Abstract
It is now generally accepted that aging and eventual death of multicellular organisms is to a large extent related to macromolecular damage by mitochondrially produced reactive oxygen species, mostly affecting long-lived postmitotic cells, such as neurons and cardiac myocytes. These cells are rarely or not at all replaced during life and can be as old as the whole organism. The inherent inability of autophagy and other cellular-degradation mechanisms to remove damaged structures completely results in the progressive accumulation of garbage, including cytosolic protein aggregates, defective mitochondria, and lipofuscin, an intralysosomal indigestible material. In this review, we stress the importance of crosstalk between mitochondria and lysosomes in aging. The slow accumulation of lipofuscin within lysosomes seems to depress autophagy, resulting in reduced turnover of effective mitochondria. The latter not only are functionally deficient but also produce increased amounts of reactive oxygen species, prompting lipofuscinogenesis. Moreover, defective and enlarged mitochondria are poorly autophagocytosed and constitute a growing population of badly functioning organelles that do not fuse and exchange their contents with normal mitochondria. The progress of these changes seems to result in enhanced oxidative stress, decreased ATP production, and collapse of the cellular catabolic machinery, which eventually is incompatible with survival. Antioxid. Redox Signal. 12, 503–535.
-
Imperfect Mitochondrial Turnover and Postmitotic Cellular Aging
Age-related accumulation of defective mitochondria within postmitotic cells
Age-related decline in autophagy and Lon protease activity accelerates mitochondrial damage
Enlarged mitochondria are resistant to degradation and do not fuse with normal ones
Mechanisms of the age-related accumulation of mitochondria with homoplasmic mtDNA mutations
I. Introduction
As can be seen from the 5,000-year-old Sumerian Gilgamesh epos, the reasons for aging have been pondered, and the fountain of eternal youth sought after, ever since the beginnings of human reflection on life and death. During the short documented period of human history that is available to us, numerous theories on biologic aging, or senescence (and how it may be prevented) have been advanced, debated, and, in most cases, rejected (156, 187, 251, 255). Now, however, some agreement seems to exist that cellular oxidation and oxygen-derived radicals contribute to biologic aging (hereafter referred to as aging), which can be defined as a progressive decline in an organism's adaptability, followed by a consequent increase in morbidity and mortality (48, 221). The oxidative-stress theory of aging, although still far from proven, is presently one of the major aging hypotheses, even though its details are vaguely outlined, the conclusions are often obscure, and attempts to prevent aging by antioxidants are so far unsuccessful (10, 98, 213).
The amalgamation for metabolic symbiosis of anaerobic methane-producing bacteria and bacterial ancestors of present-day mitochondria into a prototype chimeric eukaryotic cell resulted in a capacity for much-enhanced energy production: oxidative phosphorylation (132). In many ways, a most successful unification of two different forms of bacteria, this amalgamation created organisms with substantially better access to energy than their ancestors. The transformation, however, had the inevitable side effect of exposing early eukaryotic cells to reactive oxygen species (ROS). These species, which have electrons that escape by accident from the mitochondrial electron-transporting system as their main cause of origin, may, in the presence of redox-active transition metals, damage a large variety of macromolecules by transforming them into dysfunctional and non-degradable garbage that accumulates intracellularly. In the long run, this accumulation results in cellular functional decay and, eventually, in cell death.
All cells are not alike in this respect, however. Most pronounced age-related changes occur in long-lived postmitotic cells, such as neurons, retinal pigment epithelium (RPE), cardiac myocytes, and skeletal muscle fibers. These cells are all highly vulnerable to aging due, of course, to their intensive oxygen metabolism and a consequent extensive ROS production; this is especially true for cardiac myocytes, cortical neurons, and RPE cells (91). A no-less-important contribution to vulnerability of long-lived postmitotic cells to aging is the fact that these cells are replaced rarely, or not at all, and can thus be as old as the organism itself (19). In contrast, short-lived postmitotic cells, which are frequently replaced because of division and differentiation of stem cells (e.g., intestinal epithelial cells and peripheral blood cells), do not accumulate substantial amounts of waste during their short lifetimes. However, such short-lived postmitotic cells may alter to some extent with organismal age, possibly reflecting changes in stem and progenitor cells, even though their continuous division considerably decreases their intracellular accumulation of waste products.
Recently it was shown that the proliferation potential of stem and progenitor cells decreases with age (218, 219). Because of this deterioration, the efficiency of biologic waste dilution by cell division also decreases in stem and progenitor cells with age, accompanied by the less-frequent replacement of mature short-lived postmitotic cells. It follows that stem cells, previously believed to escape aging, acquire over time some of the properties of aged long-lived postmitotic cells, in particular increased lipofuscin-related autofluorescence, elevated carbonyl content, and enhanced oxidative stress (218, 219). Conceivably, stem and progenitor cells, along with mature short-lived postmitotic cells, may then have to rely on a defective lysosomal compartment, the function of which is hampered by the presence of lipofuscin (see Section VI.B), which affects the turnover of essential structures and macromolecules.
It should be added that stem and progenitor cells are also prone to the accumulation of mutations that are reproduced during cell division and that may result in the development of neoplasms. The majority of tumors thus arise in actively proliferating cell populations that are characterized by relatively high numbers of stem and progenitor cells. Tumor biology is, however, a separate age-related problem and not a subject of this review. A comparison between short-lived and long-lived postmitotic cells is given in Table 1.
Table 1.
Renewal and Age-related Changes of Terminally Differentiated (Postmitotic) Cells with Different Life Spans
| Characteristic | Short-lived postmitotic cells | Long-lived postmitotic cells |
|---|---|---|
| Examplesa | Mature enterocytes, peripheral blood cells | Neurons, cardiac myocytes, skeletal muscle fibers, RPE cells |
| Life span | Short, usually only days | Long, often comparable with that of the whole organism |
| Differentiation | Asymmetric division of stem cells gives rise to new stem cells and progenitor cells that divide sequentially and differentiate into mature cells | Similar to that for short-lived cells, although stem cells are scanty and differentiate rarely (more commonly in response to injury) |
| Regeneration capacity | High, usually associated with complete regeneration | Low, usually associated with incomplete regeneration, resulting in scarring |
| Malignant transformationb | Frequent, apparently due to high content of stem and progenitor cells | Rare, apparently due to low content of stem and progenitor cells |
| Senescent alterations | Minimal. Differentiated cells have a too-short life span to accumulate substantial amounts of damaged structures (waste materials). Stem and progenitor cells do not accumulate damaged structures either, because the latter are efficiently diluted by cell divisions | Pronounced. Differentiated cells have long life spans, resulting in progressive accumulation of waste materials [e.g., lipofuscin, senescent (giant) mitochondria, and aberrant proteins] |
A number of postmitotic cell types, such as mature hepatocytes or fibroblasts, show intermediate renewal and age-related characteristics and, thus, cannot be ascribed to any of the two groups.
Refers to stem and progenitor cells giving rise to differentiated cells.
Taking into consideration the plethora of symptoms that appears at advanced age, including hormonal and immunologic dysfunction, how could a decline in the function and the eventual death of postmitotic cells explain a major portion of those symptoms? Part of the answer to this question may lie in the comparatively small number of commanding neuroendocrine cells in the hypothalamus. By their production of tropic hormones, these postmitotic cells regulate the outflow of a number of secondary-order hormones from the pituitary gland, which in turn regulate a range of tertiary-order hormones from peripheral endocrine glands at the bottom of the pyramid. It is conceivable that the age-related loss of a limited number of commanders at the top of this pyramid could lead to an overthrow of the whole organism. However, further discussion of the hormonal and immune systems and their relation to aging is not within the scope of this review.
Aging may thus be assumed to be, to a large extent, a result of the deterioration of long-lived postmitotic cells due to their limited renewal capacity, even if oxidative damage to the components of connective tissues, which normally are recycled by matrix metalloproteinases (86), also contributes to the aging process. The modification of connective tissue components, making them non-degradable, results from metal-dependent oxidation, or from glycation with secondary Amadori rearrangements into advanced glycation end products (AGEs). In either case, degeneration of the cartilages and ligaments, decreased elasticity of the skin and arteries, and hardening of the lens with resulting presbyopia occur. Furthermore, age-related defects in the remodeling of connective tissues contributes to declines in bone structural integrity, back problems, and the development of arthritic joints (248, 250). Even if these age-related problems are not life threatening, they can be sources of much frustration for the elderly, who may be confronted by them each time they get out of bed, take a look in the mirror, find their blood pressure elevated, or try to read a book without glasses. Hence, the recent finding that the elasticity, at least partly, may be restored by agents that break cross-links, including those of AGEs (268), may be of great clinical significance.
How does oxidation damage postmitotic cells? Basically, as long as oxidative injuries can be properly repaired, no axiomatic need exists for such damage to occur. All cells, postmitotic ones included, are fantastic self-repairing machines that turn over and reuse the building blocks of their macromolecular constituents. However, the occurrence of age-related damage implies that the cellular renewal mechanisms are not perfect (i.e., not all damaged structures are being removed and, as a result, they gradually accumulate in the cell). The decline in vigor seems significantly to accelerate at old age, suggesting that the defective turnover and repair of damaged structures, preventing successful rejuvenation, progresses with age.
It is known that aging is characterized by the increasing accumulation in long-lived postmitotic cells of dysfunctional, usually enlarged (sometimes called giant) mitochondria, lipofuscin-loaded lysosomes, and oxidatively modified cytosolic proteins and lipids. Damaged proteins often accumulate in the form of indigestible aggregates, termed aggresomes (92, 215). Since the emergence of the oxidative stress or free radical theory of aging, such alterations have been considered the result of a gradual accumulation of oxidatively injured macromolecules. Some other theories, such as the somatic mutation theory of aging (33, 54) and the error catastrophe theory (175), emphasized instead the role of the erroneous synthesis of macromolecules in aging. Later studies, however, did not show any substantial increase in the occurrence of synthetic errors with age (83, 97). Although somatic mutations do accumulate, they cannot explain the variety of changes associated with aging (120). Apparently, the role of somatic mutations is mostly restricted to the increased frequency of malignant neoplasms with age (see earlier).
Because damaged structures obviously would not accumulate if they were being perfectly removed, it can be reasoned that it is not the formation of dysfunctional and oxidized proteins and lipids that creates all the multifaceted problems that exist for aged long-lived postmitotic cells, but rather the malfunction of catabolic enzymes, such as the cytosolic proteasomes and calpains and the host of lysosomal enzymes that cannot completely degrade damaged structures. With this line of reasoning, aging, together with a number of neurodegenerative diseases, is starting to be considered a catabolic disorder.
Having provided a general description of the current understanding of aging, this review now focuses on mitochondrial and lysosomal features of importance for aging, the interplay and cross-talk between mitochondria and the lysosomal compartment, and summarizes the evidence behind the mitochondrial–lysosomal axis theory of aging (30). In essence, this hypothesis suggests that depressed macroautophagy secondary to the accumulation of lipofuscin inside the lysosomal compartment results in prolongation of mitochondrial life span with accumulation of enlarged functionally effete mitochondria, and ensuing decline in ATP production, increased formation of ROS, accelerated formation of lipofuscin and, finally, lysosomal labilization with activation of the apoptotic or necrotic pathways. These alterations at the cellular level inevitably lead to progressive functional decline, decreased adaptability, and an increased probability of disease and death for the organism.
II. ROS, Mitochondrial Damage, and Aging
A. Biomolecular damage under normal conditions
Soon after the important discovery that ROS, including the superoxide anion radical (O2•–) and hydroxyl radical (HO•), both of which are short-lived with half-lives of 10−6 and 10−9 seconds, respectively, form within living cells as a consequence of normal respiration (94), Denham Harman (98) postulated that biologic aging (senescence) occurs because of the accumulation of oxidatively damaged macromolecules. This theory, called “the free radical theory of aging,” although initially poorly accepted, has gathered an increasing number of followers over time as more supporting evidence has been presented. Today, the role of free radicals as important contributors to aging is considered most likely, and extensive studies on various biologic species ranging from yeast to humans are in support, although a final confirmation is still lacking (10, 87, 167, 200, 213). The free radical theory of aging, which points to an intrinsic mechanism underlying age-related molecular damage, does not in any way exclude that other factors may also be involved in the aging process (e.g., evolution, somatic mutations, errors in protein synthesis, accumulation of waste products, neuroendocrine and immunologic disturbances). The possibility that many mechanisms may contribute to the aging process is reflected in the existing numerous theories of aging (some of them having only historic value), which are systematized in a number of reviews (156, 187, 255).
The process of cellular respiration is tightly associated with the electron-transport chain and the transfer of electrons from substrates (e.g., NADPH from complex II) to the final acceptor (molecular oxygen) in complex IV. The electron transport is associated with translocation of protons from the mitochondrial matrix to the mitochondrial intermembrane space, which originates a membrane potential. This potential is coupled with phosphorylation of ADP to form ATP at complex V. Both free radicals and other ROS form continuously because of unavoidable electron leakage from mitochondrial complexes during electron transport and reductive one-electron transfer processes in the cytosol. The addition of one electron per oxygen molecule yields the superoxide anion radical, O2•–, which in itself, or after dismutation to hydrogen peroxide, may be toxic to some enzymes, particularly mitochondrial aconitase (261). Most superoxide is, however, converted to hydrogen peroxide by superoxide dismutases (SODs). Mammalian cells contain cytosolic Cu, Zn-SOD, whereas mitochondria contain Mn-SOD in their matrix as well as Cu, Zn-SOD in their intermembranous space (81). An extracellular form of SOD exists. Although the dismutation of superoxide to hydrogen peroxide is by itself a very rapid spontaneous process, catalysis by SODs increases the rate of superoxide dismutation to hydrogen peroxide and oxygen ∼1,000-fold. Once hydrogen peroxide is formed, it is rapidly transformed into water. In the mitochondrial matrix, this takes place mainly by the peroxiredoxin/thioredoxin system (267) and, to some degree, by glutathione peroxidase, whereas glutathione peroxidase and catalase work in concert in the cytosol to degrade hydrogen peroxide.
Hydrogen peroxide is an important signaling molecule that regulates most cytosolic redox activity (220). However, if not eliminated, it can also react with Fe(II) during Fenton-type reactions, resulting in the formation of the very reactive hydroxyl radical. In addition, superoxide can directly reduce Fe(III) to Fe(II), which further contributes to the creation of HO• or the likewise reactive ferryl or perferryl radicals. All of these radicals attack surrounding biomolecules (i.e., nucleic acids, proteins, and lipids) at their very place of formation (i.e., in direct relation to Fe(II) catalysis), resulting in damage to biomolecules with attached low-mass iron (94). Although most of the hydrogen peroxide is eliminated by glutathione peroxidase and catalase, some of it remains and may diffuse for some distance (e.g., to the lysosomal compartment, which lacks hydrogen peroxide–degrading enzymes). Because lysosomes not only lack these enzymes, but also are rich in reactive iron as a consequence of the degradation of ferruginous materials (see Section VI.A), the formation of these radicals takes place mainly inside these organelles. This may result in lysosomal rupture, followed by damage to cytosolic structures as well as to nuclear and mitochondrial DNA as a result of the relocation of redox-active iron and hydrolytic enzymes (64, 127).
It may be assumed that the reason nature has found it necessary to speed up by 1,000 times the already rapid spontaneous dismutation of superoxide to hydrogen peroxide is that the capacity of superoxide to reduce Fe(III) to Fe(II) is a very dangerous one, allowing the formation of hydroxyl radicals if hydrogen peroxide is available (see earlier). Basic metabolic pathways involved in ROS production are schematically presented in Fig. 1.
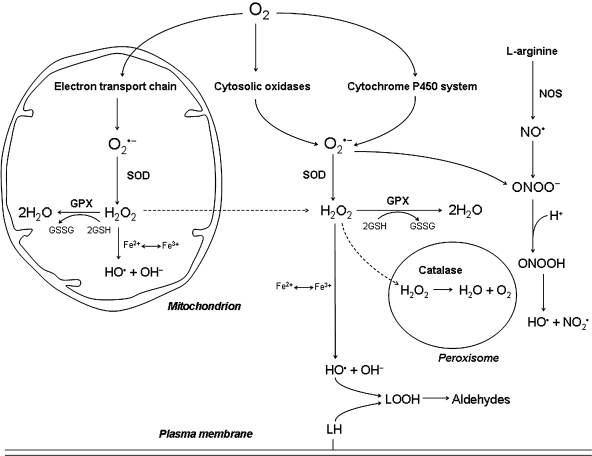
FIG. 1.
Metabolic pathways involved in the production of cellular ROS. Superoxide anion radicals (O2•−) are produced mainly in mitochondria as a result of electron leak from the electron-transport chain and to a lesser extent in the cytosol, because of the activity of one-electron transfer oxidases and the cytochrome P450 system. Superoxide rapidly dismutates spontaneously to hydrogen peroxide (H2O2), but this reaction is further increased 1,000-fold by mitochondrial and cytosolic forms of superoxide dismutase (SOD). This indicates that superoxide is a dangerous molecule, probably because of its capacity to reduce Fe(III) to Fe(II). Hydrogen peroxide, an uncharged molecule, diffuses freely within the cell. Most hydrogen peroxide is eliminated by cytosolic and mitochondrial glutathione peroxidase (GPX), as well as by catalase in peroxisomes. In the presence of redox-active iron, hydrogen peroxide is homolytically cleaved under the formation of highly reactive hydroxyl radicals (HO•; the Fenton reaction). Hydroxyl radicals can damage a variety of biomolecules, including nucleic acids, proteins, and lipids. By reacting with polyunsaturated fatty acids, they initiate a chain reaction, resulting in the formation of aldehydes that can cause additional macromolecular damage. The reaction between superoxide and nitric oxide (NO•, formed from l-arginine in the presence of nitric oxide synthase, NOS), produces peroxinitrite (ONOO−), which can generate a hydroxyl radical at acidic pH (e.g., in the lysosomal compartment). This possibility is provided by the fact that nitric oxide (which is uncharged and thus passes biologic membranes) can diffuse into the lysosomes, where it may react with superoxide derived from autophagocytosed mitochondria that are under degradation. Continuous arrows, transformation; dashed arrows, diffusion of substances.
Although ROS formation apparently is the main source of oxidative damage, it is not the only one. Another important damaging mechanism is glycation [i.e., a reaction of glucose and other reducing sugars with protein amino groups, resulting in the formation of advanced glycation end products (AGEs)]. AGEs, which can bind to DNA and proteins, may in turn induce mutations and protein–protein cross-linking. The latter phenomenon is of special importance extracellularly and can cause stiffening of elastic tissues in the skin, vessels, and the eye lens (25, 82, 134). In addition, the reactive metabolite, S-adenosylmethionine, can methylate guanine, affecting the hydrogen-bonding ability of DNA bases.
Furthermore, because of their inherent instability, many macromolecules can undergo spontaneous modifications (not caused by oxidation or glycation), such as DNA-strand breaks and depurination, deamination of DNA bases, isomerization, racemization, and deamidation of protein amino acid residues, or dephosphorylation of phosphoproteins [reviewed in (102)].
B. Imperfect turnover of damaged biologic structures
Oxidatively or otherwise damaged biologic structures are either repaired (e.g., single bases in DNA molecules are replaced) or degraded and completely replaced by newly synthesized structures, as is the case for proteins, organelles, and whole cells. Proteins, predominantly short-lived ones in the nucleus and cytosol, are degraded mainly by calpains and proteasomes, whereas most long-lived proteins and all organelles are digested in the lysosomal compartment in the process called autophagy, or autophagocytosis (49, 265). It has long been known that proteins intended for degradation by proteasomes have to be tagged by ubiquitin, but it is now recognized that some ubiquitinized proteins also are degraded by autophagy and that the proteasomal and lysosomal systems for degradation can compensate for each other (177, 242). Mitochondria possess their own proteolytic system, which includes Lon, Clp-like proteases, and AAA proteases (see Section IV). Irreversibly damaged cells are removed by self-killing programs, including apoptotic (caspase-dependent) programmed cell death (PCD-I), autophagic cell death (PCD-II), or, occasionally, necrosis (PCD-III) (71). Cellular catabolic pathways are summarized in Table 2.
Table 2.
Cellular Degradation Processes
| Degradation process | Location | Enzymes involved | Targets |
|---|---|---|---|
| Cytosolic proteolysis | Cytosol | Calpains, proteasomes | Cytosolic proteins (mainly short-lived) |
| Mitochondrial proteolysis | Mitochondria | Lon, Clp-like, and AAA proteases | Mitochondrial proteins |
| Autophagy (macroautophagy, microautophagy, and chaperone-mediated autophagy) | Lysosomes | Acid hydrolases | All cytosolic macromolecules and organelles |
| Programmed cell death (PCD) | Whole cell | Effector caspases, lysosomal cathepsins, and endonucleases in PCD-I (classic apoptosis); acid hydrolases in PCD-II (autophagic cell death) and PCD-III (programmed necrosis) | All cellular components |
The renewal of long-lived postmitotic cells, which are poorly (or not at all) replaced through division and differentiation of stem cells, is practically fully dependent on intracellular degradation pathways. As pointed out earlier, the latter do not function perfectly and, as a result, damaged structures (such as defective mitochondria, lipofuscin-loaded lysosomes, and oxidized proteins) progressively accumulate in time, resulting in a diminished amount of normal cellular structures. This will make the function of the cells less efficient and decrease their adaptability. The accumulation of biologic garbage also is associated with certain toxic effects, such as increased ROS production by senescent mitochondria, or enhancement of oxidative stress with release of lysosomal enzymes by lipofuscin-loaded lysosomes (see Section VI). These changes result in progressive functional decline of postmitotic cells, such as neurons, cardiac myocytes, and skeletal muscle fibers, making an aged organism fragile and unable to withstand stress. The lack of robustness that characterizes the aged individual is thus reflected on the cellular level.
Consistent with the idea that aging is largely dependent on the ultimate degeneration of long-lived postmitotic cells, a primitive cnidarian animal, Hydra vulgaris, has been shown to escape aging for 4 years in a controlled laboratory environment (150). The most plausible explanation for the absence of aging in hydra is that this animal, as well as other cnidarians, totally lacks long-lived postmitotic cells. All cells of cnidarian animals are continuously replaced through the division and differentiation of interstitial stem cells. Interestingly, all higher animals, which evolved later than cnidarians, contain postmitotic cells, and therefore have limited life spans. It is possible that the appearance of long-lived postmitotic cells, in particular, long-lived neurons, came along evolutionarily because this trait was associated with certain advantages, providing for better evolutionary fitness. Cnidarians are known to possess a primitive nervous system, consisting of a network of dissociated short-lived neurons. These animals can react only nonspecifically on external stimuli and do not develop conditioned responses. In contrast, higher animals have a more-developed nervous system, consisting of long-lived postmitotic neurons, allowing conditioned responses, and consequently, providing better adaptation to their environment. Apparently, the presence of long-lived neurons promoted the development of long-term memory, associated with conditioning. The price for this better evolutionary fitness was, however, a limited life span because of unavoidable postmitotic cellular aging. This hypothesis is described in detail elsewhere (234).
C. Major targets of ROS attack: mitochondria and lysosomes
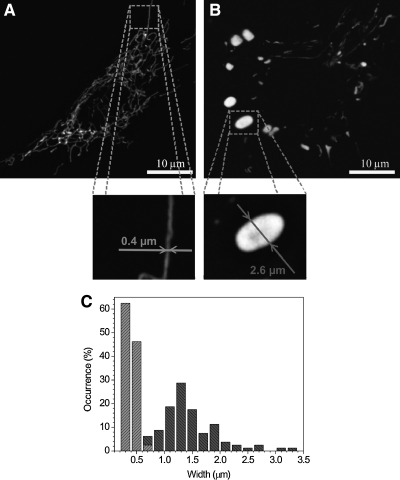
As the main sites of endogenously generated ROS, mitochondria are the logical and major targets of ROS attack, which, in combination with the insufficient degradation and replacement of damaged mitochondria, results in their pronounced alterations with age. The mitochondria of aged postmitotic cells are usually enlarged, sometimes enormously so (and are occasionally called giant mitochondria), and are functionally insufficient (see also Section VII). Abnormal mitochondria of aged cardiac myocytes are shown in Figs. 2 and 3.
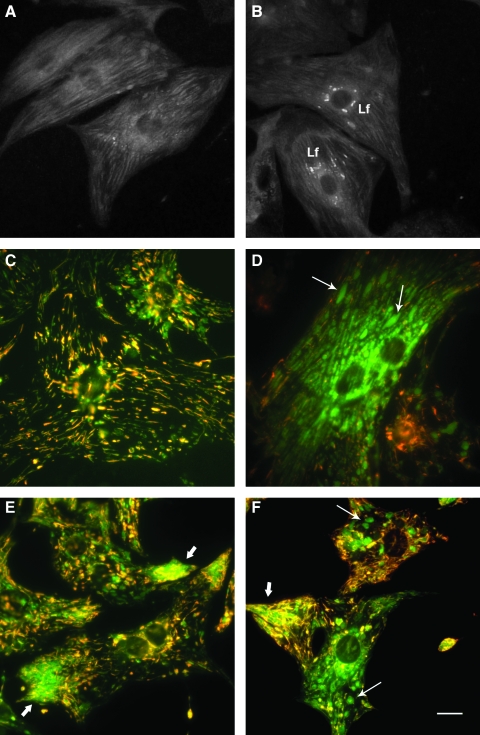
FIG. 2.
Lipofuscin accumulation and mitochondrial damage in neonatal rat cardiac myocytes. (A, B) Confocal laser scanning images (488-nm excitation) of formaldehyde-fixed cells aged 1 and 4 weeks, respectively. Lf, lipofuscin granules. (C, D) Fluorescence microscopy (blue excitation) of cardiac myocytes (aged 17 days and 3 months, respectively) vitally stained with mitochondrial tracker JC-1. Note the abundant enlarged “green” mitochondria with low membrane potential (thin arrows) and a lesser amount of slender “red” mitochondria with normal membrane potential in (D) versus (C). (E, F) The 17-day-old cells, exposed to autophagy inhibitor 3-methyladenine for 12 days, contain some enlarged mitochondria (thin arrows), as well as prominent aggregates of small mitochondria (thick arrows), many of which show a low membrane potential. Bar, 10 μm.
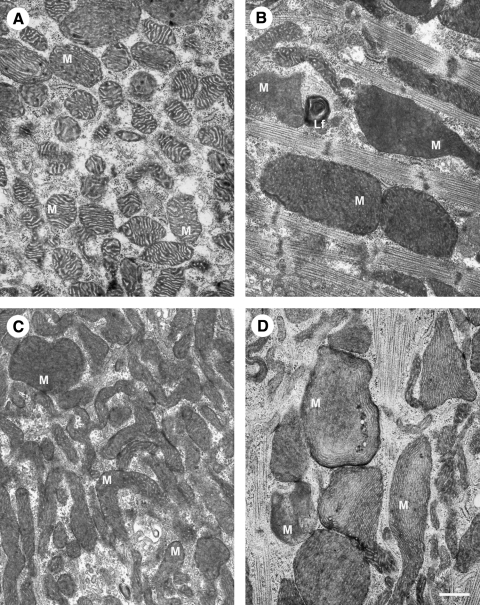
FIG. 3.
Ultrastructural mitochondrial changes associated with aging and inhibition of autophagy. (A, B) Electron microscopy images of neonatal rat cardiac myocytes cultured for 1 and 4 weeks, respectively. The aged cells contain enlarged (giant) mitochondria with irregular cristae and dense matrix. (C, D) The 17-day-old cardiac myocytes, exposed to 3-methyladenine for 12 days, accumulate numerous small, as well as some large senescent-like mitochondria (compare with Fig. 2). M, mitochondria; Lf, lipofuscin. Bar, 500 nm.
The role of mitochondria in aging is reflected in the refinements of the free radical theory of aging that stresses the importance of mitochondrial ROS production and oxidative damage to mitochondrial components in the overall role of ROS in aging (57, 99). Mitochondrial proteins are affected not only because of direct oxidative damage, but also as a consequence of oxidant-induced mutations in the mitochondrial DNA (mtDNA) that codes for 13 proteins involved in oxidative phosphorylation (53). Some changes in mitochondrial proteins arise from damage to the nuclear DNA. These changes would apparently affect all cellular mitochondria, whereas mtDNA mutations would change only a portion of the mitochondria, those mitochondria with clonally expanded mutated DNA (see Section VII.C). It is believed that the properties of mtDNA, which is a circular bacterial type not protected by histones, probably has a poor repair capacity compared with nuclear DNA, thereby contributing to its high vulnerability to ROS (176). Although recent findings suggest that the repair mechanisms of mtDNA are more advanced than originally thought (66), age-related damage to mtDNA is well established (176).
Homozygous knockout mice expressing defective mtDNA polymerase provide strong evidence for the role of mitochondrial damage in aging (244). These animals show a dramatically increased rate of mtDNA mutations and are characterized by premature development of age-related phenotypic alterations of various organs, as well as by reduced life span. A later publication from the same group (243) showed that these prematurely aging mice having defective mtDNA polymerase produce normal amounts of ROS, which seems to be in opposition to the free radical theory of aging. However, considering that, in normal aging, damage to mtDNA occurs secondary to ROS production, it is predictable that the induction of mitochondrial damage in a different way (e.g., by disturbing the function of mtDNA polymerase) may as well result in the development of senescence-like alterations. It should be also kept in mind that because oxidatively damaged structures (including defective mitochondria) accumulate because of insufficient clearance mechanisms, the increase of oxidative stress is not a necessary requirement for aging to occur. Damaged structures would accumulate anyway, although with a lower rate, at constant or even decreasing levels of oxidative stress.
Recently, the mitochondrial oxidative stress theory of aging has been somewhat challenged by the finding that Mclk1+/− mice, with a reduced activity of a mitochondrial enzyme necessary for ubiquinone synthesis, were characterized by increased hydrogen peroxide production and elevated protein carbonyl levels (indicative of protein oxidation) in hepatocyte mitochondria, but still lived longer than the wild-type animals (133). The Mclk1+/− mutants, however, showed reduced carbonyl and isoprostane levels in the nonmitochondrial cytoplasmic compartments, suggesting decreased oxidative damage to proteins and lipids. The positive changes in the nonmitochondrial part of the cytoplasm, probably involving lysosomal proteins and lipids, may to some extent explain this paradoxic finding. Another possible explanation of these results may be that an enhanced production of mitochondrial ROS induces upregulation of stress proteins, such as HSP70, which, after autophagy, reduce the concentration of lysosomal redox-active iron. This in turn would depress lysosomal formation of lipofuscin and prevent failing autophagy and reduced cellular “self-cleaning” (126, 128, 129) (see Section VI.A of this review). It should be pointed out that the importance of these-mentioned results for the understanding of the free radical theory of aging is diminished by the fact that the oxidative-stress parameters were assessed in hepatocytes, which are much less affected by age than are long-lived postmitotic cells, such as cardiac myocytes and neurons.
It should be also mentioned that mitochondria are involved in the synthesis of heme (including that of the mitochondrial inner membrane protein cytochrome c) and most iron–sulfur clusters (199). It is possible that some mitochondrial iron is in reactive form and able to support Fenton-type reactions. If so, the combination of redox-active iron with internally formed ROS would contribute to mitochondrial damage.
For the same reason, lysosomes are also sensitive to oxidative stress, leading to a gradual accumulation of the intralysosomal indigestible material, lipofuscin, paralleled by a decline in the lysosomal degradative function (30). However, compared with the generally accepted role of mitochondrial decay in aging, the roles of lysosomal malfunction and the cross-talk between lysosomes and mitochondria in aging remain less recognized.
The progress of age-related mitochondrial degeneration is, to a large extent, dependent on the failure of mitochondrial-turnover mechanisms, including (a) mitochondriogenesis or the generation of more mitochondrial mass, (b) mitochondrial fusion and fission, (c) the monitoring of protein folding and assembly by molecular chaperones and energy-dependent proteases, and (d) the removal of severely damaged mitochondria by autophagy (119). These mechanisms, as well as their age-associated malfunction, are described more in detail later.
III. Mitochondrial Fusion, Fission, and Biogenesis
A. The role of mitochondrial dynamics
Mitochondria are dynamic organelles that are continuously fusing and dividing (Fig. 4). The harmonious balance of these two opposing processes is responsible for the prevailing morphologic features of mitochondria, their distribution, inheritance, and function (61). When the mitochondrial fusion is blocked, the normal tubular network of mitochondria transforms into fragmented mitochondria (40, 41, 90, 173), whereas the blocking of the opposing process, mitochondrial fission, results in elongated, interconnected mitochondrial tubules (211, 217, 263). Likewise, the overexpression of fusion proteins, such as mitofusin 2 (Mfn2), results in the formation of large mitochondria or long mitochondrial tubules, whereas the overexpression of fission proteins, such as the dynamin-related protein 1 (Drp1) or mitochondrial fission protein 1 (Fis1), results in the formation of small fragmented mitochondria (179).
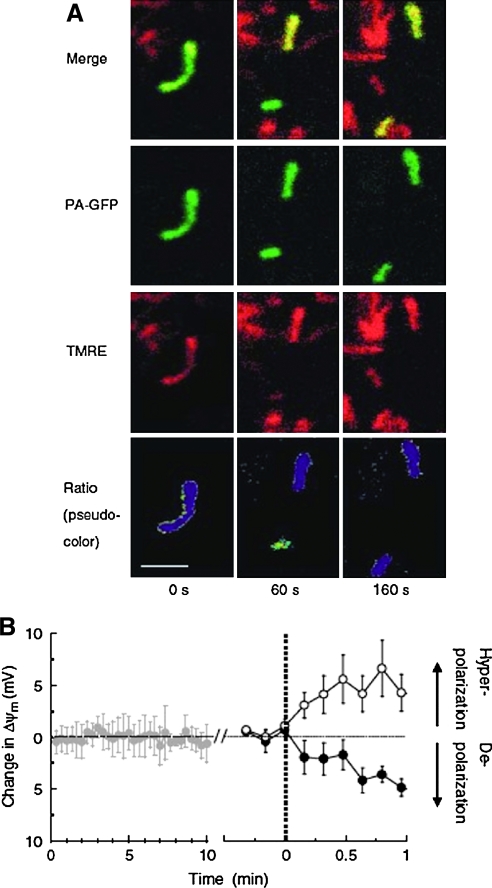
FIG. 4.
Mitochondrial fission leads to two daughter mitochondria with different membrane potentials. (A) GFP fluorescence identifies mitochondria. TMRE (tetramethylrhodamine ethyl ester) is used to calculate the membrane potential. The pseudocolor images at the bottom are used to identify the initial and daughter mitochondria. (B) Average membrane potential before (left, gray) and after fission (right, solid and empty circles denote depolarized and hyperpolarized mitochondria, respectively) are shown. Reprinted from Twig et al. (246), with permission from Macmillan Publishers Ltd. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
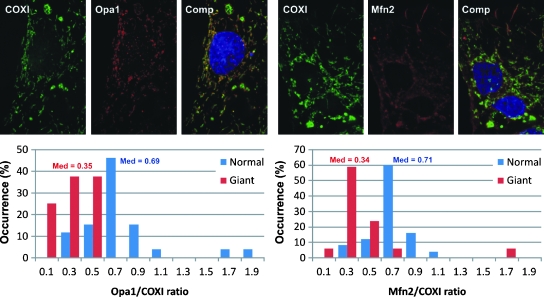
Once the delicate balance between fusion and fission is lost, not only is the morphology altered, but various mitochondrial and cellular functions are changed as well. When fusion is decreased, as in Mfn-null or OPA1 (optic atrophy protein 1)-depleted cells, fragmentation of the mitochondrial network is followed by reduced glucose oxidation, decreased mitochondrial respiration, and diminished mitochondrial membrane potential (179). This process is reversible if respiration can be restored by the reintroduction of the affected proteins (40, 42).
Mitochondrial fusion and fission is also intimately associated with mitochondria-mediated apoptosis. (178). At least two proteins involved in mitochondrial fusion appear to protect cells from apoptosis (112) by controlling the remodeling of mitochondrial cristae (47, 80). Mitochondrial fission resulting from the overexpression of Drp1 protects the cells against Ca-mediated apoptosis by interrupting intramitochondrial Ca2+ waves and reducing overall mitochondrial Ca2+ uptake (228). Conversely, a different fission protein, hFis1 (human homologue of Fis1), seems to promote apoptosis (108, 140).
Last, mitochondrial fusion and fission is related to the energetic sources of the cell. When oxidative phosphorylation is compromised (e.g., when glycolysis is the main source of ATP), mitochondria appear punctuated or vesicular. Conversely, when glycolysis is blocked, tubular mitochondria form (16). These observations do not seem to conform to the findings that senescent cells accumulate enlarged mitochondria with decreased inner membrane potential and consequent decreased ATP production (169). Apparently, mechanisms that control mitochondrial morphology depending on cellular energetic status are different from those involved in age-related mitochondrial alterations (see Section VII). One should not underestimate the intimate relation between mitochondrial structure and bioenergetics. This topic is reviewed in an excellent way by Benard and Rossignol (16).
In this section, we discuss mitochondrial fusion, fission, and biogenesis, basic processes involved in mitochondrial dynamics, dysregulation of which may be responsible for changes in mitochondrial turnover that accompany aging (see further Section VII). Mechanisms of mitochondrial fusion and fission are schematically presented in Figs. 5 and 6.
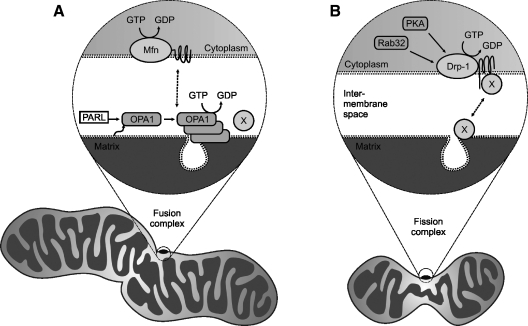
FIG. 5.
Schematic models of mitochondrial fusion and fission. (A) Mitochondrial fusion. The outer membrane protein, Mfn, and the inner-membrane protein, OPA1, regulate mitochondrial fusion in mammalian cells. PARL cleaves the transmembrane domain of OPA1 and activates it, which may result in oligomerization and assembly in large complexes responsible for mitochondrial remodeling and cristae junction formation. (B) Mitochondrial fission. Drp-1, the key component of mitochondrial fission, is localized in the cytoplasm, but may be translocated to the mitochondrial outer membrane, triggered by an unknown signal, where it binds to other proteins and forms large circular complexes. These complexes then send signals to and from the inner membrane to coordinate the fission of both membranes, which is believed to be regulated by Rab32 and PKA. X, unidentified factors involved in fusion and fission. A complete description of the processes is given in (20).
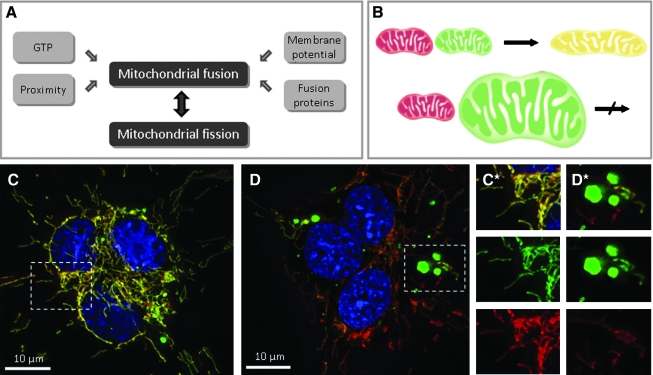
FIG. 6.
Mitochondrial fusion in mammalian cells. (A) Mitochondrial morphology is controlled by two opposing processes, fusion and fission. For mitochondrial fusion to occur, mitochondria must be in close contact. It also requires mitochondrial fusion proteins (see Fig. 5A for details), functional mitochondrial inner membrane potential, and low concentrations of GTP. (B) Fusion of two mitochondria labeled with different fluorescent proteins (e.g., GFP and DsRed2) results in the formation of a single mitochondrion with intermixed mitochondrial contents of the parent mitochondria. Giant mitochondria do not appear to fuse with normal mitochondria or with each other. (C) A fluorescence-microscopy image of a polykaryon formed by fusion of cells containing mitochondria labeled with GFP and DsRed2. Four hours after fusion, most mitochondria have fused with others and exchanged their mitochondrial matrix components, containing both fluorescent labels appearing as a yellow color in the composite image. The nuclei were counterstained with DAPI (blue). (D) A cell containing giant mitochondria labeled with mitochondria-targeted GFP were fused with other cells containing normal mitochondria labeled with DsRed2. Giant mitochondria remain single-labeled even 8 h after fusion, whereas normal mitochondria show colocalization of both fluorescent probes. (C*, D*) Enlarged sections of the images (C) and (D), respectively, with both the composite image and green and red components of the same image. Reprinted from Navratil et al. (169) with permission from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
B. Mitochondrial fusion
Mitochondrial fusion resembles virus-mediated fusion or SNARE-dependent (soluble N-ethylmaleimide–sensitive factor-attachment receptor) membrane fusion, but is unique in the sense that it requires the coordinated fusion of the inner and outer mitochondrial membranes and is more complicated than was initially thought (38).
The core components of the mitochondrial fusion machinery were first identified in Drosophila and yeast. Fuzzy onion (Fzo) protein and its yeast counterpart Fzo1 were the first proteins shown to play a role in mitochondrial fusion (93). This discovery ignited studies of mitochondrial fusion and fission in mammalian systems, in which the key players are proteins with a largely conserved genetic background, work that resulted in the identification of several mammalian orthologues. Fusion proteins in mammals are typically large GTPases localized in the mitochondrial membranes. Figure 5A illustrates key players in the fusion process. For example, Mfn1 (mitofusin 1) and Mfn2 are closely related mammalian homologues, which play an important role in the fusion of the outer mitochondrial membrane (40, 41). They contain long transmembrane domains with both the C and N termini protruding from the outer membrane into the cytosol and require low levels of GTP and mitochondrial membrane potential for mitochondrial fusion to occur (195). Although Mfn1 is believed to be involved primarily in mitochondrial tethering, Mfn1 and Mfn2 play similar roles and can, under certain conditions, function interchangeably. Cells underexpressing Mfn1 can restore their mitochondrial fusion activity by overexpressing Mfn2, and vice versa. It has been shown that the requirements for the expression of either of the mitofusins are tissue dependent (104).
OPA1 is another member of the mitochondrial-fusion protein family, and it was initially identified as a protein involved in dominant optic atrophy, an autosomally inherited disease resulting in vision loss (2). OPA1 is localized in the intermembrane space and is associated with the inner mitochondrial membrane (173). The role of OPA1 remains largely unknown, but it may be directly involved in inner membrane fusion, controlling the shape and structure of cristae. Overexpression of OPA1 can result in either mitochondrial fragmentation or elongation, depending on the experimental model used (40). It also can cause mitochondrial fragmentation while retaining its fusion activity, making it unique among mitochondrial fusion proteins. These clues point to the special role that OPA1 plays in mitochondrial fusion. It has been suggested that regulation of the OPA1 function may be due to posttranslational modifications of this protein or to the regulation of its function by mitochondrial proteases (38).
Impaired fusion capacity of depolarized or otherwise damaged mitochondria has been reported (106, 141, 147, 152, 157, 159). It is, then, not surprising that such mitochondria often show OPA1 degradation, which probably underlies an impaired fusion and any subsequent exchange of mitochondrial components (69, 105, 141, 158, 214). A decreased OPA1 expression is one of the apparent reasons for the inability of senescent-like enlarged mitochondria to fuse and exchange their contents with normal mitochondria (see Section VII). Fusion may be necessary for maintaining and restoring mitochondrial function by facilitating the stochastic redistribution of soluble and membrane components of normal and defective mitochondria (38, 41, 61, 174). Indeed, clear evidence indicates that the mixing of mitochondrial components occurs after fusion (6, 34, 113). However, the ability of mitochondria to fuse may be hampered above a certain threshold of mitochondrial damage, suggesting that mitochondrial defects, including age-related ones (Section VII), cannot be completely eliminated by fusion with functionally normal mitochondria.
C. Mitochondrial fission
Mitochondrial fission is the functional counterpart of fusion, but very little is known about its underlying molecular mechanisms. Drp1 is the key component in this process (see Fig. 5B). It is expressed largely in the cytosol, but also forms punctuate expression foci in the inner mitochondrial membrane (211). The punctuate expression of Drp1 determines the location of future fission sites on mitochondrial tubules. As in the case of mitochondrial fusion, many insights into the mechanism of mitochondrial fission have been derived from yeast studies. Drp1 is also a member of the GTPase family, which means that it requires GTP to initiate the constriction of mitochondria at the fission sites. In addition, other cellular processes, such as the organization, division, and distribution of mitochondrial DNA, rely on the functional fission machinery. Fis1 is another component of the fission machinery responsible for the recruitment of Drp1 into mitochondria. Unlike Drp1, it is uniformly expressed in the outer mitochondrial membrane, with most of the protein facing the cytosol (108). Other proteins that are thought to play a role in mitochondrial fission include endophilin B1, mitochondrial protein 18 (MTP18), ganglioside-induced differentiation-associated protein (GDAP), mitochondrial rho- (Miro), and mitochondrial rho-2 (Miro-2) proteins (223). Unlike mitochondrial fusion, fission does not require a regular membrane potential because it can be induced by a collapse of the membrane potential resulting from ATP depletion due to the inhibition of ATP synthase or Na/K ATPase (180).
Fission appears to aid in the elimination of damaged mitochondria, thereby delaying the onset of age-related damage. Recent findings indicate that fission is useful for the segregation of irreversibly damaged, depolarized, fusion-incompetent mitochondria, as well as for their subsequent elimination by autophagy, as is illustrated in Fig. 7 (73, 182). Fission induction due to exposure to an NO donor (50–200 μM S-nitrosocystein) results in fragmentation and the accumulation of mitochondria in autophagosomes (12). Most surprisingly, commonly fusion triggers fission that produces two metabolically different daughter units. Such daughter mitochondria have different membranous structures (12), their DNA is not equally redistributed (6, 12), and one of the two in the pair has a reduced membrane potential. The depolarized daughter mitochondria are less likely to re-fuse and are more likely to become autophagocytosed (246).
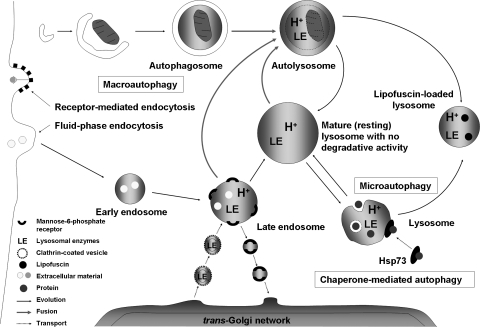
FIG. 7.
Autophagic pathways. The cell takes up extracellular material by invagination of the plasma membrane (endocytosis), thereby forming early endosomes, which mature into acidified late endosomes. The latter receive lysosomal enzymes by fusion with secretory vesicles from the trans-Golgi network (TGN). Further maturation leads to lysosome formation. Cytosolic macromolecules may be directly engulfed by invaginations of the lysosomal membrane (microautophagy), whereas organelles (e.g., mitochondria) are being enclosed by a newly formed phagophore, resulting in the formation of an autophagosome (macroautophagy), which then fuses with either a late endosome or a lysosome (or, perhaps, with secretory vesicles from the TGN), forming an autophagolysosome. Certain proteins are delivered to lysosomes with the help of chaperones, such as Hsp73 (chaperone-mediated autophagy).
Although the molecular mechanisms behind the segregation of defective mitochondrial components through asymmetric fission are in need of further investigation, these important findings have already contributed to the understanding of mitochondrial changes associated with aging.
D. Mitochondrial biogenesis
Mitochondriogenesis, or mitochondrial biogenesis, is a vital process in mitochondrial turnover. It is believed to involve ∼1,000 genes and to affect 20% of the cellular proteins (4, 79, 136, 137, 168, 260). As nonfunctional mitochondria are eliminated by autophagy, mitochondrial biogenesis is needed to sustain energy production and physiological homeostasis in the cell. Factors that regulate mitochondrial biogenesis include the levels of nutrients available, the presence or absence of hormones, temperature, exercise, hypoxia, stress, and aging. Mitochondriogenesis has been shown to decrease with age (see Section VII), contributing to progressive mitochondrial decay (143). We later present a summary of the role of various factors involved in mitochondrial biogenesis.
Thyroid and steroid hormones regulate the expression of the nuclear genes responsible for expression of a large number of mitochondrial proteins. An increase in the levels of the thyroid hormone thyroxine causes hyperplasia and increases the number and mass of mitochondria in liver and cardiac muscle (88, 259). Steroid hormones also affect mitochondrial biogenesis in adipose tissue by either inhibiting (testosterone) or promoting (progesterone) the expression of transcription factors involved in this process (193).
According to López-Lluch et al. (143), the transcription factors involved in mitochondrial biogenesis belong to three groups: ubiquitous transcription factors, nuclear respiratory factors, and coactivators. Among the latter group, the transcription factor most important to mitochondriogenesis is the proliferator-activated receptor γ coactivator-1α (PGC-1α) (143). Although the contribution of the various factors affecting mitochondrial biogenesis cannot be separated at the present time, PGC-1α has emerged as the common intracellular mediator for mitochondrial biogenesis. PGC-1α acts by coordinating and modulating the activity of other transcription factors (e.g., nuclear transcription factors such as the nuclear respiratory factor 1, NRF-1, peroxisome proliferator–activated receptors such as PPARα, and the mitochondrial transcription factor mtTFA) that are involved in mitochondrial biogenesis (184), or by interacting at DNA sites, where it promotes the recruitment of additional coactivators such as the steroid receptor coactivator-1 (SRC-1), which itself alters the DNA structure to make it more available for processing by the transcriptional machinery (183).
The number of known activators of PGC-1α is extensive (143). They include nitric oxide, the cAMP response element–binding protein (CREB), and AMP-activated kinase (AMPK). The latter is a cellular energy sensor that links mitochondriogenesis to several aging-associated processes such as insulin resistance, obesity, and decreased fatty acid catabolism. Caloric restriction (CR), physical exertion, and resveratrol also activate PGC-1α. NO regulates PGC-1α both directly and indirectly (14, 131). Direct regulation by NO occurs through upregulation of transcription factors by cyclic GMP, which in turn leads to the expression of sirtuins [silent information regulation 2 (Sir2) proteins] that deacetylate PGC-1α, making it more active (24, 172). Other compounds such as resveratrol also activate SIRT1 (sirtuin homologue 1) and cause PGC-1α to increase in hepatocyte and myocyte systems (14, 131).
A few reports described negative regulators of PGC-1α. These regulators include RIP140, which interacts with the nuclear receptors (e.g., retinoic X receptor) blocking the interactions of such receptors with PPAR receptors (257). Other negative regulators include p160 myoglobin-binding protein, glucose, GC5N acetyl transferase, and a mutant form of Huntingtin (143). The presence of both positive and negative regulators of PGC-1α is evidence of a complex system that coordinates mitochondrial biogenesis with the energetic demands of the cells.
IV. Mitochondrial Proteolytic Systems
To maintain active mitochondria, cells are equipped with multiple mechanisms for their repair and turnover (see Table 2). As pointed out later, mitochondrial autophagy is a mechanism for getting rid of severely damaged organelles. The advancement of mitochondrial damage can, however, also be lessened by the proteolytic machinery of the mitochondria, involving the ATP-dependent matrix proteases that are subdivided into the Lon (LonA and LonB subfamilies), Clp-like, and AAA proteases. The existence of this proteolytic system extends the half-life of mitochondria, apparently by decreasing demands for the more energy-consuming autophagic degradation.
Only Lon proteases are known to eliminate oxidatively modified proteins (21, 32, 114, 148), whereas the physiologic functions of the Clp-like and AAA proteases are still less well understood. The importance of Lon proteases is demonstrated by the fact that the downregulation of human LonA proteases results in apoptotic cell death within a few days (22), whereas a less-dramatic downregulation results in the accumulation of the same type of large and malformed mitochondria that are normally found in aged postmitotic cells. These findings suggest a strong correlation between aging and Lon protease dysfunction.
The Lon proteases are encoded by nuclear DNA and are composed of three phylogenetically well-conserved domains (107, 164). The N domain interacts, together with the middle one (called AAA+), which also binds ATP, with the protein to be degraded, whereas the third domain (P) contains the active site (3). Interestingly, the Lon proteases are normally bound to mitochondrial DNA and are released and activated by oxidative stress (31, 138, 145). When conditions for oxidative damage to mitochondrial proteins emerge, it is possible that mitochondrial degradation ensues.
Lon-like proteolytic activity has been found to be considerably inhibited or even inactivated in old rats (7) and more so in postmitotic cells than in replicating ones. Mitochondria from old mice contain increased amounts of oxidized proteins, especially aconitase, reflecting an age-related decrease in Lon activity and suggesting that aconitase is easily oxidized by ROS. Interestingly, aconitase inactivation is significantly less pronounced in CR animals and in animals exposed to the CR-mimetic drug resveratrol. This polyphenol, like many other plant-derived compounds with supposed antioxidant activity, is also an iron chelator (authors' unpublished observations), which suggests that it may act by chelating Fe(II) into a complex where iron is nonredox active, thereby preventing the formation of hydroxyl radicals through Fenton-type reactions. The well-known time-dependent accumulations of iron and other heavy metals in postmitotic cells (129) may be an important factor behind the inactivation of Lon proteases that is found in aged individuals. This hypothesis about the toxic effects of accumulated iron is further supported by the mitochondria-revitalization effect of another iron chelator, N-tert-butyl hydroxylamine (252).
V. Mitochondrial Turnover by Autophagy
A. The main functions of the lysosomal compartment
The lysosomal compartment is crucial for cell maintenance and has a variety of important functions, including endocytic uptake of materials from the outside and autophagic degradation of damaged mitochondria and other organelles, such as ribosomes, endoplasmic reticulum, and the proteasome microorganelles, as well as numerous, mostly long-lived, proteins (see Table 2). Consequently, lysosomes exist in all kinds of plant and animal cells, except erythrocytes, which have a very specialized function and a minimal turnover of their constituents. Inside the lysosomal compartment, the degradation of endocytosed or autophagocytosed materials takes place in an acidic environment (pH ∼ 4–5), which is maintained by ATP-dependent proton pumps present in the lysosomal membrane. Such pumps also are present in the plasma membrane, especially in tumor cells, in which anaerobic glycolysis results in significant production of lactic acid (the Warburg effect). These pumps are then needed for the active export of protons to avoid cellular acidification (13, 204, 254). Consequently, many tumor cells, especially the highly malignant and rapidly growing ones, surround themselves with a microenvironment with a low pH.
After synthesis in the endoplasmic reticulum, lysosomal hydrolases are tagged with mannose-6-phosphate (MP) at the cis-Golgi area and then enclosed in transport vesicles (sometimes named primary lysosomes, although they have a neutral pH) in the trans-Golgi network (TGN) with the help of MP receptors. The vesicles containing the newly produced hydrolases are then transported to slightly acidic (pH∼6) late endosomes, which arise from early endosomes containing endocytosed material. The lysosomal hydrolases are then activated when they release MP receptors that are recirculated to the Golgi apparatus. Finally, the late endosomes mature to lysosomes that lack MP receptors, are rich in acid hydrolases, have a pH of 4–5, and contain material to be degraded.
The acidic lysosomal compartment contains a wide spectrum of hydrolytic enzymes, which play a major role in the intracellular recycling of proteins, polysaccharides, phospholipids, and other biomolecules. Lysosomal proteases (cathepsins) are apparently the most important group of these enzymes. Lysosomal cathepsins can be categorized as cysteine (cathepsins B, C, F, H, K, L, O, S, V, W, and X), aspartic (cathepsins D and E) and serine (cathepsin G) proteases (75, 125, 245). They have their pH optima ∼5, although several of them remain active in a neutral milieu, in a timeframe varying from minutes (cathepsin L) to hours (cathepsin S) (65).
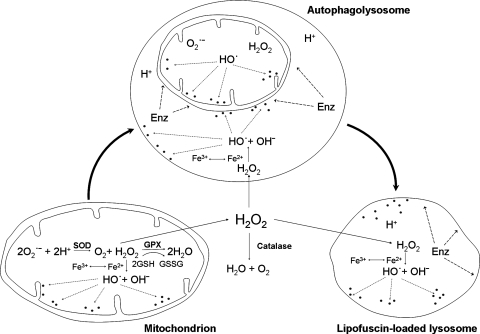
Lysosomes fuse with autophagosomes/endosomes to form “hybrid” organelles containing material in the course of degradation that originates both from the outside and inside of the cell. After completed degradation of the enclosed material, lysosomes turn into “resting” organelles, which in the electron microscope look homogeneous and moderately electron dense. They are then ready for new rounds of fusion. The pronounced fusion and fission activity that is such a typical characteristic of the lysosomal compartment (146) allows lytic enzymes and other lysosomal contents to be distributed between different lysosomes (Fig. 7).
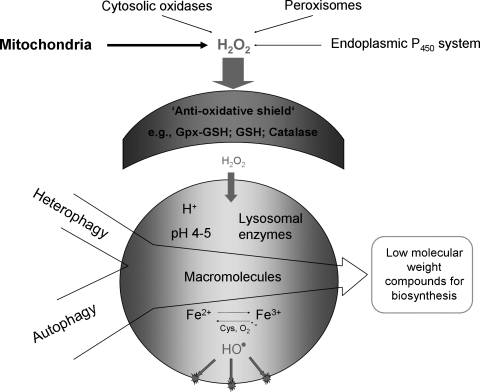
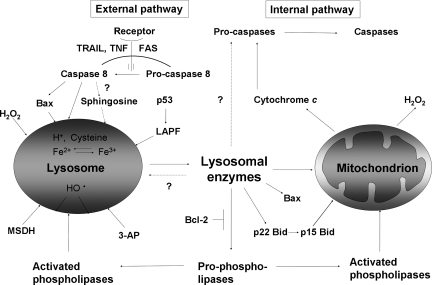
Because of autophagy of iron-containing macromolecules, such as ferritin and mitochondrial complexes, the lysosomal compartment is rich in iron (Fig. 8) that partly exists in redox-active form, making lysosomes sensitive to oxidative stress through intralysosomal Fenton-type reactions (this section). The hydroxyl radicals that form may give rise to peroxidation of material under degradation, resulting in lipofuscin formation (this section) or, if substantial, in lysosomal membrane permeabilization (LMP) (Fig. 9). Lysosomal destabilization, with relocalization to the cytosol of potent hydrolytic enzymes and low-mass iron, is able to induce either apoptosis or necrosis. As is schematically shown in Fig. 10, cross-talking between lysosomes and mitochondria is an important process in apoptosis, and either of these organelles may induce the process. Obviously, a concerted and balanced action of lysosomal cathepsins and cytosolic caspases is required for typical apoptosis, whereas a domination of cathepsins will give rise to necrosis (129).
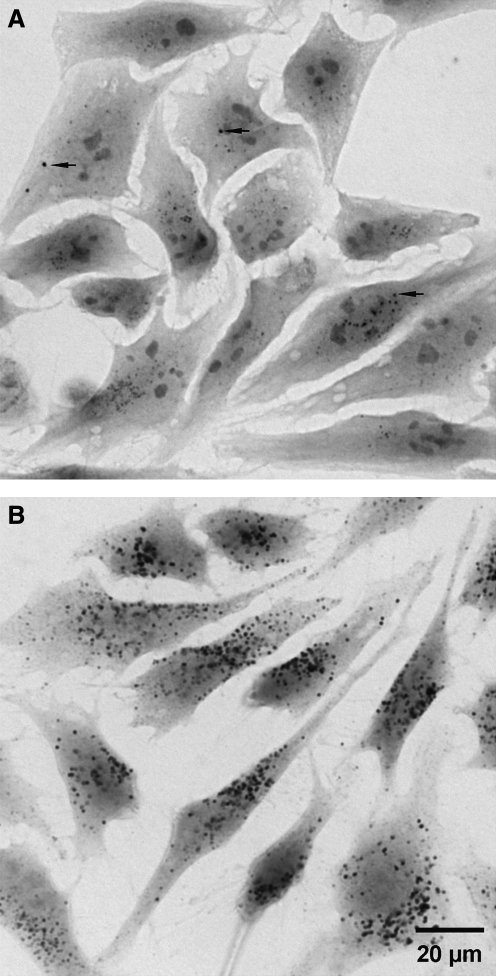
FIG. 8.
Demonstration of lysosomal iron in HeLa cells by using the sensitive cytochemical sulfide silver method. Glutaraldehyde-fixed specimens are exposed to ammonium sulfide at pH∼12 and then developed in a colloid-protected (gum arabic) solution containing silver lactate and the reducing agent hydroquinone. Tiny silver particles precipitate and gradually enlarge to a size visible by light microscopy. The process is akin to physical development of a photographic plate. After a short development time of 25 min (A), only very iron-rich lysosomes are visible (arrows). These lysosomes most probably correspond to autophagolysosomes that are engaged in the degradation of iron-containing material, such as ferrritin or mitochondrial complexes. After 40 min of development (B), a strong general lysosomal pattern is seen, reflecting the fact that most lysosomes contain some low-mass iron.
FIG. 9.
Results of intralysosomal formation of hydroxyl radicals. Hydrogen peroxide is formed normally, mainly from mitochondria. It is efficiently inactivated by the cell's antioxidative shield. Only a small portion of this oxidant manages to diffuse into lysosomes, a compartment rich in cystein and redox-active iron, the latter originating from the degradation of a variety of iron-containing proteins. Hydrogen peroxide and iron react in the Fenton reaction, yielding hydroxyl radicals. This process gives rise to intralysosomal oxidation/peroxidation with resulting damage to the lysosomal membrane and macromolecules undergoing autophagic degradation. Some oxidation products polymerize and become undegradable (lipofuscin) and accumulate in lysosomes of long-lived postmitotic cells, which do not dilute the pigment by division.
FIG. 10.
Lysosomal–mitochondrial cross-talk. Lysosomes are involved in the external as well as in the internal apoptotic pathway. In the external pathway, lysosomal destabilization can be mediated by caspase 8, either directly or indirectly, through activation of Bax or by ceramide that is converted into sphingosine, which is a lysosomotropic detergent. P53 can also destabilize lysosomes through the recently discovered LAPF protein. A variety of synthetic lysosomotropic agents (e.g., MSDH or 3-aminopropanal) can labilize lysosomes. Furthermore, the lysosomal membrane can be peroxidized and subsequently ruptured by hydroxyl radicals that originate from Fenton reaction between hydrogen peroxide and intralysosomal redox-active iron. Released lysosomal enzymes can further damage lysosomes either directly or through activation of phospholipases. The internal apoptotic pathway is activated through mitochondrial damage. This could be the result of activation of Bax or Bid, phospholipases, or lysosomal enzymes with subsequent cytochrome c release and the start of the caspase cascade, leading to apoptosis.
After receptor-mediated endocytosis, the initially plasma membrane–bound receptors are often, but not always, returned to the plasma membrane, whereas the ligands are mostly propagated down the lysosomal compartment. One exception to this “rule of ligand degradation” is transferrin, which is returned to the plasma membrane together with its receptor, whereas the iron which is bound to transferrin is released into the late endosomes because of their acidic environment (pH∼6) and transported to the cytosol by transport proteins such as Nramp [reviewed in (129)].
The processing and presentation of antigens in immunocompetent cells is dependent on a form of endocytotic–exocytotic activity, whereas autophagic degradation is vital not only for the normal turnover of cellular constituents, but also for the elimination of damaged structures and cytosolic microorganisms that have invaded the cell. Some cell types are able to exocytose lysosomal contents or even intact lysosomes (secretory lysosomes) [reviewed in (146)]. It has been recognized that tumor cells often secrete lysosomal proteases, which, in combination with the previously described acidification of their surroundings that enhances the activity of lysosomal proteolytic enzymes, may help them to infiltrate and metastasize (58, 144, 151, 165, 181, 189, 249). The acidic microenvironment around highly malignant and rapidly growing tumor cells may be used in tumor therapy. Drugs (for example, weak acids) that are deprotonated in the neutral environment of normal cells, and consequently charged, are less likely to pass the plasma membrane of normal cells. Conversely, the same drug would remain protonated and uncharged in the acidic microenvironment around malignant cells and thus be able to pass through their plasma membranes unhindered. An example of such compounds that specifically enter malignant cells is the weak acid α-tocopheryl succinate (α-TOS). The principle behind the enhanced uptake of this compound and other weak acids by tumor cells that acidify their surroundings can easily be demonstrated by exposing cells in culture to α-TOS at a different pH of the medium. At a slightly acidic pH of the medium (∼6), cells are much more affected than they are at neutral pH (170).
As pointed out earlier, lysosomes fuse with autophagosomes, or deliver part of their content (“kiss-and-run”), to form autophagolysosomes (Fig. 7). Here a variety of organelles and proteins are degraded into their building blocks, which in turn are reused by the anabolic machinery of the cell after their transport to the cytosol [reviewed in (122, 241)]. From a physiological point of view, the lysosomal compartment can be looked upon as a box, built of vacuoles that constantly fuse and divide, that receives enzymes from the TGN and substrates from either the outside or the inside of the cell. After substrate degradation inside individual lysosomes, the products diffuse or are actively transported to the cytosol for reutilization.
Because many iron-containing macromolecules are degraded intralysosomally, low-mass iron is released inside the lysosomal compartment. Because the lysosomes also contain reducing agents (for example, glutathione), ascorbic acid, and the amino acid cysteine, some low-mass iron exists as Fe(II) with the capacity to generate highly reactive radicals if exposed to hydrogen peroxide (reviewed in ref. 129). As a result, lysosomes are very sensitive to oxidative stress, and their membranes easily peroxidized and permeabilized by the radicals that are formed secondary to the Fenton-type reactions taking place in the lysosomes. The rupture of lysosomes (induction of LMP) with relocation of the lytic enzymes results in apoptosis or necrosis, depending on the magnitude of this relocation (reviewed in ref. 28). It is of importance to recognize that lysosomes contain different amounts of redox-active iron. This is because some of them have recently been active in autophagic degradation, whereas others have not. A threshold for LMP seems to exist after oxidative stress, probably because of the high concentration of vitamin E within lysosomal membranes (253). It also is important to remember that LMP after oxidative stress is not an instantaneous process but rather requires some time, because peroxidation and the ensuing fragmentation of lysosomal membranes require time (5).
Keeping the concentration of redox-active iron in lysosomes as low as possible is consequently important for the survival of cells at oxidative stress. The rapid transport of low-mass iron from lysosomes to the cytosol is thus important, as well as ways of temporarily binding iron in a non–redox-active form (129).
B. Autophagy
Autophagy is a nonstop biologic renewal mechanism providing lysosomal degradation of the cell's own constituents. It represents one of the main pathways for the turnover and reutilization of worn-out long-lived proteins and organelles and is a perfectly normal process. Interestingly, the multicatalytic proteinase complexes, proteasomes, which also play an important role in the turnover of macromolecules, are themselves degraded by autophagy (53). The implication of this is that hampered autophagy might result in defective proteasomes, because they, in common with mitochondria and other organelles, are then not properly renewed. The mechanisms involved in the formation of the autophagic double membrane (the phagophore), the inclusion of materials to be degraded, and the fusion of autophagosomes and lysosomes were mostly recently worked out as a result of the discovery in yeast of a large family of phylogenetically well-preserved autophagy-related genes (ATG) (122, 207, 227, 265).
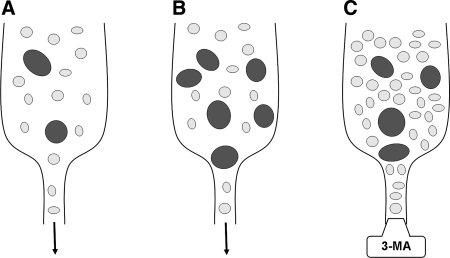
To date, three different mechanisms of autophagy have been described in mammalian cells: macroautophagy (also known as just autophagy), microautophagy, and chaperone-mediated autophagy (CMA). Macroautophagy, which, in at least a subset of cases is a nonselective process (202), involves the sequestration within a double membrane–bounded vacuole of portions of the cytosol, including aggresomes, dysfunctional mitochondria or proteasomes, as well as long-lived soluble proteins. The initially formed sequestration vacuole is devoid of lysosomal enzymes and is termed an autophagosome. In consecutive steps, it fuses with lysosomes and with other sequestration vacuoles, eventually resulting in the formation of an autophagolysosome (also called autolysosome), within which the degradation of the cargo and the recycling of amino acids and other monomeric molecules occurs (49, 129, 265); see Fig. 7.
Macroautophagy is the most universal type of autophagy, being involved in the degradation of practically any type of cellular material. It becomes activated under stress conditions, such as starvation, to generate ATP and essential building blocks by means of the nonspecific degradation of organelles and cytosolic macromolecules that are not critical for the survival of the cell (233).
Microautophagy is probably also involved in the turnover of lysosomes themselves, as suggested by the fact that fibroblasts exposed to the sequestration inhibitor 3-methyladenine accumulate large numbers of altered lysosomes containing a lipofuscin-like material (222). In support of this view, lysosomes with active hydrolytic enzymes have been found within autophagosomes (162). Moreover, by immunoelectron microscopy, lysosomal integral membrane proteins have been demonstrated to exist inside lysosomes and not only in the surrounding membranes (11). Such membrane fragments are particularly abundant within lysosomes of I-cell disease fibroblasts that lack active acid hydrolases (198).
Microautophagy involves the invagination of portions of the lysosomal membrane into the lumen of the lysosome/vacuole, resulting in the internalization of cytosolic compartments. A variation on this process, known as “piecemeal microautophagy of the nucleus” or PMN, has also been recently described in yeast, in which a small nonessential section of the nucleus is “pinched off” at nucleus–vacuole (NV) junctions (for a review, see ref. 130). Microautophagy is also a possible means of lysosomal membrane turnover, apparently working in parallel with macroautophagy.
CMA is a selective mechanism of lysosomal degradation, specific for soluble cytosolic proteins, which contain a targeting motif biochemically related to the penta-peptide KFERQ (for Lys-Phe-Glu-Arg-Gln). Unlike other forms of mammalian autophagy, CMA does not require vesicle formation or major changes in the lysosomal membrane to proceed: rather, substrate proteins directly cross the lysosomal membrane to reach the lumen, where they are rapidly degraded. This pathway requires the cooperation of cytosolic and lysosomal chaperones, including cytosolic hsc70 (cyt-hsc70), lysosomal hsc70 (lys-hsc70), LAMP-2A, and co-chaperones including Hsp90, Hsp40, Hip, Hop, and Bag-1 (1). The upregulation of CMA is observed in response to nutritional starvation and mild stress induced by toxic compounds or oxidants (49, 265).
Subsequent to cellular damage, reparative autophagy follows, by which altered and malfunctioning structures are replaced. Such reparative autophagy is commonly seen, for example, after ionizing irradiation, virus infection, and hypoxic or oxidative stress (18, 115). Interestingly, the postpartum period of starvation is overbridged by a period of enhanced autophagy in the liver, explaining why certain mutations that hinder autophagy are lethal (262). Recent evidence suggests that regular day-long periods of starvation may, by stimulating autophagy, help to “keep cells clean” and be beneficial (51).
C. Autophagic degradation of mitochondria (mitophagy)
Autophagy is the most efficient mitochondrial turnover mechanism, providing for the complete removal of irreversibly damaged mitochondria (mitophagy). It is believed that mitochondria are normally replaced every 2–4 weeks in rat brain, heart, liver, and kidneys (161), although recent studies have shown that the turnover rate might be considerably higher (163). Mitophagy is particularly important for long-lived postmitotic cells, whose mitochondria have pronounced oxidative damage (see Section II.C). Under stable, normal environmental conditions, the biogenesis of mitochondria through mitochondrial fission is balanced by mitophagy, resulting in a relatively constant number of mitochondria within postmitotic cells. As a result of fission, oxidatively damaged mitochondrial biomolecules are diluted, as are also damaged components of dividing cells. Mitophagy, in turn, prevents an excessive accumulation of mitochondria. The regulatory link between mitochondrial fission and mitophagy follows from a recent study showing that the pro-fission mitochondrial protein Fis1 induces mitochondrial fragmentation and the formation of autophagic vacuoles that contain mitochondria (89).
As mentioned earlier, oxidant-induced injury to mitochondrial components initiates asymmetric mitochondrial fission, generating daughter mitochondria with unequal inner membrane potentials (i.e., with different respiratory capacities and, probably, with different degrees of oxidative damage) (12, 247); see Fig. 4. Conceivably, asymmetric mitochondrial fission would provide for the selective removal by mitophagy of damaged mitochondria with abnormal membrane potentials, but whether this hypothesis is correct remains to be seen.
Mitophagy is apparently less critical for the survival of constantly proliferating cells, in which mitochondria formed during fission are distributed between daughter cells, preventing the excessive accumulation of damaged mitochondria. It was found that, in cultured pancreatic β-cells, the frequency of mitochondrial fission events is 5 to 10 times higher than the frequency of mitophagy (40, 45). The hypothesis that mitophagy is of particular importance for postmitotic cells is strengthened by our observation that the exposure of cultured rat cardiac myocytes to an inhibitor of autophagic sequestration, 3-methyladenine, induces excessive accumulation of defective mitochondria, followed by degeneration and death (237).
The adaptive (reparative) role of mitophagy increases under stress conditions, in particular with the oxidative stress associated with increased mitochondrial damage (142). Mitophagy is also an essential component of the programmed cell death (PCD-2, or autophagic cell death) that develops in the presence of excessive cellular damage (44, 118).
Questions yet unanswered are whether mitophagy is selective and how mitochondria are targeted for degradation. Early studies performed on isolated hepatocytes exposed to amino acid starvation showed that macroautophagy is a nonselective process (202), suggesting that the degradation of mitochondria occurs randomly. This is probably true for starving cells, in which autophagy activation is a rescue mechanism, using the cells' own constituents to generate energy and limited vital anabolism. All the functions of starving cells, perhaps with the exception of autophagy, are downregulated, with many mitochondria, including normal ones, becoming unnecessary.
However, increasing evidence suggests that both under normal and some pathologic conditions, mitochondria are selectively removed by autophagy. For example, the autophagic removal of mitochondria has been found essential for the maturation of yolk sack–derived embryonic erythroid cells (229). Furthermore, it has been shown that the mitochondria of degenerating embryonic flight muscles, as well as sperm mitochondria undergoing degradation after oocyte fertilization (the cell's mitochondria are all of maternal origin), are tagged by ubiquitin (55, 208, 226). It remains unclear what types of damage result in mitochondrial ubiquitination, and to what extent ubiquitin is required for mitophagy.
Recently, a number of new protein molecules involved in mitochondrial autophagy were discovered. The mitochondrial outer membrane protein, Uth1p (mitochondrial outer membrane and cell wall–localized SUN family member required for mitochondrial autophagy), was found essential for mitophagy in yeast (121). In addition, it was shown that a yeast mitochondrial intermembrane-space protein phosphatase homologue, Aup1p, is required for efficient stationary-phase mitophagy and cell survival (230). Several mitophagy-specific proteins have been demonstrated to exist in mammalian cells. A Bcl-2 family member protein, Bnip3, seems to trigger mitochondrial autophagy in mouse embryo fibroblasts that are exposed to hypoxia (203) and in neonatal rat cardiac myocytes during ischemia/reperfusion-like injury as well (95). A Bnip3-like protein, Bnip3L (also called Nix), has been found to play an essential role in mitophagy associated with erythroid maturation in mice (197). The relation between these mitophagy-related proteins and ubiquitination remains unresolved.
Although the molecular mechanisms responsible for selective mitochondrial degradation remain to be further elucidated, extensive evidence indicates that it is oxidant-induced mitochondrial damage and related mitochondrial permeability transition with decreased inner-membrane potential that often initiate a sequence of events resulting in mitophagy (reviewed in ref. 119, 246). As noted earlier, this kind of mitochondrial damage is an important characteristic of aging and a variety of pathologies.
VI. Lipofuscin Formation and Its Influence on Autophagy
A. Influence of labile iron and ROS on lipofuscin formation
Lipofuscin (age pigment) is a nondegradable, yellowish-brown, autofluorescent, polymeric compound that slowly accumulates within aging postmitotic cells at a rate that is inversely correlated with species longevity (76, 123) and reviewed in refs. 29 and 232. This interesting fact in and of itself suggests that lipofuscin accumulation may be hazardous to cells.
As can be seen from the previous sections, the aging of a multicellular organism depends largely on alterations occurring in long-lived postmitotic cells, such as neurons, cardiac myocytes, and RPE cells, whereas replicative aging due to telomere shortening most probably is a mechanism mainly for preventing malignant transformation (206). Long-lived postmitotic cells are very rarely (or never) replaced through the division and differentiation of stem cells, thus allowing lipofuscin and other biologic waste materials (such as irreversibly damaged mitochondria and aberrant proteins) to accumulate and gradually replace normal structures, leading to functional decay and cell death (241). It should be noted that lipofuscin also accumulates in cultured cells undergoing replicative senescence (associated with a progressive decline in the cellular proliferation rate), or in cells whose proliferation is inhibited by pronounced density-dependent inhibition of growth (reviewed in ref. 29). This suggests that lipofuscin can potentially form in various cell types, but only long-lived nondividing cells accumulate significant amounts of the pigment.
It is now generally accepted that the aging of long-lived postmitotic cells is at least partly induced by endogenously formed ROS, affecting various cellular structures, but mainly mitochondria and lysosomes (10, 98, 124, 128, 132, 212, 239). Lipofuscin formation is one of the most important manifestations of ROS-induced damage that occurs within the lysosomal compartment (27).
Although rapid and effective, lysosomal (autophagic) degradation is not completely perfect. Even under normal conditions, some iron-catalyzed peroxidation occurs intralysosomally (as pointed out, lysosomes are rich in redox-active iron), resulting in oxidative modification of the autophagocytosed material, making it resistant to the hydrolytic activity of lysosomal enzymes. If cells do not divide, this material progressively accumulates in the form of lipofuscin inclusions. Lysosomes receive a wide variety of autophagocytosed subcellular structures, most importantly mitochondria, which are rich in lipidaceous membrane components and iron-containing proteins, such as cytochrome c. That lipofuscin/ceroid to a large extent originates from mitochondrial components is proven by the presence of the ATP-synthase subunit c in age pigment or ceroid granules (72). In Alzheimer disease (AD), large amounts of mitochondrial lipoic acid have been found associated with lipofuscin, which indicates pronounced mitochondrial autophagy (166). Thus mitochondria not only are the main generators of ROS, triggering lipofuscinogenesis, but also are a major source of the macromolecules from which lipofuscin forms.
Lipofuscinogenesis has pronounced similarities to the formation of advanced glycation end products (AGEs) that is mainly involved in the aging of connective tissue components leading to wrinkling of the skin, cataract, the stiffening of blood vessels, etc. (see earlier). These effects are thought to be a consequence of alterations in extracellular matrix proteins, which undergo polymerization and lose their elasticity because of glycosylation with accompanying Maillard reactions (the binding of carbonyls of reducing sugars to amino groups of proteins) and ensuing Amadori reorganization (the formation of new carbonyls within the complex, thus allowing the binding of additional amino groups and polymerization of proteins to stiff, plastic-like structures) (60). The difference between formation of AGEs and lipofuscin is that no sugars are involved in the formation of the latter, because then the linking aldehydes are produced by degradation of oxidized lipids. Basically, lipofuscin may be regarded as a nondegradable plastic-like polymer that slowly matures by intramolecular reorganization. It has been found that lipofuscin contains oxidized proteins in which tyrosine residues have been replaced by DOPA (3,4-dihydroxy-l-phenylalanine, an oxidized form of tyrosine) (67, 117, 191, 201), secondary to an oxidation that most probably is mediated by redox-active iron. Because DOPA, being a hydroquinone-type structure, is capable of redox cycling (190), it is conceivable that lipofuscin, because of its oxidized protein residues, produces superoxide and hydrogen peroxide. This production might result in the labilization of the surrounding membrane, especially because lipofuscin is also rich in loosely bound iron. Consequently, lipofuscin-loaded lysosomes may be sites of pronounced Fenton-type reactions and be especially sensitive to oxidative stress (26, 29, 110).
Oxidized proteins are usually considered degraded by the proteasome system secondary to ubiquitination, but recent studies have shown that such proteins may also undergo autophagic degradation. Studies using a cell line with a thermolabile ubiquitin-conjugating enzyme showed that oxidized proteins were still being degraded at a normal rate, even in the absence of functioning ubiquitin-conjugating enzymes (209). Although it is not clear to what extent other pathways for ubiquitin conjugation could have been active, the latter finding suggests that the proteasomal pathway is not the only one involved in the degradation of oxidized proteins (109).
By using an approach in which oxidatively modified proteins were generated in vitro by allowing cells to incorporate DOPA into proteins, it was demonstrated that mildly modified proteins were efficiently degraded by proteasomes, because this process could be inhibited by the specific proteasome inhibitor, lactacystin (192). By increasing the amount of DOPA incorporated into proteins, it was, however, possible to generate proteins that were heavily modified and eventually to generate lysosomal autofluorescent lipofuscin aggregates in cells (68). Moreover, by using inhibitors of the proteasome and lysosomal proteases, it was found that the degradation of the more highly modified aggregate-prone DOPA-containing proteins began to switch from the proteasomal to the lysosomal pathway (192). This “cooperation between proteasomes and lysosomes” may suggest that substantially modified proteins are no longer substrates for proteasomes and, therefore, must be redirected to the endosomal–lysosomal pathway. It also was shown that soluble and ubiquitinated misfolded proteins localize to proteasome-rich perinuclear sites, whereas terminally aggregated proteins are sequestered in autophagic vacuoles (111). Further evidence for cross-talk between the proteasomes and lysosomes was reported for human RPE cells, in which an accumulation of perinuclear ubiquitin-, Hsp70-, and LAMP2-positive aggregates occurred in response to MG-132 (a proteasome inhibitor), with the aggregates being removed after cessation of inhibition by a mechanism thought to involve autophagy (196). In an earlier study, increased levels of lipofuscin-like autofluorescence were found in cultured SH-SY5Y neuroblastoma cells exposed to the proteasome inhibitor MG-115 (224). It is to be noted that the accumulation of lipofuscin-like material and other protein aggregates, considered an effect of proteasome inhibition, may depend also on the inhibition of lysosomal functions. MG-115 and MG-132, which belong to the peptide aldehyde proteasome inhibitors, are known to suppress not only proteasomes but also lysosomal cathepsins (39, 160). Even stronger support for proteasomal–lysosomal crosstalk was provided by a study using a highly specific proteasome inhibitor, MG-262. When exposed to this substance, cultured human fibroblasts showed increased lipofuscin accumulation (especially pronounced under hyperoxic conditions), suggesting that modified proteins that were not degraded by proteasomes underwent autophagic degradation, contributing to lipofuscinogenesis (242).
In professional scavengers, such as RPE cells and macrophages (foam cells) in atheroma, a large portion of lipofuscin (or ceroid) originates from endocytosed material (135, 171). Depending on the nature of the autophagocytosed/endocytosed material, the composition of lipofuscin varies among different types of postmitotic cells, and no chemical formula can be given for this complex substance that seems to be composed mainly of cross-linked protein and lipid residues. It should be pointed out, however, that all forms of lipofuscin contain considerable amounts of redox-active iron, which, as was pointed out in the Introduction, sensitizes lipofuscin-loaded cells to oxidative stress (reviewed in ref. 129). Figure 11 shows the basic mechanisms behind lipofuscinogenesis.
FIG. 11.
Mechanisms of lipofuscin formation. Superoxide (O2•−) forms mainly in mitochondria as a side product of biologic respiration. It is converted into hydrogen peroxide (H2O2) by superoxide dismutase (SOD). Hydrogen peroxide is further homolytically split, yielding the hydroxyl radical (HO•), in the presence of ferrous iron (the Fenton reaction). Hydroxyl radicals damage surrounding macromolecules, while H2O2 diffuses throughout the cell. Oxidatively damaged macromolecules (parts of mitochondria and other cellular structures) enter lysosomes through autophagy. In the autophagolysosomes, which are rich in iron, more hydroxyl radicals form, causing oxidative damage to autophagocytosed material, resulting in its polymerization and undegradability (i.e., lipofuscin formation). Actions of lysosomal enzymes (Enz) and reactive oxygen species are indicated as dashed arrows. Black dots, oxidatively damaged macromolecules, including components of lipofuscin. Bold curved arrows, the sequence of events.
B. Consequences of the nondegradability of lipofuscin
The accumulation of lipofuscin within the lysosomal compartment apparently compromises autophagic degradative capacity, prolonging the half-lives of long-lived proteins and organelles and creating a situation in which cells are forced to exercise their functions with less-than-perfect tools (Fig. 12). Consistent with this theory, the capacity for autophagic degradation is found to be diminished in aged lipofuscin-loaded cells (52, 231, 236), which may lead to some serious consequences. For example, the delayed degradation of mitochondria would result in increased damage by self-produced ROS, additionally contributing to lipofuscinogenesis and perhaps inducing apoptotic cell death by LMP.
FIG. 12.
The accumulation of “waste” is a consequence of imperfect autophagy. Lysosomal enzymes are produced in the trans-Golgi network (TGN) and by secretory vesicles transported to late endosomes that acidify and maturate into lysosomes (see Fig. 7), which in turn fuse with autophagosomes (APSs). The continual fusion and fission of the lysosomal vacuoles ensures the distribution of acid hydrolases within the lysosomal compartment, including APS. In contrast to a young cell (A) that has only few lysosomes containing the undegradable age-pigment lipofuscin (Lf), senescent postmitotic cells (B) contain large numbers of Lf-containing lysosomes, to which more and more lysosomal enzymes are directed in a useless effort to degrade lipofuscin. These lysosomal enzymes are lost for useful purposes (e.g., for the degradation of newly autophagocytosed material), resulting in a delayed turnover and the accumulation of waste products. Damaged/dysfunctional mitochondria are indicated by dark shading.
Recently, an elegant study on Caenorhabditis elegans added new evidence for the hypothesis that lipofuscin accumulation is causally related to aging and the deterioration of postmitotic cells. Fortunately, these tiny nematodes are transparent, which permits lipofuscin to be measured directly with spectrofluorimetry in vivo. It was found that mutant nematodes that live either longer or shorter than the wild-type animal accumulate lipofuscin at a slower or quicker pace, respectively. It also was found that calorie-restricted worms lived longer and accumulated lipofuscin more slowly than did animals fed ad libitum. Finally, when wild-type siblings that aged differently, as evaluated by changes in their motility capacity, were compared, it was found that the still mobile and youthful ones at day 11 of their life spans contained only 25% of the lipofuscin that was found in severely motility-impaired siblings of the same age. This implies that lipofuscin accumulation reflects biologic rather than chronologic age (84).
Besides accumulating the intralysosomal “waste” material lipofuscin, aging postmitotic cells also form extralysosomal “garbage,” such as damaged dysfunctional mitochondria and indigestible protein aggregates (aggresomes) that for some reason are not efficiently autophagocytosed or degraded by the proteasome pathway (reviewed in refs. 233 and 235). Aged mitochondria are enlarged and show considerably reduced fusion and fission activity. Their autophagy may be prevented by their size, because the autophagy of large structures is apparently energy consuming, and autophagosomes seem to have an upper volume limit (169, 241). These most important phenomena are discussed in detail in Section VII of this review.
The accumulation of aberrant proteins within aging postmitotic cells is a consequence of both ROS-induced damage and the incomplete degradation of altered protein molecules. Although damaged proteins may partially preserve their functions, their enzymatic activity per unit mass declines (186, 216). Aberrant proteins often show a tendency to aggregate. Lewy bodies and neurofibrillary tangles (composed of α-synuclein or the hyperphosphorylated protein tau, respectively) are characteristic examples of such aggregates (17, 96).
The importance of autophagy for the removal of protein aggregates and delaying aging was demonstrated in a recent study on Drosophila (210). During normal aging of the fly, the expression of the Atg8a gene (which is of importance for autophagy) decreases in neurons, resulting in the accumulation of aggregates of ubiquitinated proteins. When the expression of Atg8a was upregulated, the aggregates disappeared, and the flies showed an increased resistance to oxidative stress, as well as a more than 50% prolonged life span.
C. Disease-related accumulation of intralysosomal and extralysosomal waste
When, as a result of damage, proteins and other macromolecules undergo modifications that make them indigestible, or when lysosomal degradation is suppressed, or autophagy increases greatly as a result of reparative efforts (reparative autophagy), the accumulation of intralysosomal waste may occur more rapidly than in normal aging. The amount of nondegradable material can increase, for example, as a result of enhanced damage to cellular structures due to various types of stress, ionizing irradiation, intoxications, or malnutrition (85). Lysosomal functions can also be suppressed as a result of the administration of drugs, such as the lysosomotropic agent chloroquine that increases the lysosomal pH and thereby depresses the activity of lysosomal hydrolytic enzymes, or in a large variety of lysosomal-storage diseases that are associated with genetic defects of specific lysosomal hydrolases (256). Intralysosomal waste material formed under these conditions is usually called “ceroid” or “ceroid-type lipofuscin.” Ceroid also forms because of increased sensitivity to oxidation, such as in vitamin E deficiency (78). This material has, however, practically the same physicochemical properties (i.e., natural yellow-brown color, autofluorescence, electron density after osmium fixation, resistance to hydrolysis) and basic mechanisms of formation (oxidant-induced, aldehyde-mediated intra- and intermolecular cross-linking) as true, age-related, lipofuscin.
Because of the variable composition of lysosomal pigment in aging and different diseases, as well as in different tissues, a distinction between lipofuscin and ceroid can reasonably be made only from an etiologic viewpoint, but not with respect to their properties and chemistry (29). As ceroid forms more quickly than lipofuscin, the latter is perhaps just a more-restructured and advanced polymer (compare its formation with the Amadori reorganization of glycosylated proteins).
Juvenile neuronal lipofuscinosis is associated with lysosomal lipofuscin/ceroid accumulation and is a nontypical example of a lysosomal storage disease in the sense that no enzyme deficiency has been demonstrated. This fatal disease rather seems to be caused by a mutation of a lysosomal membrane protein of importance for the fusion between lysosomes and autophagosomes. As a result, the influx of degrading enzymes to autophagosomes is slow (35), allowing more time for peroxidation and polymerization of lysosomal contents into lipofuscin/ceroid. In cell cultures exposed to lysosomal protease inhibitors, this scenario is reproduced, and degradation is belated. Enhanced lipofuscin production is also an effect of the exposure of cells in culture to oxidative stress [e.g., growing cells at 40% ambient oxygen (reviewed in refs. 29 and 232)].
In the progressive eye condition known as age-related macular degeneration (AMD), the accumulation of lipofuscin within retinal pigment epithelial (RPE) cells in the macular area of the retina is a warning sign of developing disease (77). LF accumulation, with ensuing RPE cell damage, results in the build-up of deposits in the Bruch membrane (an inflammatory process with prominent genetic involvement) and in choroidal neovascularization. Exactly how LF accumulation inside RPE cells results in their degeneration and the eventual death of the RPE cells themselves, as well as the photoreceptors that they are supporting, is not understood in detail. Findings from RPE cells in culture, however, have provided some information: LF acts as a sensitizer to blue light by causing singlet oxygen formation with ensuing membrane destabilization, the release of hydrolytic enzymes to the cytosol, and resulting apoptosis. Moreover, lysosomal LF accumulation prevents normal RPE phagocytic activity (258). This is probably a result of a misdirection of newly produced lysosomal enzymes to lipofuscin-loaded lysosomes at the expense of late endosomes and autophagosomes (Fig. 12).
To better understand the molecular mechanisms behind AMD, and, hopefully, to improve our ability to interfere with this common disease, it is necessary to understand how RPE cells for years are able to handle the extremely high demands on their capacity for lysosomal degradation without being overwhelmed by LF accumulation. It has been suggested that iron overload may exacerbate AMD: (a) postmortem AMD-affected eyes showed an excess of both chelatable and nonchelatable iron in the RPE cells and in the Bruch membrane, including drusen; (b) the iron-carrier protein transferrin is upregulated at both the mRNA and protein levels in patients with AMD compared with controls (43); and (c) mice with RPE iron overload resulting from a deficiency of the iron exporters ceruloplasmin and hephaestin develop retinal degeneration with some features of AMD, including sub-RPE deposits and subretinal neovascularization. These findings point to a dysfunctional regulation of lysosomal labile iron in AMD. The same may be true for other neurologic disorders in which abnormal accumulation of lipofuscin occurs in the lysosomal compartment.
Extralysosomal deposition of oxidized proteins is reported in age-related pathologies, including neurodegenerative disorders such as AD (225) and Parkinson diseases (PD) (191), atherosclerosis and cataractogenesis (59), and diabetic complications (15). Interestingly, the increased levels of oxidized proteins that are normally observable in tissues from older animals are less prominent after CR, suggesting that semistarvation may lead to a reduction in the extent of protein damage (266).
Multiple neurodegenerative disorders, including AD, PD, and Huntington disease (HD), are characterized by selective neuronal loss concomitant with the accumulation of intraneuronal proteinaceous inclusion bodies. These include the amyloid plaques and neurofibrillary tangles consisting of the hyperphosphorylated protein tau in AD, Lewy bodies consisting of α-synuclein in PD, and nuclear inclusions in the polyglutamine-repeat diseases, such as HD. Intracellular inclusions are thought to form when the proteasome capacity is overwhelmed, or, in the case of familial neurodegeneration, in the presence of mutated α-synuclein (153). When misfolded proteins accumulate in sufficient quantity, they are prone to aggregation. Pathologic inclusions have been shown to contain the core protein as well as ubiquitin (Ub) moieties, chaperones and components of the ubiquitin-proteasome system (UPS), thus implicating the UPS in disease progression.
The accumulation of α-synuclein, Ub, and other proteins in Lewy bodies contained within degenerating dopaminergic neurons in idiopathic PD suggests that the inhibition of normal/abnormal protein degradation may contribute to neuronal death (154). In support of this, McNaught and co-workers (154) reported a reduction in all three catalytic activities of the 20/26S proteasome in the substantia nigra in PD by 39%, 42%, and 33% for chymotryptic, tryptic, and caspase-like activity, respectively. Direct proteasome inhibition in cultured rat ventral mesencephalic cells and PC12 cells also led to the formation of α-synuclein/ubiquitin-containing intracytoplasmic inclusion bodies and the preferential degeneration of dopaminergic neurons, further implicating UPS dysfunction in PD (155). Support for the dysfunctional UPS hypothesis is provided by rare hereditary forms of PD, in which mutations in the genes encoding parkin (an E3 Ub-ligase) and ubiquitin c-terminal hydrolase L1 or UCH-L1 (a deubiquitinylating enzyme), both components of the UPS, are closely linked to disease progression.
VII. Imperfect Mitochondrial Turnover and Postmitotic Cellular Aging
A. Age-related accumulation of defective mitochondria within postmitotic cells
The number of defective (senescent) mitochondria within long-lived postmitotic cells progressively increases with age. These mitochondria show structural deterioration, such as swelling, loss of cristae, or destruction of the inner membrane, often combined with mitochondrial enlargement, leading to the formation of the so-called giant mitochondria (Figs. 2 and 3). These structural changes underlie mitochondrial functional deficiencies such as decreased ATP production [reviewed in (240)].
Senescent mitochondria appear in initially young and healthy postmitotic cells, where they slowly accumulate with time. This suggests that early mitochondrial changes are stochastic by nature and perhaps reflect insufficient mtDNA repair, the inadequate degradation of damaged proteins by Lon, Clp-like, and AAA-proteases, as well as imperfect mitophagy. Because occasional senescent mitochondria are found early in the life span of postmitotic cells, the malfunction of mitochondrial renewal mechanisms is obviously not a consequence of aging but rather an inherent characteristic. Another example of the inherent insufficiency of cellular homeostasis is the formation of oxidatively modified, undegradable material (lipofuscin) within lysosomes (29, 30). Even if the initial changes in mitochondria and lysosomes obviously are not caused by aging, they accumulate as a function of time and, moreover, trigger additional (secondary) pathogenic mechanisms responsible for mitochondrial and lysosomal aging. According to the extensive experimental evidence, these mechanisms are associated either with an age-related decrease in the degradation of defective mitochondria and their components, or with the increased replication of damaged organelles. The involvement of additional pathogenic mechanisms, which are described later, suggests that the course of aging accelerates with time.
B. Age-related decline in autophagy and Lon protease activity accelerates mitochondrial damage
Decreased lysosomal degradative capacity and, in particular, decreased mitophagy is one of the most apparent sources of increased mitochondrial damage with age. Autophagy has been shown to decline in aged rat and mouse hepatocytes (63, 231). In a recent study, an age-related decrease in autophagic degradation was found for rat hepatocyte mitochondria containing oxidatively damaged mtDNA (as assessed by the increased 8OHdG levels). When autophagy was stimulated by the antilipolytic agent 3,5-dimethylpyrazole, the content of 8OHdG decreased to the levels comparable to those in young animals (37). The loading of lysosomes with lipofuscin is probably one of the most important contributors to the decline of autophagy in aged cells (see also Section VI). As predicted by the mitochondrial–lysosomal axis theory of aging (30), lipofuscin-loaded lysosomes would consume a major part of the newly produced lysosomal hydrolases that, however, cannot digest the undegradable material. As a result, a lesser amount of lysosomal enzymes remains available for autophagic degradation, including mitophagy. Consequently, damaged mitochondria accumulate, producing increased levels of ROS and leading to further lipofuscin accumulation. Senescent mitochondria and lipofuscin-loaded lysosomes gradually replace normal organelles, finally resulting in cell death due to a lack of ATP. In agreement with this theory, heavily lipofuscin-loaded growth-arrested human fibroblasts showed significantly decreased autophagy, and they poorly survived amino acid starvation (236). Furthermore, in aged neonatal rat cardiac myocytes, the amount of lipofuscin positively correlates with mitochondrial damage and ROS formation (238).
Lon protease expression has also been shown to be affected by oxidative stress and aging, leading to the accumulation of carbonylated mitochondrial proteins in mouse skeletal muscles (23). Because Lon proteases are coded for by nuclear genes, their downregulation may take place independent of any initial mitochondrial damage.
C. Enlarged mitochondria are resistant to degradation and do not fuse with normal ones
A number of the mechanisms involved in the progress of mitochondrial alterations with age are triggered by primary changes in the mitochondria themselves. One such change may be mitochondrial enlargement. Because autophagy is an energy-dependent process, the degradation of large organelles is obviously more demanding than that of small ones. The initial enlargement of some mitochondria is quite predictable, for example, because of the oxidative damage to mitochondrial membranes and proteins that can disturb fission. Indeed, the aging of cultured Chang cells, which were kept at low oxidative stress, was associated with the accumulation of enlarged mitochondria and was paralleled by the downregulation of Fis1 (264). Furthermore, siRNA depletion of Fis1 in cultured Chang and HeLa cells caused the formation of enlarged and flattened mitochondria with decreased membrane potential and elevated ROS production, resulting in DNA damage and increased β-galactosidase activity (139). The latter is most probably not an age-specific process, but rather reflects the generally enhanced production of lysosomal enzymes and reduced cell growth (205).
Larger mitochondria would have a lesser chance to be autophagocytosed and would thus undergo progressive degeneration and enlargement, resulting in the gradual appearance of giant mitochondria; this, along with lipofuscin accumulation within postmitotic cells, is an age-related, irreversible phenomenon. That mitochondrial size does matter for the propensity of mitochondria to be autophagocytosed follows from the fact that the inhibition of autophagy with 3-methyladenine in neonatal rat cardiac myocytes results in a dramatic accumulation of small-sized mitochondria with low membrane potential and only a moderate increase in the number of large (giant) senescent-like mitochondria (237); see Figs. 2 and 3. The most likely interpretation of these results is that mitochondria that are excluded from recycling by 3-methyladenine administration accumulate in quantities reflecting their normal turnover rates. The gradual accumulation of large mitochondria that occurs during normal aging resembles, to some extent, a bottleneck phenomenon: only mitochondria that are small enough are autophagocytosed [i.e., pass the bottleneck (233)]; see Fig. 13. Consistent with our results, the accumulation of small depolarized mitochondria was observed in INS1 cells exposed to 3-methyladenine, as well as in autophagy-deficient Beclin 1 RNAi H4 cells and ATG5−/− mouse embryonic fibroblasts (246).
FIG. 13.
The bottleneck model of age-related accumulation of giant mitochondria. Autophagy of large mitochondria is more complicated than that of small ones. This results in progressive accumulation within long-lived postmitotic cells of enlarged (giant) mitochondria, which do not “pass the bottleneck.” (A, B) Young and senescent cells, respectively. Inhibition of autophagic sequestration with 3-methyladenine (C), which suppresses the turnover of all mitochondria independent of their size, results in the accumulation of mitochondria in quantities reflecting their turnover rates. Consequently, cells accumulate numerous small mitochondria and only few large, senescent-like mitochondria.
The irreversibility of an age-related mitochondrial enlargement also follows from our recent observation that the population of senescent-like giant mitochondria formed within cultured rat myoblast cells exposed to 3-methyladenine does not fuse and exchange their contents with normal mitochondria (see Figs. 6, 14, and 15) A decreased mitochondrial inner-membrane potential and downregulation of Mfn2 and OPA1 are the most apparent reasons for the inability of giant mitochondria to fuse (169). Thus, giant mitochondria seem progressively to amass with age because they do not fuse or divide or they are removed by macroautophagy.
FIG. 14.
Morphology of normal and giant mitochondria in L6 rat myoblast cells. (A) Fluorescence-microscopy image of a myoblast cell containing normal mitochondria (up to 0.5 μm wide). (B) Several mitochondria are dramatically enlarged. The highlighted mitochondrion is 4.5 μm long and 2.6 μm wide. (C) The width distributions of 80 normal (light bars) and 80 giant (dark bars) mitochondria as determined by analysis of fluorescent images in SimplePCI 5.3 software. The distributions were normalized by dividing the number of hits in each bin (bin size, 0.2 μm) by 80 (i.e., the total number of mitochondria analyzed). Reprinted from Navratil et al. (169) with permission from Elsevier.
FIG. 15.
Expression of mitochondrial fusion proteins in giant mitochondria. L6 myoblasts treated with 5 mM 3-MA were immunostained for cytochrome c oxidase subunit I (COXI, green) and mitochondrial fusion proteins OPA1 and Mfn2 (red). The nuclei are counterstained with DAPI in the composite images. Relative OPA1 and Mfn2 expression levels were assessed by measuring the red fluorescence signal normalized by the green COXI fluorescence in overlapping areas. Bottom plots show differences in the distributions of Opa1/COXI (left) and Mfn2/COXI (right) ratios between normal (blue) and giant (red) mitochondria. For each distribution a median value (Med) is reported. Modified from Navratil et al. (169) with permission from Elsevier. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
That cells cannot rid themselves of giant mitochondria is consistent with earlier observations of the impaired fusion capacity of damaged mitochondria with increased OPA1 degradation (see Section III.B).
D. Mechanisms of the age-related accumulation of mitochondria with homoplasmic mtDNA mutations
It has been found that aging postmitotic cells often accumulate homoplasmic mtDNA point mutations and deletions, resulting in the gradual replacement of all normal mitochondria with mutated ones (116, 176). Mitochondria with homoplasmic mutations have also been found to occupy atrophic segments of the ragged muscle fibers typical of aged individuals (36). The amassing of mitochondria with defective mtDNA was earlier described for a variety of pathologies, mainly myopathies and cardiomyopathies (reviewed in refs. 62 and 149). It was hypothesized that the accumulation of mitochondria with single-type mtDNA mutations within postmitotic cells is because mutated mtDNA is associated with defective respiration, resulting in decreased oxidative damage to mitochondrial membranes. As a result, such mitochondria would be less targeted by autophagy than normal ones (56). This theory, called SOS (for “survival of the slowest”) requires, however, some confirmation that mitochondria are indeed being targeted for autophagy based on the degree of damage to their membranes. Moreover, the accumulating evidence that mitochondria with decreased membrane potential are preferentially selected for degradation (see earlier) is contradictory to the SOS hypothesis.
Another still unproven explanation for the accumulation of mutated mitochondria within aged or diseased postmitotic cells is that mutant mtDNA has a replicative advantage over normal mtDNA, resulting in the clonal expansion of mitochondria with mutated DNA (176). Although it has been suggested that deleted mtDNA might replicate more easily because of its shorter length, the propagation of mtDNA point mutations cannot be explained from this premise (57). Yet, an important argument in favor of the clonal expansion hypothesis is the accumulation within malignant tumor cells of mitochondria with homoplasmic mtDNA mutations (103). These cells and their mitochondria replicate indefinitely, which excludes the preferential accumulation of mitochondria with single-type mutations because of their limited autophagy, as was predicted by the SOS hypothesis that describes the age-related accumulation of defective mitochondria specifically in long-lived postmitotic cells.
E. Decreased mitochondrial biogenesis in aged cells
Age-related decrease of mitochondrial biogenesis (143) is related, at least in part, to diminished AMPK activity (185, 188). AMPK appears to be the key cellular energy sensor, linking decreased mitochondriogenesis to several aging-associated changes, including insulin resistance and deficient lipid metabolism (185, 188). With aging, a decline in PGC-1α expression levels also occurs, with the latter being slowed by CR (8, 100). Treatment with hexarelin or resveratrol rescues mitochondrial biogenesis and lipid metabolism by inducing a higher turnover of aged and damaged organelles (194). It should be mentioned that the changes in mitochondriogenesis are tissue specific, being more dramatic in the central nervous system (143), which is consistent with the generally higher susceptibility of neurons and other postmitotic cells to the aging process.
The relation between proteins involved in the regulation of mitochondrial dynamics and age-related mitochondrial changes is not understood. An important fact in this regard is that the processing of the fusion protein OPA1 may be regulated by cellular ATP levels (9), thereby providing a connection with aging- or disease-associated bioenergetic decline. It was found that depolarization of mitochondria is followed by the proteolytic cleavage of OPA1 long (l)-isoforms (69, 90, 105, 214), resulting in a diminished capacity for fusion and ensuing mitochondrial fragmentation (69, 246). The dependence of OPA1 proteolysis on ATP concentration may explain how even small changes in the membrane potential can change mitochondrial morphology (101). Mitochondrial energetics regulates OPA1 levels through the presenilin-associated rhomboid-like protease (PARL), AAA-proteases, and AFG3L2-proteases (46, 70, 105). The short form of OPA1 has been detected in cells with dysfunctional respiration and in patients with cardiomyopathies. It would be interesting to investigate whether the short form of OPA1 has also increased levels in the aging tissue because of the ATP deficiency of senescent cells. This regulatory mechanism would result in mitochondrial fragmentation and autophagic turnover of mitochondria. However, the ability of postmitotic cells to renew their mitochondria would progressively decline because of decreasing autophagic capacity and the increasing proportion of enlarged senescent mitochondria that can neither fuse nor divide (169).
Possible mechanisms involved in age-related mitochondrial damage are summarized in Table 3.
Table 3.
Potential Mechanisms Involved in Age-related Mitochondrial Changes due to Oxidative Damage
| Mechanism | Cellular manifestations |
|---|---|
| 1. Decreased capacity of cellular-degradation mechanisms | |
| A. Decreased mitophagy due to lipofuscin loading of lysosomes | A. Accumulation of defective, mainly enlarged mitochondria combined with extensive lipofuscin loading of lysosomes |
| B. Decreased activity of mitochondrial proteases | B. Intramitochondrial accumulation of oxidatively modified proteins |
| 2. Decreased susceptibility of mitochondria and their components to degradation | |
| A. Mitochondrial enlargement (presumably due to impaired fission) interferes with mitophagy | A. Accumulation of enlarged (giant), functionally defective mitochondria |
| B. Modifications of mitochondrial proteins make them resistant to Lon, Clp-like, and AAA proteases | B. Intramitochondrial accumulation of oxidatively modified proteins |
| C. Defective respiration may lessen oxidative damage to mitochondrial membranes, resulting in decreased targeting of mitochondria for autophagy | C. Accumulation of mitochondria with reduced respiration, presumably with homoplasmic mtDNA mutations |
| 3. Increased replication (clonal expansion) of defective mitochondria | 3. Accumulation of mitochondria with homoplasmic mtDNA mutations |
| 4. Decreased mitochondrial biogenesis | 4. Lack of normal mitochondria |
Detailed explanations and references are given in the text.
VIII. Summary and Conclusions
Mitochondria are the power-generating factories of the cell, but they are also generators of ROS. These reactive species are probably responsible for at least a major part of the macromolecular damage that gradually accumulates and results in mitochondrial malfunction. Mitochondrial failure deprives the cell of ATP and increases the production of ROS, which eventually leads to cell death. Age-related mitochondrial damage mainly affects postmitotic cells, such as neurons and myocardial cells, which are rarely or not at all replaced because of the division or differentiation of stem cells. Mitochondria possess a potent proteolytic self-repair system, as well as a capacity to fuse and divide. During fusion, mitochondria can probably renew themselves by exchanging material with other mitochondria, whereas mitochondrial division enhances the dilution of damaged macromolecular components, which apparently prevents the aging of actively proliferating cells. In addition, mitochondria can divide asymmetrically into two daughter organelles, one of which contains most of the defects. This allows a selective mitophagy (macroautophagy of mitochondria) of those organelles that are damaged beyond the possibility of effective repair. If this system worked perfectly, the cell would remain vital and operating well. In postmitotic cells, however, over time, a slow accumulation of poorly functioning mitochondria occurs; these often take the phenotype of giant mitochondria with low membrane potential. The reason that these dysfunctional mitochondria accumulate seems to be an increasing incapability to divide, in combination with insufficient autophagic degradation. The regulation of mitochondrial fusion and fission is just beginning to be at least partly understood, whereas the influence of accumulated lipofuscin on the process of macroautophagy, as a result of extensive studies of C. elegans and cells in culture during the last few years (50, 74, 122, 129), is somewhat better understood. The new findings point to the existence of a mitochondrial–lysosomal cross-talk in which formation of ROS by mitochondria gives rise to peroxidation of autophagocytosed lysosomal contents under degradation. The result is a slow accumulation of lipofuscin in the lysosomal compartment of long-lived postmitotic cells; this is catalyzed by the redox-active iron that is released during the degradation of autophagocytosed ferruginous materials. Lipofuscin can neither be degraded nor exocytosed, to any substantial extent. Lipofuscin accumulation, in turn, seems to depress the capacity of lysosomes to degrade autophagocytosed materials because of their futile endeavor to break down the plastic-like, undegradable pigment. Moreover, the transport and location of an increasing amount of newly produced lysosomal enzymes to lipofuscin-loaded lysosomes seems to create a situation in which autophagic vacuoles become equipped with an insufficient amount of degrading capacity, thereby forcing the cell to use damaged structures, including mitochondria, longer than is optimal. It is envisioned that further research focused on mitochondrial–lysosomal interactions will add to our knowledge about aging and age-related pathologies, as well as suggest new strategies for antiaging intervention.
Abbreviations Used
- AD
Alzheimer's disease
- AGE
advanced glycation end product
- AMD
age-related macular degeneration
- APS
autophagosome
- ATG
autophagy-related gene
- Aup1p
yeast mitochondrial protein phosphatase homologue
- Bnip3L
Bcl2/adenovirus E1B 19-kDa interacting protein 3
- CMA
chaperone-mediated autophagy
- CR
caloric restriction
- DOPA
3,4-dihydroxy-l-phenylalanine
- Drp1
dynamin-related protein 1
- Fis1
mitochondrial fission protein 1
- Fzo
fuzzy onion protein
- GDAP
ganglioside-induced differentiation-associated protein
- HD
Huntington's disease
- Hsp70
heat-shock protein 70
- LAMP2
lysosome-associated membrane protein 2
- LF
lipofuscin
- LMP
lysosomal membrane permeabilization
- 3-MA
3-methyladenine
- Mfn1
mitofusin 1
- Mfn2
mitofusin 2
- Miro
mitochondrial rho protein
- Miro-2
mitochondrial rho-2 protein
- MP
mannose phosphate
- MTP18
mitochondrial protein 18
- mtTFA
mitochondrial transcription factor A
- Nramp
natural resistance–associated macrophage protein
- NRF1
nuclear respiratory factor 1
- OPA1
optic atrophy protein 1
- PARL
presenilin-associated rhomboid-like protein
- PCD
programmed cell death
- PD
Parkinson's disease
- PGC-1α
peroxisome proliferators–activated receptor γ, coactivator 1 α
- RIP140
nuclear receptor–interacting protein
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
- SIRT1
sirtuin (silent mating-type information regulation 2 homologue) 1 (C. cerevisiae)
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment receptor
- SOD
superoxide dismutase
- SOS hypothesis
“survival of the slowest” hypothesis
- TGN
trans-Golgi network
- α-TOS
α-tocopheryl succinate
- Ub
ubiquitin
- UPS
ubiquitin–proteasome system
- Uth1p
mitochondrial outer membrane and cell wall–localized SUN family member required for mitochondrial autophagy
Footnotes
Reviewing Editors: Enrique Cadenas, Sandra Cardoso, Joanne Clark, Mark Hannink, Sergio Papa, George Perry, Rodrigue Rossignol, and Raj S. Sohal
Acknowledgments
We thank Carol Makkyla for proofreading the manuscript. E.A.A. thanks NIH R01-AG20866 for support.
References
- 1.Agarraberes FA. Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114:2491–2499. doi: 10.1242/jcs.114.13.2491. [DOI] [PubMed] [Google Scholar]
- 2.Alexander C. Votruba M. Pesch UE. Thiselton DL. Mayer S. Moore A. Rodriguez M. Kellner U. Leo-Kottler B. Auburger G. Bhattacharya SS. Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 3.Amerik A. Antonov VK. Ostroumova NI. Rotanova TV. Chistiakova LG. [Cloning, structure and expression of the full-size lon gene in Escherichia coli coding for ATP-dependent La-proteinase] Bioorg Khim. 1990;16:869–880. [PubMed] [Google Scholar]
- 4.Annex BH. Kraus WE. Dohm GL. Williams RS. Mitochondrial biogenesis in striated muscles: rapid induction of citrate synthase mRNA by nerve stimulation. Am J Physiol. 1991;260:C266–C270. doi: 10.1152/ajpcell.1991.260.2.C266. [DOI] [PubMed] [Google Scholar]
- 5.Antunes F. Cadenas E. Brunk UT. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J. 2001;356:549–555. doi: 10.1042/0264-6021:3560549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arimura S. Yamamoto J. Aida GP. Nakazono M. Tsutsumi N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc Natl Acad Sci U S A. 2004;101:7805–7808. doi: 10.1073/pnas.0401077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakala H. Delaval E. Hamelin M. Bismuth J. Borot-Laloi C. Corman B. Friguet B. Changes in rat liver mitochondria with aging: Lon protease-like reactivity and N(epsilon)-carboxymethyllysine accumulation in the matrix. Eur J Biochem. 2003;270:2295–2302. doi: 10.1046/j.1432-1033.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- 8.Baker DJ. Betik AC. Krause DJ. Hepple RT. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. J Gerontol A Biol Sci Med Sci. 2006;61:675–684. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- 9.Baricault L. Segui B. Guegand L. Olichon A. Valette A. Larminat F. Lenaers G. OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res. 2007;313:3800–3808. doi: 10.1016/j.yexcr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Barja G. Free radicals and aging. Trends Neurosci. 2004;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Barriocanal JG. Bonifacino JS. Yuan L. Sandoval IV. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986;261:16755–16763. [PubMed] [Google Scholar]
- 12.Barsoum MJ. Yuan H. Gerencser AA. Liot G. Kushnareva Y. Graber S. Kovacs I. Lee WD. Waggoner J. Cui J. White AD. Bossy B. Martinou JC. Youle RJ. Lipton SA. Ellisman MH. Perkins GA. Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartrons R. Caro J. Hypoxia, glucose metabolism and the Warburg's effect. J Bioenerg Biomembr. 2007;39:223–229. doi: 10.1007/s10863-007-9080-3. [DOI] [PubMed] [Google Scholar]
- 14.Baur JA. Pearson KJ. Price NL. Jamieson HA. Lerin C. Kalra A. Prabhu VV. Allard JS. Lopez-Lluch G. Lewis K. Pistell PJ. Poosala S. Becker KG. Boss O. Gwinn D. Wang M. Ramaswamy S. Fishbein KW. Spencer RG. Lakatta EG. Le Couteur D. Shaw RJ. Navas P. Puigserver P. Ingram DK. de Cabo R. Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baynes JW. Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Benard G. Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal. 2008;10:1313–1342. doi: 10.1089/ars.2007.2000. [DOI] [PubMed] [Google Scholar]
- 17.Bennett MC. The role of alpha-synuclein in neurodegenerative diseases. Pharmacol Ther. 2005;105:311–331. doi: 10.1016/j.pharmthera.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Bergamini E. Autophagy: a cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. Mol Aspects Med. 2006;27:403–410. doi: 10.1016/j.mam.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Bhardwaj RD. Curtis MA. Spalding KL. Buchholz BA. Fink D. Bjork-Eriksson T. Nordborg C. Gage FH. Druid H. Eriksson PS. Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bossy-Wetzel E. Barsoum MJ. Godzik A. Schwarzenbacher R. Lipton SA. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr Opin Cell Biol. 2003;15:706–716. doi: 10.1016/j.ceb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Bota DA. Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 22.Bota DA. Ngo JK. Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic Biol Med. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Bota DA. Van Remmen H. Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 24.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 26.Brun A. Brunk U. Heavy metal localization and age related accumulation in the rat nervous system: a histochemical and atomic absorption spectrophotometric study. Histochemie. 1973;34:333–342. doi: 10.1007/BF00306305. [DOI] [PubMed] [Google Scholar]
- 27.Brunk UT. Jones CB. Sohal RS. A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res. 1992;275:395–403. doi: 10.1016/0921-8734(92)90042-n. [DOI] [PubMed] [Google Scholar]
- 28.Brunk UT. Neuzil J. Eaton JW. Lysosomal involvement in apoptosis. Redox Rep. 2001;6:91–97. doi: 10.1179/135100001101536094. [DOI] [PubMed] [Google Scholar]
- 29.Brunk UT. Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell functions. Free Radic Biol Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 30.Brunk UT. Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 31.Bulteau AL. Lundberg KC. Ikeda-Saito M. Isaya G. Szweda LI. Reversible redox-dependent modulation of mitochondrial aconitase and proteolytic activity during in vivo cardiac ischemia/reperfusion. Proc Natl Acad Sci U S A. 2005;102:5987–5991. doi: 10.1073/pnas.0501519102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulteau AL. Szweda LI. Friguet B. Mitochondrial protein oxidation and degradation in response to oxidative stress and aging. Exp Gerontol. 2006;41:653–657. doi: 10.1016/j.exger.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Burnet FM. A genetic interpretation of ageing. Lancet. 1973;2:480–483. doi: 10.1016/s0140-6736(73)92074-6. [DOI] [PubMed] [Google Scholar]
- 34.Busch KB. Bereiter-Hahn J. Wittig I. Schagger H. Jendrach M. Mitochondrial dynamics generate equal distribution but patchwork localization of respiratory complex I. Mol Membr Biol. 2006;23:509–520. doi: 10.1080/09687860600877292. [DOI] [PubMed] [Google Scholar]
- 35.Cao Y. Espinola JA. Fossale E. Massey AC. Cuervo AM. MacDonald ME. Cotman SL. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J Biol Chem. 2006;281:20483–20493. doi: 10.1074/jbc.M602180200. [DOI] [PubMed] [Google Scholar]
- 36.Cao Z. Wanagat J. McKiernan SH. Aiken JM. Mitochondrial DNA deletion mutations are concomitant with ragged red regions of individual, aged muscle fibers: analysis by laser-capture microdissection. Nucleic Acids Res. 2001;29:4502–4508. doi: 10.1093/nar/29.21.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavallini G. Donati A. Taddei M. Bergamini E. Evidence for selective mitochondrial autophagy and failure in aging. Autophagy. 2007;3:26–27. doi: 10.4161/auto.3268. [DOI] [PubMed] [Google Scholar]
- 38.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan D. Hideshima T. Mitsiades C. Richardson P. Anderson KC. Proteasome inhibitor therapy in multiple myeloma. Mol Cancer Ther. 2005;4:686–692. doi: 10.1158/1535-7163.MCT-04-0338. [DOI] [PubMed] [Google Scholar]
- 40.Chen H. Chomyn A. Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 41.Chen H. Detmer SA. Ewald AJ. Griffin EE. Fraser SE. Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H. McCaffery JM. Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Chowers I. Wong R. Dentchev T. Farkas RH. Iacovelli J. Gunatilaka TL. Medeiros NE. Presley JB. Campochiaro PA. Curcio CA. Dunaief JL. Zack DJ. The iron carrier transferrin is upregulated in retinas from patients with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:2135–2140. doi: 10.1167/iovs.05-1135. [DOI] [PubMed] [Google Scholar]
- 44.Chu CT. Zhu J. Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy. 2007;3:663–666. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cipolat S. Martins de Brito O. Dal Zilio B. Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cipolat S. Rudka T. Hartmann D. Costa V. Serneels L. Craessaerts K. Metzger K. Frezza C. Annaert W. D'Adamio L. Derks C. Dejaegere T. Pellegrini L. D'Hooge R. Scorrano L. De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Cipolat S. Scorrano L. To fuse and to protect: a novel role for CED-9 in mitochondrial morphology reveals an ancient function. Cell Death Differ. 2006;13:1833–1834. doi: 10.1038/sj.cdd.4402005. [DOI] [PubMed] [Google Scholar]
- 48.Comfort A. Ageing: the biology of senescence. New. 1979;York:Elsevier. [Google Scholar]
- 49.Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- 50.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuervo AM. Bergamini E. Brunk UT. Droge W. Ffrench M. Terman A. Autophagy and aging: the importance of maintaining “clean” cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 52.Cuervo AM. Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 53.Cuervo AM. Palmer A. Rivett AJ. Knecht E. Degradation of proteasomes by lysosomes in rat liver. Eur J Biochem. 1995;227:792–800. doi: 10.1111/j.1432-1033.1995.tb20203.x. [DOI] [PubMed] [Google Scholar]
- 54.Curtis HJ. A composite theory of aging. Gerontologist. 1966;6:143–149. doi: 10.1093/geront/6.3_part_1.143. [DOI] [PubMed] [Google Scholar]
- 55.Davis WL. Jacoby BH. Goodman DB. Immunolocalization of ubiquitin in degenerating insect flight muscle. Histochem J. 1994;26:298–305. doi: 10.1007/BF00157762. [DOI] [PubMed] [Google Scholar]
- 56.de Grey AD. A proposed refinement of the mitochondrial free radical theory of aging. Bioessays. 1997;19:161–166. doi: 10.1002/bies.950190211. [DOI] [PubMed] [Google Scholar]
- 57.de Grey ADNJ. The mitochondrial free radical theory of aging. Austin, TX: RG Landes; 1999. [Google Scholar]
- 58.de Milito A. Fais S. Tumor acidity: chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779–786. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 59.Dean RT. Dunlop R. Hume P. Rodgers KJ. Proteolytic “defences” and the accumulation of oxidized polypeptides in cataractogenesis and atherogenesis. Biochem Soc Symp. 2003;70:135–146. doi: 10.1042/bss0700135. [DOI] [PubMed] [Google Scholar]
- 60.DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004;4:301–305. doi: 10.1016/j.coph.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Detmer SA. Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 62.DiMauro S. Mitochondrial myopathies. Curr Opin Rheumatol. 2006;18:636–641. doi: 10.1097/01.bor.0000245729.17759.f2. [DOI] [PubMed] [Google Scholar]
- 63.Donati A. Cavallini G. Paradiso C. Vittorini S. Pollera M. Gori Z. Bergamini E. Age-related changes in the autophagic proteolysis of rat isolated liver cells: effects of antiaging dietary restrictions. J Gerontol A Biol Sci Med Sci. 2001;56:B375–B383. doi: 10.1093/gerona/56.9.b375. [DOI] [PubMed] [Google Scholar]
- 64.Doulias PT. Christoforidis S. Brunk UT. Galaris D. Endosomal and lysosomal effects of desferrioxamine: protection of HeLa cells from hydrogen peroxide-induced DNA damage and induction of cell-cycle arrest. Free Radic Biol Med. 2003;35:719–728. doi: 10.1016/s0891-5849(03)00396-4. [DOI] [PubMed] [Google Scholar]
- 65.Droga-Mazovec G. Bojic L. Petelin A. Ivanova S. Romih R. Repnik U. Salvesen GS. Stoka V. Turk V. Turk B. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J Biol Chem. 2008;283:19140–19150. doi: 10.1074/jbc.M802513200. [DOI] [PubMed] [Google Scholar]
- 66.Druzhyna NM. Wilson GL. LeDoux SP. Mitochondrial DNA repair in aging and disease. Mech Ageing Dev. 2008;129:383–390. doi: 10.1016/j.mad.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunlop RA. Brunk UT. Rodgers KJ. Oxidized proteins: mechanisms of removal and consequences of accumulation. IUBMB Life. 2009;61:522–527. doi: 10.1002/iub.189. [DOI] [PubMed] [Google Scholar]
- 68.Dunlop RA. Dean RT. Rodgers KJ. The impact of specific oxidized amino acids on protein turnover in J774 cells. Biochem J. 2008;410:131–140. doi: 10.1042/BJ20070161. [DOI] [PubMed] [Google Scholar]
- 69.Duvezin-Caubet S. Jagasia R. Wagener J. Hofmann S. Trifunovic A. Hansson A. Chomyn A. Bauer MF. Attardi G. Larsson NG. Neupert W. Reichert AS. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 70.Duvezin-Caubet S. Koppen M. Wagener J. Zick M. Israel L. Bernacchia A. Jagasia R. Rugarli EI. Imhof A. Neupert W. Langer T. Reichert AS. OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell. 2007;18:3582–3590. doi: 10.1091/mbc.E07-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edinger AL. Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Elleder M. Sokolova J. Hrebicek M. Follow-up study of subunit c of mitochondrial ATP synthase (SCMAS) in Batten disease and in unrelated lysosomal disorders. Acta Neuropathol. 1997;93:379–390. doi: 10.1007/s004010050629. [DOI] [PubMed] [Google Scholar]
- 73.Elmore SP. Qian T. Grissom SF. Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 74.Eskelinen EL. New insights into the mechanisms of macroautophagy in mammalian cells. Int Rev Cell Mol Biol. 2008;266:207–247. doi: 10.1016/S1937-6448(07)66005-5. [DOI] [PubMed] [Google Scholar]
- 75.Eskelinen EL. Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–673. doi: 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 76.Essner E. Novikoff AB. Human hepatocellular pigments and lysosomes. J Ultrastruct Res. 1960;3:374–391. doi: 10.1016/s0022-5320(60)90016-2. [DOI] [PubMed] [Google Scholar]
- 77.Ethen CM. Reilly C. Feng X. Olsen TW. Ferrington DA. Age-related macular degeneration and retinal protein modification by 4-hydroxy-2-nonenal. Invest Ophthalmol Vis Sci. 2007;48:3469–3479. doi: 10.1167/iovs.06-1058. [DOI] [PubMed] [Google Scholar]
- 78.Fattoretti P. Bertoni-Freddari C. Casoli T. Di Stefano G. Solazzi M. Corvi E. Morphometry of age pigment (lipofuscin) and of ceroid pigment deposits associated with vitamin E deficiency. Arch Gerontol Geriatr. 2002;34:263–268. doi: 10.1016/s0167-4943(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 79.Freyssenet D. Berthon P. Denis C. Mitochondrial biogenesis in skeletal muscle in response to endurance exercises. Arch Physiol Biochem. 1996;104:129–141. doi: 10.1076/apab.104.2.129.12878. [DOI] [PubMed] [Google Scholar]
- 80.Frezza C. Cipolat S. Martins de Brito O. Micaroni M. Beznoussenko GV. Rudka T. Bartoli D. Polishuck RS. Danial NN. De Strooper B. Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 81.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 82.Furber JD. Extracellular glycation crosslinks: prospects for removal. Rejuvenation Res. 2006;9:274–278. doi: 10.1089/rej.2006.9.274. [DOI] [PubMed] [Google Scholar]
- 83.Gallant J. Palmer L. Error propagation in viable cells. Mech Ageing Dev. 1979;10:27–38. doi: 10.1016/0047-6374(79)90068-x. [DOI] [PubMed] [Google Scholar]
- 84.Gerstbrein B. Stamatas G. Kollias N. Driscoll M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 2005;4:127–137. doi: 10.1111/j.1474-9726.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- 85.Ghadially FN. Ultrastructural pathology of the cell and matrix. London: Butterworths; 1975. [Google Scholar]
- 86.Ghaffari A. Kilani RT. Ghahary A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J Invest Dermatol. 2009;129:340–347. doi: 10.1038/jid.2008.253. [DOI] [PubMed] [Google Scholar]
- 87.Golden TR. Hinerfeld DA. Melov S. Oxidative stress and aging: beyond correlation. Aging Cell. 2002;1:117–123. doi: 10.1046/j.1474-9728.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 88.Goldenthal MJ. Weiss HR. Marin-Garcia J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol Cell Biochem. 2004;265:97–106. doi: 10.1023/b:mcbi.0000044321.17680.a2. [DOI] [PubMed] [Google Scholar]
- 89.Gomes LC. Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 90.Griparic L. van der Wel NN. Orozco IJ. Peters PJ. van der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem. 2004;279:18792–18798. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- 91.Grote J. Tissue respiration. In: Schmidt RF, editor; Thews G, editor. Human physiology. Berlin: Springer-Verlag; 1989. pp. 598–613. [Google Scholar]
- 92.Grune T. Jung T. Merker K. Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and “aggresomes” during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 93.Hales KG. Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 94.Halliwell B. Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 1999. [Google Scholar]
- 95.Hamacher-Brady A. Brady NR. Logue SE. Sayen MR. Jinno M. Kirshenbaum LA. Gottlieb RA. Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 96.Hardy J. Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 97.Harley CB. Pollard JW. Chamberlain JW. Stanners CP. Goldstein S. Protein synthetic errors do not increase during aging of cultured human fibroblasts. Proc Natl Acad Sci U S A. 1980;77:1885–1889. doi: 10.1073/pnas.77.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;211:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 99.Harman D. The biologic clock: the mitochondria? J Am Geriat Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 100.Hepple RT. Baker DJ. McConkey M. Murynka T. Norris R. Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation Res. 2006;9:219–222. doi: 10.1089/rej.2006.9.219. [DOI] [PubMed] [Google Scholar]
- 101.Herlan M. Bornhovd C. Hell K. Neupert W. Reichert AS. Alternative topogenesis of Mgm1 and mitochondrial morphology depend on ATP and a functional import motor. J Cell Biol. 2004;165:167–173. doi: 10.1083/jcb.200403022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hipkiss AR. Non-oxidative modification of DNA and proteins. In: von Zglinicki T., editor. Aging at the molecular level. Dordrecht: Kluwer; 2003. pp. 145–177. [Google Scholar]
- 103.Hochhauser D. Relevance of mitochondrial DNA in cancer. Lancet. 2000;356:181–182. doi: 10.1016/S0140-6736(00)02475-2. [DOI] [PubMed] [Google Scholar]
- 104.Ishihara N. Eura Y. Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 105.Ishihara N. Fujita Y. Oka T. Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishihara N. Jofuku A. Eura Y. Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem Biophys Res Commun. 2003;301:891–898. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 107.Iyer LM. Leipe DD. Koonin EV. Aravind L. Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol. 2004;146:11–31. doi: 10.1016/j.jsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 108.James DI. Parone PA. Mattenberger Y. Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 109.Jariel-Encontre I. Bossis G. Piechaczyk M. Ubiquitin-independent degradation of proteins by the proteasome. Biochim Biophys Acta. 2008;1786:153–177. doi: 10.1016/j.bbcan.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 110.Jolly RD. Douglas BV. Davey PM. Roiri JE. Lipofuscin in bovine muscle and brain: a model for studying age pigment. Gerontology. 1995;41:283–295. doi: 10.1159/000213750. [DOI] [PubMed] [Google Scholar]
- 111.Kaganovich D. Kopito R. Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karbowski M. Arnoult D. Chen H. Chan DC. Smith CL. Youle RJ. Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol. 2004;164:493–499. doi: 10.1083/jcb.200309082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karbowski M. Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 114.Kaser M. Langer T. Protein degradation in mitochondria. Semin Cell Dev Biol. 2000;11:181–190. doi: 10.1006/scdb.2000.0166. [DOI] [PubMed] [Google Scholar]
- 115.Kaushik S. Cuervo AM. Autophagy as a cell-repair mechanism: activation of chaperone-mediated autophagy during oxidative stress. Mol Aspects Med. 2006;27:444–454. doi: 10.1016/j.mam.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khrapko K. Bodyak N. Thilly WG. van Orsouw NJ. Zhang X. Coller HA. Perls TT. Upton M. Vijg J. Wei JY. Cell-by-cell scanning of whole mitochondrial genomes in aged human heart reveals a significant fraction of myocytes with clonally expanded deletions. Nucleic Acids Res. 1999;27:2434–2441. doi: 10.1093/nar/27.11.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kikugawa K. Kato T. Beppu M. Hayasaka A. Fluorescent and cross-linked proteins formed by free radical and aldehyde species generated during lipid oxidation. Adv Exp Med Biol. 1989;266:345–356; discussion 357. doi: 10.1007/978-1-4899-5339-1_25. [DOI] [PubMed] [Google Scholar]
- 118.Kim EH. Choi KS. A critical role of superoxide anion in selenite-induced mitophagic cell death. Autophagy. 2008;4:76–78. doi: 10.4161/auto.5119. [DOI] [PubMed] [Google Scholar]
- 119.Kim I. Rodriguez-Enriquez S. Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kirkwood TB. DNA, mutations and aging. Mutat Res. 1989;219:1–7. doi: 10.1016/0921-8734(89)90035-0. [DOI] [PubMed] [Google Scholar]
- 121.Kissova I. Deffieu M. Manon S. Camougrand N. Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem. 2004;279:39068–39074. doi: 10.1074/jbc.M406960200. [DOI] [PubMed] [Google Scholar]
- 122.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 123.Koneff H. Beiträge zur Kenntniss der Nervenzellen der peripheren Ganglien. Mitt Naturforsch Gesellsch Bern. 1886;1143–1168:13–44. [Google Scholar]
- 124.Ku HH. Brunk UT. Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic Biol Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- 125.Kuester D. Lippert H. Roessner A. Krueger S. The cathepsin family and their role in colorectal cancer. Pathol Res Pract. 2008;204:491–500. doi: 10.1016/j.prp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 126.Kurz T. Brunk UT. Autophagy of HSP70 and chelation of lysosomal iron in a non-redox-active form. Autophagy. 2009;5:93–95. doi: 10.4161/auto.5.1.7248. [DOI] [PubMed] [Google Scholar]
- 127.Kurz T. Leake A. Von Zglinicki T. Brunk UT. Relocalized redox-active lysosomal iron is an important mediator of oxidative-stress-induced DNA damage. Biochem J. 2004;378:1039–1045. doi: 10.1042/BJ20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kurz T. Terman A. Brunk UT. Autophagy, ageing and apoptosis: the role of oxidative stress and lysosomal iron. Arch Biochem Biophys. 2007;462:220–230. doi: 10.1016/j.abb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 129.Kurz T. Terman A. Gustafsson B. Brunk UT. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol. 2008;129:389–406. doi: 10.1007/s00418-008-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kvam E. Goldfarb DS. Nucleus-vacuole junctions and piecemeal microautophagy of the nucleus in S. cerevisiae. Autophagy. 2007;3:85–92. doi: 10.4161/auto.3586. [DOI] [PubMed] [Google Scholar]
- 131.Lagouge M. Argmann C. Gerhart-Hines Z. Meziane H. Lerin C. Daussin F. Messadeq N. Milne J. Lambert P. Elliott P. Geny B. Laakso M. Puigserver P. Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 132.Lane N. Power, sex, suicide: mitochondria and the meaning of life. New York: Oxford University Press; 2005. [Google Scholar]
- 133.Lapointe J. Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J Biol Chem. 2008;283:26217–2627. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee AT. Cerami A. Role of glycation in aging. Ann N Y Acad Sci. 1992;663:63–70. doi: 10.1111/j.1749-6632.1992.tb38649.x. [DOI] [PubMed] [Google Scholar]
- 135.Lee FY. Lee TS. Pan CC. Huang AL. Chau LY. Colocalization of iron and ceroid in human atherosclerotic lesions. Atherosclerosis. 1998;138:281–288. doi: 10.1016/s0021-9150(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 136.Lee HC. Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005;37:822–834. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 137.Lee HC. Yin PH. Chi CW. Wei YH. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci. 2002;9:517–526. doi: 10.1007/BF02254978. [DOI] [PubMed] [Google Scholar]
- 138.Lee I. Suzuki CK. Functional mechanics of the ATP-dependent Lon protease: lessons from endogenous protein and synthetic peptide substrates. Biochim Biophys Acta. 2008;1784:727–735. doi: 10.1016/j.bbapap.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee S. Jeong SY. Lim WC. Kim S. Park YY. Sun X. Youle RJ. Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 140.Lee YJ. Jeong SY. Karbowski M. Smith CL. Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Legros F. Lombes A. Frachon P. Rojo M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 143.Lopez-Lluch G. Irusta PM. Navas P. de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lorenzo K. Ton P. Clark JL. Coulibaly S. Mach L. Invasive properties of murine squamous carcinoma cells: secretion of matrix-degrading cathepsins is attributable to a deficiency in the mannose 6-phosphate/insulin-like growth factor II receptor. Cancer Res. 2000;60:4070–4076. [PubMed] [Google Scholar]
- 145.Lu B. Yadav S. Shah PG. Liu T. Tian B. Pukszta S. Villaluna N. Kutejova E. Newlon CS. Santos JH. Suzuki CK. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J Biol Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- 146.Luzio JP. Pryor PR. Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 147.Malka F. Guillery O. Cifuentes-Diaz C. Guillou E. Belenguer P. Lombes A. Rojo M. Separate fusion of outer and inner mitochondrial membranes. EMBO Rep. 2005;6:853–839. doi: 10.1038/sj.embor.7400488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marcillat O. Zhang Y. Lin SW. Davies KJ. Mitochondria contain a proteolytic system which can recognize and degrade oxidatively-denatured proteins. Biochem J. 1988;254:677–683. doi: 10.1042/bj2540677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marin-Garcia J. Goldenthal MJ. Moe GW. Mitochondrial pathology in cardiac failure. Cardiovasc Res. 2001;49:17–26. doi: 10.1016/s0008-6363(00)00241-8. [DOI] [PubMed] [Google Scholar]
- 150.Martinez DE. Mortality patterns suggest lack of senescence in hydra. Exp Gerontol. 1998;33:217–225. doi: 10.1016/s0531-5565(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 151.Martinez-Zaguilan R. Lynch RM. Martinez GM. Gillies RJ. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol. 1993;265:C1015–C1029. doi: 10.1152/ajpcell.1993.265.4.C1015. [DOI] [PubMed] [Google Scholar]
- 152.Mattenberger Y. James DI. Martinou JC. Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin. FEBS Lett. 2003;538:53–59. doi: 10.1016/s0014-5793(03)00124-8. [DOI] [PubMed] [Google Scholar]
- 153.McNaught KS. Belizaire R. Isacson O. Jenner P. Olanow CW. Altered proteasomal function in sporadic Parkinson's disease. Exp Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 154.McNaught KS. Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson's disease. Neurosci Lett. 2001;297:191–194. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- 155.McNaught KS. Mytilineou C. Jnobaptiste R. Yabut J. Shashidharan P. Jennert P. Olanow CW. Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J Neurochem. 2002;81:301–306. doi: 10.1046/j.1471-4159.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 156.Medvedev ZA. An attempt at a rational classification of theories of ageing. Biol Rev Camb Philos Soc. 1990;65:375–398. doi: 10.1111/j.1469-185x.1990.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 157.Meeusen S. DeVay R. Block J. Cassidy-Stone A. Wayson S. McCaffery JM. Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 158.Meeusen S. McCaffery JM. Nunnari J. Mitochondrial fusion intermediates revealed in vitro. Science. 2004;305:1747–1752. doi: 10.1126/science.1100612. [DOI] [PubMed] [Google Scholar]
- 159.Meeusen SL. Nunnari J. How mitochondria fuse. Curr Opin Cell Biol. 2005;17:389–394. doi: 10.1016/j.ceb.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 160.Mellgren RL. Specificities of cell permeant peptidyl inhibitors for the proteinase activities of mu-calpain and the 20 S proteasome. J Biol Chem. 1997;272:29899–29903. doi: 10.1074/jbc.272.47.29899. [DOI] [PubMed] [Google Scholar]
- 161.Menzies RA. Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. J Biol Chem. 1971;246:2425–2429. [PubMed] [Google Scholar]
- 162.Miettinen R. Reunanen H. Vinblastine-induced autophagocytosis in cultured fibroblasts. Comp Biochem Physiol C. 1991;99:29–34. doi: 10.1016/0742-8413(91)90070-a. [DOI] [PubMed] [Google Scholar]
- 163.Miwa S. Lawless C. von Zglinicki T. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell. 2008;7:920–923. doi: 10.1111/j.1474-9726.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mogk A. Dougan D. Weibezahn J. Schlieker C. Turgay K. Bukau B. Broad yet high substrate specificity: the challenge of AAA+ proteins. J Struct Biol. 2004;146:90–98. doi: 10.1016/j.jsb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 165.Montcourrier P. Silver I. Farnoud R. Bird I. Rochefort H. Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin Exp Metastasis. 1997;15:382–392. doi: 10.1023/a:1018446104071. [DOI] [PubMed] [Google Scholar]
- 166.Moreira PI. Siedlak SL. Wang X. Santos MS. Oliveira CR. Tabaton M. Nunomura A. Szweda LI. Aliev G. Smith MA. Zhu X. Perry G. Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy. 2007;3:614–615. doi: 10.4161/auto.4872. [DOI] [PubMed] [Google Scholar]
- 167.Muller FL. Lustgarten MS. Jang Y. Richardson A. Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 168.Nagino M. Tanaka M. Nishikimi M. Nimura Y. Kubota H. Kanai M. Kato T. Ozawa T. Stimulated rat liver mitochondrial biogenesis after partial hepatectomy. Cancer Res. 1989;49:4913–4918. [PubMed] [Google Scholar]
- 169.Navratil M. Terman A. Arriaga EA. Giant mitochondria do not fuse and exchange their contents with normal mitochondria. Exp Cell Res. 2008;314:164–172. doi: 10.1016/j.yexcr.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 170.Neuzil J. Zhao M. Ostermann G. Sticha M. Gellert N. Weber C. Eaton JW. Brunk UT. Alpha-tocopheryl succinate, an agent with in vivo anti-tumour activity, induces apoptosis by causing lysosomal instability. Biochem J. 2002;362:709–715. doi: 10.1042/0264-6021:3620709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nilsson SE. Sundelin SP. Wihlmark U. Brunk UT. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol. 2003;106:13–16. doi: 10.1023/a:1022419606629. [DOI] [PubMed] [Google Scholar]
- 172.Nisoli E. Tonello C. Cardile A. Cozzi V. Bracale R. Tedesco L. Falcone S. Valerio A. Cantoni O. Clementi E. Moncada S. Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 173.Olichon A. Baricault L. Gas N. Guillou E. Valette A. Belenguer P. Lenaers G. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 174.Ono T. Isobe K. Nakada K. Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–275. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]
- 175.Orgel LE. Ageing of clones of mammalian cells. Nature. 1973;243:441–445. doi: 10.1038/243441a0. [DOI] [PubMed] [Google Scholar]
- 176.Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997;77:425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- 177.Pandey UB. Nie Z. Batlevi Y. McCray BA. Ritson GP. Nedelsky NB. Schwartz SL. DiProspero NA. Knight MA. Schuldiner O. Padmanabhan R. Hild M. Berry DL. Garza D. Hubbert CC. Yao TP. Baehrecke EH. Taylor JP. HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature. 2007;447:859–863. doi: 10.1038/nature05853. [DOI] [PubMed] [Google Scholar]
- 178.Perfettini JL. Roumier T. Kroemer G. Mitochondrial fusion and fission in the control of apoptosis. Trends Cell Biol. 2005;15:179–183. doi: 10.1016/j.tcb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 179.Pich S. Bach D. Briones P. Liesa M. Camps M. Testar X. Palacin M. Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005;14:1405–1415. doi: 10.1093/hmg/ddi149. [DOI] [PubMed] [Google Scholar]
- 180.Pletjushkina OY. Lyamzaev KG. Popova EN. Nepryakhina OK. Ivanova OY. Domnina LV. Chernyak BV. Skulachev VP. Effect of oxidative stress on dynamics of mitochondrial reticulum. Biochim Biophys Acta. 2006;1757:518–524. doi: 10.1016/j.bbabio.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 181.Podgorski I. Sloane BF. Cathepsin B and its role(s) in cancer progression. Biochem Soc Symp. 2003;70:263–276. doi: 10.1042/bss0700263. [DOI] [PubMed] [Google Scholar]
- 182.Priault M. Salin B. Schaeffer J. Vallette FM. di Rago JP. Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005;12:1613–1621. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 183.Puigserver P. Adelmant G. Wu Z. Fan M. Xu J. O'Malley B. Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 184.Puigserver P. Wu Z. Park CW. Graves R. Wright M. Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 185.Qiang W. Weiqiang K. Qing Z. Pengju Z. Yi L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp Mol Med. 2007;39:535–543. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- 186.Rattan SI. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol. 1996;31:33–47. doi: 10.1016/0531-5565(95)02022-5. [DOI] [PubMed] [Google Scholar]
- 187.Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 188.Reznick RM. Zong H. Li J. Morino K. Moore IK. Yu HJ. Liu ZX. Dong J. Mustard KJ. Hawley SA. Befroy D. Pypaert M. Hardie DG. Young LH. Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rochefort H. Capony F. Garcia M. Cathepsin D: a protease involved in breast cancer metastasis. Cancer Metastasis Rev. 1990;9:321–331. doi: 10.1007/BF00049522. [DOI] [PubMed] [Google Scholar]
- 190.Rodgers KJ. Dean RT. Metabolism of protein-bound DOPA in mammals. Int J Biochem Cell Biol. 2000;32:945–955. doi: 10.1016/s1357-2725(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 191.Rodgers KJ. Hume PM. Morris JG. Dean RT. Evidence for L-dopa incorporation into cell proteins in patients treated with levodopa. J Neurochem. 2006;98:1061–1067. doi: 10.1111/j.1471-4159.2006.03941.x. [DOI] [PubMed] [Google Scholar]
- 192.Rodgers KJ. Wang H. Fu S. Dean RT. Biosynthetic incorporation of oxidized amino acids into proteins and their cellular proteolysis. Free Radic Biol Med. 2002;32:766–775. doi: 10.1016/s0891-5849(02)00768-2. [DOI] [PubMed] [Google Scholar]
- 193.Rodriguez-Cuenca S. Monjo M. Gianotti M. Proenza AM. Roca P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab. 2007;292:E340–E346. doi: 10.1152/ajpendo.00175.2006. [DOI] [PubMed] [Google Scholar]
- 194.Rohrbach S. Niemann B. Abushouk AM. Holtz J. Caloric restriction and mitochondrial function in the ageing myocardium. Exp Gerontol. 2006;41:525–531. doi: 10.1016/j.exger.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 195.Rojo M. Legros F. Chateau D. Lombes A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115:1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 196.Ryhanen T. Hyttinen JM. Kopitz J. Rilla K. Kuusisto E. Mannermaa E. Viiri J. Holmberg CI. Immonen I. Meri S. Parkkinen J. Eskelinen EL. Uusitalo H. Salminen A. Kaarniranta K. Crosstalk between Hsp70 molecular chaperone, lysosomes and proteasomes in autophagy-mediated proteolysis in human retinal pigment epithelial cells. J Cell Mol Med. doi: 10.1111/j.1582-4934.2008.00577.x. (in press) doi:10.1111/j.1582–4934.2008.00577.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sandoval H. Thiagarajan P. Dasgupta SK. Schumacher A. Prchal JT. Chen M. Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sandoval IV. Chen JW. Yuan L. August JT. Lysosomal integral membrane glycoproteins are expressed at high levels in the inclusion bodies of I-cell disease fibroblasts. Arch Biochem Biophys. 1989;271:157–167. doi: 10.1016/0003-9861(89)90266-x. [DOI] [PubMed] [Google Scholar]
- 199.Sano S. Inoue S. Tanabe Y. Sumiya C. Koike S. Significance of mitochondria for porphyrin and heme biosynthesis. Science. 1959;129:275–276. doi: 10.1126/science.129.3344.275. [DOI] [PubMed] [Google Scholar]
- 200.Sanz A. Pamplona R. Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- 201.Schutt F. Bergmann M. Holz FG. Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- 202.Seglen PO. Gordon PB. Holen I. Non-selective autophagy. Semin Cell Biol. 1990;1:441–448. [PubMed] [Google Scholar]
- 203.Semenza GL. Mitochondrial autophagy: life and breath of the cell. Autophagy. 2008;4:534–536. doi: 10.4161/auto.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Semenza GL. Artemov D. Bedi A. Bhujwalla Z. Chiles K. Feldser D. Laughner E. Ravi R. Simons J. Taghavi P. Zhong H. The metabolism of tumours: 70 years later. Novartis Found Symp. 2001;240:251–260. [PubMed] [Google Scholar]
- 205.Severino J. Allen RG. Balin S. Balin A. Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–171. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 206.Shay JW. Wright WE. Senescence and immortalization: role of telomeres and telomerase. Carcinogenesis. 2005;26:867–874. doi: 10.1093/carcin/bgh296. [DOI] [PubMed] [Google Scholar]
- 207.Shintani T. Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shitara H. Kaneda H. Sato A. Inoue K. Ogura A. Yonekawa H. Hayashi JI. Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis. Genetics. 2000;156:1277–1284. doi: 10.1093/genetics/156.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shringarpure R. Grune T. Mehlhase J. Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 210.Simonsen A. Cumming RC. Brech A. Isakson P. Schubert DR. Finley KD. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4:176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 211.Smirnova E. Griparic L. Shurland DL. van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sohal RS. Mockett RJ. Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- 213.Sohal RS. Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Song Z. Chen H. Fiket M. Alexander C. Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 216.Stadtman ER. Starke-Reed PE. Oliver CN. Carney JM. Floyd RA. Protein modification in aging. EXS. 1992;62:64–72. doi: 10.1007/978-3-0348-7460-1_7. [DOI] [PubMed] [Google Scholar]
- 217.Stojanovski D. Koutsopoulos OS. Okamoto K. Ryan MT. Levels of human Fis1 at the mitochondrial outer membrane regulate mitochondrial morphology. J Cell Sci. 2004;117:1201–1210. doi: 10.1242/jcs.01058. [DOI] [PubMed] [Google Scholar]
- 218.Stolzing A. Jones E. McGonagle D. Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 219.Stolzing A. Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 220.Stone JR. Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 221.Strehler BL. Time, cells, and aging. New York: Academic Press; 1977. [Google Scholar]
- 222.Stroikin Y. Dalen H. Lööf S. Terman A. Inhibition of autophagy with 3-methyladenine results in impaired turnover of lysosomes and accumulation of lipofuscin-like material. Eur J Cell Biol. 2004;83:583–590. doi: 10.1078/0171-9335-00433. [DOI] [PubMed] [Google Scholar]
- 223.Suen DF. Norris KL. Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sullivan PG. Dragicevic NB. Deng JH. Bai Y. Dimayuga E. Ding Q. Chen Q. Bruce-Keller AJ. Keller JN. Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J Biol Chem. 2004;279:20699–20707. doi: 10.1074/jbc.M313579200. [DOI] [PubMed] [Google Scholar]
- 225.Sultana R. Perluigi M. Butterfield DA. Protein oxidation and lipid peroxidation in brain of subjects with Alzheimer's disease: insights into mechanism of neurodegeneration from redox proteomics. Antioxid Redox Signal. 2006;8:2021–2037. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- 226.Sutovsky P. Moreno RD. Ramalho-Santos J. Dominko T. Simerly C. Schatten G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol Reprod. 2000;63:582–590. doi: 10.1095/biolreprod63.2.582. [DOI] [PubMed] [Google Scholar]
- 227.Suzuki K. Ohsumi Y. Molecular machinery of autophagosome formation in yeast, Saccharomyces cerevisiae. FEBS Lett. 2007;581:2156–2161. doi: 10.1016/j.febslet.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 228.Szabadkai G. Simoni AM. Chami M. Wieckowski MR. Youle RJ. Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 229.Takano-Ohmuro H. Mukaida M. Kominami E. Morioka K. Autophagy in embryonic erythroid cells: its role in maturation. Eur J Cell Biol. 2000;79:759–764. doi: 10.1078/0171-9335-00096. [DOI] [PubMed] [Google Scholar]
- 230.Tal R. Winter G. Ecker N. Klionsky DJ. Abeliovich H. Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J Biol Chem. 2007;282:5617–5624. doi: 10.1074/jbc.M605940200. [DOI] [PubMed] [Google Scholar]
- 231.Terman A. The effect of age on formation and elimination of autophagic vacuoles in mouse hepatocytes. Gerontology. 1995;41:319–326. doi: 10.1159/000213753. [DOI] [PubMed] [Google Scholar]
- 232.Terman A. Brunk UT. Lipofuscin: mechanisms of formation and increase with age. APMIS. 1998;106:265–276. doi: 10.1111/j.1699-0463.1998.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 233.Terman A. Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;68:355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 234.Terman A. Brunk UT. Is aging the price for memory? Biogerontology. 2005;6:205–210. doi: 10.1007/s10522-005-7956-3. [DOI] [PubMed] [Google Scholar]
- 235.Terman A. Brunk UT. Oxidative stress, accumulation of biological “garbage,” and aging. Antioxid Redox Signal. 2006;8:197–204. doi: 10.1089/ars.2006.8.197. [DOI] [PubMed] [Google Scholar]
- 236.Terman A. Dalen H. Brunk UT. Ceroid/lipofuscin-loaded human fibroblasts show decreased survival time and diminished autophagocytosis during amino acid starvation. Exp Gerontol. 1999;34:943–957. doi: 10.1016/s0531-5565(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 237.Terman A. Dalen H. Eaton JW. Neuzil J. Brunk UT. Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol. 2003;38:863–876. doi: 10.1016/s0531-5565(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 238.Terman A. Dalen H. Eaton JW. Neuzil J. Brunk UT. Aging of cardiac myocytes in culture: oxidative stress, lipofuscin accumulation, and mitochondrial turnover. Ann N Y Acad Sci. 2004;1019:70–77. doi: 10.1196/annals.1297.015. [DOI] [PubMed] [Google Scholar]
- 239.Terman A. Gustafsson B. Brunk UT. The lysosomal-mitochondrial axis theory of postmitotic aging and cell death. Chem Biol Interact. 2006;163:29–37. doi: 10.1016/j.cbi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 240.Terman A. Gustafsson B. Brunk UT. Mitochondrial damage and intralysosomal degradation in cellular aging. Mol Aspects Med. 2006;27:471–482. doi: 10.1016/j.mam.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 241.Terman A. Gustafsson B. Brunk UT. Autophagy, organelles and ageing. J Pathol. 2007;211:134–143. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- 242.Terman A. Sandberg S. Proteasome inhibition enhances lipofuscin formation. Ann N Y Acad Sci. 2002;973:309–312. doi: 10.1111/j.1749-6632.2002.tb04657.x. [DOI] [PubMed] [Google Scholar]
- 243.Trifunovic A. Hansson A. Wredenberg A. Rovio AT. Dufour E. Khvorostov I. Spelbrink JN. Wibom R. Jacobs HT. Larsson NG. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Trifunovic A. Wredenberg A. Falkenberg M. Spelbrink JN. Rovio AT. Bruder CE. Bohlooly YM. Gidlof S. Oldfors A. Wibom R. Tornell J. Jacobs HT. Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 245.Turk V. Turk B. Guncar G. Turk D. Kos J. Lysosomal cathepsins: structure: role in antigen processing and presentation, and cancer. Adv Enzyme Regul. 2002;42:285–303. doi: 10.1016/s0065-2571(01)00034-6. [DOI] [PubMed] [Google Scholar]
- 246.Twig G. Elorza A. Molina AJ. Mohamed H. Wikstrom JD. Walzer G. Stiles L. Haigh SE. Katz S. Las G. Alroy J. Wu M. Py BF. Yuan J. Deeney JT. Corkey BE. Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Twig G. Hyde B. Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.van der Kraan PM. van den Berg WB. Osteoarthritis in the context of ageing and evolution: loss of chondrocyte differentiation block during ageing. Ageing Res Rev. 2008;7:106–113. doi: 10.1016/j.arr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 249.Victor BC. Sloane BF. Cysteine cathepsin non-inhibitory binding partners: modulating intracellular trafficking and function. Biol Chem. 2007;388:1131–1140. doi: 10.1515/BC.2007.150. [DOI] [PubMed] [Google Scholar]
- 250.Viguet-Carrin S. Roux JP. Arlot ME. Merabet Z. Leeming DJ. Byrjalsen I. Delmas PD. Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–1079. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 251.Vijg J. Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Voloboueva LA. Killilea DW. Atamna H. Ames BN. N-tert-butyl hydroxylamine, a mitochondrial antioxidant, protects human retinal pigment epithelial cells from iron overload: relevance to macular degeneration. FASEB J. 2007;21:4077–4086. doi: 10.1096/fj.07-8396com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang X. Quinn PJ. Vitamin E and its function in membranes. Prog Lipid Res. 1999;38:309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 254.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 255.Weinert BT. Timiras PS. Invited review: theories of aging. J Appl Physiol. 2003;95:1706–1716. doi: 10.1152/japplphysiol.00288.2003. [DOI] [PubMed] [Google Scholar]
- 256.Wenger DA. Coppola S. Liu SL. Insights into the diagnosis and treatment of lysosomal storage diseases. Arch Neurol. 2003;60:322–328. doi: 10.1001/archneur.60.3.322. [DOI] [PubMed] [Google Scholar]
- 257.White R. Morganstein D. Christian M. Seth A. Herzog B. Parker MG. Role of RIP140 in metabolic tissues: connections to disease. FEBS Lett. 2008;582:39–45. doi: 10.1016/j.febslet.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 258.Wihlmark U. Wrigstad A. Roberg K. Nilsson SE. Brunk UT. Lipofuscin accumulation in cultured retinal pigment epithelial cells causes enhanced sensitivity to blue light irradiation. Free Radic Biol Med. 1997;22:1229–1234. doi: 10.1016/s0891-5849(96)00555-2. [DOI] [PubMed] [Google Scholar]
- 259.Wooten WL. Cascarano J. The effect of thyroid hormone on mitochondrial biogenesis and cellular hyperplasia. J Bioenerg Biomembr. 1980;12:1–12. doi: 10.1007/BF00745009. [DOI] [PubMed] [Google Scholar]
- 260.Wu CW. Ping YH. Yen JC. Chang CY. Wang SF. Yeh CL. Chi CW. Lee HC. Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol. 2007;220:243–251. doi: 10.1016/j.taap.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 261.Yan LJ. Levine RL. Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc Natl Acad Sci U S A. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yin XM. Ding WX. Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 263.Yoon Y. Krueger EW. Oswald BJ. McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yoon YS. Yoon DS. Lim IK. Yoon SH. Chung HY. Rojo M. Malka F. Jou MJ. Martinou JC. Yoon G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol. 2006;209:468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- 265.Yorimitsu T. Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang C. Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zhang H. Go YM. Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 268.Zieman SJ. Melenovsky V. Clattenburg L. Corretti MC. Capriotti A. Gerstenblith G. Kass DA. Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. J Hypertens. 2007;25:577–583. doi: 10.1097/HJH.0b013e328013e7dd. [DOI] [PubMed] [Google Scholar]