Abstract
Introduction
Male sexual dysfunction is a common complication of diabetes (DM), but the relative impact of erectile dysfunction (ED), orgasmic dysfunction (OD), and/or decreased libido (DL) on global sexual bother has not been assessed.
Aim
To assess the relationship between ED, OD, and DL and overall sexual satisfaction in men with type 1 DM, and determine which form of dysfunction causes the most bother.
Methods
The study cohort consisted of 713 men with type 1 DM who completed the Diabetes Control and Complication Trial and then participated in the follow-up Epidemiology of Diabetes Interventions and Complications Study. In year 10 of EDIC, 583 (83%) completed a validated instrument assessing ED, OD, and DL and the bother these conditions cause. Statistical tests determined the concordance of function and bother in each domain, and the impact of each domain on overall sexual satisfaction.
Main Outcome Measures
Patient-reported outcomes using responses to individual items of the International Index of Erectile Function (IIEF).
Results
ED was present in 34%, OD in 20%, and DL in 55%. When correlated with overall sexual satisfaction, ED had the highest weighted kappa (0.84, 95% confidence interval [CI] = 0.80–0.87), while OD (0.57, 95% CI = 0.51–0.63) and DL (0.55, 95%CI = 0.48–0.62) were considerably lower. Furthermore, the single item assessing confidence in getting and keeping an erection had the strongest correlation with overall sexual bother as well as specific erectile bother.
Conclusions
ED, OD, and DL are highly prevalent in men with long-standing type I diabetes. All three sexual dysfunctions cause bother in men with DM, but ED causes more general sexual bother and likely has a greater overall impact on quality of life. Our data underscore the importance of asking men with DM about their sexual function and point to the need for further research to investigate disorders of orgasm and desire.
Keywords: Male Sexual Dysfunction, Diabetes, Erectile Dysfunction, Questionnaire
Introduction
Male sexual dysfunction is a well-known complication of diabetes mellitus [1,2], and is also quite common in the general population of older men [3]. Studies of male sexual dysfunction, however, tend to focus primarily on erectile dysfunction [4]. This may not provide a complete portrait of the patient’s disease-specific health-related quality of life, as male sexual function is a mutidimensional construct comprising orgasmic/ejaculatory function and desire/libido in addition to penile erection. Most epidemiological studies of male sexual dysfunction, particularly those in men with DM, have not comprehensively assessed these other domains of sexual function.
One reason why male sexual dysfunction has not been comprehensively assessed in epidemiologic studies is the need to use multi-item scales to assess the various sexual domains and other items of interest. This often results in unusually long questionnaires that are difficult for older patients to complete. In turn, this leads to increased respondent burden, missing data, and problematic selection bias that may negatively impact the validity of study findings. To this end, it may be advantageous to attempt to capture sexual dysfunction using either a single or as few items as possible. Because erectile dysfunction is, in many respects, the easiest domain for the patient to report upon and the investigator to objectively assess, this is the domain that has been most widely studied. While this has resulted in a good understanding of the incidence of erectile function in men with sexual dysfunction, it has limited our understanding of the other elements of male sexual dysfunction.
Although prior studies have attempted to identify a single proxy item for erectile function in men [5,6], they have not assessed the influence of that single item on overall sexual bother. Furthermore, none of these studies captured ejaculatory or libido dysfunction, which may also affect sexual bother. Corona et al. [7] captured libido dysfunction, as well as erectile dysfunction, but this group failed to assess the relationship between these dysfunctions and bother. Furthermore, the study was limited by selection bias, as the entire cohort was assembled from patients who presented for sexual dysfunction treatment at an andrology clinic. To adequately address the question at hand, subject must be drawn for a more general population, and both function and bother must be assessed. Gill and Feinstein [8] have documented the importance of incorporating both the degree of dysfunction and also the amount of bother, as this provides the most comprehensive understanding of the relationship between the medical condition under study and the patient’s quality of life.
Aims
The goal of the current study was to utilize patient-centered data on male sexual function to determine the prevalence of erectile, orgasmic/ejaculatory, and libido/desire dysfunction in a cohort of men with type I DM. Importantly, we attempted to determine the differential impact on each of these domains on global sexual bother. Finally, we sought to identify a single item that could accurately assess global sexual dysfunction and bother in large epidemiologic studies. To achieve these goals, we analyzed International Index of Erectile Function (IIEF) [9] data from men who participated in the UroEDIC study, a cross-sectional ancillary study of The Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Intervention and Complication (EDIC) Study.
Materials and Methods
The DCCT and EDIC Study
The DCCT randomly assigned 761 male and 680 female patients with type 1 diabetes to intensive or conventional therapy, treating them for a mean of 6.5 years between 1983 and 1993 [10]. The primary prevention cohort consisted of subjects with no retinopathy or neuropathy, urinary albumin excretion rate (AER) <40 mg per 24 hours, and diabetes duration of 1–5 years. The secondary intervention cohort consisted of 715 subjects who had nonproliferative retinopathy, urinary AER ≤200 mg per 24 hours, and diabetes duration of 1–15 years. Individuals were excluded if they had hypertension (defined by systolic ≥140 or diastolic ≥90 mm Hg), symptomatic ischemic heart disease, or symptomatic peripheral neuropathy.
In 1994, of the surviving cohort of 746 men enrolled in DCCT at closeout, 720 (96%) elected to participate in the annual examinations of the EDIC follow-up study [11]. During year 10 of EDIC, of the 713 men still active in the trial, 591 (83%) men agreed to participate in UroEDIC, an ancillary study of urological symptoms. Of these, 571 men provided data on erectile function and comprise the study population for the current analyses. The institutional review board of each participating center approved the study, and the Federal Government issued a Certificate of Confidentiality.
DCCT Intervention and Other Therapies
Intensive treatment consisted of insulin administered three or more times per day by injection or by continuous subcutaneous infusion with an external pump, with dose adjustments based on at least four self-monitored glucose measurements per day. Daily glucose goals were 70–120 mg per deciliter (3.9–6.7 mmol per liter) before meals and peak levels of less than 180 mg per deciliter (10.0 mmol per liter) after meals. The goal for hemoglobin A1c was less than 6.05%—2 standard deviation (SD) above the mean value for persons without diabetes. Conventional therapy had no glucose goals beyond those needed to prevent symptoms of hyperglycemia and hypoglycemia, and consisted of one or two daily injections of insulin.
The subjects were randomized to receive either intensive or conventional therapy. Intensive treatment consisted of insulin administered three or more times per day by injection or by continuous subcutaneous infusion with an external pump. Conventional therapy consisted of one or two daily insulin injections. Treatment goals have been previously described [10]. The DCCT was terminated in 1993, when the principal study question concerning treatment effects had been answered. The mean duration of follow-up in DCCT was 6.5 years. In 1994, 1,394 DCCT subjects (96% of the original cohort) agreed to participate in the EDIC follow up study that included annual examinations and completion of a standardized questionnaire for complication status [11].
Main Outcome Measures
As part of UroEDIC, all the male subjects completed the IIEF, a validated and reliable questionnaire that assesses sexual function [9]. Additional validated questions measuring symptom impact were also included [12].
Of the 591 men who consented to participate in the UroEDIC study, 571 (97%) completed all of the ED questions. An additional 14 (2%) subjects completed at least part of the questionnaire, and were included in portions of the analysis when possible. Six (1%) of the UroEDIC subjects did not complete any of the ED items, and, therefore, were excluded from this analysis of sexual function. The 571 UroEDIC men who provided complete information with respect to their sexual function and bother were comparable to 142 men who did not participate in UroEDIC, with the exception that the participants had a lower mean blood pressure at EDIC Year 10 (94.4 vs. 92.3 mm Hg, P = 0.025).
Definitions of Male Sexual Dysfunction
Sexual dysfunction was defined a priori using the complete functional domain scales of the iief for erectile function (EF), desire, and orgasmic function. Therefore, responses to the IIEF were initially scored according to scoring instructions, which have been published previously [9]. Erectile dysfunction was defined as a score of <20 on the IIEF EF domain. We elected to use a more conservative definition of ED to ensure that we captured truly clinically significant ED in our cohort of men with diabetes in the general population. To this end, we included men with “normal” erectile function, those with mild ED, and some with mild/moderate ED according to Cappelleri et al. [13] in the no ED group. It should be noted that Cappelleri et al. proposed a cut-off of 22 in their original analysis as a suggested threshold, although they cautioned that “clinical validation with selfrated assessments of ED severity is warranted” to ensure the validity of this threshold. Low sexual desire was defined as a score <7 on the desire domain of the IIEF. Orgasmic Dysfunction was defined as score <7 on the orgasmic domain of the IIEF. Because there are no published studies defining the optimal threshold for defining clinically meaningful dysfunction with these scales, the choice of these thresholds was based upon two clinicians review of the instrument (HBW and DFP) to ensure the face validity of the definition.
Statistical Analysis
The primary outcome of interest in this cross-sectional study was sexual function as defined by responses to items in individual functional domains of the IIEF. Descriptive statistics were used to described baseline characteristics and overall sexual function of the cohort. To assess the relationship of functional items to bother (the degree to which the subject feels that dysfunction is a problem) in the individual sexual domains of erection, desire and ejaculation, Spearman’s correlation coefficient was calculated. Testing of statistical significance was performed using the Cochran–Mantel–Haenszel test for correlation. Similarly, the relationship between individual function items and the global sexual bother domain was assessed using similar methods. Finally, concordance between responses to individual items in certain functional and bother domains were compared using the weighted kappa statistic for multilevel categorical variables. All analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC, USA).
Results
Table 1 presents the clinical characteristics of the 571 men who completed the entire IIEF. The majority of subjects were over age 40 (78%), with most of these being between age 40 and 49 (300, 53% of total). Most of the men were white and were married. The mean duration of diabetes for the cohort was 22.1 years. The time weighted mean hemoglobin A1c level for the cohort over the course of the DCCT/EDIC studies was 8.07%. Not surprisingly, a considerable number of men with diabetes in the UroEDIC study reported sexual dysfunction of some form. The mean score in the erectile function domain of the IIEF was 21.3 (SD = 10.5, range 0–30), 7.1 (SD = 1.9, range 2–10) in the sexual desire domain, and 8.3 (SD = 2.9, range 0–10) in the orgasmic function domain. As shown in Table 1, while 58% of the cohort reported no erectile dysfunction, 34% reported an IIEF score from 0 to 20, and 9% reported that they were not sexually active in the past month. More than half the cohort (55%) reported decreased sexual desire, and 20% reported abnormal ejaculatory function. Individual responses to the sexual bother items are shown in Table 2. The majority of men reported no problem in the global sexual bother domain or the individual domains of erectile, desire/libido, or ejaculatory bother.
Table 1.
Clinical characteristics and sexual function of 571 men with diabetes who completed the International Index of Erectile Function (IIEF) in its entirety as part of the uroEDIC study
| Category | Response | n (%) |
|---|---|---|
| Age | ||
| 20–29 | 3 (1) | |
| 30–39 | 128 (22) | |
| 40–49 | 300 (53) | |
| 50 or more | 140 (25) | |
| Race | ||
| White | 551 (96) | |
| Other | 20 (4) | |
| Married | ||
| Yes | 431 (75) | |
| No | 125 (22) | |
| Other/no response | 15 (34) | |
| Retinopathy | ||
| None or nonproliferative (ETDRS <12) | 340 (60) | |
| Proliferative (ETDRS ≥12) | 231 (40) | |
| Nephropathy | ||
| None (albumin excretion rate [AER] <40 mg/24 hour) | 411 (72) | |
| Microalbuminuria (40 ≤ AER < 300) | 102 (18) | |
| Albuminuria (AER ≥300) | 58 (10) | |
| Hypertension (sitting sBP ≥140 mm Hg and/or dBP ≥90 mm Hg or use of antihypertensive medication) | ||
| No | 313 (55) | |
| Yes | 258 (45) | |
| Peripheral neuropathy ever during DCCT and EDIC* | ||
| No | 384 (67) | |
| Yes | 187 (33) | |
| Coronary calcification | ||
| None | 357 (63) | |
| Any | 214 (37) | |
| Erectile dysfunction | ||
| Not sexually active | 49 (9) | |
| Yes (IIEF score 0–20) | 193 (34) | |
| No (IIEF score 21 or higher) | 329 (58) | |
| Sexual desire score | ||
| 0–7 (abnormal) | 315 (55) | |
| 8 or higher (normal) | 256 (45) | |
| Orgasmic function score | ||
| 0–7 (abnormal) | 114 (20) | |
| 8 or higher (normal) | 457 (80) | |
Defined in DCCT by the presence of definite clinically evident distal symmetrical polyneuropathy and an abnormal nerve conduction study or in EDIC by MNSI >6 positive responses on the questionnaire or a score >2 on the examination.
DCCT = Diabetes Control and Complications Trial; EDIC = Epidemiology of Diabetes Intervention and Complication; ETDRS = Early Treatment Diabetic Retinopathy Study; MNSI = Michigan Neuropathy Screening Index.
Table 2.
Distribution of individual responses to the bother/quality of life items in the 585 men who completed this portion of the survey in the UroEDIC study (percentages may not add up to 100% due to rounding error)
| Item | Response | Number responding |
Percentage responding |
|---|---|---|---|
| Global sexual bother: | No problem | 322 | 55 |
| Overall, how big a problem has your sexual function been for you during the last 4 weeks? |
Very small problem | 87 | 15 |
| Small problem | 61 | 10 | |
| Moderate problem | 69 | 12 | |
| Big problem | 46 | 8 | |
| Erectile bother: | No problem | 339 | 58 |
| Overall, how big a problem has your erectile function been for you during the last 4 weeks? |
Very small problem | 81 | 14 |
| Small problem | 46 | 8 | |
| Moderate problem | 69 | 12 | |
| Big problem | 50 | 9 | |
| Desire/libido bother: | No problem | 379 | 65 |
| Overall, how big a problem has your sexual desire been for you during the last 4 weeks? |
Very small problem | 87 | 15 |
| Small problem | 50 | 9 | |
| Moderate problem | 54 | 9 | |
| Big problem | 15 | 3 | |
| Ejaculation bother: | No problem | 413 | 71 |
| Overall, how big a problem has your orgasm and ejaculation been for you during the last 4 weeks? |
Very small problem | 69 | 12 |
| Small problem | 37 | 6 | |
| Moderate problem | 40 | 7 | |
| Big problem | 26 | 4 |
EDIC = Epidemiology of Diabetes Intervention and Complication.
Prior to exploring the effect of the individual sexual domains (erection, desire/libido, and orgasm) on the global sexual domain, we assessed the relationship of individual items within each domain with function in that particular domain. The goal of this analysis was to determine which individual functional items correlated most closely with patient bother. The results of these analyses are shown in Table 3. In the erectile domain, the item that queries the subject to rate his confidence that he could get and keep an erection correlated quite highly with the degree of bother in the erectile domain (Spearman’s correlation coefficient = 0.80, P = 0.02). The other items in the erectile domain correlated well with bother, with correlation coefficients varying from 0.52 to 0.68 (all P < 0.05); none approached the degree of correlation between the “confidence” item and erectile bother. Interestingly, as shown in Table 3, functional items in the desire and orgasm domains did correlate with bother in those domains, but much less closely.
Table 3.
Correlation between individual items and their related bother domains in 571 men with diabetes who completed the International Index of Erectile Function as part of the UroEDIC study
| Specific bother domain |
Individual item: Over the past 4 weeks … | Number of respondents |
Spearman’s correlation coefficient |
P value |
|---|---|---|---|---|
| Erectile | Item 1: How often were you able to get an erection during sexual activity? | 583 | 0.64 | <0.001 |
| Erectile | Item 2: When you had erections with sexual stimulation, how often were your erections hard enough for penetration? |
583 | 0.68 | <0.001 |
| Erectile | Item 3: When you attempted sexual intercourse, how often were you able to penetrate (enter) your partner? |
582 | 0.53 | <0.001 |
| Erectile | Item 4: During sexual intercourse, how often were you able to maintain your erection after you had penetrated (entered) your partner? |
582 | 0.54 | <0.001 |
| Erectile | Item 5: During sexual intercourse, how difficult was it to maintain your erection to completion of intercourse? |
579 | 0.58 | <0.001 |
| Erectile | Item 6: How would you rate your confidence that you get and keep your erection? |
571 | 0.80 | <0.001 |
| Desire/libido | Item 7: How often have you felt sexual desire? | 584 | 0.28 | <0.001 |
| Desire/libido | Item 8: How would you rate your level of sexual desire? | 585 | 0.43 | <0.001 |
| Ejaculatory | Item 9: When you had sexual stimulation or intercourse, how often did you ejaculate? |
587 | 0.43 | <0.001 |
| Ejaculatory | Item 10: When you had sexual stimulation or intercourse, how often did you have the feeling of orgasm or climax? |
584 | 0.45 | <0.001 |
| Ejaculatory | Item 11: Do you ejaculate? | 582 | 0.47 | <0.001 |
EDIC = Epidemiology of Diabetes Intervention and Complication.
To assess the relationship of erectile, desire, and orgasmic bother with the global sexual bother domain, we performed weighted kappa tests of concordance. As shown in Table 4, erectile bother had the highest concordance with global sexual bother (weighted kappa = 0.83). While concordance rates for responses to the items on bother due to sexual desire and orgasm/ejaculation were acceptable (0.53 and 0.56, respectively), they were both substantially lower than that seen for problems due to erectile function.
Table 4.
Concordance between individual erectile, desire, and ejaculatory bother domains and global sexual bother domain in 571 men with diabetes who completed the International Index of Erectile Function as part of the UroEDIC study
| Individual domain | Weighted kappa |
95% confidence interval |
|---|---|---|
| Erectile bother | 0.83 | 0.79–0.86 |
| Desire/libido bother | 0.53 | 0.47–0.59 |
| Ejaculatory bother | 0.56 | 0.51–0.62 |
EDIC = Epidemiology of Diabetes Intervention and Complication.
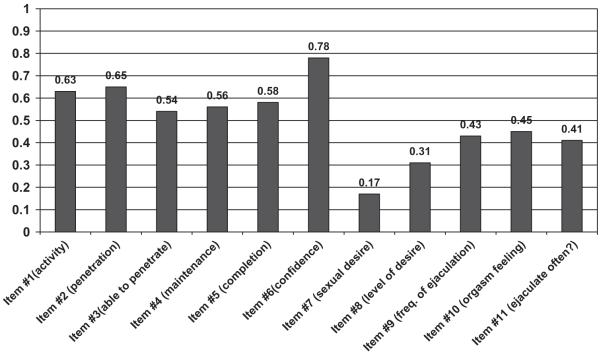
Given the findings that the functional item on confidence in erections correlated most closely with bother in the erectile domain, and that erectile bother was the bother domain most closely related to overall sexual bother, the final analysis sought to assess the relationship between the various functional items and a single global sexual bother item. It was our hypothesis that of all the functional items, the erectile confidence item would most closely correlate with the global sexual bother item. As shown in Figure 1, the results confirm this hypothesis. Of the 11 functional domain items on the IIEF, the erectile confidence item correlated most closely with global sexual bother (Spearman’s correlation coefficient = 0.78, P < 0.001). In fact, as shown in Figure 1, correlation coefficients for each of the erectile function items were higher than those seen for the libido/desire or orgasmic/ejaculatory function items.
Figure 1.
Correlation between individual items and their related bother domains in men with diabetes who completed the International Index of Erectile Function as part of the UroEDIC study. EDIC = Epidemiology of Diabetes Intervention and Complication.
Discussion
Data from UroEDIC indicate that sexual dysfunction is common even in relatively young men with type I DM. This finding is not surprising and confirms the work of others [3,14,15]. Unlike other studies, however, UroEDIC provides greater detail into the individual forms of sexual dysfunction that occur in these patients. Some-what surprisingly, decreased libido (55% of cohort) is a more common finding than either ED (34%) or orgasmic dysfunction (20%). Prior studies have tended to group men with type I and II diabetes into a single population, and have found a lower prevalence of decreased sexual drive. For example, Burke et al. [16] analyzed data from Olmsted County, Minnesota, and found that 40% of men with diabetes reported decreased sexual drive. These patients, however, were considerably older (mean age 53.1 years) than the UroEDIC cohort. In a subgroup analysis from the Olmsted County database, only 22% of men age 40–49 and 33% age 50–59 reported decreased sexual libido. Similarly, while 31% of the Olmsted County cohort reported ejaculatory dysfunction, younger men (11% of age 40–49 and 21% of men age 50–59) reported considerably less.
The data from the UroEDIC study help us to understand which of the three individual domains of sexual dysfunction influence global sexual bother the most in these patients. Specifically, it appears as though ED has a greater influence on global sexual bother than either decreased libido or ejaculatory problems, although ED is less common than decreased libido. In addition to the ED’s influence on sexual bother, it has also been shown to be an important predictor of future coronary artery disease in men with diabetes [17,18]. It should be noted that previous studies have noted that certain forms of ejaculatory dysfunction, specifically premature ejaculation, have a significant impact on sexual bother as well and that most men only seek help for ejaculatory dysfunction after they have ED [19-21]. However, all these studies use cohorts assembled from men presenting to andrology clinics for treatment, and are not as applicable to the general population as the current study. Because the UroEDIC dataset is restricted to type I diabetes and the subjects are younger, it probably represents a more accurate portrait of the prevalence of the various forms of male sexual dysfunction in men with type I diabetes, and possibly the more general population.
Data from the uroEDIC study demonstrate that a single item that assesses the confidence that a man has in his ability to get and keep and erection can be used as a proxy item for global sexual function and bother in large observational studies. This item correlates well with bother due to erectile problems, and, more importantly, with global sexual bother. In fact, of the 11 items of the IIEF, the confidence item has the highest correlation with global sexual bother. This is not to say that it is not important to assess function and bother in the OD or DL domains. Rather, in large clinical and epidemiologic studies of male sexual dysfunction, where long questionnaires may result in increased respondent burden and missing data, it may be reasonable to use the single confidence item from the IIEF as a proxy for sexual function and bother. It should also be noted that it is probably best to reserve the use the single confidence item for assessment of sexual dysfunction to large epidemiological studies, which are susceptible to respondent bias if longer multi-item questionnaires are employed. In smaller clinical trial settings, it preferable to utilize a more detailed assessment tool, such as the full IIEF.
Previous large epidemiologic studies have used both multi-attribute scales and single items to assess male sexual dysfunction [6,22,23]. Those which have used multi-attribute scales have explored outcomes in the erectile, desire/libido and ejaculation domains. For example, the Multinational Survey on the Aging Male (MSAM-7) [22] included the IIEF, and the National Health and Social Life Survey (NHSLS) [23] included an interviewer-administered 7-item scale that addressed all of these domains. While the response rate for the NHSLS was an acceptable 79%, this was likely related to the use of face-to-face interviews, which is not always feasible in large studies. The MSAM-7 study [22] used patient-completed paper instruments that were returned by mail, and evaluable response rates were much lower (37%), due at least in part to the length of the questionnaires and the sensitive nature of the topics studied. This illustrates the potential for selection bias due to missing data that may be associated with the use of long multi-attribute scales in large, epidemiologic studies.
Others have used single items to capture male sexual function, but have primarily focused upon erectile function, failing to capture either dysfunction in the other sexual domains or the degree of bother that the dysfunction may cause. Numerous researchers have used data from the Massachusetts Male Aging Study (MMAS) [24] to document the relationship between an interviewer-administered single item on erectile function and both clinician assessment of ED and multi-item scales assessing ED. O’Donnell and colleagues [5] compared patient responses to this single with urologist examination in 139 men from the MMAS. They found a high correlation between the interviewer-administered item and clinician evaluation (0.80). Derby and colleagues [6] compared responses to a single item assessing erections with summary scores from the IIEF or the Brief Male Sexual Function Inventory in 505 men in the MMAS and found correlation coefficients ranging from 0.71 to 0.78. These reports document that single erectile function items can be used to accurately categorize the degree of erectile dysfunction in large epidemiologic studies. However, these studies fail to demonstrate that a single item on erectile function can serve as a proxy for other forms of male sexual dysfunction or can incorporate degree of global sexual bother that a patient experiences.
The current study builds upon the existing literature by addressing these limitations. Specifically, the UroEDIC analysis demonstrates that a single item that assesses the subjects’ confidence in getting and keeping an erection can assess both erectile function but also erectile and global sexual bother. This item is inherently different from prior single items in that earlier items tend to focus on the frequency of erection and/or the severity of erectile dysfunction rather than the subject’s perception of his ability to have an erection. In an analysis of the DCCT/EDIC cohort, the item appears to have some face validity. Men who reported microvascular complications, such as retinopathy or nephropathy, were more like to report sexual dysfunction as measured by the single item than men without these complications (59 vs. 35% and 23 vs. 6%, respectively [25]. In addition, the current study also documents that the erectile bother domain is the sexual domain that most closely correlates with global sexual bother. Therefore, a single item that is closely correlated with both the erectile and global sexual bother domains may also serve as a proxy for bother from ejaculatory and desire/libido dysfunction.
While the current study has the advantage of using validated and reliable instruments and having an impressive response rate, it is not without its limitations. Specifically, all of the subjects in the current study have type I diabetes, which may affect generalizability of the study to the larger population of men with type II diabetes. Penson and colleagues [14] have previously shown that men with diabetes and erectile dysfunction present with more severe erectile dysfunction than the general population. However, no differences were noted in orgasmic function, sexual desire, or overall sexual satisfaction between impotent men with diabetes and men with erectile dysfunction presumably due to other causes. While this earlier study [14] does not conclusively prove that impotent men with diabetes perceive their sexuality the same way as impotent men without diabetes, it does provide preliminary evidence that many of the quality of life effects are similar. In addition, the cross-sectional study design prevents us from observing longitudinal changes in erectile function to further strengthen our contention that the single item identified functions well over time. Acknowledging these limitations—while it is probably reasonable to conclude that the single item that assesses a man’s confidence in his ability to get and maintain an erection will perform equally well in the general population of men with erectile dysfunction—additional studies documenting this are needed.
In addition, the present study does not include any information on possible underlying hypogonadism in these patients. Serum testosterone assessment was not included in either the DCCT or the EDIC study. Prior studies of hypogonadism in men with type II diabetes have documented that at least 17% of men over the age of 30 with type II diabetes have serum total testosterone levels below 8 nmol/L [26]. Others have noted similar findings [27]. However, it should be noted that profound differences exist between the androgen milieu of type I and type II diabetes, as shown in a recent study [28]. In contrast to type II diabetes with frequent occurrence of hypogonadotrophic hypogonadism, type 1 diabetes is associated with normal total testosterone concentrations. Free testosterone, luteinizing hormone, and follicle-stimulating hormone concentrations also tended to be normal. While it is likely that many of the patients in UroEDIC who suffer from decreased libido have abnormally low testosterone levels that would provide an organic explanation for their dysfunction, some of the observed decreased sexual drive may be psychogenic in nature. Further research is needed on this topic.
Conclusions
The current study documents that decreased libido, orgasmic dysfunction and erectile dysfunction are all common in men with type I diabetes. Contrary to what one might expect, decreased libido, as opposed to ED, was the most common form of sexual dysfunction noted in these patients. However, ED had a greater impact on global sexual bother than DL or OD. These data also indicate that a single validated item that assesses the confidence that a man has in his ability to get and keep an erection can be used as a proxy item for global sexual function and bother in large observational studies. Of all of the items on the IIEF, the confidence item correlates most closely not just with erectile bother, but with global sexual bother. Administration of this single patient-completed item in epidemiologic studies will provide researchers with a composite estimate of both of erectile function and global sexual bother, while minimizing respondent burden and selection bias and avoiding the use of interviewer-administered surveys.
Acknowledgments
Supported by contracts with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases and by the General Clinical Research Centers Program, National Center for Research Resources.
Footnotes
Conflict of Interest: None.
Statement of Authorship
-
Conception and DesignDavid F. Penson; Hunter Wessells
-
Acquisition of DataPatricia Cleary
-
Analysis and Interpretation of DataDavid F. Penson; Hunter Wessells; Patricia Cleary; Brandy N. Rutledge
-
Drafting the ArticleDavid F. Penson; Hunter Wessells; Brandy N. Rutledge
-
Revising It for Intellectual ContentDavid F. Penson; Hunter Wessells; Brandy N. Rutledge; Patricia Cleary
-
Final Approval of the Completed ArticleDavid F. Penson; Hunter Wessells; Brandy N. Rutledge; Patricia Cleary
References
- 1.Schiel R, Muller UA. Prevalence of sexual disorders in a selection-free diabetic population (JEVIN) Diabetes Res Clin Pract. 1999;44:115–21. doi: 10.1016/s0168-8227(99)00012-1. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo-Tamola J, Chitaley K. Type 2 diabetes mellitus and erectile dysfunction. J Sex Med. 2009;6:916–26. doi: 10.1111/j.1743-6109.2008.01116.x. [DOI] [PubMed] [Google Scholar]
- 3.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. Jama. 1999;281:537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 4.Latini DM, Penson DF, Lubeck DP, Wallace KL, Henning JM, Lue TF. Longitudinal differences in disease specific quality of life in men with erectile dysfunction: results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction study. J Urol. 2003;169:1437–42. doi: 10.1097/01.ju.0000049203.33463.9e. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell AB, Araujo AB, Goldstein I, McKinlay JB. The validity of a single-question self-report of erectile dysfunction. Results from the Massachusetts Male Aging Study. J Gen Intern Med. 2005;20:515–9. doi: 10.1111/j.1525-1497.2005.0076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology. 2000;56:302–6. doi: 10.1016/s0090-4295(00)00614-2. [DOI] [PubMed] [Google Scholar]
- 7.Corona G, Mannucci E, Mansani R, Petrone L, Bartolini M, Giommi R, Forti G, Maggi M. Organic, relational and psychological factors in erectile dysfunction in men with diabetes mellitus. Eur Urol. 2004;46:222–8. doi: 10.1016/j.eururo.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Gill TM, Feinstein AR. A critical appraisal of the quality-of-life measurements. JAMA. 1994;272:619–26. [PubMed] [Google Scholar]
- 9.Rosen R, Riley A, Wagner G, Osterloh I, Kirk-patrick J, Mishra A. The International Index of Erectile Function(IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan ME, Thompson CS, Dashwood MR, Khan MA, Jeremy JY, Morgan RJ, Mikhailidis DP. Nitric oxide and penile erection: Is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43:658–65. doi: 10.1016/s0008-6363(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 12.Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159–66. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–51. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 14.Penson DF, Latini DM, Lubeck DP, Wallace KL, Henning JM, Lue TF. Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care. 2003;26:1093–9. doi: 10.2337/diacare.26.4.1093. [DOI] [PubMed] [Google Scholar]
- 15.Malavige LS, Jayaratne SD, Kathriarachchi ST, Sivayogan S, Fernando DJ, Levy JC. Erectile dysfunction among men with diabetes is strongly associated with premature ejaculation and reduced libido. J Sex Med. 2008;5:2125–34. doi: 10.1111/j.1743-6109.2008.00907.x. [DOI] [PubMed] [Google Scholar]
- 16.Burke JP, Jacobson DJ, McGree ME, Nehra A, Roberts RO, Girman CJ, Lieber MM, Jacobsen SJ. Diabetes and sexual dysfunction: Results from the Olmsted county study of urinary symptoms and health status among men. J Urol. 2007;177:1438–42. doi: 10.1016/j.juro.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 17.Ma RC, So WY, Yang X, Yu LW, Kong AP, Ko GT, Chow CC, Cockram CS, Chan JC, Tong PC. Erectile dysfunction predicts coronary heart disease in type 2 diabetes. J Am Coll Cardiol. 2008;51:2045–50. doi: 10.1016/j.jacc.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 18.Chew KK, Bremner A, Jamrozik K, Earle C, Stuckey B. Male erectile dysfunction and cardiovascular disease: Is there an intimate nexus? J Sex Med. 2008;5:928–34. doi: 10.1111/j.1743-6109.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 19.Corona G, Petrone L, Mannucci E, Jannini EA, Mansani R, Magini A, Giommi R, Forti G, Maggi M. Psycho-biological correlates of rapid ejaculation in patients attending an andrologic unit for sexual dysfunctions. Eur Urol. 2004;46:615–22. doi: 10.1016/j.eururo.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Corona G, Petrone L, Mannucci E, Magini A, Lotti F, Ricca V, Chiarini V, Forti G, Maggi M. Assessment of the relational factor in male patients consulting for sexual dysfunction: the concept of couple sexual dysfunction. J Androl. 2006;27:795–801. doi: 10.2164/jandrol.106.000638. [DOI] [PubMed] [Google Scholar]
- 21.Waldinger MD. The neurobiological approach to premature ejaculation. J Urol. 2002;168:2359–67. doi: 10.1016/S0022-5347(05)64146-8. [DOI] [PubMed] [Google Scholar]
- 22.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, O’Leary MP, Puppo P, Robertson C, Giuliano F. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–49. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Laumann EO, Paik A, Rosen RC. The epidemiology of erectile dysfunction: results from the National Health and Social Life Survey. Int J Impot Res. 1999;11:S60–4. doi: 10.1038/sj.ijir.3900487. [DOI] [PubMed] [Google Scholar]
- 24.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Construction of a surrogate variable for impotence in the Massachusetts Male Aging Study. J Clin Epidemiol. 1994;47:457–67. doi: 10.1016/0895-4356(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 25.Wessells HB, Penson DF, Cleary P, Rutledge BN, Lachin JM, Chan KL, McVary KT, Schade D, Sarma A. Effect of intensive glycemic therapy on erectile function in men with type 1 diabetes in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Ann Intern Med. 2009 in press. [Google Scholar]
- 26.Corona G, Mannucci E, Petrone L, Ricca V, Balercia C, Mansani R, Chiarini V, Giommi R, Forti G, Maggi M. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res. 2006;18:190–7. doi: 10.1038/sj.ijir.3901391. [DOI] [PubMed] [Google Scholar]
- 27.Corona G, Mannucci E, Petrone L, Balercia G, Paggi F, Fisher AD, Lotti F, Chiarini V, Fedele D, Forti G, Maggi M. NCEP-ATPIII-defined metabolic syndrome, type 2 diabetes mellitus, and prevalence of hypogonadism in male patients with sexual dysfunction. J Sex Med. 2007;4:1038–45. doi: 10.1111/j.1743-6109.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 28.Tomar R, Dhindsa S, Chaudhuri A, Mohanty P, Garg R, Dandona P. Contrasting testosterone concentrations in type 1 and type 2 diabetes. Diabetes Care. 2006;29:1120–2. doi: 10.2337/diacare.2951120. [DOI] [PubMed] [Google Scholar]