Abstract
Hypoxic exposure causes pulmonary vasoconstriction, which serves as a critical physiologic process that ensures regional alveolar ventilation and pulmonary perfusion in the lungs, but may become an essential pathologic factor leading to pulmonary hypertension. Although the molecular mechanisms underlying hypoxic pulmonary vasoconstriction and associated pulmonary hypertension are uncertain, increasing evidence indicates that hypoxia can result in a significant increase in intracellular reactive oxygen species concentration ([ROS]i) through the mitochondrial electron-transport chain in pulmonary artery smooth muscle cells (PASMCs). The increased mitochondrial ROS subsequently activate protein kinase C-ɛ (PKCɛ) and NADPH oxidase (Nox), providing positive mechanisms that further increase [ROS]i. ROS may directly cause extracellular Ca2+ influx by inhibiting voltage-dependent K+ (KV) channels and opening of store-operated Ca2+ (SOC) channels, as well as intracellular Ca2+ release by activating ryanodine receptors (RyRs), leading to an increase in intracellular Ca2+ concentration ([Ca2+]i) and associated contraction. In concert with ROS, PKCɛ may also affect KV channels, SOC channels, and RyRs, contributing to hypoxic Ca2+ and contractile responses in PASMCs. Antioxid. Redox Signal. 11, 611–623.
Introduction
It is well known that pulmonary arteries constrict in response to hypoxic exposure (<60 mm Hg Po2). Hypoxia-induced pulmonary vasoconstriction serves as an important physiologic process that preserves the sufficient matching of regional alveolar ventilation and pulmonary perfusion in the lungs, thereby allowing sufficient oxygenation of the blood. In contrast, systemic arteries normally do not contract or even dilate in response to hypoxia to retain fairly constant blood flow to fulfill cellular metabolic demand in important organs. Despite having a unique physiologic significance, hypoxic pulmonary vasoconstriction, if sustained, may serve as a key pathologic factor leading to pulmonary hypertension and even heart failure.
The cellular and molecular mechanisms underlying the unique hypoxic pulmonary vasoconstriction and associated pulmonary hypertension remain largely elusive; however, we and many other investigators recently provided extensive evidence showing that hypoxia results in a large increase in intracellular reactive oxygen species concentration ([ROS]i) in pulmonary artery smooth muscle cells (PASMCs) (10, 26, 34, 43, 47, 61, 69, 70, 97, 98, 102, 103), which is consistent with the contribution of ROS to the initiation or maintenance or both of numerous physiologic and pathologic cellular responses in virtually all types of cells. It also should be noted that the hypoxic decrease in [ROS]i has been reported (4, 49, 50, 53). Intracellular ROS can be generated by multiple resources, including the mitochondrial electron-transport chain (ETC), NADPH oxidase (Nox), xanthine oxidase, cyclooxygenase, and cytochrome P450. Among these resources, the mitochondrial ETC and Nox (4, 37, 47, 50, 61, 69, 70, 97, 102, 104, 109) have been shown to be essential for the hypoxic increase or decrease in [ROS]i in PASMCs.
A number of publications suggest that the hypoxic increase or decrease in [ROS]i can directly affect the activity of ion channels, leading to a large increase in intracellular Ca2+ concentration ([Ca2+]i) in PASMCs. For instance, hypoxia may inhibit voltage-dependent K+ (KV) channels by affecting [ROS]i (4, 20, 50). Presumably, hypoxic inhibition of KV channels would result in membrane depolarization, activation of voltage-dependent Ca2+ (CaV) channels, and extracellular Ca2+ influx, resulting in an increase in [Ca2+]i. ROS also may activate ryanodine receptors/Ca2+-release channels (RyRs) to induce Ca2+ release from the sarcoplasmic reticulum (SR), contributing to the hypoxic increase in [Ca2+]i in PASMCs (40, 66). As an increase in [Ca2+]i is a most important factor for cell contraction, recent studies have demonstrated that the hypoxic increase in [Ca2+]i and contraction are intimately related in PASMCs (66, 72, 73). Pharmacologic and genetic interventions that inhibit or eliminate the hypoxic increase in [Ca2+]i can correspondingly inhibit or eliminate the hypoxic contraction (38, 69, 70, 122). Moreover, hypoxia normally causes neither an increase in [Ca2+]i nor a contraction in systemic (e.g., cerebral and mesenteric) artery SMCs (69, 91, 99). In addition to the direct effect, ROS also may activate intermediate signaling molecules, such as protein kinase C-ɛ (PKCɛ), to regulate specific ion channels in concert with ROS, contributing to the hypoxic increase in [Ca2+]i and associated contraction in PASMCs (69). It is interesting to note that our recent work revealed that mitochondrial ROS-dependent activation of PKCɛ can significantly augment Nox activity and lead to a further increase in intracellular ROS generation, which provides a positive-feedback mechanism to augment intracellular ROS generation further, contributing to the hypoxic increase in [ROS]i and [Ca2+]i as well (70).
In this review, we summarize recent progress in the study of signaling mechanisms underlying the hypoxic ROS generation and attendant Ca2+ responses in PASMCs, particularly highlighting our own and others' latest work in the identification of specific sources, signaling cascades, and effective targets for ROS during hypoxic stimulation.
Hypoxia Causes a Significant Increase in [ROS]i
ROS function as important signaling molecules mediating many physiologic and pathologic processes in virtually all types of cells. To explore the potential important role of ROS in hypoxic responses in PASMCS, Archer and his colleagues (4) examined the effect of hypoxia on intracellular ROS generation in isolated rat lungs by using lucigenin, a chemiluminescence reagent that is often used to assess superoxide anion (O2−) production. As shown in Table 1, they found that an acute hypoxic exposure for minutes causes a significant decrease in lucigenin-derived chemiluminescence, indicating a decrease in [ROS]i. By using lucigenin chiefly for measuring O2− generation, dichlorodihydrofluorescein (H2DCF) for hydrogen peroxide (H2O2), dihydroethidium for O2−, AmplexRed for H2O2 (or a combination of these), their associated research groups further confirmed findings that acute hypoxia for minutes decreases [ROS]i in freshly isolated rat pulmonary arteries and PASMCs (50), and hypoxia for hours decreases [ROS]i in passaged human PASMCs (49). Consistent with the hypoxic reduction in [ROS]i, acute hypoxia results in a decrease in lucigenin-derived chemiluminescence in microsome-enriched fractions of calf pulmonary arteries (53). In contrast, with lucigenin, H2DCF, dihydroethidium, and electron paramagnetic resonance, numerous research groups revealed that acute hypoxia increases, rather decreases, ROS generation in isolated rabbit and lamb lungs, rat and dog pulmonary arteries, and cultured calf, dog, and rat PASMCs (10, 26, 27, 34, 42–44, 47, 61, 97, 102, 103). Intriguingly, a study using dihydroethidium found that acute hypoxic exposure for minutes significantly and rapidly decreases, but for hours, markedly increases [ROS]i in passaged human PASMCs (114). We looked at the effect of acute hypoxia on [ROS]i in freshly isolated mouse PASMCs by using multiple approaches, including H2DCF/DA (mainly for measuring H2O2), cytochrome c reduction assay and lucigenin (for O2−), and RedoxSensor Red CC-1 (for both O2− and H2O2). Our data indicate that acute hypoxia for minutes brings about a large increase in [ROS]i (69, 70, 97).
Table 1.
Summary of Previous Reports on the Effect of Hypoxia on [ROS]i in Isolated Lungs, Pulmonary Arteries, and PASMCs
| Authors | Hypoxia | [ROS]i | Preparations | Detection methods | References |
|---|---|---|---|---|---|
| Archer et al. | Minutes | Decrease | Isolated rat lungs, pulmonary arteries, and PASMCs | AmplexRed, dihydroethidium, H2DCF, Lucigenin | (4; 50) |
| Jernigan et al. | Minutes Weeks | IncreaseIncrease | Isolated rat pulmonary arteries | H2DCF | (26; 27) |
| Killilea et al. | Minutes | Increase | Cultured rat PASMCs | H2DCF | (34) |
| Liu et al. | Minutes Weeks | Increase | Isolated porcine pulmonary arteries | Electron paramagnetic resonance, H2DCF, Lucigenin | (43) |
| Marshall et al. | Minutes | Increase | Cultured calf PASMCs | Lucigenin | (47) |
| Mehta et al. | Hours | Decrease | Cultured human PASMCs | Amplexred, Dihydroethidium, H2DCF, Lucigenin | (49) |
| Mittal et al. | Weeks | Increase | Isolated mouse pulmonary arteries | Dihydroethidium | (51) |
| Mohazzab and Wolin | Minutes | Decrease | Microsome-enriched fractions of calf pulmonary arteries | Lucigenin | (53) |
| Paddenberget al. | Minutes | Increase | Isolated mouse pulmonary arteries and cultured rabbit PASMCs | H2DCF | (61) |
| Rathore et al. | Minutes | Increase | Isolated mouse PASMCs | Cytochrome c reduction assay, H2DCF | (69; 70) |
| Wang et al. | Minutes | Increase | Isolated mouse PASMCs | H2DCF, lucigenin, RedoxSensor Red CC-1 | (97) |
| Wang et al. | Hours | Increase | Isolated rat pulmonary arteries | Dihydroethidium | (98) |
| Waypa et al. | Minutes | Increase | Cultured rat PASMCs | Fluorescence resonance energy transfer probe, H2DCF | (102; 103) |
| Wu et al. | Minutes Hours | DecreaseIncrease | Cultured human PASMCs | Dihydroethidium | (114) |
These previous controversial findings with conventional ROS-detection methods have been attributed to the use of freshly isolated and cultured cells (56). Cultured cells undergo significant changes in expression levels and functional roles of hypoxia-responsible molecules in PASMCs (57, 122). Conversely, it should be noted that both the hypoxic decrease and increase in [ROS]i were observed in cultured PASMCs, as described earlier. Similar findings were made in freshly isolated PASMCs as well. Apparently, the increased generation of intracellular ROS by acute hypoxia in cultured PASMCs is not due to the cell culture per se; rather, they still retain indispensable parts of the hypoxia-sensing machinery. A concern also is expressed about the experimental findings in isolated lungs, because lungs are composed of a variety of cell types, which may produce distinct responses to hypoxic stimulation (78). However, this cannot well explain the observed hypoxic decrease and increase in [ROS]i in isolated lungs. Despite “everyone can be right” (100) or “ROS up, no way” (106), a recent report of using a novel, ratiometric, redox-sensitive fluorescence resonance energy-transfer probe demonstrates that acute hypoxia augments ROS signaling in isolated rat PASMCs (103). Consistent with this report, by using the newly developed, specific H2O2 biosensor HyPer (9), our more recent study reveals that acute hypoxia results in an increase in H2O2 generation in isolated mouse PASMCs (36). Nevertheless, a general agreement exists that chronic hypoxia increases [ROS]i in lungs, pulmonary arteries, and PASMCs (26, 27, 42, 51, 114). [ROS]i is most likely to be increased, playing an essential role in hypoxic responses in PASMCs.
Mitochondrial Electron-chain Transport Serves as a Primary Hypoxic Sensor That Initiates ROS Generation, Leading to an Increase in [ROS]i
In vascular SMCs, one of the major resources for intracellular ROS generation is the mitochondrial ETC, wherein ROS can be generated at complex I, II, and III, with complex III appearing to be the main site. Archer and his colleagues (4, 50) reported that the complex I inhibitor rotenone and complex III postubisemiquinone site–inhibitor antimycin-A mimic and subsequently block the acute hypoxic decrease in [ROS]i in isolated rat lungs and PASMCs (4, 50). Conversely, Waypa et al. (102–104) showed that rotenone and the complex III preubisemiquinone-site inhibitor myxothiazol block, but do not mimic, the acute hypoxic responses in cultured rat PASMCs. These investigators also found that antimycin-A neither mimics nor inhibits the hypoxic effect. Similar observations were obtained in isolated rat pulmonary arteries (37) and rabbit lungs (109). In support, our recent studies reveal that multiple, structurally distinctive complex I inhibitor rotenone and methylphenylpyridinium iodide, complex II inhibitor nitropropionic acid and tenoyltrifluoroacetone, as well as the complex III preubisemiquinone-site inhibitor myxothiazol all do not mimic, but significantly block, the acute hypoxic increase in [ROS]i in freshly isolated mouse PASMCs (69, 70, 97). Moreover, antimycin-A and the complex IV inhibitor sodium azide neither mimic nor block the acute hypoxic response. The preventive effect of the complex I, II, and III preubisemiquinone-site inhibitors, but not the complex III postubisemiquinone-site and complex IV inhibitors, on the acute hypoxic increase in [ROS]i also were observed in vascular cells of isolated mouse lung slices (61). Collectively, the mitochondrial ETC molecules before the complex III ubisemiquinone site may act as a functional unit that serves to increase generation of ROS in PASMCs.
To complement pharmacologic studies, we and other investigators have begun to look at the effect of genetic inhibition of mitochondrial ROS generation on the hypoxic response. In mitochondria, O2− is rapidly converted to H2O2 by manganese superoxide dismutase; H2O2 is then degraded by glutathione peroxidase-1 (Gpx1) in mitochondria and the cytosol, as well as by catalase in the cytosol. Perceptibly, overexpression and deletion of these endogenous antioxidant molecules may specifically modify intracellular ROS levels and associated hypoxic responses in PASMCS. In agreement with this view, our recent study reveals that Gpx1 gene overexpression to augment ROS removal attenuates the acute hypoxic increase in [ROS]i in freshly isolated mouse PASMCs, whereas Gpx1 gene deletion to prevent ROS removal has the opposite effect (97). Similarly, adenoviral overexpression of mitochondrial catalase and Gpx1 attenuate the acute hypoxia-induced changes in the ROS signaling in cultured rat PASMCs (103). We also found that the hypoxic response is inhibited in PASMCs from mice with catalase gene overexpression (97).
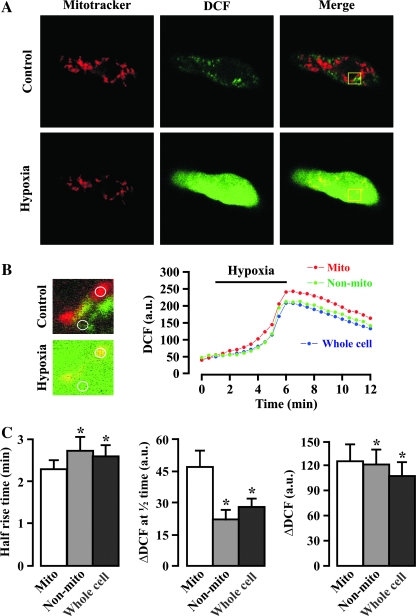
Further to provide evidence for the initial role of mitochondria in the hypoxic increase in [ROS]i in PASMCs, we examined and compared the acute hypoxic increase in ROS generation in mitochondrial and nonmitochondrial areas of freshly isolated mouse PASMCs by using the specific mitochondrial marker MitoTracker and ROS-sensitive fluorescent dye H2DCF. The results are shown in Fig. 1, indicating that the acute hypoxic increase in ROS generation occurs significantly earlier in mitochondrial areas than in nonmitochondrial areas. Additionally, the hypoxic increase in ROS generation is greater in the former areas than in the latter (97). We also recently showed that acute hypoxia results in a large increase in ROS generation in isolated mitochondria from mouse PASMCs (36). These findings further suggest that the mitochondrial ETC is an important primary hypoxic sensor that initiates ROS generation, leading to an increase in mitochondrial ROS generation ([ROS]m) and then [ROS]i in PASMCs.
FIG. 1.
Hypoxia-induced increase in ROS generation in mitochondria precedes that in nonmitochondrial areas in freshly isolated mouse pulmonary artery smooth muscle cells. (A) Original images show MitoTracker Deep Red 633 staining (shown as red) and DCF fluorescence (green) in a myocyte before and after hypoxia for 5 min. The superimposition of both MitoTracker staining and DCF fluorescence images produced yellow, indicating the hypoxic increase in ROS generation in mitochondria. (B) Extracted images were taken from the area indicated by the box in the cell shown in (A). The mitochondrial and nonmitochondrial area taken to measure the hypoxic increase in DCF fluorescence in the extracted images is indicated by a circle. Traces show the time course for the hypoxic response in mitochondrial (Mito), nonmitochondrial (Non-mito), and whole-cell areas. (C) Bar graph summarizes the mean half-rise time, amplitude of hypoxic increase in DCF fluorescence at the half-rise time (ΔDCF at half time), and maximal hypoxic increase in DCF fluorescence (ΔDCF) in mitochondrial, nonmitochondrial, and whole-cell areas. Data were obtained from 10 cells from five independent experiments. *p < 0.05 compared with mitochondrial areas. The figure is cited with permission from Wang et al. (97). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
NADPH Oxidase Is Involved in the Hypoxic Increase in [ROS]i
NADPH oxidase (Nox) is believed to be another important source for the generation of intracellular ROS in vascular SMCs. The active form of Nox is normally composed of various subunits, dependent on the cell type. In phagocytic cells, Nox is well characterized to include the membrane-bound subunits p22phox and gp91phox (Nox2) subunits, as well as the cytosolic subunits p47phox and p67phox; the association of these cytosolic and membrane-bound subunits is required for the assembly of the active Nox. Previous studies with RT-PCR showed mRNA expression of gp91phox, p22phox, p47phox, p67phox, as well as the gp91phox analogues Nox1 and Nox4 in mouse lung tissues (51) and Nox4 in rabbit lungs (110). Immunofluorescence staining shows the presence of Nox4 protein in isolated human pulmonary arteries and cultured human PASMCs (85), as well as in human lung tissues (51). The existence of Nox 4 in human lungs has been shown by Western blotting (51). With Western blot analysis, we recently showed that the well-characterized, major phagocytic Nox membrane-bound subunit p22phox, as well as the cytosolic subunits p47phox and p67phox, are expressed in endothelium-denuded mouse pulmonary arteries. Although the phagocytic Nox membrane-bound subunit gp91phox protein expression is not detected in pulmonary and mesenteric arteries, its analogues, Nox1 and Nox4 proteins, are observed (70).
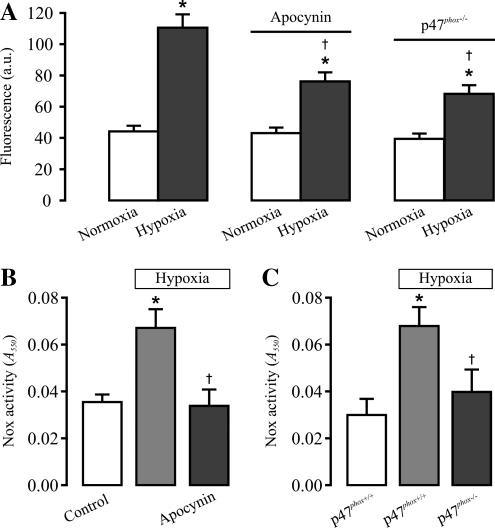
Our study also demonstrated that acute hypoxia for minutes causes an increase in Nox activity and translocation of p47phox, a major component of Nox, from the cytosol to the plasma membrane in endothelium-denuded mouse pulmonary arteries (70). These results suggest that Nox may contribute to the hypoxic ROS generation in PASMCs. In favor of this view, treatment with a Nox inhibitor apocynin blocks the hypoxic increase in [ROS]i in freshly isolated mouse PASMCs and Nox activity in mouse pulmonary arteries. The hypoxic increase in [ROS]i and Nox activity are significantly prevented as well in p47phox-/- mouse PASMCs (Fig. 2) (70). In support of this, the Nox inhibitor diphenyleneiodonium also inhibits the hypoxic increase in [ROS]i in cultured calf PASMCs (47). Nox is another important source for the hypoxic generation of intracellular ROS in PASMCs. However, it should be noted that hypoxia may inhibit the Nox-dependent generation of intracellular ROS in a microsome-enriched fraction of calf pulmonary arteries (52).
FIG. 2.
Pharmacologic and genetic inhibition of NADPH oxidase significantly attenuates the hypoxic increase in [ROS]i in freshly isolated mouse pulmonary artery smooth muscle cells. (A) Effects of the Nox inhibitor apocynin and p47phox gene deletion on hypoxic increase in [ROS]i (DCF fluorescence). DCF fluorescence was recorded before (normoxia) and after hypoxia for 5 min in control (p47phox+/+) cells, in p47phox+/+ cells pretreated with apocynin (1 μM) for 10 min, and in p47phox−/− cells. Data are presented as mean ± SEM from 21 to 23 cells in four independent experiments. *p < 0.05 compared with normoxia (before hypoxia); †p < 0.05 compared with hypoxia alone. (B) Effects of apocynin on the hypoxic increase in Nox activity in mouse pulmonary arteries. The activity of Nox was determined in arteries treated with normoxia, hypoxia for 5 min, and apocynin (1 μM) for 10 min plus hypoxia for 5 min. Data are presented as mean ± SEM from three independent experiments. *p < 0.05 compared with control (normoxia); †p < 0.05 compared with hypoxia. (C) Effects of p47phox gene deletion on the hypoxic increase in Nox activity. Data are presented as mean ± SEM from three independent experiments. *p < 0.05 compared with control (normoxia, p47phox+/+); †p < 0.05 compared with hypoxia (p47phox+/+). The figure is cited with permission from Rathore et al. (70).
Role of NADPH Oxidase in the Hypoxic Increase in [ROS]i Is Mediated by the Mitochondrial ROS–Protein Kinase C-ɛ Signaling Axis
Protein kinase C (PKC) can activate Nox to increase [ROS]i, participating in a variety of cellular responses in vascular SMCs (81, 101). The PKC family consists of 12 isoforms, which can be categorized into three groups based on their structure and activation in vitro: the conventional PKCs (α, β1, β2, and γ) that are sensitive to Ca2+ and diacylglycerol (DAG); novel PKCs (δ, ɛ, η, θ, μ, and υ) that are sensitive only to DAG; and atypical PKCs (ζ and ι) that are sensitive to neither Ca2+ nor DAG. Damron et al. (17) reported that PKC isoforms (PKCα, PKCδ, PKCɛ, PKCζ, PKCι, and PKCυ) are expressed in cultured canine PASMCs. Our recent data reveal that PKCɛ protein is expressed in endothelium-denuded mouse pulmonary arteries; acute hypoxia for minutes significantly augments the total activity of PKC and PKCɛ (69). We more recently found that pharmacologic and genetic inhibition of PKCɛ blocks the hypoxia-induced increase in [ROS]i in freshly isolated mouse PASMCs (70). These findings suggest that the Nox-dependent intracellular ROS generation may be mediated by PKCɛ.
In support of the role of PKCɛ in Nox-dependent ROS generation, we showed that the conventional/novel PKC inhibitor chelerythrine and specific PKCɛ peptide inhibitor block the hypoxic increase in Nox activity in mouse pulmonary arteries, whereas the conventional PKC blocker Gö6796 has no effect. Our recent data further reveal that the hypoxic activation of Nox is prevented in PKCɛ−/− mouse pulmonary arteries, and PKCɛ activation with PMA mimics the hypoxic response, leading to an increase of Nox activity in pulmonary arteries. This is the first report demonstrating the PKCɛ-dependent Nox activation as a mediator of hypoxic-induced increase in [ROS]i in PASMCs.
As the mitochondrial ETC may function as a primary oxygen sensor in the initiation of hypoxic ROS generation, we explored whether the role of PKCɛ is secondary to the increased generation of mitochondrial ROS and unveiled that pharmacologic inhibition of mitochondrial ROS generation with rotenone and myxothiazol both significantly prevent acute hypoxia inducing an increase in PKCɛ activity in mouse pulmonary arteries (69). Overexpression of Gpx1 to enhance ROS removal in mitochondria and the cytosol significantly inhibits the acute hypoxic increase in Nox activity, whereas Gpx1 gene deletion has the opposite effect. Consistent with these results, exogenous application of H2O2 mimics the hypoxic response, bringing about an increase in PKCɛ activity. These data, together with the findings that specific inhibition of PKCɛ activation by pharmacologic agents and gene deletion abolishes the hypoxic activation of Nox and associated ROS generation, emphasize that the acute hypoxia-induced, Nox-dependent ROS generation is secondary to the mitochondrial ROS–PKCɛ signaling axis, which provides a unique positive-feedback mechanism contributing to the hypoxic increase in [ROS]i in PASMCs.
An Increase in [ROS]i through the Mitochondrial ROS–PKCɛ–Nox Signaling Axis Is Critical for the Hypoxic Increase in [Ca2+]i and Associated Contraction in PASMCs
In agreement with the concept that an increase in [ROS]i is essential for hypoxic responses in PASMCs, exogenous application of H2O2 for minutes, similar to acute hypoxia, induces an increase in [Ca2+]i in cultured rat PASMCs (40, 104) and isolated rat pulmonary arteries (66). Exogenous O2− and H2O2 cause pulmonary vasoconstriction in isolated rat pulmonary arteries as well (11, 30, 31, 35, 63, 66, 71, 77, 80, 113, 116). However, H2O2 also was found to dilate isolated calf pulmonary arteries (12).
Parallel to the effect on [ROS]i, earlier studies by Archer's group (4, 50) reported that application of rotenone or antimycin-A mimics and then blocks the acute hypoxic contraction in isolated rat pulmonary arteries and lungs. Waypa et al. (102, 104) found that rotenone and myxothiazol, but not antimycin A, block the acute hypoxia-induced vasoconstriction in isolated rat lungs, and contraction as well as increase in [Ca2+]i in cultured rat PASMCs; however, these inhibitors do not mimic the acute hypoxic responses (102, 104). Other research groups also discovered that rotenone or myxothiazol produces a similar inhibitory effect on the acute hypoxic contraction in isolated rat pulmonary arteries (37) and rabbit lungs (109). Our recent work indicates that various, structurally different mitochondrial complex I, II, and III preubisemiquinone-site inhibitors, including rotenone and methylphenylpyridinium iodide, nitroproprionic acid, tenoyltrifluoroacetone, and myxothiazol, all block, but do not reproduce the hypoxic increase in [Ca2+]i and associated contraction in freshly isolated mouse PASMCs (69, 70, 97).
Interestingly, we found that pharmacologic inhibition of the complex I and II with tenoyltrifluoroacetone and tenoyltrifluoroacetone, complex I and III with methylphenylpyridinium iodide and myxothiazol, and complex II and III with tenoyltrifluoroacetone and myxothiazol do not produce an additive inhibitory effect on the acute hypoxic increase in [Ca2+]i in mouse PASMCs (97). These findings further support the view that, in response to hypoxia, the mitochondrial complex molecules before the ubisemiquinone site in the complex III may operate as a functional unit to increase mitochondrial ROS generation, leading to an increase in [ROS]i and [Ca2+]i, as well as contraction in PASMCs.
Complementing these pharmacologic effects, a previous report showed that adenoviral overexpression of mitochondrial or cytosolic Gpx1 (or both) and catalase attenuate the acute hypoxic increase in [Ca2+]i in cultured rat PASMCs (103). We also found that Gpx1 gene overexpression to promote ROS removal inhibits the acute hypoxic increase in [Ca2+]i and contraction in freshly isolated mouse PASMCs, whereas Gpx1 gene deletion to inhibit ROS removal has the opposite effect. Catalase gene overexpression to enhance intracellular ROS clearance produces an inhibitory effect as well (97).
Our comparable study revealed that inhibition of PKCɛ with the conventional/novel PKC inhibitor chelerythrine or specific peptide inhibitor not only significantly diminishes the acute hypoxic increase in [ROS]i, but also attenuates the hypoxic increase in [Ca2+]i and contraction in mouse PASMCs; the hypoxic ROS, Ca2+, and contractile responses are all blocked in PKCɛ−/− mouse PASMCs as well (69). In support, numerous previous reports also showed that the PKC inhibitors H7, bisindolylmaleimide, calphostin C, and chelerythrine prevent, whereas the PKC activators PMA, thymelation, and farnesylthiotriazole mimic and subsequently block the acute hypoxic vasoconstriction in isolated canine and rabbit lungs, as well as isolated rat pulmonary arteries (8, 28, 60, 89, 111). Furthermore, the acute hypoxic vasoconstriction is inhibited in isolated lungs from PKCɛ−/− mice (41). Consistent with the role of PKCɛ as a signaling molecule downstream of mitochondrial ROS, H2O2-induced pulmonary vasoconstriction in isolated rat pulmonary arteries has been found to be blocked by PKC inhibitors (29).
Similar to the inhibition of mitochondrial ETC and PKCɛ activity, pharmacologic inhibition of Nox by DPI has been found to block comparably the acute hypoxic increase in [ROS]i in PASMCs and vasoconstriction in isolated pulmonary arteries (47). Moreover, a number of publications show that Nox inhibition by DPI, iodonium diphenyl, and aminoethylbenzenesulfonyl fluoride all reduce the acute hypoxia-induced increase in [Ca2+]i and contraction in cultured rat PASMCs (118), and vasoconstriction in isolated calf pulmonary arteries (52) and in rabbit and rat lungs (22, 88, 110). However, the specificity of iodonium compounds as Nox inhibitors has been disputed, because these agents can inhibit the mitochondrial ETC in heart cells and voltage-dependent Ca2+ currents in PASMCs (68, 107). Whereas gp91phox-/- mice show normal or reduced acute hypoxic responses (6, 42, 44), acute hypoxic vasoconstriction is inhibited in p47phox-/- mice (112). We recently demonstrated that apocynin, a more-specific Nox blocker, significantly reduces the acute hypoxic increase in [Ca2+]i in freshly isolated mouse PASMCS, and p47phox gene deletion produces a similar inhibitory effect (70). These results further support the view that the Nox-dependent increase in intracellular ROS generation contributes to the hypoxic increase in [Ca2+]i and contraction in PASMCs.
ROS-dependent, Hypoxic Increases in [Ca2+]i and Associated Contraction Are Mediated by Multiple Ion Channels in PASMCs
ROS-dependent, hypoxic increases in [Ca2+]i and associated contraction in PASMCs are likely to be mediated by multiple ion channels, particularly KV channels, SOC channels, and RyRs/Ca2+ release channels. Major recent advances in our understanding of the role of these ion channels in hypoxic Ca2+ and contractile responses are reviewed.
Involvement of voltage-dependent K+ channels
KV channels are important for control of the membrane potential and intracellular Ca2+ homeostasis, thereby playing a significant role in the regulation of vascular cell contraction. Extensive publications demonstrate that both acute and chronic hypoxia significantly inhibit KV channels in PAMSCs, which may cause membrane depolarization, CaV channel opening, and extracellular Ca2+ influx, mediating the hypoxic increase in [Ca2+]i and associated contraction (3, 48, 84). Rather surprisingly, no patch-clamp studies directly examine the effect of hypoxia on CaV channels in the cultured or freshly isolated rat, human, or mouse PASMCs. It also was noted that the hypoxic increase in [Ca2+]i and associated contraction in PASMCs are preserved in the presence of KV channel blockers, CaV channel blockers, and high extracellular K+, as well as in the absence of extracellular Ca2+ (under conditions in which Ca2+ influx through CaV channels is eliminated) (18, 19, 23, 73, 79, 82).
KV channels normally consist of α and β subunits. The α subunits form an actual ion-conducting pore, whereas the β subunits do not conduct ions on their own, but rather modulate the channel activity. Based on sequence homology of the hydrophobic transmembrane domains, the KV channel α subunits can be divided into 12 classes, designated KV1 to 12. KVα1.1, 1.2, 1.5, 1.6, 2.1, 4.3, and 9.3 channels have been shown to be hypoxia sensitive in functional activity or expression level or both, potentially participating in hypoxic responses in PASMCs (5, 7, 16, 24, 62, 64, 67, 93, 96). It is worth pointing out that previous studies that tried to determine the molecular identity of hypoxia-sensitive KV channel members in PASMCs yielded conflicting results. Archer et al. (7) suggested that acute hypoxic inhibition of KV currents in rat PASMCs is primarily caused by KV2.1 block, but not KV1.5, although they later reported that KV1.5 plays a key role in acute hypoxic inhibition of KV currents (5, 67). A study using anti-KV2.1 antibody indicates that KV2.1 channels may be a major hypoxic target in rat PASMCs (24). However, other investigators have not been able to detect KV1.5 mRNA and KV2.1 protein in rabbit or rat PASMCs (16, 62).
It has been reported that rotenone and antimycin-A mimic and subsequently inhibit the acute hypoxia-induced [ROS]i reduction, KV-current inhibition, and contraction in isolated rat PASMCs (4, 50). Intriguingly, a recent study also showed that the multiple mitochondrial complex inhibitors attenuate KV currents and shift KV current activation to more-negative membrane voltages in rat PASMCs (20). Complementing the effect of mitochondrial inhibitors, a membrane permeable to hydrogen peroxide, t-butyl hydroperoxide, was found to inhibit KV currents in rat PASMCs (13). These results suggest that KV channels may be involved in ROS-dependent, hypoxic increase in [Ca2+]i and contraction in PASMCs.
However, it was reported that neither removal of extracellular Ca2+ nor treatment with nifedipine to block CaV channels inhibits H2O2-induced increase in [Ca2+]i in cultured rat PASMCs (40). Similarly, the use of the CaV channel blocker verapamil or removal of extracellular Ca2+ does not affect H2O2-evoked increase in [Ca2+]i and contraction in isolated rat pulmonary arteries (66). A lack of the role of extracellular Ca2+ removal in H2O2-induced contraction in pulmonary arteries also was observed by other investigators (63, 80). Further studies are needed to resolve the reported inconsistent findings and to demonstrate further the role of KV channel inhibition in ROS-dependent, hypoxic Ca2+ and contractile responses in PASMCs (78, 86, 90, 100).
Evidence also indicates that hypoxia may inhibit KV channels by activating PKCɛ, contributing to the hypoxic increase in [Ca2+]i and to contraction in PASMCs. A previous study showed that application of 4-aminopyridine to block KV channels significantly augments hypoxic vasoconstriction in isolated lungs from PKCɛ−/− mice, and Kv3.1b channel protein expression is increased in PKCɛ−/− mouse lungs (41). These results indicate that PKCɛ may downregulate the expression and activity of KV channels (such as Kv3.1b), participating in hypoxic responses in PASMCs. In support of this view, a previous report showed that PKC activation with PMA inhibits KV currents in rat PASMCs, and this inhibition can be blocked by the selective PKC inhibitor bis-indolylmaleimide (119). Inhibition of KV currents by endothelin-1 in cultured human PASMCs is also reversed by bis-indolylmaleimide and the general PKC inhibitor staurosporine (83). Similarly, Cogolludo et al. (14) reported that thromboxane A2–induced inhibition of KV currents in isolated rat PASMCs are attenuated by the general PKC inhibitors staurosporine, calphostin C, and Gö6983; however, the effect of thromboxane A2 is not blocked by bis-indolylmaleimide or the conventional PKC inhibitor Gö6976 and can be prevented by the selective PKCζ pseudosubstrate inhibitor (14). These investigators further showed that Gö6976 blocks serotonin-evoked inhibition of native KV currents in rat PASMCs and human KV1.5 currents stably expressed in LTK cells (15), and PKCζ gene deletion prevents thromboxane A2–induced inhibition of KV currents in isolated mouse PASMCs (54). These data further support the concept that PKCɛ is involved in the hypoxic inhibition of KV channels and also suggest that PKCɛ and PKCζ may differentially mediate agonist-induced responses in PASMCs.
Role of store-operated Ca2+ channels
ROS-dependent, hypoxic increase in [Ca2+]i may result from extracellular Ca2+ influx due to activation of SOC channels in PASMCs. Pharmacologic studies showed that pretreatment with La3+ to inhibit SOC channels or cyclopiazonic acid to deplete SR Ca2+ significantly inhibits hypoxic vasoconstriction in isolated rat pulmonary arteries (73) and rat lungs (105). Acute hypoxia also significantly increases extracellular Ca2+ influx via SOC channels in pig, rabbit, and rat PASMCs (32, 39, 45, 58, 59, 94, 95).
The major molecular candidates for SOC channels are likely to be canonic transient receptor potential (TRPC) channels. These channels include seven members named TRPC1–7, each encoded by a different gene. All seven TRPC channels have been found to be expressed in mRNA or protein levels or both in pulmonary arteries, among which, TRPC1 and TRPC6 channels are likely to be involved in the acute and chronic hypoxic increase in [Ca2+]i and contraction in PAMSCs (33, 39, 95, 108).
It is interesting to note that H2O2-induced increase in [Ca2+]i in PASMCs does not appear to be related to TRPC channels because the general channel blockers La3+ and SKF-96365 fail to produce an inhibitory effect in cultured rat PASMCs. In addition, H2O2 does not affect Mn2+-induced quenching of fura-2 fluorescence, a typical indicator of the opening of TRPC-encoded SOC channels (40). Similarly, H2O2-evoked vasoconstriction in isolated pulmonary arteries is not affected by SKF-96365 (66). Moreover, H2O2-induced increases in [Ca2+]i in cultured rat PASMCs and isolated rat pulmonary arteries are not inhibited by removal of extracellular Ca2+ (40, 66).
Despite the lack of direct experimental evidence for the effect of PKC on TRPC channels in PASMCs, previous studies reported that SOC channels in systemic vascular (e.g., coronary artery, mesenteric artery, and portal vein) SMCs are activated by the PKC activators phorbol ester phorbol 12,13-dibutyrate and 1-oleoyl-2-acetyl-sn-glycerol, as well as a PKC catalytic subunit, whereas they are inhibited by the PKC inhibitor chelerythrine (2, 74, 75). TRPC1 channels have been shown to be phosphorylated by PKCα, regulating store-operated Ca2+ entry in human endothelial cells (1). Moreover, PKCα is known to participate in the activation of SOCs in mesangial cells (46). Thus, it is interesting to determine whether a similar mechanism exits in PASMCs.
Contribution of ryanodine receptors/Ca2+ release channels
The Ca2+ release from the SR via RyRs is a major component of Ca2+ signaling in vascular SMCs. The role of RyRs in the hypoxic Ca2+ release and associated contraction in PASMCs has received extensive attention. Numerous publications have shown that the depletion of SR Ca2+ with caffeine (through activation of RyRs) reduces or abolishes the acute hypoxia-induced increase in [Ca2+]i in cultured rat and freshly isolated canine and rat PASMCs (65, 76, 99) and vasoconstriction in isolated canine and rabbit pulmonary arteries (19, 25). Similarly, ryanodine, an agent that binds with high affinity to RyRs, largely inhibits the acute hypoxic Ca2+ response in cultured cat PASMCs (91) and vasoconstriction in isolated rat lungs and canine and rabbit pulmonary arteries (19, 25, 55). Other RyR antagonists, such as ruthenium red, tetracaine, and dantrolene, also significantly block the acute hypoxic increase in [Ca2+]i in freshly isolated rat PASMCs and vasoconstriction in pulmonary arteries (122). These data suggest that RyRs are key targets for acute hypoxia, by which hypoxia may induce Ca2+ release and associated contraction in PASMCs. The importance of RyR-mediated Ca2+ release in hypoxic responses in PASMCs is reinforced by the findings that hypoxic inhibition of KV channels is likely to be secondary to Ca2+ release from the SR (21, 65, 92). Moreover, hypoxic Ca2+ release through RyRs may result in the opening of SOC channels, which causes not only extracellular Ca2+ influx through the opening channels, but also may result in membrane depolarization, activation of CaV channels, and further Ca2+ influx, providing a positive-feedback mechanism that enhances hypoxic increase in [Ca2+]i and contraction in PASMCs (58, 59).
Consistent with the potentially important role of RyRs in ROS-dependent, hypoxic Ca2+ and contractile responses, a previous study showed that treatment with ryanodine (50 μM) to block RyRs significantly inhibits H2O2-evoked, initial rapid increase in [Ca2+]i in cultured rat PASMCS (40). In support, ryanodine and dantrolene abolish or greatly suppress an H2O2-induced increase in [Ca2+]i and vasoconstriction in isolated rat pulmonary arteries (66).
Three distinct gene-encoded subtypes of RyRs (RyR1, RyR2, and RyR3) are expressed in mammalian cells. By using real-time quantitative RT-PCR, we showed that RyR1, RyR2, and RyR3 are all expressed in freshly isolated rat and mouse PASMCs (121, 122). In support of our findings, other investigators reported that all three RyR subtype mRNAs are present in rat intralobar pulmonary arteries (117). Expression of RyR1, RyR2, and RyR3 proteins also was observed in freshly isolated rat PASMCs by using immunofluorescence staining (122) and in isolated pulmonary arteries by using Western blot analysis (117).
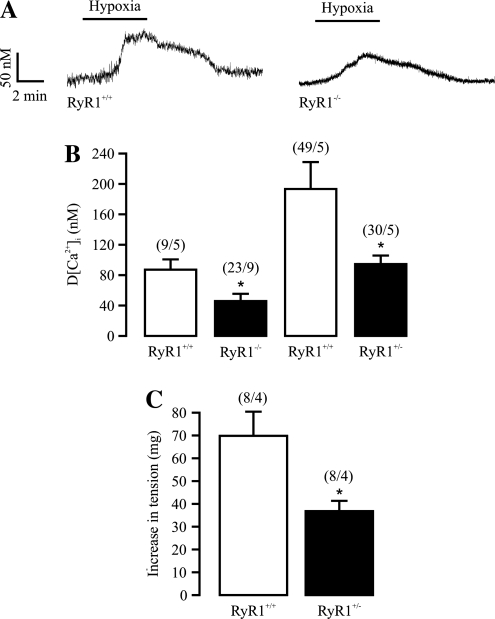
We have started to explore the potential role of individual subtypes of RyRs in hypoxic responses by using genetically manipulated mice. As shown in Fig. 3, our studies revealed that acute hypoxia induces a much smaller increase in [Ca2+]i in freshly isolated PASMCs from embryonic RyR1−/− mice at day 17, compared with wild-type mice. A decreased Ca2+ response also was observed in adult RyR1+/− mouse PASMCs. Moreover, acute hypoxic vasoconstriction is inhibited in pulmonary arteries from adult RyR1+/− mice (38). The acute hypoxic increase in [Ca2+]i in PASMCs and vasoconstriction in pulmonary arteries are significantly diminished in adult RyR3−/− mice as well (122). As FK506 binding protein with a molecular mass of 12.6 kDa (FKBP12.6) is known to bind to and regulate RyR2 (115), we used FKBP12.6−/− mice as a unique tool in determining the potential role of RyR2 in hypoxic responses; we found that that the acute hypoxia-induced increase in [Ca2+]i in PASMCs and vasoconstriction in isolated pulmonary arteries are both enhanced in FKBP12.6−/− mice (120). Collectively, all three RyR subtypes are involved in the hypoxic Ca2+ release and contraction in PASMCs.
FIG. 3.
RyR1 mediates the hypoxic increase in [Ca2+]i and contraction in pulmonary artery smooth muscle cells. (A) Original recordings show that hypoxic exposure for 5 min induced an increase in [Ca2+]i in an embryonic RyR1+/+ and RyR1−/− mouse PASMC. (B) Bar graphs summarize the hypoxic increase in [Ca2+]i in embryonic RyR1+/+, embryonic RyR1−/−, adult RyR+/−, and adult RyR1+/+ PASMCs. *p < 0.05 compared with RyR1+/+ cells. Numbers in parentheses indicate the numbers of cells and mice tested. (C) Summary of hypoxic vasoconstriction in adult RyR1+/+ and RyR+/− mouse pulmonary arteries. *p < 0.05 compared with RyR1+/+ pulmonary arteries. The figure is cited with permission from Li et al. (38).
It has been reported that addition of catalytic PKC phosphorylates RyR2 in canine cardiac microsomes. The observed extent of PKC-dependent phosphorylation of RyR2 is comparable to the level of PKC-dependent increase in the activity of RyR2 determined by [3H]ryanodine-binding assay (87). By analogy, PKC is likely to regulate RyRs directly to mediate hypoxic Ca2+ release in PASMCs; however, this view must be demonstrated by further studies.
Conclusions
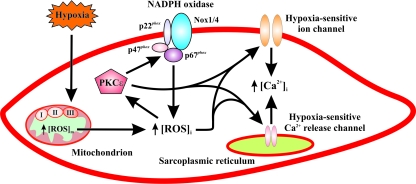
Based on our recent studies and previous publications, we present a schematic diagram, as illustrated in Fig. 4, to conclude that the mitochondrial ETC may function as a hypoxic sensor in PASMCs, by which hypoxia can significantly enhance [ROS]m, leading to an initial, large increase in [ROS]i. The increased [ROS]i subsequently activates the intermediate signaling molecules PKCɛ and Nox, providing a positive-feedback mechanism to increase further the hypoxic generation of intracellular ROS. As a consequence, ROS and PKCɛ synergistically result in the inhibition of plasmalemmal KV channels (hypoxic effectors), opening of CaV channels, and extracellular Ca2+ influx, contributing to the hypoxic increase in [Ca2+]i and associated contraction. In addition, ROS and PKCɛ may activate plasmalemmal SOC channels. As important hypoxic effectors, the opened SOC channels not only allow extracellular Ca2+ to enter the cell, but also cause membrane depolarization, leading to the further opening of CaV channels and extracellular Ca2+ influx. Moreover, both ROS and PKCɛ can in concert activate RyR1, RyR2, RyR3 or all three, inducing Ca2+ release from the SR, as an important process for the hypoxic Ca2+ and contractile responses in PASMCs. Interestingly, available evidence suggests that the hypoxic inhibition of KV channels and activation of SOC channels are likely to be secondary to SR Ca2+ release. Finally, it is worth noting that, despite recent progress in the field, we are still far from fully understanding the cellular and molecular mechanisms responsible for hypoxic increases in [Ca2+]i and associated contraction in PASMCs. For instance, it is unclear how the mitochondrial ETC senses hypoxia in PASMCs. To what extent each of the individual ion channels contributes to the hypoxic Ca2+ response remains to be determined. Thus, further studies are necessary to answer these fundamental questions and also to resolve the reported inconsistent findings.
FIG. 4.
A schematic diagram illustrating the signaling mechanisms for ROS-dependent, hypoxic increase in [Ca2+]i and associated contraction in pulmonary artery smooth muscle cells. This includes the potential important primary hypoxic sensor mitochondrial ETC, intermediate signaling molecules ROS, PKCɛ, and Nox, as well as effectors hypoxia-sensitive plasmalemmal ion channels (e.g., KV channels and SOC channels) and sarcolemmal Ca2+ release channels (RyRs). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Abbreviations Used
- [Ca2+]i
intracellular Ca2+ concentration
- CaV
voltage-dependent Ca2+
- ETC
electron-transport chain
- FKBP12.6
FK506-binding protein with a molecular mass of 12.6 kDa
- Gpx1
glutathione peroxidase-1
- H2DCF
dichlorodihydrofluorescein
- H2O2
hydrogen peroxide
- KV
voltage-dependent K+
- Nox
NADPH oxidase
- O2−
superoxide anion
- PASMC
pulmonary artery smooth muscle cell
- PKC
protein kinase C
- [ROS]i
intracellular reactive oxygen species concentration
- RyR
ryanodine receptor
- SOC
store-operated Ca2+
- SR
sarcoplasmic reticulum
- TRPC
canonic transient receptor potential
Acknowledgments
Our work presented in this article was supported by Scientist Development Grant, Established Investigator Award, and Grant-in-Aid from the American Heart Association, Research Grant from the American Lung Association, and R01 Research Grants from the National Institutes of Health.
Author Disclosure Statement
None of the authors has a financial interest in the subject of this article.
References
- 1.Ahmmed GU. Mehta D. Vogel S. Holinstat M. Paria BC. Tiruppathi C. Malik AB. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem. 2004;279:20941–20949. doi: 10.1074/jbc.M313975200. [DOI] [PubMed] [Google Scholar]
- 2.Albert AP. Large WA. Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J Physiol. 2002;544:113–125. doi: 10.1113/jphysiol.2002.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SL. Gomberg-Maitland M. Maitland ML. Rich S. Garcia JG. Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL. Huang J. Henry T. Peterson D. Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL. London B. Hampl V. Wu X. Nsair A. Puttagunta L. Hashimoto K. Waite RE. Michelakis ED. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J. 2001;15:1801–1803. doi: 10.1096/fj.00-0649fje. [DOI] [PubMed] [Google Scholar]
- 6.Archer SL. Reeve HL. Michelakis E. Puttagunta L. Waite R. Nelson DP. Dinauer MC. Weir EK. O2 sensing is preserved in mice lacking the gp91phox subunit of NADPH oxidase. Proc Natl Acad Sci U S A. 1999;96:7944–7949. doi: 10.1073/pnas.96.14.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archer SL. Souil E. Dinh-Xuan AT. Schremmer B. Mercier JC. El Yaagoubi A. Nguyen-Huu L. Reeve HL. Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest. 1998;101:2319–2330. doi: 10.1172/JCI333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barman SA. Potassium channels modulate canine pulmonary vasoreactivity to protein kinase C activation. Am J Physiol. 1999;277:L558–L565. doi: 10.1152/ajplung.1999.277.3.L558. [DOI] [PubMed] [Google Scholar]
- 9.Belousov VV. Fradkov AF. Lukyanov KA. Staroverov DB. Shakhbazov KS. Terskikh AV. Lukyanov S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 10.Brennan LA. Steinhorn RH. Wedgwood S. Mata-Greenwood E. Roark EA. Russell JA. Black SM. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 11.Burghuber OC. Strife R. Zirolli J. Mathias MM. Murphy RC. Reeves JT. Voelkel NF. Hydrogen peroxide induced pulmonary vasoconstriction in isolated rat lungs is attenuated by U60,257, a leucotriene synthesis blocker. Wien Klin Wochenschr. 1986;98:117–119. [PubMed] [Google Scholar]
- 12.Burke TM. Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol. 1987;252:H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- 13.Cogolludo A. Frazziano G. Cobeno L. Moreno L. Lodi F. Villamor E. Tamargo J. Perez-Vizcaino F. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann N Y Acad Sci. 2006;1091:41–51. doi: 10.1196/annals.1378.053. [DOI] [PubMed] [Google Scholar]
- 14.Cogolludo A. Moreno L. Bosca L. Tamargo J. Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Cζ. Circ Res. 2003;93:656–663. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- 15.Cogolludo A. Moreno L. Lodi F. Frazziano G. Cobeno L. Tamargo J. Perez-Vizcaino F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res. 2006;98:931–938. doi: 10.1161/01.RES.0000216858.04599.e1. [DOI] [PubMed] [Google Scholar]
- 16.Coppock EA. Tamkun MM. Differential expression of KV channel alpha- and beta-subunits in the bovine pulmonary arterial circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1350–L1360. doi: 10.1152/ajplung.2001.281.6.L1350. [DOI] [PubMed] [Google Scholar]
- 17.Damron DS. Nadim HS. Hong SJ. Darvish A. Murray PA. Intracellular translocation of PKC isoforms in canine pulmonary artery smooth muscle cells by ANG II. Am J Physiol. 1998;274:L278–L288. doi: 10.1152/ajplung.1998.274.2.L278. [DOI] [PubMed] [Google Scholar]
- 18.Demiryurek AT. Wadsworth RM. Kane KA. Peacock AJ. The role of endothelium in hypoxic constriction of human pulmonary artery rings. Am Rev Respir Dis. 1993;147:283–290. doi: 10.1164/ajrccm/147.2.283. [DOI] [PubMed] [Google Scholar]
- 19.Dipp M. Nye PC. Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001;281:L318–L325. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- 20.Firth AL. Yuill KH. Smirnov SV. Mitochondria-dependent regulation of Kv currents in rat pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L61–L70. doi: 10.1152/ajplung.90243.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelband CH. Gelband H. Ca2+ release from intracellular stores is an initial step in hypoxic pulmonary vasoconstriction of rat pulmonary artery resistance vessels. Circulation. 1997;96:3647–3654. doi: 10.1161/01.cir.96.10.3647. [DOI] [PubMed] [Google Scholar]
- 22.Grimminger F. Weissmann N. Spriestersbach R. Becker E. Rosseau S. Seeger W. Effects of NADPH oxidase inhibitors on hypoxic vasoconstriction in buffer-perfused rabbit lungs. Am J Physiol. 1995;268:L747–L752. doi: 10.1152/ajplung.1995.268.5.L747. [DOI] [PubMed] [Google Scholar]
- 23.Hasunuma K. Rodman DM. McMurtry IF. Effects of K+ channel blockers on vascular tone in the perfused rat lung. Am Rev Respir Dis. 1991;144:884–887. doi: 10.1164/ajrccm/144.4.884. [DOI] [PubMed] [Google Scholar]
- 24.Hogg DS. Davies AR. McMurray G. Kozlowski RZ. KV2.1 channels mediate hypoxic inhibition of I(KV) in native pulmonary arterial smooth muscle cells of the rat. Cardiovasc Res. 2002;55:349–360. doi: 10.1016/s0008-6363(02)00411-x. [DOI] [PubMed] [Google Scholar]
- 25.Jabr RI. Toland H. Gelband CH. Wang XX. Hume JR. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol. 1997;122:21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jernigan NL. Resta TC. Walker BR. Contribution of oxygen radicals to altered NO-dependent pulmonary vasodilation in acute and chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L947–L955. doi: 10.1152/ajplung.00215.2003. [DOI] [PubMed] [Google Scholar]
- 27.Jernigan NL. Walker BR. Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin N. Packer CS. Rhoades RA. Pulmonary arterial hypoxic contraction: signal transduction. Am J Physiol. 1992;263:L73–L78. doi: 10.1152/ajplung.1992.263.1.L73. [DOI] [PubMed] [Google Scholar]
- 29.Jin N. Packer CS. Rhoades RA. Reactive oxygen-mediated contraction in pulmonary arterial smooth muscle: cellular mechanisms. Can J Physiol Pharmacol. 1991;69:383–388. doi: 10.1139/y91-058. [DOI] [PubMed] [Google Scholar]
- 30.Jin N. Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol. 1997;272:H2686–H2692. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- 31.Jones RD. Thompson JS. Morice AH. The effect of hydrogen peroxide on hypoxia, prostaglandin F2 alpha and potassium chloride induced contractions in isolated rat pulmonary arteries. Pulmon Pharmacol Ther. 1997;10:37–42. doi: 10.1006/pupt.1997.0071. [DOI] [PubMed] [Google Scholar]
- 32.Kang TM. Park MK. Uhm DY. Effects of hypoxia and mitochondrial inhibition on the capacitative calcium entry in rabbit pulmonary arterial smooth muscle cells. Life Sci. 2003;72:1467–1479. doi: 10.1016/s0024-3205(02)02441-4. [DOI] [PubMed] [Google Scholar]
- 33.Keseru B. Barbosa-Sicard E. Popp R. Fisslthaler B. Dietrich A. Gudermann T. Hammock BD. Falck JR. Weissmann N. Busse R. Fleming I. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J. 2008;22:4306–4315. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killilea DW. Hester R. Balczon R. Babal P. Gillespie MN. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L408–L412. doi: 10.1152/ajplung.2000.279.2.L408. [DOI] [PubMed] [Google Scholar]
- 35.Kjaeve J. Vaage J. Bjertnaes L. Toxic oxygen metabolites induce vasoconstriction and bronchoconstriction in isolated, plasma-perfused rat lungs. Acta Anaesthesiol Scand. 1991;35:65–70. doi: 10.1111/j.1399-6576.1991.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 36.Korde AS. Wang YX. Mitochondrial rieske protein, are you a real hypoxic sensor in pulmonary artery smooth muscle cells? FASEB J. 2008;22:11174. [Google Scholar]
- 37.Leach RM. Hill HM. Snetkov VA. Robertson TP. Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li XQ. Zheng YM. Rathore R. Ma J. Takeshima H. Wang YX. Genetic evidence for functional role of ryanodine receptor 1 in pulmonary artery smooth muscle cells. Pflugers Arch. 2009;457:771–783. doi: 10.1007/s00424-008-0556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin MJ. Leung GP. Zhang WM. Yang XR. Yip KP. Tse CM. Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res. 2004;95:496–505. doi: 10.1161/01.RES.0000138952.16382.ad. [DOI] [PubMed] [Google Scholar]
- 40.Lin MJ. Yang XR. Cao YN. Sham JS. Hydrogen peroxide-induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1598–L1608. doi: 10.1152/ajplung.00323.2006. [DOI] [PubMed] [Google Scholar]
- 41.Littler CM. Morris KG., Jr Fagan KA. McMurtry IF. Messing RO. Dempsey EC. Protein kinase C-ɛ-null mice have decreased hypoxic pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol. 2003;284:H1321–H1331. doi: 10.1152/ajpheart.00795.2002. [DOI] [PubMed] [Google Scholar]
- 42.Liu JQ. Erbynn EM. Folz RJ. Chronic hypoxia-enhanced murine pulmonary vasoconstriction: role of superoxide and gp91phox. Chest. 2005;128:594S–596S. doi: 10.1378/chest.128.6_suppl.594S. [DOI] [PubMed] [Google Scholar]
- 43.Liu JQ. Sham JS. Shimoda LA. Kuppusamy P. Sylvester JT. Hypoxic constriction and reactive oxygen species in porcine distal pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2003;285:L322–L333. doi: 10.1152/ajplung.00337.2002. [DOI] [PubMed] [Google Scholar]
- 44.Liu JQ. Zelko IN. Erbynn EM. Sham JS. Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 45.Lu W. Wang J. Shimoda LA. Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L104–L113. doi: 10.1152/ajplung.00058.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma R. Kudlacek PE. Sansom SC. Protein kinase Cα participates in activation of store-operated Ca2+ channels in human glomerular mesangial cells. Am J Physiol Cell Physiol. 2002;283:C1390–C1398. doi: 10.1152/ajpcell.00141.2002. [DOI] [PubMed] [Google Scholar]
- 47.Marshall C. Mamary AJ. Verhoeven AJ. Marshall BE. Pulmonary artery NADPH-oxidase is activated in hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 1996;15:633–644. doi: 10.1165/ajrcmb.15.5.8918370. [DOI] [PubMed] [Google Scholar]
- 48.Mauban JR. Remillard CV. Yuan JX. Hypoxic pulmonary vasoconstriction: role of ion channels. J Appl Physiol. 2005;98:415–420. doi: 10.1152/japplphysiol.00732.2004. [DOI] [PubMed] [Google Scholar]
- 49.Mehta JP. Campian JL. Guardiola J. Cabrera JA. Weir EK. Eaton JW. Generation of oxidants by hypoxic human pulmonary and coronary smooth-muscle cells. Chest. 2008;133:1410–1414. doi: 10.1378/chest.07-2984. [DOI] [PubMed] [Google Scholar]
- 50.Michelakis ED. Hampl V. Nsair A. Wu X. Harry G. Haromy A. Gurtu R. Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- 51.Mittal M. Roth M. Konig P. Hofmann S. Dony E. Goyal P. Selbitz AC. Schermuly RT. Ghofrani HA. Kwapiszewska G. Kummer W. Klepetko W. Hoda MA. Fink L. Hanze J. Seeger W. Grimminger F. Schmidt HH. Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 52.Mohazzab KM. Fayngersh RP. Kaminski PM. Wolin MS. Potential role of NADH oxidoreductase-derived reactive O2 species in calf pulmonary arterial PO2-elicited responses. Am J Physiol. 1995;269:L637–L644. doi: 10.1152/ajplung.1995.269.5.L637. [DOI] [PubMed] [Google Scholar]
- 53.Mohazzab KM. Wolin MS. Properties of a superoxide anion-generating microsomal NADH oxidoreductase, a potential pulmonary artery PO2 sensor. Am J Physiol. 1994;267:L823–L831. doi: 10.1152/ajplung.1994.267.6.L823. [DOI] [PubMed] [Google Scholar]
- 54.Moreno L. Frazziano G. Cogolludo A. Cobeno L. Tamargo J. Perez-Vizcaino F. Role of protein kinase Cζ and its adaptor protein p62 in voltage-gated potassium channel modulation in pulmonary arteries. Mol Pharmacol. 2007;72:1301–1309. doi: 10.1124/mol.107.037002. [DOI] [PubMed] [Google Scholar]
- 55.Morio Y. McMurtry IF. Ca2+ release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92:527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- 56.Moudgil R. Michelakis ED. Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- 57.Ng LC. Kyle BD. Lennox AR. Shen XM. Hatton WJ. Hume JR. Cell culture alters Ca2+ entry pathways activated by store-depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2008;294:C313–C323. doi: 10.1152/ajpcell.00258.2007. [DOI] [PubMed] [Google Scholar]
- 58.Ng LC. Wilson SM. Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563:409–419. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng LC. Wilson SM. McAllister CE. Hume JR. Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells. Br J Pharmacol. 2007;152:101–111. doi: 10.1038/sj.bjp.0707357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orton EC. Raffestin B. McMurtry IF. Protein kinase C influences rat pulmonary vascular reactivity. Am Rev Respir Dis. 1990;141:654–658. doi: 10.1164/ajrccm/141.3.654. [DOI] [PubMed] [Google Scholar]
- 61.Paddenberg R. Ishaq B. Goldenberg A. Faulhammer P. Rose F. Weissmann N. Braun-Dullaeus RC. Kummer W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol. 2003;284:L710–L719. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- 62.Patel AJ. Lazdunski M. Honore E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J. 1997;16:6615–6625. doi: 10.1093/emboj/16.22.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pelaez NJ. Braun TR. Paul RJ. Meiss RA. Packer CS. H(2)O(2) mediates Ca2+- and MLC20 phosphorylation-independent contraction in intact and permeabilized vascular muscle. Am J Physiol Heart Circ Physiol. 2000;279:H1185–H1193. doi: 10.1152/ajpheart.2000.279.3.H1185. [DOI] [PubMed] [Google Scholar]
- 64.Platoshyn O. Yu Y. Golovina VA. McDaniel SS. Krick S. Li L. Wang JY. Rubin LJ. Yuan JX. Chronic hypoxia decreases KV channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L801–L812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- 65.Post JM. Gelband CH. Hume JR. [Ca2+]i inhibition of K+ channels in canine pulmonary artery: novel mechanism for hypoxia-induced membrane depolarization. Circ Res. 1995;77:131–139. doi: 10.1161/01.res.77.1.131. [DOI] [PubMed] [Google Scholar]
- 66.Pourmahram GE. Snetkov VA. Shaifta Y. Drndarski S. Knock GA. Aaronson PI. Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med. 2008;45:1468–1476. doi: 10.1016/j.freeradbiomed.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 67.Pozeg ZI. Michelakis ED. McMurtry MS. Thebaud B. Wu XC. Dyck JR. Hashimoto K. Wang S. Moudgil R. Harry G. Sultanian R. Koshal A. Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 68.Ragan CI. Bloxham DP. Specific labelling of a constituent polypeptide of bovine heart mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone reductase by the inhibitor diphenyleneiodonium. Biochem J. 1977;163:605–615. doi: 10.1042/bj1630605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rathore R. Zheng YM. Li XQ. Wang QS. Liu QH. Ginnan R. Singer HA. Ho YS. Wang YX. Mitochondrial ROS-PKCɛ signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochem Biophys Res Commun. 2006;351:784–790. doi: 10.1016/j.bbrc.2006.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rathore R. Zheng YM. Niu CF. Liu QH. Korde A. Ho YS. Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCɛ signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008;45:1223–1231. doi: 10.1016/j.freeradbiomed.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhoades RA. Packer CS. Roepke DA. Jin N. Meiss RA. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol. 1990;68:1581–1589. doi: 10.1139/y90-241. [DOI] [PubMed] [Google Scholar]
- 72.Robertson TP. Aaronson PI. Ward JP. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol. 1995;268:H301–H307. doi: 10.1152/ajpheart.1995.268.1.H301. [DOI] [PubMed] [Google Scholar]
- 73.Robertson TP. Hague D. Aaronson PI. Ward JP. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saleh SN. Albert AP. Peppiatt CM. Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol. 2006;577:479–495. doi: 10.1113/jphysiol.2006.119305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saleh SN. Albert AP. Peppiatt-Wildman CM. Large WA. Diverse properties of store-operated TRPC channels activated by protein kinase C in vascular myocytes. J Physiol. 2008;586:2463–2476. doi: 10.1113/jphysiol.2008.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salvaterra CG. Goldman WF. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol. 1993;264:L323–L328. doi: 10.1152/ajplung.1993.264.3.L323. [DOI] [PubMed] [Google Scholar]
- 77.Seeger W. Suttorp N. Schmidt F. Neuhof H. The glutathione redox cycle as a defense system against hydrogen-peroxide-induced prostanoid formation and vasoconstriction in rabbit lungs. Am Rev Respir Dis. 1986;133:1029–1036. doi: 10.1164/arrd.1986.133.6.1029. [DOI] [PubMed] [Google Scholar]
- 78.Sham JS. Hypoxic pulmonary vasoconstriction: ups and downs of reactive oxygen species. Circ Res. 2002;91:649–651. doi: 10.1161/01.res.0000039065.10754.de. [DOI] [PubMed] [Google Scholar]
- 79.Sham JS. Crenshaw BR Jr. Deng LH. Shimoda LA. Sylvester JT. Effects of hypoxia in porcine pulmonary arterial myocytes: roles of KV channel and endothelin-1. Am J Physiol Lung Cell Mol Physiol. 2000;279:L262–L272. doi: 10.1152/ajplung.2000.279.2.L262. [DOI] [PubMed] [Google Scholar]
- 80.Sheehan DW. Giese EC. Gugino SF. Russell JA. Characterization and mechanisms of H2O2-induced contractions of pulmonary arteries. Am J Physiol. 1993;264:H1542–H1547. doi: 10.1152/ajpheart.1993.264.5.H1542. [DOI] [PubMed] [Google Scholar]
- 81.Shen GX. Selective protein kinase C inhibitors and their applications. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:301–307. doi: 10.2174/1568006033481375. [DOI] [PubMed] [Google Scholar]
- 82.Shimoda LA. Sham JS. Shimoda TH. Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol. 2000;279:L884–L894. doi: 10.1152/ajplung.2000.279.5.L884. [DOI] [PubMed] [Google Scholar]
- 83.Shimoda LA. Sylvester JT. Booth GM. Shimoda TH. Meeker S. Undem BJ. Sham JS. Inhibition of voltage-gated K+ currents by endothelin-1 in human pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1115–L1122. doi: 10.1152/ajplung.2001.281.5.L1115. [DOI] [PubMed] [Google Scholar]
- 84.Shimoda LA. Wang J. Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation. 2006;13:657–670. doi: 10.1080/10739680600930305. [DOI] [PubMed] [Google Scholar]
- 85.Sturrock A. Cahill B. Norman K. Huecksteadt TP. Hill K. Sanders K. Karwande SV. Stringham JC. Bull DA. Gleich M. Kennedy TP. Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 86.Sylvester JT. Hypoxic pulmonary vasoconstriction: a radical view. Circ Res. 2001;88:1228–1230. doi: 10.1161/hh1201.093167. [DOI] [PubMed] [Google Scholar]
- 87.Takasago T. Imagawa T. Furukawa K. Ogurusu T. Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem (Tokyo) 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- 88.Thomas HM., III Carson RC. Fried ED. Novitch RS. Inhibition of hypoxic pulmonary vasoconstriction by diphenyleneiodonium. Biochem Pharmacol. 1991;42:R9–R12. doi: 10.1016/0006-2952(91)90440-g. [DOI] [PubMed] [Google Scholar]
- 89.Tsai BM. Wang M. Pitcher JM. Meldrum KK. Meldrum DR. Hypoxic pulmonary vasoconstriction and pulmonary artery tissue cytokine expression are mediated by protein kinase C. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1215–L1219. doi: 10.1152/ajplung.00179.2004. [DOI] [PubMed] [Google Scholar]
- 90.Turner JL. Kozlowski RZ. Relationship between membrane potential, delayed rectifier K+ currents and hypoxia in rat pulmonary arterial myocytes. Exp Physiol. 1997;82:629–645. doi: 10.1113/expphysiol.1997.sp004052. [DOI] [PubMed] [Google Scholar]
- 91.Vadula MS. Kleinman JG. Madden JA. Effect of hypoxia and norepinephrine on cytoplasmic free Ca2+ in pulmonary and cerebral arterial myocytes. Am J Physiol. 1993;265:L591–L597. doi: 10.1152/ajplung.1993.265.6.L591. [DOI] [PubMed] [Google Scholar]
- 92.Vandier C. Delpech M. Bonnet P. Spontaneous transient outward currents and delayed rectifier K+ current: effects of hypoxia. Am J Physiol. 1998;275:L145–L154. doi: 10.1152/ajplung.1998.275.1.L145. [DOI] [PubMed] [Google Scholar]
- 93.Wang J. Juhaszova M. Rubin LJ. Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel alpha subunits in pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:2347–2353. doi: 10.1172/JCI119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J. Shimoda LA. Weigand L. Wang W. Sun D. Sylvester JT. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1059–L1069. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- 95.Wang J. Weigand L. Lu W. Sylvester JT. Semenza GL. Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res. 2006;98:1528–1537. doi: 10.1161/01.RES.0000227551.68124.98. [DOI] [PubMed] [Google Scholar]
- 96.Wang J. Weigand L. Wang W. Sylvester JT. Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2005;266:L1049–L1058. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- 97.Wang QS. Zheng YM. Dong L. Ho YS. Guo Z. Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X. Tong M. Chinta S. Raj JU. Gao Y. Hypoxia-induced reactive oxygen species downregulate ETB receptor-mediated contraction of rat pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2006;290:L570–L578. doi: 10.1152/ajplung.00262.2005. [DOI] [PubMed] [Google Scholar]
- 99.Wang YX. Zheng YM. Abdullaev II. Kotlikoff MI. Metabolic inhibition with cyanide induces intracellular calcium release in pulmonary artery myocytes and Xenopus oocytes. Am J Physiol Cell Physiol. 2003;284:C378–C88. doi: 10.1152/ajpcell.00260.2002. [DOI] [PubMed] [Google Scholar]
- 100.Ward JP. Aaronson PI. Mechanisms of hypoxic pulmonary vasoconstriction: can anyone be right? Respir Physiol. 1999;115:261–271. doi: 10.1016/s0034-5687(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 101.Ward JP. Knock GA. Snetkov VA. Aaronson PI. Protein kinases in vascular smooth muscle tone: role in the pulmonary vasculature and hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2004;104:207–231. doi: 10.1016/j.pharmthera.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 102.Waypa GB. Chandel NS. Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 103.Waypa GB. Guzy R. Mungai PT. Mack MM. Marks JD. Roe MW. Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- 104.Waypa GB. Marks JD. Mack MM. Boriboun C. Mungai PT. Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. doi: 10.1161/01.res.0000036751.04896.f1. [DOI] [PubMed] [Google Scholar]
- 105.Weigand LA. Wang J. Shimoda LA. Sham JS. Sylvester JT. Inhibitors of capacitative calcium entry block hypoxic pulmonary artery vasoconstriction (HPV) in isolated rat lungs. Am J Respir Crit Care Med. 2003;167:A698. [Google Scholar]
- 106.Weir EK. Archer SL. Counterpoint: hypoxic pulmonary vasoconstriction is not mediated by increased production of reactive oxygen species. J Appl Physiol. 2006;101:995–998. doi: 10.1152/japplphysiol.00480a.2006. [DOI] [PubMed] [Google Scholar]
- 107.Weir EK. Wyatt CN. Reeve HL. Huang J. Archer SL. Peers C. Diphenyleneiodonium inhibits both potassium and calcium currents in isolated pulmonary artery smooth muscle cells. J Appl Physiol. 1994;76:2611–2615. doi: 10.1152/jappl.1994.76.6.2611. [DOI] [PubMed] [Google Scholar]
- 108.Weissmann N. Dietrich A. Fuchs B. Kalwa H. Ay M. Dumitrascu R. Olschewski A. Storch U. Schnitzler M. Ghofrani HA. Schermuly RT. Pinkenburg O. Seeger W. Grimminger F. Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Weissmann N. Ebert N. Ahrens M. Ghofrani HA. Schermuly RT. Hanze J. Fink L. Rose F. Conzen J. Seeger W. Grimminger F. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. Am J Respir Cell Mol Biol. 2003;29:721–732. doi: 10.1165/rcmb.2002-0217OC. [DOI] [PubMed] [Google Scholar]
- 110.Weissmann N. Tadic A. Hanze J. Rose F. Winterhalder S. Nollen M. Schermuly RT. Ghofrani HA. Seeger W. Grimminger F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase- derived H2O2? Am J Physiol Lung Cell Mol Physiol. 2000;279:L683–L690. doi: 10.1152/ajplung.2000.279.4.L683. [DOI] [PubMed] [Google Scholar]
- 111.Weissmann N. Voswinckel R. Hardebusch T. Rosseau S. Ghofrani HA. Schermuly R. Seeger W. Grimminger F. Evidence for a role of protein kinase C in hypoxic pulmonary vasoconstriction. Am J Physiol. 1999;276:L90–L95. doi: 10.1152/ajplung.1999.276.1.L90. [DOI] [PubMed] [Google Scholar]
- 112.Weissmann N. Zeller S. Schafer RU. Turowski C. Ay M. Quanz K. Ghofrani HA. Schermuly RT. Fink L. Seeger W. Grimminger F. Impact of mitochondria and NADPH oxidases on acute and sustained hypoxic pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2006;34:505–513. doi: 10.1165/rcmb.2005-0337OC. [DOI] [PubMed] [Google Scholar]
- 113.Wilhelm J. Herget J. Role of ion fluxes in hydrogen peroxide pulmonary vasoconstriction. Physiol Res. 1995;44:31–37. [PubMed] [Google Scholar]
- 114.Wu W. Platoshyn O. Firth AL. Yuan JX. Hypoxia divergently regulates production of reactive oxygen species in human pulmonary and coronary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L952–L959. doi: 10.1152/ajplung.00203.2007. [DOI] [PubMed] [Google Scholar]
- 115.Xin HB. Senbonmatsu T. Cheng DS. Wang YX. Copello JA. Ji GJ. Collier ML. Deng KY. Jeyakumar LH. Magnuson MA. Inagami T. Kotlikoff MI. Fleischer S. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature. 2002;416:334–337. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- 116.Yamaguchi K. Asano K. Mori M. Takasugi T. Fujita H. Suzuki Y. Kawashiro T. Constriction and dilatation of pulmonary arterial ring by hydrogen peroxide: importance of prostanoids. Adv Exp Med Biol. 1994;361:457–463. doi: 10.1007/978-1-4615-1875-4_80. [DOI] [PubMed] [Google Scholar]
- 117.Yang XR. Lin MJ. Yip KP. Jeyakumar LH. Fleischer S. Leung GP. Sham JS. Multiple ryanodine receptor subtypes and heterogeneous ryanodine receptor-gated Ca2+ stores in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L338–L348. doi: 10.1152/ajplung.00328.2004. [DOI] [PubMed] [Google Scholar]
- 118.Zhang F. Carson RC. Zhang H. Gibson G. Thomas HM III. Pulmonary artery smooth muscle cell [Ca2+]i and contraction: responses to diphenyleneiodonium and hypoxia. Am J Physiol. 1997;273:L603–L611. doi: 10.1152/ajplung.1997.273.3.L603. [DOI] [PubMed] [Google Scholar]
- 119.Zhang YC. Ni W. Zhang ZK. Xu YJ. The effect of protein kinase C on voltage-gated potassium channel in pulmonary artery smooth muscle cells from rats exposed to chronic hypoxia. Chin Med J (Engl ) 2004;117:19–23. [PubMed] [Google Scholar]
- 120.Zheng YM. Mei QB. Wang QS. Abdullaev I. Lai FA. Xin HB. Kotlikoff MI. Wang YX. Role of FKBP12.6 in hypoxia- and norepinephrine-induced Ca2+ release and contraction in pulmonary artery myocytes. Cell Calcium. 2004;35:345–355. doi: 10.1016/j.ceca.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 121.Zheng YM. Wang QS. Liu QH. Rathore R. Yadav V. Wang YX. Heterogeneous gene expression and functional activity of ryanodine receptors in resistance and conduit pulmonary as well as mesenteric artery smooth muscle cells. J Vasc Res. 2008;45:469–479. doi: 10.1159/000127438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zheng YM. Wang QS. Rathore R. Zhang WH. Mazurkiewicz JE. Sorrentino V. Singer HA. Kotlikoff MI. Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol. 2005;125:427–440. doi: 10.1085/jgp.200409232. [DOI] [PMC free article] [PubMed] [Google Scholar]