Abstract
Aims
Prior studies have shown that HAART is associated with decreased HIV viral load in the lungs. The correlation between antiretroviral exposure in bronchoalveolar lavage (BAL) fluid and virologic response was evaluated in patients starting HAART and enrolled in The AIDS Clinical Trial Group Protocol 723.
Materials & methods
A total of 24 subjects underwent blood and BAL sampling prior to starting HAART, and after 4 and 24 weeks of HAART. Drug concentrations and HIV RNA were measured in paired plasma and BAL samples.
Results
Antiretroviral drugs, including efavirenz, were detectable in BAL fluid of HIV-infected subjects beginning HAART. Efavirenz was also associated with a higher likelihood of clearing HIV RNA from the lungs.
Conclusion
These results suggest the excellent pulmonary virologic response to antiretroviral therapy may, in part, be due to penetration of antiretroviral drugs into the alveolar compartment.
Keywords: bronchoalveolar lavage, drug concentration, efavirenz, HAART, HIV RNA, lung
Pulmonary complications are a significant source of morbidity and mortality in subjects infected with HIV [1,2]. However, the spectrum of pulmonary diseases in HIV infection has been dramatically altered in the HAART era [3]. It is well established that HAART is able to significantly reduce HIV viremia [4]. We recently published the results of a longitudinal study demonstrating that HAART is also associated with a significant decrease in HIV viral load in acellular bronchoalveolar lavage (BAL) fluid and BAL cells [5]. In this report we have examined the ability to measure antiretroviral drugs in BAL and correlated this ability with the pulmonary virologic response.
Patients & methods
The AIDS Clinical Trials Group Protocol 723 (ACTG 723) was a multicenter, prospective, longitudinal study that assessed the effect of HAART on pulmonary viral burden and immune function. Details about the study design have been previously published [5]. Briefly, HIV-infected subjects scheduled to begin HAART had blood drawn and underwent bronchoscopy with BAL before starting therapy and again at 4 and 24 weeks after HAART was initiated. A specific HAART regimen was not required. However, subjects had to be starting a regimen consisting of at least three drugs and designed to reduce plasma HIV to undetectable levels.
Bronchoscopy with BAL was performed as previously described [6]. The last dose of antiretroviral medication was administered in the evening prior to bronchoscopy (on average 12 h prior to the procedure). Lavage fluid was filtered through sterile gauze and BAL cells pelleted by centrifugation. The supernatant was saved as the acellular BAL fraction at −70°C. Plasma was prepared from peripheral blood. Measurement of plasma and acellular BAL HIV viral load and cellular HIV RNA and DNA in BAL cells was performed as previously described [5].
The extracellular concentration of antiretroviral drugs in undiluted plasma and two-times concentrated BAL fluid was measured using high-performance liquid chromatography as previously described [7–9]. The dynamic range of the assays for all drugs was 10–2000 ng/ml. This lower limit is two- to 200-fold lower than the clinically relevant plasma trough concentrations of the antiretroviral drugs evaluated. Assays were performed for the nucleoside/tide reverse transcriptase inhibitors (NRTIs) lamivudine (3TC), didanosine, stavudine, zidovudine, abacavir, emtricitabine and tenofovir; the non-NRTI (NNRTI) efavirenz (EFV); and the protease inhibitors (PI) ritonavir, nelfinavir, amprenavir and lopinavir. All BAL drug concentrations were adjusted for dilution by expressing the concentration per ml of epithelial lining fluid as previously described [5,10].
The ability to detect different drug classes in BAL was summarized and reported in proportions treating week 4 and week 24 measurements as independent. Each different drug concentration in an individual was also treated as independent data. The relationship between receiving different antiretroviral drugs and control of virus in the pulmonary compartment was assessed using Fisher’s exact test. Receiver operating characteristic (ROC) curves were generated to examine the relationship between plasma concentrations of 3TC and EFV and the ability to detect the drug in BAL fluid. All reported p-values are two-sided. p-values of 0.05 or under were considered significant.
Results
Subject follow-up & characteristics
Subject follow-up and characteristics have been described elsewhere [5]. Frozen plasma and BAL fluid from all three time points were available for 24 subjects who make up the population for this report. All subjects had measurable plasma concentrations of at least one of their prescribed drugs at 4 weeks. At 24 weeks, 22 out of 24 subjects had measurable plasma concentrations of at least one of their prescribed drugs. In two individuals, none of the antiretroviral drugs prescribed were detected in plasma (after being detectable at week 4), which probably reflects nonadherence.
Detection of antiretroviral drugs in plasma & BAL fluid
Table 1 shows the ability to detect various antiretroviral drugs in plasma and BAL fluid at 4 and 24 weeks after beginning HAART. Plasma drug concentrations were easily quantifiable in most subjects. However, owing to the dilution of lung fluid during lavage, BAL drug concentrations were most often detectable but below the 10 ng/ml limit of quantification.
Table 1.
Detectable antiretroviral drug concentrations in bronchoalveolar lavage and plasma by drug class.
| Drug class | Drug | Lung |
Blood |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | % | 24 weeks | % | Total (%) | 4 weeks | % | 24 weeks | % | Total (%) | ||
| NRTI | 3TC | 13/22 | 43 | 14/22 | 45 | 44 | 21/21 | 83 | 20/22 | 74 | 79 |
| DDI | 0/1 | 43 | 0/1 | 45 | 44 | 0/1 | 83 | 0/1 | 74 | 79 | |
| D4T | 0/3 | 43 | 0/3 | 45 | 44 | 2/3 | 83 | 1/3 | 74 | 79 | |
| ZDV | 5/16 | 43 | 5/16 | 45 | 44 | 12/16 | 83 | 10/16 | 74 | 79 | |
| ABC | 5/12 | 43 | 5/12 | 45 | 44 | 8/10 | 83 | 6/9 | 74 | 79 | |
| FTC | 1/1 | 43 | 1/1 | 45 | 44 | 1/1 | 83 | 1/1 | 74 | 79 | |
| TDF | 0/1 | 43 | 0/1 | 45 | 44 | 1/1 | 83 | 1/1 | 74 | 79 | |
| NNRTI | EFV | 10/16 | 63 | 10/16 | 63 | 63 | 14/16 | 88 | 16/16 | 100 | 94 |
| PI | RTV | 1/5 | 54 | 1/5 | 38 | 46 | 5/5 | 100 | 5/5 | 85 | 92 |
| NFV | 4/4 | 54 | 2/4 | 38 | 46 | 4/4 | 100 | 2/4 | 85 | 92 | |
| APV | 2/3 | 54 | 2/3 | 38 | 46 | 3/3 | 100 | 3/3 | 85 | 92 | |
| LPV | 0/1 | 54 | 0/1 | 38 | 46 | 1/1 | 100 | 1/1 | 85 | 92 | |
3TC: Lamivudine; ABC: Abacavir; APV: Amprenavir; D4T: Stavudine; DDI: Didanosine; EFV: Efavirenz; FTC: Emtricitabine; LPV: Lopinavir; NFV: Nelfinavir; NNRTI: Non-nucleoside reverse transcriptase inhibitor; NRTI: Nucleoside/tide reverse transcriptase inhibitors; PI: Protease inhibitor; RTV: Ritonavir; TDF: Tenofovir; ZDV: Zidovudine.
For the NNRTI EFV, 20 out of 32 measurements (63%) contained detectable drug concentrations in BAL fluid. For NRTIs, 49 out of 112 measurements (44%) had detectable concentrations, and for PIs, 12 out of 26 measurements (46%) had detectable concentrations. In those instances where simultaneous plasma and BAL concentrations could be measured (n = 23), after correcting for BAL dilution, the concentration of antiretroviral agents in the epithelial lining fluid was similar to concentrations found in plasma. Specifically, BAL concentrations of NRTIs were 60–73% that of plasma, NNRTIs were 31% that of plasma, and PIs were 66–200% that of plasma.
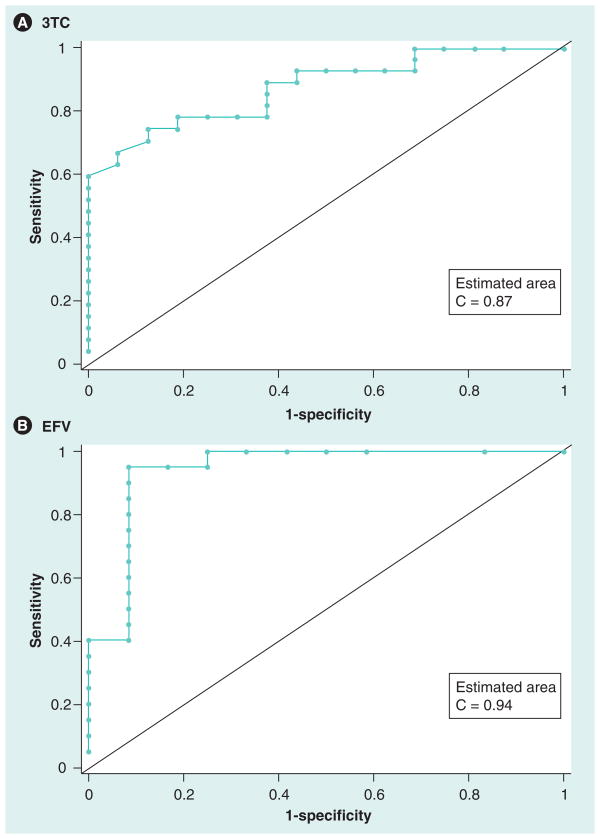
Enough individuals were on 3TC and EFV to generate ROC curves to explore plasma ‘thresholds’, which predicted the ability to detect drug in the lung. Repeated measures over time were treated as independent observations. For 3TC, there were 43 measurements from 21 individuals and for EFV there were 32 measurements from 16 individuals. Figure 1 shows the ROC curves for plasma drug concentration and the ability to detect drugs in the lungs for 3TC and EFV. The areas under the ROC curves were 0.87 and 0.94 for 3TC and EFV, respectively (p < 0.0001 for both). The optimal plasma cut-offs suggested by the data were 178 ng/ml or less for 3TC and 2234 ng/ml or less for EFV. Therefore, a subject with a trough 3TC plasma concentration greater than 178 ng/ml would have an 87% chance of detecting 3TC in the lungs. Similarly, a subject with a trough EFV plasma concentration greater than 2234 ng/ml would have a 94% chance of detecting EFV in the lungs.
Figure 1. Receiver operating characteristic curves for plasma drug concentration and the ability to detect drugs in the lungs for lamivudine (A) and efavirenz (B).
The areas under the receiver operating characteristic curves were 0.87 and 0.94 for 3TC and EFV, respectively (p < 0.0001 for both). The optimal plasma cut-offs suggested by the data are 178 ng/ml or less for 3TC and 2234 ng/ml or less for EFV.
EFV: Efavirenz; 3TC: Lamivudine.
Relationship between control of pulmonary viral load & antiretroviral therapy
Based on persistence of RNA in acellular BAL fluid or BAL cells at 24 weeks or a rebound of RNA in the plasma at 24 weeks, there were seven lung ‘failures’ and five plasma ‘failures’. Interestingly, four of the five plasma failures were also associated with lung failures. Three other patients had lung failure despite good control of plasma viremia.
The relationship between antiretroviral drug treatment and virologic failure in the lungs is shown in Table 2. In this cohort all subjects received NRTIs. Thus, the overall lung failure rate in subjects who received NRTIs was 29% (seven out of 24). Four out of nine subjects receiving PIs had lung failure, giving a failure rate of 44%. Thus, there was no relationship between lung failure and receiving NRTIs or PIs. By contrast, 16 subjects received EFV. There were only two lung failures in this group. Eight subjects did not receive EFV. There were five lung failures in this group. Subjects who received EFV had a significantly lower chance of lung failure than subjects who did not receive EFV (13 vs 63%; p = 0.02).
Table 2.
Relationship between antiretroviral drugs and virologic failure in the lungs.
| Drug class | Subjects (n) | Total failure rate in subjects on drug (%) | Total failure rate in subjects not on drug (%) | Failure rate and detectable drug in BAL fluid (%) | Failure rate and undetectable drug in BAL fluid (%) |
|---|---|---|---|---|---|
| NRTI | 24 | 7/24 (29) | – | 4/16 (25) | 3/8 (38) |
| NNRTI | 16 | 2/16 (13)† | 5/8 (63) | 1/10 (10) | 1/6 (17) |
| PI | 9 | 4/9 (44) | 3/15 (20) | 3/5 (60) | 1/4 (25) |
p = 0.02 for comparison of lung failure in those taking NNRTI versus those not taking NNRTI.
BAL: Bronchoalveolar lavage; NNRTI: Non-nucleoside/tide reverse transcriptase inhibitor; NRTI: Nucleoside/tide reverse transcriptase inhibitor; PI: Protease inhibitor.
The ability to detect antiretroviral drugs in BAL did not predict lung failure (Table 2). For each class of drug the failure rate was similar regardless of whether the drug was detectable in BAL or not. In fact, in four of seven subjects with lung failure, all drugs in the HAART regimen were detectable in BAL fluid.
Discussion
In this report we have demonstrated that antiretroviral drugs are detectable in BAL fluid of HIV-infected subjects beginning HAART. This is especially true for the NNRTI EFV. Furthermore, taking EFV is associated with a higher likelihood of clearing HIV RNA from the lungs. These results suggest that the excellent pulmonary virologic response to antiretroviral therapy previously reported [5] may, in part, be due to penetration of antiretroviral drugs into the alveolar compartment.
Since subjects had to be nil by mouth for at least 8 h prior to bronchoscopy, the ability to detect drug concentrations with the BAL sampling strategy was in part dependent on the plasma half-life. The half-life of the NRTIs and protease inhibitors range from 3 to 10 h. By contrast, EFV has the longest half-life of all the drugs studied (40–55 h). Thus, it is not surprising that EFV was the most readily detectable antiretroviral agent in BAL. Interestingly, 3TC and emtricitabine have longer half-lives compared with other NRTIs (5–7 h and 10 h, respectively), and these agents were the most readily detectable NRTIs in BAL. Likewise, the protease inhibitors nelfinavir (5 h) h) and amprenavir (7–11 h) were also more readily detected in BAL. Thus, the plasma half-life of antiretroviral drugs appears to affect the ability to detect them in the pulmonary compartment.
In the lungs, lipid solubility also plays an important role in drug pharmacology. It is well known that lipophilic drugs preferentially penetrate the lungs and, in fact, this forms the rationale for encapsulating certain hydrophilic drugs in liposomes (i.e., amphotericin) to promote delivery to the pulmonary compartment [11]. In this regard it is intriguing that the highly lipid soluble drug EFV appeared to enter BAL most efficiently and was associated with control of HIV in the alveolar compartment.
While HIV manifestations in many different organs have been altered by HAART [12], not all compartments have a similar virologic response. The gastrointestinal tract in particular appears to respond less well to antiretroviral therapy, with evidence of ongoing viral replication even in patients with a good plasma virologic response [13]. Similarly, penetration into cerebral spinal fluid is variable, depending on the drug studied [14]. Interestingly, EFV is one of the more effective drugs in penetrating spinal fluid, a trait which has been attributed to its lipophilic property [14]. It will be important to determine whether failure of a particular compartment to respond appropriately to HAART reflects poor drug penetration of that tissue.
There are limitations to our findings in this exploratory study. Since subjects were studied at the end of the dosing interval, we probably underestimated the ability of antiretroviral drugs to enter BAL. Second, we cannot draw conclusions on how well antiretroviral drugs penetrate into the lung over the entire dosing interval. Third, we did not measure drug concentrations in cell pellets and, thus, cannot directly assess intracellular drug exposure. Finally, repeated measurements over time and with different drugs in the same individual were analyzed as independent observations. Thus, a mixed effects model would be more appropriate if the sample size was much larger. Nevertheless, these results demonstrate that antiretroviral drugs are measurable in BAL and justify future studies examining the pharmacokinetics of these drugs in the pulmonary compartment.
Conclusion & future perspective
Antiretroviral drugs are detectable in BAL fluid of HIV-infected subjects on HAART. Patients on EFV are more likely to have cleared HIV RNA from the lungs on follow-up testing. Further studies are needed to better define the pharmacokinetics of antiretroviral drugs in the lungs and to determine if the excellent pulmonary response to HAART can be directly attributed to drug penetration into the alveolar compartment.
Executive summary
Pulmonary complications are a significant source of morbidity and mortality in subjects infected with HIV.
HAART is associated with a significant decrease in HIV viral load in acellular bronchoalveolar lavage (BAL) fluid and cells.
In those instances where simultaneous plasma and BAL concentrations could be measured, after correcting for BAL dilution, the concentration of antiretroviral agents in the epithelial lining fluid was similar to concentrations found in plasma.
Subjects who received efavirenz had a significantly lower chance of lung failure than subjects who did not receive efavirenz (13 vs 63%; p = 0.02).
These results suggest that the excellent pulmonary virologic response to antiretroviral therapy previously reported may, in part, be due to penetration of antiretroviral drugs into the alveolar compartment.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors were supported in part by the Adult AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases (NIH; AI 38858); the Statistical and Data Analysis Center (AI 38855); the individual AIDS Clinical Trials Units at Indiana University (AI 25859), New York University (AI 27665), University of Alabama at Birmingham (AI 32775), and the University of Washington (AI 27664); General Clinical Research Center Awards funded by the National Center for Research Resources at Indiana University (MO1 RR750) and New York University (MO1 RR00096); The University of North Carolina Center for AIDS Research (AI50410); RO1 HL59834 (HLT) and HL057879 (MW). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part II. Am Rev Respir Dis. 1990;141:1582–1598. doi: 10.1164/ajrccm/141.6.1582. [DOI] [PubMed] [Google Scholar]
- 2.Murray JF, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990;141:1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AJ, O’Donnell AE. Pulmonary manifestations of HIV infection in the era of highly active antiretroviral therapy. Chest. 2001;120:1888–1893. doi: 10.1378/chest.120.6.1888. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SC, Gerber JG. Advances in HIV/AIDS therapy. Adv Intern Med. 2000;45:1–40. [PubMed] [Google Scholar]
- 5.Twigg HL, III, Weiden M, Valentine F, et al. Effect of highly active antiretroviral therapy on viral burden in the lungs of HIV-infected subjects. J Infect Dis. 2008;197:109–116. doi: 10.1086/523766. [DOI] [PubMed] [Google Scholar]
- 6.Twigg HL, III, Soliman DM, Day RB, et al. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am J Respir Crit Care Med. 1999;159:1439–1444. doi: 10.1164/ajrccm.159.5.9808031. [DOI] [PubMed] [Google Scholar]
- 7.Choi SO, Rezk NL, Kashuba AD. High-performance liquid chromatography assay for the determination of the HIV-protease inhibitor tipranavir in human plasma in combination with nine other antiretroviral medications. J Pharm Biomed Anal. 2007;43:1562–1567. doi: 10.1016/j.jpba.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Rezk NL, Crutchley RD, Kashuba AD. Simultaneous quantification of emtricitabine and tenofovir in human plasma using high-performance liquid chromatography after solid phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;822:201–208. doi: 10.1016/j.jchromb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Rezk NL, Tidwell RR, Kashuba AD. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:137–147. doi: 10.1016/s1570-0232(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 10.Rennard SI, Basset G, Lecossier D, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 11.Pea F, Viale P. The antimicrobial therapy puzzle: could pharmacokinetic–pharmacodynamic relationships be helpful in addressing the issue of appropriate pneumonia treatment in critically ill patients? Clin Infect Dis. 2006;42:1764–1771. doi: 10.1086/504383. [DOI] [PubMed] [Google Scholar]
- 12.Torre D, Speranza F, Martegani R. Impact of highly active antiretroviral therapy on organ-specific manifestations of HIV-1 infection. HIV Med. 2005;6:66–78. doi: 10.1111/j.1468-1293.2005.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boffito M, Pillay D, Wilkins E. Management of advanced HIV disease: resistance, antiretroviral brain penetration and malignancies. Int J Clin Pract. 2006;60:1098–1106. doi: 10.1111/j.1742-1241.2006.01073.x. [DOI] [PubMed] [Google Scholar]