Abstract
In this report, we show that affinity purified human anti-Delta Opioid Receptor (DOR) autoantibodies from IVIG are specific to DOR and possess agonistic properties displayed by their ability to dramatically decrease forskolin stimulated cAMP accumulation. Anti-DOR autoantibody also caused phosphorylation of the opioid receptor. Anti-DOR autoantibody treatment showed a significant reduction in CXCR4 gene expression as well as surface protein expression. In contrast, anti-DOR autoantibody treatment significantly upregulated CCR5 gene and protein expression. The presence of anti-DOR autoantibodies in IVIG and their potent immunomodulatory activity is further evidence to support the crosstalk between the neuroendocrine and immune system.
Keywords: Opioid receptors, autoantibodies, immunomodulation
1. Introduction
Endogenous opioid peptides and their receptors are involved in critical physiological functions such as analgesia, regulation of the autonomic nervous system and neuroendocrine activities, respiration and gastrointestinal motility (Mellon and Bayer, 1998, Tegeder and Geisslinger, 2004). The endogenous biological activities of the opioid neuropeptides endorphins, enkephalins and dynorphin is mediated by three classes of opioid receptors named μ MOR, δ DOR and κ KOR opioid receptors that belong to the seven transmembrane G-protein coupled receptor (GPCR) superfamily (McCarthy et al., 2001, Sharp et al., 1998c, Williams et al., 2001). In addition to their role in the modulation of the pain networks, opioids also influence certain parameters of both the innate and adaptive immune system (House et al., 1996, Roy et al., 2006, Vallejo et al., 2004). Apart from cells of the central and peripheral nervous system, immune cells, particularly under stressful conditions, produce endogenous opioids such as enkaphalins locally at the site of inflammation (Bidlack, 2000, Mellon and Bayer, 1998, Shahabi et al., 2003). The opioids elicit analgesia by acting on the peripheral sensory nerve terminals, and also exert a range of immunomodulatory effects on T cell responses (Shahabi et al., 2003, Shahabi et al., 2006). Numerous studies have demonstrated the presence of opioid receptors on peripheral blood lymphocytes (Shahabi et al., 2000, Sharp et al., 2001, Sharp et al., 1998b, Shen et al., 2005). The distribution of the opioid receptors on various types of cells such as monocytes, macrophages and lymphocytes differ, and their expression can be modulated by factors such as the cytokine microenvironment, and the stage and state of cell differentiation and activation (Peterson et al., 1998, Sharp et al., 1998c, Shen et al., 2005). Various groups have shown that activation of the T cell through the TCR significantly upregulates both the percentage of T cells that express DOR as well as the number of DORs expressed by each T cell (Miller, 1996, Nguyen and Miller, 2002, Shahabi et al., 2000). DOR agonists exert a range of immunomodulatory effects on T cell responses that include, but are not limited to, T cell proliferation, cytokine production, chemotaxis, thymic T cell selection, opioid mediated modulation of the release of chemokines and expression and/or functionality of chemokine receptors on leukocytes (Benard et al., 2008, Happel et al., 2008, Jaume et al., 2007, Hebert, 2008, Finley et al., 2008b, House et al., 1996).
Apart from the expression of the opioid receptors on immune cells, another piece of evidence that supports the bidirectional network of communication between the opiate and immune system is the discovery that IgG autoantibodies directed against the human MOR are commonly present in the serum of healthy individuals (Mace et al., 1999a, Mace et al., 2002). These autoantibodies display an agonistic activity, demonstrated by the inhibition of forskolin stimulated cAMP accumulation. The autoantibodies bind to the first and third extracellular loops known to be involved in ligand binding and activation of the GPCR (Mace et al., 1999b, Mace et al., 2002, Dietrich et al., 1998, Mace et al., 2001). The earlier dogma of horror autotoxicus, according to which all autoantibodies were considered to contribute to autoimmune disease, has been replaced by the knowledge that the presence of a low level of circulating serum autoantibodies is, in fact, a hallmark of a healthy immune system (Quintana and Cohen, 2004, Shoenfeld and Toubi, 2005). While the exact function of this subset of antibodies remains to be clearly elucidated, it is believed that autoantibodies contribute to the maintenance of immune homeostasis.
In this paper, we used the peptide approach to affinity purify anti-DOR loop 1 and anti-DOR loop 3 antibodies from intravenous immunoglobulin (IVIG), a therapeutic blood product that contains purified IgG isolated from the plasma of thousands of healthy donors. Affinity purified anti-DOR autoantibodies specifically bind to DOR and activate the downstream signaling pathway. More interestingly, the anti-DOR autoantibodies possess diverse immunomodulatory or regulatory functions as seen by the modulation of chemokine receptor expression on the surface of immune cells. We also confirm the presence of autoantibodies against MOR, and demonstrate the presence of antibodies to DOR external loops 1 and 3 in healthy individuals, systemic lupus erythematosus (SLE) and HIV patient sera.
2. Materials and Methods
2.1. ELISA
SLE patient serum and healthy individual serum samples were provided by Dr. Miranda Adelman, Arizona Arthritis Center, University of Arizona. HIV patient serum was provided by Dr. Nafees Ahmad, Department of Immunobiology, University of Arizona. Peripheral blood samples were obtained from volunteers in accordance to protocols approved by the Internal Review Board (IRB) at the University of Arizona. We tested the serum of 4 healthy individuals, 7 SLE patients and 13 HIV patients. ELISA plates were coated with peptides corresponding to DOR loop1, DOR loop3 (10 μg/ml), MOR loop1 and MOR loop 3 (5 μg/ml) in carbonate buffer, overnight at 37°C. Sequences; DOR Loop 1- AKYLMETWPFGELLCK, DOR Loop 3- WTLVDIDRRDPLVVAA, MOR Loop 1-VNYLMGTWPFGTILCK, MOR Loop3-IKALVTIPETTFQTVS. Peptides were obtained from Chemicon, USA. Serum was added in 2-fold dilution. Following primary incubation, plates were washed and then treated with HRP conjugated rabbit anti-human IgG (DAKO). Following the final washing step, plates were developed with TMB ELISA substrate (Pierce) and the reaction stopped after 5 min with the addition of 1N HCl. Absorbance was read at 450 nm.
2.2. Purification of anti-DOR loop 1 and 3 autoantibodies
A sepharose affinity column (NHS-activated Sepharose™ 4 Fast Flow, Amersham Biosciences, Uppsala, Sweden) coupled with peptides corresponding to the amino acid sequences of extracellular loops 1 and 3 of the human delta opioid receptor was made. IgG obtained from normal donors was used as the source for human IgG (Sandoglobulin™, Sandoz Pharmaceuticals Corporation). The lyophilized IgG was reconstituted with PBS and run over the column. Following this, the column was washed with 10 volumes of pre-elution buffer (0.5X TBS) to remove unbound antibody. The anti-DOR antibody was eluted with 100 mM glycine (pH 2.7) and eluted fractions were neutralized with 2M Tris. Several rounds of purification were performed and eluted fractions pooled. The antibodies were dialyzed against PBS overnight at 4°C and further run through a desalting column (D-Salt Polyacrylamide Desalting columns, Thermo Fisher Scientific, Rockford, IL, USA).
2.3. Cell lines
The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: 174×CEM from Dr. Peter Cresswell. CEM×174 is a CD4+ cell line that is a fusion product of human B cell line 721.174 and human T cell line CEM (Salter et al., 1985). The hybrid cell line is positive for all three subtypes of opioid receptors and expresses chemokine receptors CXCR4 and CCR5 (Miyagi et al., 2000, Suzuki et al., 2002). The cell line was maintained in Iscove’s Modified Dulbecco’s Medium containing 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin.
The CHO cell line stably transfected with human DOR (CHO DOR cells) was a gift from Dr. Henry Yamamura (University of Arizona). The cell line was maintained in Ham’s F-12 media supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 500 μg/ml G418.
2.4. Binding assays
2.4.1 Flow cytometry
CEM×174 cells (1×106) were labeled with affinity purified anti-DOR loop 1 and 3 antibodies at 4°C for 45 min. The cells were then washed (1% BSA, 0.1% sodium azide in PBS) and a secondary antibody, goat anti-human IgG-PE (Southern Biotech, USA) was added to the cells for one hour at 4°C. The cells were washed and then fixed using 4% paraformaldehyde (Cytofix, BD Biosciences). Secondary antibody alone labeled cells were used a control. The cells were analyzed in a LSR II cytometer (BD Biosciences).
2.4.2 Western Blot
CHO cells and CHO DOR cells were lysed using lysis buffer (300 mM NaCl, 50 mM Tris, 0.5% Triton-X-100, pH 7.4) containing 1% protease inhibitor cocktail and 0.1% phosphatase inhibitor cocktails I and II (Sigma). Cell lysate was boiled for 5 min with reducing sample buffer. Approximately 20 μg of whole protein per well (as determined by BCA assay) was run on a 10% denaturing SDS-PAGE gel. The proteins were transferred to a PVDF membrane (Millipore). The membrane was incubated with affinity purified primary anti-DOR antibody (1μg/ml) or unpurified IVIG (1μg/ml) for one hour and then incubated with the secondary antibody, HRP conjugated rabbit anti-human IgG (1 in 4000 dilution, Pierce). Beta-actin (1in 1000 dilution, Cell Signaling Technology®) and a HRP conjugated goat anti-rabbit secondary antibody (1:2500, Pierce) was used as a loading control. The SuperSignal West Dura chemiluminescent kit (Pierce) was used to detect reactive bands.
2.5. Inhibition of forskolin stimulated cAMP accumulation
DOR transfected CHO cells were plated at 1×105 in a 24 well plate in Hams F-12 media containing 10%FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 500 μg/ml G418. The supernatant was removed 24 hours later, and serum-free media was added 6 hours prior to assay. Cells were incubated for 20 min with 100 mM of phosphodiesterase inhibitor (3-isobutyl-1-methylxanthine, Sigma). In the last 10 min of the assay, 20 μM forskolin (Sigma) was added to indicated wells, in the presence or absence of 10 μM [D-Pen2, D-Pen5] enkephalin (DPDPE, Sigma) or 50 μg of anti-DOR autoantibody. The supernatant was removed, cells lysed and cAMP (pmol.min−1.mg−1 of protein) measured using the CatchPoint cAMP Assay kit (Molecular Devices) as per manufacturer’s instructions.
2.6. Desensitization assay
CEM×174 (1×106) cells were treated with 10 μM DPDPE, or 50μg affinity purified anti-DOR Ab, or left untreated for 10 and 30 minutes at 37°C in serum free media. After treatment cells were lysed, protein concentration determined and approximately 10μg of total protein was resolved by 10% denaturing SDS PAGE gels. The protein was transferred to PVDF membranes. Phosphorylation of serine 363 of the δ-opioid receptor was measured by Western blots using a rabbit primary antibody specific for phospho-S363 of the δ-opioid receptor (1:1000, Cell Signaling Technology®) and a HRP conjugated goat anti-rabbit secondary antibody (1:1000, Pierce). Bands were detected using SuperSignal West Dura chemiluminescent kit (Pierce).
2.7. Real-time PCR
CEM×174 cells (1× 106) were incubated for 24 hours with either 10 μM DPDPE, 100 μg anti-DOR Ab, or left untreated. Total RNA was extracted using TRIzol (Invitrogen) from the CEM×174 cells. cDNA synthesis was performed using qScript™ cDNA synthesis kit (Quanta Biosciences) as per manufacturer’s instructions. Primers specific for human CCR5, CXCR4, Tata binding protein were purchased from Applied Biosystems. Real-time PCR was performed using PerfeCTa QPCR SuperMix (Quanta Biosciences) according to the manufacturer’s instructions. A Bio-Rad iCycler was used for performing the real-time PCR.
2.8. PBMC isolation
Human PBMCs were isolated from whole blood obtained from two healthy donors. Whole blood was diluted 1 in 2 with PBS and then layered on Histopaque (Sigma –Aldrich) and centrifuged at 400 × g for 30 minutes at 25°C. The buffy coat was carefully isolated and washed 3X in 4 volumes of PBS (10% FBS) by centrifuging at 400 × g for 15 minutes. Cells were counted and resuspended at 105 per ml of IMDM, 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin.
2.9. Flow cytometry analysis
CEM×174 cells and human PBMCs were treated with 10μM DPDPE, 100 μg anti-DOR antibody or left untreated. Forty-eight hours after treatment, cells were analyzed by flow cytometry for surface expression of chemokine receptors using anti-CXCR4-PECy7, anti-CCR7-PECy7 (eBiosciences) and anti-CCR5-FITC (R&D Systems). Cells were gated on the lymphocyte population using forward and side scatter. Paired t test of the mean fluorescence intensities was used to determine p values.
3. Results
3.1. Affinity purification of anti-DOR autoantibodies
Following reports of the presence of anti-MOR antibodies in the serum of healthy individuals (Mace et al., 1999b, Mace et al., 1999a, Mace et al., 2002), we focused upon autoantibody activity to DOR, a receptor shown to be expressed on peripheral blood cells, including T and B lymphocytes (Miller, 1996, Nguyen and Miller, 2002, Sharp, 2004, Sharp et al., 2001, Shen et al., 2005). Various groups have shown that the first and the third extracellular loops of the opioid receptors are critical for ligand binding specificity and functional activation of the receptor (Dietrich et al., 1998, Fadhil et al., 2004, Li et al., 1996, Mace et al., 1999b, Varga et al., 1996, Varga et al., 2004). Therefore, to characterize autoantibody activity to DOR, we prepared a sepharose affinity column coupled with peptides corresponding to the first and third extracellular loops of DOR. We ran IVIG over the affinity column and bound antibodies were eluted, dialyzed and desalted before use.
3.2. Affinity purified anti-DOR autoantibodies show specificity in binding to DOR
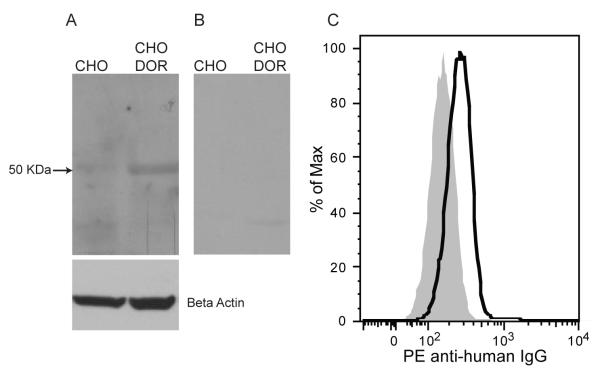
We used three cell lines to characterize the binding specificity and signaling pathway activated by the affinity purified anti-DOR antibody, CHO cell line, DOR transfected CHO (DOR CHO, expressing only DOR) cell line and the human lymphocyte cell line CEM×174 (expressing all three subtypes of opioid receptors). DOR CHO and CEM×174 cell lines have previously been shown to express opioid receptors (Miyagi et al., 2000, Navratilova et al., 2005, Suzuki et al., 2002). In western blots, a band corresponding to ~50kDa was detected in the DOR CHO cell lysate using the affinity purified anti-DOR antibodies, whereas no band was detected in the untransfected CHO cell line (Fig. 1A). We detected no bands when the lysates were incubated with unpurified IVIG at the same concentration of the affinity purified anti-DOR antibodies, confirming the specificity of binding of the affinity purified anti-DOR antibodies (Fig 1B). Using flow cytometry, we also showed that the affinity purified anti-DOR Ab binds to CEM×174 cells (Fig. 1C).
FIGURE 1.
Binding of affinity purified anti-DOR antibodies to DOR. A, B, Western blot. Lanes 1 and 2 correspond to protein from the cell lysate of CHO and CHO DOR respectively. Affinity purified anti-DOR Abs were used as primary antibody followed by a HRP conjugated rabbit anti-human IgG secondary antibody. The ~50kDa band corresponds to DOR monomer (A). We confirmed that the 50kDa band corresponded to DOR using a specific anti-DOR Ab (Abcam), results not shown. Unpurified IVIG was used as primary antibody, followed by a HRP conjugated rabbit anti-human IgG secondary antibody. No bands were detected (B). Beta-actin was used as loading control. C, Flow cytometry analysis of DOR antibody binding to CEM×174 cell line. CEM×174 cells (1×106) were incubated with primary anti-DOR autoantibody followed by secondary PE labeled goat anti-human IgG. Solid grey histogram indicates background binding of the secondary antibody.
3.3. Anti-DOR autoantibodies function as DOR agonists
Ligands that bind specifically to the opioid receptor and initiate signaling are referred to as agonists, whereas antagonists are ligands that bind to the cognate receptors with great affinity but do not elicit biological activity, effectively dampening an agonist mediated response. Upon agonist binding to the opioid receptor, the receptor associated Gαi proteins inhibit adenylyl cyclase activity resulting in a decrease of cellular cAMP (Hebert, 2008, Mace et al., 1999a, Sharp, 2006, Sharp et al., 1998b). In order to characterize the agonistic properties of the anti-DOR autoantibodies, we tested for the ability of the antibody to inhibit forskolin stimulated cAMP accumulation in DOR transfected CHO cells, expressing only DOR. Basal cAMP levels in untreated cells were used as a reference point. As shown in Fig. 2, anti-DOR autoantibodies dramatically reduced forskolin stimulated cAMP accumulation. In the absence of forskolin treatment, DPDPE alone reduced cAMP levels to 13.7±8.9% (p<0.05), whereas anti-DOR autoantibody treatment reduced cAMP levels to 31.5±23.3% (p=0.15). Forskolin treatment alone stimulated cAMP levels to 354.5±4.9% compared to untreated cells. Anti-DOR autoantibody addition to forskolin stimulated cells reduced cAMP levels to 114±4.24% (p<0.05) where as the addition of DPDPE reduced cAMP levels to 100% (p<0.01). These results show that anti-DOR autoantibodies are specific, potent agonists, displaying activity comparable to DPDPE, a DOR specific synthetic agonist.
FIGURE 2.
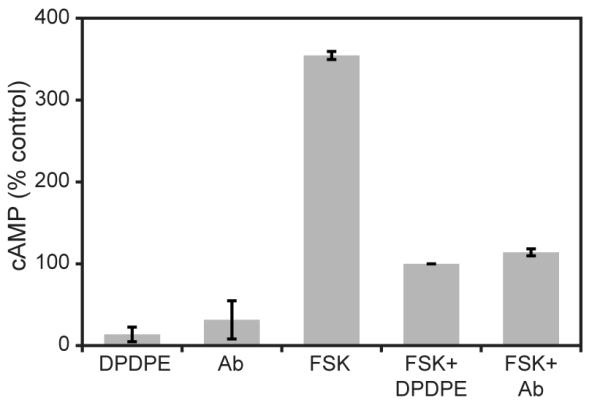
Inhibition of forskolin stimulated cAMP accumulation by anti-DOR autoantibodies. CHO DOR cells were pretreated with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine for 20 min and 20 μM forskolin was added in the last 10 min, with or without 10 μM DPDPE or 50 μg anti-DOR autoantibody. The reaction was carried out at 37°C and stopped 10 min after the addition of forskolin. Protein concentrations per well were determined using the BCA assay, and cAMP measured as described in the Materials and Methods section. Results are expressed as a percentage of the untreated control and represent mean ±SD of at least four independent experiments. In the absence of forskolin treatment, DPDPE alone reduced cAMP levels to 13.7±8.9% (p<0.05), whereas anti-DOR autoantibody treatment reduced cAMP levels to 31.5±23.3% (p=0.15). Forskolin alone stimulated intracellular cAMP levels to 354.5±4.9% compared to untreated cells. The addition of anti-DOR autoantibody to stimulated cells reduced cAMP levels to 114±4.24% (p<0.05) while DPDPE reduced the levels to 100% (p<0.01). Statistical significance was calculated using paired t-test.
There are various levels of regulation controlling the agonist stimulated GPCR response. One of the most tightly controlled events is the extremely rapid process of desensitization that occurs following the activation of the receptor inactivation signaling pathway. Desensitization usually involves the phosphorylation of a Serine/Threonine residue in an intracellular loop, or at the carboxy-terminal of the receptor. This process may be followed by the recruitment of arrestins and sequestration and internalization of the receptor into clathrin coated vesicles. The serine residue at position 363 is one of the primary phosporylation sites involved in agonist mediated desensitization of DOR (Navratilova et al., 2005, Varga et al., 2004, Varga et al., 2002, Steele et al., 2002). As shown in Fig. 3, the affinity purified anti-DOR binds specifically to DOR and induces phosphorylation of the Ser 363 residue in a manner comparable to DPDPE, further demonstrating the agonistic activity of the anti-DOR autoantibodies.
FIGURE 3.
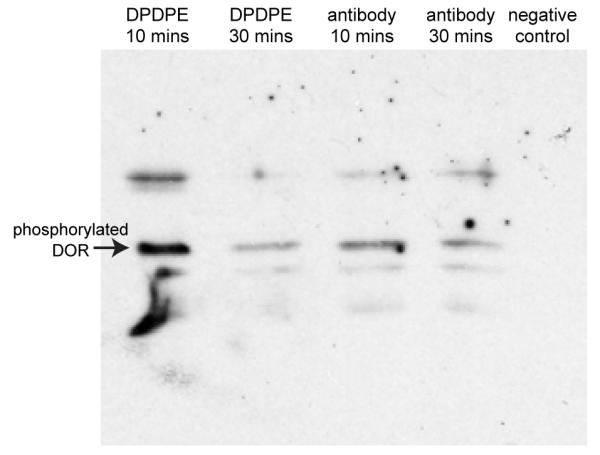
Anti-DOR autoantibodies induce phosphorylation of DOR at Ser 363. CEM×174 cells were incubated with 10 μM DPDPE or 100μg anti-DOR Ab for 10 And 30 min in serum-free media. Cells were lysed and 10 μg of total protein was run on 10% denaturing SDS-PAGE gels and transferred to a PVDF membrane. Western blot analysis was performed using an anti-phospho Ser 363 DOR antibody. Anti-DOR autoantibodies cause significant phosphorylation of the receptor at 10 min and 30 min. DPDPE causes phosphorylation of DOR as early as 10 min that decreases significantly by 30 min.
The above results demonstrate the feasibility of using peptides corresponding to extracellular loops 1 and 3 to isolate DOR specific antibodies that function as agonists.
3.4. Immunomodulation by anti-DOR autoantibodies
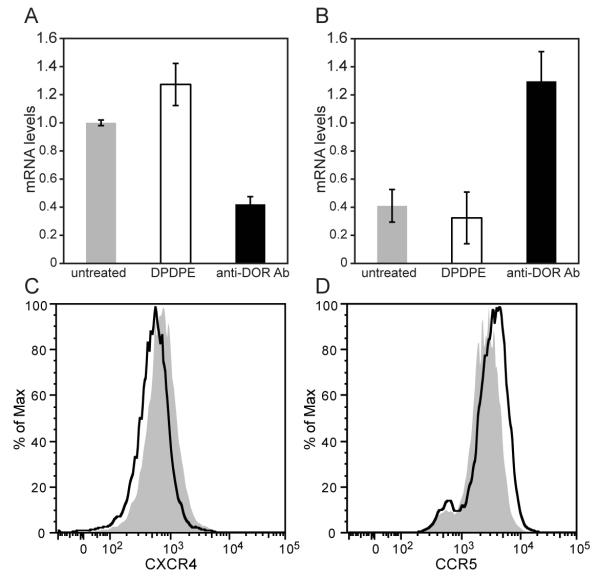
Opioid receptors and chemokine receptors are both members of the seven transmembrane GPCR superfamily, and there are a number of reports that detail the influence of opioids on chemokine receptor expression (Finley et al., 2008b, Hebert, 2008, Hereld and Jin, 2008, Pello et al., 2008, Finley et al., 2008a). The opioid mediated modulation of chemokine receptors CCR5 and CXCR4 has interested researchers because of their critical role in promoting HIV infection and progression of disease (Chen et al., 2004, Miyagi et al., 2000, Sharp et al., 1998a, Steele et al., 2003, Szabo et al., 2003, Vallejo et al., 2004, Finley et al., 2008a). A substantial percentage of HIV-1 infected individuals include intravenous drug abuses that abuse opioids (Vallejo et al., 2004). For example, MOR is known to heterodimerize with CCR5, and the binding of a ligand to its cognate receptor results in heterologous desensitization of the other receptor (Chen et al., 2004, Suzuki et al., 2002, Szabo et al., 2003). DOR is known to heterodimerize with the chemokine receptor CXCR4, however, the addition of the opioid ligand has been shown not to desensitize the chemokine receptor (Chen et al., 2004, Rogers et al., 2000). Interestingly, the addition of ligands to both of the receptors not only stabilizes the heterodimer but also causes them to become silent complexes that do not signal (Steele et al., 2002, Finley et al., 2008a, Grimm et al., 1998). The opioid receptor heterodimerization with chemokine receptors causes subtle changes in their expression that varies according to the different heterodimer formations. Using human PBMCs, it has been shown that DAMGO, a MOR specific agonist can increase the expression of chemokine receptors CCR5 and CXCR4 at the protein and mRNA level (Happel et al., 2008, Miyagi et al., 2000). Based on these reports that detailed the association of chemokine receptors CCR5 and CXCR4 with the opioid receptors, we decided to investigate whether the autoantibodies to the opioid receptor could modulate certain parameters of the immune system in a way that the exogenous, synthetic ligands do. In particular, to investigate the immunomodulatory properties of the anti-DOR autoantibodies, we looked at the ability of the autoantibodies to influence CXCR4 and CCR5 chemokine receptor expression, we incubated CEM×174 cells, or human PBMCs, with 10μM DPDPE or 100 μg affinity purified anti-DOR autoantibody. Real-time PCR of the CEM×174 cell line after 24 hours shows that anti-DOR autoantibody treatment decreased CXCR4 gene expression by approximately two-fold compared to the untreated controls (Fig. 4A, 0.42±0.05 vs. 1±0.02, p<0.05). In contrast, anti-DOR autoantibody treatment had a converse effect on CCR5 gene expression. While the synthetic DOR agonist DPDPE had no effect on CCR5 gene expression, the anti-DOR antibody treatment produced a three-fold increase in CCR5 gene expression (Fig. 4B, 3.24±0.53 vs. 1.025±0.29, p< 0.01). We confirmed that the anti-opioid receptor antibody treatment also modulated protein expression by analyzing CXCR4 and CCR5 surface protein expression at 48 hours post treatment using flow cytometry analysis. DPDPE did not cause any changes in surface receptor expression (data not shown). Consistent with results of the real-time PCR assay, anti-DOR autoantibody treatment caused a decrease in CXCR4 expression (Fig. 4C) and an increase in CCR5 expression (Fig. 4D).
FIGURE 4.
Immunomodulation of chemokine receptor expression by anti-DOR autoantibodies. A, B, Real-time PCR analysis. CEM×174 cells (1×106) were incubated for 24 hours with 10 μM DPDPE or 100 μg anti-DOR autoantibodies. Total RNA was extracted from the cells and real-time PCR was performed using human specific primers for CXCR4, CCR5 and TATA box binding protein. Values were normalized against TATA box binding protein. There was a significant reduction in CXCR4 gene expression by anti-DOR antibody treated cells as compared to untreated cells (A, p<0.05). In contrast, anti-DOR antibody treatment significantly upregulated CCR5 expression (B, p<0.01). Paired t-test was performed to determine p values. Data is representative of three independent experiments. C, D, Flow cytometry analysis. CEM×174 cells were plated at 1×105 cells/well. The cells were treated with 10 μM DPDPE or 100 μg anti-DOR autoantibody. After 48 hours, cells were analyzed by flow cytometry for surface expression of chemokine receptors. Treated and untreated cells are shown in black and grey histograms, respectively. Treatment with anti-DOR Ab reduced surface CXCR4 expression (C), while increasing CCR5 expression (D). There was no change in the expression of chemokine receptors after DPDPE treatment (data not shown).
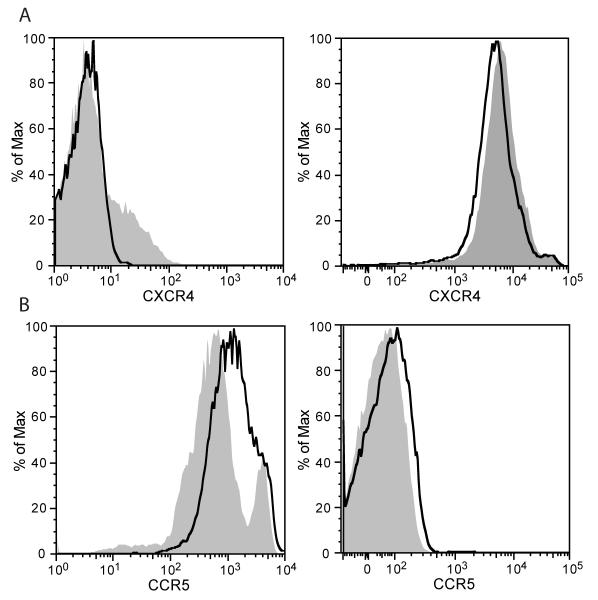
The same experiment was performed using PBMCs isolated from healthy donors and the surface expression of three chemokine receptors (CXCR4, CCR5 and CCR7) was analyzed 48 hours after treatment. The PBMCs treated with anti-DOR autoantibody showed a significant decrease in CXCR4 expression as compared to untreated cells (Fig. 5A, p<0.05). We could detect no difference in CXCR4 expression between the DPDPE treated and untreated PBMCs (data not shown). On the other hand, there was a significant increase in the expression of CCR5 in PBMCs treated with anti-DOR autoantibody as compared to untreated cells (Fig. 5B, p<0.05). Again, we saw no difference in expression of CCR5 between the DPDPE treated and untreated PBMCs (data not shown). In order to investigate the specificity of the anti-DOR autoantibody mediated modulation of chemokine receptor expression, we looked at another chemokine receptor CCR7, a receptor critical for the homing of immune cells into the draining lymph node. We could not detect any changes in CCR7 expression between untreated, DPDPE treated or anti-DOR autoantibody treated cells (data not shown).
FIGURE 5.
Anti-DOR autoantibody modulates expression of CCR5 and CXCR4 on human PBMCs. PBMCs were plated at 1×105 cells/well. The cells were treated with 10 μM DPDPE, 100 μg anti-DOR antibody or left untreated. After 48 hours cells were analyzed by flow cytometry for surface expression of chemokine receptors. Solid grey histograms represent untreated cells, while open black histograms reflect cells treated with anti-DOR autoantibody. A, Treatment with anti-DOR autoantibody reduced surface CXCR4 expression (p<0.05). B, Treatment with anti-DOR autoantibody increased CCR5 expression (p<0.05). There was no change in expression of chemokine receptors after DPDPE treatment (results not shown). Left and right panels of the figure reflect results of two healthy donors. Statistical significance of the mean fluorescence intensity values was determined by using the paired t-test.
3.5. Anti-DOR autoantibodies are present in the sera of healthy individuals, SLE and HIV patients
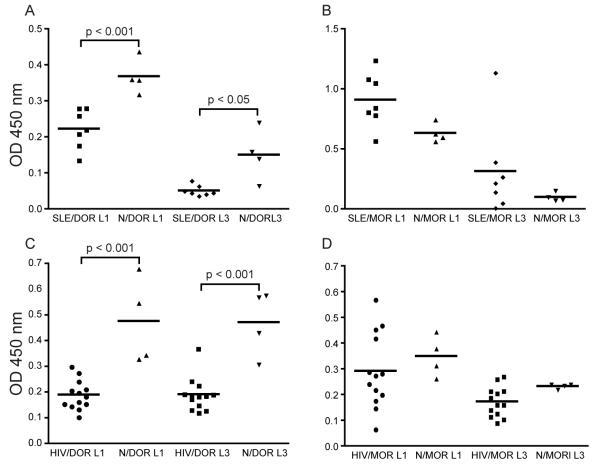
Since our peptide approach was successful in isolating antibodies against DOR that show specific binding to the receptor as well as agonistic activity, we set out to characterize autoantibody activity to opioid receptors in the serum of healthy individuals, as well as individuals with SLE and HIV. We used peptides corresponding to the loop 1 and loop 3 regions of both DOR and MOR as antigens. Using ELISA, we demonstrate the presence of autoantibodies to DOR in the sera of healthy individuals and SLE and HIV patients. We also confirmed the presence of autoantibodies to MOR in normal sera, and extend the findings to SLE and HIV patients. We tested the serum of 4 healthy individuals, 7 SLE patients and 13 HIV patients. Autoantibody binding to DOR loop peptides 1 and 3 is higher in healthy donors as compared to SLE sera (Fig. 6A). In healthy individuals, the mean absorbance against DOR loop 1 was 0.36 ±0.04 whereas it was 0.22 ± 0.05 in SLE serum (p<0.001). The mean absorbance in healthy serum was 0.15 ± 0.07 against DOR loop 3 as compared to 0.04 ± 0.01 in SLE sera (p<0.05). The trend was reversed in autoantibody binding to the MOR loop peptides 1 and 3 (Fig. 6B), where we saw higher binding in SLE serum as compared to healthy individuals. However, the difference was not significant. We also compared the binding of autoantibodies to these peptides between healthy and HIV patient serum. Here we saw significantly higher binding to DOR loop 1 peptide in healthy serum compared to HIV serum (Fig. 6C, 0.47 ± 0.16 vs. 0.19 ± 0.05, p<0.001). We observed the same trend in autoantibody binding to DOR loop 3 peptide. Healthy serum showed a mean absorbance of 0.47± 0.12 compared to 0.19 ± 0.06 in HIV (p<0.001, Fig. 6C). There was no significant difference in autoantibody binding to the MOR loop 1 and 3 peptides between healthy and HIV patient sera (Fig. 6D).
FIGURE 6.
Autoantibodies to DOR loops 1 and 3 are present in the sera of healthy individuals, SLE and HIV patients. Plates were coated with peptides corresponding to DOR and MOR loop 1 (L1) and 3 (L3) peptides. We tested the serum of 4 healthy individuals (N), 7 SLE patients and 13 HIV patients. Values reflect mean absorbance at 450nm ± s.d. of serum samples diluted 1 in 400. SLE serum samples show significantly lower binding to DOR loops 1 and 3 than normal sera (A, p<0.05). The converse is seen in binding to MOR loops, where SLE sera show higher binding than normal sera (B). HIV sera show significantly lower binding to DOR loops 1 and 3 than normal sera (C, p<0.001). The trend is reversed in binding to MOR loops 1 and 3 (D). Statistical significance was determined with a one-way analysis of variance, Bonferroni’s post-test was used to obtain p-values for the indicated pairwise comparisons.
4. Discussion
We affinity purified anti-DOR autoantibodies from IVIG that bind specifically to DOR. The antibodies display agonistic activity as seen by their ability to inhibit forskolin stimulated cAMP accumulation, as well as receptor desensitization via phosphorylation of DOR. The antibodies also elicit immunomodulatory effects as seen by the changes in expression of chemokine receptors CXCR4 and CCR5. We also report the presence of anti-delta opioid receptor antibodies in the sera of healthy individuals, SLE and HIV patients.
Opioid receptors belong to the seven transmembrane Gαi-protein coupled receptor superfamily. The binding of opioid agonists to the receptor results in inhibition of adenylate cyclase activity, inhibition of calcium channels and an array of diverse downstream signaling events (Sharp, 2006, Tegeder and Geisslinger, 2004). Dietrich et al. showed that IVIG contains a fraction of autoantibodies to the mu opioid receptor that behave as agonists by binding to the first and third extracellular loops of the receptor (Mace et al., 1999b, Mace et al., 1999a, Mace et al., 2002, Mace et al., 2001, Dietrich et al., 1998).
Here, we show that anti-DOR autoantibodies in IVIG, that have been affinity purified against the extracellular loop 1 and 3 peptides, bind with a high degree of specificity to the DOR protein. The anti-DOR autoantibodies effectively engage the opioid receptor and display potent agonistic properties. This is demonstrated by the inhibition of cAMP accumulation in cells treated with anti-DOR autoantibody, as well as the characteristic phosphorylation of DOR at Ser 363, an important step in agonist mediated desensitization and down-regulation of the receptor.
We set out to investigate the immunomodulatory properties of affinity purified anti-DOR autoantibodies that would further support the cross-talk between two seemingly disparate systems; the neuroendocrine and immune system (Bidlack, 2000, Mellon and Bayer, 1998, Roy et al., 2006, Sharp, 2006). The opioid modulation of chemokine receptors, especially CCR5 and CXCR4 has garnered interest in researchers because of their central role in promoting HIV-1 infection and progression of disease. Many research groups have shown that signaling through the MOR has profound effects on chemokine CCL2, CCL5 and CXCL10 expression, chemokine receptor CCR5, CXCR4 expression as well as susceptibility to HIV-1 infection (Chen et al., 2004, Miyagi et al., 2000, Sharp et al., 1998a, Steele et al., 2003, Szabo et al., 2003, Vallejo et al., 2004, Finley et al., 2008a). Our data shows that while both DPDPE and the anti-DOR autoantibody bind to the receptor, they elicit very different modulatory responses with respect to changes in chemokine receptor expression. In CEM×174 cells as well as PBMCs treated with anti-DOR autoantibody, there was a downregulation of CXCR4 gene expression and surface protein expression. In contrast, DPDPE, a DOR specific synthetic agonist, does not cause changes in CXCR4 expression. DPDPE is a small molecule agonist that has been conclusively shown not to induce CXCR4 desensitization and internalization (Pello et al., 2008). It has been proposed that CXCR4 and DOR form silent non-functional heterodimers that are stabilized by the addition of both the ligands, but the addition of only one ligand at a time, i.e. either the chemokine or opioid ligand, disrupts the heterodimers and promotes homodimer formation (Hereld and Jin, 2008, Pello et al., 2008). However, it may be, that unlike DPDPE, anti-DOR autoantibodies binding to the opioid receptor causes a conformational change to the opioid-chemokine heterodimer resulting in receptor internalization. In contrast to the results with CXCR4, in cells treated with anti-DOR autoantibodies, there was a significant increase in CCR5 expression, both at the gene expression and protein expression level, as seen in the RT-PCR and FACS analysis. This result was unlike DPDPE treatment that did not result in modulating CCR5 expression. The differences in the patterns of chemokine receptor expression modulation seen as a result of anti-DOR autoantibody, or DPDPE, binding to the opioid receptor may be due to the differences in binding patterns of the agonists and/or the strength of signaling. It would be interesting to see if the changes in chemokine receptor expression caused by the anti-DOR autoantibody treatment result in tangible downstream effects such as changes in cell migration pathways and susceptibility to HIV-1 infection. While the exact mechanism by which anti-DOR autoantibodies modulate the expression of chemokine receptors CCR5 and CXCR4 remains unclear, a growing body of evidence indicates that factors such as cytokine microenvironment and heterodimeric desensitization may play a role (Finley et al., 2008b, Grimm et al., 1998, Happel et al., 2008). Modulation of chemokine receptor expression on T cells has important implications in the alteration of T cell migration patterns between the peripheral inflamed tissues and draining lymph nodes, thereby modulating immune response (Hebert, 2008, Steele et al., 2002, Suzuki et al., 2002, Finley et al., 2008a). The ability of anti-DOR antibodies to alter chemokine expression patterns may well influence these parameters of the immune system. While looking at the levels of anti-DOR autoantibodies in the serum of healthy individuals as compared to those with disease, we found that autoantibody levels to DOR are higher in healthy individuals as compared to patients with HIV and SLE. Since we are looking at IgG levels, the contribution of T cells must be taken into account. HIV infection leads to reduced T cell numbers, which could be one of the reasons we see lower levels in HIV patients. However, this does not explain the phenomena in SLE patients, where the immune system is conversely in a hyper-reactive state. It must also be noted that the trend with respect to anti-MOR autoantibodies was reversed in SLE patients where we see higher binding activity than seen in healthy individuals. It is possible that agonistic anti-MOR antibodies serve to stimulate the Th2/humoral arm of the immune system, in a manner similar to synthetic MOR agonists (Greeneltch et al., 2005, Roy et al., 2004), whereas anti-DOR autoantibodies may function to stimulate the cell-mediated/Th1 arm. In SLE patients therefore, elevated levels of anti-MOR Abs might exacerbate disease, while upregulating the anti-DOR autoantibodies might help deviate to a Th1-type immune response. The difference in the levels of these antibodies between healthy individuals and those with diseases such as HIV and SLE is indirect evidence for an active role of the immune system in the selection and maintenance of these antibodies.
IVIG is used regularly as a therapeutic blood product for the treatment of immunodeficiencies as well as autoimmune diseases (Hartung, 2008, Kazatchkine and Kaveri, 2001, Vani et al., 2008). To a large extent the mechanisms by which IVIG acts is unknown, though it is accepted that the beneficial effects are elicited via a complex interaction of multiple factors some of which include the Fc-receptor mediated effects, regulation of the idiotypic networks, and modulation of dendritic cell maturation and function, induction of granulocyte cell death via ligation of siglecs, modulation of T and B cells as well as the complement system. (Arumugam et al., 2007, Blank et al., 2007, Dussault et al., 2008, Ephrem et al., 2008, Kessel et al., 2007, Samuelsson et al., 2001, Shoenfeld et al., 2002, Seite et al., 2008, von Gunten and Simon, 2008). IVIG has also been shown to contain antibodies to functional receptors on immune cells such as the human TCR, cytokines and cytokine receptors, CD4, CD5, HLA class I molecules, RGD adhesion motifs, CCR5, CD40 and Fas (Aukrust et al., 1994, Hurez et al., 1994, Marchalonis et al., 1992, Negi et al., 2007, Prasad et al., 1998, Svenson et al., 1998, Vassilev et al., 1993, Vassilev et al., 1999, Kaveri et al., 1996). Among the many pathways activated by the antibodies in IVIG, the induction of apoptosis in human lymphocytes and monocytes, and the modulation of apoptosis through CD95 is determined in part, by the concurrent presence of agonistic and antagonistic anti-Fas antibodies in IVIG, as well as the concentration of the antibodies (Prasad et al., 1998, Viard et al., 1998, Reipert et al., 2008). Natural antibodies against CCR5, a coreceptor for HIV-1 infection, present in IVIG, mucosa and breast milk have been shown to successfully block HIV-1 infection, and transport across the human epithelial cells, setting the stage to exploit the anti-CCR5 antibodies as a natural, passive component of the combinatorial therapeutic regimen required to fight HIV-1 infection (Eslahpazir et al., 2008, Bouhlal et al., 2005, Bomsel et al., 2007, Bouhlal et al., 2001).
Our results show that anti-DOR antibodies are a component of IVIG, and the ability to modulate chemokine receptor expression may be one of the pieces of the puzzle in the mechanism by which IVIG exerts its diverse effects.
In summary, our data supports the presence of a dynamic, bi-directional communication network between the neuroendocrine and immune systems. Apart from the immunomodulatory role of the anti-DOR autoantibodies, it will be interesting to see if they have an anti-nociceptive role in the peripheral sensory system, further cementing their role in bridging these two systems.
Acknowledgements
This work was supported by a grant from the Arizona Biomedical Research Commission Contract Number 0711 and National Institute of Health Grant NIDA 330726. We thank Carol Haussler for the maintenance of the CHO DOR cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Parvathi Ranganathan, Department of Immunobiology, University of Arizona, 1656 E. Mabel Street, PO Box 245221, Tucson, Arizona 85724..
Hao Chen, Department of Immunobiology, University of Arizona, 1656 E. Mabel Street, PO Box 245221, Tucson, Arizona 85724..
Samuel F. Schluter, Department of Immunobiology, University of Arizona, 1656 E. Mabel Street, PO Box 245221, Tucson, Arizona 85724..
References
- Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, Magnus T, Chan SL, Jo DG, Ouyang X, Fairlie DP, Granger DN, Vortmeyer A, Basta M, Mattson MP. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci U S A. 2007;104:14104–9. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust P, Froland SS, Liabakk NB, Muller F, Nordoy I, Haug C, Espevik T. Release of cytokines, soluble cytokine receptors, and interleukin-1 receptor antagonist after intravenous immunoglobulin administration in vivo. Blood. 1994;84:2136–43. [PubMed] [Google Scholar]
- Benard A, Boue J, Chapey E, Jaume M, Gomes B, Dietrich G. Delta opioid receptors mediate chemotaxis in bone marrow-derived dendritic cells. J Neuroimmunol. 2008;197:21–8. doi: 10.1016/j.jneuroim.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Bidlack JM. Detection and function of opioid receptors on cells from the immune system. Clin Diagn Lab Immunol. 2000;7:719–23. doi: 10.1128/cdli.7.5.719-723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M, Anafi L, Zandman-Goddard G, Krause I, Goldman S, Shalev E, Cervera R, Font J, Fridkin M, Thiesen HJ, Shoenfeld Y. The efficacy of specific IVIG anti-idiotypic antibodies in antiphospholipid syndrome (APS): trophoblast invasiveness and APS animal model. Int Immunol. 2007;19:857–65. doi: 10.1093/intimm/dxm052. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Pastori C, Tudor D, Alberti C, Garcia S, Ferrari D, Lazzarin A, Lopalco L. Natural mucosal antibodies reactive with first extracellular loop of CCR5 inhibit HIV-1 transport across human epithelial cells. AIDS. 2007;21:13–22. doi: 10.1097/QAD.0b013e328011049b. [DOI] [PubMed] [Google Scholar]
- Bouhlal H, Hocini H, Quillent-Gregoire C, Donkova V, Rose S, Amara A, Longhi R, Haeffner-Cavaillon N, Beretta A, Kaveri SV, Kazatchkine MD. Antibodies to C-C chemokine receptor 5 in normal human IgG block infection of macrophages and lymphocytes with primary R5-tropic strains of HIV-1. J Immunol. 2001;166:7606–11. doi: 10.4049/jimmunol.166.12.7606. [DOI] [PubMed] [Google Scholar]
- Bouhlal H, Latry V, Requena M, Aubry S, Kaveri SV, Kazatchkine MD, Belec L, Hocini H. Natural antibodies to CCR5 from breast milk block infection of macrophages and dendritic cells with primary R5-tropic HIV-1. J Immunol. 2005;174:7202–9. doi: 10.4049/jimmunol.174.11.7202. [DOI] [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–86. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Dietrich G, Gaibelet G, Capeyrou R, Butour JL, Pontet F, Emorine LJ. Implication of the first and third extracellular loops of the mu-opioid receptor in the formation of the ligand binding site: a study using chimeric mu-opioid/angiotensin receptors. J Neurochem. 1998;70:2106–11. doi: 10.1046/j.1471-4159.1998.70052106.x. [DOI] [PubMed] [Google Scholar]
- Dussault N, Ducas E, Racine C, Jacques A, Pare I, Cote S, Neron S. Immunomodulation of human B cells following treatment with intravenous immunoglobulins involves increased phosphorylation of extracellular signal-regulated kinases 1 and 2. Int Immunol. 2008;20:1369–79. doi: 10.1093/intimm/dxn090. [DOI] [PubMed] [Google Scholar]
- Ephrem A, Chamat S, Miquel C, Fisson S, Mouthon L, Caligiuri G, Delignat S, Elluru S, Bayry J, Lacroix-Desmazes S, Cohen JL, Salomon BL, Kazatchkine MD, Kaveri SV, Misra N. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111:715–22. doi: 10.1182/blood-2007-03-079947. [DOI] [PubMed] [Google Scholar]
- Eslahpazir J, Jenabian MA, Bouhlal H, Hocini H, Carbonneil C, Gresenguet G, Keou FX, Legoff J, Saidi H, Requena M, Nasreddine N, De Dieu Longo J, Kaveri SV, Belec L. Infection of macrophages and dendritic cells with primary R5-tropic human immunodeficiency virus type 1 inhibited by natural polyreactive anti-CCR5 antibodies purified from cervicovaginal secretions. Clin Vaccine Immunol. 2008;15:872–84. doi: 10.1128/CVI.00463-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadhil I, Schmidt R, Walpole C, Carpenter KA. Exploring deltorphin II binding to the third extracellular loop of the delta-opioid receptor. J Biol Chem. 2004;279:21069–77. doi: 10.1074/jbc.M311468200. [DOI] [PubMed] [Google Scholar]
- Finley MJ, Chen X, Bardi G, Davey P, Geller EB, Zhang L, Adler MW, Rogers TJ. Bi-directional heterologous desensitization between the major HIV-1 co-receptor CXCR4 and the kappa-opioid receptor. J Neuroimmunol. 2008a;197:114–23. doi: 10.1016/j.jneuroim.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008b;252:146–54. doi: 10.1016/j.cellimm.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeneltch KM, Kelly-Welch AE, Shi Y, Keegan AD. Chronic morphine treatment promotes specific Th2 cytokine production by murine T cells in vitro via a Fas/Fas ligand-dependent mechanism. J Immunol. 2005;175:4999–5005. doi: 10.4049/jimmunol.175.8.4999. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1998;188:317–25. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel C, Steele AD, Finley MJ, Kutzler MA, Rogers TJ. DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-beta1. J Leukoc Biol. 2008;83:956–63. doi: 10.1189/jlb.1007685. [DOI] [PubMed] [Google Scholar]
- Hartung HP. Advances in the understanding of the mechanism of action of IVIg. J Neurol. 2008;255(Suppl 3):3–6. doi: 10.1007/s00415-008-3002-0. [DOI] [PubMed] [Google Scholar]
- Hebert TE. Opioid and chemokine receptor heterodimers: arranged marriages or dangerous liaisons? Biochem J. 2008;412:e7–9. doi: 10.1042/BJ20080620. [DOI] [PubMed] [Google Scholar]
- Hereld D, Jin T. Slamming the DOR on chemokine receptor signaling: heterodimerization silences ligand-occupied CXCR4 and delta-opioid receptors. Eur J Immunol. 2008;38:334–7. doi: 10.1002/eji.200738101. [DOI] [PubMed] [Google Scholar]
- House RV, Thomas PT, Bhargava HN. A comparative study of immunomodulation produced by in vitro exposure to delta opioid receptor agonist peptides. Peptides. 1996;17:75–81. doi: 10.1016/0196-9781(95)02051-9. [DOI] [PubMed] [Google Scholar]
- Hurez V, Kaveri SV, Mouhoub A, Dietrich G, Mani JC, Klatzmann D, Kazatchkine MD. Anti-CD4 activity of normal human immunoglobulin G for therapeutic use. (Intravenous immunoglobulin, IVIg) Ther Immunol. 1994;1:269–77. [PubMed] [Google Scholar]
- Jaume M, Laffont S, Chapey E, Blanpied C, Dietrich G. Opioid receptor blockade increases the number of lymphocytes without altering T cell response in draining lymph nodes in vivo. J Neuroimmunol. 2007;188:95–102. doi: 10.1016/j.jneuroim.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Kaveri S, Vassilev T, Hurez V, Lengagne R, Lefranc C, Cot S, Pouletty P, Glotz D, Kazatchkine MD. Antibodies to a conserved region of HLA class I molecules, capable of modulating CD8 T cell-mediated function, are present in pooled normal immunoglobulin for therapeutic use. J Clin Invest. 1996;97:865–9. doi: 10.1172/JCI118488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747–55. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- Kessel A, Ammuri H, Peri R, Pavlotzky ER, Blank M, Shoenfeld Y, Toubi E. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol. 2007;179:5571–5. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- Li X, Varga EV, Stropova D, Zalewska T, Malatynska E, Knapp RJ, Roeske WR, Yamamura HI. delta-Opioid receptor: the third extracellular loop determines naltrindole selectivity. Eur J Pharmacol. 1996;300:R1–2. doi: 10.1016/0014-2999(96)00098-2. [DOI] [PubMed] [Google Scholar]
- Mace G, Blanpied C, Emorine LJ, Druet P, Dietrich G. Isolation and characterization of natural human IgG with a morphine-like activity. Eur J Immunol. 1999a;29:997–1003. doi: 10.1002/(SICI)1521-4141(199903)29:03<997::AID-IMMU997>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Mace G, Blanpied C, Emorine LJ, Druet P, Dietrich G. Morphine-like activity of natural human IgG autoantibodies is because of binding to the first and third extracellular loops of the mu-opioid receptor. J Biol Chem. 1999b;274:20079–82. doi: 10.1074/jbc.274.29.20079. [DOI] [PubMed] [Google Scholar]
- Mace G, Jaume M, Blanpied C, Stephan L, Coudert JD, Druet P, Dietrich G. Anti-mu-opioid-receptor IgG antibodies are commonly present in serum from healthy blood donors: evidence for a role in apoptotic immune cell death. Blood. 2002;100:3261–8. doi: 10.1182/blood-2002-01-0055. [DOI] [PubMed] [Google Scholar]
- Mace G, Jaume M, Druet E, Blanpied C, Nguyen C, Druet P, Dietrich G. Identification of mu-opioid receptor epitopes recognized by agonistic IgG. Biochem Biophys Res Commun. 2001;280:1142–7. doi: 10.1006/bbrc.2001.4258. [DOI] [PubMed] [Google Scholar]
- Marchalonis JJ, Kaymaz H, Dedeoglu F, Schluter SF, Yocum DE, Edmundson AB. Human autoantibodies reactive with synthetic autoantigens from T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1992;89:3325–9. doi: 10.1073/pnas.89.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–23. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83:19–28. doi: 10.1016/s0165-5728(97)00217-8. [DOI] [PubMed] [Google Scholar]
- Miller B. delta opioid receptor expression is induced by concanavalin A in CD4+ T cells. J Immunol. 1996;157:5324–8. [PubMed] [Google Scholar]
- Miyagi T, Chuang LF, Doi RH, Carlos MP, Torres JV, Chuang RY. Morphine induces gene expression of CCR5 in human CEM×174 lymphocytes. J Biol Chem. 2000;275:31305–10. doi: 10.1074/jbc.M001269200. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Eaton MC, Stropova D, Varga EV, Vanderah TW, Roeske WR, Yamamura HI. Morphine promotes phosphorylation of the human delta-opioid receptor at serine 363. Eur J Pharmacol. 2005;519:212–4. doi: 10.1016/j.ejphar.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, Lacroix-Desmazes S, Bayry J, Kaveri SV. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27:233–45. doi: 10.1007/s10875-007-9088-9. [DOI] [PubMed] [Google Scholar]
- Nguyen K, Miller BC. CD28 costimulation induces delta opioid receptor expression during anti-CD3 activation of T cells. J Immunol. 2002;168:4440–5. doi: 10.4049/jimmunol.168.9.4440. [DOI] [PubMed] [Google Scholar]
- Pello OM, Martinez-Munoz L, Parrillas V, Serrano A, Rodriguez-Frade JM, Toro MJ, Lucas P, Monterrubio M, Martinez AC, Mellado M. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–49. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–9. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Prasad NK, Papoff G, Zeuner A, Bonnin E, Kazatchkine MD, Ruberti G, Kaveri SV. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J Immunol. 1998;161:3781–90. [PubMed] [Google Scholar]
- Quintana FJ, Cohen IR. The natural autoantibody repertoire and autoimmune disease. Biomed Pharmacother. 2004;58:276–81. doi: 10.1016/j.biopha.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Reipert BM, Stellamor MT, Poell M, Ilas J, Sasgary M, Reipert S, Zimmermann K, Ehrlich H, Schwarz HP. Variation of anti-Fas antibodies in different lots of intravenous immunoglobulin. Vox Sang. 2008;94:334–41. doi: 10.1111/j.1423-0410.2008.001036.x. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann N Y Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Gupta S, Charboneau R, Loh HH, Barke RA. Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet. J Neuroimmunol. 2004;147:78–81. doi: 10.1016/j.jneuroim.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang J, Kelschenbach J, Koodie L, Martin J. Modulation of Immune Function by Morphine: Implications for Susceptibility to Infection. J Neuroimmune Pharmacology. 2006;1:77–89. doi: 10.1007/s11481-005-9009-8. [DOI] [PubMed] [Google Scholar]
- Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21:235–46. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- Seite JF, Shoenfeld Y, Youinou P, Hillion S. What is the contents of the magic draft IVIg? Autoimmun Rev. 2008;7:435–9. doi: 10.1016/j.autrev.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Shahabi NA, Mcallen K, Matta SG, Sharp BM. Expression of delta opioid receptors by splenocytes from SEB-treated mice and effects on phosphorylation of MAP kinase. Cell Immunol. 2000;205:84–93. doi: 10.1006/cimm.2000.1717. [DOI] [PubMed] [Google Scholar]
- Shahabi NA, Mcallen K, Sharp BM. Phosphorylation of activating transcription factor in murine splenocytes through delta opioid receptors. Cell Immunol. 2003;221:122–7. doi: 10.1016/s0008-8749(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Shahabi NA, Mcallen K, Sharp BM. delta opioid receptors stimulate Akt-dependent phosphorylation of c-jun in T cells. J Pharmacol Exp Ther. 2006;316:933–9. doi: 10.1124/jpet.105.091447. [DOI] [PubMed] [Google Scholar]
- Sharp BM. Opioid receptor expression and function. J Neuroimmunol. 2004;147:3–5. doi: 10.1016/j.jneuroim.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Sharp BM. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav Immun. 2006;20:9–14. doi: 10.1016/j.bbi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Gekker G, Li MD, Chao CC, Peterson PK. Delta-opioid suppression of human immunodeficiency virus-1 expression in T cells (Jurkat) Biochem Pharmacol. 1998a;56:289–92. doi: 10.1016/s0006-2952(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Mcallen K, Gekker G, Shahabi NA, Peterson PK. Immunofluorescence detection of delta opioid receptors (DOR) on human peripheral blood CD4+ T cells and DOR-dependent suppression of HIV-1 expression. J Immunol. 2001;167:1097–102. doi: 10.4049/jimmunol.167.2.1097. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Mckean DJ, Mcallen K, Shahabi NA. Signaling through delta opioid receptors on murine splenic T cells and stably transfected Jurkat cells. Ann N Y Acad Sci. 1998b;840:420–4. doi: 10.1111/j.1749-6632.1998.tb09580.x. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998c;83:45–56. [PubMed] [Google Scholar]
- Shen H, Aeschlimann A, Reisch N, Gay RE, Simmen BR, Michel BA, Gay S, Sprott H. Kappa and delta opioid receptors are expressed but down-regulated in fibroblast-like synoviocytes of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2005;52:1402–10. doi: 10.1002/art.21141. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Rauova L, Gilburd B, Kvapil F, Goldberg I, Kopolovic J, Rovensky J, Blank M. Efficacy of IVIG affinity-purified anti-double-stranded DNA anti-idiotypic antibodies in the treatment of an experimental murine model of systemic lupus erythematosus. Int Immunol. 2002;14:1303–11. doi: 10.1093/intimm/dxf099. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y, Toubi E. Protective autoantibodies: role in homeostasis, clinical importance, and therapeutic potential. Arthritis Rheum. 2005;52:2599–606. doi: 10.1002/art.21252. [DOI] [PubMed] [Google Scholar]
- Steele AD, Henderson EE, Rogers TJ. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology. 2003;309:99–107. doi: 10.1016/s0042-6822(03)00015-1. [DOI] [PubMed] [Google Scholar]
- Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 2002;13:209–22. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280:192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- Svenson M, Hansen MB, Ross C, Diamant M, Rieneck K, Nielsen H, Bendtzen K. Antibody to granulocyte-macrophage colony-stimulating factor is a dominant anti-cytokine activity in human IgG preparations. Blood. 1998;91:2054–61. [PubMed] [Google Scholar]
- Szabo I, Wetzel MA, Zhang N, Steele AD, Kaminsky DE, Chen C, Liu-Chen LY, Bednar F, Henderson EE, Howard OM, Oppenheim JJ, Rogers TJ. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J Leukoc Biol. 2003;74:1074–82. doi: 10.1189/jlb.0203067. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival--unraveling mechanisms and revealing new indications. Pharmacol Rev. 2004;56:351–69. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- Vallejo R, De Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther. 2004;11:354–65. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- Vani J, Elluru S, Negi VS, Lacroix-Desmazes S, Kazatchkine MD, Bayary J, Kaveri SV. Role of natural antibodies in immune homeostasis: IVIg perspective. Autoimmun Rev. 2008;7:440–4. doi: 10.1016/j.autrev.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Varga EV, Li X, Stropova D, Zalewska T, Landsman RS, Knapp RJ, Malatynska E, Kawai K, Mizusura A, Nagase H, Calderon SN, Rice K, Hruby VJ, Roeske WR, Yamamura HI. The third extracellular loop of the human delta-opioid receptor determines the selectivity of delta-opioid agonists. Mol Pharmacol. 1996;50:1619–24. [PubMed] [Google Scholar]
- Varga EV, Navratilova E, Stropova D, Jambrosic J, Roeske WR, Yamamura HI. Agonist-specific regulation of the delta-opioid receptor. Life Sci. 2004;76:599–612. doi: 10.1016/j.lfs.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Varga EV, Rubenzik M, Grife V, Sugiyama M, Stropova D, Roeske WR, Yamamura HI. Involvement of Raf-1 in chronic delta-opioid receptor agonist-mediated adenylyl cyclase superactivation. Eur J Pharmacol. 2002;451:101–2. doi: 10.1016/s0014-2999(02)02220-3. [DOI] [PubMed] [Google Scholar]
- Vassilev T, Gelin C, Kaveri SV, Zilber MT, Boumsell L, Kazatchkine MD. Antibodies to the CD5 molecule in normal human immunoglobulins for therapeutic use (intravenous immunoglobulins, IVIg) Clin Exp Immunol. 1993;92:369–72. doi: 10.1111/j.1365-2249.1993.tb03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev TL, Kazatchkine MD, Van Huyen JP, Mekrache M, Bonnin E, Mani JC, Lecroubier C, Korinth D, Baruch D, Schriever F, Kaveri SV. Inhibition of cell adhesion by antibodies to Arg-Gly-Asp (RGD) in normal immunoglobulin for therapeutic use (intravenous immunoglobulin, IVIg) Blood. 1999;93:3624–31. [PubMed] [Google Scholar]
- Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, Hunziker T, Saurat JH, Tschopp J, French LE. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998;282:490–3. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- Von Gunten S, Simon HU. Natural anti-Siglec autoantibodies mediate potential immunoregulatory mechanisms: implications for the clinical use of intravenous immunoglobulins (IVIg) Autoimmun Rev. 2008;7:453–6. doi: 10.1016/j.autrev.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]