Abstract
Objectives
Dysbindin (DTNBP1) has been identified as a susceptibility gene for schizophrenia (SZ) through a positional approach. However, a variety of single nucleotide polymorphisms (SNPs) and haplotypes, in different parts of the gene, have been reported to be associated in different samples, and a precise molecular mechanism of disease remains to be defined. We have performed an association study with two well-characterized family samples not previously investigated at the DTNBP1 locus.
Methods
We examined 646 subjects in 136 families with SZ, largely of European ancestry (EA), genotyping 26 SNPs in DTNBP1.
Results
Three correlated markers (rs875462, rs760666, and rs7758659) at the 3′ region of DTNBP1 showed evidence for association to SZ (p = 0.004), observed in both the EA (p = 0.031) and the African American (AA) subset (p = 0.045) with the same over-transmitted allele. The most significant haplotype in our study was rs7758659-rs3213207 (global p = 0.0015), with rs3213207 being the most frequently reported associated marker in previous studies. A non-conservative missense variant (Pro272Ser) in the 3′ region of DTNBP1 that may impair DTNBP1 function was more common in SZ probands (8.2%) than in founders (5%) and in dbSNP (2.1%), but did not reach statistical significance.
Conclusion
Our results provide evidence for an association of SZ with SNPs at the 3′ end of DTNBP1 in the samples studied.
Key Words: Single nucleotide polymorphism, Haplotype, Linkage disequilibrium, Complex disorder, Dystrobrevin binding protein 1, Schizophrenia, Association
Introduction
A locus for SZ in chromosome 6p24-p22 has been supported by some [1,2,3,4] but not all linkage studies [5,6,7]. Straub et al. reported four SNPs from exons 1–5 of the 140 kb DTNBP1 (at 6p22.3), and several 3-marker haplotypes (p = 0.008–0.0001), to be associated with SZ [8]. In vitro functional studies of DTNBP1 have suggested that DTNBP1 may influence exocytotic glutamate release presynaptically [9]. Patients with SZ were reported to have decreased DTNBP1 in the glutamatergic terminal of the hippocampus and dorsolateral prefrontal cortex [10], and risk haplotypes for SZ were also shown to be associated with reduced DTNBP1 expression in human cerebral cortex [11]. Convergent effects of several putative SZ susceptibility genes (including DTNBP1) upon glutamate synapses have been hypothesized as a possible model for the pathogenesis of SZ [12].
So far (November 2006), 19 association studies of DTNBP1 and SZ have been published, of 25 independent samples in a variety of populations (table 1) [8, 9,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Although a number of studies showed supportive evidence for the association between DTNBP1 and SZ, these reports also showed considerable disagreement between the associated markers, alleles, and haplotypes. It has been suggested that the inconsistencies across studies were population dependent [30]. However, a recent analysis of the reported associated haplotypes concluded that the conflicting results across EA studies could not be attributed to population differences because the genetic architecture at DTNBP1 locus is similar in EA samples [31].
Table 1.
Complex results from previous association studies on DTNBP1a
| Markersb | Distance to next marker (kb)c | SNP | Origin of the samples studied |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irish |
UK/Irish |
Scottish |
German and Israeli |
Polish |
Bulgarian |
Swedish |
Australian |
Canadian |
US-EA |
US-Hispanic |
US-AA |
African |
Indian South |
Chinese Han |
Japanese |
Korean |
|||||||||||||
| Straub 2002d: 270 families | Morris 2003: 219 cases vs. 231controls | van den Oord 2003e: 268 families | Williams 2004: 708 cases vs. 711 controls | Li 2005 f: 580 cases and 620 controls | Schwab 2003: 203 families | Fallin 2005: 274 Ashkenazi Jewish trios Van Den Bogaert 2003: 418 cases vs. 285 controls | Van Den Bogaert 2003: 294 cases vs. 113 controls | Kirov 2004: 488 trios | Van Den Bogaert 2003: 142 cases vs. 272 controls | Holliday 2006: 41 families and 194 cases vs. 180 controls | De Luca 2005: 117 families (106 EA, 2 AA and 9Asian) | Funke 2004: 258 cases vs. 467 controls | Hall 2004: 210 trios | Wood 2006g: 451 cases vs. 291 controls | Gornick 2005h:102 families | Funke 2004: 51 cases vs. 32 controls | Funke 2004: 215 cases vs. 74 controls | Hall 2004: 233 families | Holliday 2006: 197 families | Tang 2003i:233 trios | Li 2005f:638 nuclear families | Numakawa 2004: 670 cases vs. 588 controls | Tochigi 2006: 314 cases vs. 314 controls | Joo 2006: 194 cases vs. 351 controls | |||||
| rs1474588 | P1730 | 42.9 | C/G | ns | |||||||||||||||||||||||||
| rs760659 | P1283 | 18.5 | G/A | ns | |||||||||||||||||||||||||
| rs1047631 | P3230 | 1.4 | A/G | ns | ns | ns | ns | ns | ns | ns | ns | ns | |||||||||||||||||
| rs742106 | P1328 | 23 | C/T | ns | ns | ns | ns | ns | ns | ns | ns | ns | |||||||||||||||||
| rs1040410 | 25.6 | C/T | ns | ns | ns | ||||||||||||||||||||||||
| rs742105 | P1333 | 13.1 | G/A | 0.0099 (A) | ns | ns | ns | ns | |||||||||||||||||||||
| rs12524251 | P3236 | 3 | T/C | ns | ns | ns | |||||||||||||||||||||||
| rs760666 | P1287 | 31.7 | C/T | ns | ns | ns | ns | ns | ns | ns | ns | ns | |||||||||||||||||
| rs2619539 | P1655 | 6.7 | C/G | 0.0059 (G) | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||||||||
| rs16876738 | P2381 | 0.2 | G/C | ns | |||||||||||||||||||||||||
| rs12525702 | 0.3 | G/A | |||||||||||||||||||||||||||
| rs3213207 | P1635 | 1.1 | A/G | 9E-05 (G) | ns | ns | ns | ns | 0.0052 (A) | ns | ns | ns | 9E-04 (A) | ns | ns | ns | ns | ns | 0.02 (A) | 0.001 (G) | ns | ns | |||||||
| rs2619542 | 4.2 | G/A | ns | ||||||||||||||||||||||||||
| rs1011313 | P1325 | 0.2 | G/A | ns | ns | ns | ns | 0.0092 (G) | ns | ns | ns | ns | 0.032 (A) | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||||||
| rs6924627 | P3762 | 0.6 | G/A | ns | |||||||||||||||||||||||||
| rs2619550 | 15.6 | C/G | ns | ||||||||||||||||||||||||||
| rs2619528 | P1765 | 1 | G/A | ns | 0.013 (A) | ns | 0.014 (G) | ns | ns | 0.017 (A) | ns | 0.017 (A) | ns | ns | ns | 0.002 (G) | ns | ||||||||||||
| rs2005976 | P1757 | 0.3 | G/A | ns | ns | 0.012 (A) | ns | ns | ns | ns | 0.0013 (G) | ns | ns | ||||||||||||||||
| rs760761 | P1320 | 2.5 | C/T | 0.0004 (T) | ns | 0.016 (T) | ns | 7E-04 (C) | ns | ns | ns | ns | ns | ns | ns | 0.035 (T) | ns | ns | 0.027 (T) | ns | ns | ||||||||
| rs2619522 | P1763 | 3.4 | T/G | ns | ns | ns | 0.03 (T) | ns | ns | 0.017 (G) | ns | ns | 0.024 (G) | ns | ns | ns | ns | ns | 0.022 (G) | ns | ns | ||||||||
| rs1018381 | P1578 | 1.1 | C/T | ns | ns | ns | ns | ns | ns | ns | ns | ns | 0.003 (T) | ns | ns | ns | ns | ns | ns | ns | |||||||||
| rs1474605 | P1792 | 0.7 | A/G | 0.003 (G) | ns | ns | |||||||||||||||||||||||
| rs1997679 | P1795 | 2 | C/T | ns | ns | ns | |||||||||||||||||||||||
| rs909706 | P1583 | 2.2 | A/G | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ||||||||||||
| rs11558324 | P3521 | 1.3 | A/G | 0.01 (A) | |||||||||||||||||||||||||
| rs2619537 | P3587 | 0.4 | T/C | ns | |||||||||||||||||||||||||
| rs2743852 | 0.4 | C/G | ns | ns | (A) | ||||||||||||||||||||||||
| rs2619538 | SNP A | 4.1 | A/T | ns | ns | ns | ns | ns | ns | 0.025 | ns | ||||||||||||||||||
| rs742206 | P3593 | 0.2 | A/G | ns | |||||||||||||||||||||||||
| rs885773 | P1586 | 95.2 | C/T | ns | |||||||||||||||||||||||||
| rs441539 | P1294 | 109.4 | A/G | 0.018 (G) | |||||||||||||||||||||||||
| rs1000117 | P1140 | N/A | A/C | ns | |||||||||||||||||||||||||
The previous association studies are grouped by sample origin. First author, year and sample sizes are listed. Nominal p value and associated allele are shown if significant. ns = Not significant; EA = European Ancestry; AA = African Ancestry.
Bold markers were selected in the current study. rs numbers are in the first column, and alias if any in the second column. Markers are in the order from the 3 to the 5 flanking regions. SNP = Nu-cleotide changes listed as major allele/minor allele. Allele nucleotides were converted to a unified format by ensuring they were from the minus strand since DTNBP1 is transcribed from the minus strand.
The position for the first marker is nucleotide 15,569,688 in the UCSC May 2004 freeze of chromosome 6.
270 Irish high density families; TRANSMIT p values for diagnosis category D1–D2 for all individuals are shown (i.e., ‘core’ SZ – in der) [8].
268 Irish high density schizophrenia families (the same collection as that in the initial report), here also for diagnosis category D1–D2; for this table, TRANSMIT TDT p values are listed (one triad per family) [17].
Haplotypes rs2619538-rs909706 (global p = 0.011) and rs760761-rs2005976 (global p = 0.002) in the Scottish sample, and rs2005976-rs2619528 (global p = 0.00000138) in the Chinese sample were significantly associated with illness [50].
4 SNPs between P1586 and P1294 in 5 and 2 SNPs between P1283 and P3280 in 3 were also tested, but not significant [25].
102 childhood onset psychosis (72 SZ and 30 Psychosis Not Otherwise Specified) patients and their families from the US (primarily EA) [24]. this case
rs760666 and rs3213207 were excluded because MAF < 0.05. Haplotype rs742106-rs2619539-SZ, simple SZ, or poor outcome schizoaffective disor-rs2619522-rs1018381-rs909706 was significant (global p = 0.00072) [14].
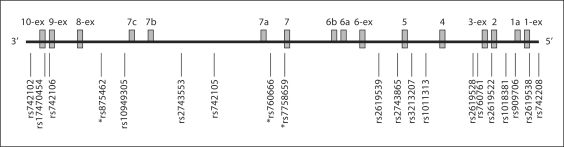
Another explanation of the inconsistent association reports (table 1) is that multiple variants in DTNBP1 might confer susceptibility to SZ [30, 32]. Most replication studies tested predominantly markers in the 5′ region of DTNBP1 (table 1); testing SZ association using a set of markers that also has good coverage at the 3′ end of the gene may help to explain or unify the divergent evidence for association. Furthermore, testing coding variants, especially missense SNPs, may also add valuable information. In a mutation scan of exons of DTNBP1 in 50 Chinese Han SZ patients and of the promoter region in 94 such patients, no mutations were detected [33]. Another mutation scan of exons and promoters in 14 familial EA SZ patients found 2 untranslated exonic SNPs, along with 9 SNPs in putative promoter regions, but no mutations in the coding sequences; these 11 SNPs were not associated (nominal p >0.05) with SZ in 552 cases and 552 controls [19]. When all 15 alternatively spliced mRNA transcripts from AceView (www.ncbi.nlm.nih.gov/IEB/Research/Acembly/; update November 2004) are considered (fig. 1), 7 missense SNPs can be defined from a total of 10 coding SNPs available in a search of dbSNP (www.ncbi.nlm.nih.gov/projects/SNP/; build 123) and the literature (table 2). None of these coding variants have so far been tested in previous association studies.
Fig. 1.
Alternative transcripts for DTNBP1 and relative positions of the tested SNPs in this study. Exons are numbered in the order of the typical 10-exon transcript variant (NM_032122). 1a, 6a, b, 7a–c indicate cryptic exons. 1-ex, 3-ex, 6-ex, 8-ex, 9-ex, and 10-ex represent exons with variable boundaries. All the information for 15 transcripts is taken from AceView, an integrated view of human genes reconstructed by co-alignment of all publicly available mRNAs and expressed sequence tags (ESTs) on the genome sequence, updated on November 29, 2004 (www.ncbi.nlm.nih.gov/IEB/Research/Acembly/). Positively associated SNPs are indicated by an asterisk.
Table 2.
FBAT results for DTNBP1 coding variants
| SNPa | Genomic positionb | Exonc | Nucleotide changesd | Amino acid changes | Sourcee |
MAFf |
FBAT p value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| dbSNP | Li et al., 2003 | dbSNP | founders | |||||||
| rs9476887 | 15,770,870 | 1-ex | C/T | Pro/Leu | + | N/A | NT | N/A | ||
| N/A | A162G | 15,746,014 | 4 | G/A | Arg/Arg | + | N/A | NT | N/A | |
| N/A | C307T | 15,735,601 | 5 | C/T | Gln/Stop | + | N/A | NT | N/A | |
| rs6926401 | 15,693,988 | 7a | C/A | Pro/His | + | N/A | 0.06 | 0.90 | ||
| rs16876589 | 15,641,476 | 8 | G/A | Gly/Asp | + | 0.005 | 0.00 | N/A | ||
| rs16876573 | 15,632,677 | 9-ex | A/G | Pro/Pro | + | 0.062 | 0.04 | 0.23 | ||
| rs16876571 | 15,632,658 | 9-ex | C/T | His/Tyr | + | 0.028 | 0.03 | 0.09 | ||
| rs16876569 | 15,632,640 | 9-ex | G/A | Ala/Thr | + | 0.006 | 0.004 | 1.00 | ||
| rs17470454 | C814T | 15,631,427 | 10 | C/T | Pro/Ser | + | + | 0.021 | 0.05 | 0.53 |
| N/A | A874G | 15,631,367 | 10 | A/G | Arg/Gly | + | N/A | 0.02 | 0.88 | |
rs number is listed first, and any other names follow from Li et al. [51].
Position on UCSC Human Genome draft May 2004 freeze.
Exon number named in the order of typical 10-exon transcript shown in figure 1 (NM_032122); -ex means extended exon; 7a is a cryptic exon.
Listed as major allele/minor allele on the minus strand.
Source of SNP is dbSNP and/or Li et al. [51] as indicated by a plus sign.
MAF = Minor allele frequency. Allele frequency is taken from dbSNP or from the founders of our family sample. N/A = Not available; NT = not tested because of failure of the genotyping assay design.
We present here a family based association study of DTNBP1 and SZ in two independently collected US SZ data sets. We have tested 26 SNPs, including 9 previously reported associated SNPs, 10 other SNPs to increase map coverage (particularly at the 3′ portion of the gene), and 7 additional coding SNPs. We found evidence for association with SZ at the 3′ end of DTNBP1.
Materials and Methods
Subjects and Phenotyping
Our total sample is comprised of 136 families ascertained from all over the US of EA (72%) and AA (18%) descent (online suppl. table 1, www.karger.com/doi10.1159/000101961). This sample was approximately equally derived from two collections, the NIMH-IRP (Intramural Research Program, also known as the Clinical Neurogenetics or CNG sample) [34, 35] and the NIMH-GI (Genetics Initiative – Part I) [36]. This combined set contained 319 genotyped individuals diagnosed as SZ (n = 273) or schizoaffective disorder (n = 46) via the criteria of the Diagnostic and Statistical Manual of Mental Disorders, third edition, revised (DSM-IIIR) [37]. Besides the affected probands (n = 136), most of the additional genotyped individuals were siblings (n = 258), parents (n = 207), or other relatives (n = 45) of the probands; genotyping these additional individuals, 183 of whom were themselves affected, made the sample more informative for the family-based association testing. Our group and others have used these families in previous linkage studies, although no suggestive or significant linkage evidence was found for 6p [34, 38, 39]. There is no overlap with any other US sample used in previous association studies with DTNBP1 and SZ. The Institutional Review Board of the Evanston Northwestern Healthcare Research Institute approved the study.
As estimated by TDT-PC (transmission disequilibrium test – power calculator) software [40], our sample has about 87% power to detect a locus with a relative risk (RR) of 2 at a significance level of 5%; but power drops to 44% for an RR of 1.4 [41].
SNP Selection and Genotyping
A total of 26 SNPs in DTNBP1 were genotyped by either TaqMan or SNPlex. Out of 10 previously reported as associated SNPs (table 1), 9 SNPs were successfully genotyped (rs742105, rs2619539, rs3213207, rs1011313, rs2619528, rs2619522, rs760761, rs1018381, and rs2619538); rs2005976 was not selected since it is tagged by the genotyped rs2619522 with r2 = 0.94 [8]. To increase map coverage, we typed 3 previously known (but not associated) SNPs (rs742106, rs760666, and rs909706), and 7 other previously untested SNPs (rs742102, rs875462, rs10949305, rs2743553, rs7758659, rs2743865, and rs742208). We also genotyped 7 out of 10 coding SNPs (3 failed assay designs; SNP-flanking DNA sequences were drawn from the UCSC Genome draft, genome.ucsc.edu; May 2004 freeze) (table 2). Thirteen SNPs (rs17470454, rs1094305, rs742105, rs7758659, rs1018381, rs16876589, rs6926401, rs16876573, rs16876571, rs16876569, A874G, rs760761, and rs2619538) were genotyped with TaqMan on an Applied Biosystems (ABI) Prism 7900 instrument. Thirteen other SNPs (rs742102, rs742106, rs875462, rs2743553, rs760666, rs2619539, rs2743865, rs3213207, rs1011313, rs2619528, rs2619522, rs909706, and rs742208) were genotyped with SNPlex following standard procedures.
Genotyping Cleaning
Genotypes were checked for Mendelian inconsistencies and unlikely recombinants via MERLIN [42]. All Mendelian inconsistencies were removed by zeroing out the genotypes for the involved individuals in that family for that SNP. For the genotypes that were flagged as ‘unlikely’ recombinants (p < 0.01), we manually checked the raw genotyping traces to search for questionable genotypes (e.g., obviously external to the genotype clusters, very low signal intensity, etc.) and questionable genotypes were zeroed. The average genotyping completion rate was 98.4% (95.7–99.4%). The total genotyping error rate was 0.54%, including 15 Mendelian errors and 55 unlikely recombinations out of 12,911 non-zero genotypes. Detected error rates for individual SNPs ranged from 0.16 to 1.30%. All the genotyping errors were blanked. Given our high genotyping completion rate and low genotyping error rates, we did not attempt second-pass genotyping for these zeroed errors. All the markers were in Hardy-Weinberg equilibrium.
Inter-Marker LD Analysis
LD between SNPs was estimated with Haploview 3.0 [43] using the genotypes from unrelated founders. The standard D’, LOD, and r2 were derived in the EA and AA subsets separately. HapMap genotype data were downloaded from HapMap (www.hapmap.org).
Association Analysis
For association analyses, we used FBAT v1.5.5 [44, 45]. Alleles and haplotypes were tested for association if there were at least 5 informative families; in our data this corresponds to testing alleles and haplotypes with frequencies over 3%. We limited the number of multi-locus systems tested by using a stepwise procedure, and limited the number of multi-locus tests to the combinations including the SNP with highest single Z score value.
Results
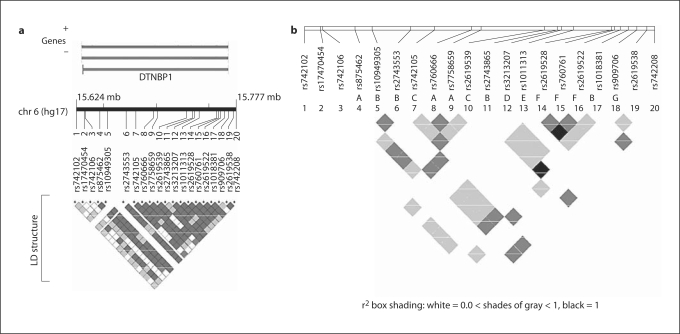
Initially, 20 SNPs in −140 kb of the genomic sequence of DTNBP1 were analyzed (all the missense SNPs, except for rs17470454, were analyzed separately because of their low minor allele frequency, MAF). Consistent with previous studies [8, 13, 17], our LD analyses in EA founders showed only one long LD block (defined by D′ >0.80 and LOD >2, about 122 kb from rs875462 to rs909706) spanning almost the whole DTNBP1 gene. The r2 estimates were variable (fig. 2); grouping SNPs with r2 >0.8 [46] yielded 7 bins (A–G) [47] of high LD, where any SNP can be a proxy (r2 >0.8) of all remaining SNPs within the bin (note that one SNP that is not tagged by other SNPs constitutes a one SNP bin). The bins often overlapped (fig. 2b). Bin B spans approximately 110 kb, encompassing rs1094305, rs2743553, rs2743865, and rs1018381. In total, 12 out of the 20 analyzed SNPs were tag SNPs.
Fig. 2.
LD structure of DTNBP1 in EA founders. a Pair wise LD structure is shown as matrix according to D' and LOD at the bottom. Genomic positions are according to the NCBI Build 35 of the human genome assembly (genome.ucsc.edu; May 2004 freeze). We generated the figure by the LocusView program (www.broad.mit.edu/mpg/locusview/). b r2 among different markers. Within the long LD block (D'), seven LD bins measured by r2 are indicated with A to G.
The LD patterns and allele frequencies of the tested markers were similar in EA founders and in AA founders, although LD was weaker in AAs (data not shown). We have performed association analyses in the EA and AA subsets separately, in addition to a combined analysis. Single-locus FBAT results for the 12 tag SNPs (r2 <0.8) are summarized in table 3. We found evidence for association with allele G of rs7758659 on the 3′ end of DTNBP1 in both the EA subset (p = 0.031) and the smaller AA subset (p = 0.045). Since the over-transmitted alleles for all the tested SNPs were the same in EA and AA, we also performed association tests in the combined sample. Only rs7758659 remained associated after Bonferroni correction (p = 0.004; p = 0.048 when corrected by 12, the number of tag SNPs). Because rs7758659 tags two other SNPs, rs875462 and rs760666 (bin A with r2 >0.94; fig. 2b), the association is also present (and with similar significance) with each of these SNPs (online suppl. table 2).
Table 3.
FBAT results for 12 DTNBP1 tag SNPsa
| Markersb | Distancec | SNP | Allele | All families |
EA Subset |
AA Subset |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq. | Z score | p value | Freq. | Z score | p value | Freq. | Z score | p value | |||||
| rs742102 | 6,815 | C/T | T | 0.04 | 1.07 | 0.28 | 0.03 | 1.01 | 0.31 | 0.03 | N/A | N/A | |
| rs17470454 | 1,032 | C/T | C | 0.95 | 0.67 | 0.5 | 0.93 | 0.63 | 0.53 | 0.98 | N/A | N/A | |
| rs742106 | P1328 | 68,760 | C/T | T | 0.31 | 0.26 | 0.79 | 0.33 | 0.64 | 0.52 | 0.20 | −0.27 | 0.79 |
| rs7758659 | 27,615 | G/A | G | 0.81 | 2.84 | 0.004 | 0.77 | 2.16 | 0.03 | 0.92 | 2.00 | 0.045 | |
| rs2619539 | P1655 | 5,448 | C/G | G | 0.4 | 0.55 | 0.58 | 0.44 | 0.43 | 0.67 | 0.45 | 0.343 | 0.73 |
| rs2743865 | 1,799 | C/T | T | 0.17 | 0.87 | 0.38 | 0.1 | 0.47 | 0.64 | 0.10 | 0.47 | 0.64 | |
| rs3213207 | P1635 | 5,330 | A/G | A | 0.9 | 0.55 | 0.58 | 0.87 | 0.64 | 0.52 | 0.98 | N/A | N/A |
| rs1011313 | P1325 | 20,217 | G/A | A | 0.09 | 0.14 | 0.89 | 0.1 | −0.31 | 0.76 | 0.02 | N/A | N/A |
| rs2619522 | P1763 | 7,222 | T/G | T | 0.72 | 0.22 | 0.83 | 0.77 | 0.82 | 0.41 | 0.51 | −0.32 | 0.75 |
| rs909706 | P1583 | 4,338 | A/G | G | 0.31 | 1.33 | 0.18 | 0.32 | 1.43 | 0.15 | 0.20 | −0.62 | 0.53 |
| rs2619538 | SNP A | 3,452 | A/T | T | 0.43 | 0.58 | 0.56 | 0.42 | 1.09 | 0.27 | 0.37 | −0.61 | 0.54 |
| rs742208 | N/A | T/C | C | 0.17 | 1.00 | 0.32 | 0.11 | −0.12 | 0.90 | 0.43 | 1.35 | 0.18 | |
Results are shown in the whole sample, and EA and AA subsets. N/A = Not available due to the small number of AA families; SNP = nucleotide changes listed as major allele/minor allele. Allele nucleotides were converted to a unified format by ensuring they were from the minus strand. Nominal p value and associated allele are shown. Freq. = Frequency of the more often transmitted allele. Significant SNP (rs7758659) row is bold.
rs numbers are in the first column, and alias if any in the second column. Markers are in the order from the 3 to the 5 flanking regions.
Distance to next marker in base pairs (bp). The position for the first marker is nucleotide 15,624,612 in the UCSC May 2004 freeze of chromosome 6.
We performed haplotypic analyses using the 12 tag SNPs, starting by analyzing all two-marker systems anchored with rs7758659 (table 4). In the EA subset, 3 out of the 11 two-marker combinations gave global p values <0.05. The most significant two-marker system was rs7758659-rs2619522 (global p = 0.016). In the AA subset, only one two-marker system, rs7758659-rs3213207, showed nominal significance (global p = 0.02; the corresponding global p value in the EA families was 0.03). In the combined dataset, 9 of the 11 two-marker combinations gave global p values <0.05. The most significant two-marker haplotype was found with rs7758659-rs3213207 (global p = 0.0015; the only two-marker combination with higher significance than rs7758659, p = 0.004, by itself). rs3213207 (also known as P1635 [1]) is the most frequently reported associated SNP, albeit with the associated alleles being different across those studies (table 1). Haplotypic analyses with a three-marker system anchored to rs7758659-rs3213207 did not increase significance (data not shown). We have also extended haplotypic analysis to 7 markers from rs875462 to rs909706 (tag SNPs: rs7758659, rs2619539, rs2743865, rs3213207, rs1011313, rs2619522, and rs909706; each representing one bin, A-G). Ninety-eight percent of the haplotypes were represented by only 6 common (frequency >5%) haplotypes. None of those 6 common haplotypes yielded a global p value or haplotypic p value <0.05, with haplotype G-C-C-A-A-T-A (frequency = 20%) having the lowest p value of 0.08.
Table 4.
FBAT results for two-marker haplotypes anchoring with rs7758659 in DTNBP1a
| Markers | Haplotype | All families |
EA subset |
||||||
|---|---|---|---|---|---|---|---|---|---|
| frequency | Z score | p value | global p | frequency | Z score | p value | global p | ||
| rs742102-rs7758659 | C-G | 0.78 | 2.33 | 0.020 | 0.029 | 0.76 | 1.82 | 0.068 | 0.15 |
| rs17470454-rs7758659 | C-G | 0.75 | 3.13 | 0.0018 | 0.0081 | 0.71 | 2.50 | 0.012 | 0.047 |
| rs742106-rs7758659 | C-G | 0.51 | 1.86 | 0.063 | 0.017 | 0.46 | 0.97 | 0.33 | 0.12 |
| rs7758659-rs2619539 | G-C | 0.37 | 1.80 | 0.071 | 0.009 | 0.30 | 1.52 | 0.13 | 0.067 |
| rs7758659-rs2743865 | G-C | 0.65 | 1.98 | 0.047 | 0.013 | 0.69 | 1.74 | 0.082 | 0.11 |
| rs7758659-rs3213207 | G-A | 0.69 | 3.28 | 0.0010 | 0.0015 | 0.64 | 2.49 | 0.013 | 0.031 |
| rs7758659-rs1011313 | G-G | 0.72 | 2.27 | 0.023 | 0.017 | 0.68 | 2.15 | 0.032 | 0.066 |
| rs7758659-rs2619522 | G-T | 0.53 | 2.59 | 0.010 | 0.0073 | 0.54 | 2.72 | 0.0065 | 0.016 |
| rs7758659-rs909706 | G-G | 0.30 | 1.52 | 0.13 | 0.018 | 0.32 | 1.46 | 0.14 | 0.12 |
| rs7758659-rs2619538 | G-T | 0.38 | 1.19 | 0.23 | 0.067 | 0.38 | 1.29 | 0.20 | 0.24 |
| rs7758659-rs742208 | G-T | 0.64 | 1.58 | 0.11 | 0.073 | 0.68 | 1.49 | 0.14 | 0.36 |
Only haplotypes anchoring with rs7758659 were tested, and only the most over-transmitted haplotypes are listed. All the significant haplotypes contain allele G of rs7758659, consistent with the result of single marker analyses.
Finally, we tested association with the coding SNPs in DTNBP1 (not included in haplotype analysis due to low MAF). None of the 7 genotyped coding SNPs showed association with SZ (p values >0.09; table 2) or co-segregated with disease. We also searched for aggregations of missense SNPs within families, but found no evidence of this. However, it is worth noting that the MAF of rs17470454 (C814T; Pro272Ser) was higher in founders in our samples (5%) than that in dbSNP (2%); interestingly, the MAF in EA probands was found to be even higher (8.2%) than in founders, though no transmission distortion was found (p = 0.53; table 2).
Discussion
We have studied 26 SNPs in DTNBP1, including 6 missense variants. Our results support an association of SZ with three highly correlated markers in the 3′ region of DTNBP1 (rs875462, rs760666, and rs7758659). The association was observed in both EA and AA subsets with the same over-transmitted allele (G) at rs7758659. rs875462 and rs7758659 were previously untested, while rs760666 (or P1287 [8]) had been previously tested, but no evidence for association was reported (table 1). Interestingly, rs7758659 is near the boundary of exon 7 and intron 7 (being 49 bp away from exon 7), suggesting the possibility that it might affect splicing.
Association Evidence for SNPs Located at the 3′ Region of the DTNBP1 Gene
The four initially reported associated markers[8] and an associated haplotype that spans a promoter SNP, rs2619538 (SNP A) [19], are all located in the 5′ region of the DTNBP1. All other replication studies limited their tests to regions in the 5′ portion of the gene, as it has been speculated that the potential causative polymorphism(s) lie in the 5′ region of DTNBP1. With a more comprehensive set of markers than in previous studies, we did not observe evidence of association with SNPs located in the 5′ region of the gene; instead, we found evidence for association of SZ with SNPs located in the 3′ region of DTNBP1. However, it may not be possible to define the location of a true disease variant in a long region with strong LD (as in DTNBP1) [48]. One could argue that rs875462, rs760666, and rs7758659 that were found associated to SZ in our study are, in fact, in LD with some other untested variants in the 5′ region of the gene. On the other hand, it is also conceivable that associated markers located in the 5′ region of the gene may actually reflect associations with causative loci in the 3′ region. Indeed, the LD pattern of DTNBP1 allows for this possibility (fig. 2b). For instance, rs1018381 in the 5′region of the gene has been reported to be associated with SZ [15, 20], but this SNP is highly correlated (r2 >0.90) with three other SNPs in bin B (fig. 2b), two of which (rs1094305 and rs2743553) are in the 3′ region of DTNBP1.
Missense Variants in DTNBP1 May Still Confer Susceptibility to SZ
We have tested 7 DTNBP1 coding variants, but none of them showed association with SZ (table 2). It is nonetheless worth noting that some of these missense SNPs are in LD with some previously reported associated SNPs: rs3213207 (in bin D) was found in our data moderately correlated (r2 = 0.52) to rs17470454 (C814T; Pro272Ser; not tested in any of the previous studies). The MAF of rs17470454 was higher in SZ probands than in founders and dbSNP, albeit not reaching statistical significance. The substitution of a proline for a serine at rs17470454 is predicted by PolyPhen (tux.embl-heidelberg.de/ramensky/index.shtml) to be a non-conservative change with potential for impairing DTNBP1 function [49]. Therefore, rs17470454 might (together with other still unrecognized functional SNPs) underlie the previously observed association between DTNBP1 and SZ [8, 9, 13, 17, 18, 23].
In conclusion, our results provide evidence for an association of SNPs at the 3′ end of DTNBP1 and SZ in both EA and AA samples, but not at the 5′ region of DTNBP1. However, our sample has limited statistical power and some true loci might not have been detected. Furthermore, in the absence of a molecular hypothesis that can provide a clear mechanistic explanation, association tests should be considered ‘indirect’, and this severely constrains the statistical power. Though we genotyped a denser marker map than other studies (table 1), the SNP set we have used only captures 76% of all the common SNPs of DTNBP1 (MAF >0.05) currently available in HapMap Phase II (release #20) at r2 >0.8 (data not shown). A total of 42 SNPs (based on all the known variants in DTNBP1) would be needed to capture all alleles at DTNBP1 locus [31]. A critical experiment would require a large sample with sufficient statistical power and genotyping of a comprehensive and sufficiently dense SNP set.
Supplementary Material
Subjects and Phenotyping
FBAT Results for All 20 Markers of DTNBP1a
Acknowledgements
The National Institute of Mental Health (NIMH) Schizophrenia Genetics Initiative families, data and biomaterials were collected in three projects. From 1991–1997, the Principal Investigators and Co-Investigators were: Harvard University, Boston, Mass., USA, U01 MH46318, Ming T. Tsuang, MD, PhD, DSc, Stephen Faraone, PhD, and John Pepple, PhD; Washington University, St. Louis, Mo., USA, U01 MH46276, C. Robert Cloninger, MD, Theodore Reich, MD, and Dragan Svrakic, MD; Columbia University, New York, N.Y., USA, U01 MH46289, Charles Kaufmann, MD, Dolores Malaspina, MD, and Jill Harkavy Friedman, PhD. Research Career Development Awards (to J.D. and A.R.S.) at the Evanston Northwestern Healthcare Research Institute supported this work.
Supplementary information is available at Human Heredity's website.
References
- 1.Straub RE, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, O'Neill FA, Walsh D, Kendler KS. Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes. Mol Psychiatry. 2002;7:542–559. doi: 10.1038/sj.mp.4001051. [DOI] [PubMed] [Google Scholar]
- 2.Moises HW, Yang L, Kristbjarnarson H, Wiese C, Byerley W, Macciardi F, Arolt V, Blackwood D, Liu X, Sjogren B, Aschauer HN, Hwu HG, Jang K, Livesley WJ, Kennedy JL, Zoega T, Ivarsson O, Bui MT, Yu MH, Havsteen B, Commenges D, Weissenbach J, Schwinger E, Gottesman II, Pakstis AJ, Wetterberg L, Kidd KK, Helgason T. An international two-stage genome-wide search for schizophrenia susceptibility genes. Nat Genet. 1995;11:321–324. doi: 10.1038/ng1195-321. [DOI] [PubMed] [Google Scholar]
- 3.Schwab SG, Hallmayer J, Albus M, Lerer B, Eckstein GN, Borrmann M, Segman RH, Hanses C, Freymann J, Yakir A, Trixler M, Falkai P, Rietschel M, Maier W, Wildenauer DB. A genome-wide autosomal screen for schizophrenia susceptibility loci in 71 families with affected siblings: support for loci on chromosome 10p and 6. Mol Psychiatry. 2000;5:638–649. doi: 10.1038/sj.mp.4000791. [DOI] [PubMed] [Google Scholar]
- 4.Brzustowicz LM, Honer WG, Chow EW, Hogan J, Hodgkinson K, Bassett AS. Use of a quantitative trait to map a locus associated with severity of positive symptoms in familial schizophrenia to chromosome 6p. Am J Hum Genet. 1997;61:1388–1396. doi: 10.1086/301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLisi LE, Shaw SH, Crow TJ, Shields G, Smith AB, Larach VW, Wellman N, Loftus J, Nanthakumar B, Razi K, Stewart J, Comazzi M, Vita A, Heffner T, Sherrington R. A genome-wide scan for linkage to chromosomal regions in 382 sibling pairs with schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:803–812. doi: 10.1176/appi.ajp.159.5.803. [DOI] [PubMed] [Google Scholar]
- 6.Williams NM, Norton N, Williams H, Ekholm B, Hamshere ML, Lindblom Y, Chowdari KV, Cardno AG, Zammit S, Jones LA, Murphy KC, Sanders RD, McCarthy G, Gray MY, Jones G, Holmans P, Nimgaonkar V, Adolfson R, Osby U, Terenius L, Sedvall G, O'Donovan MC, Owen MJ. A systematic genomewide linkage study in 353 sib pairs with schizophrenia. Am J Hum Genet. 2003;73:1355–1367. doi: 10.1086/380206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez BK, Duan J, Sanders AR, Hinrichs AL, Jin CH, Hou C, Buccola NG, Hale N, Weilbaecher AN, Nertney DA, Olincy A, Green S, Schaffer AW, Smith CJ, Hannah DE, Rice JP, Cox NJ, Martinez M, Mowry BJ, Amin F, Silverman JM, Black DW, Byerley WF, Crowe RR, Freedman R, Cloninger CR, Levinson DF, Gejman PV. Genomewide linkage scan of 409 European-Ancestry and African American families with schizophrenia: Suggestive evidence of linkage at 8p23.3-p21.2 and 11p13.1-q14.1 in the combined sample. Am J Hum Genet. 2006;78:315–333. doi: 10.1086/500272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C, Wormley B, Sadek H, Kadambi B, Cesare AJ, Gibberman A, Wang X, O'Neill FA, Walsh D, Kendler KS. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, Ozaki N, Taguchi T, Tatsumi M, Kamijima K, Straub RE, Weinberger DR, Kunugi H, Hashimoto R. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13:2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- 10.Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 11.Bray NJ, Preece A, Williams NM, Moskvina V, Buckland PR, Owen MJ, O'Donovan MC. Haplotypes at the dystrobrevin binding protein 1 (DTNBP1) gene locus mediate risk for schizophrenia through reduced DTNBP1 expression. Hum Mol Genet. 2005;14:1947–1954. doi: 10.1093/hmg/ddi199. [DOI] [PubMed] [Google Scholar]
- 12.Owen MJ, Williams NM, O'Donovan MC. Dysbindin-1 and schizophrenia: from genetics to neuropathology. J Clin Invest. 2004;113:1255–1257. doi: 10.1172/JCI21470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M, Lerer B, Rietschel M, Trixler M, Maier W, Wildenauer DB. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72:185–190. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang JX, Zhou J, Fan JB, Li XW, Shi YY, Gu NF, Feng GY, Xing YL, Shi JG, He L. Family-based association study of DTNBP1 in 6p22.3 and schizophrenia. Mol Psychiatry. 2003;8:717–718. doi: 10.1038/sj.mp.4001287. [DOI] [PubMed] [Google Scholar]
- 15.Van Den Bogaert A, Schumacher J, Schulze TG, Otte AC, Ohlraun S, Kovalenko S, Becker T, Freudenberg J, Jonsson EG, Mattila-Evenden M, Sedvall GC, Czerski PM, Kapelski P, Hauser J, Maier W, Rietschel M, Propping P, Nothen MM, Cichon S. The DTNBP1 (dysbindin) gene contributes to schizophrenia, depending on family history of the disease. Am J Hum Genet. 2003;73:1438–1443. doi: 10.1086/379928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris DW, McGhee KA, Schwaiger S, Scully P, Quinn J, Meagher D, Waddington JL, Gill M, Corvin AP. No evidence for association of the dysbindin gene (DTNBP1) with schizophrenia in an Irish population-based study. Schizophr Res. 2003;60:167–172. doi: 10.1016/s0920-9964(02)00527-3. [DOI] [PubMed] [Google Scholar]
- 17.Van Den Oord EJ, Sullivan PF, Jiang Y, Walsh D, O'Neill FA, Kendler KS, Riley BP. Identification of a high-risk haplotype for the dystrobrevin binding protein 1 (DTNBP1) gene in the Irish study of high-density schizophrenia families. Mol Psychiatry. 2003;8:499–510. doi: 10.1038/sj.mp.4001263. [DOI] [PubMed] [Google Scholar]
- 18.Kirov G, Ivanov D, Williams NM, Preece A, Nikolov I, Milev R, Koleva S, Dimitrova A, Toncheva D, O'Donovan MC, Owen MJ. Strong evidence for association between the dystrobrevin binding protein 1 gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from Bulgaria. Biol Psychiatry. 2004;55:971–975. doi: 10.1016/j.biopsych.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 19.Williams NM, Preece A, Morris DW, Spurlock G, Bray NJ, Stephens M, Norton N, Williams H, Clement M, Dwyer S, Curran C, Wilkinson J, Moskvina V, Waddington JL, Gill M, Corvin AP, Zammit S, Kirov G, Owen MJ, O'Donovan MC. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1) Arch Gen Psychiatry. 2004;61:336–344. doi: 10.1001/archpsyc.61.4.336. [DOI] [PubMed] [Google Scholar]
- 20.Funke B, Finn CT, Plocik AM, Lake S, DeRosse P, Kane JM, Kucherlapati R, Malhotra AK. Association of the DTNBP1 locus with schizophrenia in a US population. Am J Hum Genet. 2004;75:891–898. doi: 10.1086/425279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall D, Gogos JA, Karayiorgou M. The contribution of three strong candidate schizophrenia susceptibility genes in demographically distinct populations. Genes Brain Behav. 2004;3:240–248. doi: 10.1111/j.1601-183X.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 22.De Luca V, Voineskos D, Shinkai T, Wong G, Kennedy JL. Untranslated region haplotype in dysbindin gene: analysis in schizophrenia. J Neural Transm. 2005;112:1263–1267. doi: 10.1007/s00702-005-0338-9. [DOI] [PubMed] [Google Scholar]
- 23.Li T, Zhang F, Liu X, Sun X, Sham PC, Crombie C, Ma X, Wang Q, Meng H, Deng W, Yates P, Hu X, Walker N, Murray RM, St Clair D, Collier DA. Identifying potential risk haplotypes for schizophrenia at the DTNBP1 locus in Han Chinese and Scottish populations. Mol Psychiatry. 2005;10:1037–1044. doi: 10.1038/sj.mp.4001718. [DOI] [PubMed] [Google Scholar]
- 24.Gornick MC, Addington AM, Sporn A, Gogtay N, Greenstein D, Lenane M, Gochman P, Ordonez A, Balkissoon R, Vakkalanka R, Weinberger DR, Rapoport JL, Straub RE. Dysbindin (DTNBP1, 6p22.3) is associated with childhood-onset psychosis and endophenotypes measured by the Premorbid Adjustment Scale (PAS) J Autism Dev Disord. 2005;35:831–838. doi: 10.1007/s10803-005-0028-3. [DOI] [PubMed] [Google Scholar]
- 25.Wood LS, Pickering EH, Dechairo BM: Significant support for DAO as a Schizophrenia Susceptibility Locus: Examination of five genes putatively associated with schizophrenia. Biol Psychiatry 2006 (Epub ahead of print). [DOI] [PubMed]
- 26.Joo EJ, Lee KY, Jeong SH, Ahn YM, Koo YJ, Kim YS. The dysbindin gene (DTNBP1) and schizophrenia: no support for an association in the Korean population. Neurosci Lett. 2006;407:101–106. doi: 10.1016/j.neulet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Tochigi M, Zhang X, Ohashi J, Hibino H, Otowa T, Rogers M, Kato T, Okazaki Y, Kato N, Tokunaga K, Sasaki T. Association study of the dysbindin (DTNBP1) gene in schizophrenia from the Japanese population. Neurosci Res. 2006;56:154–158. doi: 10.1016/j.neures.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, Steel G, Nestadt G, Liang KY, Huganir RL, Valle D, Pulver AE. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holliday EG, Handoko HY, James MR, McGrath JJ, Nertney DA, Tirupati S, Thara R, Levinson DF, Hayward NK, Mowry BJ, Nyholt DR. Association study of the dystrobrevin-binding gene with schizophrenia in Australian and Indian samples. Twin Res Hum Genet. 2006;9:531–539. doi: 10.1375/183242706778025035. [DOI] [PubMed] [Google Scholar]
- 30.Williams NM, O'Donovan MC, Owen MJ. Is the dysbindin gene (DTNBP1) a susceptibility gene for schizophrenia? Schizophr Bull. 2005;31:800–805. doi: 10.1093/schbul/sbi061. [DOI] [PubMed] [Google Scholar]
- 31.Mutsuddi M, Morris DW, Waggoner SG, Daly MJ, Scolnick EM, Sklar P. Analysis of high-resolution HapMap of DTNBP1 (Dysbindin) suggests no consistency between reported common variant associations and schizophrenia. Am J Hum Genet. 2006;79:903–909. doi: 10.1086/508942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendler KS. Schizophrenia genetics and dysbindin: a corner turned? Am J Psychiatry. 2004;161:1533–1536. doi: 10.1176/appi.ajp.161.9.1533. [DOI] [PubMed] [Google Scholar]
- 33.Liao HM, Chen CH. Mutation analysis of the human dystrobrevin-binding protein 1 gene in schizophrenic patients. Schizophr Res. 2004;71:185–189. doi: 10.1016/j.schres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Cao Q, Martinez M, Zhang J, Sanders AR, Badner JA, Cravchik A, Markey CJ, Beshah E, Guroff JJ, Maxwell ME, Kazuba DM, Whiten R, Goldin LR, Gershon ES, Gejman PV. Suggestive evidence for a schizophrenia susceptibility locus on chromosome 6q and a confirmation in an independent series of pedigrees. Genomics. 1997;43:1–8. doi: 10.1006/geno.1997.4815. [DOI] [PubMed] [Google Scholar]
- 35.Gejman PV, Sanders AR, Badner JA, Cao Q, Zhang J. Linkage analysis of schizophrenia to chromosome 15. Am J Med Genet. 2001;105:789–793. doi: 10.1002/ajmg.1552. [DOI] [PubMed] [Google Scholar]
- 36.Cloninger CR, Kaufmann CA, Faraone SV, Malaspina D, Svrakic DM, Harkavy-Friedman J, Suarez BK, Matise TC, Shore D, Lee H, Hampe CL, Wynne D, Drain C, Markel PD, Zambuto CT, Schmitt K, Tsuang MT. Genome-wide search for schizophrenia susceptibility loci: the NIMH Genetics Initiative and Millennium Consortium. Am J Med Genet. 1998;81:275–281. [PubMed] [Google Scholar]
- 37.APA . Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 38.Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD, Meyer J, Zambuto CT, Schmitt K, Matise TC, Harkavy Friedman JM, Hampe C, Lee H, Shore D, Wynne D, Faraone SV, Tsuang MT, Cloninger CR. NIMH Genetics Initiative Millenium Schizophrenia Consortium: linkage analysis of African-American pedigrees. Am J Med Genet. 1998;81:282–289. [PubMed] [Google Scholar]
- 39.Faraone SV, Matise T, Svrakic D, Pepple J, Malaspina D, Suarez B, Hampe C, Zambuto CT, Schmitt K, Meyer J, Markel P, Lee H, Harkavy Friedman J, Kaufmann C, Cloninger CR, Tsuang MT. Genome scan of European-American schizophrenia pedigrees: results of the NIMH Genetics Initiative and Millennium Consortium. Am J Med Genet. 1998;81:290–295. [PubMed] [Google Scholar]
- 40.Chen WM, Deng HW. A general and accurate approach for computing the statistical power of the transmission disequilibrium test for complex disease genes. Genetic Epidemiology. 2001;21:53–67. doi: 10.1002/gepi.1018. [DOI] [PubMed] [Google Scholar]
- 41.Duan J, Martinez M, Sanders AR, Hou C, Krasner AJ, Schwartz DB, Gejman PV. Neuregulin 1 (NRG1) and schizophrenia: Analysis of a US family sample and the evidence in the balance. Psychol Med. 2005;35:1599–1610. doi: 10.1017/S0033291705005428. [DOI] [PubMed] [Google Scholar]
- 42.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin – rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 43.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 44.Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(suppl 1):S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Rabinowitz D, Laird N. A unified approach to adjusting association tests for population admixture with arbitrary pedigree structure and arbitrary missing marker information. Hum Hered. 2000;50:211–223. doi: 10.1159/000022918. [DOI] [PubMed] [Google Scholar]
- 46.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinds DA, Stuve LL, Nilsen GB, Halperin E, Eskin E, Ballinger DG, Frazer KA, Cox DR. Whole-genome patterns of common DNA variation in three human populations. Science. 2005;307:1072–1079. doi: 10.1126/science.1105436. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence R, Evans DM, Morris AP, Ke X, Hunt S, Paolucci M, Ragoussis J, Deloukas P, Bentley D, Cardon LR. Genetically indistinguishable SNPs and their influence on inferring the location of disease-associated variants. Genome Res. 2005;15:1503–1510. doi: 10.1101/gr.4217605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li T, Stefansson H, Gudfinnsson E, Cai G, Liu X, Murray RM, Steinthorsdottir V, Januel D, Gudnadottir VG, Petursson H, Ingason A, Gulcher JR, Stefansson K, Collier DA. Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Molecular Psychiatry. 2004;9:698–704. doi: 10.1038/sj.mp.4001485. [DOI] [PubMed] [Google Scholar]
- 51.Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, Jenkins NA, Amato D, Roe BA, Starcevic M, Dell'Angelica EC, Elliott RW, Mishra V, Kingsmore SF, Paylor RE, Swank RT. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subjects and Phenotyping
FBAT Results for All 20 Markers of DTNBP1a