Abstract
Inflammation and oxidative stress contribute to the pathology of many diseases, but specific therapeutic targets remain elusive. Oxidative stress, generated by excessive reactive oxygen species (ROS), promotes cardiovascular disease. However, the precise mechanism of how ROS deteriorate vascular function and promote vascular remodeling in vivo has not been clearly elucidated. Cyclophilin A (CyPA) is a 20 kD chaperone protein that is secreted from vascular smooth muscle cells (VSMC) in response to ROS, and stimulates VSMC proliferation and inflammatory cell migration in vitro and in vivo. CyPA (both intracellular and extracellular) contributes to inflammation and atherosclerosis by promoting endothelial cell (EC) apoptosis and EC expression of leukocyte adhesion molecules, stimulating leukocyte migration, enhancing T helper cell type 1 (Th1) responses, increasing proliferation of macrophages and vascular smooth muscle cells (VSMC), and increasing pro-inflammatory signal transduction in VSMC. We tested the hypothesis that CyPA contributes to cardiovascular diseases by analyzing several genetic interventions that include the CyPA knockout mouse and the CyPA overexpressing transgenic mouse (VSMC-Tg). CyPA plays a crucial role in VSMC proliferation/migration and inflammatory cell recruitment, resulting in cardiovascular diseases in vivo. Antioxid. Redox Signal. 12, 675–682.
Introduction
Vascular smooth muscle cells (VSMC) are among the most plastic of all cells in their ability to respond to different stimuli. Autocrine/paracrine growth factors from VSMC have been mentioned for long time as important mechanisms that mediate varying cellular responses in vascular remodeling (5). The concept of VSMC auto/paracrine growth factors was first mentioned 30 years ago (10, 11, 23, 32). Dzau (18) and Nilsson (52) used the term autocrine growth to describe increased expression of VSMC growth factors. It has now become clear that almost all VSMC growth factors elicit auto/paracrine growth pathways. Recent evidence suggests that many other stimuli that modulate VSMC function including reactive oxygen species (ROS) promote VSMC growth by inducing auto/paracrine growth mechanisms, as reviewed by Taniyama and Griendling (72). ROS increase cell proliferation, mediate agonist-induced hypertrophy, and also induce apoptosis in a concentration-dependent manner (27). It has now become clear that ROS plays a crucial role for VSMC proliferation both directly and indirectly by inducing auto/paracrine growth mechanisms.
The major topics that will be addressed in this review are a series of projects that were performed in our laboratory for >15 years. Our questions are as follows: (a) Why do ROS promote VSMC growth? (b) Does ROS-induced VSMC growth utilize auto/paracrine growth mechanisms? (c) What might be the secreted factors that explain the ROS-induced VSMC growth? (d) What are the mechanisms involved for ROS-induced secretion of growth factor? and finally, (e) Do the ROS-induced factors actually contribute to vascular remodeling in vivo? In order to answer these questions, we performed studies and identified cyclophilin A (CyPA) as auto/paracrine growth factor released from VSMC in response to increased levels of ROS. To further demonstrate the role of CyPA for VSMC growth and vascular remodeling in vivo, we studied CyPA knockout and VSMC-specific CyPA overexpressing mouse. By using these transgenic mice, we elucidated that CyPA mediates a variety of cardiovascular diseases including vascular intimal thickness and abdominal aortic aneurysms (AAA).
CyPA as a Molecular Chaperone with Enzymatic Properties
Cyclophilins are a family of highly conserved and ubiquitous proteins termed immunophilins (48). The most abundant cyclophilin is cyclophilin A (CyPA) (21), which is widely distributed in almost all tissues in prokaryotes and eukaryotes. In 1984, CyPA was identified as the main target for the immunosuppressive drug cyclosporine A (CsA) (6, 30, 31, 66). In humans, CyPA was found in all organs and the CyPA concentration may account for as much as 0.1–0.4% of total protein in the cell (49, 59, 60). CyPA was abundant in cytosolic extracts from lymphocytes and to had a high affinity for CsA (30). CyPA was also shown to be a part of a cytosolic heat-shock protein–immunophilin chaperone complex that includes caveolin and cholesterol (75). Due to its enzymatic properties, cellular localization, and role in protein folding, CyPA belongs to a diverse set of proteins that are termed molecular chaperones. Since CyPA catalyzes the cis–trans isomerization of peptidyl–prolyl bonds of certain proteins (PPIase activity), CyPA acts as an acceleration factor in protein folding and assembly. When protein peptide binds, the trans-state is much more favored compared with cis-state (>100 times). Therefore, unfolded proteins need an extremely longer time for trans–cis isomerization, which is a rate limiting step in the folding of proteins (34). The first demonstration of this activity in vitro was the delayed maturation of collagen by blocking PPIase activity with cyclosporine A (CsA) (67). In addition to the role for protein folding, the PPIase activity of CyPA has recently been demonstrated to have a variety of roles, including intracellular trafficking (79), signal transduction, and transcription regulation (41).
Following the identification of CyPA, several other members of the cyclophilins were cloned and characterized. Cyclophilin B (CyPB) (55), Cyclophilin C (CyPC) (64), and Cyclophilin D (CyPD) (4), were found to be less abundant and localized not only in cytosol but also in membranes and subcellular organelles because of the presence of hydrophobic N-terminal as well as C-terminal extensions. Human CyPB and murine CyPC are localized to endoplasmic reticulum (4). CyPD is localized to mitochondria, is an integral part of the mitochondrial permeability transition complex, and plays a crucial role in apoptosis. A more detailed classification of the different cyclophilins has been reviewed elsewhere (21).
Identification of Extracellular CyPA As a Secreted Protein That Promotes VSMC Growth
Production of intracellular ROS, such as superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH·), have been implicated in the pathogenesis of cardiovascular disease, in part by promoting VSMC proliferation (1, 3, 53). Changes in vascular redox state are a common pathway involved in the pathogenesis of atherosclerosis, aortic aneurysms, and vascular restenosis after angioplasty. ROS can be very harmful, especially under conditions where their production is enhanced which then exceeds cellular antioxidant defenses. ROS target cellular biomolecules and cause severe damage such as lipid peroxidation, protein oxidation/inactivation, and DNA damage/mutations. However, while high levels of ROS might be very dangerous to cells and their content, controlled ROS levels (physiological levels) are important for the regulation of cell functions and cell fate (proliferation/death). For example, H2O2 is also important for endothelial cell (EC) function and vascular relaxation in a very low concentration (50). In the vascular wall, ROS are generated by several mechanisms, including NADPH oxidases, xanthine oxidase, the mitochondrial respiratory chain, lipoxygenases, and nitric oxide synthases (13). ROS formation can be stimulated by mechanical forces (e.g., stretch, pressure, shear stress), environmental factors (such as hypoxia), secreted factors coupled to tyrosine kinase receptors (e.g., platelet derived growth factor, PDGF) (70), and secreted factors coupled to G protein-coupled receptors such as angiotensin II, AngII (26). Initial studies in our laboratory demonstrated that ROS stimulate cultured VSMC proliferation and activate intracellular kinases such as ERK1/2 that is associated with cell growth (3, 57).
In particular, we found that activation of ERK1/2 by a ROS generator, napthoquinolinedione (LY83583) was biphasic: an early peak of ERK1/2 activity was present at 5–10 min, whereas a delayed ERK1/2 activation appeared after 2 h. One logical mechanism for the delayed ERK1/2 activation was the response to the secreted oxidative stress-induced factors (SOXF), which show autocrine/paracrine signals. In order to identify the presence of SOXF, we evaluated the ability of conditioned medium for ERK1/2 activation. ERK1/2 activity was significantly increased by conditioned medium from VSMC treated with LY83583. We analyzed the proteins released into the medium in response to LY83583. Approximately 35 secreted proteins were detected, and to purify SOXF we used sequential chromatography and finally found that CyPA is one of the major SOXF (44). To study the role of intracellular ROS in CyPA function, we studied cells transfected with nox1 (homolog of the NADPH oxidase catalytic subunit) to stimulate ROS production. CyPA expression and secretion were increased, and antioxidants blocked the secretion of CyPA from nox1-transfected cells (37). To provide further evidence that CyPA is a SOXF, human recombinant CyPA was tested on VSMC cultures. Human recombinant CyPA stimulated ERK1/2 activity and DNA synthesis in VSMC in a concentration-dependent manner (37). Thus, we concluded that CyPA is a novel VSMC growth factor that may substantially contribute to the growth promoting activity of ROS in VSMC.
Mechanism of VSMC Growth and CyPA Receptor
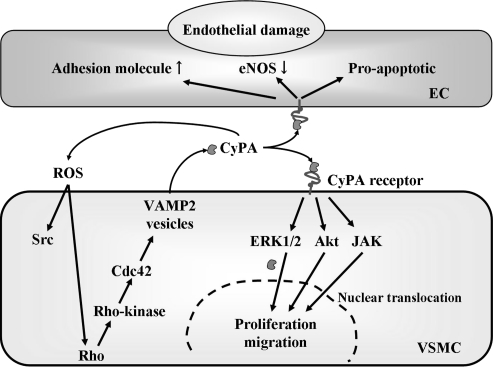
There are many publications regarding intracellular functions of CyPA. It may be relevant to note that CyPA intracellular functions include roles as immunophilins that interact with calcineurin, as components of a caveolin–cholesterol–cyclophilin complex, and as components of cell cycle (48). Our model for CyPA action is cell type specific (Fig. 1). In VSMC, ROS such as superoxide activate a pathway containing vesicles that results in secretion of CyPA (71). CyPA stimulates ERK1/2, Akt, and JAK in VSMC that contribute to DNA synthesis, proliferation, and migration (Fig. 1) (37). In EC, CyPA largely activates proinflammatory pathways including increased expression of VCAM-1 and E-selectin (36) (Fig. 1). Despite the mounting evidence that cyclophilins serve multiple intracellular and extracellular functions, surprisingly little is known regarding the effect on specific receptors. Several molecules have been proposed to serve as extracellular receptors for CyPA, including extracellular matrix metalloproteinase inducer (EMMPRIN) (56, 68), CD14 (2), and CD91 (7). To date, none of these proteins have unequivocally been proved to mediate all the cellular events associate with CyPA.
FIG. 1.
Proposed mechanisms for ROS-induced CyPA secretion and autocrine/paracrine growth signal in VSMC. ROS-induced CyPA secretion requires an active process including vesicle formation, vesicle transport to the plasma membrane (requiring myosin II), docking, and fusion (requiring actin cytoskeleton remodeling). Rho, Rho kinase, and Cdc42 contribute to the myosin II activation and actin remodeling. Secreted extracellular CyPA activates ERK1/2, Akt, and JAK in an autocrine/paracrine manner, which promotes proliferation and migration of VSMC. On the other hand, in EC, extracellular CyPA activates ERK1/2, JNK, and p38 and promotes adhesion molecule expression including VCAM-1.
Mechanism of ROS-Induced CyPA Secretion
Several growth factors are secreted from VSMC in response to various stimuli. However, the intracellular trafficking mechanisms that regulate secretion of these growth factors are not well understood. The best studied secretory pathway in VSMC involves tissue factor, the initiator of the clotting cascade. Tissue factor is released in microparticles that bud from the VSMC plasma membrane (63). We harvested medium from LY83583-exposed VSMC and performed high-speed centrifugation onto a 60% sucrose cushion. Although virtually all of the tissue factor activity (>95%) has been shown to be precipitated by this technique (63), we did not find CyPA in microparticles, supporting the concept that CyPA might be released by vesicle pathway (Fig. 1). In fact, CyPA was secreted from VSMC via a highly regulated pathway that involves vesicle transport and plasma membrane binding (Fig. 1). Rho GTPases, including RhoA, Cdc42, and Rac1, are key regulators in signaling pathways linked to actin cytoskeletal rearrangement (46). The Rho GTPases play a central role in vesicular trafficking pathways by controlling organization of the actin cytoskeleton. It has been reported that active participation of Rho GTPases is required for secretion. Consistently, we showed that expression of dominant-negative mutants of RhoA and Cdc42 inhibited ROS-induced CyPA secretion, suggesting that both RhoA- and Cdc42-dependent signaling events regulate CyPA secretion. Myosin II is involved in secretory mechanisms as a motor for vesicle transport (51). Rho-kinase, a downstream effector of RhoA, mediates myosin II activation via phosphorylation and inactivation of myosin II light chain phosphatase (40). We also demonstrated that Rho-kinase inhibitor reduced ROS-induced CyPA secretion (71). These results suggest that myosin II-mediated vesicle transport is required for CyPA secretion from VSMC.
This novel secretory pathway for CyPA involves Rho GTPase-myosin II activation/actin remodeling regulated vesicle transport, docking, and a fusion process. Moreover, CyPA was transported to the plasma membrane and colocalized with vesicle-associated membrane protein (VAMP) in response to ROS stimulation. In particular, gene silencing of VAMP-2 by siRNA significantly inhibited ROS-induced CyPA secretion. These results support the hypothesis that CyPA is secreted from VSMC through a process requiring ROS production and vesicle formation.
CyPA as a VSMC Growth Factor for Vascular Remodeling In Vivo
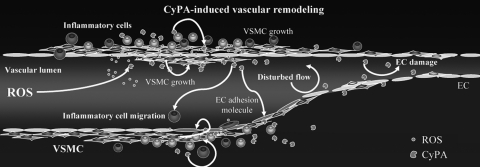
It has become clear that increases in ROS represent one of the pathogenic mechanisms for vascular disease (24, 43). ROS have been implicated in the pathogenesis of neointima formation in part by promoting VSMC growth (3, 57), as well as stimulating pro-inflammatory events (20, 45, 58). As discussed above, CyPA is secreted in response to ROS in vitro (37, 44, 71). We demonstrated that extracellular CyPA stimulates pro-inflammatory signals in EC, including expression of E-selectin and vascular cell adhesion molecule (VCAM)-1 (Fig. 1) (36). In addition to effects on vascular cells, CyPA has been shown to be a chemoattractant for inflammatory cells (15, 39) and promotes activation of matrix metalloproteinases (MMPs) (39, 80). Therefore, CyPA could be a key mediator that affects EC, VSMC, and inflammatory cell function during oxidative stress in vivo (Fig. 2).
FIG. 2.
Cyclophilin A (CyPA) is a novel growth factor that mediates VSMC growth under oxidative stress. Decreased blood flow increases reactive oxygen species (ROS) generation which induces secretion of CyPA. Secreted CyPA promotes vascular smooth muscle cells (VSMC) proliferation, endothelial adhesion molecule expression, and inflammatory cell migration, resulting in the vascular remodeling.
To strengthen the link between flow cessation, CyPA expression, and cell growth, we observed the time course and distribution of CyPA expression in carotids after ligation (61). We found that CyPA expression is dramatically increased with a time course that paralleled neointima formation, suggesting an important role for CyPA in the cell response to oxidative stress induced by vascular injury. In parallel with CyPA expression, carotid ligation induced phosphorylation of ERK1/2 in WT carotids, which was significantly less in CyPA−/− carotids, consistent with the reduced number of Ki67+ cells in ligated CyPA−/− carotids. The distribution of Ki67+ cells closely overlapped with areas of highest CyPA expression, especially in the rapidly proliferating neointimal cells in WT mice. Co-localization of CyPA, αSMA, and Masson–Trichrome staining revealed that CyPA expression was especially elevated in VSMC. To prove further the contribution of VSMC-derived CyPA to vascular remodeling, we prepared VSMC-specific CyPA transgenic mice (VSMC-Tg). VSMC-Tg mice exhibited no significant change in sham carotids, while ligated carotids showed increases of 217% in intimal area, 32% in medial area, and 140% in I/M ratio, compared with control mice expressing normal levels of CyPA. The observation that VSMC-specific CyPA overexpression not only increased the medial area but also the intimal area suggests that VSMC-derived extracellular CyPA promotes the proliferation and migration of VSMC via a paracrine manner. The tremendous vascular proliferation resulting from VSMC-specific overexpression of CyPA suggests a major contribution for VSMC-derived CyPA in vascular remodeling.
CyPA is expressed by all cell types participating in vascular pathology (35). Additionally, extracellular CyPA has recently been found to induce IL-6 release in inflammatory cells (54). Moreover, CyPA function using monocyte/macrophage cell lines revealed that CyPA induces the expression of cytokines-chemokines such as TNFα, IL-8, MCP-1, IL-1β, and matrix metalloproteinase (MMP)-9 through a pathway that is dependent on NF-kB activation (39). In our carotid ligation model, we observed significant accumulation of CD45+ inflammatory cells in the intima in ligated CyPA−/− carotids and VSMC-specific overexpression of CyPA (VSMC-Tg) further enhanced the accumulation of inflammatory cells in ligated carotids, supporting the important role for CyPA in mediating the recruitment of inflammatory cells.
In the context of flow cessation, a predominant Th2 inflammatory response could limit vascular remodeling. In fact, an important aspect of CyPA biology is its effects on T cell function by regulating calcineurin and Itk (a Tec family tyrosine kinase) (14). Consequently, in CyPA−/− mice there is increased responsiveness of T cells with a predominant Th2 response (14). CyPA also regulates T cell function by the effects of its peptidyl-prolyl isomerase (PPIase) activity on calcineurin. Cyclosporine A (CsA) binds to the site of PPIase activity and by inhibiting PPIase prevents T cell proliferation, thereby explaining the anti-inflammatory properties of CsA (30).
We propose that ROS generated locally by inflammatory cells causes VSMC to release CyPA, which would promote a recruitment of inflammatory cells that releasing several pro-inflammatory cytokines contribute to the intima thickening in this model. In addition, CyPA could regulate the proteolytic activity necessary for the migration of inflammatory cells through the activation of matrix metalloproteinases (MMPs) (39, 80).
These results were the first direct demonstration that CyPA contributes to vascular remodeling in vivo (61). This study revealed three important pathologic consequences of CyPA activity in vivo. First, VSMC-derived secreted CyPA is mitogenic by virtue of its ability to promote VSMC proliferation. Second, secreted extracellular CyPA is pro-inflammatory because it stimulates the recruitment of inflammatory cells. Third, secreted CyPA can further promote a pathological setting, exacerbating the generation of intracellular ROS in VSMC derived from mouse aorta.
CyPA as a MMP Activator That Initiates Aortic Aneurysm Formation
In the cardiovascular system, abdominal aortic aneurysm (AAA) formation results from chronic inflammation of the aortic wall, associated with decreased medial VSMC, and progressive destruction of structural components, particularly the elastic lamina. Key mechanisms include VSMC senescence (42), oxidative stress (25, 72), increased local production of proinflammatory cytokines (8), and increased activities of matrix metalloproteinases (MMPs) (69, 77) that degrade extracellular matrix. In animal models of AAA, genetic and pharmacological inhibition of ROS production (22, 73) and MMPs (47, 74) suppressed development of aneurysms. AngII infusion into ApoE−/− mice for 4 weeks promotes AAA formation (16, 17). In this animal model, the AngII type 1 (AT1) receptor in the vascular wall, not in inflammatory cells, is required for the initiation of AngII-induced AAAs (9). Furthermore, treatment with an AT1 receptor blocker significantly suppressed aneurysm formation in ApoE−/− mice (29).
As discussed above, ROS stimulate secretion of CyPA from VSMC, and that extracellular CyPA stimulates VSMC migration and proliferation (37, 44). Extracellular CyPA also stimulates EC adhesion molecule expression, and is a chemoattractant for inflammatory cells (37, 38, 71). Furthermore, CyPA is upregulated in patients with rheumatoid arthritis and implied for its crucial role for MMP activation (39). Therefore, we hypothesized that VSMC-derived CyPA augments AngII-induced ROS production, MMP activation, and inflammatory cell recruitment into the aortic VSMC. As expected, AAA formation in the AngII-induced ApoE−/− model was completely prevented in the CyPA−/− background (62). We also demonstrated that CyPA is highly expressed in the aorta of patients with AAA, and co-localizes with active form of MMPs. Based on these findings, we propose that AngII induces ROS and MMP activation via a CyPA-dependent pathway, a novel mechanism for induction of AAA formation by AngII.
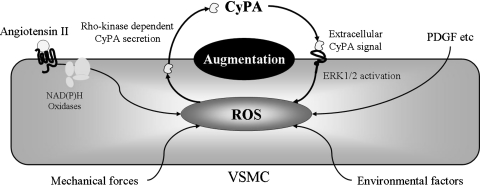
Our data suggest that extracellular CyPA and its signaling pathways are novel targets for AAA therapy and potentially other cardiovascular diseases associated with inflammation. In addition, extracellular CyPA induces ROS production in VSMC, which is consistent with our previous report that extracellular CyPA stimulates at least three signaling pathways (ERK1/2, Akt, and JAK) in VSMC (37) that have been shown to be important for ROS production (25, 72). All these data are a proof-of-concept that CyPA plays a crucial role in VSMC through ROS generation. ROS-induced CyPA secretion augments ROS production synergistically (Fig. 3). AngII induces the generation of ROS and promotes the secretion of CyPA. Then, secreted CyPA, acting as a proinflammatory cytokine, synergistically augments AngII-mediated ROS production, contributing to the onset of vascular inflammatory cell migration and AAA formation.
FIG. 3.
ROS-induced secretion of Cyclophilin A synergistically augments ROS production. ROS inducer such as angiotensin II, mechanical stress, and environmental factors, promotes Cyclophilin A (CyPA) secretion from vascular smooth muscle cells (VSMC). Secreted CyPA activates ERK1/2 and promotes ROS production, contributing to the augmentation of ROS production.
Summary and Future Directions
Numerous basic and clinical studies have clearly identified that ROS has a major role in the development of cardiovascular diseases. However, we still have no strong therapeutic strategy for clinical benefits of antioxidant administration. One potential reason for those could be a crucial role of ROS (especially H2O2) for intracellular signaling pathways that is also important for vascular functions in a very low concentration (50).
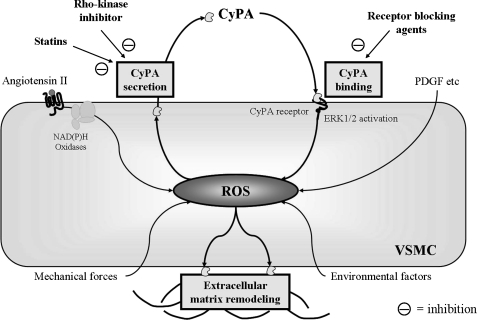
The identification of CyPA as a mediator of tissue damage associated with inflammation and oxidative stress provides insight into the mechanisms of several therapies. For example, the Rho-kinase inhibitor Y27632, and simvastatin significantly reduced CyPA secretion from VSMC. Rho-kinase is an important therapeutic target in cardiovascular disease (65) and Rho-kinase inhibition has been reported to reduce AngII-induced AAA formation (76). Moreover, AT1a receptor blockers and ACE inhibitors have been shown to prevent AAA formation in mice (9, 19, 29) and reduced CyPA secretion may partially contribute to the therapeutic effect of these drugs on AAA formation (62). EMMPRIN, a putative CyPA receptor, was identified as a tumor cell membrane protein that is expressed in VSMC, is activated by ROS, and stimulates MMP production (28). A recent article demonstrated ROS-dependent increases in EMMPRIN (33), which may be activated by binding of extracellular CyPA (78). Moreover, it has been demonstrated that EMMPRIN is strongly expressed in human AAA lesions (12). Therefore, it is logical to propose that agents which prevent CyPA binding to its receptors may have therapeutic potential (Fig. 4).
FIG. 4.
Potential targets for novel therapeutic efficacy targeting on CyPA-mediated cardiovascular diseases. We focus on blocking (a) CyPA secretion and (b) CyPA binding on CyPA receptor(s). We have shown that Rho-kinase inhibitor and statins inhibit the secretion of CyPA from VSMC. Additionally, we have recently found several candidates for potential molecules that mediate cell signaling by extracellular CyPA stimulation.
Because inflammation and oxidative stress contribute to tissue damage in several situations such as ischemia-reperfusion injury in the brain, heart, and kidney, future studies of CyPA-mediated function in appropriate models may reveal a significant role in other diseases.
By blocking this malignant cycle that augments ROS production through CyPA autocrine/paracrine signaling pathway, we may have a novel therapeutic tool for controlling cardiovascular diseases in the near future.
Abbreviations Used
- AAA
abdominal aortic aneurysms
- CsA
cyclosporine A
- CyPA
cyclophilin A
- CyPB
cyclophilin B
- CyPC
cyclophilin C
- CyPD
cyclophilin D
- EC
endothelial cell
- PPIase
peptidyl-prolyl cis-trans isomerase
- VCAM
vascular cell adhesion molecule
- VSMC
vascular smooth muscle cells
Acknowledgments
This work was supported by National Institutes of Health Grant HL49192 (to B.C. Berk), Internal Grant of the University of Salerno (to P. Nigro), and Japan Heart Foundation/Bayer Yakuhin Research Grant Abroad (to K. Satoh), Japan Heart Foundation/Novartis Grant for Research Award on Molecular and Cellular Cardiology (to K. Satoh), and the grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (to K. Satoh). We are grateful to the Aab Cardiovascular Research Institute members for useful suggestions.
References
- 1.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. .Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25:155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 2.Asea A. Kraeft SK. Kurt–Jones EA. Stevenson MA. Chen LB. Finberg RW. Koo GC. Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 3.Baas AS. Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2− in vascular smooth muscle cells. Circ Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Bergsma DJ. Eder C. Gross M. Kersten H. Sylvester D. Appelbaum E. Cusimano D. Livi GP. McLaughlin MM. Kasyan K, et al. The cyclophilin multigene family of peptidyl-prolyl isomerases. Characterization of three separate human isoforms. J Biol Chem. 1991;266:23204–23214. [PubMed] [Google Scholar]
- 5.Berk BC. Vascular smooth muscle growth: Autocrine growth mechanisms. Physiol Rev. 2001;81:999–1030. doi: 10.1152/physrev.2001.81.3.999. [DOI] [PubMed] [Google Scholar]
- 6.Bierer BE. Cyclosporin A, FK506, and rapamycin: binding to immunophilins and biological action. Chem Immunol. 1994;59:128–155. [PubMed] [Google Scholar]
- 7.Binder RJ. Han DK. Srivastava PK. CD91: A receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 8.Bruemmer D. Collins AR. Noh G. Wang W. Territo M. Arias–Magallona S. Fishbein MC. Blaschke F. Kintscher U. Graf K. Law RE. Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassis LA. Rateri DL. Lu H. Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–384. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 10.Castellot JJ., Jr. Karnovsky MJ. Spiegelman BM. Potent stimulation of vascular endothelial cell growth by differentiated 3T3 adipocytes. Proc Natl Acad Sci USA. 1980;77:6007–6011. doi: 10.1073/pnas.77.10.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamley–Campbell JH. Campbell GR. What controls smooth muscle phenotype? Atherosclerosis. 1981;40:347–357. doi: 10.1016/0021-9150(81)90145-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen XF. Wang JA. Hou J. Gui C. Tang LJ. Chen XQ. Xie XJ. Jiang JJ. Cai JF. Chen HS. Lu HS. Chen H. Extracellular matrix metalloproteinase inducer (EMMPRIN) is present in smooth muscle cells of human aneurysmal aorta and is induced by angiotensin II in vitro. Clin Sci. 2008;40:567–571. doi: 10.1042/CS20080235. [DOI] [PubMed] [Google Scholar]
- 13.Clempus RE. Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colgan J. Asmal M. Neagu M. Yu B. Schneidkraut J. Lee Y. Sokolskaja E. Andreotti A. Luban J. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21:189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Damsker JM. Bukrinsky MI. Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007;82:613–618. doi: 10.1189/jlb.0506317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daugherty A. Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 17.Daugherty A. Manning MW. Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzau VJ. Vascular renin-angiotensin: A possible autocrine or paracrine system in control of vascular function. J Cardiovasc Pharmacol. 1984;6:S377–382. [PubMed] [Google Scholar]
- 19.Ejiri J. Inoue N. Tsukube T. Munezane T. Hino Y. Kobayashi S. Hirata K. Kawashima S. Imajoh-Ohmi S. Hayashi Y. Yokozaki H. Okita Y. Yokoyama M. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: Protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc Res. 2003;59:988–996. doi: 10.1016/s0008-6363(03)00523-6. [DOI] [PubMed] [Google Scholar]
- 20.Festa A. D'Agostino R. Howard G. Mykkanen L. Tracy RP. Haffner SM. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000;58:1703–1710. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 21.Galat A. Metcalfe SM. Peptidylproline cis/trans isomerases. Prog Biophys Mol Biol. 1995;63:67–118. doi: 10.1016/0079-6107(94)00009-x. [DOI] [PubMed] [Google Scholar]
- 22.Gavazzi G. Deffert C. Trocme C. Schappi M. Herrmann FR. Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007;50:189–196. doi: 10.1161/HYPERTENSIONAHA.107.089706. [DOI] [PubMed] [Google Scholar]
- 23.Gospodarowicz D. Moran J. Braun D. Birdwell C. Clonal growth of bovine vascular endothelial cells: Fibroblast growth factor as a survival agent. Proc Natl Acad Sci USA. 1976;73:4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griendling KK. FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: Basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 25.Griendling KK. FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 26.Griendling KK. Minieri CA. Ollerenshaw JD. Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activation in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK. Ushio–Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998;132:9–15. doi: 10.1016/s0022-2143(98)90019-1. [DOI] [PubMed] [Google Scholar]
- 28.Guo H. Majmudar G. Jensen TC. Biswas C. Toole BP. Gordon MK. Characterization of the gene for human EMMPRIN, a tumor cell surface inducer of matrix metalloproteinases. Gene. 1998;220:99–108. doi: 10.1016/s0378-1119(98)00400-4. [DOI] [PubMed] [Google Scholar]
- 29.Habashi JP. Judge DP. Holm TM. Cohn RD. Loeys BL. Cooper TK. Myers L. Klein EC. Liu G. Calvi C. Podowski M. Neptune ER. Halushka MK. Bedja D. Gabrielson K. Rifkin DB. Carta L. Ramirez F. Huso DL. Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handschumacher RE. Harding MW. Rice J. Drugge RJ. Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 31.Harding MW. Handschumacher RE. Speicher DW. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986;261:8547–8555. [PubMed] [Google Scholar]
- 32.Harker LA. Ross R. Pathogenesis of arterial vascular disease. Semin Thromb Hemost. 1979;5:274–292. doi: 10.1055/s-0028-1087159. [DOI] [PubMed] [Google Scholar]
- 33.Haug C. Lenz C. Diaz F. Bachem MG. Oxidized low-density lipoproteins stimulate extracellular matrix metalloproteinase Inducer (EMMPRIN) release by coronary smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:1823–1829. doi: 10.1161/01.ATV.0000142806.59283.11. [DOI] [PubMed] [Google Scholar]
- 34.Herzberg O. Moult J. Analysis of the steric strain in the polypeptide backbone of protein molecules. Proteins. 1991;11:223–229. doi: 10.1002/prot.340110307. [DOI] [PubMed] [Google Scholar]
- 35.Jin ZG. Berk BC. SOXF: Redox mediators of vascular smooth muscle cell growth. Heart. 2004;90:488–490. doi: 10.1136/hrt.2003.029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin ZG. Lungu AO. Xie L. Wang M. Wong C. Berk BC. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186–1191. doi: 10.1161/01.ATV.0000130664.51010.28. [DOI] [PubMed] [Google Scholar]
- 37.Jin ZG. Melaragno MG. Liao DF. Yan C. Haendeler J. Suh YA. Lambeth JD. Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 38.Khromykh LM. Kulikova NL. Anfalova TV. Muranova TA. Abramov VM. Vasiliev AM. Khlebnikov VS. Kazansky DB. Cyclophilin A produced by thymocytes regulates the migration of murine bone marrow cells. Cell Immunol. 2007;249:46–53. doi: 10.1016/j.cellimm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Kim H. Kim WJ. Jeon ST. Koh EM. Cha HS. Ahn KS. Lee WH. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol. 2005;116:217–224. doi: 10.1016/j.clim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Kimura K. Ito M. Amano M. Chihara K. Fukata Y. Nakafuku M. Yamamori B. Feng J. Nakano T. Okawa K. Iwamatsu A. Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 41.Krummrei U. Bang R. Schmidtchen R. Brune K. Bang H. Cyclophilin-A is a zinc-dependent DNA binding protein in macrophages. FEBS Lett. 1995;371:47–51. doi: 10.1016/0014-5793(95)00815-q. [DOI] [PubMed] [Google Scholar]
- 42.Kunieda T. Minamino T. Nishi J. Tateno K. Oyama T. Katsuno T. Miyauchi H. Orimo M. Okada S. Takamura M. Nagai T. Kaneko S. Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 43.Leopold JA. Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1332–1340. doi: 10.1161/01.ATV.0000163846.51473.09. [DOI] [PubMed] [Google Scholar]
- 44.Liao DF. Jin ZG. Baas AS. Daum G. Gygi SP. Aebersold R. Berk BC. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 45.Libby P. Ridker PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999;100:1148–1150. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- 46.Mackay DJ. Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 47.Manning MW. Cassis LA. Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 48.Marks AR. Cellular functions of immunophilins. Physiol Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 49.Marks WH. Harding MW. Handschumacher R. Marks C. Lorber MI. The immunochemical distribution of cyclophilin in normal mammalian tissues. Transplantation. 1991;52:340–345. doi: 10.1097/00007890-199108000-00030. [DOI] [PubMed] [Google Scholar]
- 50.Matoba T. Shimokawa H. Nakashima M. Hirakawa Y. Mukai Y. Hirano K. Kanaide H. Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;107:23–25. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neco P. Giner D. Viniegra S. Borges R. Villarroel A. Gutierrez LM. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J BIol Chem. 2004;279:27450–27457. doi: 10.1074/jbc.M311462200. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson J. Sjolund M. Palmberg L. Thyberg J. Heldin CH. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci USA. 1985;82:4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omar HA. Cherry PD. Mortelliti MP. Burke–Wolin T. Wolin MS. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ Res. 1991;69:601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- 54.Payeli SK. Schiene–Fischer C. Steffel J. Camici GG. Rozenberg I. Luscher TF. Tanner FC. Cyclophilin A differentially activates monocytes and endothelial cells Role of purity, activity, and endotoxin contamination in commercial preparations. Atherosclerosis. 2008;28:705–710. doi: 10.1016/j.atherosclerosis.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 55.Price ER. Zydowsky LD. Jin MJ. Baker CH. McKeon FD. Walsh CT. Human cyclophilin B: A second cyclophilin gene encodes a peptidyl- prolyl isomerase with a signal sequence. Proc Natl Acad Sci USA. 1991;88:1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pushkarsky T. Zybarth G. Dubrovsky L. Yurchenko V. Tang H. Guo H. Toole B. Sherry B. Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci USA. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao GN. Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 58.Ross R. Atherosclerosis—An inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 59.Ryffel B. Brockhaus M. Greiner B. Mihatsch MJ. Gudat F. Tumour necrosis factor receptor distribution in human lymphoid tissue. Immunology. 1991;74:446–452. [PMC free article] [PubMed] [Google Scholar]
- 60.Sarris AH. Harding MW. Jiang TR. Aftab D. Handschumacher R. Immunofluorescent localization and immunochemical determination of cyclophilin-A with specific rabbit antisera. Transplantation. 1992;54:904–910. doi: 10.1097/00007890-199211000-00026. [DOI] [PubMed] [Google Scholar]
- 61.Satoh K. Matoba T. Suzuki J. O'Dell MR. Nigro P. Cui Z. Mohan A. Pan S. Li L. Jin ZG. Yan C. Abe J. Berk BC. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008;117:3088–3098. doi: 10.1161/CIRCULATIONAHA.107.756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satoh K. Nigro P. Matoba T. O'Dell MR. Cui Z. Shi X. Mohan A. Yan C. Abe J. Illig KA. Berk BC. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schecter AD. Spirn B. Rossikhina M. Giesen PL. Bogdanov V. Fallon JT. Fisher EA. Schnapp LM. Nemerson Y. Taubman MB. Release of active tissue factor by human arterial smooth muscle cells. Circ Res. 2000;87:126–132. doi: 10.1161/01.res.87.2.126. [DOI] [PubMed] [Google Scholar]
- 64.Schneider H. Charara N. Schmitz R. Wehrli S. Mikol V. Zurini MG. Quesniaux VF. Movva NR. Human cyclophilin C: primary structure, tissue distribution, and determination of binding specificity for cyclosporins. Biochemistry. 1994;33:8218–8224. doi: 10.1021/bi00193a007. [DOI] [PubMed] [Google Scholar]
- 65.Shimokawa H. Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 66.Siekierka JJ. Hung SH. Poe M. Lin CS. Sigal NH. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989;341:755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- 67.Steinmann B. Bruckner P. Superti-Furga A. Cyclosporin A slows collagen triple-helix formation in vivo: Indirect evidence for a physiologic role of peptidyl-prolyl cis-trans-isomerase. J Biol Chem. 1991;266:1299–1303. [PubMed] [Google Scholar]
- 68.Sun J. Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–2281. [PubMed] [Google Scholar]
- 69.Sun J. Sukhova GK. Wolters PJ. Yang M. Kitamoto S. Libby P. MacFarlane LA. Mallen-St Clair J. Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- 70.Sundaresan M. Yu ZX. Ferrans VJ. Irani K. Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 71.Suzuki J. Jin ZG. Meoli DF. Matoba T. Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 72.Taniyama Y. Griendling KK. Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 73.Thomas M. Gavrila D. McCormick ML. Miller FJ., Jr. Daugherty A. Cassis LA. Dellsperger KC. Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson RW. Baxter BT. MMP inhibition in abdominal aortic aneurysms. Ann NY Acad Sci. 1999;878:159–178. doi: 10.1111/j.1749-6632.1999.tb07682.x. [DOI] [PubMed] [Google Scholar]
- 75.Uittenbogaard A. Ying Y. Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- 76.Wang YX. Martin–McNulty B. da Cunha V. Vincelette J. Lu X. Feng Q. Halks–Miller M. Mahmoudi M. Schroeder M. Subramanyam B. Tseng JL. Deng GD. Schirm S. Johns A. Kauser K. Dole WP. Light DR. Fasudil, a Rho-kinase inhibitor, attenuates angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice by inhibiting apoptosis and proteolysis. Circulation. 2005;111:2219–2226. doi: 10.1161/01.CIR.0000163544.17221.BE. [DOI] [PubMed] [Google Scholar]
- 77.Yoshimura K. Aoki H. Ikeda Y. Fujii K. Akiyama N. Furutani A. Hoshii Y. Tanaka N. Ricci R. Ishihara T. Esato K. Hamano K. Matsuzaki M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 78.Yurchenko V. Zybarth G. O'Connor M. Dai WW. Franchin G. Hao T. Guo H. Hung HC. Toole B. Gallay P. Sherry B. Bukrinsky M. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002;277:22959–22965. doi: 10.1074/jbc.M201593200. [DOI] [PubMed] [Google Scholar]
- 79.Zhu C. Wang X. Deinum J. Huang Z. Gao J. Modjtahedi N. Neagu MR. Nilsson M. Eriksson PS. Hagberg H. Luban J. Kroemer G. Blomgren K. Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med. 2007;204:1741–1748. doi: 10.1084/jem.20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu P. Ding J. Zhou J. Dong WJ. Fan CM. Chen ZN. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: Its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023–1033. doi: 10.1186/ar1778. [DOI] [PMC free article] [PubMed] [Google Scholar]