Abstract
Parkinson's disease (PD) researchers have pioneered the use of cell-based therapies (CBTs) in the central nervous system. CBTs for PD were originally envisioned as a way to replace the dopaminergic nigral neurons lost with the disease. Several sources of catecholaminergic cells, including autografts of adrenal medulla and allografts or xenografts of mesencephalic fetal tissue, were successfully assessed in animal models, but their clinical translation has yielded poor results and much controversy. Recent breakthroughs on cell biology are helping to develop novel cell lines that could be used for regenerative medicine. Their future successful clinical application depends on identifying and solving the problems encountered in previous CBTs trials. In this review, we critically analyze past CBTs' clinical translation, the impact of the host in graft survival, and the role of preclinical studies and emerging new cell lines. We propose that the prediction of clinical results from preclinical studies requires experimental designs that allow blind data acquisition and statistical analysis, assessment of the therapy in models that parallel clinical conditions, looking for sources of complications or side effects, and limiting optimism bias when reporting outcomes. Antioxid. Redox Signal. 11, 2189–2208.
Introduction
Parkinson's disease (PD) is a chronic, progressive neurodegenerative disorder of unknown etiology that affects 1% of the population over 55 years of age. It is characterized by specific movement dysfunction that includes resting tremor, bradykinesia (slowing of voluntary movements), altered gait, muscular rigidity, postural instability, and flat unemotional, fixed facial expression. The pathological hallmarks of the disease are the loss of dopamine (DA)-producing neurons in the substantia nigra pars compacta (SNpc) and the presence of intracytoplasmic inclusions called Lewy bodies that are formed primarily from alpha-synuclein and ubiquitin. Chronic inflammation and signs of oxidative stress have been observed in the brains of PD patients (151). A definitive cause of PD is not clear, but risks factors such as increasing age and exposure to environmental toxins have been linked to the sporadic form of the disease. The identification of alpha-synuclein genetic mutations in familial cases (118, 147) has renewed the search for a genetic component in PD and has brought attention to the role of the intracytoplasmic accumulation of proteins in neurodegeneration. Other genetic mutations have since been implicated in both dominant and recessive occurrences of parkinsonism: a heterozygous leucine-rich repeat kinase 2 (LRRK2) mutation in cases of autosomal dominant parkinsonism (39, 78) and three specific genes (parkin, PINK1, and DJ-1) in autosomal recessive, early-onset parkinsonism (157, 101).
The loss of dopaminergic neurons in the SNpc results in decreased levels of DA in the striatum (composed of the caudate nucleus and putamen). This is the primary area of projection of these neurons and part of the network that modulates motor function. DA replacement, mainly by oral administration of L-DOPA or DA agonists, has become the mainstay of pharmaceutical and adjunctive therapeutic treatment. As the disease progresses, new symptoms become evident, a number of which do not respond to DA replacement (21, 88). With continued administration, the period in which L-DOPA is efficacious (“on time”) decreases and abnormal movements (dyskinesias) related to the treatment appear (16). Deep brain stimulation (DBS) of the globus pallidus interna (Gpi) or the subthalamic nucleus are effective surgical treatment options that restore functional balance in the motor neural network; yet, as the number of patients treated with DBS increases, so do the reports of surgical and nonsurgical complications resulting from this approved therapy (e.g., ref. 167). Clinical trials attempting to replace lost dopaminergic nigral neurons (these trials will be discussed later in this review) did not produce significant improvement in PD symptoms and were associated with serious side effects.
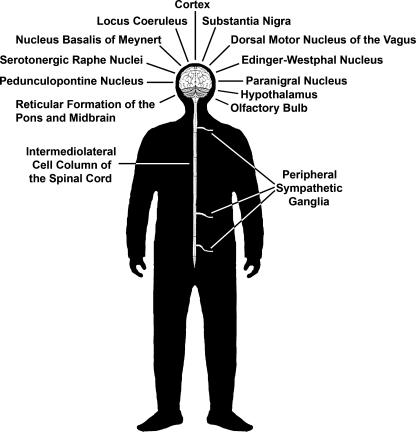
PD also presents with a variety of nonmotor symptoms, such as autonomic dysfunction and sleep disturbances (88). These are not responsive to treatments targeting DA loss and are associated with the presence of Lewy bodies and neurodegeneration of areas in the central and peripheral nervous system different from the dopaminergic nigrostriatal nuclei (Fig. 1). This newly acquired appreciation of PD complexity, in conjunction with the limitations of current therapies and the failure of cell replacement trials, has shifted the emphasis of the field from cell replacement of DA neurons to the development of neuroprotective strategies aiming to slow or halt PD progression in the SNpc and beyond. The search for biomarkers that will provide early identification of PD and allow implementation of intervention, as well as an unbiased assessment of disease progression, are new research priorities.
FIG. 1.
Areas of the central and peripheral nervous system presenting pathology in PD.
What are cell-based therapies (CBTs) and what role can they play in our current understanding of PD? CBTs can be defined as a class of therapeutic agents that are intended for implantation, transplantation, infusion, or transfer into a human recipient. They include cells and tissues, as well as cellular and tissue-based products (65). Classified in this fashion, CBTs can provide solutions to PD that go beyond replacing lost DA neurons, additionally helping to produce and deliver treatments.
In this article, we first examine the findings of past CBT preclinical and clinical trials (Fig. 2), ongoing studies and new cell sources. We then analyze the encountered problems. We conclude by proposing strategies to help predict, and overcome the hurdles inherent to, potential clinical issues associated with the application of cell-based technologies for treating PD.
FIG. 2.
Timeline of major events shaping the development of CBTs for PD. Refer to text for a more comprehensive list.
Repairing Nigral Cell Loss: The Original Goal of CBTs
The death of dopaminergic nigral neurons and concomitant loss of striatal DA have been at the epicenter of PD research since its identification in the early 1960s (17, 46), in accordance with Tretiakoff's 1919 thesis (165a). This stems from the fact that the typical and most disabling PD symptoms (motor dysfunction) are associated with nigral neurodegeneration and have been shown to be responsive to DA therapy. Attempting to replace the lost cells with functioning equivalent cells was the original application of CBTs: the possibility of repairing the circuit was the ultimate goal of neurotransplantation. This idea was supported by the belief that the brain was a post-mitotic organ that, after reaching full development, did not have the capacity for self-repair. New neurons were not believed to develop in the adult brain. At that time, knowledge of trophic factors and their potential to stimulate plastic changes was limited. The first report of successful “brain transplants” was produced by Dunn in 1917 (44), after brain tissue was grafted from one newborn rat into another (Fig. 2). In the late 1970s, Bjorklund and colleagues (18) suggested the conceptual basis of CBTs for cell replacement in PD. The investigators used rats that received unilateral administration of the dopaminergic neurotoxin 6-hydroxy-dopamine (6-OHDA) in the medial forebrain bundle. They assessed improvements in the hemiparkinsonian syndrome by measuring changes in the direction and intensity of the rotations induced by dopaminergic agents. The investigators showed that transplantation of mesencephalic fetal tissue into the striatum led to increased neuronal survival and amelioration of circling behaviors (18).
Investigators proposing the clinical application of CBTs realized that translation would require testing their efforts in hosts with larger brains and more complex anatomy and behavior than rodents (28). The identification of the human dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was a discovery that revolutionized PD research and helped to develop the application of CBTs (37, 89). MPTP can cross the blood–brain barrier and is transformed in the brain into its toxic metabolite MPP+ by the enzyme monoamine oxidase B (MAO B). In monkeys, MPTP can be administered by different routes (e.g., i.v., i.m., intracarotid artery), and the characteristics of the resultant PD syndrome depend on the method of infusion. MPTP toxicity was also tested in rodents and found to affect mice, as they, like human and nonhuman primates, have high levels of intracerebral MAO B. (119).
While administration of 6-OHDA in rats and MPTP in mice produces DA nigrostriatal loss, the induced symptoms in rodents are not entirely comparable with human PD. In contrast, nonhuman primates intoxicated with MPTP develop a PD-like state that closely resembles the sporadic form of the disease (23, 48, 90). Another issue of importance for CBT evaluation is that, compared to monkeys, rodents' brain size and complexity allows for limited evaluation of surgical targets and cell distribution. Furthermore, laboratory rats and mice are highly inbred and, as such, their immunological response to foreign cells is compromised in relation to other species. The investigators pioneering grafting techniques used primarily MPTP to induce parkinsonism in monkeys in order to test fetal monkey tissue transplants in a DA-depleted host. Ultimately, they were simultaneously establishing a new model of PD while developing original transplant methods.
As fetal tissue availability is limited and accompanied by ethical considerations based on its origin, investigators searched for alternative sources of DA-releasing cells. Autologous grafts of adrenal medullary tissue were proposed with the rationale that self-transplants would be immunologically compatible and devoid of the risk of disease transfer between donor and host. Success would then depend upon the patients' overall health and ability to overcome two major surgeries: the first, an abdominal procedure to extract the adrenal medulla, and the second, a neurosurgical procedure to introduce the graft into the striatum. The selection of the striatal target was to ensure prompt DA release in the primary area affected by nigral cell loss. In 1981, Freed et al. (58) showed graft survival in rats. In 1984, Morihisa and colleagues (111) transplanted adrenal medullary tissue into MPTP-treated parkinsonian rhesus monkeys and showed limited cell survival in four animals (10‱300 cells per subject). In this study, the investigators also transplanted fetal cells, but they did not survive. In follow-up experiments, poor survival of transplanted adrenal tissue was also found (117). Though the parkinsonian signs ameliorated, researchers hypothesized that the intense host-derived dopaminergic sprouting in the transplanted area was responsible for the behavioral improvements. In later studies, increased graft survival was observed when chromaffin cells were purified using a Percoll gradient that diminished the content of endothelial components (135). In 1987, Madrazo and colleagues reported the first clinical use of adrenal medullary autografts (to the caudate nucleus). The investigators showed dramatic improvement of PD symptoms in 2 patients. Following these promising results, several similar case studies were undertaken, reporting different, less favorable outcomes (121). In 1989, a multicenter study of 19 patients (61) showed minimal temporary improvements in behavioral deficits following adrenal medullary grafting. This was recorded as decreased mean severity of “off” time (time when the positive effects of pharmacological treatment wear off) as assessed by both the Activities of Daily Living subscale of the Unified Parkinson's Disease Rating Scale (UPDRS) and the Schwab and England scale. Despite this evidence, the dosage of antiparkinsonian medications could not be decreased and postoperative morbidity was considerable. The investigators concluded that the application of the procedure outside of research centers was premature due to the relative high-risk and low benefit for patients. Postmortem results of the clinical cases confirmed the findings of poor cell survival and localized sprouting observed in the nonhuman primate experiments.
Throughout this time, fetal tissue research continued. Mesencephalic fetal tissue grafts were first performed in parkinsonian monkeys by Bakay and colleagues in 1985 (8) and Redmond and colleagues in 1986 (125). They were followed by several reports of fetal tissue transplants performed in young adult monkeys (Table 1). The emphases of these initial studies were on optimizing the grafting technique, analyzing the effect of donor age, refining harvesting techniques, tissue preservation, and tissue preparation. Most studies used a limited number of animals that received MPTP or 6-OHDA to induce DA depletion and a severe PD syndrome. The surgical target was primarily the caudate nucleus. Transplanted monkeys were not immunosuppressed or treated with L-DOPA. These studies successfully demonstrated feasibility of the technique and cell survival. Amelioration of the parkinsonian syndrome was also documented.
Table 1.
Comparative List of Preclinical Nonhuman Primate Studies
| Reference | Host monkey species | Cell species/type | Transplant location | Model | n | Immunosup. | L-DOPA | Behavioral test |
|---|---|---|---|---|---|---|---|---|
| Bakay et al., 1985 (7) | Rhesus | Rhesus/fetal SN | Cau | MPTP systemic | 2 | None | None | General behavior |
| Redmond et al., 1986 (125) | Vervet | Vervet/fetal SN | Cau | MPTP systemic | 3 | None | None | Clinical rating |
| Vervet/fetal SN | Cerebellum | Naïve | 1 | None | None | Clinical rating | ||
| Sladek et al., 1986 (141) | Vervet | Vervet/fetal SN | Cau | MPTP systemic | 2 | None | None | None (morphological analysis) |
| Vervet/fetal SN, locus coeruleus, subcoreuleus and hypothalamus | Cau, cingulate Cx | MPTP systemic | 1 | None | None | |||
| None | None | MPTP systemic | 5 | None | None | |||
| Collier et al., 1987 (35) | Vervet | Vervet/fetal SN and olfactory bulb | Cau | MPTP systemic | 1 | None | None | General behavior (analysis cryopreservation) |
| Naïve | 3 | None | None | |||||
| Redmond et al., 1988 (123) | Vervet | Human/fetal SN | Cau | Naïve | 3 | CSA 15 mg/kg | None | General behavior (analysis cryopreservation) |
| Dubach et al., 1988 (43) | Long tailed macaque | Long tailed macaque/fetal SN | Cau, Pu | MPTP intra-nigral | 1 | None | None | Rotation: spontaneous |
| Fine et al., 1988 (54) | Common marmoset | Common marmoset/fetal SN | Pu | MPTP systemic | 4 | None | None | Rotation: spontaneous, apomorphine |
| Bankiewicz et al., 1990 (11) | Rhesus | Rhesus/fetal SN | Cau | MPTP systemic | 1 | None | None | Rotation: spontaneous, apomorphine, amphetamine |
| None | None | MPTP systemic | 3 | None | None | General behavior | ||
| Rhesus/fetal SN | Cau | MPTP intracarotid artery | 3 | None | None | |||
| None | None | MPTP intracarotid artery | 5 | None | None | |||
| Annett et al., 1990 (3) | Common marmoset | Common marmoset/fetal SN | Cau, Pu, Acc | 6-OHDA nigrostriatal bundle | 6 | None | None | Rotation: spontaneous, apomorphine, amphetamine |
| None | 6-OHDA nigrostriatal bundle | 4 | None | None | ||||
| Taylor et al., 1990 (158) | Vervet | Vervet/fetal SN | Cau | MPTP systemic | 2 | None | None | Object Retrieval Detour Task |
| Vervet/fetal SN | Cx | MPTP systemic | 1 | None | None | |||
| Vervet/fetal SN | Cau | Naïve | 1 | None | None | |||
| Taylor et al., 1991 (159) | Vervet | Vervet/fetal SN | Cau | MPTP systemic | 4 | None | None | Clinical rating |
| Vervet/fetal cerebellum | Cau | MPTP systemic | 1 | None | None | |||
| Vervet/fetal SN | Cx | MPTP systemic | 1 | None | None | |||
| Vervet/fetal SN | Cau | Naïve | 3 | None | None | |||
| Sladek et al., 1993 (142) | Vervet | Vervet/fetal SN (young) | Cau | MPTP systemic | 2 | None | None | None (evaluation of gestational age) |
| Vervet/fetal SN (older) | Cau | MPTP systemic | 1 | None | None | |||
| Annett et al., 1994 (4) | Common marmoset | Common marmoset/fetal SN | Cau, Pu, Acc (U) | 6-OHDA nigrostriatal bundle | 6 | None | None | Rotation: spontaneous, apomorphine, amphetamine hand skilled task, neglect and position bias |
| None | 6-OHDA nigrostriatal bundle | 4 | None | None | ||||
| None | None | Sham lesioned and naive | 5 | None | None | |||
| Elsworth et al., 1994 (47) | Vervet | Vervet/fetal SN | Cau | MPTP systemic | 1 | None | None | Clinical rating (evaluation of DA transporter radioligands) |
| None | None | MPTP systemic | 2 | None | None | |||
| Collier et al., 1994 (34) | Vervet | Vervet/fetal SN/saphenous nerve in polymer | Cau | MPTP systemic (asymptomatic) | 5 | None | None | Clinical Rating |
| Vervet/fetal SN/polymer | Cau | MPTP systemic (asymptomatic) | 5 | None | None |
Studies evaluating DA fetal transplants were published between 1985 (year of first report) and 1994 (year when NIH-sponsored double-blind clinical trials started).
Acc, nucleus accumbens; Cau, caudate nucleus; CX, cortex; Pu, putamen nucleus; SN, substantia nigra.
In 1988 (2‱3 years after the first monkey experiments were published), the first human case reports were released (69, 98, 108). A number of small clinical trials followed showing variable degrees of success (121). The different surgical techniques, age of fetuses, and methods of cell preparation made it difficult to attempt comparisons across multiple studies and draw any viable conclusions. The Core Assessment Program for Intracerebral Transplantation (CAPIT) was created to improve the rigor and comparability when evaluating the efficacy of future studies. It was clear that there was a need for trials in which there was a control group and a standardized system for fetal tissue selection/preparation, selection of host targets, medication management, and overall assessment methods.
Starting in 1994, the National Institutes of Health (NIH) funded two prospective, double-blind, randomized control trials. Both used rigorous scientific methods to assess the efficacy and safety of transplanting fetal mesencephalic grafts to treat PD, including the use of surgical sham operations to assess the placebo effect of the procedures.
In 2001, Curt Freed and Stanley Fahn published the results of their clinical trial (57). Forty patients were treated: 20 received bilateral postcommissural putaminal grafts of ventral mesencephalic fetal neurons, and 20 had a sham procedure consisting of a burr hole drilled into their skull. None of the patients were immunosuppressed. The fetal cells were cultured for 1 week prior to transplantation. Patient reports on activity of daily living (recorded at home for 12 months) were the primary endpoint for evaluation of treatment outcome. The results of the study did not show significant differences between treatment groups. Further analysis revealed that patients younger than 60 years old performed significantly better in the subjective reports as well as in the secondary measure, the Unified Parkinson's Disease Rating Scale (UPDRS), at 1 year (28% improvement) and 3 years (38% improvement) post-transplantation. Seventeen of the 20 patients in the transplantation group showed extensive in vivo survival of transplanted neurons, as evidenced by increased 18F fluorodopa uptake on positron emission tomography (PET). This finding was confirmed in postmortem examinations. An unexpected outcome was the occurrence in 5 fetal grafted patients of dystonia and dyskinesias that did not ameliorate with reduction or cessation of L-DOPA treatment. These uncontrolled, abnormal movements that were not associated with L-DOPA administration were named “runaway” dyskinesias.
The outcome of a subsequent trial, led by Warren Olanow and Thomas Freeman, was highly anticipated, and in 2003 results were released (114). Thirty-four patients were treated: 11 received bilateral postcommissural putaminal grafts of ventral mesencephalic fetal neurons from one donor fetus, 12 from four donor fetuses, and 11 received bilateral sham procedures. All patients underwent oral cyclosporine (CsA) immunosuppression that started 2 weeks prior to surgery and continued for 6 months after grafting. The patients were followed for 2 years and the primary endpoint was the UPDRS motor subscore. Similar to the Freed and colleagues trial, this study failed to meet the primary endpoint, revealing no significant differences in the effect of treatments between groups. Younger patients and those with less severe PD at baseline showed improvement in the UPDRS score. Patients with four donor grafts had more 18F fluorodopa uptake on PET. “Runaway” dyskinesias were observed in half the graft-treated patients by one year post transplant. Similar to the Freed and Fahn trial, the dyskinesias did not improve with reduction or cessation of dopamine replacement treatment.
Other Clinical Trials for Cell Replacement in PD
While the fetal tissue trials were ongoing, investigators assessed a number of alternative sources of dopaminergic neurons, from other animal species, cadaveric human and autologous. Their aim was to overcome the practical and ethical limitations of using fetal cells for large-scale clinical applications.
Porcine xenograft
Cells equivalent to human fetal mesencephalic neurons obtained from animal sources were proposed as an alternative to human fetal tissue. Problems associated with xenografts include the high risk of graft rejection and the possibility of introducing into the human host viruses to which they are not immune. Aware of these issues, investigators developed immunosuppression protocols and screening assays in order to pursue this line of research. One candidate species was porcine, as pigs have large brains that resemble primate organization and have long been used in research studies. Porcine mesencephalic dopaminergic fetal neurons were grafted into PD rats (59). The transplanted cells survived and improvements in motor function were observed. A Phase I clinical trial (55) in 12 subjects followed. The patients were treated with two different immunosuppressive regimes: cyclosporine (CsA) or monoclonal antibodies against major histocompatibility complex (MHC). No graft-to-host transmission of porcine diseases was found. Both groups showed improvement during the “off” period in UPDRS scores, but there was individual variability in response. An 18F FDOPA PET scan did not show an increase in uptake, suggesting that the cells were lost. In a similar double-blind placebo-controlled study, 10 subjects received bilateral grafts and 8 had sham surgeries. Patients received short-term prednisone treatment and long-term CsA immunosuppression. None of the patients developed disabling dyskinesias or titers against porcine endogenous retrovirus (PERV), but no significant improvements in PD symptoms were documented. The mesencephalic fetal porcine cells presented similar challenges to success, as did the human fetal tissue grafts, with the added complication of a magnified immunological reaction triggered by the xenograft (129).
Human retinal pigmented epithelial cells
Human retinal pigmented epithelial (hRPE) cells produce L-DOPA as a step in the production of eumelanin; they also produce DA and can be obtained from cadaveric sources. These characteristics suggested they might be suitable as an alternative cell type for DA replacement. Preclinical trials showed that transplantation of hRPE attached to gel microcarriers (Spheramine®, Titan Pharmaceuticals, Inc.) into the striatum of parkinsonian rats and monkeys improved motor function. Cell survival was observed, as well as a mild inflammatory reaction (42, 170). Based on these results, a pilot study in six patients was performed. None of the patients received immunosuppression. Improvements in UPDRS were observed at 6 months and continued to 12 months; 3 patients showed lower dyskinesia scale scores as compared to baseline (9). As safety and tolerability were documented, a double blind clinical trial (phase IIb) was initiated. The trial, however, was halted after the 12 months follow up, as the results did not meet the study's primary or key secondary endpoint, that is, no significant differences were detected between the Spheramine and sham surgery arms of the study (http://www.medicalnewstoday.com/articles/113908.php).
Autologous sympathetic ganglion and carotid cell bodies
The idea of using autologous transplants to treat PD was not abandoned after the failed adrenal medulla trials. Instead, other sources of catecholaminergic-producing cells that might be more easily obtained were investigated. Autologous sympathetic ganglion cell grafts were tested in a PD rat model showing improvement of rotational behavior and cell survival (113). A trial of 35 patients with advanced PD followed; only about half showed improvements in bradykinesia and gait disturbances that persisted 3 years following transplantation (76). No serious complications were observed, although all patients showed mild ptosis (drooping eyelids) related to the loss of sympathetic innervation to the superior tarsal muscle.
Carotid cell bodies have also been proposed as an alternative source of dopaminergic cells. These neural crest-derived cells produce DA and release it when they sense low oxygen levels. In rat and monkey PD models, striatal grafts of carotid body cells improved motor behavior (66, 104, 159). A clinical trial in 13 patients showed initial success, though there was variability between cases: Ten of 12 blindly video-analyzed patients showed amelioration of motor symptoms and “off” period dyskinesias were not observed. Three years after the transplants, 3 of 6 patients maintained improvement. A nonsignificant 5% increase in mean putaminal 18F FDOPA uptake was observed but there was an inverse relationship between clinical amelioration and annual decline in putaminal 18F FDOPA uptake. Investigators suggested that the positive effects of the carotid body grafts were mediated by trophic factor release, instead of being associated with increased striatal DA as predicted (6, 110).
Ex Vivo Gene Therapy for PD
Gene transfer has emerged as an approach to chronically and locally deliver therapeutic molecules into the brain. Different methods have been evaluated to treat PD models since the early 1990s. Though delivery can be achieved by infusion using cannulae and pumps, risks such as infection of the lines, tissue redistribution, and the necessity of refilling the reservoir are considerable. Liposomal delivery is an alternative delivery method, but it has its own inherent risks. With this type of delivery, it is difficult to predict inflammatory and other reactions in the brain, as well as the fact that additional intracerebral injections would be needed, as the therapeutic success for PD may require chronic delivery. In response to these risks, more direct alternatives were developed and utilized. Ex vivo (manipulation of live cells in a rigorously controlled environment outside the organism) and in vivo (direct injection of viral vectors into the host) gene therapy methods have been developed. (48) While a number of in vivo gene therapy trials for PD are ongoing, clinical translation of ex vivo methods for PD still awaits (52).
Ex vivo gene transfer emerged as a possible way to “manufacture” a cell that could deliver a therapeutic molecule while being a safer alternative to in vivo gene therapy. The benefits of this method are that the transfected cells can be monitored before transplantation for the effects of viral infection and the production of a foreign protein; thus the possibility of introducing a live virus into the host is very low. In addition, the efficacy of the transfection and protein expression can be tested and optimal batches selected for transplantation. Similar to in vivo gene therapy, ex vivo transplants can incorporate regulatable promoters to stop gene expression in case of unwanted side effects. Another added advantage is that the efficacy of the system can be evaluated in vitro as, until now, regulatable systems could be “leaky.” An alternative method (only advisable for ex vivo therapy) is the novel, genetically engineered kill-switch to terminate the cells and completely stop product synthesis. The type of cell and the vector system used for grafting are critical factors for delivering therapeutic levels of the transgene product to the desired target. While vector development has improved over the years, appropriate sources of cells have been limited until recently.
The first ex vivo strategies were aimed at classical DA replacement (148). For example, autologous fibroblasts were genetically engineered to produce tyrosine hydroxylase (the limiting enzyme for DA production (e.g., ref. 10). Following the identification of glial cell line-derived neurotrophic factor (GDNF) as a potent DA trophic factor, ex vivo strategies to deliver GDNF were developed. Encapsulated GDNF producing C2C12 cells were transplanted in MPTP-treated baboons (83). The monkeys showed temporary improvements of their parkinsonian symptoms but the problem of a low and variable protein production by the encapsulated cells remained. This was likely due to limited cell survival inside the semi-permeable polymer. Developments in cell biology are providing new cell sources for ex vivo gene therapy (see below). An example is the application of human neuroprogenitor (hNP) cells for delivery of trophic factors. These cells have been shown to survive and locally produce GDNF in the brain of parkinsonian immunosuppressed rats and monkeys (Fig. 3) (4, 14). Ex vivo delivery of insulin-like growth factor-1 (IGF-1) to parkinsonian rats has also yielded positive results in the rodent models (45) and is being analyzed as an alternative to GDNF due to the controversial results of direct protein delivery trials (137). Despite these positive outcomes, the immune reaction against human cells in xenografts has halted the clinical translation for PD until more compatible cell sources are developed.
FIG. 3.
Ex vivo gene therapy using human neuroprogenitor cells. hNP cells were transfected with lentiviral vectors encoding for glial derived neurotrophic factor (GDNF) and transplanted into the striatum of a cynomolgus monkey intoxicated with a single intracarotid artery injection of MPTP. Used with permission from ref. 42.
Breakthroughs in Neurobiology and Cell Biology Affect the Use of CBTs for PD
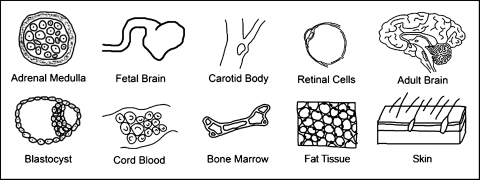
The search for the best cell to treat PD is ongoing. New cell sources have emerged as the result of biological breakthroughs and development of new technologies (Figs. 4 and 5, Table 2). These events, added to the accumulated information from the failed clinical trials, are reshaping how CBTs are conceptualized.
FIG. 4.
Sources of cells for PD CBTs.
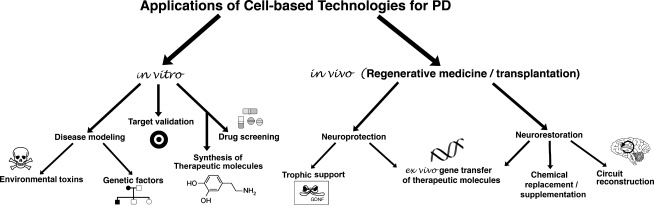
FIG. 5.
Possible applications of cell-based technologies for PD. CBTs for PD are traditionally envisioned as cell replacement strategies aiming for circuit restoration. New developments in gene transfer, stem cells, and tissue culture techniques are expanding CBTs' role in vivo as a mean for localized delivery of therapeutic molecules that can be spontaneously secreted or genetically engineered to be produced. In vitro, cell cultures are proposed as tools to help understand PD, develop and screen new therapies.
Table 2.
Comparison Between New Cell Types Currently Proposed for Use on CBTs for PD and Other Neurological Disorders
| Cell Type | Advantages | Disadvantages |
|---|---|---|
| NPCs | • Multipotent • Proven capacity to differentiate into DA phenotypes • Multiple cell lines |
• Fetal origin • Grafting requires immunosuppression |
| Bone marrow | • Multipotent • Availability • Multiple cell lines • Can be genetically matched to male or female donor |
• Difficult to preserve long term self-renewal • Challenge to differentiate • Low yield |
| Cord blood | • Multipotent • Availability • Multiple cell lines |
• Difficult to preserve long term self-renewal • Grafting may require immunosuppression • Challenge to differentiate • Low yield |
| Fat tissue | • Multipotent • Availability • Multiple cell lines |
• Difficult to preserve long term self-renewal • Grafting requires immunosuppression • Challenge to differentiate • Low yield |
| ES (pre-implantation blastocyst) | • Pluripotent • Multiple cell lines • Proven capacity to differentiate to DA phenotypes |
• Embryonic origin • Potential teratoma formation • Challenge to maintain pluripotency • Culture necessities (e.g. feeders) • Mutations |
| ES – parthenogenetic | • Pluripotent • Proven capacity to differentiate to DA phenotypes • Can be genetically matched to female donor |
• Requires unfertilized oocyte • Spontaneous differentiation |
| ES – somatic cell nuclear transfer (cloning) | • Pluripotent • Can be genetically matched to male or female donor |
• Requires unfertilized oocyte • Low success rate • Genetic instability • Potential teratoma formation • Can create implantable embryo • High cost |
| ES – altered nuclear transfer | • Pluripotent • Can be genetically matched to male or female donor • Inability to create implantable embryo |
• Requires unfertilized oocyte • Low success rate • Genetic instability • Potential teratoma formation • High cost |
| iPS | • Pluripotent • Source availability • Ability to differentiate • Low cost • Can be genetically matched to male or female donor |
• Retrovirally-induced cancers/tumor formation |
ES, embryonic stem cells; iPS, induced pluripotent stem cells; NPCs, neuroprogenitor stem cells.
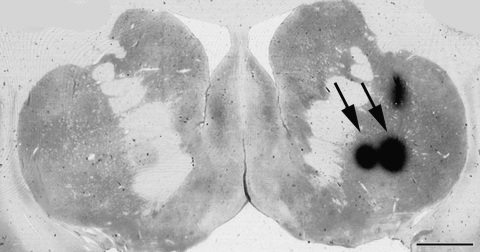
A viable cell candidate for neuroregenerative medicine appears to be human neuroprogenitor (hNP) cells (166). Though hNP cells are limited in ways that stem cells are not (mainly pluripotency and self-renewal), they remain a valuable tool in regenerative research. Their potential ability to migrate to areas in the body where needed makes them ideal for many therapies. hNP cells proliferate in the germinal layers of the brain and can multiply to create cell lines. These multipotent cells can then be differentiated into a DA phenotype in vitro (133). Transplants of hNP cells to rodents were able to survive and migrate to areas affected by PD (143). Studies in MPTP-treated monkeys have shown behavioral improvements, cell survival, and migration, while hNP cell progeny seemed to differentiate in vivo into DA-like neurons and glial phenotypes (19, 20, 122). The properties of the hNP cells differ between cell lines and the species in which they are transplanted, exemplified by the fact that migration is not always observed (48). Transplants of hNP cells into rats and monkeys were designed with clinical translation in mind, following USA Food and Drug Administration (FDA) recommendations, which state that the same product proposed for use in humans should first be tested in animals. As such, investigators working with rodent or monkey models were confronted with the dilemma of using human cells (the product to be used in clinical patients) and risking minimal cell survival, or testing cells from the same species as the host and losing translational value. Animals receiving xenografts were treated with immunosuppressants, an immune reaction was still present (Fig. 6).
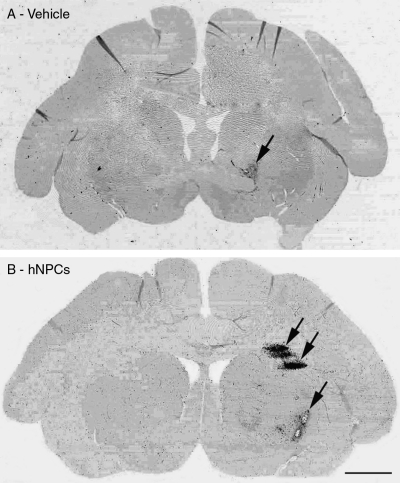
FIG. 6.
Immunological reaction to intracerebral injections. CD68 expression (marker for microglia/macrophages; arrows) is observed after MRI-guided stereotaxic intracerebral injections of: (A) Vehicle (cell culture media), (B) hNP cell transplants.
Although hNP cells are typically obtained from the germinal layer of a fetal brain, the birth of new neurons, or neurogenesis, in the adult nonhuman and human primate brain has also been documented (49, 62). Astrocytes in the subventricular zone of the human adult brain seem to have the potential to generate neuroprogenitor cells (130). These reports, plus earlier studies in rodents, shattered the previous conception that the mature brain cannot produce new cells (1, 25, 79, 81). This new finding, combined with the discovery of GDNF as a potent dopaminergic trophic factor for DA neurons (60, 97), created the possibility of curing PD by helping the brain heal itself rather than through replacing its dying cells. The option of recruiting hNP cells already present in the human brain is an interesting possibility, but its utility has not yet been proven (120). As an alternative, investigators are assessing the prospect of using hNP cells present in the adult brain from biopsies or cadavers.
Other alternative cells have been investigated for their therapeutic potential and the possibility of transdifferentiation to multiple cell types, specifically neural lineage. Promising cell sources have been shown to be stem cells derived from bone marrow (BM), umbilical cord blood (UCB), and adipose adult stromal tissue (ADAS) (50, 95, 107). These types of cells can be endogenously sourced, retaining the possibility for autologous transplantation. In addition to their use as transplantable cell sources, BM- and UCB-derived stem cells have been used in vitro to support embryonic stem (ES) cell growth (138, 179). BM- and UCB-derived stem cells remain challenging as sources for transplantation due to difficulties involved with their proliferation. To be a feasible option, the cells must maintain long term self-renewal, be genetically stable, and not spontaneously differentiate. McCoy and colleagues (107) reported neuronal differentiation in vitro of ADAS cells for transplantation and examined in rodents if the cells provided any neuroprotective benefits against the PD-inducing neurotoxin 6-OHDA. The differentiated cells' results were measured against naïve grafts of ADAS cells: both were shown to have DA neuroprotective effects in hemiparkinsonian rats. Levy and colleagues (95) transplanted neural-induced human BM mesenchymal stromal cells into hemi-parkinsonian rats to find both behavioral improvement as well as the presence of dopaminergic precursors in grafted sites. The researchers compared induced cells to naïve and found the effect of the neural-induced cells significant. Further investigation and analysis must be undertaken to demonstrate the safety and efficacy of these cell types and their ability to present a stable phenotype and survive long term in vivo.
Embryonic stem (ES) cells obtained from blastocysts have the potential to become any cell of the body. In 1995, the first nonhuman primate ES cells were isolated (161, 162), followed in 1998 by human ES (hES; 160) cells. The development of hES lines signified unlimited sources of human cells for studies in developmental biology, drug discovery, transplantation, and regenerative medicine. The first desired application of ES cells for PD was to obtain DA neurons for cell replacement. In 2000, mouse ES cells were differentiated into a DA phenotype (92), followed a few years later by human (116, 174) and nonhuman primate ES cells (154). As midbrain DA neurons are the primary cells lost in PD, they can be generated from ES cells and a sorting process can be used to select and filter out these specific DA neurons. There are multiple benefits to the specific selection of midbrain DA neuron from ES cell culture, including decreased risk of tumor or teratoma formation and the possibility that grafts containing large portions of midbrain DA neurons promote greater attenuation of PD symptoms. This suggests that the selection for midbrain-specific DA neurons could be a determining factor in the success of the transplanted cells. The transcription factor Pitx3 has been used successfully to select for midbrain DA neurons. In conjunction with fluorescence-activated cell sorting (FACS), specificity can be achieved in a differentiating ES cell culture (67). Similar work has been done to investigate the role of Lmx1a and Msx1 in the generation of midbrain-specific DA neurons from ES cells in culture (2, 26). It has also been shown that human DA neurons can be generated in coculture with PA6 cells, with the resultant cells expressing the appropriate markers and displaying the functional properties of mature DA neurons (24, 182). Despite in vitro success, transplantation of differentiated ES cells proved more difficult. The subsequent funding support and research efforts were devoted to improving hES-derived DA neurons. Successful grafts were performed in parkinsonian rats (175). Transplantation of hES cells into MPTP-treated monkeys was attempted with limited cell survival, possibly due to host brain immune reaction against the xenografts. Allographic transplantation of monkey ES cell-derived DA neurons into the same species produced a better outcome, though it was associated with limited cell survival (154). Additionally, the necessity at the time for the presence of mouse fibroblast feeder cells to maintain stem cells in culture was an added immunological complication. Advances in ES cell-derived DA cell replacement languished as problems accumulated. In the meantime, investigators using ES cells sought to find more efficient ways to produce DA neurons and solve the problems of intracerebral graft survival in addition to the other challenges identified by the fetal tissue trials. Although cell lines were available, the ethical dilemma concerning the origin of ES was still unavoidable, limiting the further development of hES-based clinical therapies. USA federal funding for hES was limited to research performed in authorized cell lines, limiting the chances for creating new ones.
Parthenogenesis, somatic cell nuclear transfer, and altered somatic cell nuclear transfer came to be as potential means of bypassing the ethical implication of destroying a human embryo to obtain hES (80). Parthenogenesis is an asexual form of reproduction where development of embryos occurs without fertilization by a male. In the absence of paternal DNA, mammalian eggs cannot fully develop but can provide blastocysts to generate parthenogenesis-derived ES cell lines that could be differentiated into a desired phenotype. This possibility has been proven using cynomolgus monkey cells. Using the cyno-1 cell line, investigators differentiated the ES cells into dopaminergic neurons (116) and these cells were successfully transplanted into parkinsonian rats (132). This method has the potential to generate an immunological match to a female donor. Another method is somatic cell nuclear transfer, or therapeutic cloning. Although it has been achieved in mammalian species, it has a very low success rate and is still associated with the ethical issue of being able to produce an implantable embryo that could fully develop. This method has been used to generate ES cell-derived DA neurons that were then grafted into parkinsonian mice with the advantage of a perfect immunological match to the host (152). The controversial work of Hwang et al. suggested that patient-specific somatic cell nuclear transfer can be used to develop lines from patients suffering from injury or disease; thus indicating the future application of such methods to study in vitro the mechanisms of patient-specific disease progression (71). Altered nuclear transfer evolved as a more acceptable alternative to cloning, as the cells are genetically engineered to inhibit embryo implantation as a result of generating defective trophoblasts (70).
A new direction in ES technology came in the form of the development of feeder-independent culture methods (102) that negated the risk of inducing cross-species contamination if cells were used in humans. Yet their benefits were curtailed as federal funding required the use of authorized ES cell lines, which had already been exposed to mouse feeder cells. Other cell sources were as controversial as the original hES cells, or presented challenges in their method of production that would have to be solved prior to clinical translation. Interestingly, in January 2009, the FDA approved the first clinical trial using human embryonic stem cell derivatives. Geron Corp., a biotechnology company based in California, will study the safety of stem cell-based treatment of spinal cord injuries in up to 10 patients (112). The results of this trial will directly influence the future of CBTs for PD. Investigators will be closely watching this initial foray into clinical trials for safety and efficacy. It should be mentioned that a few months later (March 9, 2009) the USA President B. Obama signed the “Stem Cell Executive Order and Scientific Integrity Presidential Memorandum” that lifted the 8-year ban on federal funding for embryonic stem cell research (http://www.whitehouse.gov/the_press_office/Remarks-of-the-President-As-Prepared-for-Delivery-Signing-of-Stem-Cell-Executive-Order-and-Scientific-Integrity-Presidential-Memorandum/). Investigators and stem cell advocates celebrated this presidential decision that will hopefully promote advances in CBTs.
The most promising discovery for CBTs has been the creation of induced pluripotent stem (iPS) cells from skin fibroblasts (155, 177), by finding a combination of genes capable of de-differentiating cells committed to an adult phenotype. This technology resolved critical ethical questions associated with the use of embryos, cloning, and the availability of oocytes. Successful iPS generation from macaque fibroblasts has also been achieved and the cells further induced into a neural lineage (100). A report that motor neurons and astroglia derived from iPS cells originating from an 82-year old female with familial (carrying a SOD-1 mutation) amyotrophic lateral sclerosis (ALS) demonstrated that it is feasible to obtain iPS cells from the fibroblasts of aged patients with progressive neurodegenerative disease (40). Researchers have recently been able to obtain iPS cells from PD patients using viral reprogramming factor-free methods and differentiate them into dopaminergic neurons (145).
Considerations such as genetic background (identification of patients with familial PD) may lead to the development of “healthy” iPS cell lines with major histocompatibility complex (MHC) characteristics to match individual patient needs. By using iPS cell-derived transplants, investigators will have a chance to assess the impact that immunological matching has on cell survival, function, circuit integration, and synaptogenesis (171). Despite their immense potential, iPS cell transplants will still have to overcome all the major hurdles presented in other grafts: survival, staying differentiated, and remaining genetically stable. Investigators are working to find approaches that can inhibit persistent cell proliferation and limit heterogeneity of cells in the transplants. Cell enrichment approaches are being tested to drive DA differentiation and cell selection prior to transplantation in order to eliminate the transplant variability seen in fetal tissue trials (73, 74, 75, 82). The continued knowledge gained with ES cells will facilitate progress in iPS discovery. Yet, the true advantage of iPS cell technologies may be the ability to evaluate the behavior of the cells in culture. This allows for assessment on a molecular and cellular level that is specific to individual PD patients. Continued future production of neurons or glia from PD patients will help us understand PD mechanisms of cell death and life and the relative roles of genetics and environment in PD. This would allow for the screening of drugs and tailoring of patient-specific therapies (Fig. 7) (31, 115). These advances have the potential to benefit PD patients far beyond repair of the DA nigrostriatal system.
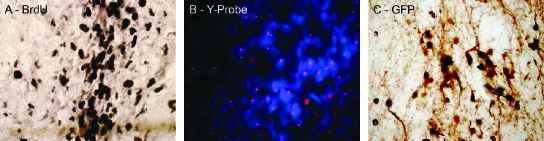
FIG. 7.
Methods of labeling and identification of human cells grafted into the brain of nonhuman primates (A) Immunohistochemistry against bromodeoxyuridine (BrdU). hNP cells were incubated with BrdU before transplantation into the striatum of a cynomolgus monkey (used with permission from ref. 112). (B) hNP cells from a male donor were transplanted in a female cynomolgus monkey host brain and identified using a probe against the Y chromosome (pink dots) (used with permission from ref. 42). (C) immunohistochemistry against Green Fluorescent Protein (GFP)–labeled hES cells transplanted into the striatum of a rhesus monkey.
The Role of Preclinical Studies in the Application of Past and fFuture CBTs
While new cell sources present exciting opportunities to treat PD, the success of clinical translation of therapies that require intracerebral grafting depends on learning from failed trials, accepting the limitations of current animal models (as none of them show the full spectrum of symptoms or the slow progression of PD) and performing critical preclinical research.
In the past, every CBT has demonstrated marginal clinical benefits when the trials reached a phase II stage that requires double-blind, placebo-controlled methods. The negative results of the two large NIH-sponsored clinical trials (57, 114) had the biggest impact on the use of CBTs for the treatment of PD. They confirmed the complexity of attempting cell replacement and circuit repair and showed that CBTs had more limitations and side effects than the designers had originally anticipated. Although some patients improved, the results showed low efficacy and high risk of the treatment compared to other approved therapies, such as DBS. It should be mentioned that before the trials were funded, some researchers expressed their concerns that the transplant methods were not well enough understood for adequately powered clinical trials and that a failure was highly possible (33, 172).
The main setbacks in the NIH-funded fetal tissue trials were the individual variability in the response to the grafts and the runaway dyskinesias. Analysis of the accumulated case reports leading to the phase II fetal trials shows that the transplants improved PD signs, but there was individual variability in the magnitude of the response and the incidence of complications, such as dystonia (64) and dyskinesias (38, 77, 84). The developed techniques ensured graft survival, as documented in postmortem cases. In some, the graft or the host appeared to extend processes and form synapses (86). Yet, the individual differences in the clinical response suggest that several grafts were not producing enough DA to improve PD motor signs. This could have been due to heterogeneity of the transplanted cells that define low yield of ideal fetal DA nigral neurons. The grafted cells could have been lost due to insufficient immunosuppression or inappropriate targeting. Another possibility is that other areas of the brain not targeted by the transplants, such as the caudate nucleus, were missing DA. In contrast, the presence of runaway dyskinesias raises the possibility of localized overproduction of DA. Comparison between the results of the open label and the double-blind clinical trials raises the concern that “optimism bias” by the researchers accelerated the clinical application, probably stimulated by the scarcity of alternative treatments for advanced PD patients. [It should be noted that the FDA approved DBS as a treatment for PD in 2002; http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm083894.htm].
There are several reasons that may explain why the preclinical studies failed to predict the negative clinical results. It should be reiterated that investigators developing grafting techniques were also pioneering the use of the MPTP monkey model. As such, preclinical studies demonstrated feasibility of grafting fetal cells and the proposed methods allowed intracerebral cell survival of allogeneic dopaminergic fetal tissue. Amelioration of the monkeys' parkinsonism was also documented. It is clear that there were limitations in the experimental designs and dissociation between the animal studies and the human trials (Table 1). For example, the number of animals per treatment group was usually small and did not allow for blind acquisition of data and statistical power analysis. Most nonhuman primate studies targeted the grafts to the caudate nucleus, yet the clinical cases were grafted into the postcommisural putamen. Understanding and quantifying the difference in striatal volume between human and nonhuman primates is essential to the clinical translation of therapies (176). The ability to predict these ratios could greatly enhance accuracy in designing therapies for clinical use based on the preliminary nonhuman primate research. The nonhuman primate research leading to fetal tissue trials had emphasized optimizing transplantation methods but neglected the effect that the condition of the host could have on the graft. Young adult monkeys were used and whether old age (similar to that of the PD population) affected the efficacy of the grafts was never tested. Most patients in the control or treatment groups of the clinical trials received chronic L-DOPA treatment before and after the transplants. Although some rodent results suggested that chronic L-DOPA affected graft success (178), in monkeys the effects of this widely used treatment on graft survival and dyskinesia onset were not evaluated and the efficacy of the grafts was not compared to optimal anti-parkinsonian dosage. None of the animals in the preclinical trials received immunosuppression (with the exception of three monkeys receiving human fetal cells; 123) and a systematic assessment of changes within the host brain and the possible effects on the graft were missing.
New animal studies have been published to address issues such as target areas for transplantation, the effect of suspensions versus solid grafts (126), and the effect of different enriched culture media to improve cell survival and decrease graft cell heterogeneity (108). Multiple implantation sites including caudate nucleus and globus pallidus can improve anti-parkinsonian efficacy as well as address the issue that PD involves more than nigrostriatal DA loss (3–5, 36). Development of models of dyskinesias in rodents (103) and monkeys (91) are helping researchers understand the nature of runaway dyskinesias, primarily what characteristics of the graft and host response affect the efficacy of the transplant and possible subsequent development of the abnormal movements (29, 30, 144). The prediction of clinical results from preclinical studies depends on using experimental designs that allow blind data acquisition and statistical analysis, assessing the therapy in investigational conditions that parallel clinical situations, looking for potential sources of complications or side effects and limiting optimism bias when reporting outcomes.
The “Host Factor” Affects Graft Survival and Function
The results of fetal tissue trials, as well as the other CBT trials, all reflect that CBTs' success depends not only on the viability of the implanted cells, but also upon various host factors.
Evidence of the importance of the host environment was presented in 1997 in a report analyzing the brains of two fetal tissue-treated patients that died from unrelated causes. The investigators described the presence of infiltrates of microglia/macrophages, B, and T cells around the graft site (87). This observation suggested that the host brain was immunologically responding to the grafted cells, in a fashion consistent with chronic rejection (129, 156). Since the grafts appeared healthy at the time of transplantation, researchers proposed two distinct possibilities: that the infiltrates could affect the long-term viability and function of the grafts or that the immune cells could be producing trophic factors to aid in the survival and integration of the graft. When the results of the NIH-trials were released, the concept that the host was negatively affecting the graft became a possibility.
More recently, two different postmortem reports of long-term (11‱16 years) grafted patients described three cases in which some engrafted cells developed PD pathology, observed as the presence of Lewy bodies (22, 85, 96, 106). Proposed mechanisms for development of these intracytoplasmic inclusions are nigral high levels of oxidative stress, chronic inflammation, and excitotoxicity, or host-to-graft disease propagation by prion-like activity. Another possibility is that alpha-synuclein may be excreted by local affected cells, taken up by grafted cells inducing intracytoplasmic accumulations that could impair neuronal function. These findings suggest that grafts may only be a temporary solution, as the transplanted cells may also become “parkinsonian”.
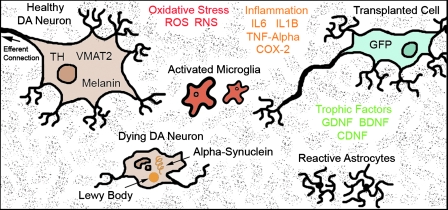
Based on these findings, it can be argued that the PD brain may be a hostile environment for resident and transplanted DA neurons (Fig. 8). Graft rejection may be facilitated by a local milieu already in a reactive state. Within this context, long-term successful transplants require a modification of their surroundings, such as the use of co-grafts (34), localized delivery of trophic factors by gene therapy or by systematic administration of neuroprotective agents. These interventions could provide novel means of facilitating graft survival (151) and, in the case of systemic treatments, simultaneously affect other systems beyond nigrostriatal DA; possibly improving the overall signs of PD.
FIG. 8.
The microenvironment of the PD brain for host and transplanted cells.
Summary and Perspective
This review reflected on past preclinical and clinical CBT trials, and the current understanding of PD, in order to gain insight that can facilitate the future successful translation of appropriate cell-based technologies.
The clinical results strongly suggest the need for preclinical and clinical investigators to have close, true interactions. This approach facilitates the identification of problems that arise during the clinical translation of CBTs. In that context, experimental designs in the laboratory should strive to replicate as closely as possible the targeted human condition, including medications, with the purpose of developing accurate predictors of therapeutic effects. This is especially important as it is an unavoidable incongruence that while MPTP-treated nonhuman primates are currently the best model for PD in preclinical trials, neurotoxic treatment does not identically replicate the slow, progressive degenerative process of PD. The use of experimental outcome measures that mirror clinical ones will aid in data sharing between basic and clinical scientists. Clinicians directing the human translation have to understand the conditions and methods utilized in preclinical research, as well as their limitations (e.g., lack of chronic L-DOPA treatment). It is useless to preclinically develop treatment protocols (e.g., for caudate targets) that are ultimately different in the clinical setting. Experiments with a small sample of animals is an efficient way to assess feasibility of a certain therapy (a common practice in nonhuman primate research due to the cost and availability of subjects); however, evaluation of efficacy requires statistically powered experiments, randomized assignment of the subjects, and blind collection and analysis of the data. Comparisons between the efficacy of a new treatment and conventional treatments, like L-DOPA, need to be part of the preclinical evaluation. Moreover, the selection of a CBT for clinical translation has to be weighed (magnitude of the positive effects and possible complications) against current treatments available (like DBS) and other therapies in the pipeline. Clinical translation of invasive procedures cannot be rushed. Optimism bias in scientific reporting is an issue that should be addressed during peer reviews of material.
Circuit repair is more complex than was previously assumed. A combination of strategies aiming to avoid immunological reaction, modify the PD microenvironment, support cell survival, and guide synaptogenesis may be required. Solving the issue of graft rejection will depend on improving immunosuppression techniques and/or developing cell lines that match the immunological makeup of the host. In that regard, iPS cells may provide ethically acceptable autologous cells that eliminate or reduce this variable. CBTs have the potential to be used to identify treatment targets, as well as to deliver trophic factors for regenerative therapy, or in co-grafts aiding in graft survival. Additionally, the transplantation of nigral neurons into a target area (as done in most studies), rather than in the area of origin for the neurons in a properly functioning brain may not be the most effective approach. Teams of cell biologists and bioengineers are working to devise in vivo methods of guiding transplanted cells to their targets and assisting circuit reconstruction.
CBTs for PD are evolving as products of a greater understanding of the disease, enhanced by developments in neurological and cell biology. Breakthroughs, such as iPS cells, have the potential to help solve the PD puzzle, allowing for the development of individualized medical treatments and the ability to target PD as a whole in each patient. A shift in the way CBTs are conceived for the treatment of PD has begun, and its future success depends on learning from the pioneers of the past.
Abbreviations Used
- B
bilateral
- BrdU
bromodeoxyuridine
- Cau
caudate nucleus
- CBT
cell-based therapy
- CsA
cyclosporine A
- Cx
cortex
- DA
dopamine
- DBS
deep brain stimulation
- ES
embryonic stem
- FACS
fluorescence-activated cell sorting
- F-DOPA
[18F] flourodopa
- GDNF
glial cell line-derived neurotrophic factor
- GFP
green fluorescent protein
- GPi
globus pallidus interna
- hNP
human neural progenitor
- hRPE
human retinal pigment epithelial
- iPS
induced pluripotent stem
- L-DOPA
levo-dihydroxyphenylalanin
- Lmx1a
LIM homeobox transcription factor 1, alpha
- LRRK2
leucine-rich repeat kinase 2
- MHC
major histocompatibility complex
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
2-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Msx1
MSh homeobox homolog 1 protein
- 6-OHDA
6-hydroxy-dopamine
- PD
Parkinson's disease
- PET
positron emission tomography
- PINK1
PTEN induced putative kinase 1
- Pitx3
paired-like homeodomain transcription factor 3
- Pu
putamen
- Snack
substantia nigra pars compacta
- U
unilateral
- UPDRS
Unified Parkinson's Disease Rating Scale
Acknowledgments
This work was supported in part by National Institutes of Health Grant P51 RR000167 to the Wisconsin National Primate Research Center, University of Wisconsin–Madison and the Parkinson's Disease Foundation. The authors respectfully acknowledge Jordana Lenon for editing assistance.
References
- 1.Altman J. Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Andersson E. Tryggvason U. Deng Q. Friling S. Alekseenko Z. Robert B. Perlmann T. Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Annett LE. Dunnett SB. Martel FL. Rogers DC. Ridley RM. Baker HF. Marsden CD. A functional assessment of embryonic dopaminergic grafts in the marmoset. Prog Brain Res. 1990;82:535–542. doi: 10.1016/s0079-6123(08)62644-8. [DOI] [PubMed] [Google Scholar]
- 4.Annett LE. Martel FL. Rogers DC. Ridley RM. Baker HF. Dunnett SB. Behavioral assessment of the effects of embryonic nigral grafts in marmosets with unilateral 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 1994;125:228–246. doi: 10.1006/exnr.1994.1026. [DOI] [PubMed] [Google Scholar]
- 5.Annett LE. Reading PJ. Tharumaratnam D. Abrous DN. Torres EM. Dunnett SB. Conditioning versus priming of dopaminergic grafts by amphetamine. Exp Brain Res. 1994;93:46–54. doi: 10.1007/BF00227779. [DOI] [PubMed] [Google Scholar]
- 6.Arjona V. Mínguez–Castellanos A. Montoro RJ. Ortega A. Escamilla F. Toledo–Aral JJ. Pardal R. Méndez–Ferrer S. Martín JM. Pérez M. Katati MJ. Valencia E. García T. López–Barneo J. Autotransplantation of human carotid body cell aggregates for treatment of Parkinson's disease. Clinical Studies. 2003;53:321–330. doi: 10.1227/01.neu.0000073315.88827.72. [DOI] [PubMed] [Google Scholar]
- 7.Bakay RA. Barrow DL. Fiandaca MS. Iuvone PM. Schiff A. Collins DC. Biochemical and behavioral correction of MPTP parkinson-like syndrome by fetal cell transplantation. Ann Y Acad Sci. 1987;495:623–640. doi: 10.1111/j.1749-6632.1987.tb23705.x. [DOI] [PubMed] [Google Scholar]
- 8.Bakay RA. Fiandaca MS. Barrow DL. Schiff A. Collins DC. Preliminary report on the use of fetal tissue transplantation to correct MPTP-induced parkinsonian-like syndrome in primates. Appl Neurophysiol. 1985;48:358–361. doi: 10.1159/000101157. [DOI] [PubMed] [Google Scholar]
- 9.Bakay RA. Raiser CD. Stover NP. Subramian T. Cornfeldt ML. Schweikert AW. Allen RC. Watts R. Implantation of Spheramine in advanced Parkinson's disease (PD) Front Biosci. 2004;9:592–602. doi: 10.2741/1217. [DOI] [PubMed] [Google Scholar]
- 10.Bankiewicz KS. Bringas J. Pivirotto P. Kutzscher E. Nagy D. Emborg ME. Technique for bilateral intracranial implantation of cells in monkeys using an automated delivery system. Cell Transplant. 2000;9:595–607. doi: 10.1177/096368970000900505. [DOI] [PubMed] [Google Scholar]
- 11.Bankiewicz KS. Plunkett RJ. Jacobowitz DM. Porrino L. di Porzio U. London WT. Kopin IJ. Oldfield EH. The effect of fetal mesencephalon implants on primate MPTP-induced parkinsonism: histochemical and behavioral studies. J Neurosurg. 1990;72:231–244. doi: 10.3171/jns.1990.72.2.0231. [DOI] [PubMed] [Google Scholar]
- 12.Bankiewicz KS. Plunkett RJ. Kophin IJ. Jacobwitz DM. London WT. Oldfield EH. Transient behavioral recovery in hemiparkinsonian primates after adrenal medullary allografts. Prog Brain Res. 1988;78:543–549. doi: 10.1016/s0079-6123(08)60329-5. [DOI] [PubMed] [Google Scholar]
- 13.Bankiewicz KS. Plunkett RJ. Mefford I. Kopin IJ. Oldfield EH. Behavioral recovery from MPTP-induced parkinsonism in monkeys after intracerebral tissue implants is not related to CSF concentrations of dopamine metabolites. Prog Brain Res. 1990;82:561–571. doi: 10.1016/s0079-6123(08)62646-1. [DOI] [PubMed] [Google Scholar]
- 14.Behrstock S. Ebert A. McHugh J. Vosberg S. Moore J. Schneider B. Capowski E. Hei D. Kordower J. Aebischer P. Svendsen CN. Human neural progenitors deliver glial cell line-derived neurotrophic factor to parkinsonism rodents and aged primates. Gene Ther. 2006;13:379–388. doi: 10.1038/sj.gt.3302679. [DOI] [PubMed] [Google Scholar]
- 15.Ben–Hur T. Idelson M. Khaner H. Pera M. Reinhartz E. Itzik A. Reubinoff BE. Transplantation of human embryonic stem cell-derived neural progenitors improves behavioral deficit in Parkinsonian Rats. Stem Cells. 2004;22:1246–1255. doi: 10.1634/stemcells.2004-0094. [DOI] [PubMed] [Google Scholar]
- 16.Bezard E. Brotchie JM. Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2:577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- 17.Birkmayer W. Hornykiewicz O. Der 1-3,4 Dioxyphenylalanin (D-dopa)-Effekt bei der Parkinson-Akinese. Wein Klin Wochenschr. 1961;73:787–788. [PubMed] [Google Scholar]
- 18.Bjorklund A. Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by Intracerebral nigral transplants. Brain Res. 1979;177:555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- 19.Bjugstad KB. Redmond DE. Teng YD. Elsworth JD. Roth RH. Blanchard BC. Snyder EY. Sladek JR., Jr Neural stem cells implanted into MPTP-treated monkeys increase the size of endogenous tyrosine hydroxylase-positive cells found in the striatum: a return to control measures. Cell Trans. 2005;14:183–192. doi: 10.3727/000000005783983098. [DOI] [PubMed] [Google Scholar]
- 20.Bjugstad KB. Teng YD. Redmond DE. Elsworth JD. Roth RH. Cornelius SK. Snyder EY. Sladek JR. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson's disease. Exp Neurol. 2008;211:362–369. doi: 10.1016/j.expneurol.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braak H. Del Tredici K. Rub U. de Vos RA. Jansen Steur EN. Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 22.Brundin P. Li JY. Holton JL. Lindvall O. Revesz T. Research in motion: the enigma of Parkinson's disease pathology spread. Nature Reviews Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- 23.Burns RS. Chiueh CC. Markey SP. Ebert MH. Jacobowitz DM. Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA. 1983;80:4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buytaert–Hoefen KA. Alvarez E. Freed CR. Generation of tyrosine hydroxylase positive neurons from human embryonic stem cells after coculture with cellular substrates and exposure to GDNF. Stem Cells. 2004;22:669–674. doi: 10.1634/stemcells.22-5-669. [DOI] [PubMed] [Google Scholar]
- 25.Buzanska L. Machaj EK. Zablocka B. Pojda Z. Domanska–Janik K. Human cord blood-derived cells attain neuronal and glial features in vitro. J Cell Sci. 2002;115:2131–2138. doi: 10.1242/jcs.115.10.2131. [DOI] [PubMed] [Google Scholar]
- 26.Cai J. Donaldson A. Yang M. German MS. Enikolopov G. Iacovitti L. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson's disease model. Stem Cells. 2009;27:220–229. doi: 10.1634/stemcells.2008-0734. [DOI] [PubMed] [Google Scholar]
- 27.Cameron HA. Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 28.Capitanio JP. Emborg ME. Contributions of nonhuman primates to neuroscience research. Lancet. 2008;371:1126–1135. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson T. Winkler C. Lundblad M. Cenci MA. Kirik D. Graft replacement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol Dis. 2006;21:657–668. doi: 10.1016/j.nbd.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Carta M. Carlsson T. Munoz A. Kirik D. Bjorklund A. Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog Brain Res. 2008;172:465–478. doi: 10.1016/S0079-6123(08)00922-9. [DOI] [PubMed] [Google Scholar]
- 31.Chamberlain SJ. Li XJ. Lalande M. Induced pluripotent stem (iPS) cells as in vitro models of human neurogenetic disorders. Neurogenetics. 2008;9:227–235. doi: 10.1007/s10048-008-0147-z. [DOI] [PubMed] [Google Scholar]
- 32.Chung S. Shin B-S. Hwang M. Lardaro T. Kang UJ. Isacson O. Kim K-S. Neural precursors derived from embryonic stem cells, but not those from fetal ventral mesencephalon, maintain the potential to differentiate into dopaminergic neurons after expansion in vitro. Stem Cells. 2006;24:1583–1593. doi: 10.1634/stemcells.2005-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. New fight over fetal tissue grafts. Science. 1994;263:600–601. doi: 10.1126/science.8303261. [DOI] [PubMed] [Google Scholar]
- 34.Collier TJ. Elsworth JD. Taylor JR. Sladek JR., Jr Roth RH. Redmond DE., Jr Peripheral nerve-dopamine neuron co-grafts in MPTP-treated monkeys: Augmentation of tyrosine hydroxylase-positive fiber staining and dopamine content in host systems. Neurosci. 1994;61:875–889. doi: 10.1016/0306-4522(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 35.Collier TJ. Redmond DE., Jr Sladek CD. Gallagher MJ. Roth RH. Sladek JR., Jr Intracerebral grafting and culture of cryopreserved primate dopamine neurons. Brain Res. 1987;436:363–366. doi: 10.1016/0006-8993(87)91680-5. [DOI] [PubMed] [Google Scholar]
- 36.Collier TJ. Sortwell CE. Elsworth JD. Taylor JR. Roth RH. Sladek JR., Jr Redmond DE., Jr Embryonic fetal mesencephalic grafts to the substantia nigra of MPTP-treated monkeys: feasibility relevant to multiple-target grafting as a therapy for Parkinson's disease. J Comp Neurol. 2002;21:320–330. doi: 10.1002/cne.10108. [DOI] [PubMed] [Google Scholar]
- 37.Davis GC. Williams AC. Markey SP. Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1:249–254. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- 38.Defer GL. Geny C. Ricolfi F. Fenelon G. Monfort JC. Remy P. Villafane G. Jeny R. Samson Y. Keravel Y. Gaston A. Degos JD. Peschanski M. Cesaro P. Nguyen JP. Long-term outcome of unilaterally transplanted parkinsonian patients: I. Clinical approach. Brain. 1996;119:41–50. doi: 10.1093/brain/119.1.41. [DOI] [PubMed] [Google Scholar]
- 39.Di Fonzo A. Rohe CF. Ferreira J. Chien HF. Vacca L. Stocchi F. Guedes L. Fabrizio E. Manfredi M. Vanacore N. Goldwurm S. Breedveld G. Sampaio C. Meco G. Barbosa E. Oostra BA. Bonifati V Italian Parkinson Genetics Network. A frequent LRRK2 gene mutation associated with autosomal dominant Parkinson's disease. Lancet. 2005;365:412–415. doi: 10.1016/S0140-6736(05)17829-5. [DOI] [PubMed] [Google Scholar]
- 40.Dimos JT. Rodolfa KT. Niakan KK. Weisenthal LM. Mitsumoto H. Chung W. Croft GF. Saphier G. Leibel R. Goland R. Wichterle H. Henderson CE. Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 41.Dismore JH. Manhart C. Raineri R Jacoby DB. Moore A. No evidence for infection of human cells with porcine endogenous retrovirus (PERV) after exposure to porcine fetal neuronal cells. Transplantation. 2000;70:1382–1389. doi: 10.1097/00007890-200011150-00020. [DOI] [PubMed] [Google Scholar]
- 42.Doudet DJ. Cornfeldt ML. Honey CR. Schweikert AW. Allen RC. PET imaging of implanted human retinal pigment epithelial cells in the MPTP-induced primate model of Parkinson's disease. Exp Neurol. 2004;189:361–368. doi: 10.1016/j.expneurol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Dubach M. Schmidt RH. Martin R. German DC. Bowden DM. Transplant improves hemiparkinsonian syndrome in nonhuman primate: Intracerebral injection, rotometry, tyrosine hydroxylase immunohistochemistry. Prog Brain Res. 1988;78:491–496. doi: 10.1016/s0079-6123(08)60322-2. [DOI] [PubMed] [Google Scholar]
- 44.Dunn EH. Primary and secondary findings in a series of attempts to transplant cerebral cortex in the albino rat. J Comp Neurol. 1917;27:565–582. [Google Scholar]
- 45.Ebert AD. Beres AJ. Barber AE. Svendson CN. Human neural progenitor cells over-expressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson's disease. Exp Neurol. 2008;209:213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Ehringer H. Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3-hydroxytyramin) im Gerhirn des Menschens und ihr Verhalten bei Erkrankugen des extrapyramidalen Systems. Klin Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 47.Elsworth JD. al-Tikriti MS. Sladek JR., Jr Taylor JR. Innis RB. Redmond DE., Jr Roth RH. Novel radioligands for the dopamine transporter demonstrate the presence of intrastriatal nigral grafts in the MPTP-treated monkey: correlation with improved behavioral function. Exp Neurol. 1994;126:300–304. doi: 10.1006/exnr.1994.1068. [DOI] [PubMed] [Google Scholar]
- 48.Emborg ME. Ebert AD. Moirano J. Peng S. Suzuki M. Capowski E. Joers V. Roitberg BZ. Aebischer P. Svendsen CN. GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus moneys. Cell Transplant. 2008;17:383–395. [PubMed] [Google Scholar]
- 49.Eriksson PS. Perfilieva E. Bjork–Eriksson T. Alborn AM. Nodborg C. Peterson DA. Gage FH. Neurogensis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 50.Fallahi–Sichani M. Soleimani M. Najafi SMA. Kiani J. Arefian E. Atashi A. In vitro differentiation of cord blood unrestricted somatic stem cells expressing-associated genes into neuron-like cells. Cell Bio Intl. 2007;31:299–307. doi: 10.1016/j.cellbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Feigin A. Eidelberg D. Gene transfer therapy for neurodegenerative disorders. Mov Disord. 2007;22:1223–1228. doi: 10.1002/mds.21423. [DOI] [PubMed] [Google Scholar]
- 52.Fiandaca M. Forsayeth J. Bankiewicz K. Current status of gene therapy trials for Parkinson's disease. Exp Neurol. 2008;209:51–57. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Fiandaca MS. Kordower JH. Hansen JT. Jiao SS. Gash DM. Adrenal Medullary autografts into the basal ganglia of Cebuc monkeys: injury-induced regeneration. Exp Neurol. 1988;102:76–91. doi: 10.1016/0014-4886(88)90080-5. [DOI] [PubMed] [Google Scholar]
- 54.Fine A. Hunt SP. Oertel WH. Nomoto M. Chong PN. Bond A. Waters C. Temlett JA. Annett L. Dunnett S. Transplantation of embryonic marmoset dopaminergic neurons to the corpus striatum of marmosets rendered parkinsonian by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Prog Brain Res. 1988;78:479–489. doi: 10.1016/s0079-6123(08)60321-0. [DOI] [PubMed] [Google Scholar]
- 55.Fink JS. Schumacher JM. Ellias SL. Paler EP. Saint–Hilaire M. Shannon K. Penn R. Starr P. VanHorne C. Knott HS. Dempsey PK. Fischman AJ. Raineri R. Manhart C. Dinsmore J. Isacson O. Porcine xenografts in Parkinson's disease and Huntington's disease patients: preliminary results. Cell Transp. 2000;9:273–278. doi: 10.1177/096368970000900212. [DOI] [PubMed] [Google Scholar]
- 56.Flores J. Cepeda IL. Cornfeldt ML. O'Kusky JR. Doudet DJ. Characterization and survival of long-term implants of human retinal pigment epithelial cells attached to gelatin microcarriers in a model of Parkinson's disease. J Neuropathol Exp Neurol. 2007;66:585–596. doi: 10.1097/nen.0b013e318093e53a. [DOI] [PubMed] [Google Scholar]
- 57.Freed CR. Greene PE. Breeze RE. Tsai WY. DuMouchel W. Kao R. Dillon S. Winfield H. Culver S. Trojanowski JQ. Eidelberg D. Fahn S. Transplantation of embryonic dopamine neurons for treatment of severe Parkinson's disease. NEJM. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 58.Freed WJ. Morihisia JM. Spoor E. Hoffer BJ. Olson L. Seiger A. Wyatt RJ. Transplanted adrenal chromaffin cells in rat brain reduce lesion-induced rotational behaviour. Nature. 1981;292:351–352. doi: 10.1038/292351a0. [DOI] [PubMed] [Google Scholar]
- 59.Galpern WR. Burns LH. Deacon TW. Dinsmore J. Isacson O. Xenotransplantation of porcine fetal ventral mesencephalon in a rat model of Parkinson's disease: Functional recovery and graft morphology. Exp Neurol. 1996;140:1–13. doi: 10.1006/exnr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 60.Gash DM. Zhang Z. Ovadia A. Cass WA. Yi A. Simmerman L. Russell D. Martin D. Lapchak PA. Collins F. Hoffer BJ. Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 61.Goetz CG. Olanow CW. Koller WC. Penn RD. Cahill D. Morantz R. Stebbins G. Tanner CM. Klawans HL. Shannon KM. Multicenter study of autologus adrenal medullary transplantation to the corpus striatum in patients with advanced Parkinson's disease. NEJM. 1989;321:324–327. doi: 10.1056/NEJM198902093200601. [DOI] [PubMed] [Google Scholar]
- 62.Gould E. Reeves AJ. Graziano MS. Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 63.Hagell P. Piccini P. Bjorklund A. Brundin P. Rehncrona S. Widner H. Crabb L. Pavese N. Oertel WH. Quinn N. Brooks DJ. Lindvall O. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 64.Hagell P. Widner H. Clinical rating of dyskinesias in Parkinson's disease: Use and reliability of a new rating scale. Mov Disord. 1999;14:488–455. doi: 10.1002/1531-8257(199905)14:3<448::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 65.Halme DG. Kessler DA. FDA regulation of stem-cell-based therapies. NEJM. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 66.Hao G. Yao Y. Wang J. Zhang L. Viroonchatapan N. Wang ZZ. Intrastriatal grafting of glomus cells ameliorates behavioral defects of Parkinsonian rats. Physiol Behav. 2002;77:519–525. doi: 10.1016/s0031-9384(02)00938-1. [DOI] [PubMed] [Google Scholar]
- 67.Hedlund E. Pruszak J. Lardaro T. Ludwig W. Vinuela A. Kin K-S. Isacson O. Embryonic stem cell-derived Pitx3-enhanced green fluorescent protein midbrain dopamine neurons survive enrichment by fluorescence-activated cell sorting and function in an animal model of Parkinson's disease. Stem Cells. 2008;26:1526–1536. doi: 10.1634/stemcells.2007-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hellman MA. Panet H. Barhum Y. Melamed E. Offen D. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hdroxydopamine-lesioned rodents. Neurosci Lett. 2006;395:124–128. doi: 10.1016/j.neulet.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 69.Hitchcock ER. Clough C. Hughes R. Kenny B. Embryos and Parkinson's disease. Lancet. 1988;1:1274. doi: 10.1016/s0140-6736(88)92088-0. [DOI] [PubMed] [Google Scholar]
- 70.Hurlbut WB. Altered nuclear transfer: A way forward for embryonic stem cell research. Stem Cell Rev. 2005;1:293–300. doi: 10.1385/SCR:1:4:293. [DOI] [PubMed] [Google Scholar]
- 71.Hwang WS. Roh SI. Lee BC. Kang SK. Kwon DK. Kim S. Kim SJ. Park SW. Kwon HS. Lee CK. Lee JB. Kim JM. Ahn C. Paek SH. Chang SS. Koo JJ. Yoon HS. Hwang JH. Hwang YY. Park YS Oh SK. Kim HS. Park JH. Moon SY. Schatten G. Patient-specific embryonic stem cells derived from human SCNT blastocysts. Science. 2005;308:1777–1783. doi: 10.1126/science.1112286. [DOI] [PubMed] [Google Scholar]
- 72.Iacovitti L. Donaldson AE. Marshall CE. Suon S. Yang M. Protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: studies in vitro and in vivo. Brain Res. 2007;1127:19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iacovitti L. Stull ND. Johnston K. Melatonin rescues dopamine neurons from cell death in tissue culture models of oxidative stress. Brain Res. 1997;768:317–326. doi: 10.1016/s0006-8993(97)00668-9. [DOI] [PubMed] [Google Scholar]
- 74.Iacovitti L. Stull ND. Mishizen A. Neurotransmitters, KCl and antioxidants rescue striatal neurons from apoptotic cell death in culture. Brain Res. 1999;816:276–285. doi: 10.1016/s0006-8993(98)00955-x. [DOI] [PubMed] [Google Scholar]
- 75.Isacson O. Costantini L. Schumacher JM. Cicchetti F. Chung S. Kim K. Cell implantation therapies for Parkinson's disease using neural stem, transgenic or xenogeneic donor cells. Parkinsonism Relat Disord. 2001;7:205–212. doi: 10.1016/s1353-8020(00)00059-6. [DOI] [PubMed] [Google Scholar]
- 76.Itakura T. Uematsu Y. Nakao N. Nakai E. Nakai K. Transplantation of autologous sympathetic ganglion into the brain with Parkinson's disease: Long-term follow-up of 35 cases. Stereotact Funct Neurosurg. 1997;69:112–115. doi: 10.1159/000099860. [DOI] [PubMed] [Google Scholar]
- 77.Jacques DB. Kopyov OV. Eagle KS. Carter T. Lieberman A. Outcomes and complications of fetal tissue transplantation in Parkinson's disease. Stereotact Funct Neurosurg. 1999;72:219–224. doi: 10.1159/000029729. [DOI] [PubMed] [Google Scholar]
- 78.Kachergus J. Mata IF. Hulihan M. Taylor JP. Lincoln S. Aasly J. Gibson JM. Ross OA. Lynch T. Wiley J. Payami H. Nutt J. Maraganore DM. Czyzewski K. Styczynska M. Wszolek ZK. Farrer MJ. Toft M. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: Evidence of a common founder across European populations. Am J Hum Genet. 2005;76:672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaplan MS. Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 80.Kastenberg ZJ. Odorico JS. Alternative sources of pluripotency: science, ethics, and stem cells. Transplant Rev. 2008;22:215–222. doi: 10.1016/j.trre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Kempermann G. Kuhn HG. Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 82.Kim SU. Park IH. Kim TH. Kim KS. Choi HB. Hong SH. Bang JH. Lee MA. Joo IS. Lee CS. Kim YS. Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson's disease. Neuropathology. 2006;26:129–140. doi: 10.1111/j.1440-1789.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 83.Kishima H. Poyot T. Bloch J. Dauguet J. Conde F. Dolle F. Hinen F. Pralong W. Palfi S. Deglon N. Aebischer P. Hantraye P. Encapsulated GDN-producing C2C12 cells for Parkinson's disease: A pre-clinical study in chronic MPTP-treated baboons. Neuroiol Dis. 2004;16:428–439. doi: 10.1016/j.nbd.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 84.Kopyov O. Jacques DS. Lieberman A. Duma CM. Rogers RL. Outcome following intrastriatal fetal mesencephalic grafts for Parkinson's patients is directly related to the volume of grafted tissue. Exp Neurol. 1997;146:536–545. doi: 10.1006/exnr.1997.6577. [DOI] [PubMed] [Google Scholar]
- 85.Kordower JH. Chu Y. Hauser RA. Freeman TB. Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 86.Kordower J. Freeman T. Snow B. Vingerhoets F. Mufson E. Sanberg P. Hjauser RA. Smith DA. Nauert GM. Perl DP, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- 87.Kordower JH. Styren S. Clarke M. DeKosky ST. Olanow CW. Freeman TB. Fetal grafting for Parkinson's disease: Expression of immune markers in two patients with functional fetal nigral implants. Cell Transplant. 1997;6(3):213–9. doi: 10.1177/096368979700600304. [DOI] [PubMed] [Google Scholar]
- 88.Langston JW. The Parkinson's complex: Parkinsonism is just the tip of the iceberg. Annals Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 89.Langston JW. Ballard P. Tetrud JW. Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 90.Langston JW. Langston EB. Irwin I. MPTP-induced parkinsonism in human and non-human primates: Clinical and experimental aspects. Acta Neurol Scand Suppl. 1984;100:49–54. [PubMed] [Google Scholar]
- 91.Langston JW. Quik M. Petzinger G. Jakowec M. Di Monte DA. Investigating levodopa-induced dyskinesias in the parkinsonsian primate. Ann Neurol. 2000;47:S79–89. [PubMed] [Google Scholar]
- 92.Lee SH. Lumelsky N. Studer L. Auerbach JM. McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 93.Leranth C. Roth RH. Elsworth JD. Naftolin F. Horvath TL. Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: Implications for Parkinson's disease and memory. J Neuroscience. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leranth C. Sladek J., Jr Roth R. Redmond D., Jr Efferent synaptic connections of dopaminergic neurons grafted into the caudate nucleus of experimental induced parkinsonian monkeys are different than those of control animals. Exp Brain Res. 1998;123:323–333. doi: 10.1007/s002210050575. [DOI] [PubMed] [Google Scholar]
- 95.Levy YS. Bahat–Stroomza M. Barzilay R. Burshtein A. Bulvik S. Barhum Y. Panet H. Melamed E. Offen D. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson's disease. Cytotherapy. 2008;10:340–352. doi: 10.1080/14653240802021330. [DOI] [PubMed] [Google Scholar]
- 96.Li JY. Englund E. Holton JL. Soulet D. Hagell P. Lees AJ. Lashley T. Quinn NP. Rehncrona S. Bjorklund A. Widner H. Revesz T. Lindvall O. Brundin P. Lewy bodeies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 97.Lin LF. Doherty DH. Lile JD. Bektesh S. Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 98.Lindvall O. Brundin P. Widner H. Rehncrona S. Gustavii B. Frackowiak R. Leenders KL. Sawle G. Rothwell JC. Marsden CD, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990;247:574–577. doi: 10.1126/science.2105529. [DOI] [PubMed] [Google Scholar]
- 99.Lindvall O. Rehncrona S. Brundin P. Gustavii B. Astedt B. Widner H. Lindholm T. Bjorklund A. Leenders KL. Rothwell JC, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson's disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989;46(6):615–31. doi: 10.1001/archneur.1989.00520420033021. [DOI] [PubMed] [Google Scholar]
- 100.Liu H. Zhu F. Yong J. Zhang P. Hou P. Li H. Jiang W. Cai J. Liu M. Cui K. Qu X. Xiang T. Lu D. Gao G. Ji W. Ding M. Deng H. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3:587–590. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 101.Lucking CB. Durr A. Bonifati V. Vaughan J. De Michele G. Gasser T. Harhangi BS. Meco G. Denefle P. Wood NW. Agid Y. Brice A French Parkinson's Disease Genetics Study Group; European Consortium on Genetic Susceptibility in Parkinson's Disease. Association between early-onset Parkinson's disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- 102.Ludwig TE. Bergendahl V. Levenstein MS. Yu J. Probasco MD. Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 103.Lundblad M. Picconi B. Lindgren H. Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 104.Luquin MR. Montoro RJ. Guillen J. Saldise L. Insausti R. Del Rio J. Lopez–Barneo J. Recovery of chronic parkinsonian monkeys by autotransplants of carotid body cell aggregates into putamen. Neuron. 1999;22:743–750. doi: 10.1016/s0896-6273(00)80733-3. [DOI] [PubMed] [Google Scholar]
- 105.Madrazo I. Drucker–Colin R. Diaz V. Martinez–Mata J. Torres C. Becerril JJ. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson's disease. N Engl J Med. 1987;316:831–834. doi: 10.1056/NEJM198704023161402. [DOI] [PubMed] [Google Scholar]
- 106.Marras C. McDermott MP. Rochon PA. Tanner CM. Naglie G. Lang AE Parkinson Study Group DATATOP Investigators. Predictors of deterioration in health-related quality of life in Parkinson's disease: Results from the DATATOP trial. Mov Disord. 2008;23:653–659. doi: 10.1002/mds.21853. [DOI] [PubMed] [Google Scholar]
- 107.McCoy MK. Martinez TN. Ruhn KA. Wrage PC. Keefer EW. Botterman BR. Tansey KE. Autologous transplants of Adipose-Derived Adult Stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson's disease. Exp Neurol. 2008;210:14–29. doi: 10.1016/j.expneurol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendez I. Vinuela A. Astradsson A. Mukhida K. Hallett P. Robertson H. Tierney T. Holness R. Dagher A. Trojanowski J. Isacson O. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nature Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mezey E. Key S. Vogelsang G. Szalayova I. Lange GD. Crain B. Transplanted bone marrow generates new neurons in human brains. Proc Natl Acad Sci USA. 2003;100:1364–1369. doi: 10.1073/pnas.0336479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minguez-Castellanos A. Escamilla–Sevilla F. Hotton GR. Toledo–Aral JJ. Ortega–Moreno A. Mendez–Ferrer S. Martin–Linares JM. Katati MJ. Mir P. Villadiego J. Meersmans M. Perez–Garcia M. Brooks DJ. Arjona V. Lopez–Barneo J. Carotid body autotransplantation in Parkinson's disease: A clinical and positron emission tomography study. J Neurol Neurosurg Psychiatry. 2007;78:825–831. doi: 10.1136/jnnp.2006.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morihisa JM. Nakamura RK. Freed WJ. Mishkin M. Wyatt RJ. Adrenal medulla grafts survive and exhibit catecholamine-specific fluorescence in the primate brain. Exp Neurol. 1984;84:643–653. doi: 10.1016/0014-4886(84)90211-5. [DOI] [PubMed] [Google Scholar]
- 112.Mundy A. Winslow R. First Embryonic Stem-Cell Trial Gets Approval from the FDA. Wall Street Journal. 2009 Jan 23;:A12. [Google Scholar]
- 113.Nakao N. Itakura T. Uematsu Y. Komai N. Transplantation of cultured sympathetic ganglionic neurons into parkinsonian rat brain: Survival and function of graft. Acta Neurochir. 1995;133:61–67. doi: 10.1007/BF01404950. [DOI] [PubMed] [Google Scholar]
- 114.Olanow CW. Goetz CG. Kordower JH. Stoessl AJ. Sossi V. Brin MF. Shannon KM. Nauert GM. Perl DP. Godbold J. Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 115.Paquette JA. Kumpf SW. Streck RD. Thomson JJ. Chapin RE. Stedman DB. Assessment of the Embryonic Stem Cell Test and application and use in the pharmaceutical industry. Birth Defects Res B Dev Reprod Toxicol. 2008;83:104–111. doi: 10.1002/bdrb.20148. [DOI] [PubMed] [Google Scholar]
- 116.Perrier AL. Tabar V. Barberi T. Rubio ME. Bruses J. Topf N. Harrison NL. Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Plunkett RJ. Bankiewicz KS. Cummins AC. Miletich RS. Schwartz JP. Oldfield EH. Long-term evaluation of hemiparkinsonian monkeys after adrenal autografting or cavitation alone. J Neurosurg. 1990;73:918–926. doi: 10.3171/jns.1990.73.6.0918. [DOI] [PubMed] [Google Scholar]
- 118.Polymeropoulos MH. Lavedan C. Leroy E. Ide SE. Dehejia A. Dutra A. Pike B. Root H. Rubenstein J. Boyer R. Stenroos ES. Chandrasekharappa S. Athanassiadou A. Papapetropoulos T. Johnson WG. Lazzarini AM. Duvoisin RC. Di Iorio G. Golbe LI. Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 119.Przedborski S. Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model. Ann NYAS. 2003;991:189–198. [PubMed] [Google Scholar]
- 120.Rakic P. Immigration denied. Nature. 2004;427:685–686. doi: 10.1038/427685a. [DOI] [PubMed] [Google Scholar]
- 121.Redmond ED., Jr Cellular replacement therapy for Parkinson's disease—Where are we today? The Neuroscientist. 2002;8:457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- 122.Redmond DE. Bjugstad KB. Teng YD. Ourednik J. Wakeman DR. Parsons XH. Gonzalez R. Blanchard BC. Kim SU. Gu Z. Lipton SA. Markakis EA. Roth RH. Elsworth JD. Sladek JR., Jr Sidman RL. Snyder EY. Behavioral improvement in a primate model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Redmond DE., Jr Naftolin F. Collier TJ. Leranth C. Robbins RJ. Cladek CD. Roth RH. Sladek JR., Jr Cryopreservation, culture, and transplantation of human fetal mesencephalic tissue into monkeys. Science. 1988;242:768–771. doi: 10.1126/science.2903552. [DOI] [PubMed] [Google Scholar]
- 124.Redmond DE., Jr Ourednik J. Ourdenik V. Roth RH. Elsworth JD. Sladek JR., Jr Teng YD. Hack M. Sidman RL. Snyder EY. Human neural stem cells survive, migrate, and differentiate into TH-positive neurons in mesencephalon of adult MPTP-treated non-human primates. Exp Neurol. 2000;164:445. [Google Scholar]
- 125.Redmond DE., Jr Sladek JR., Jr Roth RH. Collier TJ. Elsworth JD. Deutch AY. Haber S. Fetal neuronal grafts in monkeys given methylphenyltetrahydropyridine. Lancet. 1986;1:1125–1127. doi: 10.1016/s0140-6736(86)91839-8. [DOI] [PubMed] [Google Scholar]
- 126.Redmond DE., Jr Vinuela A. Kordower JH. Isacson O. Influence of cell preparation and target location on the behavioral recovery after striatal transplantation of fetal dopaminergic neurons in a primate model of Parkinson's disease. Neurolbiol Dis. 2008;29:103–116. doi: 10.1016/j.nbd.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ridley RM. Baker HF. Annett LE. Dunnett SB. Torres EM. Fine A. Behavioral assessment of the ability of intracerebral embryonic neural tissue grafts to ameliorate the effects of brain damage in marmosets. Mol Neurobiol. 1994;9:207–223. doi: 10.1007/BF02816120. [DOI] [PubMed] [Google Scholar]
- 128.Roitberg BZ. Mangubat E. Chen EY. Sugaya K. Thulborn KR. Kordower JH. Pawer A. Konecny T. Emborg ME. Survival and early differentiation of human neural stem cells transplanted in a non-human primate model of stroke. J Neurosurg. 2006;105:96–102. doi: 10.3171/jns.2006.105.1.96. [DOI] [PubMed] [Google Scholar]
- 129.Roitberg B. Urbaniak K. Emborg M. Cell transplantation for Parkinson's disease. Neurol Res. 2004;26:355–362. doi: 10.1179/016164104225017604. [DOI] [PubMed] [Google Scholar]
- 130.Sanai N. Tramontin AD. Quinones–Hinojosa A. Barbaro NM Gupta N. Kunwar S. Lawton MT. McDermott MW. Parsa AT. Manuel–Garcia Verdugo J. Berger MS. Alvarez–Buylla A. Unique astrocyte ribbon I adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 131.Sanberg PR. Willing AE. Garbuzova–Davis S. Saporta S. Liu G. Davis Sanberg C. Bickford PC. Klasko SK. Elbadri NS. Umbilical cord blood-derived stem cells and brain repair. Ann NY Acad Sci. 2005;1049:67–83. doi: 10.1196/annals.1334.008. [DOI] [PubMed] [Google Scholar]
- 132.Sanchez–Pernaute R. Lee H. Patterson M. Reske–Nielsen C. Yoshizaki T. Sonntag KC. Studer L. Isacson O. Parthenogenetic dopamine neurons from primate embryonic stem cells restore function in experimental Parkinson's disease. Brain. 2008;131:2127–2139. doi: 10.1093/brain/awn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanchez–Pernaute R. Studer L. Bankiewicz KS. Major EO. McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. J Neurosci Res. 2001;65:284–288. doi: 10.1002/jnr.1152. [DOI] [PubMed] [Google Scholar]
- 134.Sanchez–Pernaute R. Studer L. Ferrari D. Perrier A. Lee H. Vinuela A. Isacson O. Long-term survival of dopaminergic neurons derived from parthenogenetic primate embryonic stem cells (cyno-1) after transplantation. Stem Cells. 2005;23:914–922. doi: 10.1634/stemcells.2004-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schueler SB. Ortega JD. Sagen J. Kordower JH. Robust survival of isolated bovine adrenal chromaffin cells following intrastriatal transplantation: A novel hypothesis of adrenal graft viability. J Neurosci. 1993;13:4496–4510. doi: 10.1523/JNEUROSCI.13-10-04496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schulz TC. Noggle SA. Palmarini GM. Weiler DA. Lyons IG. Pensa KA. Meedeniya CB. Davidson BP. Lambert NA. Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22:1218–1238. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- 137.Sherer TB. Fiske BK. Svendson CN. Lang AE. Langston JW. Crossroads in GDNF therapy for Parkinson's disease. Mov Disord. 2006;21:136–141. doi: 10.1002/mds.20861. [DOI] [PubMed] [Google Scholar]
- 138.Shintani A. Nakao N. Kakishita K. Itakura T. Generation of dopamine neurons from embryonic stem cells in the presence of the neuralizing activity of bone marrow stromal cells derived from adult mice. J Neurosci Res. 2008;86:2829–2838. doi: 10.1002/jnr.21748. [DOI] [PubMed] [Google Scholar]
- 139.Sladek JR., Jr Bjugstad KB. Collier TJ. Bundock EA. Blanchard BC. Elsworth JD. Roth RH. Redmond DE., Jr Embryonic substantia nigra grafts show directional outgrowth to cografted striatal grafts and potential for pathway reconstruction in non-human primate. Cell Transplant. 2008;17:427–444. [PubMed] [Google Scholar]
- 140.Sladek JR., Jr Collier TJ. Elsworth JD. Roth RH. Taylor JR. Redmond DE., Jr Intrastriatal grafts from multiple donors do not result in a proportional increase of survival of dopamine neurons in non-human primates. Cell Transplant. 1998;7:87–96. doi: 10.1177/096368979800700204. [DOI] [PubMed] [Google Scholar]
- 141.Sladek JR., Jr Collier TJ. Haber SN. Roth RH. Redmond DE., Jr Survival and growth of fetal catecholamine neurons transplanted into primate brain. Brain Res Bull. 1986;17:809–818. doi: 10.1016/0361-9230(86)90092-4. [DOI] [PubMed] [Google Scholar]
- 142.Sladek JR., Jr Elsworth JD. Roth RH. Evans LE. Collier TJ. Cooper SJ. Taylor JR. Redmond DE., Jr Fetal dopamine cell survival after transplantation is dramatically improved at a critical donor gestation age in non-human primates. Exp Neurol. 1993;122:16–27. doi: 10.1006/exnr.1993.1103. [DOI] [PubMed] [Google Scholar]
- 143.Snyder EY. Macklis JD. Multipotent neural progenitor or stem-like cells may be uniquely suited for therapy for some neurodegenerative conditions. Clin Neurosci. 1995;3:310–316. [PubMed] [Google Scholar]
- 144.Soderstrom KE. Meredith G. Freeman TB. McGuire SO. Collier TJ. Wu Q. Steece–Collier K. The synaptic impact of the host immune response in a parkinsonian allograft rat model: Influence on graft-derived aberrant behaviors. Neurobiol Dis. 2008;32:229–242. doi: 10.1016/j.nbd.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soldner F. Hockemeyer D. Beard C. Gao Q. Bell GW. Cook EG. Hargus G. Blak A. Cooper O. Mitalipova M. Isacson O. Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sonntag K–C, Pruszak J.Yoshizaki T.van Arensbergen J.Sanchez-Pernaute R.Isacson O.Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist Noggin Stem Cells 25411–418.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spillantini MG. Parkinson's disease, dementia with Lewy bodies and multiple system atrophy are alpha-synucleinopathies. Parkinsonism Relat Disord. 1999;5:157–162. doi: 10.1016/s1353-8020(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 148.Suhr ST. Gage FH. Gene therapy for neurologic disease. Arch Neurol. 1993;50:1252–1268. doi: 10.1001/archneur.1993.00540110122012. [DOI] [PubMed] [Google Scholar]
- 149.Svendsen CN. Neural stem cells for brain repair. Alzheimer's Res. 1997;3:131–135. [Google Scholar]
- 150.Svendsen CN. Smith AG. New prospects for human stem-cell therapy in the nervous system. Trends Neurosci. 1999;22:357–364. doi: 10.1016/s0166-2236(99)01428-9. [DOI] [PubMed] [Google Scholar]
- 151.Swanson CR. Sesso SL. Emborg ME. Can we prevent Parkinson's disease? Front Biosci. 2009;14:1642–1660. doi: 10.2741/3331. [DOI] [PubMed] [Google Scholar]
- 152.Tabar V. Tomishima M. Panagiotakos G. Wakayama S. Menon J. Chan B. Mizutani E. Al–Shamy G. Ohta H. Wakayama T. Studer L. Therapeutic cloning in individual parkinsonian mice. Nat Med. 2008;14:379–381. doi: 10.1038/nm1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tai Y. Svendsen CN. Stem cells as a potential treatment of neurological disorders. Curr Opin Pharm. 2004;4:98–104. doi: 10.1016/j.coph.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 154.Takagi Y. Takahashi J. Saiki H. Morizane A. Hayashi T. Kishi Y. Fukuda H. Okamoto Y. Koyanagi M. Ideguchi M. Hayashi H. Imazato T. Kawasaki H. Suemori H. Omachi S. Iida H. Itoh N. Nakatsuji N. Sasai Y. Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson's primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Takahashi K. Okita K. Nakagawa M. Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 156.Tambur AR. Transplantation immunology and the central nervous system. Neurol Res. 2004;26:243–255. doi: 10.1179/016164104225013932. [DOI] [PubMed] [Google Scholar]
- 157.Tang B. Xiong H. Sun P. Zhang Y. Wang D. Hu Z. Zhu Z. Ma H. Pan Q. Xia JH. Xia K. Zhang Z. Association of PINK1 and DJ-1 confers digenic inheritance of early-onset Parkinson's disease. Hum Mol Genet. 2006;15:1816–1825. doi: 10.1093/hmg/ddl104. [DOI] [PubMed] [Google Scholar]
- 158.Taylor JR. Elsworth JD. Roth RH. Collier TJ. Sladek JR., Jr Redmond DE., Jr Improvements in MPTP-induced object retrieval deficits and behavioral deficits after fetal nigral grafting in monkeys. Prog Brain Res. 1990;82:543–559. doi: 10.1016/s0079-6123(08)62645-x. [DOI] [PubMed] [Google Scholar]
- 159.Taylor JR. Elsworth JD. Roth RH. Sladek JR., Jr Collier TJ. Redmond DE., Jr Grafting of fetal substantia nigra to striatum reverses behavioral deficits induced by MPTP in primates: A comparison with other types of grafts as controls. Exp Brain Res. 1991;85:335–348. doi: 10.1007/BF00229411. [DOI] [PubMed] [Google Scholar]
- 160.Thomson JA. Itskovitz–Eldor J. Shapiro SS. Waknitz MA. Swiergiel JJ. Marshall VS. Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 161.Thomson JA. Kalishman J. Golos TG. Durning M. Harris CP. Becker RA. Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thomson JA. Kalishman J. Golos TG. Durning M. Harris CP. Hearn JP. Pluripotet cell lines derived from common marmoset (Callithrix jacchus) blastocytes. Biol Reprod. 1996;55:254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- 163.Thomson JA. Marshall VS. Primate embryonic stem cells. Curr Top Dev Biol. 1998;38:133–165. doi: 10.1016/s0070-2153(08)60246-x. [DOI] [PubMed] [Google Scholar]
- 164.Thomson JA. Marshall VS. Trojanowski JQ. Neural differentiation of rhesus embryonic stem cells. APMIS. 1998;106:149–156. doi: 10.1111/j.1699-0463.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 165.Toledo–Aral JJ. Mendez–Ferrer S. Pardal R. Echevarria M. Lopez–Barneo J. Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid body grafted parkinsonian rats. J Neurosci. 2003;23:141–148. doi: 10.1523/JNEUROSCI.23-01-00141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165a.Tretiakoff C. These pour le doctorat en medicine. Paris: These de Paris; 1919. Contribution a l'étude de l'anatomic pathologique du locus niger de soemmering avec quelques déductions relatives à la pathologie des troubles du tonus musculaire et de la maladie de Parkinson; pp. 1–24. [Google Scholar]
- 166.Uchida N. Buck DW. He D. Reitsma MJ. Masek M. Phan TV. Tsukamoto AS. Gage FH. Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Videnovic A. Metman LV. Deep brain stimulation for Parkinson's disease: Prevalence of adverse events and need for standardized reporting. Mov Disord. 2008;23:343–349. doi: 10.1002/mds.21753. [DOI] [PubMed] [Google Scholar]
- 168.Villadiego J. Mendez–Ferrer S. Valdes–Sanches T. Silos–Santiago I. Farinas I. Lopez–Barneo J. Toledo–Aral JJ. Selective glial cell line-derived neurotrophic factor production in adult dopaminergic carotid body cells in situ and after intrastriatal transplantation. J Neurosci. 2005;25:4091–4098. doi: 10.1523/JNEUROSCI.4312-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vogel G. Parkinson's research fetal cell transplant draws fire. Science. 2001;291:260–261. doi: 10.1126/science.291.5511.2060b. [DOI] [PubMed] [Google Scholar]
- 170.Watts RL. Raiser CD. Stover NP. Cornfeldt ML. Schweikert AW. Allen RC. Subramian T. Doudet D. Honey CR. Bakay RA. Stereotaxic intrastriatal implantation of human retinal pigment epithelial (hRPE) cells attached to gelatin microcarriers: A potential new cell therapy for Parkinson's disease. J Neural Transm Suppl. 2003;65:215–227. doi: 10.1007/978-3-7091-0643-3_14. [DOI] [PubMed] [Google Scholar]
- 171.Wernig M. Zhao JP. Pruszak J. Hedlund E. Fu D. Soldner F. Broccoli V. Constantine–Paton M. Isacson O. Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci USA. 2008;15:5856–58561. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Widner H. NIH neural transplantation funding [letter] Science. 1994;263:737. doi: 10.1126/science.8303281. [DOI] [PubMed] [Google Scholar]
- 173.Williams N. Setback spurs Parkinson's disease research. Curr Biol. 2001;11:R285–286. doi: 10.1016/s0960-9822(01)00161-0. [DOI] [PubMed] [Google Scholar]
- 174.Yan Y. Yang D. Zarnowska ED. Du Z. Werbel B. Valliere C. Pearce RA. Thomson JA. Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang D. Zhang Z-J. Oldenburg M. Ayala M. Zhang S-C. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yin D. Valles FE. Fiandaca MS. Forsayeth J. Larson P. Starr P. Bankiewicz KS. Striatal volume differences between non-human and human primates. J Neurosci Methods. 2009;176:200–205. doi: 10.1016/j.jneumeth.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu J. Vodyanik MA. Smuga–Otto K. Antosiewicz–Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V. Stewart R. Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 178.Yurek DM. Steece–Collier K. Collier TJ. Sladek JR., Jr Chronic levodopa impairs the recovery of dopamine agonist-induced rotational behavior following neural grafting. Exp Brain Res. 1991;86:97–107. doi: 10.1007/BF00231044. [DOI] [PubMed] [Google Scholar]
- 179.Zhan X. Hill C. Brayton CF. Shamblott MJ. Cells derived from human umbilical cord blood support the long-term growth of undifferentiated human embryonic stem cells. Cloning Stem Cells. 2008;10:513–522. doi: 10.1089/clo.2007.0087. [DOI] [PubMed] [Google Scholar]
- 180.Zhang HL. Wu JJ. Ren HM. Wang J. Su YR. Jiang YP. Therapeutic effect of microencapsulated porcine retinal pigmented epithelial cell transplantation on rat model of Parkinson's disease. Neurosci Bull. 2007;23:137–144. doi: 10.1007/s12264-007-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang SC. Wernig M. Duncan ID. Brustle O. Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 182.Zeng X. Cai J. Chen J. Luo Y. You Z-B. Fotter E. Wang Y. Harvey B. Miura T. Backman C. Chen G-J. Rao MS. Freed WJ. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]