Abstract
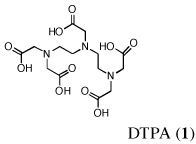
Purpose
Previous systematic structure-activity studies of the desferrithiocin (DFT) platform have allowed the design and synthesis of analogues and derivatives of DFT that retain the exceptional iron-clearing activity of the parent, while eliminating its adverse effects. We hypothesized that a similar approach could be adopted to identify DFT-related analogues that could effectively decorporate uranium.
Materials and Methods
The decorporation properties of nine DFT-related analogues were determined in a bile duct-cannulated rat model. Diethylenetriaminepentaacetic acid (DTPA) served as a positive control. Selected ligands also underwent multiple and delayed dosing regimens. Uranium excretion in urine and bile or stool was determined by inductively coupled plasma mass spectroscopy (ICP-MS); tissue levels of uranium were also assessed.
Results
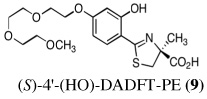
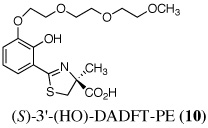
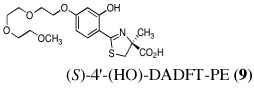
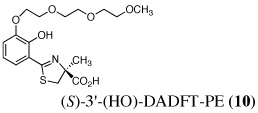
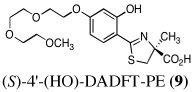
The two best clinical candidates are (S)-4,5-dihydro-2-[2-hydroxy-4-(3,6,9-trioxadecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid [(S)-4'-(HO)-DADFT-PE (9)], with a 57% reduction in kidney uranium levels on oral (p.o.) administration and (S)-4,5-dihydro-2-[2-hydroxy-3-(3,6,9-trioxadecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid [(S)-3'-(HO)-DADFT-PE (10)], with a 62% renal reduction on p.o. administration. The majority of the metal excretion promoted by these analogues is in the bile, thus further reducing kidney actinide exposure.
Conclusions
While 9 administered p.o. or subcutaneously (s.c.) immediately post-metal is an effective decorporation agent, withholding the dose (s.c.) until 4 h reduced the activity of the compound. Conversion of 9 to its isopropyl ester may circumvent this issue.
Keywords: uranium, decorporation, desferrithiocin analogues, inductively coupled plasma mass spectroscopy (ICP-MS)
Introduction
There are any number of scenarios in which uranium (U) can initiate serious health problems: inhalation, wound contamination, ingestion of contaminated water and food, etc. Although workers involved in processing nuclear fuel and weapons assembly with depleted uranium are the most at-risk, terrorist threats ranging from the so-called "dirty bomb" to the destruction of a nuclear reactor have added a new dimension to potential sources of uranium contamination.
Once absorbed, hexavalent uranium, U(VI), finds its way to the kidney and bone, causing profound damage. This actinide is particularly insidious in that it can present with two different levels of toxicity depending on the isotope–chemical or radiotoxicity. Naturally abundant uranium, 238U, is primarily chemically toxic, while enriched uranium, 235U, derives most of its deleterious effects from its radiation profile. The key is to decorporate the metal as soon as possible.
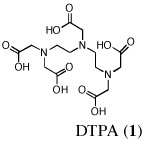
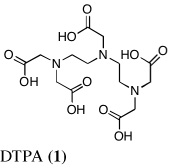
Probably the most widely accepted chelator, diethylenetriaminepentaacetic acid (DTPA) (Pentetate Calcium Trisodium Injection Package Insert, 2008) requires very prompt treatment with parenteral, e.g., intravenous (i.v.) or subcutaneous (s.c.) administration, and presents with a number of side effects associated with zinc stripping (Pippard et al., 1986, Levien et al., 2005). While parenterally administered chelators have a place, the lack of orally active ligands is of genuine concern in the case of mass exposure. It would be difficult to manage large numbers of patients requiring protracted i.v. or s.c. administration of DTPA. In a paper by G. N. Stradling, et al., the investigators examined a group of phosphorous-containing ligands, e.g., polyphosphonic acids, biophosphonates and phonoalkylphosphinates, and the hydroxypyridone (HOPO) ligand, 3,4,3-L1(1,2-HOPO), for their ability to decorporate uranium (Hengé-Napoli et al., 1998). Their results underscore the necessity of introducing the decorporation agent soon after contamination; the drug’s efficacy decreased significantly as the interval between exposure and treatment increased.
In a definitive review by K. N. Raymond (Gorden et al., 2003, Xu and Raymond, 1999) on the “Rational Design of Sequestering Agents for Plutonium and Other Actinides,” the author underscores the similarities early on between Fe(III) and various actinides, pointing out that a number of octacoordinate, hexacoordinate, and tetracoordinate catechol and HOPO ligands bind U(VI) (Durbin et al., 2000). The current study, focusing on the design, evaluation, and development of a series of desferrithiocin [(S)-4,5-dihydro-2-(3-hydroxy-2-pyridinyl)-4-methyl-4-thiazolecarboxylic acid, DFT] analogues for the decorporation of U(VI), is a direct extension of our efforts with DFT iron chelators to bring a compound through all of the developmental stages from laboratory to patient.
Recall that DFT (Naegeli and Zähner, 1980) is a natural product tridentate iron chelator that forms a tight 2:1 complex with Fe(III) (Anderegg and Räber, 1990, Hahn et al., 1990). Although the compound was shown to be an excellent deferration agent when administered orally (p.o.) to rodents (Bergeron et al., 1991) and primates (Bergeron et al., 1990, Wolfe et al., 1989), it caused severe nephrotoxicity in rats (Bergeron et al., 1993). However, the compound’s oral activity spurred structure-activity relationship (SAR) studies focused on the DFT platform aimed at identifying an orally active and safe DFT analogue (Bergeron et al., 1999a, Bergeron et al., 2003, Bergeron et al., 1999b).
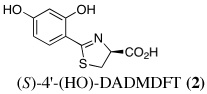
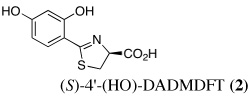
Our approach first entailed simplifying the platform. Removal of the pyridine nitrogen of DFT provided (S)-4,5-dihydro-2-(2-hydroxyphenyl)-4-methyl-4-thiazolecarboxylic acid [(S)-DADFT], the parent ligand of the desaza (DA) series. Substitution of the 4-methyl of (S)-DADFT with a hydrogen led to (S)-4,5-dihydro-2-(2-hydroxyphenyl)-4-thiazolecarboxylic acid [(S)-DADMDFT], the platform for the ensuing DADM systems. In the course of additional SAR studies, we discovered that the lipophilicity (partition between octanol and water, expressed as the log of the fraction in the octanol layer, log Papp) (Sangster, 1997) of the DFT analogues could have a profound effect on the ligand's iron-clearing efficiency (ICE), organ distribution, and toxicity profile (Bergeron et al., 2006a, Bergeron et al., 2006b). The more lipophilic a chelator, the better the ICE. However, often the more lipophilic the chelator, the more toxic it was (Bergeron et al., 2006b).
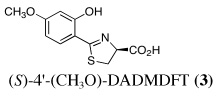
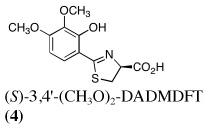
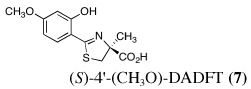
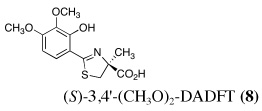
Ultimately, the SAR studies demonstrated that substituting the 4'-methoxy group of [(S)-4'-(CH3O)-DADFT (7), Table I] with a 3,6,9-trioxadecyloxy group, providing the polyether (S)-4,5-dihydro-2-[2-hydroxy-4-(3,6,9-trioxadecyloxy)phenyl]-4-methyl-4-thiazolecarboxylic acid [(S)-4'-(HO)-DADFT-PE, 9], reduced the lipophilicity from −0.70 log Papp for 7 to −1.10 for 9, and virtually eliminated nephrotoxicity in a rodent model (Bergeron et al., 2006b). As uranium’s major target of chemical toxicity is the kidney, it would be unwise to have both an actinide and a chelating agent causing renal problems. The ICE of the 4'-polyether (9) given p.o. to rats and primates was excellent (Bergeron et al., 2006b). This encouraged the synthesis of an additional ligand, the 3'-(3,6,9)-trioxadecyloxy analogue (S)-3'-(HO)-DADFT-PE, 10 (Bergeron et al., 2007), which also did not present with any nephrotoxicity and was an excellent iron-clearing device (Bergeron et al., 2008). The chelator-induced nephrotoxicity problem now seemed manageable.
Table I.
Uranium excretion and impact on renal actinide content of bile duct-cannulated rats given a single dose of DTPA or a DFT analoguea.
| Compound/Structure | n | Dose (µmolkg−1) |
Route | U Excretion (µg kg−1) |
Bile/Urine (%) |
% Administered Dose Excreted |
Renal Conc. (µg g−1) |
Reduction (%) |
|---|---|---|---|---|---|---|---|---|
| Control: Uranyl Acetate 1 mg U kg−1 SC |
5 | - | - | 208 ± 28 | 0/100 | 20.8 | 84 ± 16 | - |
 |
6 | 150 | SC | 276 ± 88 | 2/98 | 27.6 | 47 ± 9 b | 44 |
| 5 | 300 | PO | 212 ± 114 | 0/100 | 21.2 | 54 ± 11 b | 36 | |
 |
5 | 150 | SC | 185 ± 51 | 14/86 | 18.5 | 76 ± 20 | 10 |
| 3 | 300 | SC | 184 ± 14 | 45/55 | 18.4 | 79 ± 9 | 5 | |
| 4 | 300 | PO | 132 ± 30b | 23/77 | 13.2 | 59 ± 29 | 29 | |
 |
4 | 150 | SC | 238 ± 40 | 37/63 | 23.8 | 51 ± 22 b | 40 |
| 3 | 300 | SC | 327 ± 38c | 77/23 | 32.7 | 34 ± 5 b | 60 | |
| 6 | 300 | PO | 260 ± 45c | 85/15 | 26.0 | 23 ± 11 b | 73 | |
 |
4 | 150 | SC | 199 ± 24 | 14/86 | 19.9 | 71 ± 7 | 15 |
| 5 | 300 | SC | 340 ± 99 c | 84/16 | 34.0 | 9 ± 5 b | 89 | |
| 4 | 300 | PO | 270 ± 114 | 47/53 | 27.0 | 42 ± 6 b | 49 | |
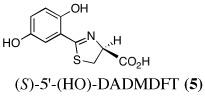 |
4 | 150 | SC | 240 ± 74 | 1/99 | 24.0 | 83 ± 3 | 0 |
| 5 | 300 | SC | 182 ± 76 | 21/79 | 18.2 | 66 ± 14 b | 21 | |
| 4 | 300 | PO | 251 ± 90 | 6/94 | 25.1 | 65 ± 10 b | 22 | |
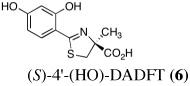 |
5 | 150 | SC | 167 ± 79 | 39/61 | 16.7 | 39 ± 25 b | 53 |
| 4 | 300 | SC | 271 ± 69 | 56/44 | 27.1 | 49 ± 11 b | 41 | |
| 4 | 300 | PO | 230 ± 102 | 41/59 | 23.0 | 27 ± 5 b | 68 | |
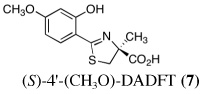 |
5 | 150 | SC | 248 ± 43 | 62/38 | 24.8 | 19 ± 4 b | 77 |
| 3 | 300 | SC | 339 ± 140 | 92/8 | 33.9 | 8 ± 2 b | 90 | |
| 5 | 300 | PO | 265 ± 83 | 89/11 | 26.5 | 5 ± 3 b | 94 | |
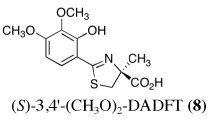 |
4 | 150 | SC | 186 ± 37 | 21/79 | 18.6 | 38 ± 14 b | 54 |
| 5 | 300 | SC | 173 ± 99 | 55/45 | 17.3 | 12 ± 8 b | 86 | |
| 4 | 300 | PO | 262 ± 85 | 50/50 | 26.2 | 12 ± 5 b | 86 | |
 |
6 | 150 | SC | 482 ± 205 c | 69/31 | 48.2 | 37 ± 16 b | 55 |
| 5 | 300 | SC | 515 ± 107 c | 84/16 | 51.5 | 29 ± 6 b | 66 | |
| 5 | 300 | PO | 435 ± 131 c | 71/29 | 43.5 | 36 ± 8 b | 57 | |
 |
3 | 150 | SC | 316 ± 58 c | 55/45 | 31.6 | 33 ± 8 b | 61 |
| 6 | 300 | SC | 314 ± 105 c | 79/21 | 31.4 | 13 ± 6 b | 85 | |
| 5 | 300 | PO | 257 ± 111 | 67/23 | 25.7 | 32 ± 20 b | 62 |
Data are presented as the mean ± the standard error of the mean; n, number of animals; p-values were generated via a one-tailed student’s t-test.
Bile duct-cannulated rats were given uranium s.c. (left flank) at a dose of 1 mg U kg−1. Immediately thereafter the rodents were given the decorporation agents s.c. (right flank) or p.o. at the doses indicated. Bile and urine samples were collected for 24 h. The kidneys were removed 24 h post-metal.
Mean is significantly less than control.
Mean is significantly greater than control.
The lack of nephrotoxicity, outstanding iron-clearing efficiency of the polyether analogues, and preliminary studies indicating DFT analogues clear U(VI) from rats (unpublished data) compelled us to closely examine the potential of nine DFT analogues (Table I)–four DADMDFT ligands (2–5) and five DADFT analogues (6–10) as uranium decorporation agents. Recall that DTPA, the drug of choice for uranium decorporation, was initially developed as an iron clearing ligand to treat patients suffering from various forms of iron overload (Kemble, 1964, Powell and Thomas, 1967). However, it was ineffective when administered orally, both as an iron chelator and a uranium decorporation agent.
Materials and methods
DTPA was obtained from Sigma-Aldrich, Milwaukee, Wi., United States. The DFT analogues (2–10) were synthesized as previously described (Bergeron et al., 2003, Bergeron et al., 2006a, Bergeron et al., 2006b, Bergeron et al., 2007). DTPA was given as its trisodium calcium salt, prepared by the addition of 3:1:1 mole ratio quantities of NaOH, DTPA, and Ca(OH)2 in saline. The remaining chelators were given as their sodium salts, prepared by the addition of 1 equiv of NaOH to a suspension of the free acid in distilled water. Samples were assessed for their metal content using inductively coupled plasma mass spectroscopy (ICP-MS), model X0675, manufactured by Thermo Electron Corporation, Winsford, England. All of the standards used for the ICP-MS were purchased from Inorganic Ventures, Christiansburg, Va., United States. Teflon syringe filters were obtained from GE Osmonics’ Labstore, Minnetonka, Mn., United States.
Uranyl acetate dihydrate was purchased from Chem-Impex International, Wood Dale, Il., United States. The actinide was solubilized by brief sonication in saline. The pH was adjusted to 6.4 using saturated sodium bicarbonate. The solution was sterilized via a 0.2 μ syringe filter, Fisher Scientific, Pittsburgh, Pa., United States. The final metal concentration was 1 mg U ml−1. Adult male Sprague-Dawley rats (375–425 g) were purchased from Harlan Sprague Dawley, Indianapolis, In., United States. The rats were given the actinide s.c. (1 ml kg−1) at a dose of 1 mg U kg−1. Note that unlike a “wound simulation,” in which the actinide and decorporation agent may be premixed in a syringe prior to administration (Paquet et al., 2000), or injected in the same location (Paquet et al., 2003), in these experiments the s.c. injections were given on the opposite sides of the body; the uranium was injected in the left flank, while the chelator was given in the right flank. This is an important distinction, as premixing/injecting both solutions in the same area could artificially overestimate the efficacy of the decorporation agent.
Animals
All animal studies were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, following a protocol that had been approved by the Institutional Animal Care and Use Committee of the University of Florida.
Single dose chelator administration regimen: bile duct-cannulated rats
Because of our experience with the behavior of the DFT analogues as iron chelators in bile duct-cannulated rats, the initial uranium decorporation studies were carried out in this model. Surgery was performed as previously described (Bergeron et al., 1990, Bergeron et al., 1991). Animals that were to be given the drugs p.o. were fasted overnight. The rats were allowed to recover from surgery for approximately 3 h before being utilized. The rats were given uranium s.c. at a dose of 1 mg U kg−1. Immediately thereafter, the animals were given a single p.o. (gavage) or s.c. dose of the chelators at 150 and/or 300 µmol kg−1 (Table I). Bile and urine were collected for 24 h. The rats were then euthanized and the kidneys, bone (femur), liver, spleen and lung were removed and frozen. Although the kidneys and bone are the tissues most affected by uranium, the other organs were evaluated for their metal content to assess unexpected chelator-induced uranium redistribution.
Multiple dose chelator administration regimen in non-bile duct-cannulated (intact) rats: urinary and fecal metal clearance
The initial screen of new compounds involved cannulating the rats' bile duct, administering a single dose of the test chelator and monitoring the urinary and biliary metal clearance for 24 h. To determine the cumulative urinary and fecal metal clearance induced by a given chelating agent over a longer interval than is possible with a bile duct-cannulated animal, DTPA and the 4'-polyether (9) were administered s.c. at a dose of 300 µmol kg−1 to metal-treated rats on a daily basis for four days. The rats were given a single s.c. injection of U(VI), 1 mg kg−1. The initial dose of the chelator was given immediately post-metal. Additional doses of the decorporation agents were given at 24-h intervals for three more days (4 doses total).
The rodents were housed in individual metabolic cages. The animals were provided with standard rodent chow and distilled water ad libitum. Urine and feces samples were collected from the metabolic cages at 24-h intervals. The rats were euthanized 24 h after the last dose of the chelators; the kidneys, bone (femur), liver, spleen and lung were removed and frozen.
Rodent bile and urine sample preparation
Bile was prepared for analysis on the ICP-MS as follows: the bile collection tube was vortexed. Immediately thereafter, a 1-mL aliquot of bile was removed and placed in a separate tube. The bile sample was digested by the addition of 1 mL of concentrated nitric acid (65%). The samples were placed in a 60-°C water bath for 4 h. Deionized, distilled water was added such that the final concentration of nitric acid was reduced to 2%. Finally, the samples were poured into a syringe and filtered using 0.45 μ, 30 mm, Teflon syringe filters. Urine was prepared the same as the bile, except that to reduce nitric acid-related foaming, the urine was diluted 1:1 with distilled water.
Rodent feces preparation
Feces samples were collected from the metabolic cages at 24-h intervals. In addition, at necropsy, any feces distal to the cecum was removed and added to the collection tube. The fecal pellets were rinsed with distilled water to remove any food particles. Additional distilled water was added to the collection tube. The tube was vortexed to form a feces slurry. The slurry was poured into a tared, acid-rinsed 250 ml round bottom. The collection tube was rinsed two times with distilled water. The rinsate was added to the round bottom. The feces slurry/rinsate was frozen in a dry ice/acetone bath and placed on a lyophilizer overnight. The round bottoms were weighed to determine the dry weight of the feces. A 1-g portion of the dried feces was weighed and placed in a 100-mL round bottom. Concentrated nitric acid (65%), 20 mL, was added and the samples were refluxed in an oil bath at 120 °C for 3 d. The concentration of nitric acid was reduced to 2% by the addition of distilled water. Finally, the samples were poured into a syringe and filtered using 0.45 μ, 30 mm, Teflon syringe filters.
Rodent tissue preparation
The rats were euthanized by exposure to carbon dioxide gas from a commercially available gas cylinder. The kidneys, bone (femur), liver, spleen and lung were removed for the determination of their uranium content. The initial step in the tissue preparation involved removing any obvious membranes or fat. A sample of each tissue (300–350 mg) was weighed and transferred to acid-washed hydrolysis (pressure) tubes. Note that the same region of each tissue was always utilized; the femur sample was taken proximally from the lesser trochanter and distally to the popliteal surface and included the bone + bone marrow. Concentrated nitric acid (65%), 1.5 mL, and 2 mL of distilled water were added. The tubes were then sealed and placed in a 120-°C oil bath for 5 h; the tubes were vented as necessary. Then, the tubes were removed from the oil bath and allowed to cool to room temperature. The temperature of the oil bath was decreased to 100 °C. Once the samples were cooled, 0.7 mL of hydrogen peroxide (30%) was added to the hydrolysis tube. The samples were placed back in the oil bath and cooked overnight. The samples were then removed from the oil bath and allowed to cool to room temperature. The hydrolysis tubes were vortexed and the digested samples were poured into 50-mL volumetric flasks. The samples were brought to volume using distilled water. Finally, the samples were poured into a syringe and filtered using 0.45 μ, 30 mm, Teflon syringe filters.
Determination of uranium content using inductively coupled plasma mass spectroscopy (ICP-MS)
An ICP-MS, model X0675, was used to determine the actinide concentration of the samples. The operating conditions of similar equipment and the average performance obtained with environmental samples are described elsewhere (Chiappini et al., 1996). The operational parameters were optimized for the detection of uranium. 115In was added as an internal standard to each sample to correct for any instrumental drifts. Five standard solutions for the calibration curve (0, 0.1, 1, 10 and 100 ppb) were freshly prepared by the dilution of a commercially obtained standard stock solution. A blank sample (2% nitric acid) and one of the standard solutions were run for every ten test samples to check the stability of the background and to detect potential contamination. Each sample was run in triplicate; the mean value was used. Tissue uranium concentration is reported as µg g−1; urine and bile/feces metal excretion are reported as µg kg−1.
Statistical analyses
Data are presented as the mean ± the standard error of the mean; n, number of animals; p-values were generated via a one-tailed student’s t-test, in which the inequality of variances was assumed; a p-value of <0.05 was considered significant.
Results and Discussion
In the design of uranium decorporation agents, three issues demand attention: 1) the effectiveness of total nuclide decorporation; 2) ligand-promoted organ actinide decorporation versus redistribution, and 3) mode of chelator-induced metal excretion. In a preliminary study (unpublished data), bile duct-cannulated rats were given U(VI) s.c. at a dose of 2.8 mg U kg−1. Immediately thereafter, the animals were given a single 300-µmol kg−1 dose of (S)-2-(2-hydroxy-4-methoxyphenyl)-4,5-dihydro-4-thiazolecarboxylic acid [(S)-4'-(CH3O)-DADMDFT, 3], s.c., p.o., or intraperitoneally (i.p.). The drug was an effective decorporation agent via all three routes (data not shown), clearing up to 40% of the administered uranium in the bile and urine; a significant reduction in renal uranium was also observed (data not shown). Although i.p. administration of a chelator is not practical in potential human applications, and the dose of uranium (2.8 mg U kg−1) was too high because of nephrotoxicity issues in longer-term studies (Voegtlin and Hodge, 1949), these findings were sufficient to warrant a more systematic look at DFT analogues as uranium decorporation agents.
In the current study, the dose of uranium has been reduced from 2.8 mg U kg−1 to 1 mg U kg−1. Nine different DFT analogues–four DADMDFT ligands (2–5) and five DADFT analogues (6–10) have been evaluated as uranium decorporation agents in rodents (Table I). DTPA (1) served as a positive control. The first set of experiments with the ligands shown in Table I focused on a single s.c. or p.o. dose of the respective chelators at 150 µmol kg−1 and/or 300 µmol kg−1 to bile duct-cannulated rodents. The ligands were administered immediately after uranyl acetate was given s.c. at a dose of 1 mg U kg−1. Bile and urine were collected for 24 h; uranium excretion and tissue actinide content were monitored using ICP-MS.
Metal-only treated bile duct-cannulated rats spontaneously excreted 20.8% of the administered dose of uranium during the 24-h collection period; 100% of the decorporated metal was excreted in the urine (Table I). Interestingly, in terms of total uranium excretion (bile + urine), DTPA (1) was ineffective when given either s.c. (150 µmol kg−1) or p.o. (300 µmol kg−1); the dose of DTPA simply may not have been high enough. These clearance studies left us with three active desferrithiocin-based compounds, (S)-4'-(CH3O)-DADMDFT (3), (S)-4'-(HO)-DADFT-PE (9) and (S)-3'-(HO)-DADFT-PE (10). Unlike DTPA, 3, 9 and 10-promoted uranium excretion is largely biliary, further sparing the kidney. In addition, ligands 3 and 9 work well when given orally; DTPA is ineffective via this route. However, as will be discussed below, 3 redistributes the actinide to the liver and will not be pursued further. This left us with two polyether ligands 9 and 10 that have good overall decorporation properties. For example, (S)-4'-(HO)-DADFT-PE (9) cleared 43.5% of the administered uranium in the bile and urine when a single 300 µmol kg−1 dose of the ligand was given p.o. (p < 0.008), and 51.5% when it was administered s.c. at the same dose (p < 0.001), more than twice as much uranium as was spontaneously excreted by the metal-only control animals, 20.8% (Table I).
A second and equally important issue is the reduction of kidney uranium. DTPA given s.c. at a dose of 150 µmol kg−1 reduced renal uranium by 44% (p < 0.002); given p.o. at 300 µmol kg−1, it was reduced by 36% (p < 0.005). However, total uranium excretion was insignificant (Table I). The renal actinide clearance was no doubt lost in the noise. Several of the DFT analogues had a profound effect on removing uranium from the kidney (Table I). For example, ligand 7 reduces renal actinide burden by 77% and 90% when the drug is given s.c. at 150 and 300 µmol kg−1, respectively. However, like DTPA, the drug was ineffective in terms of global uranium excretion. The two best DFT candidates in terms of a trade-off between renal uranium diminution and total actinide decorporation are (S)-4'-(HO)-DADFT-PE (9), with a 57% reduction in kidney uranium levels on p.o. (300 µmol kg−1) administration and (S)-3'-(HO)-DADFT-PE (10), with a 62% renal reduction on p.o. administration at the same dose (Table I). In both instances, renal actinide decorporation after s.c. dosing (300 µmol kg−1) of the chelators was even better, 66% and 85%, respectively. While ligand 10 promotes global uranium excretion after s.c. dosing, the 4'-polyether (9) works well whether given p.o. or s.c., clearly increasing total uranium decorporation over spontaneous excretion (Table I).
Probably the most compelling reason for selecting the polyethers (S)-4'-(HO)-DADFT-PE (9) and (S)-3'-(HO)-DADFT-PE (10) as clinical candidates is their mode of metal decorporation. In both the metal-only controls and the DTPA-treated rats, what little uranium comes out is all excreted through the kidneys. With the polyether analogues (9 and 10) given p.o., the actinide excretion is now largely in the bile, 71% and 67% respectively, no longer traveling through the kidney, the most sensitive organ. Thus, should a ligand-metal exchange occur in the kidney, the level of uranium exchange with 9 or 10 is limited–most of the complex is excreted in the bile.
Actinide decorporation or redistribution in kidney, bone, liver, spleen and lung of bile duct-cannulated rats given a single dose of DTPA or a DFT analogue
Although the kidneys and bone are the tissues most affected by uranium, the liver, spleen, and lung were also evaluated for their metal content to assess if the ligands were promoting actinide redistribution. While the renal uranium concentration data was provided in Table I, it is also included in Table II to allow for a more ready analysis of uranium decorporation versus actinide redistribution to the other organs.
Table II.
Tissue uranium concentration of bile duct-cannulated rats given a single dose of DTPA or a DFT analoguea.
| Compound/Structure | n | Dose (µmol kg−1) |
Route | Kidney U Conc. (µg g−1) |
Bone (femur) U Conc. (µg g−1) |
Liver U Conc. (µg g−1) |
Spleen U Conc. (µg g−1) |
Lung U Conc. (µg g−1) |
|---|---|---|---|---|---|---|---|---|
| Control: Uranyl Acetate 1 mg U kg−1 SC |
5 | - | - | 84 ± 16 | 3.6 ± 1.2 | 0.16 ± 0.03 | 0.19 ± 0.01 | 0.13 ± 0.02 |
 |
6 | 150 | SC | 47 ± 9b | 3.1 ± 1.1 | 0.1 ± 0.0b | 0.15 ± 0.05b | 0.07 ± 0.01 b |
| 5 | 300 | PO | 54 ± 11b | 4.3 ± 1.3 | 0.2 ± 0.0 | 0.13 ± 0.03b | 0.10 ± 0.02b | |
 |
5 | 150 | SC | 76 ± 20 | 3.4 ± 0.7 | 1.3 ± 0.5c | 0.18 ± 0.06 | 0.11 ± 0.03 |
| 3 | 300 | SC | 79 ± 9 | 3.1 ± 1.5 | 3.1 ± 0.8c | 0.50 ± 0.05c | 0.30 ± 0.09c | |
| 4 | 300 | PO | 59 ± 29 | 4.7 ± 1.8 | 0.8 ± 0.4c | 0.20 ± 0.05 | 0.18 ± 0.06 | |
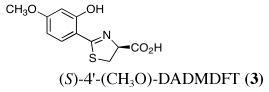 |
4 | 150 | SC | 51 ± 22b | 4.1 ± 1.6 | 9.0 ± 4.0c | 0.48 ± 0.09c | 0.32 ± 0.09c |
| 3 | 300 | SC | 34 ± 5b | 3.0 ± 1.5 | 6.6 ± 1.2c | 0.39 ± 0.07c | 0.32 ± 0.02c | |
| 6 | 300 | PO | 23 ± 11b | 3.1 ± 1.3 | 2.5 ± 0.8c | 0.29 ± 0.07c | 0.28 ± 0.10c | |
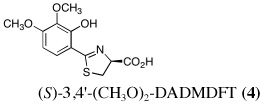 |
4 | 150 | SC | 71 ± 7 | 1.6 ± 1.1b | 2.6 ± 0.6c | 0.26 ± 0.06 | 0.13 ± 0.04 |
| 5 | 300 | SC | 9 ± 5b | 2.6 ± 1.0 | 5.5 ± 2.1c | 0.78 ± 0.19c | 0.51 ± 0.22c | |
| 4 | 300 | PO | 42 ± 6b | 2.5 ± 1.3 | 1.2 ± 0.3c | 0.34 ± 0.06c | 0.22 ± 0.04c | |
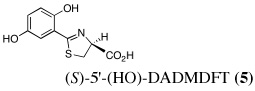 |
4 | 150 | SC | 83 ± 3 | 3.3 ± 0.5 | 0.3 ± 0.1c | 0.15 ± 0.04 | 0.13 ± 0.03 |
| 5 | 300 | SC | 66 ± 14b | 4.3 ± 0.6 | 1.1 ± 0.5c | 0.36 ± 0.10c | 0.25 ± 0.09c | |
| 4 | 300 | PO | 65 ± 10b | 3.5 ± 0.6 | 0.3 ± 0.1c | 0.25 ± 0.05 | 0.19 ± 0.11 | |
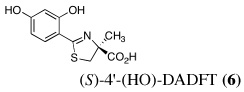 |
5 | 150 | SC | 39 ± 25b | 2.8 ± 1.1 | 4.8 ± 1.2c | 0.29 ± 0.10c | 0.19 ± 0.10 |
| 4 | 300 | SC | 49 ± 11b | 3.6 ± 0.8 | 7.7 ± 1.6c | 0.41 ± 0.04c | 0.22 ± 0.03c | |
| 4 | 300 | PO | 27 ± 5b | 5.0 ± 1.8 | 4.8 ± 1.0c | 0.26 ± 0.04c | 0.22 ± 0.10 | |
 |
5 | 150 | SC | 19 ± 4b | 2.9 ± 0.9 | 11.3 ± 3.2c | 0.39 ± 0.08c | 0.28 ± 0.04c |
| 3 | 300 | SC | 8 ± 2b | 1.8 ± 0.6b | 8.1 ± 2.4c | 0.67 ± 0.18c | 0.61 ± 0.13c | |
| 5 | 300 | PO | 5 ± 3b | 1.7 ± 0.9b | 5.1 ± 1.7c | 0.49 ± 0.44 | 0.30 ± 0.10c | |
 |
4 | 150 | SC | 38 ± 14b | 2.9 ± 0.2 | 5.7 ± 1.5c | 0.63 ± 0.03c | 0.47 ± 0.10c |
| 5 | 300 | SC | 12 ± 8b | 3.3 ± 1.6 | 5.9 ± 3.4c | 0.83 ± 0.26c | 0.86 ± 0.38c | |
| 4 | 300 | PO | 12 ± 5b | 3.5 ± 0.5 | 4.7 ± 0.7c | 1.20 ± 0.40c | 1.50 ± 0.17c | |
 |
6 | 150 | SC | 37 ± 16b | 2.1 ± 0.5b | 1.6 ± 0.9c | 0.20 ± 0.05 | 0.16 ± 0.13 |
| 5 | 300 | SC | 29 ± 6b | 1.9 ± 0.5b | 0.9 ± 0.2c | 0.18 ± 0.04 | 0.13 ± 0.02 | |
| 5 | 300 | PO | 36 ± 8b | 2.7 ± 1.2 | 0.6 ± 0.2c | 0.13 ± 0.02b | 0.10 ± 0.02b | |
 |
3 | 150 | SC | 33 ± 8b | 3.4 ± 0.1 | 1.2 ± 0.6c | 0.21 ± 0.01c | 0.09 ± 0.02b |
| 6 | 300 | SC | 13 ± 6b | 2.3 ± 0.6b | 0.8 ± 0.2c | 0.18 ± 0.03 | 0.08 ± 0.02b | |
| 5 | 300 | PO | 32 ± 20b | 3.1 ± 1.1 | 0.7 ± 0.4c | 0.16 ± 0.05 | 0.13 ± 0.04 |
Data are presented as the mean ± the standard error of the mean; n, number of animals; p-values were generated via a one-tailed student’s t-test.
Bile duct-cannulated rats were given uranium s.c. (left flank) at a dose of 1 mg U kg−1. Immediately thereafter, the rodents were given the decorporation agents s.c. (right flank) or p.o. at the doses indicated. Tissue samples were collected 24 h post-metal. Bone (femur) includes bone + bone marrow.
Mean is significantly less than control.
Mean is significantly greater than control.
Kidney
The uranium concentration in the tissues of the metal-only control rats was, as expected, highest in the kidneys, 84 ± 16 µg g−1 (Table I). DTPA given s.c. immediately post-metal at a dose of 150 µmol kg−1 reduced renal actinide content by 44% (p < 0.002); it was also effective (36% reduction) when given orally at twice the dose (p < 0.005). While 2 was completely ineffective (p.o. or s.c.) at reducing kidney metal content, DFT analogues 3–10 (with the exception of 4 and 5 at 150 µmol kg−1 s.c.) were very effective (p.o. and s.c.) at removing the actinide from this organ. In fact, ligand 7 reduced renal actinide content by 94% when given p.o. and 90% when administered s.c. at a dose of 300 µmol kg−1.
Bone (femur)
The uranium content of the femur (bone + bone marrow) of the control rats was 3.6 ± 1.2 µg g−1. While p.o. or s.c. administered DTPA was ineffective at reducing uranium content in this tissue, four of the DFT analogues (4, 7, 9, 10) were quite effective (Table II). Although there was a ≥ 50% metal reduction in the bone of the 4 (150 µmol kg−1 s.c.) and 7-treated rats (300 µmol kg−1 p.o. and s.c.), neither of these chelators were effective in terms of global uranium excretion (bile + urine); the metal may be being redistributed to other tissues. The 4'-polyether analogue 9 reduced the bone actinide content by 42% when given s.c. at 150 µmol kg−1 and by 48% when given s.c. at 300 µmol kg−1. This was matched by a significant increase in overall uranium excretion (Table I). The 3'-polyether ligand 10 was less effective, reducing bone metal content by 36%, but only when it was dosed s.c. at 300 µmol kg−1.
Liver
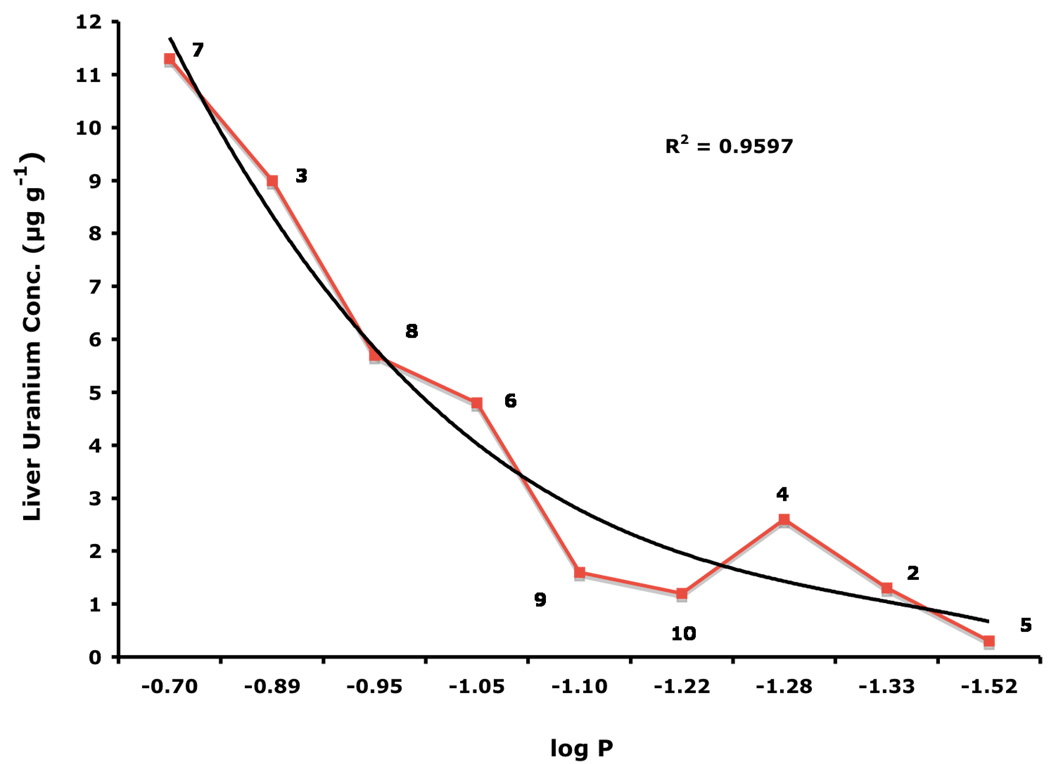
There appears to be a strong correlation between the lipophilicity (log Papp) of the DFT chelators and the amount of uranium that is found in the liver. The more lipophilic the chelator, the more likely it is to cause actinide redistribution to the liver. Interestingly, the liver uranium concentration was highest when the ligands were given s.c. at a dose of 150 µmol kg−1 (Table II). The relationship between ligand lipophilicity and liver actinide concentration is best seen in Figure 1: the least lipophilic of the DFT analogues 5, log Papp = −1.52, was associated with the least amount of the metal in the liver, 0.3 ± 0.1 µg g−1. The most lipophilic chelator, 7, log Papp =−0.70, likewise had the highest liver uranium content, 11.3 ± 3.2 µg g−1. In addition, although 7 did dramatically reduce the renal uranium content when administered p.o. at a dose of 300 µmol kg−1 (94%) or s.c. at a dose of 150 or 300 µmol kg−1, 77% and 90% respectively, there was no significant uranium excretion in the bile + urine. Thus, this ligand is clearly redistributing the actinide from the kidney to the liver. However, while the presence of uranium in the kidney is known to lead to renal failure, there is much less known about the impact of actinide burden on liver function (Gueguen et al., 2006, Craft et al., 2004).
Figure 1.
Liver uranium concentrations versus lipophilicity (log Papp) of DFT analogues 2–10. Log Papp data are expressed as the log of the fraction in the octanol layer; measurements were done in TRIS buffer, pH 7.4, using a “shake flask” direct method (Sangster, 1997). The rats were given uranium s.c. at a dose of 1 mg U kg−1. Immediately thereafter, the rodents were given a single s.c. 150-µmol kg−1 dose of the respective chelators. The uranium was injected in the left flank, while the chelator was given in the right flank. The livers were harvested 24-h post-metal.
Spleen
The uranium content in the spleen of the metal-only treated rats was 0.19 ± 0.01 µg g−1 (Table II). While DTPA given s.c. or p.o. did significantly reduce the metal content in this tissue, the same could not be said for the DFT analogues in general. With the exception of the 4'-polyether ligand 9, which was only effective when given p.o. at 300 µmol kg−1, the remaining compounds either had no impact on actinide concentration in the spleen, or actually increased these values relative to the control animals. The significance of this finding is not yet known.
Lung
The uranium content in the lung of the metal-only treated rats was 0.13 ± 0.02 µg g−1 (Table II). While DTPA given s.c. or p.o. did significantly reduce the metal content in this tissue, the same could not be said for the DFT analogues in general. Only 9 dosed p.o. at 300 µmol kg−1 or 10 given s.c. at 150 or 300 µmol kg−1 reduced the level of uranium in the lung. As with the spleen, the remaining compounds either had no impact on actinide concentration, or actually increased these values relative to the control animals. The significance of this finding is not yet known.
Multiple dose chelator administration regimen in non-bile duct-cannulated (intact) rats
To determine the cumulative urinary and fecal metal clearance induced by a given compound over a longer interval than is possible with a bile duct-cannulated animal, DTPA and the 4'-polyether (9) were administered to metal-treated rats on a daily basis for 4 d. Rats were given uranyl acetate (1 mg U kg−1) s.c. Immediately thereafter, the animals were given DTPA or 9 s.c. at a dose of 300 µmol kg−1. The rodents were housed in individual metabolic cages. The rats were given s.c. doses of the chelators at 24-h intervals for three additional days (4 doses total). Urine and feces were collected once daily. At the end of four days, uranium-only treated controls had excreted 51% of the administered dose of uranium, DTPA-treated animals excreted 53% and 9-treated animals excreted 93% (Table III). In addition, there was a 39% reduction in renal uranium with the DTPA-treated rats versus the controls (p < 0.02) and a 62% reduction in the uranium content of the kidneys of the (S)-4'-(HO)-DADFT-PE (9)-treated rats (p < 0.003). Even more exciting was the observation that, even on day 2, there was a significant amount of uranium being excreted in the feces of the 4'-polyether treated rats (p < 0.04) (Table III). Recall that the first dose of the chelator was given s.c. immediately after actinide administration. We are now in the process of running similar studies using p.o. dosing.
Table III.
Chelator-induced uranium excretion in non-bile duct-cannulated (intact) rats: Multiple dose chelator administration regimen a.
| Compound/Structure | n | Day | Urine U Excretionb (µg kg−1) |
Fecal U Excretionb (µg kg−1) |
Total U Excretion (µg kg−1) |
% Administered Dose Excreted |
Renal Conc. (µg g−1) |
Reduction (%) |
|---|---|---|---|---|---|---|---|---|
| Control: Uranyl Acetate 1 mg U kg−1 SC |
Day 1 | 295 ± 125 | 33 ± 14 | 328 ± 122 | 32.8 | |||
| 4 | Day 2 | 58 ± 8 | 32 ± 10 | 90 ± 3 | 9.0 | |||
| Day 3 | 34 ± 7 | 15 ± 5 | 49 ± 11 | 4.9 | ||||
| Day 4 | 17 ± 9 | 30 ± 16 | 47 ± 23 | 4.7 | 31 ± 6 | - | ||
 |
Day 1 | 380 ± 55 | 30 ± 19 | 410 ± 56 | 41.0 | |||
| 5 | Day 2 | 43 ± 11 | 13 ± 9 | 56 ± 16c | 5.6 | |||
| Day 3 | 29 ± 6 | 13 ± 5 | 42 ± 5 | 4.2 | ||||
| Day 4 | 14 ± 3 | 9 ± 5 | 23 ± 3 | 2.3 | 19 ± 2c | 39 | ||
 |
Day 1 | 205 ± 27 | 478 ± 181 | 683 ± 161d | 68.3 | |||
| 4 | Day 2 | 39 ± 5 | 116 ± 46 | 155 ± 48d | 15.5 | |||
| Day 3 | 23 ± 4 | 33 ± 4 | 56 ± 7 | 5.6 | ||||
| Day 4 | 11 ± 1 | 25 ± 2 | 36 ± 1 | 3.6 | 12 ± 2c | 62 | ||
Data are presented as the mean ± the standard error of the mean; n, number of animals; p-values were generated via a one-tailed student’s t-test.
Non-bile duct-cannulated (intact) rats were given uranium s.c. (left flank) at a dose of 1 mg U kg−1. The animals were given the chelators s.c. (right flank) at a dose of 300 µmol kg−1 immediately thereafter. Additional s.c. doses of DTPA or 9 were given at 24-h intervals for three more days (4 doses total).
Urine and feces were collected at 24-h intervals.
Mean is significantly less than control.
Mean is significantly greater than control.
Bile duct-cannulated rats: delayed dosing regimen
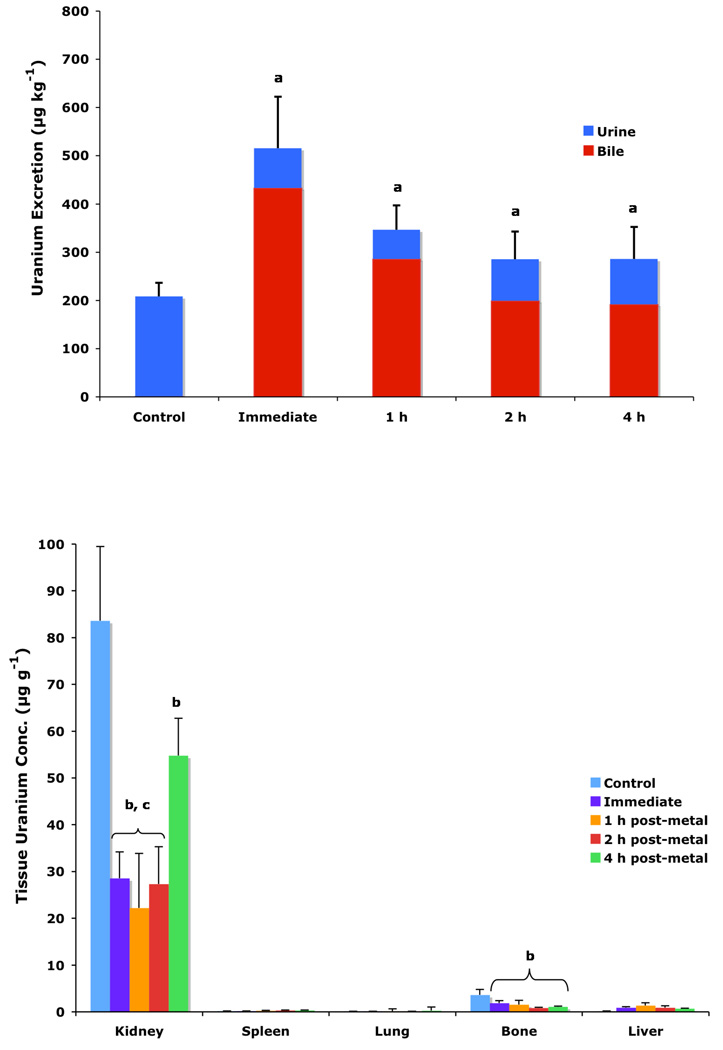
To determine if (S)-4'-(HO)-DADFT-PE (9) could retain its effectiveness if chelator administration was delayed, bile duct-cannulated rats were given uranium s.c. at a dose of 1 mg U kg−1. The rats were then housed in individual metabolic cages. The animals were given a single s.c. 300-µmol kg−1 dose of the 4'-polyether (9) 1, 2, or 4 h post-uranium. Bile and urine samples were collected for 24 h. Recall that 9 given s.c. at a dose of 300 µmol kg−1 immediately post-metal causes the excretion of nearly 52% of the administered actinide; renal uranium content is decreased by 66% (Table 1). Delaying the dose of the chelator until 1, 2, or 4 h post-uranium was also associated with effective decorporation, although the efficacy was ablated with time, Figure 2 (top). When 9 was given s.c. 1 h post-actinide, the rats excreted 34.6% of the administered uranium; this was further reduced to 28.5% and 28.6% at 2 and 4 h post-metal, respectively. Furthermore, a significant reduction in renal uranium content was observed at all three time points, 74%, 68%, and 35% at 1, 2 and 4 h, respectively, Figure 2 (bottom). Note that the kidney actinide levels were within error of each other for the immediate versus 1 and 2 h delayed dosing regimens. Although withholding the dose of 9 until 4 h post-uranium still resulted in a 35% reduction in renal metal content (p < 0.006 vs control), this was significantly less than when the chelator is given earlier after metal exposure. Finally, there was also a clear reduction in bone actinide content, even when the chelator was not given until 4 h post-metal, Figure 2 (bottom). These studies with 9 will now be repeated using p.o. dosing. In addition, the results using multiple p.o. and s.c. dosing regimens with the corresponding 3'-polyether (10) remain to be elucidated.
Figure 2.
Uranium decorporation (top) and tissue actinide content (bottom) of rats given uranium s.c. at a dose of 1 mg U kg−1. Actinide-only treated rats (n=5) served as controls. (S)-4'-(HO)-DADFT-PE (9) was then given s.c. at a dose of 300 µmol kg−1 either immediately thereafter (n=5), or not until 1 (n=3), 2 (n=5) or 4 h post-metal (n=4). The uranium was injected in the left flank, while the chelator was given in the right flank. Note that 9-treated rats excreted significantly more uranium in their urine/bile than controls (top, a), even when the drug was not given until 4 h post-metal, although its effectiveness was ablated with time. The kidney and bone actinide levels (bottom) were significantly less than controls at all times tested (b); the renal values were within error of each other (c) for the immediate versus 1 and 2 h delayed dosing regimens. Data are presented as the mean ± the standard error of the mean; n, number of animals; p-values were generated via a one-tailed student’s t-test.
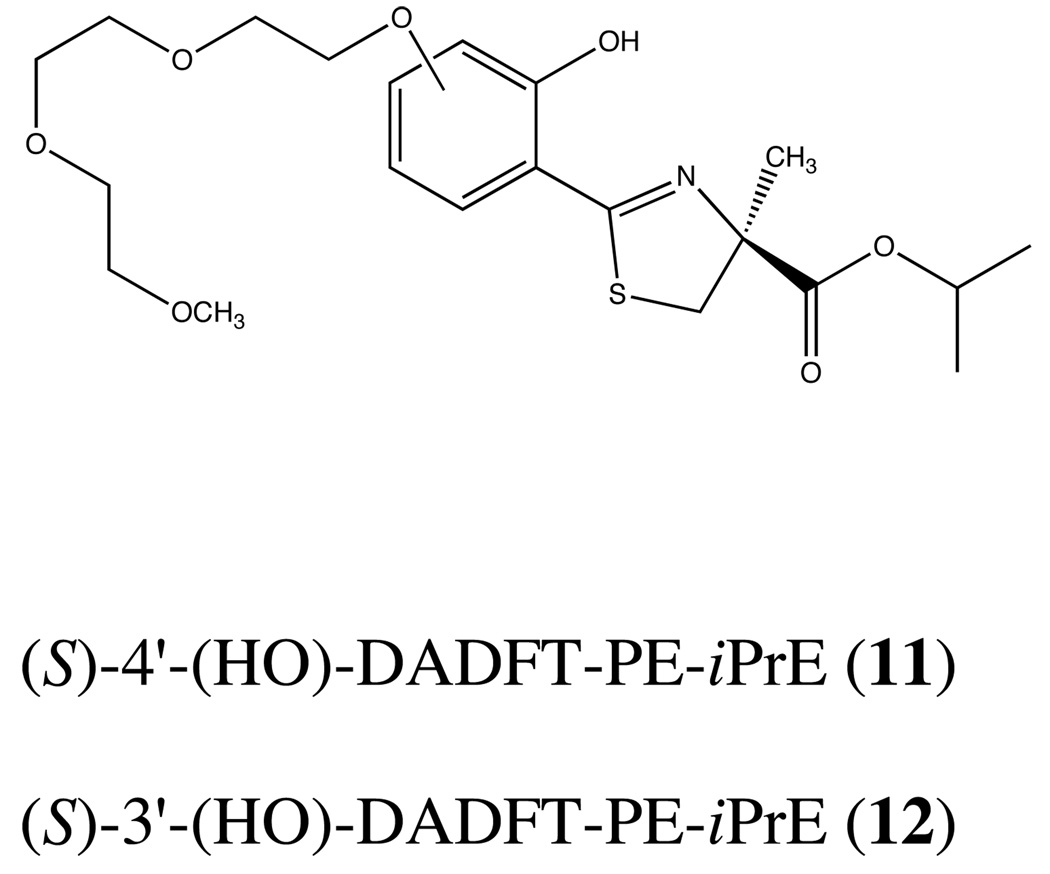
While the results of the delayed dosing regimens are quite exciting, it is clear that withholding the dose of 9 much longer than 4 h will more than likely render the compound inactive. This observation is not entirely unexpected. It has been shown repeatedly that effective actinide excretion is best achieved by prompt administration of decorporation agents (Hengé-Napoli et al., 1998, Domingo et al., 1992, Domingo et al., 1990); once the metal has gained intracellular access, it is very difficult to remove. This temporal dosing problem is also in keeping with the idea that ligands 9 and 10 do not have good intracellular access because of the negative charge on the carboxyl group at physiological pH. Once the uranium finds its way intracellularly, decorporation is impeded. This begs the question, would conversion of 9 and 10 to their corresponding isopropyl esters 11 and 12 (Figure 3) solve this problem? Preliminary evidence from our iron clearance studies with the isopropyl ester of 9 (11, Figure 4) suggests yes (Bergeron et al., 2006b).
Figure 3.
Chemical structures of the 4'- and 3'-polyether isopropyl esters, (S)-4'-(HO)-DADFT-PE-iPrE (11) and (S)-3'-(HO)-DADFT-PE-iPrE (12), respectively.
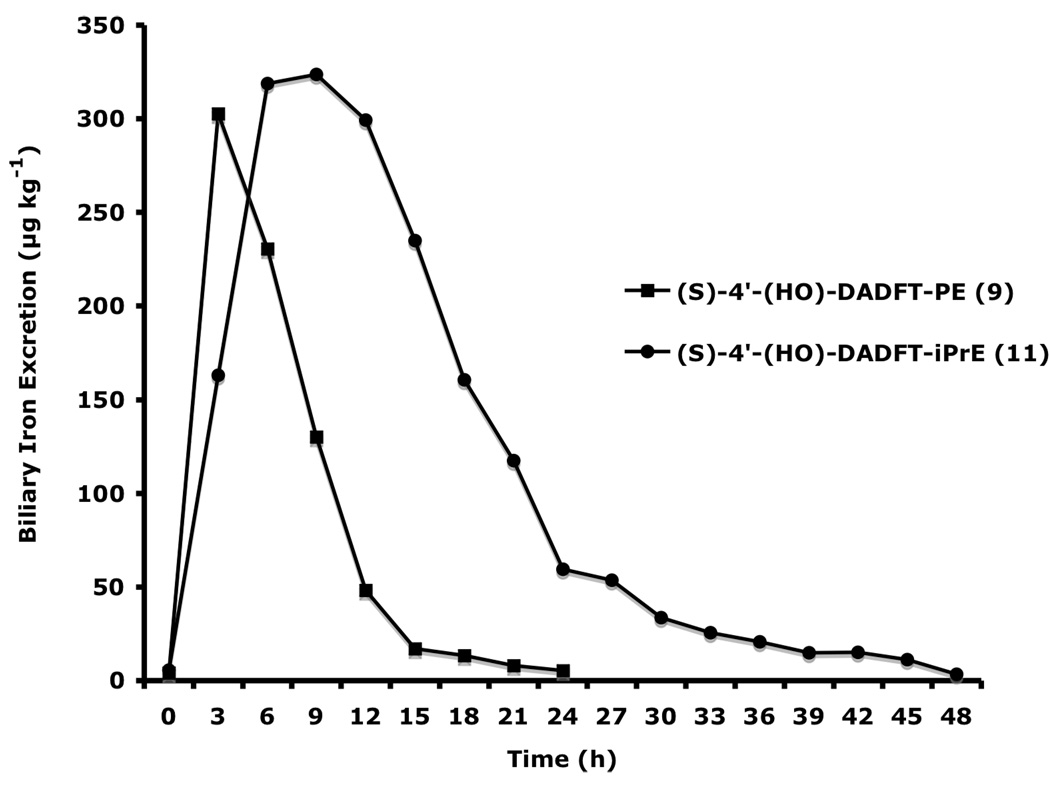
Figure 4.
Biliary ferrokinetics curves of (S)-4'-(HO)-DADFT-PE (9) and (S)-4'-(HO)-DADFT-PE-iPrE (11) given s.c. to bile duct-cannulated rats at a dose of 300 µmol kg−1. Note that, due to ongoing iron excretion, the bile samples for 11 were collected until 48 h post-drug.
Converting the 4'-polyether (9) to its isopropyl ester (S)-4'-(HO)-DADFT-PE-iPrE (11) resulted in a profound increase in iron excretion and ICE (Figure 4). Note that the ICE of the parent 4'-polyether (9) given s.c. was 8.7 ± 2.6%; the ICE of its isopropyl ester was significantly greater, 22.1 ± 7.6%, p < 0.003 (Bergeron et al., 2006b). We believe that ester 11, because it is neutral, (there is no longer a negative charge on the carboxyl group), gained intracellular access not possible with its charged parent (9). Isopropyl ester 11 clearly has a much more protracted half-life and biliary ferrokinetics clearance time than its parent (Figure 4). Both of these properties are almost certain to provide for better uranium clearance and will allow for less dependence on the timing of the ligand dosing. We will now evaluate the uranium decorporation properties of two new ligands, the isopropyl esters of 9 and 10, i.e., 11 and 12 (Figure 3). The drugs will have intracellular access and cleave to their respective parents, potentially removing otherwise inaccessible pools of uranium. This approach is particularly attractive in view of studies that show that uranium indeed gains intracellular access to the renal proximal tubules (Donnadieu-Claraz et al., 2007). Furthermore, their results strongly suggest that one of the components of uranium-induced nephrotoxicity is associated with deposition of iron in the proximal tubules. Ligands 9 and 10 both remove iron and uranium.
Toxicity assessment of (S)-4'-(HO)-DADFT-PE (9) and (S)-3'-(HO)-DADFT-PE (10)
One of the major concerns surrounding the development of chelators for actinide decorporation agents is the metal selectivity issue, which is a significant problem with DTPA. DTPA strips metals other than iron (e.g., Zn), causing significant side effects (Pippard et al., 1986, Levien et al., 2005). In the case of the 3'- and 4'-polyether analogues, it was clear that although these ligands promoted the clearance of uranium, they were also excellent iron chelators. Extending this observation to its limits, if one removes enough iron from a human, toxicity would result. However, it seems unlikely that this will be a problem, since iron replacement is easily accomplished. Nevertheless, we needed to develop some sense of the potential toxicity of these ligands surrounding iron removal.
In previous investigations we evaluated the toxicity of 9 (Bergeron et al., 2006b) and 10 (Bergeron et al., 2008) when the ligands were given orally by gavage once daily to non-iron-overloaded rats at a dose of 384 µmol kg−1 day−1 (equivalent to 100 mg kg−1 of the DFT sodium salt) for 10 d. All of the rats survived the exposure period. Extensive tissues were sent out for histopathological analysis; no drug-related abnormalities were found.
The tolerability of 9 (Bergeron et al., 2006b) and 10 (Bergeron et al., 2008) were also evaluated when given to the rodents for a much longer time period. Rats were administered 9 p.o. once daily for 30 d at a dose of 63.3 µmol kg−1 day−1, a dose that, in primates, causes the excretion of 450 µg Fe kg−1. This is the suggested iron clearance required to keep a thalassemia patient in negative iron balance (Brittenham, 1990). All of the rats survived the dosing period. Extensive tissues were sent out for histopathological analysis; no drug-related abnormalities were found. Rats were also given (S)-3'-(HO)-DADFT-PE (10) orally once daily for 28 d (Bergeron et al., 2008) at 75, 150, or 300 µmol kg−1day−1 to iron-loaded animals (350 mg Fe kg−1) as well as to rats with normal iron stores. Note that these doses are approximately 1, 2, and 4 times the dose necessary to excrete 450 µg Fe kg−1 in the primates. All of the rats survived the dosing period. Although extensive tissues were taken, due to the number of animals involved in this study (48 rats) and the expense of performing the histopathologies, we elected to send out only the kidney and bone marrow. Neither renal toxicity nor any evidence of bone marrow toxicity was observed in any of the rodents at any of the doses studied. Bone marrow samples were checked because of previous reports of other iron chelators, e.g., hydroxypyridones (Hoffbrand et al., 1998, Olivieri, 1996, Olivieri et al., 1998, Richardson, 2001) and deferasirox (2007) causing agranulocytosis. The tolerability of the isopropyl esters of 9 and 10, (S)-4'-(HO)-DADFT-PE-iPrE (11) and (S)-3'-(HO)-DADFT-PE-iPrE (12), respectively, remain to be determined.
Conclusion
A group of ten different ligands were evaluated in a rodent model for their ability to remove uranium (Table I). These chelators included DTPA as a positive control, along with nine DFT analogues, tridentate ligands already shown to clear iron from rodents and primates. The DFT analogues were given at 150 and 300 µmol kg−1 s.c. or 300 µmol kg−1 p.o. In regards to removing uranium from the kidney and bone, ligands 7, 9, and 10 looked the most promising (Table II). Unfortunately, ligand 7, the most lipophilic of the three chelators, also greatly increased liver actinide concentration (Figure 1) and was much less effective as an overall decorporation agent than 9 or 10. However, ligands 9 and 10 not only increased overall uranium excretion, they both have a profound effect on reducing renal uranium content, with modest decreases in metal concentration in the bone as well (Table II). Recall that the animals in these experiments only received a single dose of the chelators.
In a multiple dose chelator administration regimen, in which the initial dose of DTPA or the 4'-polyether (9) was given immediately post-metal, and additional doses of the chelators were given at 24-h intervals for three more days (4 doses total), metal-only control rats excreted 51% of the administered dose of the uranium in the urine and feces (Table III). The metal excretion induced by DTPA was similar, 53%. While DTPA was ineffective in terms of global uranium excretion (urine + feces), the renal uranium concentration of the DTPA-treated rats was reduced by 39% versus the control animals. However, the performance of 9 was much better. The 9-treated rats excreted 68.3% of the metal in the urine and feces during the first 24-h post-drug. Unlike DTPA, a significant amount of the metal (15.5%) was still being excreted on day 2. Uranium excretion on days 3 and 4 was similar to that of the control or DTPA treated rats. By the end of the collection period, the 9-treated rats had excreted 93% of the administered dose of the actinide in the urine and feces; there was also a 62% reduction in kidney uranium concentration (Table III). However, what remained unclear was how long after exposure to the actinide would the drug remain of value.
In a preliminary experiment, 9 was given to bile duct-cannulated rats 1, 2, or 4 h post-uranium; the data were compared to what happens when the chelator was given immediately after the actinide (Figure 2). In each instance, uranium excretion continued, although it was more ablated with time. This suggests that, as time passes, the actinide becomes intracellularly bound and therefore much less accessible. We believe that this obstacle can be overcome with a ligand capable of intracellular transport, i.e., esters. Our iron clearance work with the isopropyl ester of 9 (11, Figure 4) is in keeping with this idea.
There are four compelling reasons to pursue the DFT polyether analogues (S)-4'-(HO)-DADFT-PE (9) and (S)-3'-(HO)-DADFT-PE (10) as candidates for uranium decorporation: (1) Both of these ligands substantially reduce the total body burden of uranium when given p.o. and/or s.c. (Table I). (2) Both of these ligands have a significant effect on reducing uranium in the kidney–the main target organ. (3) Both ligands have a profound effect on changing the route of actinide excretion relative to DTPA (1), which has 100% renal excretion; both 9 and 10 given p.o. clear approximately 70% of the uranium in the bile. This reduces the chance of ligand-metal exchange in the kidney and decreases the potential for nephrotoxicity. (4) The toxicity profiles for both ligands look outstanding.
Thus, we have identified at least one ligand (9) that clears uranium globally, as well as from the kidney and the bone (the results with the corresponding 3'-polyether (10) remain to be elucidated). In addition, the dosing regimens will now involve a closer look at higher doses of 9 and 10, further verifying their p.o. activity, and an investigation of the appropriate esters. Nevertheless, at the very least, 9 represents an excellent prophylactic decorporation agent.
Acknowledgements
The project described was supported by Grant Number R01AI074068 from the National Institute Of Allergy And Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy And Infectious Diseases or the National Institutes of Health. We thank Elizabeth M. Nelson, Tanaya Lindstrom and Pam Lipsky for their technical assistance and Miranda E. Coger for her editorial and organizational support.
References
- Anderegg G, Räber M. Metal complex formation of a new siderophore desferrithiocin and of three related ligands. Journal of the Chemical Society, Chemical Communications. 1990:1194–1196. [Google Scholar]
- Bergeron RJ, Streiff RR, Creary EA, Daniels RD, King W, Jr, Luchetta G, Wiegand J, Moerker T, Peter HH. A comparative study of the iron-clearing properties of desferrithiocin analogues with desferrioxamine B in a Cebus monkey model. Blood. 1993;81:2166–2173. [PubMed] [Google Scholar]
- Bergeron RJ, Streiff RR, Wiegand J, Vinson JRT, Luchetta G, Evans KM, Peter H, Jenny H-B. A comparative evaluation of iron clearance models. Annals of the New York Academy of Sciences. 1990;612:378–393. doi: 10.1111/j.1749-6632.1990.tb24325.x. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Bharti N, Singh S, Rocca JR. Impact of the 3,6,9-trioxadecyloxy group on desazadesferrithiocin analogue iron clearance and organ distribution. Journal of Medicinal Chemistry. 2007;50:3302–3313. doi: 10.1021/jm070214s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Dionis JB, Egli-Karmakka M, Frei J, Huxley-Tencer A, Peter HH. Evaluation of desferrithiocin and its synthetic analogues as orally effective iron chelators. Journal of Medicinal Chemistry. 1991;34:2072–2078. doi: 10.1021/jm00111a023. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, McManis JS, Bharti N. The design, synthesis, and evaluation of organ-specific iron chelators. Journal of Medicinal Chemistry. 2006a;49:7032–7043. doi: 10.1021/jm0608816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, McManis JS, Bharti N, Singh S. Design, synthesis, and testing of non-nephrotoxic desazadesferrithiocin polyether analogues. Journal of Medicinal Chemistry. 2008 doi: 10.1021/jm800154m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, McManis JS, Bussenius J, Smith RE, Weimar WR. Methoxylation of desazadesferrithiocin analogues: enhanced iron clearing efficiency. Journal of Medicinal Chemistry. 2003;46:1470–1477. doi: 10.1021/jm020412d. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, McManis JS, McCosar BH, Weimar WR, Brittenham GM, Smith RE. Effects of C-4 stereochemistry and C-4' hydroxylation on the iron clearing efficiency and toxicity of desferrithiocin analogues. Journal of Medicinal Chemistry. 1999a;42:2432–2440. doi: 10.1021/jm990058s. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, McManis JS, Vinson JRT, Yao H, Bharti N, Rocca JR. (S)-4,5-dihydro-2-(2-hydroxy-4-hydroxyphenyl)-4-methyl-4-thiazolecarboxylic acid polyethers: a solution to nephrotoxicity. Journal of Medicinal Chemistry. 2006b;49:2772–2783. doi: 10.1021/jm0508944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron RJ, Wiegand J, Weimar WR, Vinson JRT, Bussenius J, Yao GW, McManis JS. Desazadesmethyldesferrithiocin analogues as orally effective iron chelators. Journal of Medicinal Chemistry. 1999b;42:95–108. doi: 10.1021/jm980340j. [DOI] [PubMed] [Google Scholar]
- Brittenham GM. Pyridoxal Isonicotinoyl Hydrazone (PIH): effective iron chelation after oral administration. Annals of the New York Academy of Sciences. 1990;612:315–326. doi: 10.1111/j.1749-6632.1990.tb24319.x. [DOI] [PubMed] [Google Scholar]
- Chiappini R, Taillade JM, Brebion S. Development of a high sensitivity ICP-MS for actinide measurement in the femtogram range. Journal of Analytical Atomic Spectrometry. 1996;11:497–503. [Google Scholar]
- Craft ES, Abu-Qare AW, Flaherty MM, Garofolo MC, Rincavage HL, Abou-Donia MB. Depleted and natural uranium: chemistry and toxicological effects. Journal of Toxicology and Environmental Health, Pt B. 2004;7:279–317. doi: 10.1080/10937400490452714. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Colomina MT, Llobet JM, Jones MM, Singh PK, Campbell RA. The action of chelating agents in experimental uranium intoxification in mice: variations with structure and time of administration. Fundamental and Applied Toxicology. 1992;19:350–357. doi: 10.1016/0272-0590(92)90173-f. [DOI] [PubMed] [Google Scholar]
- Domingo JL, Ortega A, Llobet JM, Corbella J. Effectiveness of chelation therapy with time after acute uranium intoxication. Fundamental and Applied Toxicology. 1990;14:18–95. doi: 10.1016/0272-0590(90)90234-b. [DOI] [PubMed] [Google Scholar]
- Donnadieu-Claraz M, Bonnehorgne M, Dhieux B, Maubert C, Cheynet M, Paquet F, Gourmelon P. Chronic exposure to uranium leads to iron accumulation in rat kidney cells. Radiation Research. 2007;167:454–464. doi: 10.1667/RR0545.1. [DOI] [PubMed] [Google Scholar]
- Durbin PW, Kullgren B, Ebbe SN, Xu J, Raymond KN. Chelating Agents for Uranium(Vi): 2. Efficacy and toxicity of tetradentate catecholate and hydroxypyridinonate ligands in mice. Health Physics. 2000;78:511–521. doi: 10.1097/00004032-200005000-00008. [DOI] [PubMed] [Google Scholar]
- Exjade Prescribing Information. 2007 [Online] http://www.pharma.us.novartis.com/product/pi/pdf/exjade.pdf.
- Gorden AE, Xu J, Raymond KN, Durbin P. Rational design of sequestering agents for plutonium and other actinides. Chemical Reviews. 2003;103:4207–4282. doi: 10.1021/cr990114x. [DOI] [PubMed] [Google Scholar]
- Gueguen Y, Souidi M, Baudelin C, Dudoignon N, Grison S, Dublineau I, Marquette C, Voisin P, Gourmelon P, Aigueperse J. Short-term hepatic effects of depleted uranium on xenobiotic and bile acid metabolizing cytochrome P450 enzymes in the rat. Archives of Toxicology. 2006;80:187–195. doi: 10.1007/s00204-005-0027-3. [DOI] [PubMed] [Google Scholar]
- Hahn FE, McMurry TJ, Hugi A, Raymond KN. Coordination Chemistry of Microbial Iron Transport. 42. Structural and spectroscopic characterization of diastereomeric Cr(III) and Co(III) complexes of desferriferrithiocin. Journal of the American Chemical Society. 1990;112:1854–1860. [Google Scholar]
- Hengé-Napoli MH, Ansoborlo E, Houpert P, Mirto H, Paquet F, Burgada R, Hodgson S, Stradling GN. Progress and trends in in vivo chelation of uranium. Radiation Protection Dosimetry. 1998;79:449–452. [Google Scholar]
- Hoffbrand AV, Al-Refaie F, Davis B, Siritanakatkul N, Jackson BFA, Cochrane J, Prescott E, Wonke B. Long-term trial of deferiprone in 51 transfusion-dependent iron overloaded patients. Blood. 1998;91:295–300. [PubMed] [Google Scholar]
- Kemble JV. The new chelating agent Ca-DTPA in the treatment of primary haemochromatosis. Guys Hospital Report. 1964;113:68–73. [PubMed] [Google Scholar]
- Levien T, Baker DE. Pentetate calcium trisodium (Ca-DTPA) and pentetate zinc trisodium (Zn-DTPA) Formulary Drug Reviews. 2005;40:65–71. [Google Scholar]
- Naegeli H-U, Zähner H. Metabolites of microorganisms. Part 193. Ferrithiocin. Helvetica Chimica Acta. 1980;63:1400–1406. [Google Scholar]
- Olivieri NF. Long-term therapy with deferiprone. Acta Haematologica. 1996;95:37–48. doi: 10.1159/000203854. [DOI] [PubMed] [Google Scholar]
- Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, Burt AD, Fleming KA. Long-term safety and effectiveness of iron-chelation therapy with deferiprone for thalassemia major. New England Journal of Medicine. 1998;339:417–423. doi: 10.1056/NEJM199808133390701. [DOI] [PubMed] [Google Scholar]
- Paquet F, Chazel V, Houpert P, Guilmette R, Muggenburg B. Efficacy of 3,4,3-L1(1,2-HOPO) for decorporation of Pu, Am and U from rats injected intramuscularly with high-fired particles of MOX. Radiation Protection Dosimetry. 2003;105:521–525. doi: 10.1093/oxfordjournals.rpd.a006296. [DOI] [PubMed] [Google Scholar]
- Paquet F, Montegue B, Ansoborlo E, Henge-Napoli MH, Houpert P, Durbin PW, Raymond K. Efficacy of 3,4,3-L1HOPO for reducing neptunium retention in the rat after simulated wound contamination. International Journal of Radiation Biology. 2000;76:113–117. doi: 10.1080/095530000139078. [DOI] [PubMed] [Google Scholar]
- Pentetate Calcium Trisodium Injection Package Insert-Instruction for Use. 2008 [Online] http://www.akorn.com/documents/pentatate/product_info/ca_dtpa_insert.pdf.
- Pippard MJ, Jackson MJ, Hoffman K, Petrou M, Modell CB. Iron chelation using subcutaneous infusions of diethylene triamine penta-acetic acid (DTPA) Scandinavian Journal of Haematology. 1986;36:466–472. doi: 10.1111/j.1600-0609.1986.tb02282.x. [DOI] [PubMed] [Google Scholar]
- Powell LW, Thomas MJ. Use of diethylenetriamine penta-acetic acid (D.T.P.A.) in the clinical assessment of total body iron stores. Journal of Clinical Pathology. 1967;20:896–904. doi: 10.1136/jcp.20.6.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DR. The controversial role of deferiprone in the treatment of thalassemia. Journal of Laboratory and Clinical Medicine. 2001;137:324–329. doi: 10.1067/mlc.2001.114105. [DOI] [PubMed] [Google Scholar]
- Sangster J. Octanol-Water Partition Coefficients: Fundamentals and Physical Chemistry. West Sussex, England: John Wiley and Sons; 1997. [Google Scholar]
- Voegtlin C, Hodge HC. Pharmacology and Toxicology of Uranium Compounds, with a Section on the Pharmacology and Toxicology of Fluorine and Hydrogen Fluoride. New York: McGraw-Hill Book Co; 1949. [Google Scholar]
- Wolfe LC, Nicolosi RJ, Renaud MM, Finger J, Hegsted M, Peter H, Nathan DG. A non-human primate model for the study of oral iron chelators. British Journal of Haematology. 1989;72:456–461. doi: 10.1111/j.1365-2141.1989.tb07732.x. [DOI] [PubMed] [Google Scholar]
- Xu J, Raymond KN. Uranyl sequestering agents: correlation of properties and efficacy with structure for UO22+ complexes of linear tetradentate 1-methyl-3-hydroxy-2(1H)-pyridinone ligands. Inorganic Chemistry. 1999;38:308–315. [Google Scholar]