Abstract
Angiotensin II (Ang II) produces inflammation and endothelial dysfunction in blood vessels. We tested the hypothesis that interleukin 10 (IL-10), an anti-inflammatory cytokine, protects against Ang II-induced vascular dysfunction. Responses of carotid arteries from wild-type and IL-10 deficient mice (IL-10−/−) were examined in vitro following overnight incubation with vehicle or Ang II (1 nmol/L). In arteries from wild-type mice, acetylcholine (an endothelium-dependent agonist) produced relaxation that was not affected by Ang II. In contrast, relaxation to acetylcholine in arteries from IL-10−/− mice was reduced by >50% by Ang II (P<0.05) and this effect was prevented by a scavenger of superoxide. Vascular superoxide increased ~2-fold (P<0.05) following treatment with Ang II in IL-10−/− mice but not in wild-type. Following systemic administration of Ang II (1.4 mg/kg per day for 10 days), Ang II produced modest impairment of endothelial function in wild-type mice but marked impairment in IL-10−/− mice (P<0.05) that was reversed by a superoxide scavenger. Increases in arterial pressure in response to Ang II were similar in wild-type and IL-10−/− mice. These findings provide the first evidence that endogenous IL-10 limits Ang II-mediated oxidative stress and vascular dysfunction both in vitro and in vivo suggesting that at least some of the protective effects of IL-10 may occur within the vessel wall.
Keywords: carotid artery, oxidative stress, inflammation, endothelium, genetically-altered mice
INTRODUCTION
The renin-angiotensin system plays a major role in vascular biology, particularly in relation to changes that occur in blood vessels with disease.1,2 While it is well established that angiotensin II (Ang II) produces oxidative stress in blood vessels,2-4 relatively little is known regarding molecular mechanisms that protect the vasculature from locally produced Ang II or during Ang II-dependent hypertension.
Components of the inflammatory response are activated within the vessel wall in diverse cardiovascular diseases including hypertension.4,5 Ang II (which plays a critical role in some forms of hypertension) activates multiple inflammatory mechanisms within vascular cells.4-7 For example, Ang II promotes activation of the transcription factor nuclear factor (NF)-κB (NF-κB), a major regulator of many inflammatory-related genes including IL-6 which is a mediator of Ang II-induced endothelial dysfunction.5,6,8-12 Recent studies suggest the anti-inflammatory cytokine interleukin-10 (IL-10)10-12 may be a key mediator of vascular protection in atherosclerosis and diabetes.13,14 Although Ang II activates components of the inflammatory cascade in vascular cells and IL-10 is a potent anti-inflammatory cytokine in other systems,10-12 nothing is known regarding the potential role of endogenous IL-10 in hypertension.
Our study had several goals. First, we used gene-targeted IL-10 deficient mice to examine the hypothesis that endogenous IL-10 inhibits Ang II-induced expression of proinflammatory cytokines (including IL-6) and vascular dysfunction. Second, in addition to testing the role of IL-10 in a model of Ang II-dependent hypertension, we examined the impact of IL-10 when the vessel wall was exposed to Ang II locally. Third, we determined if the mechanism of IL-10-mediated protection involved inhibition of Ang II-induced oxidative stress following local or systemic treatment with Ang II.
METHODS
Experimental animals
The animal protocol used in these experiments was reviewed and approved by the University of Iowa Animal Care and Use Committee. IL-10 deficient mice (IL-10−/−) in our colony have been back-crossed more than 12 generations onto the C57BL/6 strain and thus C57BL/6 mice were used as wild-type controls. Additional IL-10 deficient mice on the C57BL/6 background were obtained from the Jackson Laboratories. Mice were fed regular chow and water was available ad libitum.
For studies examining systemic effects of Ang II, mice were infused for 10 days with vehicle (saline) or a commonly used pressor dose of Ang II (1.4 mg/kg per day).15 Briefly, mice were anesthetized with ketamine and xylazine (87.5 and 12.5 mg/kg, respectively, ip) and an osmotic minipump (Alzet Model 1002, Durect Corporation, Cupertino, CA) containing either vehicle or Ang II was implanted subcutaneously as described.15 Systolic blood pressure was measured in conscious mice using tail-cuff plethysmography (BP-2000 Visitech Systems, Apex, NC).15 Mice were trained for five days, following which blood pressure was measured one day before (Day 0) and on Day 10 of vehicle or Ang II infusion. Following the last day of blood pressure measurement, carotid arteries and aorta were harvested for examination as described below.
Studies of blood vessels
Methods used to measure responses of carotid arteries in mice have been described in detail previously.16 Briefly, mice were euthanized with pentobarbital (100-150 mg/kg, ip) followed by removal of both carotid arteries and the thoracic aorta. Arteries were placed in Krebs buffer, loose connective tissue was removed and vessels were cut into rings which were then connected to force transducers to measure isometric tension in an organ bath containing Krebs solution maintained at 37°C. Resting tension was increased stepwise to reach a final tension of 0.25 g and the rings were allowed to equilibrate for 45 minutes.
Methods used for acute overnight incubation of vessels with Ang II have been described previously in detail.3 Briefly, carotid arteries and aorta from control and IL-10 deficient mice were obtained as described above and were incubated with either vehicle (ddH2O) or Ang II (1 nmol/L) for 22 hours at 37°C.
Experimental protocols
Relaxation of carotid arteries in response to acetylcholine (an endothelium-dependent agonist) and nitroprusside (an endothelium-independent agonist) was measured following submaximal precontraction using the thromboxane analog, U46619 (9,11-dideoxy-11a,9a-epoxy-methanoprostoglandin-F2α). At the end of each experiment, we obtained a full dose response curve for arteries to U46619 (0.03 to 3 μg/mL).
In some experiments, we examined the role of superoxide in mediating endothelial dysfunction in response to Ang II using scavengers of superoxide, PEG-superoxide dismutase (SOD, 300 U/ml) or Tempol (1 mM).
Measurement of superoxide
Relative superoxide levels were measured in aorta following overnight incubation with either vehicle or Ang II using lucigenin (5 μmol/L)-enhanced chemiluminescence as described previously.3,17
Quantitative real-time RT-PCR
RNA from aorta was prepared using the RNAeasy (Qiagen, Germantown, MD) method following extraction with TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription of RNA was performed using random hexamers as primers.18 Expression levels of IL-6 (TagMan primers/probe catalog # Mm00446190_m1), tumor necrosis factor-α (TNF-α, Mm00443258_m1), inducible NO synthase (iNOS, Mm00440485_m1), cyclooxygenase-2 (COX-2, Mm00478374_m1), superoxide dismutase-3 (SOD3, Mm01213380_s1), and p22phox (a component of NADPH oxidase, Mm00514478_m1)19 were determined by quantitative real-time RT-PCR using the TaqMan method.18 A housekeeping gene, ß-actin (Mm00607939_s1, whose Ct was found to be consistent among all groups), was used as an internal control for each sample, using the ΔΔCt method. TaqMan primers and probes were purchased from Applied Biosystems (Foster City, CA).
Drugs
Acetylcholine, Ang II, nitroprusside, Tempol and PEG-SOD were obtained from Sigma (St. Louis, MO) and all were dissolved in saline. U46619 was obtained from Cayman Chemical (Ann Arbor, MI) and dissolved in 100% ethanol with subsequent dilution being made with saline.
Statistical analysis
All data are expressed as means±SE. Relaxation to acetylcholine and nitroprusside is expressed as a percent relaxation to U46619-induced contraction. Comparisons of relaxation and contraction were made using analysis of variance followed by Student-Newman-Keuls post-hoc test. Differences in superoxide levels between groups were examined using unpaired t-tests. Statistical significance was accepted at P<0.05.
RESULTS
Deficiency in IL-10 promotes oxidative stress and vascular dysfunction in response to local Ang II
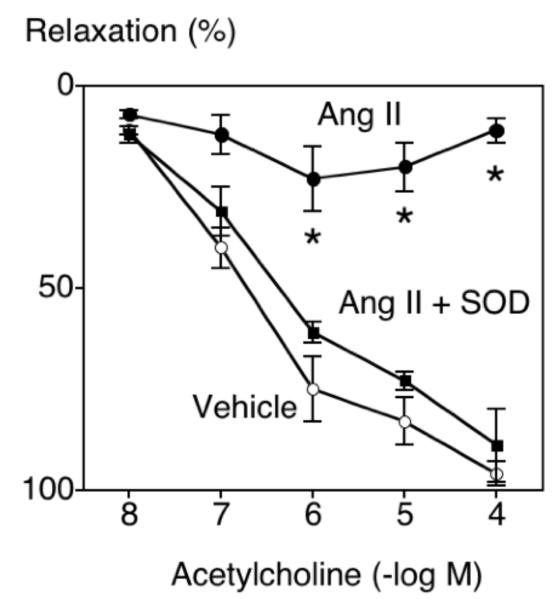
In carotid arteries from control mice, acetylcholine produced relaxation that was not affected by local treatment with 1 nmol/L Ang II (P>0.05)(Figure 1). In contrast, 1 nmol/L Ang II produced marked impairment (P<0.05) of acetylcholine-induced relaxation in arteries from IL-10 deficient mice (Figure 1). The effect of Ang II was selective for endothelium since relaxation in response to nitroprusside was similar in carotid arteries from all groups treated with vehicle or Ang II (Supplemental Figure 1, http://hyper.ahajournals.org).
Figure 1.
Relaxation of the carotid artery in response to acetylcholine in wild-type (left panel; n=11) and IL-10 deficient (right panel; n=10) mice following overnight treatment with vehicle or Ang II (1 nmol/L). Values are means±SE. *P<0.05 vs vehicle.
Superoxide levels were similar in vessels from wild-type and IL-10 deficient mice following treatment with vehicle (Figure 2). Ang II (1 nmol/L) had no effect on superoxide levels in vessels from control mice, but increased superoxide approximately 2-fold in vessels from IL-10 deficient mice (Figure 2). These findings indicate that deficiency in IL-10 does not affect vascular function under normal conditions but markedly enhances oxidative stress and endothelial dysfunction in response to a concentration of Ang II that has no effect in wild-type mice.
Figure 2.
Superoxide levels in aorta treated with vehicle or Ang II (1 nmol/L). Values are means±SE. n=11 wild-type (WT) and n=10 IL-10 deficient (KO). *P<0.05 vs vehicle.
To examine the functional importance of superoxide in this model, vascular responses in an additional group of IL-10 deficient mice were examined following incubation with vehicle, Ang II (1 nmol/L) or Ang II plus PEG-SOD. In this group of mice, Ang II again produced marked impairment of the response to acetylcholine and this impairment was almost completely prevented by PEG-SOD (Figure 3). These data suggest that endogenous IL-10 normally protects against Ang II-induced superoxide-mediated endothelial dysfunction.
Figure 3.
Relaxation of the carotid artery in response to acetylcholine in IL-10 deficient mice following overnight treatment with vehicle, Ang II (1 nmol/L), or Ang II (1 nmol/L) and PEG-SOD. Values are means±SE (n=5). *P<0.05 vs vehicle.
Vascular dysfunction in an Ang II-dependent model of hypertension is augmented in IL-10 deficient mice
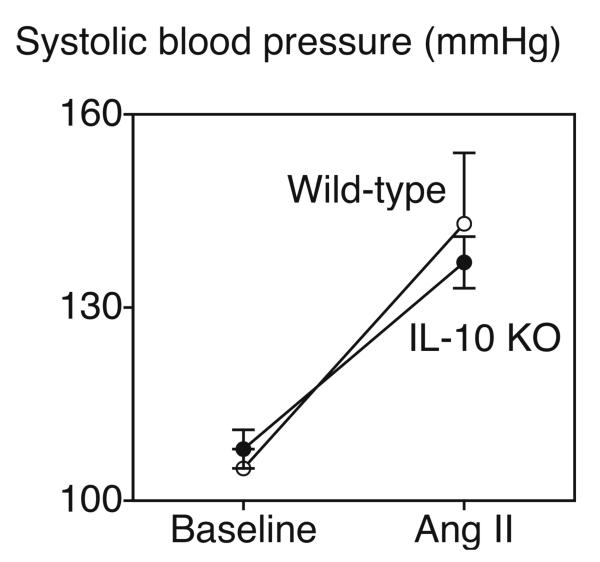
Blood pressure was similar in control and IL-10 deficient mice under baseline conditions and infusion of Ang II produced similar increases in arterial pressure in the two groups of animals (Figure 4). Consistent with previous studies, infusion of vehicle did not significantly alter blood pressure in either group (data not shown).
Figure 4.
Blood pressure in wild-type and IL-10 deficient mice (KO) under baseline conditions and following infusion of Ang II (1.4 mg/kg per day) for 10 days. Values are means±SE (n=6 and n=5 for wild-type infused with vehicle or Ang II, respectively; n=9 and n=10 for IL-10 deficient mice infused with vehicle or Ang II, respectively). *P<0.05 vs baseline.
Systemic administration of Ang II for 10 days produced modest impairment of response to acetylcholine in carotid arteries from wild-type mice (consistent with previous work)(Figure 5).15 In contrast, this same treatment produced marked impairment of this response in IL-10 deficient mice that was prevented by treatment with Tempol (Figure 5). Relaxation of the carotid artery in response to nitroprusside was similar in vehicle or Ang II-infused mice (Supplemental Figure 2, http://hyper.ahajournals.org). These findings suggest a major protective role for endogenous IL-10 in an Ang II-dependent model of hypertension.
Figure 5.
Relaxation of the carotid artery in response to acetylcholine in wild-type (left panel; n=6) and IL-10 deficient (right panel; n=10) mice following infusion of vehicle or Ang II (1.4 mg/kg per day) for 10 days. Arteries from some of the IL-10 deficient mice treated with Ang II were treated with Tempol (n=4). Values are means±SE. *P<0.05 vs vehicle.
To gain additional insight into mechanisms that may contribute to vascular dysfunction following administration of Ang II, we measured expression (mRNA levels) of several inflammatory related genes (IL-6, TNFα, iNOS and COX-2), a key antioxidant (SOD3), and a subunit of NADPH oxidases (p22phox).19 Treatment of control mice with Ang II increased vascular expression of IL-6, TNFα, iNOS and p22phox (Figure 6). These changes were selective as expression of COX-2 and SOD3 were not significantly altered (Figure 6). Expression of IL-6, TNFα, p22phox was increased further in IL-10 deficient mice treated with Ang II (Figure 6).
Figure 6.
Relative expression levels of mRNA in aorta from control (WT) and IL-10 deficient mice (KO) following infusion of vehicle or Ang II (1.4 mg/kg per day) for 10 days. IL-6, TNFα, iNOS, and p22phox were up-regulated by Ang II in WT and IL-10 KO mice (* P<0.05 versus vehicle). IL-6 was increased further in IL-10 KO mice treated with Ang II († P<0.05 versus WT treated with Ang II). Expression of TNFα and p22phox tended to increase further in IL-10 KO mice (# 0.05<P<0.1 versus WT treated with Ang II). N = 6-9 in each group.
DISCUSSION
There are several new findings in this study. First, local treatment with Ang II increased vascular superoxide and produced endothelial dysfunction in IL-10 deficient mice at a concentration that had no detectable effect in wild-type controls. Increased vascular superoxide was functionally important as impairment of endothelium-dependent relaxation was almost completely prevented with a scavenger of superoxide. With our in vitro model, these changes occurred within the vessel wall and are independent of systemic effects of Ang II or increases in blood pressure. With systemic treatment of Ang II, a similar role for endogenous IL-10 was evident as there was marked augmentation of vascular dysfunction in mice lacking IL-10 that was mediated by superoxide. Vascular expression of IL-6 increased following infusion of Ang II in control mice and increased further in IL-10 deficient mice. These findings represent the first evidence for any blood vessel that endogenous IL-10 plays a major role in preventing deleterious effects of Ang II. The data provide additional support for the concept that Ang II is proinflammatory in blood vessels and that IL-10 may be a major protective molecule in vascular disease. Importantly, the data suggest that IL-10 can exert prominent effects by acting locally within the vessel wall.
Ang II promotes oxidative stress in the vasculature
The renin-angiotensin system plays a major role in vascular biology contributing importantly to changes in vascular structure and function in hypertension and other pathophysiological conditions.1,2,4 In a variety of blood vessels and vascular cells in culture, Ang II promotes oxidative stress and endothelial dysfunction, as well as producing vascular hypertrophy and remodeling.1-4,19-23 Impairment of vascular function in response to Ang II is augmented in mice deficient in expression of CuZn-SOD and prevented by scavenging superoxide including in mice overexpressing CuZn-SOD.3 Thus, there is considerable evidence implicating oxidative stress and reactive oxygen species in mechanisms that produce vascular dysfunction in response to Ang II.
Although it is well established that Ang II produces oxidative stress in blood vessels, relatively little is known regarding molecular mechanisms that provide vascular protection from Ang II. The present study suggests that endogenous IL-10 normally protects against oxidative stress as a low concentration of Ang II increased vascular superoxide in IL-10 deficient animals, but not wild-type mice. Vascular dysfunction in response to Ang II in IL-10 deficient mice was mediated by superoxide following both local and systemic treatment with Ang II. We and others have shown previously that Ang II increases vascular superoxide in control mice and produces NADPH oxidase-dependent endothelial dysfunction.3,8,19 The present finding that treatment with Ang II increased vascular expression of p22phox, a component of several NADPH oxidases, is consistent with this concept. There is some evidence that IL-10 may inhibit expression or activity of NADPH oxidase in other models.24,25 Thus, we speculate that IL-10 may had have similar effects in the present study.
Interleukin-10 protects against Ang II-induced inflammation, oxidative stress and vascular dysfunction
Vascular inflammation is present, or components of the inflammatory response are activated, within the vessel wall in many cardiovascular diseases including hypertension. For example, Ang II activates inflammatory-related mechanisms within vascular cells, including the transcription factor NF-κB, production of proinflammatory cytokines including IL-6, as well as expression of adhesion molecules and matrix metalloproteinases.1,4,6-8 We observed similar effects in the present experiments as treatment with Ang II increased vascular expression of IL-6, TNFα, and iNOS. Because oxidative stress and inflammation are very commonly linked,5 many of these changes may be driven by Ang II-induced increases in reactive oxygen species.
Although inflammatory mechanisms are thought to be a key contributor to vascular disease, little is known about the importance of anti-inflammatory mechanisms in hypertension. Work by us and others recently led to the concept that the anti-inflammatory cytokine IL-10 may be a key mediator of vascular protection.13,14,26 For example, IL-10 attenuates increases in vascular superoxide and endothelial dysfunction during diabetes, but also protects against development of atherosclerosis and thrombosis during atherosclerosis.14,26-28 Thus, IL-10 may be part of a critical protective mechanism in diverse forms of vascular disease.
A major role for IL-10 is to inhibit expression of proinflammatory cytokines including IL-6 and TNFα.10-12 IL-10 inhibits activation of NF-κB, and many of its anti-inflammatory effects may be through this mechanism.4,6 IL-6 and TNFα are thought to be important mediators of vascular dysfunction,8,29 and IL-6 may be a key cause of Ang II-induced vascular dysfunction.8 Although such emerging evidence suggests a critical protective role for IL-10 in vascular biology, no role for endogenous IL-10 in hypertension has been defined previously. Very high levels of exogenously applied IL-10 have recently been shown to protect against Ang II-induced endothelial dysfunction in aorta from normal mice in vitro.25 In the present study, we examined the role of IL-10 as a mediator of vascular protection in two models of Ang II-dependent vascular dysfunction. Ang II increased vascular expression of IL-6 and TNFα in normal mice and deficiency in IL-10 caused a further increase in IL-6 mRNA levels (and tended to increase TNFα expression). Thus, endogenous IL-10 limits increases in IL-6, oxidative stress, and endothelial dysfunction in response to Ang II. Presumably as a consequence of the loss of both local and systemic effects, IL-10 deficient mice exhibit increased sensitivity to Ang II.
A key aspect of our findings is that some of the protective effects of IL-10 may occur within the vessel wall, independent of circulating immune cells or other mechanisms. Although there are multiple potential cellular sources of IL-10, including vascular cells,10,11 very little is known regarding the role of IL-10 within the vessel wall. Data that are available suggest that IL-10 may be produced constitutively within endothelium30,31 and that this expression may increase under pathophysiological conditions.30 Recent studied suggest that several types of inflammatory-related cells (dendritic-like cells, etc) may be present in some blood vessels and thus potentially play a role in local immune responses.32 Our findings suggest that local IL-10 may play an important protective role to limit effects of Ang II although the mechanisms involved are not clear. Other work also suggests a key role for local inflammatory mechanisms within vascular cells.32 For example, endothelial-specific inhibition of NF-κB limits vascular damage during hypertension.33
Perspectives
This study provides the first evidence that endogenous IL-10 limits Ang II-mediated increases in IL-6, oxidative stress, and vascular dysfunction. Thus, IL-10 may be a key mediator of vascular protection during hypertension and during other forms of vascular disease where Ang II plays a major role. For example, Ang II plays a key role in vascular abnormalities that occur with aging.34 Activation of components of the inflammatory response occur in vascular cells in response to Ang II and may contribute to diverse forms of vascular disease.35 The finding that endogenous IL-10, a potent anti-inflammatory cytokine, protects against Ang II-induced vascular dysfunction supports this concept. At least some of the protective effects of IL-10 may occur at the local vascular level.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL-62984, HL-38901, and NS-24621, American Heart Association grants 0230327N and 0565486Z, and by a Bugher Foundation Award in Stroke (0575092N).
References
- 1.Dzau VJ. Tissue angiotensin and pathophysiology of vascular disease. A unifying hypothesis. Hypertension. 2001;37:1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- 2.Nickenig G, Harrison DG. The AT1-type angiotensin receptor in oxidative stress and atherosclerosis. Part I: Oxidative stress and atherogenesis. Circulation. 2002;105:393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- 3.Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZnSOD in preventing angiotensin-II-induced endothelial dysfunction. Hypertension. 2005;46:1147–1153. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- 4.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci. 2007;112:375–84. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 5.Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nature Clin Pract Nephrol. 2006;2:582–93. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 6.Raines EW, Garton KJ, Ferri N. Beyond the endothelium: NF-αB regulation of smooth muscle function. Circ Res. 2004;94:706–708. doi: 10.1161/01.RES.0000125646.08156.4D. [DOI] [PubMed] [Google Scholar]
- 7.Neri Serneri GG, Boddi M, Modesti PA, Cecioni I, Coppo M, Toscano T, Papa ML, Bandinelli M, Lisi GF, Chiaverelli M. Cardiac angiotensin II participates in coronary microvessel inflammation of unstable angina and strengthens the immuno-mediated component. Circ Res. 2004;94:1630–1637. doi: 10.1161/01.RES.0000130944.49657.b8. [DOI] [PubMed] [Google Scholar]
- 8.Schrader KI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II-induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 9.Tham DM, Martin-McNulty B, Wang Y-X, Wilson DW, Vergano R, Sullivan ME, Dole W, Rutledge JC. Angiotensin II is associated with activation of NF-κB-meidated genes and down regulation of PPARs. Physiol Genomics. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 10.Goldman M, P Stordeur P. Interleukin-10 as an anti-stress cytokine. Eur Cytokine Network. 1997;8:301–302. [PubMed] [Google Scholar]
- 11.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy - a review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 12.Berg DJ, Kuehn R, Rajewsky K, Mueller W, Menon S, Davidson N, Gruenig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caligiuri G, Rudling M, Ollivier V, Jacob M-P, Michel J-B, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Molecular Medicine. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 14.Gunnett CA, Heistad DD, Faraci FM. Interleukin-10 protects endothelium-dependent relaxation during diabetes: Role of superoxide. Diabetes. 2002;51:1931–1937. doi: 10.2337/diabetes.51.6.1931. [DOI] [PubMed] [Google Scholar]
- 15.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II induced vascular dysfunction is mediated by the AT1a receptor in mice. Hypertension. 2004;43:1074–1079. doi: 10.1161/01.HYP.0000123074.89717.3d. [DOI] [PubMed] [Google Scholar]
- 16.Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol. 1998;274:H564–H570. doi: 10.1152/ajpheart.1998.274.2.H564. [DOI] [PubMed] [Google Scholar]
- 17.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 18.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol. 2002;22:611–616. doi: 10.1161/01.atv.0000012663.85364.fa. [DOI] [PubMed] [Google Scholar]
- 19.Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Molec Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- 21.Berry C, Hamilton CA, Brosnan J, Magill FG, Berg GA, McMurray JJV, Domininiczak AF. Investigation into sources of superoxide in human blood vessels. Angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101:2206–2212. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chrissobolis S, Didion SP, Kinzenbaw DA, Schrader LI, Dayal S, Lentz SR, Faraci FM. Glutathione peroxidase plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension. 2008;51:872–877. doi: 10.1161/HYPERTENSIONAHA.107.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuga S, Otsuka T, Niiro H, Nunoi H, Nemoto Y, Nakano T, Ogo T, Umei T, Niho Y. Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp Hematol. 1996;24:151–157. [PubMed] [Google Scholar]
- 25.Zemse SM, Hilgers RHP, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by Ang II in murine aortic rings. Am J Physiol. 2007;292:H3103–H3108. doi: 10.1152/ajpheart.00456.2006. [DOI] [PubMed] [Google Scholar]
- 26.Gunnett CA, Heistad DD, Berg DJ, Faraci FM. Interleukin-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol. 2000;279:H1555–H1562. doi: 10.1152/ajpheart.2000.279.4.H1555. [DOI] [PubMed] [Google Scholar]
- 27.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 28.Gunnett CA, Berg DJ, Faraci FM. Vascular effects of lipopolysaccharide are enhanced in interleukin-10-deficient mice. Stroke. 1999;30:2191–2196. doi: 10.1161/01.str.30.10.2191. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Park Y, Wu J, Chen XP, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-α in vascular dysfunction. Clinical Sci. 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hocke AC, Ermert M, Althoff A, Brell B, N'Guessan PD, Suttorp N, Ermert L. Regulation of interleukin IL-4, IL-13, IL-10, and their downstream components in lipopolysaccharide-exposed rat lungs. Comparison of the constitutive expression between rats and humans. Cytokine. 2006;33:199–211. doi: 10.1016/j.cyto.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–1197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henke N, Schmidt-Ullrich R, Dechend R, Park J-K, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific NF-κB suppression attenuates hypertension-induced renal damage. Circulation Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 34.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol. 2009;296:H1914–H1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.