Abstract
Background
Cardiac transplant arteriosclerosis, or cardiac allograft vasculopathy, remains the leading cause of graft failure and patient death in heart transplant recipients. Endothelial cell injury is crucial in the development of human atherosclerosis and may play a role in allograft vasculopathy. Glutathione-S-Transferase (GST) is known to protect endothelial cells from damage by oxidants and toxins. However, the contribution of human glutathione-S-transferase A4-4 (hGSTA4-4) to vascular cell injury and consequent transplant arteriosclerosis is unknown.
Methods
A recombinant adenoviral vector containing hGSTA4-4 gene was constructed and delivered to vascular endothelial cells in an in vivo rabbit carotid artery transplant model. Forty five days after transplantation, allografts were harvested (n = 28). Blood flow was measured by ultrasonography. In addition, grafts were analyzed by histology, morphometry, immunostaining and western blot.
Results
The severity of arteriosclerosis in hGSTA4-4 transduced allografts was compared with control by measuring degree of stenosis by neointima. Decrease in blood flow in hGSTA4-4 transduced allografts was significantly less than control allografts, which also developed greater intimal thickening and stenosis than hGSTA4-4 transduced allografts in the proximal and distal regions of the graft. Leukocyte and macrophage infiltration was reduced in hGSTA4-4 transduced carotid arteries.
Conclusion
Our data indicates that hGSTA4-4 overexpression protects the integrity of vessel wall from oxidative injury, and attenuates transplant arteriosclerosis.
Keywords: Glutathione-S-Transferase, allograft, transplant arteriosclerosis, neointima
INTRODUCTION
Cardiac allograft vasculopathy (CAV) is an unusually accelerated form of obliterative transplant arteriosclerosis that is a major cause of chronic rejection and allograft failure (1). The precise mechanism and detailed pathophysiology of CAV are not fully understood. Studies indicate that CAV results from a complicated interplay between immunologic and nonimmunologic factors resulting in repetitive vascular injury and localized sustained inflammatory response (2,3). There is growing evidence implicating oxidative stress and inflammation as players in the early endothelial damage that initiates CAV (4-6).
Glutathione-S-Transferase A4-4 (GSTA4-4) is an intracellular enzyme that mediates the conjugation of the products of lipid peroxidation (such as highly active α, β-unsaturated alkenals) in several species, including human (7). An important role for GST in modulating signaling pathways during oxidative stress has been recently recognized (8,9). Reactive alkenals, (particularly 4-hydroxynonenal, which is a relatively stable end product of lipid peroxidation and oxidative stress) are now accepted as major regulators of stress-mediated signaling involving cell growth, differentiation, transformation, apoptosis, and expression of critical cell cycle genes (10-12). Conjugation of 4-hydroxynonenal (4-HNE) with reduced glutathione (GSH) influences many signal transduction pathways and modulates the activity of several cell-surface receptors, including epithelial growth factor receptor, platelet-derived growth factor-β (PDGF-β) receptor and transforming growth factor receptor β1 (TGFR-β1) (8).
In addition, 4-HNE plays an important role in cell apoptosis (10,13). Both up-regulation of growth factor receptors and apoptosis are considered crucial in the pathogenesis of CAV (14,15). De et al, reported that the level of blood GSH in CAV patients may serve as a “cellular” marker of consumption of endothelial antioxidant defense in CAV development (5). Thus, conjugation of 4-HNE to GSH through the action of GSTs may be important in endothelial oxidative injury resulting in CAV. However, the role GSTA4-4 might play in preventing or attenuating CAV is not yet established.
We recently found that the human enzyme hGSTA4-4, which detoxifies 4-HNE and exhibits high activity in vascular tissue, acts as a major defense against oxidative stress in an endothelial cell line, and is upregulated in endothelial cells overlaying the earliest stages of the human atherosclerotic plaque (16). Our earlier work in isolated vascular smooth muscle cells (17,18) also showed that oxidative damage induced by 4-HNE is an important step in the process of human atherogenesis. Furthermore, induction of 4-HNE-metabolizing GSTs in the vascular wall is appears to be protective of both endothelial and vascular smooth muscle cells undergoing oxidative stress (17,19). Most recently, we defined an important function of hGSTA4-4 in attenuating the increased endothelial permeability induced by oxidative stress (20).
Based on the previous studies, we hypothesized that augmenting the activity of endothelial cell GSTA4-4 would protect against oxidative injury to an arterial allograft, and thus attenuate CAV. We introduced an adenoviral vector containing hGSTA4-4 into rabbit carotid arteries to evaluate the therapeutic potential of GSTs in a rabbit model of allograft arteriosclerosis. We found hGSTA4-4 transduced allografts had significantly less neointima and less stenosis of lumen than control. In addition, leukocyte and macrophage infiltration were reduced in hGSTA4-4 transduced carotid arteries. We conclude that hGSTA4-4 overexpression has the potential to protect the integrity of vessel wall from oxidative injury, and attenuate transplant arteriosclerosis. Our findings are significant for understanding the development of CAV as well as for identifying novel therapeutic targets for preventing CAV.
MATERIALS AND METHODS
Animals used in experiments
Dutch Belted and New Zealand White rabbits (3 months old, male) (Myrtles Rabbitry, Thompson Station, TN) were used to harvest carotid arteries for culture and allograft transplant surgery. All animal usage was under a protocol approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
Ex vivo gene transfer into carotid artery segments
Recombinant adenoviral vectors containing hGSTA4-4 and enhanced green fluorescent protein (eGFP) genes were used for ex vivo gene transfer to vessels. The design and construction of the adenoviral vectors were previously described (20). Briefly, hGSTA4-4 and IRES-eGFP genes were linked by an internal ribosome entry site (IRES), and the dual expression cassette was cloned into Adeno-X (Clontech, Mountain View, CA) driven by cytomegalovirus (CMV) promoter (Ad5-CMV/hGST-IRES-eGFP). For the control vector, only eGFP gene was inserted into the Adeno-X (Ad5-CMV/eGFP).
After rabbits (Dutch Belted; n = 5) were anesthetized with ketamine (50mg/kg) and xylazine (20mg/kg) intraperitoneally, carotid arteries were dissected and harvested. Carotid artery segments were flushed free of blood with Dulbecco’s phosphate-buffered saline (DPBS) and aspirated. A total of 20 μl of Ad5-CMV/hGST-IRES-eGFP or Ad5-CMV/eGFP containing 1-1.5× 1010 viral particles, or PBS were injected into carotid artery segments. Two ends of the carotid arteries were clamped. Infection was conducted at 4°C for 1 hour according to the method published (15). Vessel segments were washed with PBS and cut into 2-mm-length rings and cultured with reduced growth factor Matrigel (BD Biosciences, Franklin Lakes, NJ) and endothelial cell growth medium (Clonetics, San Diego, CA). Carotid artery tissues were maintained at 37°C in a 5% CO2 incubator (21). The vessel segments were cultured for 7 to 28 days.
Carotid artery transplantation
Carotid artery allografts were orthotopically transplanted from Dutch Belted (DB) rabbits (n = 14) to New Zealand White (NZW) rabbits (n = 28). Transplantation included three groups: allograft DPBS (n = 2) control, allograft vector control (n = 12), allograft Ad-hGST (n = 14). Because our initial data suggested little or no difference between DPBS control and adenoviral vector control, we used adenoviral vector control (n = 12) for all statistical comparisons. In addition, DB to DB isografts (n = 2) with vector control and DPBS control showed no difference. Rabbits were anesthetized as described above. Carotid arteries from both sides of DB rabbits were harvested and flushed with PBS. 20 μl of Ad5-CMV/hGST-IRES-eGFP or Ad5-CMV/eGFP viral solution containing 1010 viral particles, or PBS was injected slowly into the arterial lumen. We clamped both ends of the vessels and placed them in PBS at 4°C for 1 hour based on the technique of published work (15). After washing with PBS, 3.0 cm segments of carotid arteries were used for orthotopical transplantation into right side of NZW rabbits’ carotid arteries. NZW rabbits were heparinized (600 IU i.v) and maintained under general anesthesia with mechanical respiration during the surgery. We employed a cuff technique (22) to minimize the transplant pannus formation at the site of both the proximal and distal anastomoses. This method surgically opposes graft to host tissues, maximizes the lumen at the anastomoses, and minimizes cellular reaction due to foreign bodies (suture). At the end of the surgery, all recipient rabbits received cyclosporine A (CsA) (LC laboratories, Woburn, MA) (3mg/Kg /day) through Alzet osmotic pump intraperitoneally (IP) (Durect Cooperation, Cupertino, CA.) based on the IP dose used by Sihvola et al (14).
Ultrasonography
Rabbits were anesthetized as described above before ultrasonography. Rabbit hair on both sides of the neck was removed by Nair hair remover (Church & Dwight Co., Inc., Princeton, NJ).Ultrasound transmission gel (Parker laboratories, Inc., Fairfield, NJ) was put on the skin of rabbit neck. Spectral Doppler module type 1856 for the diagnostic ultrasound system 3535 (B& K Medical, Wilmington, MA) was used to detect the blood flow of left and right carotid arteries before transplantation and the blood flow of allografts and contralateral (native) carotid arteries of transplanted rabbits.
Histology and Immunohistochemistry
Forty five days after allograft transplantation, rabbits were anaesthetized, and both left (native) and right (transplanted) carotid arteries were exposed and excised. Each carotid specimen was flushed with cold PBS. Each vessel was divided into three equal parts, proximal, central and distal parts. All three parts of the carotid arteries were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer at 4°C for 1 hour, then embedded in OCT compound (Miles Inc., Elkhart, IN) on dry ice, and stored at −80°C. Vessels were cut into 10 μm sections using a cryostat. eGFP fluorescence was visualized under a microscope. hGSTA4-4 were labeled with chicken anti-hGSTA4-4 antibody (gift from Dr. Zimniak, University of Arkansas for Medical Sciences, Little Rock, AK, ). Mouse anti human CD141 (anti-hCD141, 1:50) (Serotec, Raleigh, NC) and mouse anti human smooth muscle actin (anti-hSMA, 1:50) (DAKO, Carpinteria, CA) were used to label endothelial cells and smooth muscle cells respectively. Mouse anti-rabbit CD45 (1:100) and mouse anti-rabbit CD11b (1:100) (Serotec) were used for detecting leukocyte common antigen and macrophage respectively. Goat anti-mouse IgG and anti-chicken IgG (Alexa Fluor 594, 1:500) (Molecular Probes, Eugene, OR) were used for visualization. Immunohistochemistry was performed according to protocols provided by manufacturers. Tissue slides were mounted with mounting medium (Vector, Burlingame, CA) and examined under a fluorescence microscope (Olympus, BX51). Morphometric analysis of vessel lumen and degree of stenosis was accomplished with Image J imaging software.
Western Blot
Tissue from the cultured vessels and both sides of carotid arteries of transplanted rabbits were homogenized, and lysed in RIPA buffer (Santa Cruz Biotechnology Inc., Santa Cruz, CA) containing 1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), Aprotinin, 20ug/ml phenylmethylsulfonyl fluoride (PMSF). The concentration of total protein was determined using BCA protein assay (Pierce, Rockford, IL). Equal amounts of protein extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide). Proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane and then incubated with chicken anti-hGSTA4-4 antibody (1:1000) and mouse anti-GFP (1:2000) (Chemicon, Temecula, CA) to detect transgene expression of hGSTA4-4 and eGFP respectively. Actin detected by mouse anti-actin antibody (1:5000) (Sigma, St. Louis, MO) served as loading control. Proteins were visualized with secondary antisera coupled to horseradish peroxidase and developed with a chemiluminescence kit (Pierce, Rockford, IL). Data was analyzed using 1D scan EX software (BD Biosciences, Scanalytics, Rockville, MD).
Statistical Analysis
All data are expressed as mean ± SEM. The morphological measurements were analyzed with the use of Image J and Sigmastat statistical analysis system software. The Student’s test was used to assess the significance of changes between two means and P < 0.05 was considered significant.
RESULTS
Transgene expression ex vivo
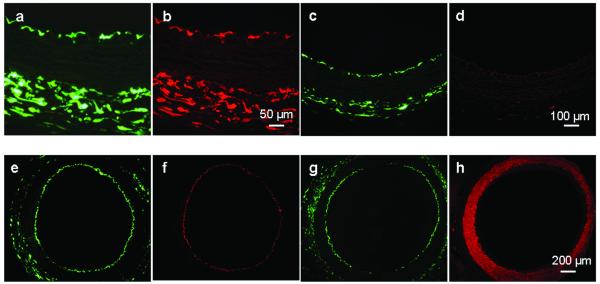
Cultured rabbit carotid arteries showed strong hGSTA4-4 and/or eGFP expression after infection with adenoviral vectors of Ad5-CMV/hGST-IRES-eGFP or Ad5-CMV/eGFP. Two to five days after infection with Ad5-hGST-IRES-eGFP, endothelial cells of the rabbit carotid arteries showed intensive eGFP fluorescence (Fig. 1a) and hGSTA4-4 expression was co-localized by immunostaining with anti hGSTA4-4 antibody (Fig. 1b). Vessels infected with Ad5-CMV/eGFP showed only eGFP expression (Fig. 1c) and no hGSTA4-4 expression (Fig. 1d). We confirmed eGFP expression (Fig. 1e) in endothelial cells by immunostaining with anti-hCD141 antibody (Fig. 1f), and excluded eGFP expression (Fig. 1g) in vascular smooth muscle cells with anti-SMA antibody (Fig. 1h). Overinjection of viral solution caused viral solution contact with outer surface of vessels, and transgene expression in adventitia, similar to the observation of others (15).
Fig.1.
Strong hGSTA4-4 and/or eGFP expression in ex vivo cultured rabbit carotid arteries. Two to five days after infection with Ad5-CMV/hGST-IRES-eGFP, endothelial cells of the rabbit carotid arteries showed intensive eGFP (a) and hGSTA4-4 expression was co-localized by immunostaining with anti-hGSTA4-4 antibody (b). Vessels infected with Ad5-CMV/eGFP showed only eGFP expression (c) and no hGSTA4-4 expression (d). eGFP expression (e) in endothelial cells was confirmed by immunostaining with anti-hCD141 antibody (f), and excluded eGFP expression (g) in vascular smooth muscle cells with anti-SMA antibody (h).
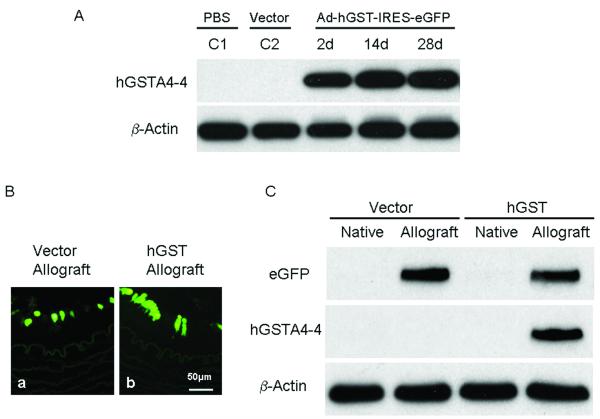
Rabbit carotid arteries retained strong eGFP fluorescence for 28 days, the longest explants were maintained in culture. This is different from the previous finding of adenovirus-mediated transgene expression in rabbit carotid arteries, which only lasted for two weeks (23). Western blot confirmed hGSTA4-4 expression in vessels transduced with hGSTA4-4 expressing adenoviral vector, but not in control vessels treated with PBS or transduced with eGFP expressing vector (Fig. 2A).
Fig. 2.
Ex vivo and in vivo hGSTA4-4 expression in rabbit carotid arteries. A: Western blot confirmed hGSTA4-4 expression in ex vivo cultured vessels infected with hGSTA4-4 expressing adenoviral vector, but not in control vessels treated with PBS (C1) or transduced with eGFP expressing vector (C2). B: eGFP expression was observed in endothelial cells of allograft infected with Ad5-CMV/eGFP (a); and eGFP expression was observed in allograft infected with Ad5-CMV/hGST-IRES-eGFP (b). Expression was co-localized with hGSTA4-4 expression proven by western blot (C). C: Efficient in vivo expression of both eGFP and hGSTA4-4 in allografts infected with adenoviral vector, Ad5-CMV/hGST-IRES-eGFP or Ad5-CMV/eGFP. In the vector control (Ad5-CMV/eGFP) group, eGFP protein expression (lane 2) was detected in allografts, but not in native carotid arteries (lane 1). There was no hGSTA4-4 expression in any carotid arteries in the vector control group (lane 1 & 2). In the hGSTA4-4 transduced group, both eGFP and hGSTA4-4 expression (lane 4) in allografts were detected, but no transgene expression was found in the native carotid arteries (lane 3).
Overexpression of hGSTA4-4 in carotid allografts in vivo
hGSTA4-4 and eGFP expression in endothelial cells of rabbit carotid allografts was determined by directly viewing with a fluorescence microscope and by western blot. The carotid allografts and native carotid vessels were harvested after 45 days of transplantation for histological study and Western blot analysis. For Western blot, vessels were homogenized in lysing buffer and total protein extracted. In the vector control (Ad5-CMV/eGFP) group, eGFP protein expression (Fig. 2B, lane 2) was detected in allografts, but not in native carotid arteries (Fig. 2B, lane 1). There was no hGSTA4-4 expression in any carotid arteries in the vector control (Ad5-CMV/eGFP) group (Fig. 2B, lane 1 & 2). In the hGSTA4-4 transduced group, both eGFP and hGSTA4-4 expression (Fig. 2B, lane 4) in allografts were detected, but no transgene expression was found in native carotid arteries (Fig. 2B, lane 3). This indicates efficient co-locating expression of both eGFP and hGSTA4-4 in allografts infected with adenoviral vector, Ad5-CMV/hGST-IRES-eGFP. Other’s work has found similar results (15); specifically the study of Wang, et al showed transgene expression in rat aorta mediated by adenoviral vector displayed the strongest expression in endothelial cells at 3 to 30 days after transplantation. The transgene expression was maintained until 60 days but started to taper by 90 days after the infection.
Ultrasonography of transplanted rabbit carotid artery
To quantify the effect of hGSTA4-4 transduction on carotid allografts, we measured the blood flow velocity in rabbit carotid arteries of both sides before transplant surgery and 45 days after transplantation by Doppler ultrasonography. Blood flow velocity was assessed with an ultrasonic probe on both carotid arteries of 12 rabbits (hGSTA4-4 transduced, n = 6; vector control, n = 6). To quantify the blood flow in each vessel, we first measure the distance traveled by tracing the area covered by the wave of velocity. The blood flow was the distance times the area of the vessel lumen. The percentage change of the blood flow between each vessel before and after surgery was calculated (hGSTA4-4 transduced, n = 6; vector control, n = 6) (Fig. 3). For the allograft side, the blood flow of hGSTA4-4 transduced allograft decreased 10.8 ± 3.1%, whereas the vector-treated allograft decreased 71.4 ± 17.4%. The difference in decrease of blood flow between these two groups is significant (p < 0.01). For the contralateral side (native), the blood flow of carotid artery increased 6.78 ± 2.5% in hGSTA4-4 group, and 28.6 ± 9.0% in vector control group (p < 0.01). These data indicate that hGSTA4-4 overexpression better preserved blood flow in the carotid arteries.
Fig. 3.
Doppler ultrasonography of carotid arteries in control and hGSTA4-4 groups before and after transplantation (hGSTA4-4 transduced, n = 6; vector control, n = 6). Comparisons of blood flow in carotid arteries (hGSTA4-4 transduced, n = 6; vector control, n = 6). For the allograft side, the blood flow of hGSTA4-4 transduced allograft decreased 10.8 ± 3.1%, whereas the vector-treated allograft decreased 71.4 ± 17.4% (p < 0.01). For the contralateral side (native), the blood flow of carotid artery increased 6.8 ± 2.5% in hGSTA4-4 group, and 28.6 ± 9.0% in vector control group (p < 0.01).
Inhibition of neointima development in carotid allografts
Carotid allografts and contralateral carotid arteries (native) were examined histologically for evidence of neointima development. Circumferential neointima formation was observed in both vector transduced and hGSTA4-4 transduced allografts 45 days after transplantation. Some segments of vector transduced allografts, especially the proximal end showed only a very small lumen (Fig. 4A, c) compared to native vessels (Fig. 4A, a). However, the proximal end of hGSTA4-4 transduced allografts had a relatively much larger lumen (Fig. 4A, d) than native carotid vessels (Fig. 4A, b). No total thrombosis of any vessel, control or transplanted, was seen.
Fig. 4.
Inhibition of neointima development in allografts by hGSTA4-4 expression. Circumferential neointima formation was observed in both vector transduced and hGSTA4-4 transduced allografts 45 days after transplantation. In vector control group, the proximal ends showed a very small lumen (A, c) compared to native vessels (A, a). The proximal end of hGSTA4-4 transduced allografts had a larger lumen (A, d) compared to the vector control group. The native vessels (A, a) of control group were dilated compared to the native vessels (A, b) in hGSTA4-4 group. The degree of stenosis was calculated (B). Proximal sections showed the greatest difference (n = 12, p < 0.01): 85% lumen stenosis was found in the proximal ends of the vector control group and only 47% lumen stenosis in hGSTA4-4 group. For the distal ends, there was 67% lumen stenosis in control allografts and 40% lumen stenosis (n = 12, p < 0.05) in hGSTA4-4 group. An apparent, but not statistically significant difference in stenosis was also found in the central parts of the allografts, with hGSTA4-4 group showing less stenosis. (**: p < 0.01; *: p < 0.05) (arrow: internal elastin layer of the vessel)
We then quantified the degree of stenosis caused by neointima in proximal, central and distal regions of allografts in the vector control group and the hGSTA4-4 transduced group. The degree of stenosis was calculated by comparing the area of circumferential neointima with the original lumen as defined by the internal elastin layer of the vessel. Ten sections from each region were serially selected from continuous sections and analyzed by Image J software (Fig. 4B). Since no significant difference was seen in lumen stenosis between allografts treated with PBS and vector (data not shown), a vector-treated group was used as control in subsequent experiments. Proximal sections showed the greatest difference; specifically, 85% lumen stenosis was found in the proximal ends of the vector control group and 47% lumen stenosis in hGSTA4-4 group (n = 12, p < 0.01). For the distal ends, lumen stenosis was 67% in control allografts and 40% in the hGSTA4-4 group (n = 12, p < 0.05). An apparent, but not statistically significant difference in stenosis was also found in the central parts of the allografts, with hGSTA4-4 group showing less stenosis. These results indicate that significant lumen stenosis in both proximal and distal ends in vector-treated allografts, caused by marked intimal thickening, is inhibited in hGSTA4-4 treated allografts by hGSTA4-4 overexpression.
Maintenance of carotid allograft architecture by hGSTA4-4 transduction
To determine if hGSTA4-4 inhibits neointima formation by reducing smooth muscle cell proliferation induced by allograft transplantation, we examined smooth muscle cells in intima by smooth muscle actin staining. As seen in Fig. 5A, b, smooth muscle cell proliferation in intima appeared markedly inhibited in hGSTA4-4 transduced allografts, compared to the exuberant smooth muscle cell proliferation in intima in vector control allografts (Fig. 5A, a). No discernible endothelial cell proliferation was observed in neointima of allografts in either control group (Fig. 5A, c) or hGSTA4-4 group (Fig. 5A, d). Moreover, smooth muscle cell actin staining showed that media smooth muscle cell loss was decreased in hGSTA4-4 transduced allografts (Fig. 5A, b) compared with vector control allografts. We found almost no actin staining in the media of control allografts (Fig. 5A, a). Furthermore, we examined the elastin layers of the media of allografts in our vector control group and hGSTA4-4 transduced group. Autofluorescence of the elastin layers was directly visualized under the fluorescence microscope (24). hGSTA4-4 transduced allografts displayed well-preserved architecture of the media with intact elastin layers (Fig. 5B, d), similar to that seen in the non-surgery (native) carotid vessel (Fig. 5B, b). The medial elastin layers were substantially disrupted in vector control allografts (Fig. 5B, c) compared with the native artery (Fig. 5B, a), which was consistent with the finding of loss of smooth muscle cells in the media (Fig. 5A, c). These findings suggest that overexpression of hGSTA4-4 protects allograft architecture by inhibiting smooth muscle cell proliferation in the intima, and preventing loss of smooth muscle cells and disruption of elastin layers in the media.
Fig. 5.
A: hGSTA4-4 inhibits smooth muscle cell (SMC) proliferation in intima and SMC loss in medium of allografts. SMC proliferation in intima detected by anti-hSMA antibody appeared markedly inhibited in hGSTA4-4 transduced allografts (b, between thin arrows), compared to the exuberant smooth muscle cell proliferation in intima in vector control allografts (a, between thin arrows). No discernible endothelial cell proliferation was observed in neointima of allografts in either control group (c) or hGSTA4-4 group (d). Actin staining showed that media SMC loss was decreased in hGSTA4-4 transduced allografts (b, between thick arrows), compared to that in control allografts (a, between thick arrows). B: Overexpression of hGSTA4-4 inhibits disruption of elastin layers of allografts. hGSTA4-4 transduced allografts displayed well-preserved architecture of the media with intact elastin layers (d), similar to that seen in the non-surgery side (native) carotid vessel (b). The medial elastin layers were substantially disrupted in vector control allografts (c) compared with the native artery (a).
Prevention of leukocyte infiltration in carotid allografts
We next examined the inflammatory response in carotid allografts, which plays a crucial role in formation of transplant arteriosclerosis. To test the effects of hGSTA4-4 on infiltration of leukocytes in allografts, sections from two groups were stained for CD45 (leukocytes) and CD 11b/c (macrophages). Infiltrating cells were mainly located in the neointima and adventitia. Heavy infiltration by CD45 positive leukocytes was seen in the neointima and adventitia of vector control allografts. Cell counts showed the mean number of leukocytes in control grafts (n = 12) was more than 10 times that detected in hGSTA4-4-transduced allografts (n = 12) (Fig. 6 a & b). Fig. 6c showed diffuse CD11b/c positive macrophages in neointima of vector control allografts. In contrast, macrophages were observed only occasionally in hGSTA4-4 transduced allografts (Fig. 6d). Hence, overexpression of hGSTA4-4 partially prevents leukocyte infiltration in transplant allografts.
Fig. 6.
Prevention of inflammatory cell infiltration in allografts. Heavy infiltration by CD45 positive leukocytes was seen in the neointima and adventitia of vector control allografts (a). Cell counts showed the mean number of leukocytes in control grafts (a) (n = 12) was more than 10 times that detected in hGSTA4-4-transduced allografts (b) (n = 12). Diffuse CD11b/c positive macrophages in neointima of vector control allografts were detected (c). In contrast, macrophages were observed only occasionally in hGSTA4-4 transduced allografts (d).
DISCUSSION
We have done extensive previous work that supports a key role for GSTA4-4 in protecting endothelial cells against cell injury induced by oxidative stress (16-20). To explore the therapeutic potential of hGSTA4-4 in preventing CAV, we examined its effect in a rabbit carotid artery transplant model. Ischemia and reperfusion injury activate the microvascular endothelium, leading to oxygen free radical formation with a subsequent inflammatory response, resulting in endothelial dysfunction, which further progresses to CAV (2,5,25,26). Hence, exploring the potentially beneficial effects of hGSTA4-4 in a model of vascular transplantation arteriosclerosis would be important for the understanding of pathological mechanism involved in allograft rejection, and the prevention of the development of CAV.
Investigators including ourselves have demonstrated that GSTA4-4 plays a role in protecting endothelial cells and vascular smooth muscle cells from damage by oxidants and toxins (16,27-29). Our earlier studies found that a rat α-class isozyme homologous to hGSTA4-4 efficiently catalyzes the conjugation of 4-HNE to GSH in smooth muscle cells of the aorta. In addition, this isozyme’s expression is up-regulated in a rat atherosclerosis model induced by the cardiovascular toxin, allylamine (28). Subsequent work from our laboratory showed that hGSTA4-4 is up-regulated in the early human atherosclerotic plaque, and mouse endothelial cells overexpressing GSTA-4 are resistant to oxidative-stress damage; this resistance is mediated via inhibition of apoptosis (16,30). Our recent experimental data also provide evidence that disruption of tight junctions mediated by oxidative stress in endothelial cells may be attenuated by hGSTA4-4 expression (20).
Allograft endothelial injury induced by oxidants and other factors can alter endothelial cell functions, predisposing them to arterial inflammation, thrombosis, vasoconstriction, and vascular smooth muscle proliferation. This cascade of events is presumed to start with endothelial damage, leading to a proliferative intimal response known as a fibrous atherosclerotic plaque (31).
In our study, we found that there was essentially no intimal proliferation in ex vivo cultured carotid arteries which had been transduced either with adenoviral control vector or hGSTA4-4 expressing vector. Rao et al. reported that adenoviral perfusion of the donor heart ex vivo did not affect the development of CAV in rat heart transplantation (32). More important, we found that intimal proliferation of allografts is significantly decreased in those expressing hGSTA4-4. We found that intimal thickening is more severe in proximal ends of allografts both in control and treated groups. This is similar to the previous findings of other researchers (14,34). We also observed that hGSTA4-4 more significantly reduced intimal lesions formation more in the proximal ends than in the distal ends of allografts. Smooth muscle cell migration and hyperplasia in intima are regulated by a variety of cytokines and growth factors (PDGF, EGF, TGF-β1, et al), which are released by damaged endothelial cells. Subsequently, leukocytes and macrophages are recruited and activated (35). A recent study has shown that application of clopidogrel after vascular transplantation results in a reduction in adhesion molecule and PDGF-β expression and is associated with attenuation of transplant arteriosclerosis (36).
With regard to our study, the inhibition of smooth muscle cell proliferation in hGSTA4-4 expressing allografts may be related to GST-mediated metabolism and removal, via GSH conjugation of 4-HNE, a major end product of oxidant stress and lipid peroxidation. This is supported by the finding in heart transplant recipients that a reduced antioxidant defense, mirrored by a reduction of GSHb1, is independently associated with the presence of angiographic CAV (5). We previously found that levels of 4-HNE decreased in GST-expressing cells (16). 4-HNE is believed to act as an intracellular signaling molecule (10-12). It modulates several cell-surface receptors, activates EGF receptor and PDGF-β receptor, and up-regulates TGF-β1 (8,10). Thus, our findings suggest that inhibition of neointimal formation may be induced by anti-oxidative effects through 4-HNE conjugated with GSH, resulting decreased activation of a variety growth factors and/ or grow factor receptors such as PDGF-β receptor.
Mounting evidence, based on animal models and human biopsy results, suggests that acute and persistent rejection-triggered apoptosis of endothelial cells plays a pivotal role in CAV (37-40). Apoptosis of endothelial cells, as well as infiltrating leukocytes and/or macrophages might be important in the early stages of CAV (41). We report in the present study that hGSTA4-4 significantly inhibits leukocyte and macrophage infiltration in neointima and adventitia of carotid artery allografts. Our previous work has shown that mGSTA4-4 inhibits endothelial cell apoptosis induced by 4-HNE, a relatively stable end product of lipid peroxidation and oxidative stress (16). 4-HNE stimulates several components in signal transduction pathways, such as Jun N-terminal kinase (JNK), promoting apoptosis (8,12). mGSTA4-4 protects endothelial cells from apoptosis through inhibiting the activation of JNK, Bax, and the caspase cascade (16). Patrick et al have reported that hGSTA4-4 down-regulated the expression of TGFα, c-jun, and other signaling molecules in hGSTA4-4 overexpressing epithelial cells (42). We suggest that the reaction of hGSTA4-4 with 4-HNE through GSH conjugation prevents endothelial apoptosis during initiating phases of CAV through the intrinsic pathway of apoptosis. This may be via down regulation of the important signaling molecules such as JNK stimulated Bax, and caspase cascade. Thus, hGSTA4-4 may prevent the adherence of leukocytes and/or monocytes to the endothelium, and migration of leukocytes and/or macrophages into the intima. Therefore, the development of CAV is inhibited in hGSTA4-4 expressing allografts. The effects of hGSTA4-4 on the activation cytochrome C of endothelial cells with oxidant stress is under investigation currently in our laboratory.
Increased vascular permeability caused by early endothelial damage, contributes to the initiation of allograft arteriosclerosis (43). In our recent studies we showed that hGSTA4-4 inhibits oxidative endothelial cell damage, thus preserving vascular permeability (20). Hence, hGSTA4-4 may attenuate CAV progress through multiple mechanisms, and preserve the function of allografts as shown by our ultrasonographic findings.
In more recent ongoing experiments we found that hGSTA4-4 inhibits the expression of important adhesion molecules in allografts (P-selectin, ICAM-1, VCAM-1 and VAP-1). These molecules promote adhesion of circulating leukocytes, which then cause endothelial damage by direct cytotoxicity (44). Thus, the mechanism through which hGSTA4-4 lesses inflammation might be related to both protection of tissue from oxidative stress and impairment of leukocyte migration. We need to further measure the level of cytokines and other inflammatory factors in allografts to explore the mechanism of anti-inflammation effects caused by GST.
In conclusion, our study has defined an important effect of hGSTA4-4 in attenuating transplant arteriosclerosis. hGSTA4-4 inhibits the development of neointimal formation, prevents the infiltration of leukocytes and macrophages, and mitigates architectural disruption of the allografts medium. Although the precise identification of the molecular mechanism involved in modulation of CAV by hGSTA4-4 is needed, our results indicate that overexpression of hGSTA4-4 is a potential novel therapeutic strategy to prevent or limit the development of transplant arteriosclerosis.
ACKNOWLEDGMENTS
This work is supported by National Institute of Health grants, HL65416 (PJB), ES-012171 (YCA) and IES 00676 (YCA); and grant ES006676 from National Institute of Environmental Health Sciences. The authors thank Brent Bell for assistance in this study.
Footnotes
This work is supported by National Institute of Health grants, HL65416 (Paul J. Boor), ES-012171 (Yogesh C. Awasthi) and IES 00676 (Yogesh C. Awasthi); and grant ES006676 from National Institute of Environmental Health Sciences (Paul J. Boor).
DISCLOSURE STATEMENT No competing financial interests exist.
No conflict of interests exists.
Reference List
- 1.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-second official adult heart transplant report--2005. J Heart Lung Transplant. 2005;24:945–955. doi: 10.1016/j.healun.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Aranda JM, Jr., Hill J. Cardiac transplant vasculopathy. Chest. 2000;118:1792–1800. doi: 10.1378/chest.118.6.1792. [DOI] [PubMed] [Google Scholar]
- 3.Vassalli G, Gallino A, Weis M, et al. Alloimmunity and nonimmunologic risk factors in cardiac allograft vasculopathy. Eur Heart J. 2003;24:1180–1188. doi: 10.1016/s0195-668x(03)00237-9. [DOI] [PubMed] [Google Scholar]
- 4.Davis SF, Yeung AC, Meredith IT, et al. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year posttransplant. Circulation. 1996;93:457–462. doi: 10.1161/01.cir.93.3.457. [DOI] [PubMed] [Google Scholar]
- 5.De CB, Bigi R, Campolo J, et al. Blood glutathione as a marker of cardiac allograft vasculopathy in heart transplant recipients. Clin Transplant. 2005;19:367–371. doi: 10.1111/j.1399-0012.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 6.Di GD, Di SP, Capecchi PL, Lazzerini PE, Pasini FL. Alteration in the redox state of plasma in heart-transplant patients with moderate hyperhomocysteinemia. J Lab Clin Med. 2003;142:21–28. doi: 10.1016/S0022-2143(03)00057-X. [DOI] [PubMed] [Google Scholar]
- 7.Hubatsch I, Ridderstrom M, Mannervik B. Human glutathione transferase A4-4: an alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem J. 1998;330(Pt 1):175–179. doi: 10.1042/bj3300175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Awasthi YC. Glutathione S-Transferases as Modulators of Signal Transduction. In: Awasthi YC, editor. Toxicology of Glutathione Transferases. Taylor & Francis CRC Press; Boca Raton: 2006. pp. 205–230. [Google Scholar]
- 10.Awasthi YC, Sharma R, Cheng JZ, et al. Role of 4-hydroxynonenal in stress-mediated apoptosis signaling. Mol Aspects Med. 2003;24:219–230. doi: 10.1016/s0098-2997(03)00017-7. [DOI] [PubMed] [Google Scholar]
- 11.Echtay KS, Esteves TC, Pakay JL, et al. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 13.Sharma RAGAYC. Physiological substrates of glutathione S-transferases. In: Awasthi YC, editor. Toxicology of Glutathione Transferases. Taylor & Francis CRC Press; Boca Raton: 2006. pp. 179–203. [Google Scholar]
- 14.Sihvola RK, Tikkanen JM, Krebs R, et al. Platelet-derived growth factor receptor inhibition reduces allograft arteriosclerosis of heart and aorta in cholesterol-fed rabbits. Transplantation. 2003;75:334–339. doi: 10.1097/01.TP.0000045056.82561.0F. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Dong C, Stevenson SC, et al. Overexpression of soluble fas attenuates transplant arteriosclerosis in rat aortic allografts. Circulation. 2002;106:1536–1542. doi: 10.1161/01.cir.0000027822.23269.07. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Yang Y, Trent MB, et al. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis. Atherosclerosis. 2004;173:211–221. doi: 10.1016/j.atherosclerosis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 17.He N, Singhal SS, Awasthi S, Zhao T, Boor PJ. Role of glutathione S-transferase 8-8 in allylamine resistance of vascular smooth muscle cells in vitro. Toxicol Appl Pharmacol. 1999;158:177–185. doi: 10.1006/taap.1999.8700. [DOI] [PubMed] [Google Scholar]
- 18.He NG, Awasthi S, Singhal SS, Trent MB, Boor PJ. The role of glutathione S-transferases as a defense against reactive electrophiles in the blood vessel wall. Toxicol Appl Pharmacol. 1998;152:83–89. doi: 10.1006/taap.1998.8511. [DOI] [PubMed] [Google Scholar]
- 19.Yang YXYPVLBP. Immunolocalization of GSTA4-4 in endothelial cells and possible role in protection against apoptosis and atherosclerosis. Society of Toxicology. 2008 Ref Type: Abstract. [Google Scholar]
- 20.Xu Y, Gong B, Yang Y, Awasthi YC, Woods M, Boor PJ. Glutathione-S-Transferase Protects against Oxidative Injury of Endothelial Cell Tight Junctions. Endothelium. 2007;14:333–343. doi: 10.1080/10623320701746263. [DOI] [PubMed] [Google Scholar]
- 21.Stiffey-Wilusz J, Boice JA, Ronan J, Fletcher AM, Anderson MS. An ex vivo angiogenesis assay utilizing commercial porcine carotid artery: modification of the rat aortic ring assay. Angiogenesis. 2001;4:3–9. doi: 10.1023/a:1016604327305. [DOI] [PubMed] [Google Scholar]
- 22.Tomita Y, Zhang QW, Uchida T, et al. A technique of cervical aortic graft transplantation in mice. J Heart Lung Transplant. 2001;20:699–702. doi: 10.1016/s1053-2498(00)00219-9. [DOI] [PubMed] [Google Scholar]
- 23.Gruchala M, Bhardwaj S, Pajusola K, et al. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- 24.Bigio IJ, Mourant JR. Ultraviolet and visible spectroscopies for tissue diagnostics: fluorescence spectroscopy and elastic-scattering spectroscopy. Phys Med Biol. 1997;42:803–814. doi: 10.1088/0031-9155/42/5/005. [DOI] [PubMed] [Google Scholar]
- 25.Day JD, Rayburn BK, Gaudin PB, et al. Cardiac allograft vasculopathy: the central pathogenetic role of ischemia-induced endothelial cell injury. J Heart Lung Transplant. 1995;14:S142–S149. [PubMed] [Google Scholar]
- 26.Hsieh GR, Schnickel GT, Garcia C, Shefizadeh A, Fishbein MC, Ardehali A. Inflammation/oxidation in chronic rejection: apolipoprotein a-i mimetic peptide reduces chronic rejection of transplanted hearts. Transplantation. 2007;84:238–243. doi: 10.1097/01.tp.0000268509.60200.ea. [DOI] [PubMed] [Google Scholar]
- 27.He NG, Singhal SS, Chaubey M, et al. Purification and characterization of a 4-hydroxynonenal metabolizing glutathione S-transferase isozyme from bovine pulmonary microvessel endothelial cells. Biochim Biophys Acta. 1996;1291:182–188. doi: 10.1016/s0304-4165(96)00064-5. [DOI] [PubMed] [Google Scholar]
- 28.Misra P, Srivastava SK, Singhal SS, Awasthi S, Awasthi YC, Boor PJ. Glutathione S-transferase 8-8 is localized in smooth muscle cells of rat aorta and is induced in an experimental model of atherosclerosis. Toxicol Appl Pharmacol. 1995;133:27–33. doi: 10.1006/taap.1995.1123. [DOI] [PubMed] [Google Scholar]
- 29.Zimniak L, Awasthi S, Srivastava SK, Zimniak P. Increased resistance to oxidative stress in transfected cultured cells overexpressing glutathione S-transferase mGSTA4-4. Toxicol Appl Pharmacol. 1997;143:221–229. doi: 10.1006/taap.1996.8070. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NF-kappaB translocation. Toxicol Appl Pharmacol. 2008;230:187–196. doi: 10.1016/j.taap.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valantine HA. Cardiac allograft vasculopathy: central role of endothelial injury leading to transplant “atheroma”. Transplantation. 2003;76:891–899. doi: 10.1097/01.TP.0000080981.90718.EB. [DOI] [PubMed] [Google Scholar]
- 32.Rao VP, Branzoli SE, Ricci D, et al. Recombinant adenoviral gene transfer does not affect cardiac allograft vasculopathy. J Heart Lung Transplant. 2007;26:1281–1285. doi: 10.1016/j.healun.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avery RK. Cardiac-allograft vasculopathy. N Engl J Med. 2003;349:829–830. doi: 10.1056/NEJMp038124. [DOI] [PubMed] [Google Scholar]
- 34.Johnson DE, Gao SZ, Schroeder JS, DeCampli WM, Billingham ME. The spectrum of coronary artery pathologic findings in human cardiac allografts. J Heart Transplant. 1989;8:349–359. [PubMed] [Google Scholar]
- 35.Autieri MV. Allograft-induced proliferation of vascular smooth muscle cells: potential targets for treating transplant vasculopathy. Curr Vasc Pharmacol. 2003;1:1–9. doi: 10.2174/1570161033386772. [DOI] [PubMed] [Google Scholar]
- 36.Abele S, Spriewald BM, Ramsperger-Gleixner M, et al. Attenuation of transplant arteriosclerosis with clopidogrel is associated with a reduction of infiltrating dendritic cells and macrophages in murine aortic allografts. Transplantation. 2009;87:207–216. doi: 10.1097/TP.0b013e3181938913. [DOI] [PubMed] [Google Scholar]
- 37.Cailhier JF, Laplante P, Hebert MJ. Endothelial apoptosis and chronic transplant vasculopathy: recent results, novel mechanisms. Am J Transplant. 2006;6:247–253. doi: 10.1111/j.1600-6143.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- 38.Choy JC, Kerjner A, Wong BW, McManus BM, Granville DJ. Perforin mediates endothelial cell death and resultant transplant vascular disease in cardiac allografts. Am J Pathol. 2004;165:127–133. doi: 10.1016/S0002-9440(10)63281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choy JC, Cruz RP, Kerjner A, et al. Granzyme B induces endothelial cell apoptosis and contributes to the development of transplant vascular disease. Am J Transplant. 2005;5:494–499. doi: 10.1111/j.1600-6143.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 40.Dong C, Wilson JE, Winters GL, McManus BM. Human transplant coronary artery disease: pathological evidence for Fas-mediated apoptotic cytotoxicity in allograft arteriopathy. Lab Invest. 1996;74:921–931. [PubMed] [Google Scholar]
- 41.Kabelitz D. Role of apoptosis in cardiac allograft vasculopathy. Z Kardiol. 2000;89(Suppl 9):IX/21–IX/23. doi: 10.1007/s003920070021. [DOI] [PubMed] [Google Scholar]
- 42.Patrick B, Li J, Jeyabal PV, et al. Depletion of 4-hydroxynonenal in hGSTA4-transfected HLE B-3 cells results in profound changes in gene expression. Biochem Biophys Res Commun. 2005;334:425–432. doi: 10.1016/j.bbrc.2005.06.099. [DOI] [PubMed] [Google Scholar]
- 43.Lai JC, Tranfield EM, Walker DC, et al. Ultrastructural evidence of early endothelial damage in coronary arteries of rat cardiac allografts. J Heart Lung Transplant. 2003;22:993–1004. doi: 10.1016/s1053-2498(02)01163-4. [DOI] [PubMed] [Google Scholar]
- 44.Calabro P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]