Abstract
The activity of the multienzyme pyruvate dehydrogenase complexes, isolated from mitochondria of beef kidney, beef heart, and pork liver, is regulated by phosphorylation and dephosphorylation. Phosphorylation and concomitant inactivation of each of the three complexes are catalyzed by an ATP-specific kinase, and dephosphorylation and concomitant reactivation are catalyzed by a phosphatase. The phosphatase has been separated from the other component enzymes of each pyruvate dehydrogenase complex, and the three phosphatases are functionally interchangeable. The kinase has been isolated from the beef kidney complex, and it is functional with the beef heart and pork liver complexes. ADP is competitive with ATP, and the ADP effect is more pronounced with the kidney kinase than with the liver and heart kinases. Pyruvate protects strongly the heart and liver pruvate dehydrogenase complexes and, to a lesser extent, the kidney complex against inactivation by ATP. Pyruvate apparently exerts its effect on the pyruvate dehydrogenase component of the complex, rather than on the kinase.
Full text
PDF

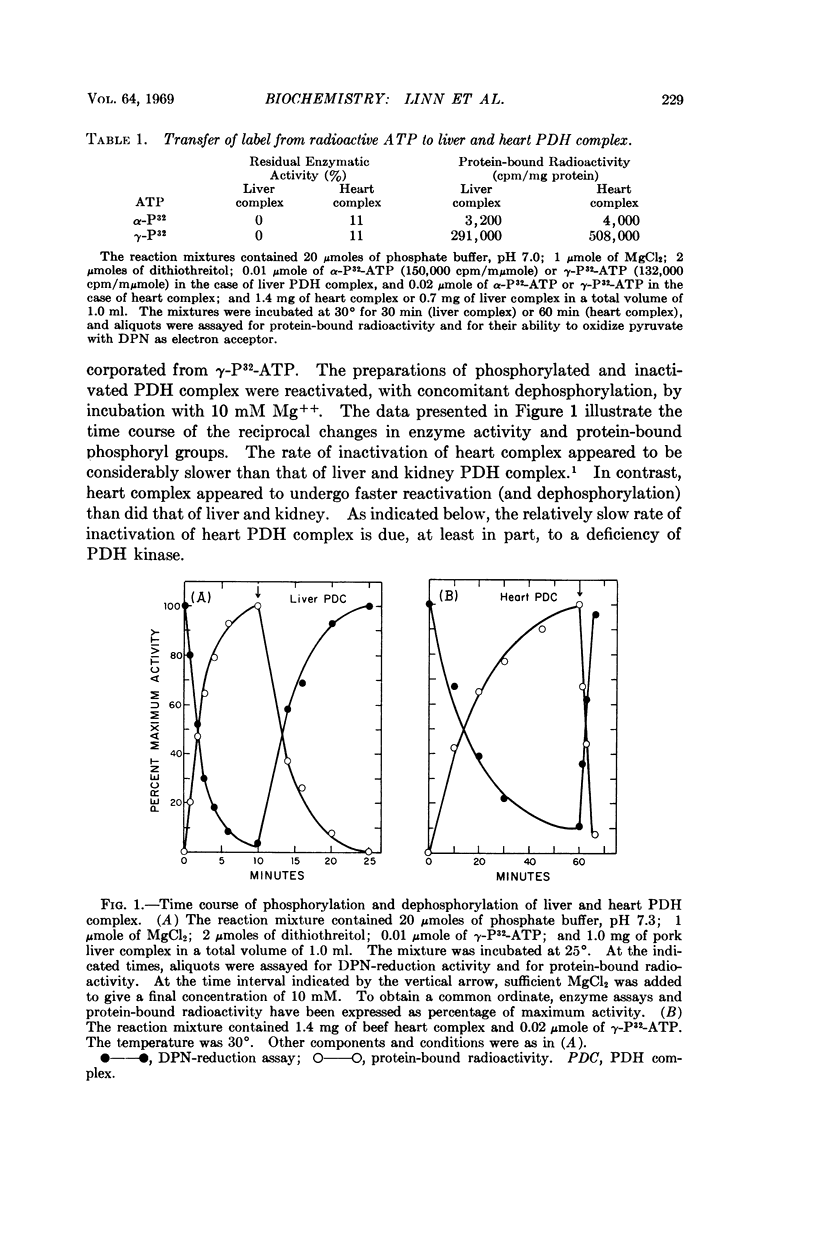


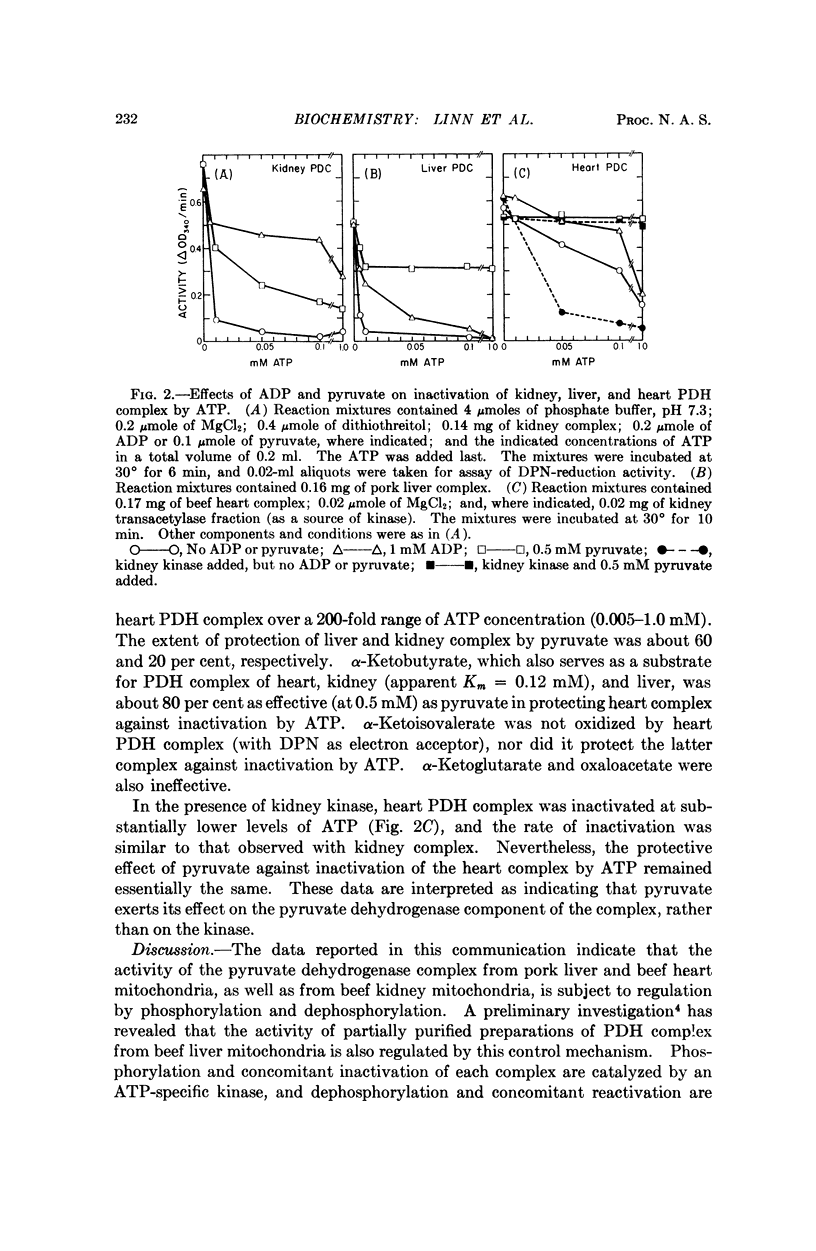


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ishikawa E., Oliver R. M., Reed L. J. Alpha-Keto acid dehydrogenase complexes, V. Macromolecular organization of pyruvate and alpha-ketoglutarate dehydrogenase complexes isolated from beef kidney mitochondria. Proc Natl Acad Sci U S A. 1966 Aug;56(2):534–541. doi: 10.1073/pnas.56.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O., Jagow-Westermann B. v. ATP-dependent inactivation of heart muscle pyruvate dehydrogenase and reactivation by Mg(++). FEBS Lett. 1969 Jun;3(4):271–274. doi: 10.1016/0014-5793(69)80156-0. [DOI] [PubMed] [Google Scholar]



