Abstract
Internalization of activated receptors to a compartment enriched with NAPDH oxidase and associated signaling molecules is expected to facilitate regulation of redox-mediated signal transduction. The aim of this study was to test the hypothesis that endocytosis is necessary for generation of reactive oxygen species (ROS) by Nox1 and for redox-dependent signaling in smooth muscle cells (SMCs). Within minutes of treatment with tumor necrosis factor (TNF)-α or thrombin, SMCs increased cellular levels of ROS that was inhibited by shRNA to Nox1. Treatment of SMC with TNF-α induced a dynamin-dependent endosomal generation of ROS, whereas thrombin-mediated ROS production did not occur within endosomes and was not prevented by dominant-negative dynamin (dn-dynamin), but instead required transactivation of the epidermal growth factor receptor (EGFR). Activation of the phosphatidylinositol 3-kinase (PI3K)-Akt-activating transcription factor-1 (ATF-1) pathway by TNF-α and thrombin were both Nox1- and dynamin-dependent. In conclusion, we show that formation of specific ligand–receptor complexes results in spatially distinct mechanisms of Nox1 activation and generation of ROS. These findings provide novel insights into the role of compartmentalization for integrating redox-dependent cell signaling. Antioxid. Redox Signal. 12, 583–593.
Introduction
NADPH oxidases are an important source of the reactive oxygen species (ROS) that participate in cellular physiologic and pathologic processes. These enzymes are comprised of two membrane-bound subunits, p22phox and one of the Nox isoforms (4). Upon activation, the Nox subunit transfers electrons from NADPH to molecular oxygen to generate superoxide. The topography of the enzyme mandates production of superoxide on the side of the membrane opposite to the cytoplasmic NADPH binding site. Recently, we and others have shown that in response to receptor activation, NAPDH oxidase-dependent activation of the transcription factor nuclear factor (NF)-ϰB is dependent on ROS generation within endosomes (32, 34). The ability to compartmentalize ROS signaling by internalization of activated receptors to a site enriched with NAPDH oxidase and associated signaling molecules is expected to facilitate regulation of redox-mediated signaling.
Endocytosis is a highly conserved process for the regulation of receptor-mediated signaling from the plasma membrane. Although traditionally considered a deactivation mechanism for receptor tyrosine kinases, increasing evidence has identified an active signaling role for endosomes. Endosome-based signaling can more intricately modify the duration and magnitude of the response initiated by ligand binding at the cell surface. In addition, endosomal membranes are biochemically suited to act as specialized signaling platforms promoting selective recruitment of assorted scaffold and signaling proteins (41, 48).
We have shown that stimulation of vascular smooth muscle cells (SMCs) with tumor necrosis factor-α (TNF-α) results in activation of NADPH oxidase and generation of ROS within early endosomes. However, it has not been determined if endocytosis is actually required for the generation of ROS and redox-dependent signaling. In addition, many existing studies of endosomal signaling have been limited by reliance upon heterologous expression of tagged proteins to define the spatiotemporal organization of signaling-protein interactions in intact cells. We have employed selective localization of ROS production to examine the coupling of receptor activation to NADPH oxidase-dependent generation of ROS and downstream cell signaling in smooth muscle cells (SMCs).
Thrombin and TNF-α are physiologically relevant, distinct agonists that when added to cultured SMC rapidly increase NADPH oxidase-dependent formation of ROS (6, 34). TNF-α is a pleiotropic inflammatory cytokine that causes diverse cellular events, including proliferation, differentiation, apoptosis, and necrosis, and has been implicated in a variety of pathological inflammatory conditions and autoimmune diseases (8). Members of the TNF ligand family exert their biological functions via interaction with the membrane receptors comprising the TNF receptor (TNFR) family. It has been proposed that TNF binding to a preformed TNFR complex can either induce a conformational change and thereby activate a signal-competent receptor complex, or can lead to the formation of higher-order receptor complexes that thereby acquire signal competence (49). Internalization of TNF-α–TNFR complexes is required for activation of some signaling pathways, whereas other signals initiated by TNF-α appear to be independent of TNFR internalization (42).
Thrombin stimulates SMCs via the proteolytic activation of the protease-activated receptor PAR1 (33). The PAR receptors are members of the 7 transmembrane G protein-coupled family of receptors (GPCR), however, their method of activation is unique. PAR1 is activated when thrombin, a serine protease, binds to and cleaves the amino-terminal extracellular domain. This cleavage unmasks a new N-terminus that serves as a tethered ligand, binding to another extracellular loop of the receptor and activating it. This irreversible proteolytic activation of PAR1 is in contrast to the reversible ligand binding that activates classical G protein-coupled receptors (45).
In this study, we confirmed the role of NADPH oxidase in TNF-α and thrombin-mediated activation of SMC, identifying Nox1 as the predominant Nox homolog responsible for production of ROS in response to both agonists. We then tested the hypothesis that endocytosis is necessary for generation of ROS by Nox1 and for the redox-dependent signaling in response to TNF-α and thrombin.
Materials and Methods
Chemicals
Fetal bovine serum (FBS), Dulbecco's Modified Eagle's Medium (DMEM), penicillin-streptomycin mixtures, glutamine, MEM amino acids solution, Trypsin/EDTA, and HEPES buffer solution were obtained from Gibco BRL (Grand Island, NY). Complete protease inhibitor cocktail was obtained from Roche Applied Science (Indianapolis, IN). Antibodies to phospho-Akt (pS473), phospho-ATF-1 (pS133, reactive with phosphorylated forms of CREB and ATF-1), and EGFR were from Cell Signaling Technology (Danvers, MA), p-EGFR (pY1068) was from BioSource Invitrogen (Carlsbad, CA), Nox1 (H-75) was from Santa Cruz Biotechnology (Santa Cruz, CA), and GAPDH from Chemicon International (Temecula, CA). Secondary horseradish peroxidase-conjugated goat anti-mouse, rabbit anti-goat, and goat anti-rabbit antibodies were from Bio-Rad Laboratories (Hercules, CA). Bio-Rad DC Protein Assay kit was used for protein measurement. Enhanced chemiluminescence (ECL) Western blotting detection reagents were from Amersham Pharmacia Biotech (Piscataway, NJ). Dihydroethidium (DHE), OxyBURST Green H2HFF-BSA (Oxyburst), 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate, acetyl ester (DCFH), and Alexa Fluor 488 10,000 MW dextran (dextran-488) were from Molecular Probes/Invitrogen Labeling and Detection (Eugene, OR). Vectashield H-1000 mounting medium was from Vector Laboratories (Burlingame, CA). GM6001 and AG1478 were from Calbiochem EMD Biosciences (Gibbstown, NJ). All other chemicals were obtained from Sigma Chemical (St. Louis, MO).
Cell culture
Medial SMCs were isolated from thoracic aorta of C57BL/6 mice by enzymatic digestion as described previously (34) and grown in DMEM supplemented with 10% (vol/vol) FBS, 10 U/ml penicillin, 10 μg/ml streptomycin, 4 mM glutamine, and 20 mM HEPES, at 37°C and 5% CO2. SMCs were confirmed by positive alpha-actin staining at second passage. All experiments were conducted using SMCs between passages 6 and 11 and 60–90% confluence. For experiments, SMCs were serum-deprived (0.2% FBS) for 24 h prior to use.
Detection of reactive oxygen species
Cells were grown on chamber slides for confocal fluorescent microscopic analysis, or in 35 mm dishes for flow cytometric analysis, serum-deprived (0.2% FBS) overnight, then incubated with one or more of the fluorescent probes prepared in phenol red free and serum free-DMEM: DHE (10 μmol/L) for detection of superoxide (35), Oxyburst (50 μg/ml) for detection of endosomal generation of ROS (34), DCFH (20 μmol/L) for detection of cellular ROS, or dextran-488 (50 μg/ml) to demonstrate endocytosis, for 5 min at 37°C, and then stimulated with TNF-α (10 ng/ml) or thrombin (2 units/ml) for 10 min. For microscopy studies, cells were then fixed in 4% paraformaldehyde for 10 min and the nucleus counterstained with ToPro-3 (Molecular Probes/Invitrogen) for 5 min, cover-slide mounted with Vectashield Mounting Medium for Fluorescence (Vector Laboratories, Burlingame, CA), and imaged with a Zeiss LSM 510 META laser scanning confocal microscope (Carl Zeiss MicroImaging, Germany). For flow cytometric analysis, after 10 min of the agonist, cells were rinsed with ice cold PBS, collected by trypsinization, centrifuged at 400 g for 5 min at 4°C, resuspended in ice-cold PBS, filtered, and kept on ice in the dark for immediate analysis. Flow cytometric data were collected on a Becton Dickinson FACS DiVa (BD Biosciences, San Jose, CA) flow cytometer and analyzed with the FlowJo software package (Tree Star, Inc., Ashland, OR) as described (34).
Adenoviral-mediated gene transfer
Adenovirus was mixed with the cationic polymer poly-L-lysine (250 molecules/virus particle) (21) and added to SMCs (70% confluence) in serum free-DMEM. After 6 h, media was supplemented to achieve 10% FBS for an additional 42 h, then the cells washed and media replaced with DMEM containing 0.2% FBS prior to use. Recombinant adenoviruses expressed a dominant-inhibitory form of dynamin (K44A) (18) (Ad-dnDyn), shRNA directed to Nox1 (Ad-shNox1), and shRNA to GFP (Ad-shGFP) as a control.
Design and construction of mouse Nox1-specific shRNA
We designed a short hairpin RNA (shRNA) construct encoding inverted sequences of 21 base pairs separated by a 6 nucleotide loop sequence to target mouse Nox1 mRNA (GenBank NM_172203 mNOX1) using siRNA selection criteria described earlier (19, 26). Five different 21-nucleotide stretch sequences were selected within the coding region of Nox1 that were 30∼50% GC-rich, with a G at the first 5’ position of each stretch for efficient transcription by the mouse U6 promoter (see below). These oligonucleotide sequences were unique to Nox1 and did not match any other sequences in the genome. A random 6-nucleotide loop sequence (5’-TATCGC) was added to the 3’ end of each stretch followed by 21-nucleotide inverted sequences. Finally, a stretch of 5 Ts' and an EcoRI restriction site was added to the 3’ end of the oligonucleotides to allow for cloning into an adenoviral (Ad) backbone plasmid containing a CMV promoter-driven reporter enhanced green fluorescent protein and a U6 promoter. The complete sequences of all 5 oligonucleotides obtained from Integrated DNA Technologies (Coralville, IA) are as follows:
GGTAACGTCAGCTATGGAGTTtatcgcAACTCCATAGCTGACGTTACCTTTTTgaattcc
GACCATAAGGGGAGTTGCAGGtatcgcCCTGCAACTCCCCTTATGGTCTTTTTgaattcc
GAGTCTTGGAAGTGGATCCTTtatcgcAAGGATCCACTTCCAAGACTCTTTTTgaattcc
GCAGAAGGTCGTGATTACCAAtatcgcTTGGTAATCACGACCTTCTGCTTTTTgaattcc
GAACAAGAGATGGAGGAATTAtatcgcTAATTCCTCCATCTCTTGTTCTTTTTgaattcc
Each shRNA oligonucleotide was cloned into the Ad-CMV-eGFP-mUpro6 plasmid (University of Iowa Gene Transfer Vector Core) sequenced to verify that they retained 100% homology to mouse Nox1. Silencing efficiency was tested by co-transfecting mouse Nox1 cDNA with each of the Nox1-shRNA plasmids in HEK-293 cells. A shRNA control plasmid that does not target any known genes in mouse genome (26) as well as a shGFP plasmid were used as a control in these experiments. Twenty four hours after transfection, total RNA was isolated and quantitative real-time PCR was performed to measure Nox1 levels. In the initial screening the construct containing siRNA sequence—GGTAACGTCAGCTATGGAGTT—yielded the greatest silencing with 71 ± 2% decrease in expression of Nox1. This construct was used to generate the adenovirus vector (Ad-shNox1) by Gene Transfer Vector Core (University of Iowa).
Western blot analysis
SMCs were grown to 80% confluence, then incubated with DMEM containing 0.2% FBS for 24 h. Cells were treated with TNF-α (10 ng/ml) or thrombin (2 units/ml) for 5 min, then lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM ethylenediamine tetraacetic acid), supplemented with phosphatase inhibitors (1 mM sodium orthovanadate and 1 mM sodium fluoride) and complete EDTA-free protease inhibitors cocktail (Roche Applied Science, Indianapolis, IN). Cell lysates were collected and centrifuged at 12,000 g for 10 min, 4°C. The lysates were mixed with SDS reducing sample buffer and boiled for 5 min before loading. Proteins (15 ug) were resolved on 12% SDS-polyacrylamide gels and transferred electrophoretically to Immobilon-P polyvinylidene difluoride membranes (Millipore Corporation, Billerica, MA). The membranes were blocked with milk blocking solution (10% non-fat dry milk in PBS with 10% glycerol, 0.9% NaCl and 0.2% Tween-20) or 5% BSA in TBS-T (10 mM Tris, pH 7.4, 100 mM NaCl, and 0.2% Tween-20) for 2 h at room temperature, and then incubated overnight at 4°C with a primary antibody against phospho-ATF-1 (1:1,000) or phospho-Akt (1:1,000), or ERK2 (1:5,000). The membranes were washed several times with 10% milk solution or 1% BSA in TBS-T and then incubated for 1 h at room temperature with appropriate horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution). After washing the membranes with N-TBS (1% NP-40 in TBS) and TBS for several times, specific immunoreactive signals were detected using the enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ) and Kodak x-ray film. Protein bands were quantitated by densitometric analysis using NIH ImageJ software. Membranes were stripped after the primary antibody and re-probed for GAPDH. Data are reported as ratios of p-ATF-1 or p-Akt to GAPDH densitometry.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Statistical comparisons were performed by Student's two-tailed t-tests or Dunnett's test as appropriate. A value of p < 0.05 was considered statistically significant.
Results
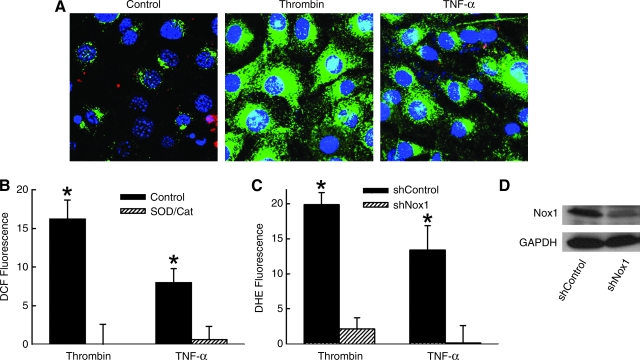
We first confirmed the ability of thrombin or TNF-α to activate SMC and generate intracellular ROS. Within minutes of treatment with thrombin or TNF-α, SMCs increased DCF fluorescence, which was confirmed to indicate cellular generation of ROS by the inhibition of fluorescence when SMCs were co-treated with superoxide dismutase (SOD, 1,000 u/ml) and catalase (500 u/ml) (Fig. 1A and B). Previous studies have implicated NADPH oxidase in both thrombin and TNF-α–induced ROS and activation of SMCs (34, 38). Expression of shRNA to Nox1 markedly inhibited DHE fluorescence in SMCs (Fig. 1C), identifying the NADPH catalytic subunit Nox1 as the primary enzymatic source of superoxide in response to both thrombin and TNF-α. Ad-shNox1 resulted in an 82 ± 4% decrease in Nox1 mRNA levels and a 27 ± 15% increase in Nox4 mRNA levels (n = 3) 48 h after gene transfer, as measured by quantitative RT-PCR and compared to Ad-shGFP treated cells. Similar treatment resulted in a significant reduction in Nox1 protein expression in SMCs (Fig. 1D).
FIG. 1.
Generation of ROS by thrombin and TNF-α in SMC is Nox1-dependent. (A) Confocal micrographs of SMCs incubated in DCFH, then treated for 10 min with TNF-α (10 ng/ml) or thrombin (2 units/ml). (B) Percent change in relative fluorescence of DCF 10 min after incubation with TNF-α or thrombin as measured by flow cytometry and compared to untreated control cells. SOD and catalase were added concurrently with the agonist. (n = 5). (C) Summary data of DHE fluorescence in SMC expressing shNox1 or shGFP (shControl) as measured by flow cytometry. (n = 4). *p < 0.05 vs. Control for respective agonist. Fluorescence is reported as percent change in relative fluorescent units per cell. (D) Western blot of SMC lysates for Nox1 and GAPDH 48 h after AdshNox1 or AdshControl. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
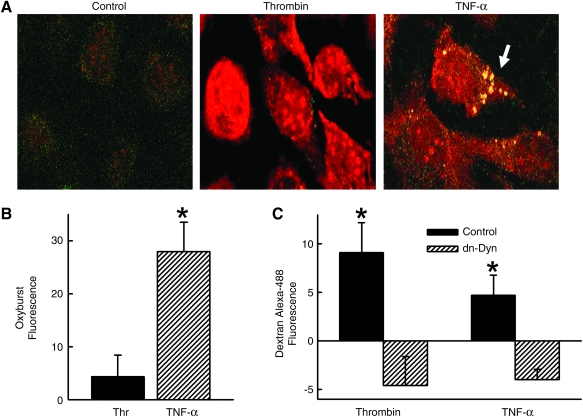
There is an increasing appreciation that endosomal membranes are important sites of receptor-initiated signal transduction (48). We examined whether Nox1-dependent generation of ROS in SMCs occurred within endosomes. For these studies, SMCs were incubated simultaneously in DHE to indicate total intracellular superoxide production and Oxyburst to identify endosomal ROS. As expected, treatment of SMCs with TNF-α for 10 min increased DHE fluorescence, consistent with increased levels of cytosolic superoxide. In addition, TNF-α resulted in the appearance of punctuate Oxyburst fluorescence, consistent with endosomal generation of ROS (Fig. 2A) as we have described previously (34). In contrast, although thrombin also increased DHE fluorescence, there was no demonstrated Oxyburst signal (Fig. 2A). The absence of endosomal ROS production by thrombin was confirmed by flow cytometric analysis of the Oxyburst signal in SMCs (Fig. 2B). This observation does not reflect a failure of thrombin to induce endocytosis in SMC, since both TNF-α and thrombin increased uptake of dextran (10,000 MW) conjugated to Alexa-488 (Fig. 2C). These findings indicate that although both TNF-α and thrombin elicit Nox1-derived superoxide production in SMCs, only TNF-α results in the endosomal localization of ROS generation.
FIG. 2.
TNF-α, but not thrombin, induces ROS production within vesicles. (A) SMCs were incubated with DHE and Oxyburst for 5 min prior to stimulation with TNF-α or thrombin. Arrow indicates Oxyburst signal within vesicles. (B) Summary data of Oxyburst fluorescence in SMC (n = 7). (C) Summary data of 10,000 MW dextran-Alexa-488 fluorescence in SMC expressing dn-dynamin 10 min after TNF-α or thrombin (n = 7). Control represents cells infected with AdshGFP. Fluorescence is reported as percent change in relative fluorescent units per cell measured by flow cytometry. *p < 0.05 vs. control for respective agonist. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
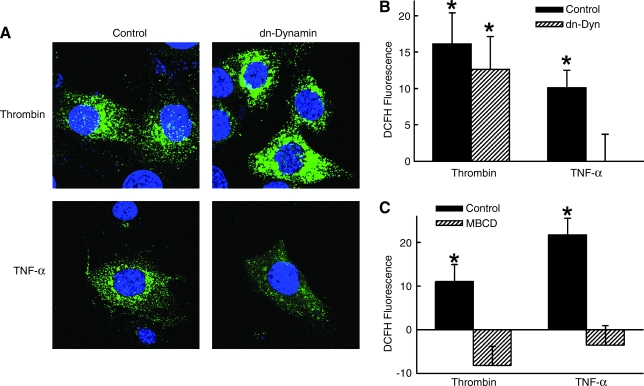
Receptor-mediated endocytosis is regulated by dynamin, a GTPase that oligomerizes around the neck of budding vesicles (12). We determined whether dynamin-dependent endocytosis was necessary for Nox1 generation of ROS by studying SMCs expressing recombinant dynamin (Ad-dnDyn) with a point mutation in the nucleotide-binding site (K44A) that prevents vesicle internalization (29). This dominant-negative dynamin (dn-dynamin) blocked SMC uptake of dextran-conjugated Alexa 488 in response to both thrombin and TNF-α (Fig. 2C). The observation that fluorescence was inhibited to levels below control cells (i.e., below zero) suggests inhibition of a basal level of endocytosis that was occurring in the absence of agonist under our experimental conditions. Having established that dn-dynamin blocks thrombin and TNF-α-mediated endocytosis in SMCs, we examined its effect on ROS generation. Expression of dn-dynamin completely prevented DCF fluorescence in response to TNF-α but had no effect on the DCF fluorescence induced by thrombin (Fig. 3A and B). These findings suggest that whereas treatment of SMC with TNF-α induces a Nox1-dependent, dynamin-dependent generation of endosomal ROS, treatment with thrombin induces a Nox1-dependent, dynamin-independent cellular generation of ROS.
FIG. 3.
Role of dynamin and lipid rafts in TNF-α and thrombin generation of ROS. SMCs expressing dn-dynamin were incubated with DCFH and fluorescence measured in response to TNF-α or thrombin by (A) microscopy or by (B) flow cytometry (n = 5). Control represents cells infected with AdshGFP. (C) SMCs were treated with 5 mM MBCD for 30 min prior to TNF-α or thrombin and DCF fluorescence measured by flow cytometry. (n = 4). *p < 0.05 vs. control for respective agonist. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Upon activation by their ligands, both TNFR1 and PAR1 translocate to lipid rafts/caveolae-enriched membrane microdomains, where they bind to signaling proteins and form a receptor-induced signaling complex (3, 31). Caveolin is necessary for targeting membrane receptors to lipid raft domains and regulating GTP loading and membrane targeting of Rac1 (53). We speculated that upon activation, Nox1 may reside within lipid raft domains along with TNFR1 and PAR1. To address this, SMCs were treated with the cholesterol-depleting agent methyl-β-cyclodextrin (MBCD) that depletes plasma membrane cholesterol and thereby disrupts the structural integrity of caveolae at the membrane surface (47). We found that unlike dn-dynamin, treatment of SMC with MBCD prevented both thrombin and TNF-α-mediated increase in ROS (Fig. 3C). This finding suggests that MBCD may block thrombin-induced ROS not by inhibiting caveolar endocytosis, but by disrupting formation of Nox1 and/or signaling complexes in caveolar-enriched areas (25).
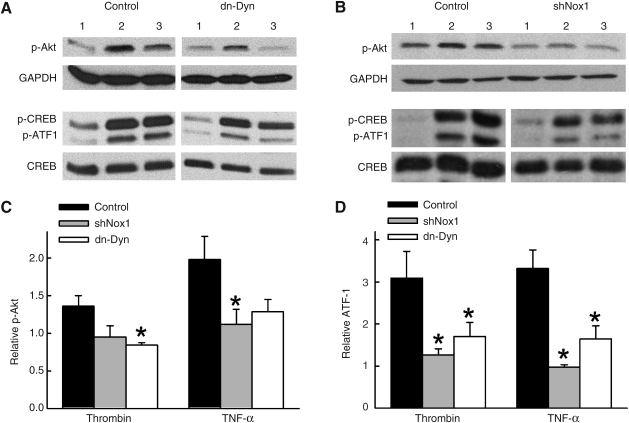
NADPH oxidase participates in the ROS-dependent activation of multiple diverse signaling pathways (10). Both TNF-α and thrombin induce SMC proliferation via stimulation of the phosphatidylinositol 3-kinase (PI3K)–Akt pathway and activating transcription factor-1 (ATF-1) (1, 7, 22, 40, 50). In a rat SMC line, inhibiting activation of PI3K-Akt prevented phosphorylation of ATF-1 (20). Based on these observations, we first examined whether activation of PI3K-Akt or ATF-1 by TNF-α and thrombin were dependent on Nox1. In vector control (Ad-shGFP)-transduced SMCs, treatment with either thrombin or TNF-α increased phosphorylation of both Akt and ATF-1. In contrast, the phosphorylation of Akt and ATF-1 were significantly inhibited in SMCs transduced with AdshNox1 (Fig. 4). We next tested the role of dynamin-dependent endocytosis in activation of Akt and ATF-1. Phosphorylation of Akt and ATF-1 by thrombin and TNF-α were inhibited in SMCs expressing dn-dynamin (Fig. 4). Closely related in structure and function to ATF-1, the transcription factor cAMP response element binding (CREB) was also phosphorylated by TNF-α and thrombin (2.2 ± 0.5 fold and 2.4 ± 0.4 fold respectively over control, n = 4, and Fig. 4). However, pretreatment with Ad-shNox1 or dn-dynamin did not significantly inhibit CREB activation to either agonist. These findings indicate that dynamin-dependent endocytosis is necessary for thrombin and TNF-α-mediated activation of Nox1-dependent signaling via PI3K/Akt-ATF-1 pathway.
FIG. 4.
Role of Nox1 and dynamin in activation of Akt and ATF-1. (A) Representative Western blots showing effects of dn-dynamin and shNox1 on phosphorylation of-Akt, CREB, and ATF-1 in SMCs collected 5 min after stimulation with TNF-α or thrombin. SMC were pre-treated with adenovirus for expression of shGFP (control), shNox1, or dn-dynamin. Numbered lanes represent 1 = no agoninst, 2 = TNF-α, and 3 = thrombin. Densitometry data were normalized to loading controls of GAPDH for p-Akt and total CREB for p-ATF1, and are summarized in (C) and (D) (n = 3–5). *p < 0.05 vs. control for respective agonist.
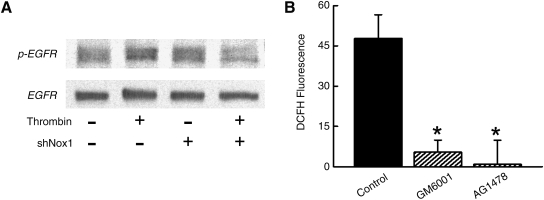
It was unexpected that dn-dynamin inhibited phosphorylation of Akt and ATF-1 by thrombin when it had no effect on the generation of thrombin-induced ROS. This finding is consistent with previous reports that receptor-mediated activation of MAPK is blocked by mutant dynamin, even in the absence of endocytosis of receptors (51). An explanation for this observation involves the activation of a receptor tyrosine kinase (RTK), such as the epidermal growth factor receptor (EGFR), in which case, endocytosis of the RTK, and not the ligand–receptor complex, is the crucial event involved in signal transduction. Relevant to our studies, PAR1 activation by thrombin induces shedding of extracellular heparin bound-EGF which then activates the EGFR (27), and inhibition of EGFR activation prevents thrombin-mediated MAPK signaling (16). Based on these reports, we hypothesized that thrombin-induced generation of ROS involved the transactivation of EGFR. As shown in Fig. 5A, and consistent with prior reports (27, 28), thrombin increased phosphorylation of EGFR. We show that the transactivation of EGFR by thrombin is inhibited by shNox1 (Fig. 5A). To assess whether the activation of EGFR contributed to generation of ROS in response to thrombin, SMCs were pre-treated with the metalloproteinase inhibitor GM6001 to prevent EGF-like ligand shedding, or with AG1478, a potent and specific tyrosine kinase inhibitor of the EGFR. Both GM6001 and AG1478 markedly inhibit thrombin-induced generation of ROS (Fig. 5B). These findings suggest that EGFR transactivation by thrombin is mediated by the extracellular shedding of EGF-like ligands and necessary for thrombin generation of intracellular ROS.
FIG. 5.
Role of EGFR in thrombin generation of ROS. (A) Representative Western blot showing phospho-EGFR and total EGFR in SMCs collected 5 min after stimulation with thrombin. SMC were pre-treated with adenovirus for expression of shGFP or shNox1. (B) SMCs were pretreated with GM6001 or AG1478 and DCFH fluorescence measured in response to thrombin by flow cytometry (n = 4). Fluorescence is reported as percent change in relative fluorescent units per cell. *p < 0.05 vs. control.
Discussion
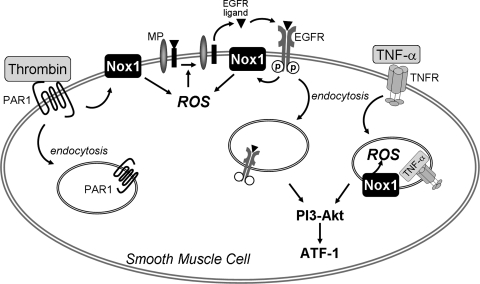
In this study, we identify two spatially-distinct mechanisms whereby receptor activation is coupled to Nox1-dependent generation of ROS and cell signaling. Whereas TNF-α induces a Nox1-dependent, dynamin-dependent endosomal generation of cellular ROS, thrombin-mediated Nox1-derived ROS production does not occur within endosomes and therefore does not require dynamin-dependent endocytosis. Instead, thrombin generation of ROS by Nox1 requires metalloproteinase shedding of EGF-like ligands and subsequent transactivation of the EGFR. In addition, we show that activation of the PI3K-Akt-ATF-1 pathway by TNF-α and thrombin is Nox1- and dynamin-dependent. This is the first study to show that specific ligand-receptor complexes result in distinct spatial generation of Nox1 ROS (Fig. 6).
FIG. 6.
Differential role for endocytosis in receptor-dependent Nox1 generation of ROS and activation of cellular signalling. In vascular SMC, TNF-α induces a Nox1-dependent, dynamin-dependent, endosomal generation of ROS with subsequent activation of PI3K-Akt-ATF-1 pathway. In contrast, thrombin activates Nox1 to transactivate the EGFR via metalloproteinase (MP)-mediated shedding of EGF-like ligands, with subsequent amplification of dynamin-independent ROS generation. Activation of PI3K-Akt-ATF-1 by thrombin is inhibited by dn-dynamin, consistent with its dependence on internalization of the activated EGFR. Internalization of receptor complexes is important in the temporal regulation of signaling.
Nox1 and Nox4, the catalytic subunits of NAPDH oxidase found in vascular SMCs, are differentially regulated by growth factors (30). Whereas Nox1 resembles the classic Nox2-based NADPH oxidase in requiring stimulus-induced translocation of cytosolic components to an active multi-protein complex for activation, Nox4 appears to be constitutively active and has a role in SMC differentiation (11). Furthermore, Nox1 and Nox4 localize to distinct intracellular compartments. Nox1 localizes with caveolin on the membrane and along the cellular margins, whereas Nox4 has been found at focal adhesions, in the endoplasmic reticulum, and in the nucleus (9, 25, 44). The recent finding that Nox4 produces mainly hydrogen peroxide, while Nox1 generates mostly superoxide also suggests that these two isoforms have distinct functions within the cell (17). We show in this study that TNF-α and thrombin both activate Nox1 to generate superoxide in SMC. Despite finding that shNox1 essentially abolished TNF-α- and thrombin-mediated ROS does not necessarily signify that the ROS is exclusively derived from Nox1-based NADPH oxidases. For example, we cannot exclude the possibility of ROS-induced ROS whereby the initial ROS derived from Nox1 subsequently induces additional ROS generation from mitochondrial or nonmitochondrial sources (5).
Although both TNF-α and thrombin activate Nox1 to increase cellular ROS, we identify important differences in the mechanisms of ROS production. If only global cellular ROS levels are assessed, for example with the commonly used oxidant sensitive fluorescent probes DCFH and DHE, both TNF-α and thrombin increase intracellular ROS (Figs. 1 and 2A). However, investigating the role of endocytosis in ROS production in response to these two ligands discloses key differences. Oxyburst consists of bovine serum albumin covalently linked to the oxidant sensitive dihydro-2’,4,5,6,7,7’-hexafluorofluorescein and thus remains extracellular unless actively internalized by the cell. In studies utilizing this reagent, we observe intracellular punctate fluorescence minutes after treatment of SMCs with TNF-α, suggesting vesicular generation of ROS. We have shown that these ROS generating vesicles contain Nox1 and the early endosomal markers Rab5 and early endosome antigen1 (34). Although thrombin clearly induces endocytosis in SMCs, as evident by uptake of dextran (Fig. 2C), these endosomes did not generate intravesicular ROS (Fig. 2B).
We examined whether endocytosis is necessary for generation of cellular ROS by TNF-α or thrombin by the expression of dn-dynamin in SMC. Dynamin is responsible for the scission of newly formed vesicles and expression of a dn-dynamin deficient in GTP hydrolysis inhibits endocytosis (14). Our finding that dn-dynamin prevented DCF fluorescence in response to TNF-α suggests that the activation of Nox1 requires endocytosis, or more likely, that in response to TNF-α, Nox1 generates ROS in endosomes. These results are consistent with the finding that mutation of the TNFR1 internalization domain, which prevents receptor internalization, also prevented TNF-α-induced ROS generation (52). Our results with TNF-α are also consistent with the observation that dn-dynamin attenuates Nox2-dependent signaling to interleukin-1β in MCF-7 cells (32).
On the other hand, dn-dynamin had no effect on thrombin-induced DCF fluorescence, despite inhibiting thrombin-induced endocytosis of dextran (Figs. 2C and 3). One possibility is that thrombin activates membrane-associated Nox1 to produce extracellular superoxide that accesses the cell by transmembrane flux via anion channels (24). Alternatively, extracellular superoxide could spontaneously or enzymatically, via the extracellular-SOD, be dismutated to hydrogen peroxide and transverse the plasma membrane by diffusion. However, these processes seem to be an inefficient way to increase intracellular ROS and poorly suited to modulate intracellular signaling events.
Another potential explanation for the failure of dn-dynamin to inhibit thrombin-induced ROS is that thrombin may activate cellular Nox1 that is not associated with the extracellular membrane and is therefore independent of internalization. For example, immunostaining of quiescent cells show Nox1 in the submembranous region, although these are poorly defined (2, 25). Alternatively, Nox1 may be internalized from the membrane by dynamin-independent mechanisms (13, 15). For example, since activated PAR1 and Nox1 are targeted to lipid rafts/caveolae membrane microdomains (3, 25), the formation of cavicles or caveosomes may be involved in the response to thrombin (36). However, caveosome formation also appears to be blocked by dn-dynamin (39). Another possibility is that in response to thrombin, SMC Nox1 is internalized by pinocytosis, which is not blocked by dn-dynamin (13). There are also mechanisms of endocystosis that appear to be dynamin-independent (15).
Until recently, endocytosis was regarded primarily as a mechanism to terminate receptor-mediated signaling by dissociation of the ligand at the acidic pH of endosomes and subsequent degradation of the receptor complex in lysosomes. In this manner, endocytosis regulates signaling by controlling the concentration of receptors and associated regulatory proteins at the cell membrane. However, endocytosis may also act to prolong signal transduction initiated with ligand binding at the cell surface and continued after internalization. Perhaps more importantly however, recent evidence implicates endosomal membranes as a scaffold to facilitate signaling events by regulating specific protein–protein interactions and activation (32, 48). With cytoskeleton-based motility, endosomes can also deliver active signaling molecules to various locations within the cell. Like other molecules activated by proteolytic mechanisms, PAR1 is used once and then discarded (37). In contrast to the TNF receptor, which can recycle to the membrane after internalization, once internalized the PAR1 is rapidly sorted to lysosomes and degraded. One interpretation of our data is that whereas TNFR requires endocytosis for redox-dependent signaling, thrombin signals from the membrane. Consistent with this, a mutant PAR1 has been shown to lose the ability to internalize without disrupting signaling (37). In fact, internalization-defective PAR1 caused persistent signaling, suggesting that internalization of activated PAR1 is critical for the temporal control but not the initiation of thrombin signaling. Although our data is consistent with this model, we did not directly examine PAR1 internalization.
In light of these observations, we examined the role of EGFR in ROS generation by thrombin. Like other G-protein coupled receptors, activation of PAR1 leads to extracellular shedding of heparin bound-EGF and subsequent transactivation of the receptor tyrosine kinase EGFR (27, 28, 54). Once activated, EGFRs serve as a docking site and integrate multiple signaling complexes. Ushio–Fukai reported that NADPH oxidase-derived ROS are critical mediators of EGFR transactivation by angiotensin II (46). We extend these findings by showing that siRNA to Nox1 reduces EGFR phosphorylation in response to thrombin (Fig. 5). In addition, we show that inhibition of EGFR activation by GM6001 or AG1478 markedly prevents thrombin-induced intracellular ROS. Together, these observations are consistent with a feed-forward mechanism in which thrombin activation of Nox1 leads to the transactivation of EGFR, which is necessary for the amplification of ROS production. A similar paradigm has been suggested for angiotensin II (43).
TNF-α and thrombin activate the PI3K-Akt signaling pathway and induce phosphorylation of ATF-1 (1, 7, 22, 40, 50). We found that both processes, the activation of PI3K-Akt and ATF-1, are dependent on Nox1 (Fig. 4). As expected, inhibition of endocytosis by dn-dynamin prevented TNF-α activation of Akt and ATF-1, consistent with the effects of dn-dynamin on TNF-α-induced ROS generation. However, dn-dynamin also inhibited phosphorylation of Akt and ATF-1 by thrombin, despite having no inhibitory effect on thrombin-induced ROS. This finding is consistent with the proposal that endocytosis of thrombin-transactivated EGFR is necessary for downstream signaling. This explanation is further supported by the evidence that; (a) mutant dynamin inhibits GPCR-mediated internalization of the activated EGFR (23), (b) inhibition of EGFR activation prevents thrombin-mediated MAPK signaling (16), and (c) receptor-mediated activation of MAPK is blocked by mutant dynamin even in the absence of endocytosis of receptors (51).
In summary, we provide evidence of spatially distinct mechanisms of receptor-mediated activation of Nox1 and generation of ROS in SMCs (Fig. 6). Exposure of SMCs to TNF-α results in a dynamin-dependent, Nox1-mediated production of endosomal ROS. In contrast, thrombin results in Nox1-induced ROS production that is dynamin-independent and not generated within endosomes, but instead requires EGFR transactivation. Although endocytosis is not required for thrombin-induced activation of Nox1, downstream signaling via this cascade is dynamin-dependent. These findings provide insight into the importance of compartmentalization and local domains for integrating redox-dependent cell signaling.
Abbreviations Used
- Ad
adenovirus
- ATF-1
activating transcription factor-1
- BSA
bovine serum albumin
- CMV
cytomegalovirus
- CREB
cAMP response element binding
- DCF
2’7-dichlorofluorescein
- DCFH
5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate, acetyl ester
- DHE
dihydroethidium
- DMEM
Dulbecco's modified Eagle's medium
- Dn-dynamin
dominant-negative dynamin
- EDTA
ethylenediaminetetraacetic acid
- EGF
epidermal growth factor
- eGFP
enhanced green fluorescent protein
- EGFR
epidermal growth factor receptor
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GFP
green fluorescent protein
- GPCR
G protein-coupled family of receptors
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MAPK
mitogen activated protein kinase
- MBCD
methyl-β-cyclodextrin
- mRNA
messenger ribonucleic acid
- NADPH
reduced nicotinamide-adenine dinucleotide phosphate
- NF-ϰB
nuclear factor kappa beta
- Nox
NADPH oxidase
- PAR1
protease-activated receptor-1
- PCR
polymerase chain reaction
- PI3K
phosphatidylinositol 3-kinase
- ROS
active oxygen species
- RTK
receptor tyrosine kinase
- SDS
sodium dodecyl sulfate
- shGFP
shRNA directed to GFP
- shNox1
shRNA directed to Nox1
- shRNA
short hairpin RNA
- siRNA
small interfering ribonucleic acid
- SMCs
smooth muscle cells
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor-α
- TNFR
TNF-α receptor
Footnotes
The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Acknowledgments
The project was supported by National Institutes of Health Grants HL062483 (FSL, FJM), HL081750 (FJM), the American Heart Association (FSL), and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (FJM).
The authors wish to thank associates of the University of Iowa Roy J. and Lucille A. Carver College of Medicine Central Microscopy Research Facility, the Flow Cytometry Facility, and the Gene Transfer Vector Core Facility of the University of Iowa Center for Gene Therapy of Cystic Fibrosis and Other Genetic Diseases (supported by NIH/NIDDK P30 DK 54759).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adiseshaiah P. Kalvakolanu DV. Reddy SP. A JNK-independent signaling pathway regulates TNF{alpha}-stimulated, c-Jun-driven FRA-1 protooncogene transcription in pulmonary epithelial cells. J Immunol. 2006;177:7193–7202. doi: 10.4049/jimmunol.177.10.7193. [DOI] [PubMed] [Google Scholar]
- 2.Ambasta RK. Kumar P. Griendling KK. Schmidt HH. Busse R. Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 3.Bae JS. Yang L. Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci USA. 2007;104:2867. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Brady NR. Hamacher-Brady A. Westerhoff HV. Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 6.Brandes RP. Viedt C. Nguyen K. Beer S. Kreuzer J. Busse R. Gorlach A. Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb Haemost. 2001;85:1104–1110. [PubMed] [Google Scholar]
- 7.Cao H. Dronadula N. Rao GN. Thrombin induces expression of FGF-2 via activation of PI3K-Akt-Fra-1 signaling axis leading to DNA synthesis and motility in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C172–182. doi: 10.1152/ajpcell.00284.2005. [DOI] [PubMed] [Google Scholar]
- 8.Chen G. Goeddel DV. TNF-R1 signaling: A beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 9.Chen K. Kirber MT. Xiao H. Yang Y. Keaney JF., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clempus RE. Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clempus RE. Sorescu D. Dikalova AE. Pounkova L. Jo P. Sorescu GP. Lassegue B. Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conner SD. Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 13.Damke H. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damke H. Binns DD. Ueda H. Schmid SL. Baba T. Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell. 2001;12:2578–2589. doi: 10.1091/mbc.12.9.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damm EM. Pelkmans L. Kartenbeck J. Mezzacasa A. Kurzchalia T. Helenius A. Clathrin- and caveolin-1-independent endocytosis: Entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168:477–488. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daub H. Ulrich Weiss F. Wallasch C. Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 17.Dikalov SI. Dikalova AE. Bikineyeva AT. Schmidt HHHW. Harrison DG. Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan D. Li Q. Kao AW. Yue Y. Pessin JE. Engelhardt JF. Dynamin is required for recombinant adeno-associated virus Type 2 infection. J Virol. 1999;73:10371–10376. doi: 10.1128/jvi.73.12.10371-10376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbashir SM. Harborth J. Weber K. Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 20.Fan C. Katsuyama M. Nishinaka T. Yabe-Nishimura C. Transactivation of the EGF receptor and a PI3 kinase-ATF-1 pathway is involved in the upregulation of NOX1, a catalytic subunit of NADPH oxidase. FEBS Lett. 2005;579:1301–1305. doi: 10.1016/j.febslet.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Fasbender A. Zabner J. Chillon M. Moninger TO. Puga AP. Davidson BL. Welsh MJ. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479–6489. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- 22.Ghosh SK. Gadiparthi L. Zeng Z-Z. Bhanoori M. Tellez C. Bar-Eli M. Rao GN. ATF-1 mediates protease-activated receptor-1 but not receptor tyrosine kinase-induced DNA synthesis in vascular smooth muscle cells. J Biol Chem. 2002;277:21325–21331. doi: 10.1074/jbc.M201608200. [DOI] [PubMed] [Google Scholar]
- 23.Grewal JS. Luttrell LM. Raymond JR. G protein-coupled receptors desensitize and down-regulate epidermal growth factor receptors in renal mesangial cells. J Biol Chem. 2001;276:27335–27344. doi: 10.1074/jbc.M103578200. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins BJ. Madesh M. Kirkpatrick CJ. Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 26.Hingtgen SD. Tian X. Yang J. Dunlay SM. Peek AS. Wu Y. Sharma RV. Engelhardt JF. Davisson RL. Nox2-containing NADPH oxidase and Akt Activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 27.Kalmes A. Vesti BR. Daum G. Abraham JA. Clowes AW. Heparin blockade of thrombin-induced smooth muscle cell migration involves inhibition of epidermal growth factor (EGF) receptor transactivation by heparin-binding EGF-like growth factor. Circ Res. 2000:92–98. doi: 10.1161/01.res.87.2.92. [DOI] [PubMed] [Google Scholar]
- 28.Kanda Y. Mizuno K. Kuroki Y. Watanabe Y. Thrombin-induced p38 mitogen-activated protein kinase activation is mediated by epidermal growth factor receptor transactivation pathway. Br J Pharmacol. 2001;132:1657–1664. doi: 10.1038/sj.bjp.0703952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kranenburg O. Verlaan I. Moolenaar WH. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J Biol Chem. 1999:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 30.Lassegue B. Clempus RE. Vascular NAD(P)H oxidases: Specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 31.Legler DF. Micheau O. Doucey M-A. Tschopp J. Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNF[alpha]-mediated NF-kB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 32.Li Q. Harraz MM. Zhou W. Zhang LN. Ding W. Zhang Y. Eggleston T. Yeaman C. Banfi B. Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNamara CA. Sarembock IJ. Gimple LW. Fenton JW., 2nd Coughlin SR. Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller FJ., Jr. Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 35.Miller FJ., Jr. Gutterman DD. Rios CD. Heistad DD. Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 36.Mundy DI. Machleidt T. Ying Y-S. Anderson RGW. Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115:4327–4339. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 37.Paing MM. Temple BRS. Trejo JA. A Tyrosine-based sorting signal regulates intracellular trafficking of protease-activated receptor-1: Multiple regulatory mechanisms for agonist-induced G-protein-coupled receptor internalization. J Biol Chem. 2004;279:21938. doi: 10.1074/jbc.M401672200. [DOI] [PubMed] [Google Scholar]
- 38.Patterson C. Ruef J. Madamanchi NR. Barry-Lane P. Hu Z. Horaist C. Ballinger CA. Brasier AR. Bode C. Runge MS. Stimulation of a vascular smooth muscle cell NAD(P)H oxidase by thrombin. Evidence that p47 phox may participate in forming this oxidase in vitro and in vivo. J Biol Chem. 1999;274:19814–19822. doi: 10.1074/jbc.274.28.19814. [DOI] [PubMed] [Google Scholar]
- 39.Pelkmans L. Kartenbeck J. Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 40.Peppel K. Zhang L. Orman ES. Hagen P-O. Amalfitano A. Brian L. Freedman NJ. Activation of vascular smooth muscle cells by TNF and PDGF: overlapping and complementary signal transduction mechanisms. Cardiovasc Res. 2005;65:674–682. doi: 10.1016/j.cardiores.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 41.Sadowski L. Pilecka I. Miaczynska M. Signaling from endosomes: Location makes a difference. Exp Cell Res. 2009;315:1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 42.Schutze S. Machleidt T. Adam D. Schwandner R. Wiegmann K. Kruse ML. Heinrich M. Wickel M. Kronke M. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J Biol Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- 43.Seshiah PN. Weber DS. Rocic P. Valppu L. Taniyama Y. Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: Upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 44.Sturrock A. Huecksteadt TP. Norman K. Sanders K. Murphy TM. Chitano P. Wilson K. Hoidal JR. Kennedy TP. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 45.Trejo J. Protease-activated receptors: New concepts in regulation of G protein-coupled receptor signaling and trafficking. J Pharmacol Exp Ther. 2003;307:437–442. doi: 10.1124/jpet.103.052100. [DOI] [PubMed] [Google Scholar]
- 46.Ushio-Fukai M. Griendling KK. Becker PL. Hilenski L. Halleran S. Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–495. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- 47.Ushio-Fukai M. Hilenski L. Santanam N. Becker PL. Ma Y. Griendling KK. Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: Role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–48275. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- 48.von Zastrow M. Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wajant H. Pfizenmaier K. Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 50.Wang AB. Li HL. Zhang R. She ZG. Chen HZ. Huang Y. Liu DP. Liang CC. A20 attenuates vascular smooth muscle cell proliferation and migration through blocking PI3k/Akt singling in vitro and in vivo. J Biomed Sci. 2007;14:357–371. doi: 10.1007/s11373-007-9150-x. [DOI] [PubMed] [Google Scholar]
- 51.Whistler JL. von Zastrow M. Dissociation of functional roles of dynamin in receptor-mediated endocytosis and mitogenic signal transduction. J Biol Chem. 1999;274:24575–24578. doi: 10.1074/jbc.274.35.24575. [DOI] [PubMed] [Google Scholar]
- 52.Woo CH. Kim TH. Choi JA. Ryu HC. Lee JE. You HJ. Bae YS. Kim JH. Inhibition of receptor internalization attenuates the TNF alpha-induced ROS generation in non-phagocytic cells. Biochem Biophys Res Commun. 2006;351:972–978. doi: 10.1016/j.bbrc.2006.10.154. [DOI] [PubMed] [Google Scholar]
- 53.Zuo L. Ushio-Fukai M. Ikeda S. Hilenski L. Patrushev N. Alexander RW. Caveolin-1 is Eessential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: Role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]
- 54.Zwick E. Hackel PO. Prenzel N. Ullrich A. The EGF receptor as central transducer of heterologous signalling systems. Trends Pharmacol Sci. 1999;20:408–412. doi: 10.1016/s0165-6147(99)01373-5. [DOI] [PubMed] [Google Scholar]