Abstract
Patients with autoimmune lymphoproliferative syndrome (ALPS) have defective lymphocyte apoptosis, with increased risk for lymphoid malignancies. Herein, we report a patient with ALPS who developed histiocytic sarcoma in a background of sinus histiocytosis and massive lymphadenopathy (SHML) or Rosai-Dorfman disease (RDD). This patient had documented ALPS type Ia with a germline missense mutation in exon 9 of the TNFRSF6 gene (973 A>T, D244V) encoding Fas (CD95/Apo-1). He presented at 10 months with hepatosplenomegaly and autoimmune hemolytic anemia and was diagnosed with ALPS. At age 6 and½, he developed classical Hodgkin lymphoma, which was treated using standard chemotherapy. Two years later, biopsy of a PET-positive axillary node showed features of ALPS and focal involvement by SHML. Thereafter, the patient continued to have continued lymphadenopathy and progressive splenomegaly, leading to exploratory surgery at age 13 for suspicion of lymphoma. Paraabdominal nodes revealed sheets of malignant-looking histiocytes with increased mitotic activity and areas of necrosis, indicative of histiocytic sarcoma. Spleen and lymph nodes also showed involvement by RDD. Both components had an identical phenotype of S-100+/CD68+/CD163+. The occurrence of malignancies involving two separate hematopoietic lineages in ALPS is not previously reported. Given the central role of defective Fas signaling in ALPS, histiocytes may be yet another lineage at risk for neoplastic transformation secondary to a block in apoptosis.
Keywords: histiocytic sarcoma, Rosai-Dorfman disease, sinus histiocytosis with massive lymphadenopathy, apoptosis, autoimmune lymphoproliferative syndrome
Autoimmune lymphoproliferative syndrome (ALPS) represents a paradigm for defective lymphocyte apoptosis leading to a plethora of clinical manifestations including autoimmune phenomena, lymphadenopathy and splenomegaly, with an increased risk of lymphoid malignancies of B-cell lineage. 20, 22 While specific defects have been identified in the gene (TNFRSF6) encoding Fas (CD95/Apo-1)5, 18, 19 in both B- and T-cells, the lymphadenopathy observed in ALPS is mainly due to an accumulation of double negative alpha beta T-cells (DNT’s) . DNT’s are also increased in the peripheral blood, and may infiltrate other sites. However, most reported cases of lymphomas in the context of ALPS have been of B-cell lineage, including both classical Hodgkin lymphoma and nodular lymphocyte predominant Hodgkin’s lymphoma.21
Benign histiocytic proliferations have been described previously by our group,13 as well others,15 in the context of ALPS, although these generally do not attain clinical significance. We previously characterized the occurrence of SHML-type S-100 positive histiocytes reminiscent of Rosai-Dorfman disease (RDD) in a cohort of 44 patients with ALPS Type Ia.13 RDD changes were more commonly found in patients with severe symptomatology, and were more common in males than females. Clinical manifestations of the RDD were generally self-limited, without progression noted.
CASE REPORT
A 13 year old boy presented in 2006 with massive splenomegaly and bulky retroperitoneal adenopathy. His past history was significant for autoimmune lymphoproliferative syndrome (ALPS) Type Ia and a proven mutation in the death domain of Fas (973 A>T:D244V). Initial clinical presenting features of the patient (NIH cohort family number 26) have been briefly reported previously by Infante et al.11 At birth (in 1993), he was mildly jaundiced; generalized lymphadenopathy and hepatosplenomegaly were noted at 10 months of age. Hemoglobin at this time was 9.7 g/dL with 5.6% reticulocytes, and positive Coombs’direct antiglobulin (DAT) test. The serum IgG was elevated at 2525mg/dL. As reported, the serum alkaline phosphatase was initially elevated at 6578 U/mL, but fell rapidly thereafter. Flow cytometry analysis performed in 1998 showed 2.1% (absolute 95/uL) α/β+, CD4−/CD8− T cells (DNTs) (normal <1%) in keeping with the history of ALPS. Ten family members of this kindred have clinical evidence of ALPS (with 3 additional mutation-positive family members having no clinical evidence of ALPS), ranging in age from 5–86 years of age. In early 2000, he was admitted with pneumonia and pleural effusion as well as exacerbation of neck adenopathy. A biopsy was performed leading to a diagnosis of classical Hodgkin lymphoma, mixed cellularity subtype (clinical stage II). Chemotherapy was instituted using a combination of MOPP and ABVD in alternating cycles, given between January and August 2001. Subsequently, he continued to have progressive splenomegaly and consequent neutropenia due to sequestration, for which he was administered low-dose (1µg/kg) G-CSF (Neupogen). Steroids were deemed a poor choice owing to the prior history of lymphoma. At the age of 9 (2002), the patient returned to the NIH prompted by a significant change in a PET scan compared to a previous study. Physical examination at this time revealed 1.5 cm axillary and supraclavicular lymph nodes as well as chronic persistent generalized adenopathy consistent with his diagnosis of ALPS. He was otherwise clinically stable. An axillary lymph node biopsy was performed, and showed no evidence of lymphoma, but exhibited changes of ALPS with associated Rosai-Dorfman disease.13 No significant life-threatening events occurred in the interim and he remained relapse-free of lymphoma until 2006, when he presented with fever and considerable interval enlargement of iliac and perirenal lymph nodes, noted by CT and FDG-PET scan (figure 1). At this time, a splenectomy was recommended owing to the massive size and also to rule out possible lymphoma. Hence, he underwent a splenectomy with simultaneous bone marrow biopsy and peri-renal abdominal lymph node biopsy. The specific lymph node was chosen for biopsy based on the maximal FDG avidity in the PET scan. This led to the diagnosis of histiocytic sarcoma arising in a background of Rosai-Dorfman disease. Because of renal compression, nephrostomy tubes were placed before beginning therapy with the anaplastic large cell lymphoma protocol ALCL-99. After the second course, the perirenal masses had decreased in size sufficient for removal of the nephrostomy tubes. He received a fourth course (cycle B2) after which the adenopathy and masses were 80% reduced in size. On 4-10-07, he underwent a mis-matched unrelated donor transplant with a busulvan/cytoxan/VP-16/Campath conditioning regimen. He was 100% engrafted on day 12. His post-BMT course was significant for graft-versus host disease (skin and intestine Grade 3), methicillin–resistant Staphylococus Aureus pneumonia and consequent pulmonary hemorrhage to which he ultimately succumbed three months after the transplant with no evidence of disease remission following chemotherapy and allo-BMT.
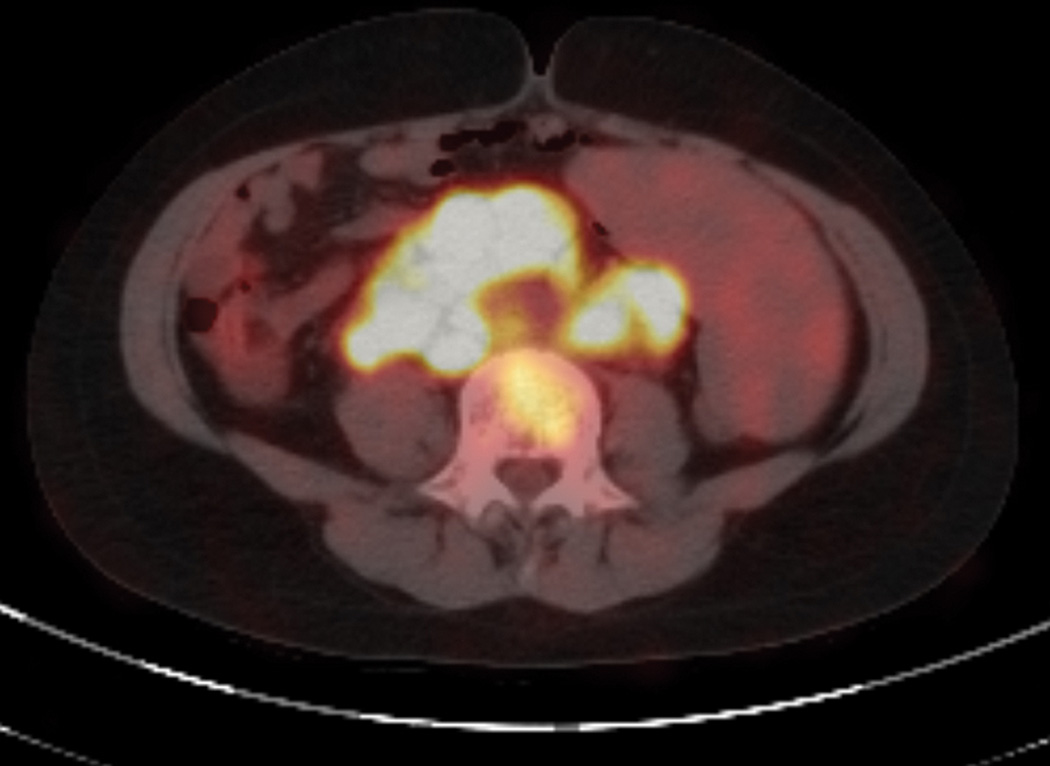
Figure 1.
Increased uptake with high SUV values in massively enlarged peri-renal/periabdominal nodes by PET-CT scan.
PATHOLOGIC FINDINGS
Histologic examination of the lymph node biopsy from 2000 demonstrated features characteristic for classical Hodgkin lymphoma, mixed cellularity subtype, which was EBV-associated (figure 2). The lymph node biopsy from 2002 showed marked paracortical expansion by lymphocytes (with markedly decreased CD45RO+ memory T-cells by immunohistochemistry and a significant DNT population corroborated by flow cytometry), plasma cells, and focal collections of histiocytes demonstrating emperipolesis, as previously reported.13 The histiocytes had abundant pale eosinophilic cytoplasm, enlarged round to oval nuclei with distinct nucleoli. Cytoplasmic vacuoles within the histiocytes contained lymphocytes and plasma cells, characteristic of sinus histiocytosis with massive lymphadenopathy (SHML), also termed Rosai-Dorfman disease (RDD) (figure 3). Ki-67 was negative in these cells.
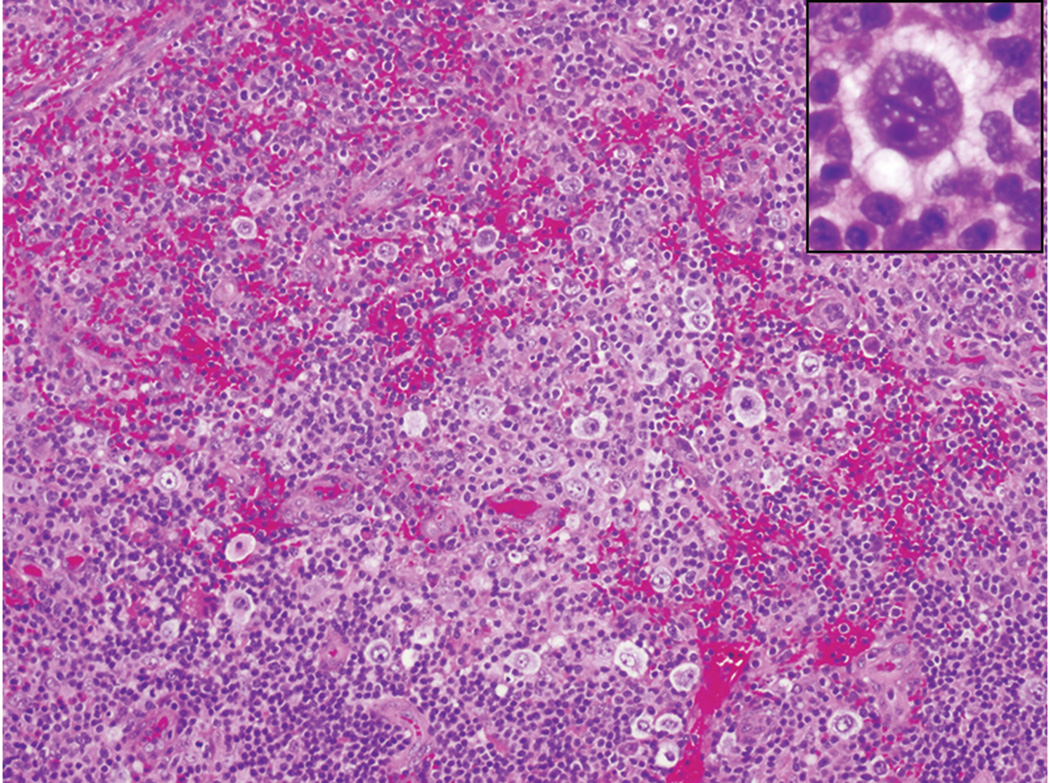
Figure 2.
Classical Hodgkin lymphoma, cervical node biopsy, age 6 and 1/2. Atypical mononuclear Hodgkin’s cells are present in an inflammatory background of lymphocytes, histiocytes, eosinophils, and plasma cells. A classical Reed-Sternberg cell is shown in inset.
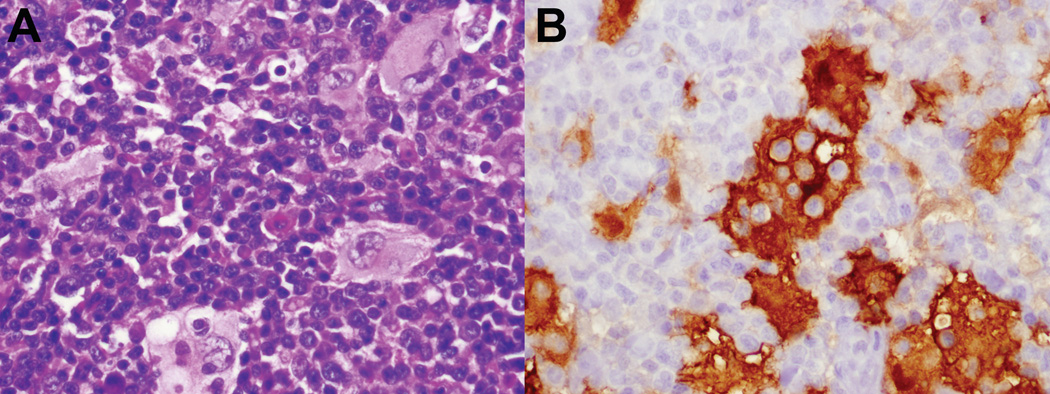
Figure 3.
Rosai-Dorfman disease, axillary node biopsy, age 9. A. Characteristic histiocytes with abundant pale cytoplasm show emperipolesis. B. Histiocytes are strongly S100-positive, highlighting engulfed lymphoid cells. (S100 immunoperoxidase). They were negative for CD1a, CD21 and langerin. (not shown)
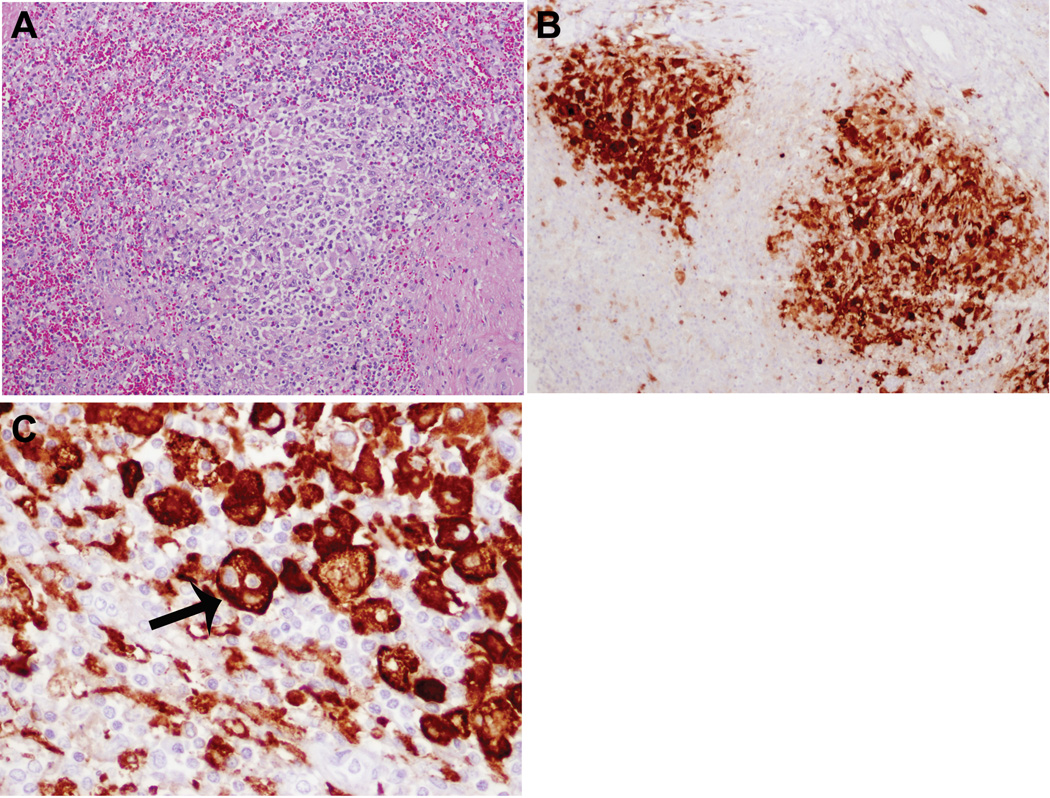
Examination of the perirenal/ paraabdominal lymph nodes from 2006 at the time of the splenectomy showed replacement by an atypical histiocytic proliferation. All the sinuses were packed with sheets of plump histiocytes containing abundant pale pink cytoplasm and convoluted and folded atypical nuclei (figure 4). In many areas, there were solid, cohesive sheets of frankly atypical histiocytes with prominent nuclear atypia, and easily observed mitotic figures with interspersed areas of necrosis, consistent with a histiocytic sarcoma. In other areas, relatively banal looking clusters of histiocytes with typical RDD morphology without significant atypia predominated the cellular milieu, admixed with plasma cells and lymphocytes in and around the post-capillary venules; prominent emperipolesis was noted in these areas (not shown). The immunophenotype of the histiocytes in the RDD areas was identical to the sarcomatous component (CD68+/CD163+/S-100+). The sarcomatous histiocytes were additionally positive for CD4 and negative for CD1a, CD21, langerin, lysozyme as well as EBV by in situ hybridization. Only rare scattered EBV-positive small lymphoid cells were identified. Ki-67 highlighted the abnormal cells, being positive in 20–40% in the sarcomatous areas. Examination of the enlarged spleen (1234 grams) showed multifocal clusters of RDD-type histiocytes with emperipolesis, consistent with SHML (figure 5). No sarcomatous changes were apparent in the spleen.
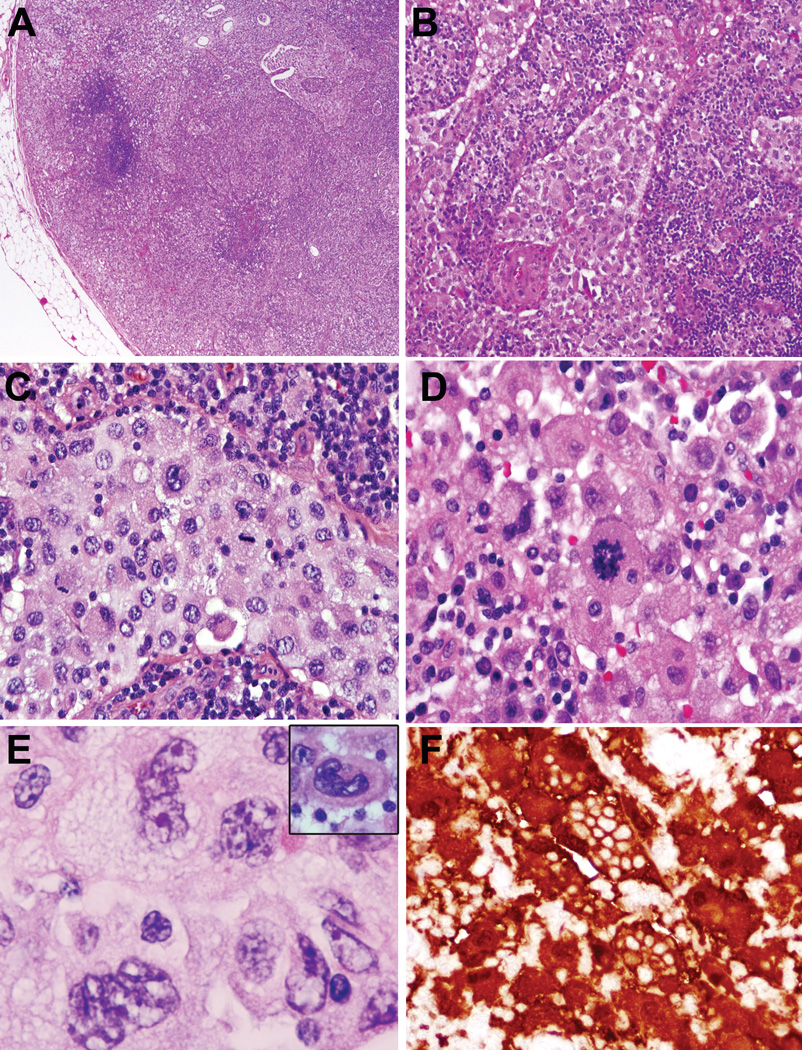
Figure 4.
Histiocytic sarcoma, para-abdominal lymph node, age 13. A. The lymph node architecture is largely effaced by a confluent growth of atypical histiocytes. In other areas, solid circumscribed nodules of atypical histiocytes were also noted (not shown) B., C. The sinuses are distended and infiltrated by cytologically atypical histiocytes. D. Pleomorphic histiocytic cells have abundant cytoplasm and easily noted mitoses.
E. Histiocytes have convoluted nuclear contours F. The atypical histiocytes are strongly positive for S-100; S-100 stain also highlights prominent emperipolesis in neoplastic cells. (S100 immunoperoxidase)
Figure 5.
Rosai Dorfman disease, spleen, age 13. A. A nodular collection of RDD histiocytes admixed with lymphocytes and plasma cells. B. Foci of RDD histiocytes are highlighted with S-100 stain, and show prominent emperipolesis (arrow) (C.) (S100 immunoperoxidase)
DISCUSSION
The transformation of RDD to a histiocytic sarcoma is a novel finding. RDD is considered a benign process, regarded as non-clonal, with morbidity and mortality in patients with RDD generally associated with involvement of multiple extranodal sites.17 To date, we are aware of only one previous report of histiocytic sarcoma arising in RDD, described by Agarwal et al.1 In the series of patients of RDD described by this group, the patient of interest was an adult who developed histiocytic sarcoma, four years after the diagnosis of RDD. No clinical evidence of autoimmune manifestations were evident in this patient (personal communication of Dr.Gujral).
In a previous study, we examined the nodal histologic findings in a cohort of 44 patients with an established diagnosis of ALPS Type Ia (affecting the Fas gene), and identified RDD lesions in 18 patients.13 Affected patients had particularly severe manifestations of ALPS, and presented at an earlier age, were more often male (15/18) than cases of ALPS Type Ia lacking the characteristic histiocytic lesion. However, the RDD changes were self-limited in these patients, did not progress to involve extranodal sites, and only in a few patients contributed significantly to the clinical manifestations of ALPS.
The presence of a morphologic continuum between the RDD areas and the sarcomatous areas, coupled with the finding of identical immunophenotypic profiles in both components is consistent with transformation of the RDD to a histiocytic sarcoma in this case. Equally unusual is the occurrence of RDD in the spleen, with only one histologically documented case of RDD involving the spleen in the western population.3 Thus, this patient differs from previously reported cases of RDD in the setting of ALPS in having widespread disease, with involvement of multiple nodal sites, as well as the spleen. Interestingly, no other extranodal sites were involved.
Straus et al. identified an increased risk for Hodgkin and B-cell lymphomas in patients with Type 1a ALPS.21 The increased risk for lymphoma in ALPS is thought to be multifactorial, related to both immune dysregulation and a block in apoptosis affecting both T and B lymphocytes. The failure of apoptotic mechanisms may conceivably extend to involve the histiocytic lineage as well, providing a plausible explanation for the histiocytic proliferation and transformation to sarcoma. Thus, the current case is further notable for the development of two independent malignancies of distinct lineages, classical Hodgkin’s lymphoma and histiocytic sarcoma, in the setting of ALPS. Although histiocytic sarcomas clonally related to B-cell lymphoma have been reported to occur rarely through a mechanism of transdifferentiation4, the histiocytic sarcoma in the present case lacked evidence of clonal immunoglobulin gene rearrangement. Thus, we can confidently exclude a clonal relationship to the initially diagnosed classical Hodgkin’s lymphoma in this patient.
A potential block in the apoptotic cascade has been implicated in the underlying pathogenesis of many B-cell neoplasms, including Hodgkin lymphoma, marginal zone lymphoma,10 and follicular lymphoma.8 Upregulation of anti-apoptotic molecules is a common theme in all these tumors. In follicular lymphoma, the prototype of this model, juxtaposition of BCL2 besides the IGH enhancer region, leads to overexpression of BCL-2 and consequent lymphoma.8, 23 In MALT lymphomas, translocation of API2 (gene coding for inhibitor of apoptosis protein-2) on chromosome 11, adjacent to the MALT1 on chromosome 18 leads to activation of the canonical NF-κB pathway by targeting NEMO for ubiquitination,24 in addition to repression of the mitochondrial apoptosis pathway by repression of Smac.9 While the exact nature of the molecular alterations that drive accumulation of histiocytic cells in ALPS and RDD is unclear, it is possible that cytokines may play a role. Notably, there is a consistent overexpression of Th2 cytokines, IL-4 and IL-10 in ALPS,7, 12 which are supportive to cells of the monocyte/macrophage lineage through inhibition of apoptosis.2 Additionally, the upregulation of M-CSF by IL-46 further supports the growth of the monocyte/macrophage lineage. Indeed, RDD histiocytes have been reported to arise from M-CSF activated histiocytes.14 The additional germline defects in Fas-induced apoptosis seen in ALPS further contribute to the accumulation of histiocytes, and may lead to their malignant transformation.
Additional evidence of the importance of the Fas/ Fas ligand pathway in the pathogenesis of the histiocytic sarcoma is provided by a recently published murine model.16 Mice that lacked expression of membrane-bound Fas-ligand (mFasL) and had abrogated mFasL-mediated apoptosis of transformed cells had severe symptoms of ALPS (lymphadenopathy, splenomegaly, and hypergammaglobulinemia) and subsequently developed histiocytic sarcomas. The prominent and early occurrence of severe clinical symptoms and subsequent development of histiocytic sarcoma in our case bears a striking similarity to this murine model.
Thus, molecular derangements underlying the ALPS phenotype, in concert with a skewed cytokine profile, might be critical in the development of RDD lesions and subsequent malignant transformation. In addition, the present case illustrates the potential for malignant transformation of multiple hematopoietic lineages, both lymphocytes and histiocytes, in patients with ALPS.
Acknowledgement
This work was supported by the intramural research programs of Center for Cancer Research, National Cancer Institute, and the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Agarwal A, Pathak S, Gujral S. Sinus histiocytosis with massive lymphadenopathy--a review of seven cases. Indian J Pathol Microbiol. 2006;49:509–515. [PubMed] [Google Scholar]
- 2.Eslick J, Scatizzi JC, Albee L, et al. IL-4 and IL-10 inhibition of spontaneous monocyte apoptosis is associated with Flip upregulation. Inflammation. 2004;28:139–145. doi: 10.1023/b:ifla.0000039560.00231.cd. [DOI] [PubMed] [Google Scholar]
- 3.Esquivel J, Krishnan J, Jundi M, et al. Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy) of the pancreas: first case report. Hepatogastroenterology. 1999;46:1202–1205. [PubMed] [Google Scholar]
- 4.Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111:5433–5439. doi: 10.1182/blood-2007-11-124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher GH, Rosenberg FJ, Straus SE, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 6.Fixe P, Praloran V. M-CSF: haematopoietic growth factor or inflammatory cytokine? Cytokine. 1998;10:32–37. doi: 10.1006/cyto.1997.0249. [DOI] [PubMed] [Google Scholar]
- 7.Fuss IJ, Strober W, Dale JK, et al. Characteristic T helper 2 T cell cytokine abnormalities in autoimmune lymphoproliferative syndrome, a syndrome marked by defective apoptosis and humoral autoimmunity. J Immunol. 1997;158:1912–1918. [PubMed] [Google Scholar]
- 8.Hockenbery DM, Zutter M, Hickey W, et al. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa Y, Suzuki H, Suzuki Y, et al. Antiapoptotic function of apoptosis inhibitor 2-MALT1 fusion protein involved in t(11;18)(q21;q21) mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2004;64:3452–3457. doi: 10.1158/0008-5472.CAN-03-3677. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki H. Mucosa-associated lymphoid tissue lymphoma: molecular pathogenesis and clinicopathological significance. Pathol Int. 2007;57:474–484. doi: 10.1111/j.1440-1827.2007.02128.x. [DOI] [PubMed] [Google Scholar]
- 11.Infante AJ, Britton HA, DeNapoli T, et al. The clinical spectrum in a large kindred with autoimmune lymphoproliferative syndrome caused by a Fas mutation that impairs lymphocyte apoptosis. J Pediatr. 1998;133:629–633. doi: 10.1016/s0022-3476(98)70102-7. [DOI] [PubMed] [Google Scholar]
- 12.Lopatin U, Yao X, Williams RK, et al. Increases in circulating and lymphoid tissue interleukin-10 in autoimmune lymphoproliferative syndrome are associated with disease expression. Blood. 2001;97:3161–3170. doi: 10.1182/blood.v97.10.3161. [DOI] [PubMed] [Google Scholar]
- 13.Maric I, Pittaluga S, Dale JK, et al. Histologic features of sinus histiocytosis with massive lymphadenopathy in patients with autoimmune lymphoproliferative syndrome. Am J Surg Pathol. 2005;29:903–911. doi: 10.1097/01.pas.0000157997.61177.08. [DOI] [PubMed] [Google Scholar]
- 14.Middel P, Hemmerlein B, Fayyazi A, et al. Sinus histiocytosis with massive lymphadenopathy: evidence for its relationship to macrophages and for a cytokine-related disorder. Histopathology. 1999;35:525–533. doi: 10.1046/j.1365-2559.1999.00746.x. [DOI] [PubMed] [Google Scholar]
- 15.Mullauer L, Emhofer J, Wohlfart S, et al. Autoimmune lymphoproliferative syndrome (ALPS) caused by Fas (CD95) mutation mimicking sarcoidosis. Am J Surg Pathol. 2008;32:329–334. doi: 10.1097/PAS.0b013e3181484f6d. [DOI] [PubMed] [Google Scholar]
- 16.O' Reilly LA, Tai L, Lee L, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulli M, Bergamaschi G, Tonon L, et al. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) Br J Haematol. 1995;91:415–418. doi: 10.1111/j.1365-2141.1995.tb05313.x. [DOI] [PubMed] [Google Scholar]
- 18.Rieux-Laucat F, Le Deist F, Hivroz C, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 19.Sneller MC, Straus SE, Jaffe ES, et al. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest. 1992;90:334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sneller MC, Wang J, Dale JK, et al. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- 21.Straus SE, Jaffe ES, Puck JM, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 22.Straus SE, Sneller M, Lenardo MJ, et al. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann Intern Med. 1999;130:591–601. doi: 10.7326/0003-4819-130-7-199904060-00020. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto Y, Cossman J, Jaffe E, et al. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 24.Zhou H, Du MQ, Dixit VM. Constitutive NF-kappaB activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell. 2005;7:425–431. doi: 10.1016/j.ccr.2005.04.012. [DOI] [PubMed] [Google Scholar]