Abstract
Hyperinsulinemia increases sympathetic nerve activity (SNA) and has been linked to cardiovascular morbidity in obesity. The rostral ventrolateral medulla (RVLM) plays a key role in the regulation of SNA and arterial blood pressure (ABP). Many sympathoexcitatory responses are mediated by glutamatergic receptor activation within the RVLM, and both the central renin-angiotensin and melanocortin systems are implicated in the sympathoexcitatory response to hyperinsulinemia. Therefore, we hypothesized that one or more of these neurotransmitters in the RVLM mediate the sympathoexcitatory response to insulin. Hyperinsulinemic-euglycemic clamps were performed in α-chloralose anesthetized, male Sprague-Dawley rats by infusion of insulin (3.75 mU/kg/min, IV) and 50% dextrose solution for 120 min. Physiological increases in plasma insulin elevated lumbar SNA with no change in renal SNA, ABP or blood glucose. Microinjection of the ionotropic glutamate receptor antagonist kynurenic acid into the RVLM significantly reduced lumbar SNA and ABP. Selective blockade of NMDA but not non-NMDA glutamate receptors resulted in similar reductions of lumbar SNA. In marked contrast, microinjection of the angiotensin II type 1 receptor antagonist losartan or the melanocortin 3/4 antagonist SHU9119 had no effect on lumbar SNA or ABP. Western blot analysis showed that insulin receptor expression is significantly lower in the RVLM than the hypothalamus, and direct microinjection of insulin into the RVLM did not significantly increase lumbar SNA. These findings suggest that hyperinsulinemia increases lumbar SNA by activation of a glutamatergic NMDA-dependent projection to the RVLM.
Keywords: Insulin, RVLM, arterial blood pressure, sympathetic nerve activity, obesity
Introduction
Compelling evidence in humans and rodents indicates that elevated sympathetic nerve activity (SNA) contributes to the pathogenesis of obesity-induced hypertension1, 2. Clinical studies indicate obese humans have increased norepinephrine spillover3, 4, elevated muscle SNA5, 6, and a greater drop in arterial blood pressure (ABP) in response to ganglionic blockade 7. Similar observations have been reported in rodent and dog models of obesity 8-10. One mechanism postulated to underlie the elevated SNA and ABP during obesity is hyperinsulinemia1, 2. Clinical studies have revealed a correlation between obesity, hypertension, and hyperinsulinemia1, 2. In both humans and rodents, acute hyperinsulinemic-euglycemic clamps selectively increase muscle or lumbar SNA, respectively11-13. These actions are mediated by a central mechanism because intracerebroventricular administration of insulin causes a similar selective increase in lumbar SNA14. In rats, chronic hyperinsulinemic-euglycemic clamps increase total peripheral resistance and ABP15. However, the neural mechanisms and pathways that mediate the sympathoexcitatory effects of insulin are poorly understood.
The rostral ventrolateral medulla (RVLM) plays a pivotal role in the regulation of SNA and ABP16. RVLM neurons project to sympathetic preganglionic neurons of the intermediolateral cell column in the thoracic and lumbar spinal cord and support basal SNA16. Electrophysiological studies in vivo have identified tonically active, bulbospinal neurons in the RVLM16. The excitability of RVLM neurons is regulated by a number of neurotransmitters including L-glutamate. Injection of L-glutamate into the RVLM increases neuronal discharge, SNA, and ABP16. Blockade of glutamate receptors in the RVLM eliminates many sympathoexcitatory reflexes 16 and lowers ABP in multiple experimental models of hypertension 17-19. Based on this evidence, we hypothesized that glutamate receptor activation in the RVLM mediates the sympathoexcitatory response to hyperinsulinemia.
In addition to glutamate, evidence from several laboratories suggests that the brain renin-angiotensin and melanocortin systems mediate the sympathoexcitatory response to insulin. In this regard, RVLM neurons express Ang II (AT1) receptors20, and injection of Ang II into the RVLM increases SNA and ABP21. Blockade of brain AT1 receptors blunts the pressor response to central hyperinsulinemia 22. Also, blockade of the renin-angiotensin system prevents insulin-induced hypertension23. On the other hand, RVLM neurons express melanocortin receptors,24 and injection of a melanocortin agonist into the RVLM increases SNA and ABP25. Interestingly, the sympathoexcitatory effect to insulin is abolished in melanocortin 4 knockout mice26. Therefore, we hypothesized that one or both of these systems may contribute to the sympathoexcitatory response during hyperinsulinemia.
Materials and Methods
Animals
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Kentucky and Pennsylvania State College of Medicine Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (250-350g; Charles River Laboratories) were housed in a temperature controlled room (22±1°C) with a 14:10-hour light:dark cycle. Rats were fed standard rat chow and given access to deionized water.
General Procedures
Rats were anesthetized with isoflurane (2-3%) and prepared for recordings of renal and lumbar SNA and ABP as described previously 27, 28. Animals were artificially ventilated with oxygen-enriched room air. End-tidal CO2 and body temperature were maintained at 4-5% and 37±1°C, respectively. After surgery, anesthesia was replaced by α-chloralose (50 mg/kg bolus, 25 mg/kg/hr, IV). The level of anesthesia was examined by the lack of a withdrawal reflex following a foot pinch. When a stable level of anesthesia was established, rats were paralyzed with gallamine triethiodide (20 mg/kg, 0.25 mL/hr, IV). Variables were allowed to stabilize for a minimum of 30 min before the experiment began.
RVLM Microinjections
RVLM microinjections were performed as described previously in our laboratory29. Initially, L-glutamate (1 nmol) was injected into the RVLM at 3 different sites separated by 300 μm in the rostral-caudal plane to identify the site that produced the largest increase in ABP; subsequent injections were performed at these coordinates. For all experiments, injections (60 nL) were performed over 5 seconds. Injection sites were marked with 0.2% rhodamine beads added to the respective drug or injected at the end of experiments.
Hyperinsulinemic-Euglycemimc Clamps
An initial set of experiments was performed to identify a physiological dose of insulin. Animals were prepared as described above, and insulin (3.75 or 7.5 mU/kg/min, 0.25 mL/hr, IV, Humulin R) and a 50% dextrose solution (0.25-1.0 mL/hr, IV) were infused for 120 min. Blood glucose was measured from a drop of arterial blood every 10 min using a standard glucometer (One Touch Ultra). The dextrose infusion rate was adjusted in order to maintain euglycemia. Control animals were infused with equal volumes of isotonic saline. Blood (0.5mL) was collected from the arterial line into microcentrifuge tubes (10 uL, 0.5 M EDTA) at baseline, 60, and 120 min. Samples were centrifuged, and plasma was stored at -80°C.
For purposes of comparison, plasma insulin levels were analyzed from a rodent model of diet-induced obesity. Male Sprague-Dawley rats (200-250g, Charles River Laboratories) were fed a low fat (LF, 10% kcal from fat; Research Diets, Inc, D12489B) or moderately high fat (32% kcal from fat; Research Diets, Inc, D12266B) diet for 13 weeks as described previously by our laboratory 10. Those on the high fat diet segregated into obesity resistant (OR) and obesity prone (OP). Rats were anesthetized and prepared as described above. Blood samples were collected from the arterial line, and insulin levels were determined by an ELISA using a commercially available kit (Millipore).
To determine the contribution of RVLM receptors to the SNA response during hyperinsulinemia, separate rats were prepared as described above. Baseline values of ABP, lumbar and renal SNA were recorded for 10 min. Then, insulin (3.75 mU/kg/min, 0.25 ml/hr, IV) and a 50% dextrose solution (0.25-1.0 ml/hr, IV) were infused for 120 min. At 90 min, one of several compounds was bilaterally microinjected into the RVLM: the ionotropic glutamate receptor antagonist kynurenic acid (KYN, 5 nmol), the NMDA receptor antagonist AP5 (5 mmol), the non-NMDA receptor antagonist NBQX (1 mmol), the AT1 receptor antagonist losartan (1 nmol), the melanocortin 3/4 receptor antagonist SHU9119 (0.03 nmol), or artificial cerebrospinal fluid (aCSF, 60nL). Doses of various receptor antagonists were based on previous studies 25, 30-33 and confirmed in preliminary experiments to block sympathoexcitatory responses to the respective agonist (data not shown).
To determine the time course of action for KYN, animals were prepared as described above, and the sciatic nerve was stimulated electrically (5 sec train, 500 μA, 20 Hz) before and 10, 20, and 30 min after KYN microinjection into the RVLM.
Central Insulin Injections
Rats were prepared as described above, and insulin (5, 0.5, 0.05, or 0.0005 μU/nL, 60 nL) was bilaterally microinjected into the RVLM. ABP and SNA were recorded for 60 min and blood glucose measured every 30 min. The insulin concentrations were based on previous studies using intracerebroventricular injection of insulin 14, 34 and re-calculated due to a minimum 10-fold dilution due to the CSF volume of the lateral and 3rd ventricles.
In a separate group of rats, intracerebroventricular cannulas were implanted in the lateral ventricle as described previously 35. Proper cannula location was verified by a positive drinking test (>3 mL in 30 min) to angiotensin II (20ng/2μL) 35. Then, rats were prepared as described above, and insulin (100 mU/2uL) was injected into the lateral ventricle. This dose of insulin has been repeatedly demonstrated to significantly elevate lumbar SNA in rodents 14, 34. Variables were recorded for 60 min, and blood glucose was measured every 30 min. At the end of experiments, cannula placement was verified again by the spread of dye (1% Evan's Blue Dye, 2μL) to the 3rd and 4th ventricle.
Western Blot Analysis of Insulin Receptors
RVLM and hypothalamic samples were collected for western blot analysis at baseline or 60 min after a hyperinsulinemic-euglycemic clamp. Rats were deeply anesthetized with 5% isoflurane and perfused transcardially with cold oxygenated aCSF (124 mmol/L NaCl, 26 mmol/L NaHCO3, 0.6 mmol/L NaH2PO4, 3mmol/L KCl, 1.6 mmol/L MgCl2, 1.5 mmol/L CaCl2, 11 mmol/L glucose, pH 7.4). The brain was rapidly removed. A chunk of the mediobasal hypothalamus defined dorsally by the top of the 3rd ventricle, laterally by the optic tract, rostrally by the optic chiasm, and caudally by the mammillary bodies was frozen and stored at -80°C. The brainstem was sectioned at 200μm in oxygenated cerebral spinal fluid (4°C) using a vibratome. The RVLM was isolated under a microscope, immediately frozen on dry ice and stored at -80°C. Western blots were performed using a rabbit polyclonal insulin receptor β (IR-β) antibody (1:1000, C-19; sc-711; Santa Cruz Biotechnology, Inc. Santa Cruz, CA.). The IR-β band intensity for each sample was quantified using NIH ImageJ (http://rsb.info.nih.gov/nih-image/) and normalized to the respective γ-tubulin band intensity (please see data supplement online at http://hyper.ahajounals.org).
Data Analysis
All data are expressed as mean±SE. Changes in integrated SNA are calculated by subtracting background noise after hexamethonium (30 mg/kg IV). For all variables, 30 sec segments at each time point were compared to three 30-second baseline period measurements. All data were analyzed by a 1- or 2- way ANOVA with repeated measures when appropriate. All post hoc tests were performed with independent or paired t tests with a layered Bonferonni correction. A P<0.05 was statistically significant.
Results
Analysis of Plasma Insulin Levels
Initial experiments were performed to identify an insulin infusion rate which produced physiological increases in plasma insulin levels. Both infusion rates significantly increased plasma insulin concentrations at 60 and 120 min (Figure 1). Plasma insulin levels were significantly greater in rats infused with 7.5 vs 3.75 mU/kg/min vs saline infusion.
Figure 1.
Plasma insulin concentrations at baseline, 60, and 120 min during a hyperinsulinemic-euglycemic clamp (3.75 mU/kg/min, n=9; 7.5 mU/kg/min, n=3) or saline infusion (n=3). Plasma insulin concentrations from LF (n=5), OR (n=5) and OP (n=6) rats were analyzed for purposes of comparison. Plasma insulin levels were not different between OP rats and control rats infused with 7.5 mU/kg/min. *Significant difference vs baseline levels (P<0.05), †Significant difference versus 3.75 mU/kg/min (P<0.05), ‡Significant difference versus LF and OR rats (P<0.05).
To compare these infusion rates to a rodent model of obesity, we analyzed plasma insulin levels from rats maintained on a low-fat or moderate high-fat diet for 13 weeks. As previously reported 10, OP rats weighed significantly more than LF or OR rats (OP: 793±13g, LF: 612±16g, OR: 596±8g). The greater body weight of OP rats was associated with a greater fat pad mass and higher adiposity index than LF or OR rats (see Table S1, http://hyper.ahajounals.org). As expected, plasma insulin levels were significantly higher in OP versus LF or OR rats (Figure 1). In fact, plasma insulin levels of OP rats were similar to those rats infused with 7.5 mU/kg/min and significantly higher than those rats infused with 3.75 mU/kg/min. Plasma insulin levels of LF and OR rats were not different versus those of rats infused with 3.75 mU/kg/min.
Blockade of Glutamatergic Receptors Reverses Sympathoexcitatory Response to Insulin
A major goal of this study was to determine whether blockade of glutamate receptors in the RVLM reversed or attenuated the sympathoexcitation during hyperinsulinemia. Figure 2 illustrates a representative example of the responses to a hyperinsulinemic-euglycemic clamp or saline infusion before and after RVLM microinjection of KYN. Group data are summarized in Figure 3. As previously reported, hyperinsulinemia selectively increased lumbar SNA12, 14, but did not affect ABP (Figure 2), blood glucose (Figure 3), renal SNA (data not shown), or heart rate (data not shown).
Figure 2.
Representative examples of ABP, mean ABP, and lumbar SNA during RVLM microinjection of KYN in rats receiving a (A) hyperinsulinemic-euglycemic clamp or (B) saline infusion. Traces for raw lumbar SNA represent (a) baseline, (b) peak infusion, and (c) post-KYN injection.
Figure 3.
Summary data of lumbar SNA, mean ABP and blood glucose during RVLM microinjection of KYN (∇) in rats receiving a hyperinsulinemic-euglycemic clamp (●, n=5) or saline infusion (□, n=5). Injection of KYN significantly reduced lumbar SNA in rats receiving a hyperinsulinemic clamp but had no effect in those receiving saline infusion. *Significant difference vs saline infused-rats (P<0.05), †Significant difference vs pre-injection or 90-min value (P<0.05).
Microinjection of KYN significantly reduced lumbar SNA in hyperinsulinemic animals but had no effect in saline-infused animals (Figures 2 and 3). In fact, lumbar SNA after injection of KYN was not different between rats infused with insulin versus saline (P>0.3). At 120 min, lumbar SNA returned to pre-injection values. Microinjection of aCSF had no effect on any variable in hyperinsulinemic or control rats (see Figure S1, http://hyper.ahajounals.org). Figures 4 summarizes the peak changes in lumbar SNA and mean ABP after injection of aCSF or KYN in hyperinsulemic or control rats. Although the hyperinsulemic-euglycemic clamp did not significantly alter ABP, microinjection of KYN significantly decreased mean ABP. Microinjection of KYN or aCSF did not alter renal SNA or heart rate (data not shown).
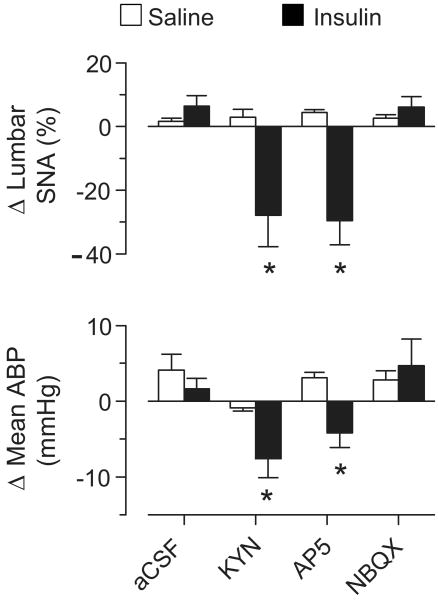
Figure 4.
Peak changes in lumbar SNA and mean ABP after bilateral microinjection of aCSF, KYN, AP5, or NBQX into the RVLM during a saline infusion (n=3-7 per group) or hyperinsulemic-euglycemic clamp (n=3-7 per group). *Significant difference vs rats infused with saline within same drug treatment or rats infused with insulin + aCSF (P<0.05)
Since lumbar SNA returned to preinjection values at 30 min after KYN injection, an additional set of experiments was performed to determine the time course of ionotropic receptor blockade by KYN. We compared the sympathoexcitatory response to activation of somatic afferents before and after RVLM injection of KYN. Prior to blockade, electrical stimulation of sciatic afferents significantly increased mean ABP, lumbar SNA, renal SNA, and heart rate (Table 1). As expected, microinjection of KYN into the RVLM significantly attenuated these responses at 10 and 20 min. However, the sympathoexcitatory responses at 30 min were not different from baseline responses.
Table 1.
Effect of Ionotropic Receptor Blockade on the Sympathoexcitatory Reflex to Activation of Somatic Afferents
| Stimulation Time | |||||
|---|---|---|---|---|---|
| Characteristic | n | Baseline | 10 min | 20 min | 30 min |
| Δ Mean ABP (mmHg) | 9 | 31±4 | 1±4 * | 6±4 * | 23±6 |
| Δ Heart Rate (BPM) | 9 | 14±2 | 1±1 * | 5±2 * | 13±3 |
| Δ Renal SNA (%) | 5 | 66±15 | 7±6 * | 26±9 * | 39±9 |
| Lumbar SNA (%) | 4 | 88±22 | 40±15* | 47±12* | 75±25 |
Values are mean ± SEM and represent changes in mean ABP, heart rate, and SNA during electrical stimulation of sciatic afferents. KYN was injected bilaterally into the RVLM at time = 0. Renal or lumbar SNA was recorded in each animal. Note that the sympathoexcitatory response was attenuated at 10 and 20 min after KYN injection but returned at 30 min.
Significant difference vs baseline values microinjection into the RVLM, P<0.05. Baseline mean ABP: 123±6 mmHg, baseline heart rate: 405±11 bpm
Blockade of NMDA but not non-NMDA Receptors Reverses the Sympathoexcitatory Response to Insulin
Since blockade of iontotropic glutamate receptors with KYN reversed the sympathoexcitatory response to insulin, an additional set of experiments was performed to identify the specific receptor subtype. RVLM microinjection of the NMDA receptor antagonist AP5 in hyperinsulinemic rats significantly reduced lumbar SNA (90 min: 142±6% vs peak: 115±9 %, P<0.05) and mean ABP (90 min: 123±6 vs peak: 116±6 mmHg, P<0.05). Interestingly, the fall in lumbar SNA and mean ABP of hyperinsulinemic rats was similar between KYN and AP5 (Figure 4). In contrast, microinjection of the non-NMDA receptor antagonist NBQX did not affect lumbar SNA (90 min: 142±12 vs peak: 148± 13 %) or mean ABP (90 min: 105± 7 vs peak: 110± 6 mmHg). AP5 and NBQX did not affect lumbar SNA or ABP in saline-infused animals.
RVLM AT1 and Melanocortin Receptors Do Not Mediate Insulin-Induced Sympathoexcitation
In contrast to blockade of glutamate receptors, microinjection of the AT1 receptor antagonist losartan or the melanocortin receptor antagonist SHU9119 did not affect the sympathoexcitatory response to hyperinsulinemia. Peak changes in lumbar SNA and ABP after microinjection of losartan or SHU9119 are illustrated in Figure 5. As expected, the hyperinsulinemic-euglycemic clamp significantly increased lumbar SNA at 90 min (P<0.01) but did not change mean ABP, renal SNA, or heart rate (data not shown). Microinjection of losartan did not decrease lumbar SNA (90 min: 138±12 vs peak: 147±12 %) or mean ABP (90 min: 111±8 vs peak: 120±8 mmHg). Similarly, microinjection of SHU9119 did not decrease lumbar SNA (90 min: 134±8 vs peak: 147±17 %) or mean ABP (90 min: 96±6 to peak: 109±5 mmHg). Losartan and SHU9119 did not affect lumbar SNA or ABP in saline-infused animals (Figure 5).
Figure 5.
Peak changes in lumbar SNA and mean ABP after bilateral microinjection of aCSF, KYN, losartan, or SHU 9119 into the RVLM during a saline infusion (n=4-9 per group) or hyperinsulinemic-euglycemic clamp (n=3-6 per group). * Significant difference vs rats infused with saline within same drug treatment or rats infused with insulin + aCSF (P<0.05)
Insulin Receptor Expression and Insulin Microinjection in the RVLM
To determine whether insulin may act directly in the RVLM to increase SNA, we analyzed insulin receptor expression and sympathetic responses to microinjection of insulin in the RVLM. Insulin receptor expression was significantly lower in the RVLM compared to the ventromedial hypothalamus (Figure 6). In fact, the IR-β band in RVLM samples was virtually absent (Figure 6) and was not altered by a hyperinsulinemic-euglycemic clamp (data not shown).
Figure 6.

(A) Examples of western blot analysis for insulin receptor β and γ-tubulin in the hypothalamus (H) and RVLM (R). Insulin receptor β expression as a ratio to γ-tubulin was significantly lower in the RVLM versus the hypothalamus (n=4 per group, *P<0.01). (B) Change in lumbar SNA and ABP after injection of insulin into the RVLM (n=3-4 per group) or lateral ventricle (n=5). *Significant difference versus aCSF or 0 insulin (P<0.01).
RVLM microinjection of insulin at any dose did not alter lumbar SNA or ABP (Figure 6). In marked contrast, injection of insulin into the lateral ventricle significantly increased lumbar SNA. Plasma glucose levels were not altered by RVLM or lateral ventricle injection of insulin (data not shown).
Histology
All injection sites were centered in the RVLM defined as the triangular region located 0 to 600 μm caudal to the caudal pole of the facial nucleus and bordered dorsally by nucleus ambiguous, medially by the inferior olive or pyramidal tracts, and laterally by the spinal trigeminal nucleus (see Figure S2, http://hyper.ahajounals.org).
Discussion
Previous studies have demonstrated that hyperinsulinemic-euglycemic clamps produce non-uniform increases in SNA11, 12, 36. However, the neural mechanisms or brain regions by which insulin acts to selectively increase lumbar SNA have not been identified. The present study provides several novel findings: 1) a hyperinsulinemic clamp with physiological increases in plasma insulin levels elevated lumbar SNA, 2) blockade of glutamatergic, and more specifically NMDA, receptors reversed the sympathoexcitatory effects of hyperinsulinemia, 3) blockade of RVLM AT1 or melanocortin 3/4 receptors did not affect the sympathoexcitatory response to insulin, 4) the RVLM has a low expression of insulin receptors, and 5) microinjection of insulin into the RVLM did not elevate lumbar SNA. Collectively, these findings suggest insulin activates a NMDA-dependent glutamatergic pathway to the RVLM to increase lumbar SNA.
To identify a physiologically relevant dose of insulin, we compared plasma insulin levels between control rats infused with insulin versus diet-induced obese rats. This model of diet-induced obesity has similar characteristics to obese humans such as activation of the renin-angiotensin system, hyperleptinemia, hyperinsulinemia, elevated sympathetic outflow, and hypertension 8, 37-40. Indeed, the plasma insulin levels of control rats infused with 3.75 mU/kg/min were significantly lower than those of OP rats. Additional data indicate that plasma insulin levels in obese Zucker rats (13-15 weeks) are not different from those of control rats infused with 3.75 mU/kg/min (see Table S2, http://hyper.ahajournals.org). Although plasma insulin levels of LF rats were significantly greater than baseline levels of control rats, this difference is likely attributed to a greater adiposity index and older age of LF rats. Collectively, these data indicate that the insulin infusion rate in the present study is physiologically relevant. Whether the elevation in circulating insulin contributes to the elevated sympathetic outflow and hypertension in these rodent models of obesity is unknown.
Glutamate neurotransmission in the RVLM mediates a number of sympathoexcitatory reflexes including the responses to hypoxia and activation of somatic afferents 16. Blockade of RVLM ionotropic glutamate receptors also lowers ABP in a number of experimental models of hypertension associated with elevated sympathetic outflow 17-19. In the present study, RVLM injection of KYN, but not losartan or SHU 9119, completely reversed the sympathoexcitatory response to hyperinsulinemia. Although lumbar SNA returned to preinjection levels at 30 min after KYN injection, this response is consistent with the time course of ionotropic receptor blockade with KYN. These findings support two important conclusions: 1) insulin activates the brain renin-angiotensin and melanocortin systems outside the RVLM (ie, hypothalamus), and 2) ionotropic glutamate receptors in the RVLM mediate the sympathoexcitatory actions to hyperinsulinemia. Subsequent experiments clearly demonstrate that NMDA receptors solely mediate this response. The ability of KYN or AP5 to reverse the sympathoexcitatory effects of hyperinsulinemia cannot be attributed to a direct modulatory role of insulin within the RVLM as insulin receptor expression is low, and direct injection of insulin into the RVLM did not alter lumbar SNA and ABP. Therefore, insulin activates a glutamatergic NMDA-dependent pathway to the RVLM to elevate SNA.
The origin of the insulin-driven glutamatergic pathway to the RVLM is not known. The sources of glutamatergic input to the RVLM have not been completely identified; however, the RVLM is densely innervated by glutamatergic neurons in the hypothalamic paraventricular nucleus 41. Interestingly, preliminary data from our laboratory indicate that inhibition of the hypothalamic paraventricular nucleus reverses the sympathoexcitatory response to hyperinsulinemia 42. Although previous studies have reported insulin receptor binding in the hypothalamic paraventricular nucleus 43, it is not known whether insulin acts directly on these neurons or elsewhere to elevate SNA. A number of other hypothalamic structures also express insulin receptors including the arcuate nucleus, ventromedial hypothalamus, and circumventricular organs of the forebrain lamina terminalis 43. However, there are no available studies that have systemically examined the contribution of these various structures to the sympathoexcitatory response to insulin and whether such neurons detect circulating insulin. To date, previous studies have demonstrated that either global inhibition of hypothalamic PI3K 34 or lesion of the anteroventral third ventricular region 13 attenuates the increase in lumbar SNA during hyperinsulinemia. Clearly, future experiments are needed to identify the neurons that detect changes in circulating insulin and how this translates into activation of a glutamatergic pathway to the RVLM to increase lumbar SNA.
In summary, the present study provides the first evidence of a specific brain region that mediates the sympathoexcitatory response to hyperinsulinemia. The results clearly demonstrate the sympathoexcitatory response to insulin depends upon activation of glutamatergic, and more specifically NMDA, receptors in the RVLM. Insulin likely acts at hypothalamic sites to increase glutamatergic drive to the RVLM as these neurons express a low level of insulin receptors and direct injection of insulin into the RVLM did not alter lumbar SNA or ABP.
Perspectives
Clinical studies have revealed a correlation between obesity, hypertension, and hyperinsulinemia 1, 2, but the role of insulin in hypertension remains controversial. In rats, acute hyperinsulinemia elevates lumbar SNA, and chronic hyperinsulinemic-euglycemic clamps increase total peripheral resistance and ABP 12, 13. In contrast, studies performed in dogs have reported that peripheral infusion of insulin did not elevate ABP 44, 45. The discrepancy between data from rats versus dogs may be explained by greater peripheral insulin sensitivity in dogs. Consistent with this notion, a hyperinsulinemic-euglycemic clamp in dogs increased, rather than decreased, cardiac output thereby indicating a systemic vasodilatory response and no change in ABP 44. Unfortunately, it is not known whether dogs exhibit a similar sympathoexcitatory response to insulin as previously reported in mice 26, 34, rats 12, 13, and humans 11, 36. Due to the absence of experimental tools to directly assess the contribution of insulin to these chronic diseases, the role of insulin in obesity-related hypertension or other disease states of hyperinsulinemia will likely remain controversial. Yet, the present findings provide a potential model to examine the pathways and mechanisms that may contribute or support the elevated SNA during obesity-related hypertension.
Supplementary Material
Acknowledgments
None.
Sources of Funding: This research was supported by a Great Rivers American Heart Association Predoctoral Fellowship (M.E.B.), American Heart Association Scientist Development Grant (S.D.S.), and a National Institutes of Health National Heart, Lung, and Blood Institute grant HL090826 (S.D.S.).
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 2.Wofford MR, Hall JE. Pathophysiology and treatment of obesity hypertension. Curr Pharm Des. 2004;10:3621–3637. doi: 10.2174/1381612043382855. [DOI] [PubMed] [Google Scholar]
- 3.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 4.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 6.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia G. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 7.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 8.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 9.Levin BE. Sympathetic activity, age, sucrose preference, and diet-induced obesity. Obes Res. 1993;1:281–287. doi: 10.1002/j.1550-8528.1993.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 10.Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension. 2007;49:640–646. doi: 10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- 11.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan DA, Balon TW, Ginsberg BH, Mark AL. Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am J Physiol Regul Integr Comp Physiol. 1993;264:R423–427. doi: 10.1152/ajpregu.1993.264.2.R423. [DOI] [PubMed] [Google Scholar]
- 13.Muntzel M, Beltz T, Mark AL, Johnson AK. Anteroventral third ventricle lesions abolish lumbar sympathetic responses to insulin. Hypertension. 1994;23:1059–1062. doi: 10.1161/01.hyp.23.6.1059. [DOI] [PubMed] [Google Scholar]
- 14.Muntzel MS, Morgan DA, Mark AL, Johnson AK. Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol. 1994;267:R1350–1355. doi: 10.1152/ajpregu.1994.267.5.R1350. [DOI] [PubMed] [Google Scholar]
- 15.Brands MW, Lee WF, Keen HL, Alonso-Galicia M, Zappe DH, Hall JE. Cardiac output and renal function during insulin hypertension in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 1996;271:R276–281. doi: 10.1152/ajpregu.1996.271.1.R276. [DOI] [PubMed] [Google Scholar]
- 16.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 17.Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- 18.Ito S, Komatsu K, Tsukamoto K, Sved AF. Tonic excitatory input to the rostral ventrolateral medulla in Dahl salt-sensitive rats. Hypertension. 2001;37:687–691. [PubMed] [Google Scholar]
- 19.Bergamaschi C, Campos RR, Schor N, Lopes OU. Role of the rostral ventrolateral medulla in maintenance of blood pressure in rats with Goldblatt hypertension. Hypertension. 1995;26:1117–1120. doi: 10.1161/01.hyp.26.6.1117. [DOI] [PubMed] [Google Scholar]
- 20.Song K, Allen AM, Paxinos G, Mendelsohn FA. Angiotensin II receptor subtypes in rat brain. Clin Exp Pharmacol Physiol. 1991;18:93–96. doi: 10.1111/j.1440-1681.1991.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 21.Dampney RA, Fontes MA, Hirooka Y, Horiuchi J, Potts PD, Tagawa T. Role of angiotensin II receptors in the regulation of vasomotor neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29:467–472. doi: 10.1046/j.1440-1681.2002.03658.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakata T, Takeda K, Hatta T, Kiyama M, Moriguchi J, Miki S, Kawa T, Morimoto S, Nakamura K, Uchida A, Itoh H, Sasaki S, Nakagawa M. Blockade of angiotensin II receptors inhibits the increase in blood pressure induced by insulin. J Cardiovasc Pharmacol. 1998;31:248–252. doi: 10.1097/00005344-199802000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Brands MW, Harrison DL, Keen HL, Gardner A, Shek EW, Hall JE. Insulin-induced hypertension in rats depends on an intact renin-angiotensin system. Hypertension. 1997;29:1014–1019. doi: 10.1161/01.hyp.29.4.1014. [DOI] [PubMed] [Google Scholar]
- 24.Adan RA, Gispen WH. Melanocortins and the brain: from effects via receptors to drug targets. Eur J Pharmacol. 2000;405:13–24. doi: 10.1016/s0014-2999(00)00537-9. [DOI] [PubMed] [Google Scholar]
- 25.Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular effects of adrenocorticotropin microinjections into the rostral ventrolateral medullary pressor area of the rat. Brain Res. 2006;1102:117–126. doi: 10.1016/j.brainres.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scislo TJ, Augustyniak RA, O'Leary DS. Differential arterial baroreflex regulation of renal, lumbar, and adrenal sympathetic nerve activity in the rat. Am J Physiol Regul Integr Comp Physiol. 1998;275:R995–R1002. doi: 10.1152/ajpregu.1998.275.4.R995. [DOI] [PubMed] [Google Scholar]
- 28.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol. 2005;563:249–263. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension. 2007;50:354–359. doi: 10.1161/HYPERTENSIONAHA.107.091843. [DOI] [PubMed] [Google Scholar]
- 30.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension. 2008;52:932–937. doi: 10.1161/HYPERTENSIONAHA.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla in centrally mediated pressor responses. Am J Physiol Heart Circ Physiol. 1994;267:H1549–1556. doi: 10.1152/ajpheart.1994.267.4.H1549. [DOI] [PubMed] [Google Scholar]
- 32.Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius in the rat. Neuroscience. 2008;153:605–617. doi: 10.1016/j.neuroscience.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- 34.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stocker SD, Smith CA, Kimbrough CM, Stricker EM, Sved AF. Elevated dietary salt suppresses renin secretion but not thirst evoked by arterial hypotension in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1521–1528. doi: 10.1152/ajpregu.00658.2002. [DOI] [PubMed] [Google Scholar]
- 36.Anderson EA, Balon TW, Hoffman RP, Sinkey CA, Mark AL. Insulin increases sympathetic activity but not blood pressure in borderline hypertensive humans. Hypertension. 1992;19:621–627. doi: 10.1161/01.hyp.19.6.621. [DOI] [PubMed] [Google Scholar]
- 37.Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R181–186. doi: 10.1152/ajpregu.00507.2004. [DOI] [PubMed] [Google Scholar]
- 38.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35:1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 39.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R412–419. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- 40.Levin BE, Triscari J, Sullivan AC. Altered sympathetic activity during development of diet-induced obesity in rat. Am J Physiol Regul Integr Comp Physiol. 1983;244:R347–355. doi: 10.1152/ajpregu.1983.244.3.R347. [DOI] [PubMed] [Google Scholar]
- 41.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–85. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stocker SD, Bardgett ME. Hypertension. American Heart Association Council for High Blood Pressure Research Meeting Abstracts; 2007. Hypothalamic paraventricular nucleus contributes to the sympathoexcitatory effects of hyperinsulinemia; p. e79. [Google Scholar]
- 43.Werther GA, Hogg A, Oldfield BJ, McKinley MJ, Figdor R, Allen AM, Mendelsohn FA. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi: 10.1210/endo-121-4-1562. [DOI] [PubMed] [Google Scholar]
- 44.Brands MW, Mizelle HL, Gaillard CA, Hildebrandt DA, Hall JE. The hemodynamic response to chronic hyperinsulinemia in conscious dogs. Am J Hypertens. 1991;4:164–168. doi: 10.1093/ajh/4.2.164. [DOI] [PubMed] [Google Scholar]
- 45.Hildebrandt DA, Smith MJ, Jr, Hall JE. Cardiovascular regulation during insulin infusion into the carotid or vertebral artery in dogs. J Hypertens. 1999;17:251–260. doi: 10.1097/00004872-199917020-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.