wnt proteins). In addition to N-linked glycosylation, which is required for Wnt3a secretion (Komekado et al., 2007), Wnt3a undergoes two types of lipid modifications that likely account for the hydrophobicity and poor solubility of Wnt proteins (Hausmann et al., 2007). The first reported lipididation was the addition of palmitate to cysteine 77 (Willert et al., 2003). Its mutation had minimal effect on Wnt3a secretion but diminished the ability of Wnt3a to activate β-catenin signaling (Galli et al., 2007; Komekado et al., 2007; Willert et al., 2003). The second identified lipididation was a palmitoleoyl attached to serine 209, and its mutation resulted in Wnt3a accumulation in the endoplasmic reticulum (ER) and failure in secretion (Takada et al., 2006).
Drosophila Wingless (Wg) is the Wnt molecule most investigated in vivo (Hausmann et al., 2007). These studies plus work in nematodes have identified genes that regulate Wnt biogenesis and secretion. Porcupine (Porc) encodes a multipass transmembrane ER protein that contains an O-acyl transferase domain suggesting a role in Wg lipid modification (Hausmann et al., 2007). Porc deficiency results in Wg and Wnt3a accumulation in the ER and diminished Wnt3a palmitoleoylation at serine 209 (Takada et al., 2006), suggesting that Porc is responsible for this particular lipidation. Whether Porc or a distinct acyltransferase is involved in Wnt3a palmitoylation at cysteine 77 remains unknown.
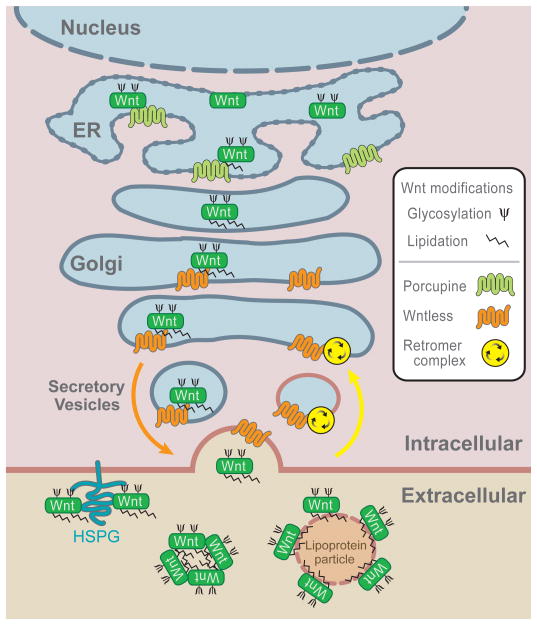
Two additional proteins/protein complexes were identified for Wg/Wnt secretion: Wntless (Wls), also known as Evenness interrupted (Evi) or Sprinter (Srt), in Drosophila and the retromer complex in nematodes (Hausmann et al., 2007). Wls is a multipass transmembrane protein that localizes to the Golgi, endocytic compartments and the plasma membrane, and is essential for Wg secretion. The retromer complex, which is composed of five subunits, was defined first in yeast. It mediates membrane protein trafficking between endosomes and the Golgi apparatus (Hausmann et al., 2007). Several groups recently reported that the retromer complex is required for retrieval/recycling of Wls from the endosome to the Golgi (Belenkaya et al., 2008; Franch-Marro et al., 2008b; Pan et al., 2008a; Port et al., 2008; Yang et al., 2008), likely mediated by direct interaction between Wls and the retromer Vps35 subunit. Loss of retromer function causes Wls to be degraded in the lysosomes and results in reduction of Wls and thus Wnt secretion. These studies led to an emerging picture of Wnt biogenesis (Figure 2). Wnt is glycosylated and lipid modified by Porc in the ER, and is escorted by Wls from the Golgi to the plasma membrane for secretion. Wls is recycled by endocytosis and trafficked back to Golgi by the retromer. Note that porc, wls and retromer mutants largely phenocopy wg/wnt mutants in flies and worms, attesting their dedicated roles in Wnt biogenesis.
Figure 2. Wnt biogenesis and secretion.
Wnts are glycosylated and lipid modified in the ER involving Porcupine, and escorted by Wntless from the Golgi to the plasma membrane for secretion. Wntless is retrieved from endocytic vescicles back to the Golgi by the retromer complex. After secretion mature Wnts bind to HSPGs and lipoprotein particles or form multimers, which can modulate Wnt gradients and facilitate long range Wnt signaling. The number of Wnt molecules bound to the lipoprotein particle and in the multimeric form were drawn arbitrarily.
Cautions need to be taken, however, when the above model is extrapolated to individual Wnt proteins. Effects of altering the two lipid-modified residues on Wg secretion and function are different from those for Wnt3a, leading to the suggestion that overall lipidation levels may be more critical (Franch-Marro et al., 2008a). Differences in the order of glycosylation and lipidation also seem to exist between Wnt3a and Wg (Komekado et al., 2007; Tanaka et al., 2002; Zhai et al., 2004). Lastly Drosophila WntD neither requires Porc and Wls for secretion nor is WntD palmitoylated at the conserved cysteine position (Ching et al., 2008).
Wnt extracellular distribution and movement
Wnt proteins can function as morphogens that are capable of both short and long range signaling, as best demonstrated for Wg. Wg lipidation raises the issue of its diffusion and distribution through the aqueous extracellular space. Indeed purified Wnt3a exhibits increased activity via artificial liposomal packaging (Morrell et al., 2008). Two distinct Wg secretory pathways for short and long range signaling have been speculated but not fully substantiated. Wg may form multimers to bury lipid modifications inside (Katanaev et al., 2008), or bind to lipoprotein particles, which may be involved in Wg long range signaling (Panakova et al., 2005) (Figure 2). The membrane microdomain protein reggie-1/flotillin-2 specifically promotes Wg long-range secretion (Katanaev et al., 2008). The Wg receptors (see below) and heparan sulfate proteoglycans (HSPGs) such as Dally and Dally-like protein have important roles in the Wg morphogen concentration via regulating Wg degradation, diffusion, endocytosis/transcytosis, and may function in Wg signaling as potential low-affinity co-receptors (Lin, 2004). Note that reggie-1/flotillin-2, lipoprotein particles, Dally and Dally-like protein are important analogously for secreted Hedgehog morphogen, which is also lipid modified (Katanaev et al., 2008; Lin, 2004; Panakova et al., 2005).
Wnt receptors: Frizzled and LRP5/6
Two distinct receptor families are critical for Wnt/β-catenin signaling (Figure 3): the Frizzled (Fz or Fzd) seven-pass transmembrane receptors (Logan and Nusse, 2004) and the LDL receptor-related proteins 5 and 6 (LRP5 and LRP6) (He et al., 2004). The Wnt-receptor relationship is best illustrated for Wg, which binds to Drosophila Fz2 (Dfz2) and Dfz1 with high affinity (1-10 nM) and requires either Fz in a redundant manner (Logan and Nusse, 2004). Wg reception also absolutely depends on Arrow, the LRP5/6 homolog (He et al., 2004). The mammalian genome harbors 10 Fz genes, most of which have variable capacities to activate β-catenin signaling when co-overexpressed with Wnt and LRP5/6 (e.g., Binnerts et al., 2007) and functional redundancy among Fz members is likely prevalent (Logan and Nusse, 2004). Between the two LRPs, LRP6 plays a more dominant role and is essential for embryogenesis whereas LRP5 is dispensable for embryogenesis but critical for adult bone homeostasis. Nonetheless LRP5 and LRP6 are partially redundant as their functions together are required for mouse gastrulation (He et al., 2004). Most data, including Wnt binding to LRP5/6 and Wnt1-Fz8-LRP6 complex formation in vitro and observations that engineered Fz-LRP5/6 proximity is sufficient to activate β-catenin signaling (Cong et al., 2004; Holmen et al., 2005; Tolwinski et al., 2003), support the model that Wnt induces the formation of Fz-LRP5/6 complex (He et al., 2004) (Figure 1). But unambiguous demonstration of this receptor complex in vivo is lacking. It is noteworthy that Wnt3a palmitoylation (at cysteine 77) is important for binding to both Fz and LRP6 (Cong et al., 2004; Komekado et al., 2007), explaining in part the importance of this lipid modification.
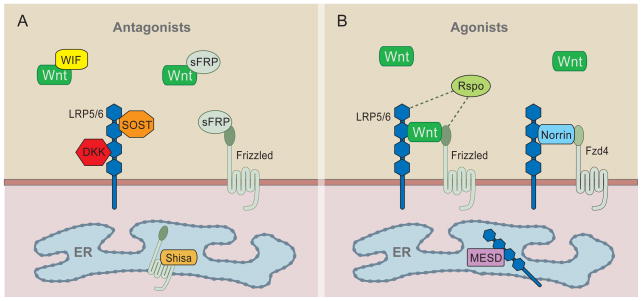
Figure 3. Secreted Wnt antagonists and agonists.
A) Antagonists. WIF and sFRP bind directly to secreted Wnts and/or Fz. DKK and SOST/WISE proteins bind LRP5/6 to prevent Fz-LRP6 complex formation. Shisa proteins trap Fz in the ER. B) Agonists. Wnts are the primary agonists and form a complex with LRP5/6 and Fz to activate signaling. Norrin acts similar to Wnt, but binds specifically to Fz4. R-spondin proteins (Rspo) act via and may bind to LRP5/6 and/or Fz receptors. In the ER, the chaperone MESD is needed for LRP5/6 maturation.
A particular Wnt may activate β-catenin and/or non-canonical pathways depending on the receptor complement (van Amerongen et al., 2008). Fz function is involved in β-catenin and non-canonical pathways. The Fz-LRP5/6 co-receptor model stipulates that a Wnt-Fz pair capable of recruiting LRP5/6 activates the β-catenin pathway, consistent with the specific requirement of LRP5/6 in Wnt/β-catenin signaling (He et al., 2004). However some evidence suggests that LRP6 antagonizes non-canonical Wnt signaling in vivo, possibly via competing for Wnt ligands (Bryja et al., 2009) or an unknown mechanism (Tahinci et al., 2007). Other Wnt receptors exist such as Ryk and ROR2, which are not required for, but in some cases may antagonize, Wnt/β-catenin signaling (van Amerongen et al., 2008).
Wnt antagonists and agonists
Several secreted protein families antagonize or modulate Wnt/β-catenin signaling (Figure 3). sFRPs (secreted Frizzled related proteins), and WIF (Wnt inhibitory protein) bind to Wnt, and in the case of sFRPs, also to Fz (Figure 3), and thereby function as Wnt antagonists for both β-catenin and non-canonical signaling (Bovolenta et al., 2008). Loss-of-function studies in mice have revealed significant redundancy for the sFRP genes (Satoh et al., 2008). The Wnt-binding property suggests that sFRPs and WIF may also regulate Wnt stability and diffusion/distribution extracellularly beyond just Wnt inhibitors. Some sFRPs have been shown to have Wnt-independent activity such as regulators of extracellular proteinases (Bovolenta et al., 2008).
Two distinct classes of Wnt inhibitors are the Dickkopf (Dkk) family and the Wise/SOST family (Figure 3). Dkk proteins, exemplified by Dkk1, are LRP5/6 ligands/antagonists and are considered specific inhibitors for Wnt/β-catenin signaling. Although two different models for Dkk1 action have been proposed (Mao et al., 2002; Semenov et al., 2001), recent biochemical and genetic studies (Ellwanger et al., 2008; Semenov et al., 2008; Wang et al., 2008) have argued against the model that Dkk1 inhibits Wnt signaling via inducing LRP6 internalization/degradation through transmembrane Kremen (Krm) proteins (Mao et al., 2002). Dkk1 disruption of Wnt-induced Fz-LRP6 complex remains a more likely mechanism (Semenov et al., 2001), with Krm playing a minor modulatory role in specific tissues (Ellwanger et al., 2008). Wise and SOST constitute another family of LRP5/6 ligands/antagonists (Itasaki et al., 2003; Li et al., 2005; Semenov et al., 2005). Like Dkk1, SOST is able to disrupt Wnt-induced Fz-LRP6 complex in vitro (Semenov et al., 2005). Both Dkk1 and SOST are strongly implicated in human diseases (see below).
Shisa proteins represent a distinct family of Wnt antagonists (Figure 3), which trap Fz proteins in the ER and prevent Fz from reaching the cell surface, thereby inhibiting Wnt signaling cell-autonomously (Yamamoto et al., 2005). Shisa proteins also antagonize FGF (fibroblast growth factor) signaling by trapping FGF receptors in the ER. Other Wnt antagonists with multivalent activities exist. Xenopus Cerberus binds to and inhibits Wnt as well as Nodal and BMP (bone morphogenetic protein) (Piccolo et al., 1999), and IGFBP-4 (Insulin-like growth-factor-binding protein-4) antagonizes Wnt signaling via binding to both Fz and LRP6, in addition to modulating IGF signaling (Zhu et al., 2008).
Norrin and R-spondin (Rspo) proteins are two families of agonists for Wnt/β-catenin signaling (Figure 3). Norrin is a specific ligand for Fz4 and acts through Fz4 and LRP5/6 during retinal vascularization (Xu et al., 2004). Rspo proteins exhibit synergy with Wnt, Fz and LRP6 (Kazanskaya et al., 2004; Kim et al., 2005; Nam et al., 2006; Wei et al., 2007), and show genetic interaction with LRP6 during embryogenesis (Bell et al., 2008), but their mechanism of action is controversial. Results that Rspo binds to both Fz and LRP6 (Nam et al., 2006), to LRP6 primarily (Wei et al., 2007), or to neither (Kazanskaya et al., 2004) have been reported. Another model suggests that Rspo is a ligand for Krm and antagonizes Dkk/Krm-mediated LRP6 internalization (Binnerts et al., 2007), but this seems unlikely given that Krm1 and Krm2 double knockout mice are viable and do not exhibit Rspo mutant phenotypes, and Rspo activates β-catenin signaling in cells lacking both Krm genes (Bell et al., 2008; Ellwanger et al., 2008). Rspo genes are often co-expressed with and depend on Wnt for expression (Kazanskaya et al., 2004), and may represent a means of positive feedback that reinforces Wnt signaling. Mutations in Norrin and Rspo genes cause distinct hereditary diseases (see below).
Wnt signaling
Wnt-off state: β-catenin phosphorylation/degradation by the Axin complex
Cytosolic β-catenin phosphorylation/degradation and its regulation by Wnt are the essence of Wnt signaling (Figure 1). The scaffolding protein Axin uses separate domains to interact with GSK3, CK1α, and β-catenin and coordinates sequential phosphorylation of β-catenin at serine 45 by CK1α and then at threonine 41, serine 37 and serine 33 by GSK3 (Kimelman and Xu, 2006). β-catenin phosphorylation at serine 33 and 37 creates a binding site for the E3 ubiquitin ligase β-Trcp, leading to β-catenin ubiquitination and degradation (Figure 4). Mutations of β-catenin at and surrounding these serine and threonine residues are frequently found in cancers, generating mutant β-catenin that escapes phosphorylation and degradation (Table 1). Axin also contains an RGS (regulator of G protein signaling) domain that interacts with APC, a large multifunctional scaffolding protein that itself binds β-catenin. These core Axin complex components (Kimelman and Xu, 2006) share a common goal of ensuring β-catenin phosphorylation and degradation. Indeed both APC and Axin are tumor suppressor genes, and APC mutations are particularly prevalent in colorectal cancer (Table 1).
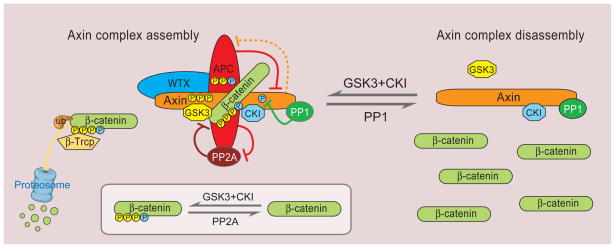
Figure 4. Regulation of Axin complex assembly for β-catenin degradation.
The core components of the Axin complex, Axin, APC, GSK3 and CK1 collectively promote β-catenin phosphorylation for degradation by β-Trcp. In addition to phosphorylating β-catenin, GSK3 (yellow) and CK1 (blue) also phosphorylate Axin and APC and enhance their binding to β-catenin and degradation complex stability, further ensuring β-catenin phosphorylation. The inset illustrates β-catenin phosphorylation (by CK1 and GSK3) and dephosphorylation (by PP2A). APC may also act to prevent PP2A dephosphorylation of β-catenin. APC paradoxically facilitates Axin degradation and possibly vise vesa (indicated by dashed line, see text). PP1 dephosphorylates Axin to antagonize CK1 phosphorylation and negatively regulates GSK3-Axin binding resulting in complex disassembly.
Table 1.
Human diseases associated with mutations of the Wnt signaling components
| Gene | Function | Human Disease | References |
|---|---|---|---|
| PORCN | + Wnt lipid modification/processing | LOF X-linked Focal dermal hypoplasia | (Grzeschik et al., 2007; Wang et al., 2007) |
| Wnt3 | + Ligands for Wnt/β-catenin signaling | LOF Tetra-amelia | (Niemann et al., 2004) |
| Wnt4 | LOF Mullerian-duct regression and viriliation | (Biason-Lauber et al., 2004) | |
| Wn5b | (?) Type II diabetes | (Kanazawa et al., 2004) | |
| Wnt7a | LOF Fuhrmann syndrome | (Woods et al., 2006) | |
| Wnt10a | LOF Odonto-onchyo-dermal hypoplasia | (Adaimy et al., 2007) | |
| Wnt10b | LOF Obesity | (Christodoulides et al., 2006) | |
| RSPO1 | + Wnt agonists | LOF XX sex reversal with palmoplantar hyperkaratosis | (Parma et al., 2006) |
| RSPO4 | LOF Autosomal recessive anonychia and hyponychia congenita | (Bergmann et al., 2006; Blaydon et al., 2006) | |
| SOST | − LRP5/6 antagonist predominantly expressed in osteocytes | LOF High bone mass, Sclerosteosis, Van Buchem disease | (Balemans et al., 2001; Balemans et al., 2002; Brunkow et al., 2001) |
| Norrin (NDP) | + Specific ligand for FZD4 and LRP5 during eye development | LOF Familial Exudative vitreoretinopathy | (Xu et al., 2004) |
| LRP5 | + Wnt co-receptors | GOF Hyperparathyroid tumors (alt. splicing) | (Bjorklund et al., 2007; Boyden et al., 2002; Gong et al., 2001; Little et al., 2002; Toomes et al., 2004) |
| GOF High bone mass | |||
| LOF Osteoporosis-pseudoglioma | |||
| LOF FEVR eye vascular defects | |||
| LRP6 | LOF Early coronary disease and osteoporosis | (Mani et al., 2007) | |
| FZD4 | + Wnt receptor | LOF Familial Exudative vitreoretinopathy | (Robitaille et al., 2002) |
| Axin1 | − Facilitates β-catenin degradation, Tumor suppressor | LOF Caudal duplication, Cancer | (Oates et al., 2006; Satoh et al., 2000) |
| Axin2 | LOF Tooth agenesis, Cancer | (Lammi et al., 2004; Liu et al., 2000) | |
| APC | − Facilitates β-catenin degradation, Tumor suppressor | LOF Familial adenomatous polyposis, Cancer | (Kinzler et al., 1991; Nishisho et al., 1991) |
| WTX | − Facilitates β-catenin degradation, Tumor suppressor | LO Wilms tumor | (Major et al., 2007; Rivera et al., 2007) |
| β-catenin(CTNNB1) | + Primary Wnt effector, Oncogene | GOF Cancer | (Korinek et al., 1997; Morin et al., 1997) |
| TCF4 (TCF7L2) | + β-catenin transcriptional partner | (?) Type II diabetes | (Florez et al., 2006; Grant et al., 2006) |
LOF: loss-of-function; GOF: gain-of-function
Several aspects of the Axin complex deserve further discussion. (i) In addition to β-catenin, GSK3 and CK1 also phosphorylate Axin and APC, leading to increased association of Axin and APC with β-catenin and thus enhanced β-catenin phosphorylation/degradation (Huang and He, 2008; Kimelman and Xu, 2006) (Figure 4). (ii) Two abundant serine/threonine phosphatases, PP1 and PP2A, both of which associate with Axin and/or APC, counteract the action of GSK3 and/or CK1 in the Axin complex. Thus PP1 dephosphorylates Axin and promotes the disassembly of the Axin complex (Luo et al., 2007), whereas PP2A dephosphorylates β-catenin (Su et al., 2008), each resulting in reduced β-catenin degradation (Figure 4). One should note that PP2A may have multiple and opposing roles in the Wnt pathway depending on the particular associated regulatory subunits and substrates (Kimelman and Xu, 2006). (iii) The assembly of the Axin complex appears to be multivalent and robust. In fly embryos that are null for Axin, expression, at physiological levels, of Axin mutants lacking either the APC-, GSK3-, or β-catenin-binding domain restores a significant degree of normal patterning, implying a quasi-functional Axin complex assembly via multivalent interactions; furthermore, some of these Axin deletion mutants can complement each other and restore fly viability, possibly via Axin dimerization or multimerization (Peterson-Nedry et al., 2008). Indeed Axin has multiple potential dimerization domains (Luo et al., 2005) and the Axin DIX domain may form multimeric polymers (Schwarz-Romond et al., 2007a). (iv) Axin concentration is exceedingly low compared to other components in Xenopus oocytes, indicating that Axin is rate limiting for the complex assembly. This feature may ensure that changes in the Axin protein level will not fluctuate the availability of GSK3 (or other components) for non-Wnt functions, thereby further insulating Wnt and other signaling events (Lee et al., 2003). It is unknown, however, whether the drastic difference between the concentration of Axin versus the other components applies universally, and whether different cells employ quantitative differences in the ratio of Axin and other components to shape their unique Wnt response kinetics (such as the speed and level of β-catenin accumulation). Indeed in Drosophila photoreceptors, APC appears to be present at minimal levels such that a 50% reduction alters the graded Wg response (Benchabane et al., 2008).
Other proteins such as WTX (Wilms tumor gene on the X chromosome) may have roles in β-catenin degradation. Loss of WTX and activating β-catenin mutations seem to have non-overlapping occurrence in Wilms tumor (a pediatric kidney cancer) (Rivera et al., 2007). WTX binds to β-catenin, Axin, APC and β-Trcp to promote β-catenin ubiquitination, although its biochemical role remains unknown (Major et al., 2007). Another Axin-binding protein Diversin can facilitate β-catenin degradation via recruiting CK1ε to phosphorylate β-catenin (Schwarz-Romond et al., 2002).
APC function and APC-Axin cross regulation
The biochemical nature of APC has been enigmatic. A recent study suggested that APC protectsβ-catenin from dephosphorylation by PP2A thereby enhancing β-catenin phosphorylation/degradation (Su et al., 2008) (Figure 4), consistent with the observation that Axin overexpression causes β-catenin degradation even in cells lacking APC function (Behrens et al., 1998). Surprisingly APC (upon phosphorylation by CK1/GSK3) and Axin bind to and compete for the same β-catenin interaction interface, leading to a proposal that APC acts as a “ratchet” to remove phosphorylated β-catenin from Axin for ubiquitination and for making Axin available for a further round of β-catenin phosphorylation (Kimelman and Xu, 2006; Xing et al., 2003). A different model was proposed based on differential β-catenin binding affinity by unphosphorylated versus phosphorylated APC (Ha et al., 2004). APC has also been shown to promote β-catenin nuclear export and to act as a chromatin-associated suppressor for β-catenin target genes, thus functioning in the nucleus (see below).
Another paradoxical observation is that APC has a positive function in physiological and ectopic Wg/Wnt signaling through the promotion of Axin degradation (Lee et al., 2003; Takacs et al., 2008) (Figure 4). One model suggests that this represents a fail-safe mechanism to buffer dramatic β-catenin fluctuations when APC levels vary (Lee et al., 2003). Thus a decrease in the APC level results in higher Axin amounts, compensating for β-catenin degradation. APC-mediated Axin degradation depends on the APC amino terminal domain that is not involved inβ-catenin degradation (Takacs et al., 2008). It is intriguing that colon cancer cells are rarely null for APC but rather retain the amino terminal half, and may have hijacked a part of this fail-safe regulation for tumorigenesis. Conversely Axin can also facilitate APC degradation upon overexpression (Choi et al., 2004), constituting perhaps the other side of the Axin-APC regulation circuit (Figure 4). Mechanisms for Axin and APC degradation, which are proteosome-dependent, have not been characterized.
Wnt-on state
Activation of Wnt receptors
Wnt signaling requires both Fz and LRP6 (or LRP5), likely through a Wnt-induced Fz-LRP6 complex (Figure 1). Wnt-induced LRP6 phosphorylation is a key event in receptor activation (Tamai et al., 2004). LRP6, LRP5 and Arrow each have five reiterated PPPSPxS motifs (P, proline; S, serine or threonine, x, a variable residue), which are essential for LRP6 function and are each transferrable to a heterologous receptor to result in constitutive β-catenin signaling (MacDonald et al., 2008; Tamai et al., 2004; Zeng et al., 2005). These dually phosphorylated PPPSPxS motifs are docking sites for the Axin complex (Davidson et al., 2005; Tamai et al., 2004; Zeng et al., 2005), thereby recruiting Axin to LRP6 upon Wnt stimulation (Mao et al., 2001) (Figure 5).
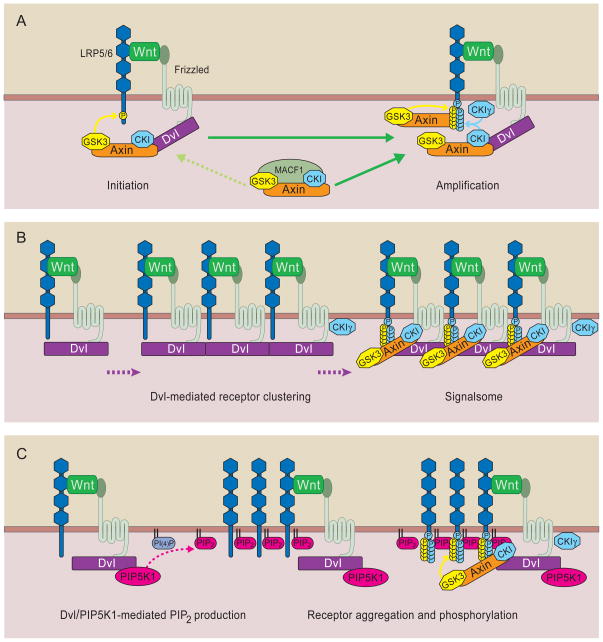
Figure 5. Models of Wnt receptor activation.
A) Initiation and Amplification. Wnt forms a complex with LRP6 and Fz-Dvl at the membrane. Dvl recruits Axin-GSK3 resulting in the phosphorylation of one or more PPPSP motifs in LRP6 (initiation). Partially phosphorylated LRP6 may be able to recruit and more efficiently bind Axin-GSK3 and promote more PPPSP phosphorylation (amplification). B) Signalsome formation via Dvl polymerization and receptor clustering. The oligiomerization property of Dvl promotes the aggregation of individual Wnt-LRP6-Fz complexes, resulting in Axin recruitment to the membrane and LRP6 phosphorylation by GSK3 and CK1. C) PI4KIIα and PIP5KI kinases, the latter of which binds directly with Dvl, promote PIP2 production and receptor clustering/phosphorylation. The configurations of receptor clustering in B and C were drawn arbitrarily. In all models, PPPSPxS motifs are sequentially phosphorylated by GSK3 and CK1, probably via CK1γ (membrane-associated) and/or CK1α and CK1ε associated with Axin and Dvl, respectively, and MACF1 may have a role in the translocation of the Axin complex to the receptors,
The kinases responsible for PPPSPxS phosphorylation have been identified unexpectedly as GSK3 and CK1 (Davidson et al., 2005; Zeng et al., 2005). Although one study argued that only CK1 phosphorylation is Wnt-induced (Davidson et al., 2005), most available data support that Wnt induces PPPSP phosphorylation (Binnerts et al., 2007; Khan et al., 2007; Pan et al., 2008b; Wei et al., 2007), which is carried out by GSK3 and primes xS phosphorylation by CK1, thereby leading to dually induced phosphorylation (Zeng et al., 2005) (Figure 5). Although potential involvement of additional kinases cannot be ruled out, experiments in GSK3α/β null cells indicate that GSK3 accounts for most, if not all, PPPSP phosphorylation (Zeng et al., 2008; Zeng et al., 2005). As in β-catenin phosphorylation, Axin-bound GSK3 appears to mediate LRP6 phosphorylation (Zeng et al., 2008). Thus PPPSPxS phosphorylation exhibits a mirror image of β-catenin phosphorylation in sequential order, in priming requirement, and importantly in functionality, but apparently by the same Axin-GSK3 complex (Huang and He, 2008) (Figure 5). This unusual mechanism, using the same kinase complex for both positive and negative regulation, is reminiscent of another morphogenetic pathway, Hedgehog signaling in Drosophila (Price, 2006), and implies a simple view that Wnt signaling regulates the two opposing activities of the Axin-GSK3 complex. One caveat is that GSK3 is genetically defined as a negative regulator of β-catenin signaling. The positive requirement of GSK3 in LRP6 activation is demonstrated when a membrane-tethered GSK3 inhibitory peptide blocks Wnt signaling (Zeng et al., 2008).
Fz function is required for Wnt-induced LRP6 phosphorylation, and forced Fz-LRP6 association is sufficient to trigger LRP6 phosphorylation (Zeng et al., 2008). Fz function is usually linked to Dsh/Dvl (Wallingford and Habas, 2005), a cytoplasmic scaffolding protein that may directly interact with Fz (Wong et al., 2003). Indeed Fz-Dvl interaction and Dvl function are critical for Wnt-induced LRP6 phosphorylation (Bilic et al., 2007; Zeng et al., 2008). As Dvl interacts with Axin (Wallingford and Habas, 2005), and is required for Axin recruitment to the plasma membrane during Wg signaling (Cliffe et al., 2003) or in Fz overexpression (Zeng et al., 2008), one model stipulates that Fz-Dvl recruitment of the Axin-GSK3 complex initiates LRP6 phosphorylation by GSK3 (Zeng et al., 2008) (Figure 5).
Several features of Wnt receptor activation deserve further discussion. (i) The observation that Axin is required for LRP6 phosphorylation, and phosphorylated LRP6 in turn recruits Axin suggests a positive feed-forward loop, potentially amplifying and ensuring the phosphorylation of all five PPPSPxS motifs (Figure 5). Indeed the phosphorylation of these motifs relies on the presence of one another, and LRP6 activity is particularly sensitive to the PPPSPxS copy number (MacDonald et al., 2008; Wolf et al., 2008). This may explain the distinct roles of Fz and LRP6/Arrow in the “initiation” (which requires both Fz and Arrow) and “amplification” (which requires Arrow only) during Wg signaling (Baig-Lewis et al., 2007) (Figure 5a). (ii) Wnt-induced clustering of Fz-LRP6 receptor has been reported that critically depend on Dvl, Axin and GSK3 for formation (see below) (Bilic et al., 2007; Schwarz-Romond et al., 2007a). Although unambiguous evidence for such aggregation under physiological conditions without overexpression remains to be shown, this “signalsome” model (Figure 5b) and the “initiation-amplification” model (Figure 5a) together provide a spatial and temporal framework for understanding Wnt receptor activation. (iii) Wnt also induces LRP6 phosphorylation by CK1γ outside the PPPSPxS motifs, in particular in a conserved S/T cluster amino-terminal to the first PPPSPxS motif (Davidson et al., 2005). This region upon phosphorylation binds to GSK3 (Piao et al., 2008), potentially accounting for observed LRP6-GSK3 interaction (Mi et al., 2006; Zeng et al., 2005). The significance of this S/T cluster to LRP6 function has not been investigated in the intact receptor, but these results imply multiple interaction interfaces among LRP6, Axin and GSK3. (iv) Wnt may also “activate” Fz, which is structurally related to G-protein coupled receptors (GPCRs). Some genetic and pharmacological evidence suggests that trimeric G proteins, specifically the Gαo and Gαq, are required downstream of Fz and probably upstream of Dvl in Wnt/β-catenin signaling (Katanaev et al., 2005; Liu et al., 2001; Liu et al., 2005). Whether G proteins are involved in Wnt/Fz/Dvl-regulated LRP6 phosphorylation is unknown.
Dvl is involved in Wnt/β-catenin and other Wnt/Fz-dependent pathways and has numerous putative binding partners (Wallingford and Habas, 2005). For example CK1ε (or CK1δ) binds to Dvl and is a potent activator of β-catenin signaling, possibly via phosphorylating Dvl, LRP6 and/or the Axin complex (Price, 2006) (Figure 5). PP2A also associates with Dvl but has a positive or negative influence on Wnt signaling depending on the associated regulatory subunit (Kimelman and Xu, 2006). In addition Dvl is subjected to proteasomal degradation via distinct ubiquitination pathways (Angers et al., 2006; Simons et al., 2005). Some of these Dvl regulation events have been suggested to switch Dvl between β-catenin and non-canonical pathways. Despite these progresses, the mechanism by which Dvl acts in Wnt/β-catenin signaling remains enigmatic. Two recent findings suggest potential new insights. (i) Polymerization/aggregation of Dvl (and Axin). Fz-Dvl and Dvl-Axin interactions are relatively weak (Schwarz-Romond et al., 2007b; Wong et al., 2003). However Dvl and Axin each harbor a homologous DIX domain that exhibit dynamic polymerization (Schwarz-Romond et al., 2007a). This unusual property is proposed to allow Dvl and Axin to form large aggregates that facilitate weak but dynamic protein interactions (Figure 5b). Indeed Wnt-induced receptor clustering requires an intact Dvl DIX domain (Bilic et al., 2007; Schwarz-Romond et al., 2007a). It is unclear whether Wnt regulates DIX-dependent polymerization, and perhaps in a related manner, Fz-Dvl or Dvl-Axin interaction. (ii) Dvl stimulation of phosphatidylinositol 4,5-bisphosphate [PtdIns (4,5)P2 or PIP2] production by sequential actions of phosphatidylinositol 4-kinase type II (PI4KIIα) and phosphatidylinositol-4-phosphate 5-kinase type I (PIP5KI) (Pan et al., 2008b). Wnt induces Dvl, via the DIX domain, to bind to and activate PIP5K, and the resulting PIP2 production is suggested to promote LRP6 clustering and phosphorylation, although the underlying mechanism remains unclear (Figure 5c). Given that PIP2 has pleiotropic functions in cells including receptor endocytosis (see below), other potential mechanisms for PIP2 in LRP6 phosphorylation remain to be explored. Nonetheless Dvl DIX polymerization and stimulation of PIP2 may act in concert to ensure LRP6 clustering/phosphorylation/activation.
Other regulatory events at or proximal to Wnt receptors
A cytoplasmic protein in vertebrates, referred to as Caprin-2, binds to LRP6 and facilitates LRP6 phosphorylation by GSK3 (Ding et al., 2008). Caprin-2 has an oligomerization domain that may enhance LRP6 aggregation, and Caprin-2 additionally may also associate with both GSK3 and Axin and promote LRP6-Axin-GSK3 complex formation (Ding et al., 2008). Besides the requirement of Dvl, recruitment of Axin to the receptor complex may involve a giant protein (600 kD), Macf1 (microtubule actin cross-linking factor 1) (Chen et al., 2006). Macf1 is a member of the spectraplakin family of proteins that link the cytoskeleton to junctional proteins. Defective gastrulation in Macf1−/− mouse embryos phenotypically resembles Lrp5/6−/− double knockout mutants. On Wnt stimulation Macf1 associates with the Axin complex (including APC) in the cytosol and with LRP6 and the Axin complex (but not APC) in the membrane fraction (Chen et al., 2006), and may shuttle Axin to LRP6 (Figure 5). This Macf1 function may be vertebrate-specific as Drosophila Macf1 (shortstop) mutants do not exhibit wg-related phenotypes.
A controversial issue concerns the involvement of receptor endocytosis in Wnt signaling. Contradicting results have been reported regarding the role of clathrin- and caveolin-dependent receptor internalization and endosomal trafficking in Wnt receptor activation and signaling (Gagliardi et al., 2008). For example, one study suggested that caveolin-mediated LRP6 endocytosis promotes Axin recruitment and is required for Wnt stabilization of β-catenin (Yamamoto et al., 2006), while another study using caveolin−/− mutant mice reached the apparently opposite conclusion (Sotgia et al., 2005). Pharmacological and molecular tools currently in use to block receptor endocytosis and trafficking have pleiotropic and sometimes non-specific effects, making interpretations of these studies complicated.
Inhibition of β-catenin phosphorylation
How receptor activation leads to inhibition of β-catenin phosphorylation remains uncertain, and available data suggest possible parallel mechanisms. In the LRP6-centric view, as constitutively activated forms of LRP6 fully activate β-catenin signaling in an apparently Fz and Dvl-independent manner (He et al., 2004), LRP6 represents the key output whereas Fz and Dvl act upstream to control LRP6 activation. On the other hand, Dsh overexpression in Drosophila or recombinant Dvl in Xenopus egg extracts can activate β-catenin signaling presumably in the absence of Arrow/LRP6 (Salic et al., 2000; Wehrli et al., 2000), and so does a GPCR-Fz chimeric protein in response to the GPCR ligand (Liu et al., 2001). These results argue that Fz/Dvl may activate β-catenin signaling independent of LRP6. The fact that nematodes have a related Wnt/β-catenin pathway (Kimble 2009) but have no LRP6 homolog may be consistent with this notion. Perhaps in Drosophila and vertebrates Wnt signaling components exist under sub-optimal levels and the two parallel branches need to operate together to counteract efficient β-catenin phosphorylation/degradation, whereas over-activation of either branch is sufficient to stabilize β-catenin.
Several biochemical mechanisms by which Wnt inhibits β-catenin phosphorylation have been suggested (Kimelman and Xu, 2006). (i) Wnt-induced and Dvl-(and Gα) -dependent Axin-GSK3 (or β-catenin) dissociation have been reported (Liu et al., 2005; Logan and Nusse, 2004). PP1 dephosphorylation of Axin also causes Axin-GSK3 dissociation (Luo et al., 2007) (Figure 4), but whether PP1 counteracts Axin complex assembly constitutively or is regulated by Wnt is unknown. (ii) Inhibition of GSK3. Phosphorylated LRP6 cytoplasmic domain or individual phospho-PPPSPxS peptides can directly inhibit GSK3 phosphorylation of β-catenin in vitro (Cselenyi et al., 2008; Piao et al., 2008; Wu et al., 2009), suggesting a potential mechanism forβ-catenin stabilization. This is consistent with the observation that upon Wnt stimulation dephosphorylated β-catenin first appears at the plasma membrane close to activated LRP6 and Axin (Hendriksen et al., 2008). (iii) Axin degradation. Overexpression of activated Wnt receptors or recombinant Dvl can lead to Axin degradation (Lee et al., 2003; Mao et al., 2001; Tolwinski et al., 2003) whereas GSK3 phosphorylation stabilizes Axin (Willert et al., 1999; Yamamoto et al., 1999). However Wnt-induced Axin degradation lags significantly behind β-catenin stabilization (Liu et al., 2005; Willert et al., 1999; Yamamoto et al., 1999), thus is unlikely to represent a primary response despite its importance. Wnt signaling elevates APC protein levels (Choi et al., 2004; Doble et al., 2007), but whether APC accumulation is responsible for Wnt-induced Axin degradation is unknown.
β-catenin nuclear function
β-catenin nuclear/cytoplasmic shuttling and retention
β-catenin stabilization results in its higher nuclear levels, but how β-catenin is shuttled to and retained in the nucleus is not well understood (Henderson and Fagotto, 2002; Stadeli et al., 2006). Earlier studies suggested that β-catenin enters the nucleus in an NLS (nuclear localization signal)- and importin-independent fashion by interacting directly with nuclear pore proteins (Henderson and Fagotto, 2002). β-catenin also exits the nucleus via export involving APC (Henderson and Fagotto, 2002), Axin (Cong and Varmus, 2004), and RanBP3 (Ran binding protein 3), which binds to β-catenin in a Ran-GTP dependent manner (Hendriksen et al., 2005). Live cell imaging suggests that while Axin and APC can enrich β-catenin in the cytoplasm and TCF and β-catenin co-activators (BCL9 and Pygopus, see below) increase nuclear β-catenin, they do not accelerate the export or import rate of β-catenin, thereby arguing for their roles in β-catenin retention rather than shuttling (Krieghoff et al., 2006). Thus β-catenin nuclear and cytoplasmic partitioning is likely the dynamic sum of both shuttling and retention between the two compartments via multiple mechanisms.
A recent study argues that Wnt-induced β-catenin stabilization is not sufficient for its nuclear accumulation, but Wnt activation of the Rac1 GTPase is required in parallel (Wu et al., 2008). Specifically Rac1, JNK2 (Jun N-terminal kinase 2) and β-catenin form a cytoplasmic complex, and JNK2 phosphorylates β-catenin (at serines 191 and 605) and promotes it nuclear translocation. Other studies have suggested a role of Rac1 and its guanine nucleotide exchange factors in β-catenin signaling, either as components of the TCF/β-catenin transcriptional complex or as an antagonistic partner of the Axin-APC complex (Esufali and Bapat, 2004; Schlessinger et al., 2009). Further studies will be required to clarify and substantiate whether/how Rac1, which participates in non-canonical Wnt signaling, is involved in the Wnt/β-catenin pathway.
TCF/LEF
The TCF/LEF family of DNA-bound transcription factors is the main partner for β-catenin in gene regulation (Arce et al., 2006; Hoppler and Kavanagh, 2007). TCF represses gene expression by interacting with the repressor Groucho (TLE1 in human), which promotes histone deacetylation and chromatin compaction; Wnt-induced β-catenin stabilization and nuclear accumulation leads TCF to complex with β-catenin, which appears to displace Groucho (Daniels and Weis, 2005) and recruits other co-activators for gene activation (Figure 1). While a single TCF gene is found in Drosophila and worm, four TCF genes, TCF1, LEF1, TCF3 and TCF4, exist in mammals. Alternative splicing and promoter usage produce a large number of TCF variants with distinct properties (Arce et al., 2006; Hoppler and Kavanagh, 2007). TCF proteins are HMG (high mobility group) DNA-binding factors, and upon binding to a DNA consensus sequence referred to as the Wnt responsive element (WRE), CCTTTGWW (W represents either T or A), they cause significant DNA bending that may alter local chromatin structure. A genome-wide analysis in colon cancer cells suggests that TCF4/β-catenin target genes are frequently “decorated” with multiple WREs, most of which are located at large distances from transcription start sites (Hatzis et al., 2008). Some TCF1 and TCF4 splicing variants harbor a second DNA-binding domain called C-clamp, which recognizes an additional GC element downstream of the typical WRE, allowing regulation of different sets of target genes (Atcha et al., 2007). These similarities and differences, combined with overlapping and unique expression patterns, underlie in part distinct and sometimes redundant functions of vertebrate/mammalian TCF genes. Note however that all major isoforms of the Drosophila TCF contain the C-clamp domain that binds to a seven base pair “Helper site” near the classic WRE, which together are required to mediate most, if not all, Wg responsive gene expression (Chang et al., 2008). Some general rules appear to describe TCF/LEF functions approximately: TCF1 and TCF4 act as both repressors and activators, LEF1 is often an activator whereas TCF3 is mostly a repressor but sometimes an activator (Arce et al., 2006; Hoppler and Kavanagh, 2007).
Three major strategies exist to regulate TCF/β-catenin transcription. (i) Alternative promoter usage in TCF-1 and LEF-1 genes produces dnTCF-1/dnLEF-1, which lack the amino-terminal β-catenin-binding domain and thus act as the endogenous dominant negative TCF/LEF (Arce et al., 2006; Hoppler and Kavanagh, 2007). Indeed the TCF-1 locus acts as an intestinal tumor suppressor primarily due to the production of dnTCF-1, which antagonizes TCF-4 in stem cell renewal. (ii) Nuclear antagonists Chibby and ICAT bind to β-catenin and disrupt β-catenin/TCF and β-catenin/co-activator interactions and promote β-catenin nuclear export (Li et al., 2008; Tago et al., 2000). Besides these devoted inhibitors, many DNA-binding transcription factors interact with β-catenin or TCF and antagonize TCF/β-catenin-dependent transcription (Supplemental Table 1). For example, KLF4 inhibition of β-catenin transcriptional activation is important for intestinal homeostasis and tumor suppression (Zhang et al., 2006). (iii) Post-translational modifications of TCF/LEF exist including phosphorylation, acetylation, sumoylation, and ubiquitination/degradation (Arce et al., 2006; Hoppler and Kavanagh, 2007). For instance, TCF-3 phosphorylation by CK1ε and LEF-1 phosphorylation by CK2 enhances their binding to β-catenin and diminishes LEF-1 binding to Groucho/TLE, whereas LEF-1 and TCF-4 phosphorylation by NLK (Nemo-like kinase) leads to less LEF/TCF/β-catenin complex binding to DNA and to LEF-1/TCF-4 degradation. LEF-1 and TCF-4 sumoylation (by the SUMO ligase PIASy) represses LEF-1 activity by targeting it to nuclear bodies but enhances TCF-4/β-catenin transcription, while CBP-mediated acetylation of TCF results in decreased TCF/β-catenin-binding in Drosophila and increased TCF nuclear retention in nematodes, both leading to transcriptional repression. These diverse modifications are often specific to individual TCF/LEF proteins, conferring differential regulation.
β-catenin associated co-activators
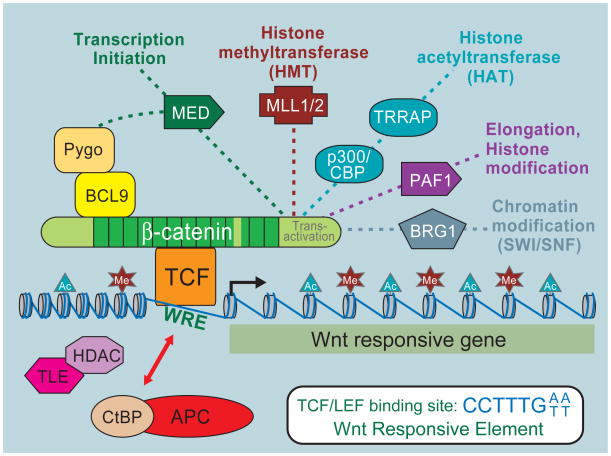
A plethora of β-catenin associated co-activators have been identified. These multi-protein complexes include BCL9 and Pygopus (Pygo), Mediator (for transcription initiation), p300/CBP and TRRAP/TIP60 histone acetyltransferases (HATs), MLL1/2 histone methyltransferases (HMTs), the SWI/SNF family of ATPases for chromatin remodeling, and the PAF1 complex for transcription elongation and histone modifications (Mosimann et al., 2009; Willert and Jones, 2006) (Figure 6). While the central Arm-repeats of β-catenin associate with TCF, and the amino-terminal Arm-repeat binds to BCL9, most of the co-activator complexes interact with the β-catenin carboxyl terminal portion (Figure 6), creating a dazzling interplay between β-catenin and the transcriptional apparatus and the chromatin. Indeed TCF/β-catenin binding to WREs leads to histone acetylation in a CBP-dependent manner over a significant genomic distance (30 kb), suggesting that local TCF/β-catenin recruitment results in widespread chromatin modifications (Parker et al., 2008). Histone H3K4 (lysine 4) trimethylation, which is indicative of active transcription, is also observed at the WRE at the c-Myc gene, a known Wnt target gene in some mammalian cells (Sierra et al., 2006), but whether this is due directly to the recruitment of HMTs by β-catenin or indirectly to reflect active promoter usage remains to be established (Parker et al., 2008). Whether various β-catenin-recruited co-activator complexes act simultaneously or in a particular sequential order remains unclear.
Figure 6. Nuclear TCF/β-catenin co-activator complexes.
Upon Wnt/β-catenin signaling activation, WRE-bound TCF/β-catenin recruits many co-activator complexes to Wnt target genes. For simplicity only a few representative complexes are illustrated. Dotted lines represent their interactions with β-catenin or between complexes. During active Wnt target gene transcription, APC promotes the exchange between β-catenin/co-activators with co-repressors CtBP, TLE and HDAC in a cyclic manner (double-headed red arrow) while TCF remains bound to the WRE. Ac and Me symbolize histone modifications, such as acetylation and methylation.
Intriguingly under a Wnt stimulated condition, while LEF1 occupies the WRE in the c-Myc gene continuously, β-catenin and its co-activators cycle on and off the WRE with a 60-minute periodicity and are replaced by Groucho/TLE1 (Sierra et al., 2006; Wang and Jones, 2006). Thus Groucho/TLE1 also acts as a repressor during Wnt responsive transcription. It is unknown how the cyclic occupancy is governed and whether this cyclic pattern between β-catenin/activators and Groucho/repressors is integral to transcription of other Wnt responsive genes, but this dynamic exchange requires APC recruitment to chromatin (Figure 6). These separate silencing (in the absence of Wnt) and repressing (during Wnt stimulation) functions of Groucho/TLE1 may apply to other repressors such as CtBP, and help to explain different modes of CtBP repressor function that have been described (Fang et al., 2006; Hamada and Bienz, 2004; Sierra et al., 2006). Curiously CtBP (Fang et al., 2006) and two related components of the SMRT/N-CoR corepressor complex, TBL1 and TBLR1 (Li and Wang, 2008), are also β-catenin co-activators for at least some Wg/Wnt responsive genes. In sum both co-activator and co-repressors appear to be active during β-catenin-mediated transcription, perhaps ensuring a dynamic chromatin environment for precise Wnt responses.
Unlike most co-activators that have general roles in transcription, BCL9 and Pygo in Drosophila are specifically required for β-catenin-dependent transcription and their biochemical functions proposed provide a glimpse of the complexity of TCF/β-catenin-coactivator interactions (Mosimann et al., 2009). (i) BCL9 and Pygo function as a “chain of activators” (Hoffmans et al., 2005). β-catenin binding to BCL9 recruits Pygo, which also interacts with Mediator (Carrera et al., 2008) (Figure 6); (ii) Pygo is constitutively nuclear and may have a role in recruiting/retaining BCL9/β-catenin in the nucleus upon Wg/Wnt signaling (Brembeck et al., 2004; Townsley et al., 2004); (iii) Pygo also co-occupies chromatin loci with and via TCF in the absence of Wg signaling (despite a lack of direct TCF-Pygo interaction), and may help capture BCL9/β-catenin for TCF at the onset of Wg signaling (de la Roche and Bienz, 2007); (iv) Pygo has a PHD (plant homology domain) that binds preferentially to dimethylated H3K4 upon interaction with BCL9 (Fiedler et al., 2008). This “histone code” recognition leads to the speculation that Pygo/BCL9 act during the transition from gene silencing to Wnt-induced transcription by participating in histone methylation changes. Alternatively Pygo/BCL9-binding to dimethylated H3K4 may provide a separate β-catenin anchor on chromatin, thereby freeing TCF for interaction with Groucho to pause/terminate transcription (Mosimann et al., 2009); (v) Pygo function is not required when Groucho activity is absent, suggesting that Pygo acts as an anti-repressor (Mieszczanek et al., 2008). Therefore either a single biochemical mechanism of Pygo underlies these diverse observations, or multiple functional properties of Pygo participate in β-catenin signaling. Perplexingly however, Pygo function is not as critical for Wnt/β-catenin signaling in mice, as Pygo1 and Pygo2 double mutants exhibit significantly milder phenotypes than expected (Schwab et al., 2007), possibly reflecting redundancy with other co-activators. Mammalian BCL9 function has also diverged compared to Drosophila, and exhibits cell type-specific and Pygo-independent roles in modulating Wnt signaling (Sustmann et al., 2008).
Nuclear functions of “cytoplasmic” Wnt signaling components
APC also acts directly on chromatin/WREs to antagonize β-catenin-mediated gene activation via promoting the exchange of co-activators with co-repressors in a stepwise and oscillating manner, as such exchange does not occur in APC mutant cancer cells (Sierra et al., 2006). How APC is recruited to chromatin is a mystery but is unlikely due to β-catenin/TCF, because APC and TCF bind to β-catenin in a mutually exclusive manner. GSK3 and β-Trcp also appear to be associated with the WRE in a cyclic fashion that synchronizes with APC but is opposite to that of β-catenin/co-activators, suggesting that they may have negative roles in TCF/β-catenin-mediated transcription (Sierra et al., 2006). Some studies have also suggested that Dvl is observed in the nucleus (Itoh et al., 2005; Torres and Nelson, 2000) and that nuclear Dvl is a component of the TCF/β-catenin complex and facilitates TCF/β-catenin interaction in conjunction with the c-Jun transcription factor (Gan et al., 2008). While provocative, some of these findings require substantiation and in particular, reconciliation with (the lack of) genetic evidence. Nonetheless the proposed nuclear functions for Dvl, APC, and perhaps GSK3 and β-Trcp in the TCF/β-catenin complex are in sync with their cytoplasmic functions in regulation of β-catenin stability (i.e., to either promote or inhibit β-catenin signaling).
β-catenin-mediated repression and other transcriptional events
Wnt signaling, via the TCF/β-catenin complex, also represses transcription. Note that this is distinct from TCF-mediated repression in the absence of β-catenin. One mechanism is competitive repression, through which TCF/β-catenin displaces or inhibits other DNA-binding transcription activators (Kahler and Westendorf, 2003; Piepenburg et al., 2000). Another mechanism is direct repression via TCF/β-catenin binding to the canonical WREs by recruiting co-repressors (Jamora et al., 2003; Theisen et al., 2007). A third mechanism is revealed by a novel TCF binding element, AGAWAW, which specifically mediates TCF/β-catenin repression in Drosophila (Blauwkamp et al., 2008). There is evidence that β-catenin is capable of recruiting co-repressors including Groucho/TLE and histone deacetylases (Olson et al., 2006), but the mechanism by which β-catenin recruits co-activators versus co-repressors is unknown. The involvement of co-factors (Theisen et al., 2007) or distinct TCF/β-catenin configurations offers potential explanations. A less understood aspect of β-catenin signaling is that many DNA-binding transcription factors, in addition to TCF/LEF, interact with β-catenin to either activate or repress transcription (Supplemental Table 1b). These β-catenin partners in principle expand significantly the gene expression programs that are regulated by Wnt/β-catenin signaling, but further substantiation of their roles in mediating Wnt signaling is required.
Wnt/β-catenin target genes and Wnt pathway self-regulation
As Wnt/β-catenin signaling regulates proliferation, fate specification and differentiation in numerous developmental stages and adult tissue homeostasis, Wnt target genes are diverse (Vlad et al., 2008) and cell- and context-specific (Logan and Nusse, 2004). An emerging feature is that Wnt signaling components including Fz, LRP6, Axin2, TCF/LEF, Naked (a Dvl antagonist), Dkk1, and Rspo, are often regulated positively or negatively by TCF/β-catenin (Chamorro et al., 2005; Kazanskaya et al., 2004; Khan et al., 2007; Logan and Nusse, 2004). Wnt induction of Axin2, Dkk1 and Naked and suppression of Fz and LRP6 constitute negative feedback loops that dampen Wnt signaling, and the suppression of Fz and LRP6 also enhances Wg/Wnt gradient formation over longer distances (Logan and Nusse, 2004). On the contrary, Wnt induction of Rspo and TCF/LEF genes constitute positive feed-forward circuits that reinforce Wnt signaling, a feature that has been exploited during colon carcinogenesis (Arce et al., 2006; Hoppler and Kavanagh, 2007). These various Wnt pathway self-regulatory loops are mostly utilized in a cell-specific manner, affording additional complexity in the control of amplitude and duration of Wnt responses. However Axin2 and Drosophila Naked genes seem to represent a few “universal” Wnt/Wg-induced target genes, respectively (Logan and Nusse, 2004).
Wnt/β-catenin signaling in diseases and potential therapeutics
Give the critical roles of Wnt/β-catenin signaling in development and homeostasis it is no surprise that mutations of the Wnt pathway components are associated with many hereditary disorders, cancer and other diseases (Table 1). These include mutations in Porcupine (PORCN), various Wnt ligands, agonists and antagonists, and have shed light on Wnt regulation of human development. For example, RSPO1 mutations result in XX sex reversal (Parma et al., 2006), a condition with similarities to patients with WNT4 mutations (Biason-Lauber et al., 2004) consistent with cooperation between Rspo and Wnt genes. Mutations in either FZ4 or LRP5 are associated with familial exudative vitreoretinopathy (FEVR) (Toomes et al., 2004), which is manifested by defective retinal vascularization that is also seen in Norrie disease carrying Norrin mutations, leading to the identification of Norrin as a ligand for Fz4 and LRP5 (Xu et al., 2004).
One fast growing field involves Wnt/β-catenin regulation of bone mass, as osteoporosis remains a global health problem. This was triggered by the discovery of LRP5 loss-of-function mutations in patients with osteoporosis pseudoglioma syndrome (OPPG), a recessive disorder characterized by low bone mass and abnormal eye vasculature (Gong et al., 2001). Conversely patients with autosomal dominant high bone mass (HBM) diseases harbor LRP5 missense (‘gain-of-function’) mutations (Boyden et al., 2002; Little et al., 2002), which are clustered in the LRP5 extracellular domain and render LRP5 resistant to binding and inhibition by the antagonist SOST (Ellies et al., 2006; Semenov and He, 2006) and DKK1 (Ai et al., 2005). Interestingly mutations in the SOST gene cause Sclerosteosis (Table 1), which resembles HBM disorders. Thus LRP5 activity correlates with bone mass likely via regulation of osteoblast (bone forming cell) proliferation, whereas SOST and DKK1, which are specifically expressed in osteocytes, negatively regulates bone mass by antagonizing LRP5. Given that reduction of sFRP1 or Dkk1 also results in increased bone mass in mice, secreted Wnt antagonists have become important drug targets for the treatment of osteoporosis (Williams and Insogna, 2008). Indeed humanized antibodies against SOST and a small molecule antagonist against sFRP1 are being tested and showed promise in increasing bone mass (Bodine et al., 2009). Antibodies against DKK1 have also been used to treat mouse models of multiple myeloma (Yaccoby et al., 2007) and osteoarthritis (Diarra et al., 2007), both of which are associated with bone tissue loss. As LRP6 also acts as a co-receptor for parathyroid hormone (PTH) (Wan et al., 2008), which is used as a drug for osteoporosis, and a new model for LRP5 function in bone mass regulation via an endocrine route (through serotonin) has recently been proposed (Yadav et al., 2008), interests in LRP5, LRP6 and their agonists and antagonists in bone homeostasis will continue to grow. Such interests likely extend to other metabolic disorders since LRP6 mutation is linked to coronary artery disease (Mani et al., 2007) (Table 1).
TCF7L2 (i.e., TCF4) is strongly implicated in type II diabetes (Grant et al., 2006), echoing the implication of WNT5B in the disease (Table 1). Although the diabetes-associated TCF7L2 gene polymorphism does not alter protein coding (as it occurs in intron 4), its association with the disease has been confirmed in numerous populations (Welters and Kulkarni, 2008). Some studies have suggested that the predisposing TCF7L2 variant causes a decrease in insulin secretion in insulin-producing pancreatic β-cells, but other pathogenic mechanisms involving additional tissues/organs or endocrine functions remain to be investigated.
Association of deregulated Wnt/β-catenin signaling with cancer has been well documented, particularly with colorectal cancer (Polakis, 2007) (Table 1). Constitutively activated β-catenin signaling, due to APC deficiency or β-catenin mutations that prevent its degradation, leads to excessive stem cell renewal/proliferation that predisposes cells to tumorigenesis. Indeed APC deletion or β-catenin activation in stem cells is essential for intestinal neoplasia (Fuchs, 2009). Blocking β-catenin signaling for cancer treatment has thus generated significant interests. Indeed the beneficial effect of non-steroidal anti-inflammatory drugs (NSAIDS) in colorectal cancer prevention and therapy has been attributed partially to the perturbation of TCF/β-catenin signaling through the ability of NSAIDS to inhibit Prostaglandin E2 production, which enhances TCF/β-catenin-dependent transcription (Castellone et al., 2005; Shao et al., 2005). Small molecules that disrupt TCF/β-catenin (Lepourcelet et al., 2004) or β-catenin/co-activator (CBP) interaction (Emami et al., 2004) and thereby block TCF/β-catenin signaling have been described. The task of disrupting TCF/β-catenin interaction specifically, however, is a difficult one since β-catenin interacts with TCF and other binding partners such as APC, Axin and E-cadherin via the same or overlapping interface (Barker and Clevers, 2006). Another potential therapeutic target is the kinase CDK8, which, as a Mediator subunit, is often amplified in and is required for β-catenin-dependent transcription and proliferation of colon cancer cells (Firestein et al., 2008; Morris et al., 2008). A new class of small molecules that inhibits β-catenin signaling has recently be identified (Chen et al., 2009), which via an unknown mechanism stabilizes the Axin protein, thereby promoting β-catenin degradation even in cancer cells that lack APC function. As discussed above, since Axin protein levels are the rate-limiting step for β-catenin degradation, manipulation of Axin stabilization represents a promising therapeutic strategy.
Many cancers that do not harbor mutations in the Wnt pathway nonetheless rely on autocrine Wnt signaling for proliferation or survival (Barker and Clevers, 2006). In fact APC mutant colon cancer cells maintain their dependence on Wnt and epigenetically silence the expression of secreted Wnt antagonists (He et al., 2005; Suzuki et al., 2004). Therefore targeting Wnt signaling upstream of TCF/β-catenin is also an important therapeutic option. Reagents against Wnt proteins such as antibodies (He et al., 2005) or a secreted Fz extracellular domain (DeAlmeida et al., 2007), which act outside the cancer cells to block Wnt-receptor interaction, show promise in certain experimental settings, as do small molecule and peptide inhibitors that antagonize Fz-Dvl interaction (Shan et al., 2005; Zhang et al., 2009). Small molecules have also been identified that inhibit Porcupine and thus prevent Wnt lipidation and secretion (Chen et al., 2009). We will likely see additional molecular and chemical agents that can interfere with different steps of Wnt/β-catenin signaling, whose complexity presents many potential therapeutic targets. The challenge will be ensuring that these agents target cancer cells without damaging normal tissue homeostasis.
Perspectives
Since the discovery of the Wnt-1 gene 27 years ago (Nusse and Varmus, 1982), Wnt/β-catenin signaling has cemented its role as a key regulatory system in biology. Studies of different animal models and human diseases have established a complex Wnt signaling network far beyond a linear pathway, with many components having multiple distinct roles and acting in different cellular compartments, and many modulators feeding into and cross-regulating within this network. The patterns of dynamic and kinetic protein phosphorylation/modification and complex assembly/disassembly are beginning to emerge. Challenges and excitement both lie ahead. (i) Novel regulators will likely continue to be identified using classical genetic, molecular, modern genomic and proteomic approaches. (ii) New analytical and imaging technologies should enable us to dissect and visualize the dynamic signaling events in vivo and to shed light on the cell biological aspects of Wnt signaling, including where, when and how signaling occurs inside the cell. (iii) Although we have obtained significant structural information on individual domains and protein interaction interfaces, atomic structures of protein complexes such as the Axin complex and ligand-receptor complexes remain daunting challenges. (iv) Additional specific small molecular inhibitors or activators with defined targets and mechanisms would provide not only leads for therapeutics but also research tools to manipulate the Wnt pathway in precise temporal and spatial manners. (v) Integration of vast amounts of information into quantitative models will allow us to predict the behavior and to study the robustness and evolvability of Wnt signaling in various biological contexts. (vi) The Wnt responsive transcriptome remains a gold mine for digging into Wnt-regulated biology. Unfolding examples include Wnt regulation of intestinal and hair follicle development/homeostasis, which has provided significant insights into stem cell biology and cancer pathogenesis (Clevers, 2006; Fuchs, 2009). As β-catenin is a co-activator for other transcription factors in addition to TCF/LEF, comparative analyses of Wnt responsive transcription programs that depend on TCF/LEF versus others will likely uncover further complexity of Wnt-regulated gene expression. (vii) β-catenin and APC are also key components in the E-cadherin cell adhesion complex and the microtubule network, but how Wnt/β-catenin signaling interacts with these cellular structures remains poorly understood. In addition, the involvement of the primary cilium, a centrosome- and microtubule-based protrusive organelle in vertebrate cells, in Wnt/β-catenin versus non-canonical Wnt signaling remains an intriguing but debated topic (Gerdes et al., 2009).
Finally the study of Wnt signaling in human diseases, and in stem cell biology and regeneration holds promises for translational medicine. In addition to cancer and osteoporosis, both of which will likely see Wnt signaling-based therapeutics moving into clinical trials or even clinics in the near future, potential links between neurological diseases (De Ferrari and Moon, 2006) and a Schizophrenia susceptibility gene product (Mao et al., 2009) to Wnt/β-catenin signaling offer new hopes for the treatment of neurological and psychiatric disorders. Manipulation of Wnt signaling for stem cell regulation also offers exciting opportunities for regenerative medicine (Clevers, 2006; Fuchs, 2009; Goessling et al., 2009; Willert et al., 2003). A better understanding of Wnt/β-catenin signaling will have broad impact on biology and medicine.
Supplementary Material
Acknowledgments
X.H acknowledges the support by the F. M. Kirby Center of Neurobiology/Children’s Hospital Boston, NIH and a scholarship from the Leukemia and Lymphoma Society. We thank Mariann Bienz, Ken M. Cadigan, Wenqing Xu and anonymous referees for comments/suggestions. We sincerely apologize to many colleagues for not being able to keep as many important primary papers in the References as we would like due to strict space constraints.
References
- Adaimy L, Chouery E, Megarbane H, Mroueh S, Delague V, Nicolas E, Belguith H, de Mazancourt P, Megarbane A. Mutation in WNT10A is associated with an autosomal recessive ectodermal dysplasia: the odonto-onycho-dermal dysplasia. American journal of human genetics. 2007;81:821–828. doi: 10.1086/520064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M, Holmen SL, Van Hul W, Williams BO, Warman ML. Reduced affinity to and inhibition by DKK1 form a common mechanism by which high bone mass-associated missense mutations in LRP5 affect canonical Wnt signaling. Molecular and cellular biology. 2005;25:4946–4955. doi: 10.1128/MCB.25.12.4946-4955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Thorpe CJ, Biechele TL, Goldenberg SJ, Zheng N, MacCoss MJ, Moon RT. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nature cell biology. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- Atcha FA, Syed A, Wu B, Hoverter NP, Yokoyama NN, Ting JH, Munguia JE, Mangalam HJ, Marsh JL, Waterman ML. A unique DNA binding domain converts T-cell factors into strong Wnt effectors. Molecular and cellular biology. 2007;27:8352–8363. doi: 10.1128/MCB.02132-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig-Lewis S, Peterson-Nedry W, Wehrli M. Wingless/Wnt signal transduction requires distinct initiation and amplification steps that both depend on Arrow/LRP. Developmental biology. 2007;306:94–111. doi: 10.1016/j.ydbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Human molecular genetics. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. Journal of medical genetics. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Developmental cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Wert SE, Mucenski ML, Scott WJ, Whitsett JA. R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development (Cambridge, England) 2008;135:1049–1058. doi: 10.1242/dev.013359. [DOI] [PubMed] [Google Scholar]
- Benchabane H, Hughes EG, Takacs CM, Baird JR, Ahmed Y. Adenomatous polyposis coli is present near the minimal level required for accurate graded responses to the Wingless morphogen. Development (Cambridge, England) 2008;135:963–971. doi: 10.1242/dev.013805. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Senderek J, Anhuf D, Thiel CT, Ekici AB, Poblete-Gutierrez P, van Steensel M, Seelow D, Nurnberg G, Schild HH, et al. Mutations in the gene encoding the Wnt-signaling component R-spondin 4 (RSPO4) cause autosomal recessive anonychia. American journal of human genetics. 2006;79:1105–1109. doi: 10.1086/509789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biason-Lauber A, Konrad D, Navratil F, Schoenle EJ. A WNT4 mutation associated with Mullerian-duct regression and virilization in a 46, XX woman. The New England journal of medicine. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, Leung JM, Liu Y, Lomas WE, Dixon M, 3rd, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14700–14705. doi: 10.1073/pnas.0702305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund P, Akerstrom G, Westin G. An LRP5 receptor with internal deletion in hyperparathyroid tumors with implications for deregulated WNT/beta-catenin signaling. PLoS medicine. 2007;4:e328. doi: 10.1371/journal.pmed.0040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwkamp TA, Chang MV, Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. Embo J. 2008;27:1436–1446. doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon DC, Ishii Y, O’Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nature genetics. 2006;38:1245–1247. doi: 10.1038/ng1883. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Stauffer B, Ponce-de-Leon H, Bhat RA, Mangine A, Seestaller-Wehr LM, Moran RA, Billiard J, Fukayama S, Komm BS, et al. A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone. 2009 doi: 10.1016/j.bone.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. Journal of cell science. 2008;121:737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. The New England journal of medicine. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9–2 in the switch between beta-catenin’s adhesive and transcriptional functions. Genes & development. 2004;18:2225–2230. doi: 10.1101/gad.317604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. American journal of human genetics. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Andersson ER, Schambony A, Esner M, Bryjova L, Biris KK, Hall AC, Kraft B, Cajanek L, Yamaguchi TP, et al. The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo. Molecular biology of the cell. 2009;20:924–936. doi: 10.1091/mbc.E08-07-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera I, Janody F, Leeds N, Duveau F, Treisman JE. Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6644–6649. doi: 10.1073/pnas.0709749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. The EMBO journal. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MV, Chang JL, Gangopadhyay A, Shearer A, Cadigan KM. Activation of wingless targets requires bipartite recognition of DNA by TCF. Curr Biol. 2008;18:1877–1881. doi: 10.1016/j.cub.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Lin CM, Lin CS, Perez-Olle R, Leung CL, Liem RK. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes & development. 2006;20:1933–1945. doi: 10.1101/gad.1411206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Hang HC, Nusse R. Lipid-independent secretion of a Drosophila Wnt protein. The Journal of biological chemistry. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Park SY, Costantini F, Jho EH, Joo CK. Adenomatous polyposis coli is down-regulated by the ubiquitin-proteasome pathway in a process facilitated by Axin. The Journal of biological chemistry. 2004;279:49188–49198. doi: 10.1074/jbc.M404655200. [DOI] [PubMed] [Google Scholar]
- Christodoulides C, Scarda A, Granzotto M, Milan G, Dalla Nora E, Keogh J, De Pergola G, Stirling H, Pannacciulli N, Sethi JK, et al. WNT10B mutations in human obesity. Diabetologia. 2006;49:678–684. doi: 10.1007/s00125-006-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–966. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development (Cambridge, England) 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2882–2887. doi: 10.1073/pnas.0307344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3’s phosphorylation of beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nature structural & molecular biology. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- de la Roche M, Bienz M. Wingless-independent association of Pygopus with dTCF target genes. Curr Biol. 2007;17:556–561. doi: 10.1016/j.cub.2007.01.063. [DOI] [PubMed] [Google Scholar]
- DeAlmeida VI, Miao L, Ernst JA, Koeppen H, Polakis P, Rubinfeld B. The soluble wnt receptor Frizzled8CRD-hFc inhibits the growth of teratocarcinomas in vivo. Cancer research. 2007;67:5371–5379. doi: 10.1158/0008-5472.CAN-07-0266. [DOI] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, et al. Dickkopf-1 is a master regulator of joint remodeling. Nature medicine. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Ding Y, Xi Y, Chen T, Wang JY, Tao DL, Wu ZL, Li YP, Li C, Zeng R, Li L. Caprin-2 enhances canonical Wnt signaling through regulating LRP5/6 phosphorylation. The Journal of cell biology. 2008;182:865–872. doi: 10.1083/jcb.200803147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Developmental cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res. 2006;21:1738–1749. doi: 10.1359/jbmr.060810. [DOI] [PubMed] [Google Scholar]
- Ellwanger K, Saito H, Clement-Lacroix P, Maltry N, Niedermeyer J, Lee WK, Baron R, Rawadi G, Westphal H, Niehrs C. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Molecular and cellular biology. 2008;28:4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esufali S, Bapat B. Cross-talk between Rac1 GTPase and dysregulated Wnt signaling pathway leads to cellular redistribution of beta-catenin and TCF/LEF-mediated transcriptional activation. Oncogene. 2004;23:8260–8271. doi: 10.1038/sj.onc.1208007. [DOI] [PubMed] [Google Scholar]
- Fang M, Li J, Blauwkamp T, Bhambhani C, Campbell N, Cadigan KM. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. The EMBO journal. 2006;25:2735–2745. doi: 10.1038/sj.emboj.7601153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M, Sanchez-Barrena MJ, Nekrasov M, Mieszczanek J, Rybin V, Muller J, Evans P, Bienz M. Decoding of methylated histone H3 tail by the Pygo-BCL9 Wnt signaling complex. Molecular cell. 2008;30:507–518. doi: 10.1016/j.molcel.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature. 2008;455:547–551. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. The New England journal of medicine. 2006;355:241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Griffith J, Maurice MM, Vincent JP. In vivo role of lipid adducts on Wingless. Journal of cell science. 2008a;121:1587–1592. doi: 10.1242/jcs.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nature cell biology. 2008b;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi M, Piddini E, Vincent JP. Endocytosis: a positive or a negative influence on Wnt signalling? Traffic (Copenhagen, Denmark) 2008;9:1–9. doi: 10.1111/j.1600-0854.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development (Cambridge, England) 2007;134:3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP, Li L. Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading to stabilization of beta-catenin-TCF interaction. J Cell Biol. 2008;180:1087–1100. doi: 10.1083/jcb.200710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nature genetics. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Grzeschik KH, Bornholdt D, Oeffner F, Konig A, del Carmen Boente M, Enders H, Fritz B, Hertl M, Grasshoff U, Hofling K, et al. Deficiency of PORCN, a regulator of Wnt signaling, is associated with focal dermal hypoplasia. Nature genetics. 2007;39:833–835. doi: 10.1038/ng2052. [DOI] [PubMed] [Google Scholar]
- Ha NC, Tonozuka T, Stamos JL, Choi HJ, Weis WI. Mechanism of phosphorylation-dependent binding of APC to beta-catenin and its role in beta-catenin degradation. Molecular cell. 2004;15:511–521. doi: 10.1016/j.molcel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Hamada F, Bienz M. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear beta-catenin from TCF. Developmental cell. 2004;7:677–685. doi: 10.1016/j.devcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Hatzis P, van der Flier LG, van Driel MA, Guryev V, Nielsen F, Denissov S, Nijman IJ, Koster J, Santo EE, Welboren W, et al. Genome-wide pattern of TCF7L2/TCF4 chromatin occupancy in colorectal cancer cells. Molecular and cellular biology. 2008;28:2732–2744. doi: 10.1128/MCB.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nature reviews. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene. 2005;24:3054–3058. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development (Cambridge, England) 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Henderson BR, Fagotto F. The ins and outs of APC and beta-catenin nuclear transport. EMBO reports. 2002;3:834–839. doi: 10.1093/embo-reports/kvf181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J, Fagotto F, van der Velde H, van Schie M, Noordermeer J, Fornerod M. RanBP3 enhances nuclear export of active (beta)-catenin independently of CRM1. The Journal of cell biology. 2005;171:785–797. doi: 10.1083/jcb.200502141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J, Jansen M, Brown CM, van der Velde H, van Ham M, Galjart N, Offerhaus GJ, Fagotto F, Fornerod M. Plasma membrane recruitment of dephosphorylated beta-catenin upon activation of the Wnt pathway. Journal of cell science. 2008;121:1793–1802. doi: 10.1242/jcs.025536. [DOI] [PubMed] [Google Scholar]
- Hoffmans R, Stadeli R, Basler K. Pygopus and legless provide essential transcriptional coactivator functions to armadillo/beta-catenin. Curr Biol. 2005;15:1207–1211. doi: 10.1016/j.cub.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Robertson SA, Zylstra CR, Williams BO. Wnt-independent activation of beta-catenin mediated by a Dkk1-Fz5 fusion protein. Biochemical and biophysical research communications. 2005;328:533–539. doi: 10.1016/j.bbrc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Kavanagh CL. Wnt signalling: variety at the core. Journal of cell science. 2007;120:385–393. doi: 10.1242/jcs.03363. [DOI] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Current opinion in cell biology. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development (Cambridge, England) 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. Journal of biology. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. The Journal of biological chemistry. 2003;278:11937–11944. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, et al. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. American journal of human genetics. 2004;75:832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Katanaev VL, Solis GP, Hausmann G, Buestorf S, Katanayeva N, Schrock Y, Stuermer CA, Basler K. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. The EMBO journal. 2008;27:509–521. doi: 10.1038/sj.emboj.7601981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Developmental cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Khan Z, Vijayakumar S, de la Torre TV, Rotolo S, Bafico A. Analysis of endogenous LRP6 function reveals a novel feedback mechanism by which Wnt negatively regulates its receptor. Molecular and cellular biology. 2007;27:7291–7301. doi: 10.1128/MCB.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hamilton SR, Hedge P, Markham A, et al. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251:1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- Komekado H, Yamamoto H, Chiba T, Kikuchi A. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. Journal of cell science. 2006;119:1453–1463. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
- Lammi L, Arte S, Somer M, Jarvinen H, Lahermo P, Thesleff I, Pirinen S, Nieminen P. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. American journal of human genetics. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS biology. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Li FQ, Mofunanya A, Harris K, Takemaru K. Chibby cooperates with 14–3-3 to regulate beta-catenin subcellular distribution and signaling activity. The Journal of cell biology. 2008;181:1141–1154. doi: 10.1083/jcb.200709091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang CY. TBL1-TBLR1 and beta-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol. 2008;10:160–169. doi: 10.1038/ncb1684. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. The Journal of biological chemistry. 2005;280:19883–19887. doi: 10.1074/jbc.M413274200. [DOI] [PubMed] [Google Scholar]
- Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development (Cambridge, England) 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. American journal of human genetics. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, Moon RT, Malbon CC. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nature genetics. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. The EMBO journal. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Zou H, Jin L, Lin S, Li Q, Ye Z, Rui H, Lin SC. Axin contains three separable domains that confer intramolecular, homodimeric, and heterodimeric interactions involved in distinct functions. The Journal of biological chemistry. 2005;280:5054–5060. doi: 10.1074/jbc.M412340200. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Yokota C, Tamai K, Zeng X, He X. Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. The Journal of biological chemistry. 2008;283:16115–16123. doi: 10.1074/jbc.M800327200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, et al. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316:1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, Flynn C, 3rd, Yuan H, Takada S, Kimelman D, Li L, et al. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Molecular cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi K, Dolan PJ, Johnson GV. The low density lipoprotein receptor-related protein 6 interacts with glycogen synthase kinase 3 and attenuates activity. The Journal of biological chemistry. 2006;281:4787–4794. doi: 10.1074/jbc.M508657200. [DOI] [PubMed] [Google Scholar]
- Mieszczanek J, de la Roche M, Bienz M. A role of Pygopus as an anti-repressor in facilitating Wnt-dependent transcription. Proceedings of the National Academy of Sciences of the United States of America; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K, Sawa H. Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends in cell biology. 2007;17:465–473. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Morrell NT, Leucht P, Zhao L, Kim JB, ten Berge D, Ponnusamy K, Carre AL, Dudek H, Zachlederova M, McElhaney M, et al. Liposomal packaging generates Wnt protein with in vivo biological activity. PLoS ONE. 2008;3:e2930. doi: 10.1371/journal.pone.0002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, Kwon EJ, Haigis KM, Naar AM, Dyson NJ. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature. 2008;455:552–556. doi: 10.1038/nature07310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nature reviews. 2009;10:276–286. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. The Journal of biological chemistry. 2006;281:13247–13257. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. American journal of human genetics. 2004;74:558–563. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Oates NA, van Vliet J, Duffy DL, Kroes HY, Martin NG, Boomsma DI, Campbell M, Coulthard MG, Whitelaw E, Chong S. Increased DNA methylation at the AXIN1 gene in a monozygotic twin from a pair discordant for a caudal duplication anomaly. American journal of human genetics. 2006;79:155–162. doi: 10.1086/505031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, et al. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Developmental cell. 2008a;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Choi SC, Wang H, Qin Y, Volpicelli-Daley L, Swan L, Lucast L, Khoo C, Zhang X, Li L, et al. Wnt3a-mediated formation of phosphatidylinositol 4,5-bisphosphate regulates LRP6 phosphorylation. Science. 2008b;321:1350–1353. doi: 10.1126/science.1160741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Parker DS, Ni YY, Chang JL, Li J, Cadigan KM. Wingless signaling induces widespread chromatin remodeling of target loci. Molecular and cellular biology. 2008;28:1815–1828. doi: 10.1128/MCB.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nature genetics. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Peterson-Nedry W, Erdeniz N, Kremer S, Yu J, Baig-Lewis S, Wehrli M. Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Developmental biology. 2008;320:226–241. doi: 10.1016/j.ydbio.2008.05.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao S, Lee SH, Kim H, Yum S, Stamos JL, Xu Y, Lee SJ, Lee J, Oh S, Han JK, et al. Direct inhibition of GSK3beta by the phosphorylated cytoplasmic domain of LRP6 in Wnt/beta-catenin signaling. PLoS ONE. 2008;3:e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenburg O, Vorbruggen G, Jackle H. Drosophila segment borders result from unilateral repression of hedgehog activity by wingless signaling. Molecular cell. 2000;6:203–209. [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Current opinion in genetics & development. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nature cell biology. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- Price MA. CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes & development. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- Rivera MN, Kim WJ, Wells J, Driscoll DR, Brannigan BW, Han M, Kim JC, Feinberg AP, Gerald WL, Vargas SO, et al. An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science. 2007;315:642–645. doi: 10.1126/science.1137509. [DOI] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nature genetics. 2002;32:326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Molecular cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nature genetics. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Satoh W, Matsuyama M, Takemura H, Aizawa S, Shimono A. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis. 2008;46:92–103. doi: 10.1002/dvg.20369. [DOI] [PubMed] [Google Scholar]
- Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes & development. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- Schwab KR, Patterson LT, Hartman HA, Song N, Lang RA, Lin X, Potter SS. Pygo1 and Pygo2 roles in Wnt signaling in mammalian kidney development. BMC biology. 2007;5:15. doi: 10.1186/1741-7007-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes & development. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nature structural & molecular biology. 2007a;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. Journal of cell science. 2007b;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. The Journal of biological chemistry. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- Semenov MV, He X. LRP5 Mutations Linked to High Bone Mass Diseases Cause Reduced LRP5 Binding and Inhibition by SOST. The Journal of biological chemistry. 2006;281:38276–38284. doi: 10.1074/jbc.M609509200. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. The Journal of biological chemistry. 2008;283:21427–21432. doi: 10.1074/jbc.M800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Shi DL, Wang J, Zheng J. Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry. 2005;44:15495–15503. doi: 10.1021/bi0512602. [DOI] [PubMed] [Google Scholar]
- Shao J, Jung C, Liu C, Sheng H. Prostaglandin E2 Stimulates the beta-catenin/T cell factor-dependent transcription in colon cancer. The Journal of biological chemistry. 2005;280:26565–26572. doi: 10.1074/jbc.M413056200. [DOI] [PubMed] [Google Scholar]
- Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes & development. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature genetics. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgia F, Williams TM, Cohen AW, Minetti C, Pestell RG, Lisanti MP. Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/beta-catenin signaling. Cell cycle (Georgetown, Tex. 2005;4:1808–1816. doi: 10.4161/cc.4.12.2198. [DOI] [PubMed] [Google Scholar]
- Stadeli R, Hoffmans R, Basler K. Transcription under the control of nuclear Arm/beta-catenin. Curr Biol. 2006;16:R378–385. doi: 10.1016/j.cub.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B. APC Is Essential for Targeting Phosphorylated beta-Catenin to the SCF(beta-TrCP) Ubiquitin Ligase. Molecular cell. 2008;32:652–661. doi: 10.1016/j.molcel.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Sustmann C, Flach H, Ebert H, Eastman Q, Grosschedl R. Cell-type-specific function of BCL9 involves a transcriptional activation domain that synergizes with beta-catenin. Molecular and cellular biology. 2008;28:3526–3537. doi: 10.1128/MCB.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nature genetics. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- Tago K, Nakamura T, Nishita M, Hyodo J, Nagai S, Murata Y, Adachi S, Ohwada S, Morishita Y, Shibuya H, et al. Inhibition of Wnt signaling by ICAT, a novel beta-catenin-interacting protein. Genes & development. 2000;14:1741–1749. [PMC free article] [PubMed] [Google Scholar]
- Tahinci E, Thorne CA, Franklin JL, Salic A, Christian KM, Lee LA, Coffey RJ, Lee E. Lrp6 is required for convergent extension during Xenopus gastrulation. Development (Cambridge, England) 2007;134:4095–4106. doi: 10.1242/dev.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs CM, Baird JR, Hughes EG, Kent SS, Benchabane H, Paik R, Ahmed Y. Dual positive and negative regulation of wingless signaling by adenomatous polyposis coli. Science. 2008;319:333–336. doi: 10.1126/science.1151232. [DOI] [PubMed] [Google Scholar]
- Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Developmental cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Molecular cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Kitagawa Y, Kadowaki T. Drosophila segment polarity gene product porcupine stimulates the posttranslational N-glycosylation of wingless in the endoplasmic reticulum. The Journal of biological chemistry. 2002;277:12816–12823. doi: 10.1074/jbc.M200187200. [DOI] [PubMed] [Google Scholar]
- Theisen H, Syed A, Nguyen BT, Lukacsovich T, Purcell J, Srivastava GP, Iron D, Gaudenz K, Nie Q, Wan FY, et al. Wingless directly represses DPP morphogen expression via an armadillo/TCF/Brinker complex. PLoS ONE. 2007;2:e142. doi: 10.1371/journal.pone.0000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Developmental cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, Craig JE, Jiang L, Yang Z, Trembath R, et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. American journal of human genetics. 2004;74:721–730. doi: 10.1086/383202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Nelson WJ. Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. The Journal of cell biology. 2000;149:1433–1442. doi: 10.1083/jcb.149.7.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nature cell biology. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Science signaling. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- Vlad A, Rohrs S, Klein-Hitpass L, Muller O. The first five years of the Wnt targetome. Cellular signalling. 2008;20:795–802. doi: 10.1016/j.cellsig.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development (Cambridge, England) 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes & development. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zhang Y, Li X, Chen L, Wang H, Wu J, Zheng J, Wu D. Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. The Journal of biological chemistry. 2008;283:23371–23375. doi: 10.1074/jbc.M802376200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Jones KA. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol. 2006;16:2239–2244. doi: 10.1016/j.cub.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Wang X, Reid Sutton V, Omar Peraza-Llanes J, Yu Z, Rosetta R, Kou YC, Eble TN, Patel A, Thaller C, Fang P, et al. Mutations in X-linked PORCN, a putative regulator of Wnt signaling, cause focal dermal hypoplasia. Nature genetics. 2007;39:836–838. doi: 10.1038/ng2057. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development (Cambridge, England) 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. The Journal of biological chemistry. 2007;282:15903–15911. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Welters HJ, Kulkarni RN. Wnt signaling: relevance to beta-cell biology and diabetes. Trends in endocrinology and metabolism: TEM. 2008;19:349–355. doi: 10.1016/j.tem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes & development. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes & development. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. Where Wnts Went: The Exploding Field of Lrp5 and Lrp6 Signaling in Bone. J Bone Miner Res. 2008 doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Palmby TR, Gavard J, Williams BO, Gutkind JS. Multiple PPPS/TP motifs act in a combinatorial fashion to transduce Wnt signaling through LRP6. FEBS Lett. 2008;582:255–261. doi: 10.1016/j.febslet.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Molecular cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods CG, Stricker S, Seemann P, Stern R, Cox J, Sherridan E, Roberts E, Springell K, Scott S, Karbani G, et al. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. American journal of human genetics. 2006;79:402–408. doi: 10.1086/506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Huang H, Garcia Abreu J, He X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE. 2009;4:e4926. doi: 10.1371/journal.pone.0004926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F. Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell. 2008;133:340–353. doi: 10.1016/j.cell.2008.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Clements WK, Kimelman D, Xu W. Crystal structure of a beta-catenin/axin complex suggests a mechanism for the beta-catenin destruction complex. Genes & development. 2003;17:2753–2764. doi: 10.1101/gad.1142603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD., Jr Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell. 2005;120:223–235. doi: 10.1016/j.cell.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. The Journal of biological chemistry. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Developmental cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Developmental cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development (Cambridge, England) 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. The Journal of biological chemistry. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, Yang VW, He X, Evers BM, et al. Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression. Molecular and cellular biology. 2006;26:2055–2064. doi: 10.1128/MCB.26.6.2055-2064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Appleton BA, Wiesmann C, Lau T, Costa M, Hannoush RN, Sidhu SS. Inhibition of Wnt signaling by Dishevelled PDZ peptides. Nature chemical biology. 2009;5:217–219. doi: 10.1038/nchembio.152. [DOI] [PubMed] [Google Scholar]
- Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.