Abstract
Purpose of review
Na+/H+ exchangers (NHEs) are ubiquitous proteins with a very wide array of physiological functions, which are summarized here with an emphasis on the most recent advances. Hypertension and organ ischemia are two disease states of paramount importance in which NHEs have been implicated. The involvement of NHEs in the pathophysiology of these disorders is incompletely understood. This paper reviews the principal findings and current hypotheses linking NHE dysfunction to hypertension and ischemia.
Recent findings
With the advent of large-scale sequencing projects and powerful in-silico analyses, we have come to know what is most likely the entire mammalian NHE gene family. Recent advances have detailed the roles of NHE proteins, exploring new functions such as anchoring, scaffolding and pH regulation of intracellular compartments. Studies of NHEs in disease models, though not conclusive to date, have contributed new evidence on the interplay of ion transporters and the delicate ion balances that may become disrupted.
Summary
This review provides the interested reader with a concise overview of NHE physiology, and addresses the implication of NHEs in the pathophysiology of hypertension and organ ischemia in light of the most recent literature.
Keywords: ischemia, Na+/Ca2+ exchanger, Na+/H+ exchanger, NCX, NHE, primary hypertension, reperfusion
Introduction
Intrinsic to life is order. The strife for low entropy is a thermodynamically costly process and the maintenance of transmembrane ion gradients requires energy. Primary active transport involves direct coupling of ionic movement to the hydrolysis of high energy phosphate bonds. The transmembrane electrochemical potential difference thus created becomes the currency of energy for secondary active transport. The ability to exchange protons (H+) for sodium ions (Na+) across lipid bilayers by a secondary active mechanism is pervasive in all living organisms. Mammalian Na+/H+ exchangers (NHEs) catalyze the countertransport of Na+ and H+ across both plasma and organellar membranes.
Peter Mitchell first suggested the existence of cation/H+ exchange in the mitochondrial membrane in a pioneering monograph in 1961 [1] and provided experimental evidence for such an exchange process [2,3]. To date, genes encoding NHEs have been cloned from the simplest prokaryotes to the most advanced multicellular eukaryotes [4••]. The human genome encodes nine NHE isoforms (SLC9A1-9 genes, NHE1-9 proteins) that have different tissue and sub cellular distributions, in addition to a putative Na+/H+ exchanger with restricted expression in spermatoza (SLC9A10 gene, sperm NHE protein). Given the ubiquity of this class of proteins (Table 1) and their extremely diverse functions (Fig. 1), it is not surprising that NHEs are implicated in multiple physiological and pathophysiological processes.
Table 1. Mammalian Na+/H+ exchanger (NHE) family.
| Protein | Human gene | Human gene locus | Human GeneID (NCBI) |
Tissue expression | Cellular distribution |
|---|---|---|---|---|---|
| NHE1 | SLC9A1 | 1p36.1–35 | 6548 | Ubiquitous, with some exceptions such as the macula densa and intercalated cells of the cortical collecting duct |
Plasma membrane; basolateral membrane of epithelial cells |
| NHE2 | SLC9A2 | 2q11.2 | 6549 | Gastrointestinal tract, skeletal muscle; less in kidney, brain, testis and uterus |
Plasma membrane; apical membrane of epithelial cells |
| NHE3 | SLC9A3 | 5p15.3 | 6550 | Gastrointestinal tract, kidney and gall bladder, epididymis; much less in brain |
Plasma membrane; apical membrane and recycling endosomes of epithelial cells |
| NHE4 | SLC9A4 | 2q12.1 | 389015 | Stomach; less in kidney and brain | Plasma membrane; basolateral membrane of epithelial cells |
| NHE5 | SLC9A5 | 16q22.1 | 6553 | Brain | Plasma membrane and recycling endosomes (synaptic vesicles) |
| NHE6 | SLC9A6 | Xq26.3 | 10479 | Ubiquitous | Intracellular (recycling endosomes) |
| NHE7 | SLC9A7 | Xp11.3–11.23 | 84679 | Ubiquitous | Intracellular (trans-Golgi network) |
| NHE8 | SLC9A8 | 20q13.13 | 23315 | Ubiquitous; luminal surface of renal and intestinal epithelia |
Intracellular (trans-Golgi network); apical membrane and recycling endosomes of epithelial cells |
| NHE9 | SLC9A9 | 3q24 | 285195 | Ubiquitous | Intracellular (recycling endosomes) |
| Sperm NHE | SLC9A10 | 3q13.2 | 285335 | Spermatozoa | Sperm flagellum |
NCBI, National Center for Biotechnology Information, US National Library of Medicine.
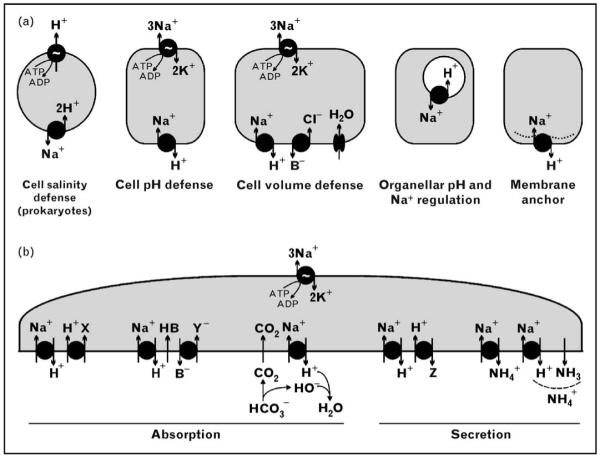
Figure 1. Functions of Na+/H+ exchangers (NHEs).
NHEs mediate the exchange of Na+ for H+ across the lipid bilayer. (a) In prokaryotes, the H+ gradient is used to energize extrusion of Na+ and thus contributes to defense against salinity. In eukaryotes, plasma membrane NHEs utilize the inward Na+ gradient to drive H+ out, hence fulfilling the role of cell pH defense. When coupled with parallel Cl−/base (B−) exchange, HB recycling, and water channels, NHEs can restore cell volume when confronted with osmotic cell shrinkage. When positioned in the lipid membrane of organelles, NHEs can regulate organellar pH and/or Na+ concentration. Finally, the plasma membrane locale of NHE can serve as a membrane anchor (non-transport function) and assembly site for the cytoskeleton and signaling complexes. (b) In specialized polarized epithelia, apical NHE isoforms (NHE3 and others) mediate several modes of Na+-coupled secretion or absorption. Coupling of NHE to H+-coupled transporters allows utilization of the Na+ gradient and conversion of H+-coupled transport to Na+-coupled transport. This is the mechanism for renal proximal tubular absorption of some organic solutes by H+-coupled cotransport (left absorption model; X stands for oligopeptides, amino acids, etc.) and for the secretion of others by H+-coupled countertransport (left secretion model; Z stands for epinephrine, norepinephrine, histamine, amiloride, etc.). NHE can also be coupled with an anion/base− (B−) exchanger with recycling of HB (center absorption model; Y stands for chloride, urate). In the case of Cl−, coupled transport leads to net NaCl transport across the membrane. The extruded H+ can also react with a buffer such as HCO3− which is converted to CO2. Diffusion of CO2 across the bilayer results in net NaHCO3 transport (right absorption model). Finally, NHE can mediate the secretion of ammonium (NH4+), both by providing the H+ necessary to trap diffused ammonia (NH3), and by directly transporting NH4+ as a substrate (right secretion model).
Mammalian Na+/H+ exchangers
The reader is referred to Table 1 for a summary of the tissue and cellular expression of NHEs. Their distribution in the kidney is detailed in Fig. 2.
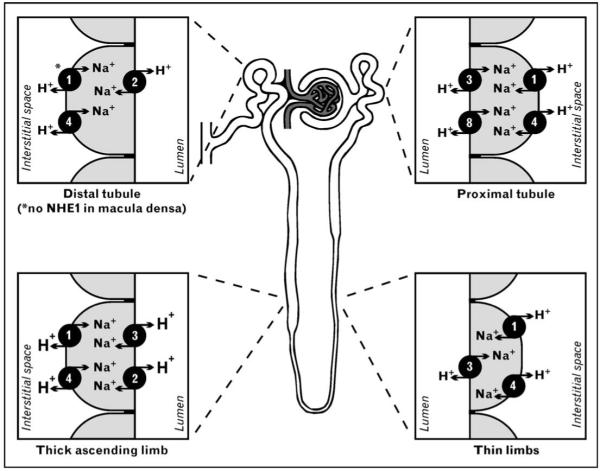
Figure 2. Na+/H+ exchanger (NHE) isoforms in the mammalian kidney.
NHE isoforms 1–4 and 6–9 are expressed in the kidney. NHE isoforms 1–4 and 8 are in the plasma membrane of renal epithelial cells, with precise localization to the apical or basolateral membrane. The apical isoforms are NHE3 and NHE8 in the proximal tubule, NHE3 and NHE2 in the thick ascending limb of the loop of Henle, and only NHE2 in the distal convoluted tubule and connecting tubule. The thin limbs lack detectable levels of apical NHEs, with the exception of a distinct subpopulation of juxtamedullary nephrons with long loops of Henle, which express low levels of apical NHE3. On the basolateral side, NHE1 and NHE4 are present in all nephron segments, with the exception of the macula densa and intercalated cells of the cortical collecting duct, where NHE4 is the only basolateral isoform. NHE isoforms 6, 7 and 9 are only in organellar membranes (not shown) [5••,6••,7•,8-10].
NHE1 was the first mammalian NHE identified, in 1989 [11], and is probably the most important isoform for intracellular pH, cell volume and intracellular sodium homeostasis. NHE1 has been shown to regulate cell cycle, proliferation, migration and adhesion, and to confer resistance to apoptosis [12,13•]. NHE1 mediates transepithelial transport in secretory parotid acinar cells [14] and may mediate ammonium reabsorption in the thick ascending limb of the loop of Henle. Apart from its ionexchange function, NHE1 interacts with the ezrin, radixin and moesin (ERM) family of actin-binding proteins, serving as a membrane anchor for the actin cytoskeleton [15] and as a scaffold for the assembly of signaling complexes [16•]. NHE1 may also modulate other transporters, such as apical membrane NHE3, possibly through actin cytoskeleton remodeling [17,18•,19•]. Further insight into NHE1 function is offered by two NHE1-defective mouse models (Table 2).
Table 2. Na+/H+ exchanger (NHE)-defective mouse models.
| Model | Genotype | Phenotype |
|---|---|---|
|
swe mouse (slow-wave epilepsy, spontaneous mutant) |
SLC9A1swe/swe nonsense mutation: Lys-442 |
Growth retardation, seizures (petit mal and grand mal), locomotor ataxia, high mortality before weaning [20] |
| NHE1−/− (knockout) | SLC9A1−/− | Same overall phenotype as swe mouse [21]; decreased parotid gland secretion [14]; resistance to experimental cardiac ischemia/reperfusion injury [22]; decreased renal HCO3− absorption [18•]; altered (mostly downregulated) expression of multiple genes in the brain [23] |
| NHE2−/− (knockout) | SLC9A2−/− | No overt disease phenotype, no renal or intestinal absorptive defects; mild degeneration of gastric parietal and zymogenic cells [24]; decreased parotid gland secretion [14] |
| NHE3−/− (knockout) | SLC9A3−/− | Hypovolemia, hypotension, mild diarrhea, mild metabolic acidosis, decreased renal absorption of Na+, fluid and HCO3−, proteinuria, increased mortality when fed a low-salt diet [25]; reduced steady- state glomerular filtration rate (evaluated in both whole kidney and single nephron), likely due to shifted tubulo-glomerular feedback sensitivity [26]; blunted flow-stimulated increase in fluid absorption in the proximal tubule (normally mediated through microvillar mechanosensing and transmitted to NHE3 by the actin cytoskeleton) [27••] |
| NHE3−/− (knockout) with small intestine rescue |
Tg(IFABP-NHE3) SLC9A3−/− | Improved compared to the NHE3 knockout (higher blood pressure, better tolerance to low-salt diet), but remains hypovolmic and hypotensive on normal-salt diet [28,29•] |
| NHE2−/− NHE3−/− (double knockout) |
SLC9A2−/− SLC9A3−/− | Same as NHE3 knockout, with no further worsening of acidosis, hypovolemia, diarrhea or proximal tubule NHE activity [30,31] |
| NHE4−/− (knockout) | SLC9A4−/− | No overt disease phenotype; slightly impaired gastric acid secretion and histological abnormalities of the gastric mucosa [32]; renal function not studied extensively |
| Sperm- NHE−/− (knockout) | SLC9A10−/− | Impaired sperm motility, male infertility [33] |
IFABP, intestinal fatty acid-binding protein promoter.
NHE2 is important for Na+ secretion in the parotid gland, and plays a role in maintaining parietal cell integrity in the stomach (Table 2). In the kidney, NHE2 functions in transepithelial NaHCO3 absorption in the distal nephron. At the macula densa NHE2 is the only luminal NHE, but its role in sodium sensing remains to be determined [34].
NHE3 differs from other isoforms in that it recycles between the plasma membrane (apical membrane of epithelia) and the endosomal compartment, and functions in both locations [35]. NHE3 activity is regulated by alterations in its intrinsic activity and by trafficking between these two compartments [36-42]. NHE3 is responsible for a significant portion of renal and intestinal Na+ absorption (Table 2). The absorptive and secretory functions of luminal membrane NHE3 in the kidney are summarized in Fig. 1b. Intracellular NHE3 has been postulated to be important for endosomal acidification in the reabsorption of filtered proteins by receptor-mediated endocytosis [43].
NHE4 is involved in intracellular pH and volume regulation, especially in some cells lacking NHE1 (Table 1). NHE4 may mediate ammonium transport from the thick ascending limb lumen to the interstitium, but this theory has not been explored with the NHE4−/− mouse. Very little is known about the function of the neuron-specific isoform NHE5. The ubiquitously expressed NHE6, NHE7 and NHE9 likely serve as organellar pH regulators [9].
NHE8 was recently identified by in-silico cloning [44]. NHE8 may recycle to the plasma membrane and be regulated by trafficking, similar to NHE3, but there are no available data as yet. In the kidney, NHE8 may functionally complement NHE3 (Fig. 2). Although NHE3−/− mice have no significant alteration in NHE8 protein expression [7•], the role of NHE8 in maintaining proximal sodium transport cannot be ruled out.
The sperm-specific NHE is a predicted hybrid protein expressed in spermatozoa, essential for sperm motility and male fertility [33]. Its Na+/H+-exchange ability has not yet been demonstrated.
In spite of the impressive amount of knowledge that we have accumulated in the last years, including the completion of the Human Genome Project, the exact identity of the protein responsible for Peter Mitchell’s mitochondrial cation/H+ exchange has remained elusive [45,46].
Na+/H+ exchangers in hypertension
The role of NHEs in hypertension is a question of pivotal importance. There is a massive body of literature on this topic that is not easily amenable to any attempts at a summary. Several broad statements can be made regarding NHEs and hypertension. The majority of findings in this vast literature are correlations of the hypertensive state with either direct measurements of NHE (activity, protein, transcript) in animal models, or surrogates of NHE (Na+/Li+ or Na+/H+ exchange in erythrocytes, lymphocytes, platelets) in humans. There is little or no reassurance that the surrogates actually reflect NHE and, even if they do, causality and significance are difficult to establish. Observations have been made in animal models of polygenic hypertension that demonstrate changes in NHE preceding the hypertensive state. These types of observation rule out NHE defects being results of hypertension but still do not distinguish the difference between causation and parallel phenomena.
The current models of possible involvement of NHEs in hypertension are centered mainly on two isoforms, NHE1 and NHE3 (Fig. 3). Transgenic overexpression of NHE1 leads to salt-sensitive hypertension in mice [47]. Primary hypertension has been associated with increased NHE1 activity measured in both experimental animals and peripheral blood cells from some human subjects with primary hypertension [48]. The NHE1 locus has been ruled out as a candidate in essential hypertension by genetic linkage analysis [49]. When present, the defect is rather due to regulatory mechanisms that are, as yet, poorly defined.
Figure 3. Theoretical links between Na+/H+ exchange overactivation and hypertension.
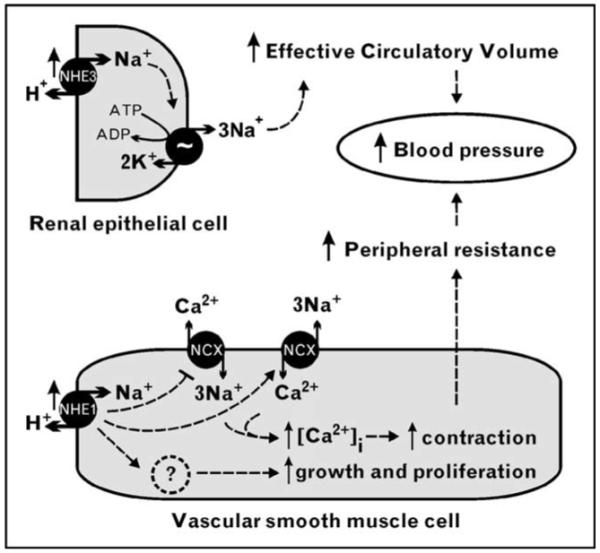
Overactivation of Na+/H+ exchanger (NHE) 3 in the kidney may lead to salt and fluid retention, increasing the effective circulatory volume. Independently or concurrently, overactivation of NHE1 in vascular smooth muscle cells (VSMCs) may lead to intracellular Na+ loading, resulting in slowing or reversal of Na+/Ca2+ exchange via Na+/Ca2+ exchanger (NCX), increased cytosolic Ca2+ and VSMC contraction. At the same time, NHE1 activity may promote VSMC growth and proliferation, the overall result being increased peripheral resistance.
One potential mechanism linking NHE1 to hypertension is overactivation of NHE1 in vascular smooth muscle cells (VSMCs), resulting in intracellular Na+ accumulation, slowing down or reversal of the Na+/Ca2+ exchanger (NCX), increased cytosolic Ca2+ concentration, and VSMC contraction. Chronically increased NHE1 activity may also drive abnormal VSMC growth and proliferation. There are several new arguments supporting the reversal of NCX exchange. The NCX inhibitor SEA0400 decreases systolic blood pressure in several rat models of salt-dependent hypertension [50•]. While NCX1−/− homozygosity is lethal, heterozygous NCX1+/− mice are viable and resistant to salt-dependent hypertension. Conversely, transgenic mice with VSMC-specific overexpression of NCX1 are more prone to high-salt-induced hypertension [50•]. However, there is no definitive evidence that NCX in VSMCs is slowed down or reversed as a result of NHE overactivity. There are alternative, non-mutually exclusive theories, such as the action of cardiotonic steroids (e.g. endogenous ouabain), which can cause intracellular Na+ accumulation by inhibition of the Na+/K+-ATPase.
Another major mechanism linking NHEs and hypertension could be renal alteration of sodium homeostasis and defective pressure natriuresis. NHE3−/− mice are hypotensive, even when small-intestine NHE3 is rescued by transgenic expression (Table 2). Conversely, overactivation of NHE3 could be a mechanism for hypertension. NHE3 has been studied in various rodent hypertension models (Table 3), and has been found to be implicated in some but not all the models. NHE3 regulatory proteins have also been studied in some models, with intriguing but inconclusive results [55]. Several polymorphic variants of NHE3 did not associate with human primary hypertension in a recent case-control study [61].
Table 3. Proximal tubular Na+/H+ exchange in hypertension rat models.
| Hypertensive model vs normotensive control | NHE3 mRNA | NHE3 protein | Na+/H+-exchange activity | Methods | Reference |
|---|---|---|---|---|---|
| Spontaneously hypertensive (SHR) vs Wistar–Kyoto (WKY) |
ND | ND | Increased | e | [51] |
| Not altered | ND | Increased | a, g | [52] | |
| Increased | Increased | Increased | a, d, g | [53] | |
| Not altered | Increased | Increased | a, d, f, g | [54] | |
| Not altered | ND | Increased | b, h | [55] | |
| Milan hypertensive (MHS) vs Milan normotensive (MNS) |
ND | ND | Not altered | i | [56] |
| ND | ND | Increased | e | [57] | |
| Not altered | Not altered | ND | c, d | [58•] | |
| Dahl salt-sensitive on high-salt vs low-salt diet |
Decreased | ND | Decreased | b, h | [55] |
| Dahl salt-sensitive vs salt-resistant Brown–Norway, on high- or low- salt diet |
ND | Not altered | ND | d | [59] |
| Unilateral renal cortical injection of phenol vs saline in Sprague–Dawley rats (model of neurogenic hypertension) |
ND | Decreased | ND | d | [60] |
Methods: a, RNA blot; b, ribonuclease-protection assay; c, real-time competitive PCR; d, immunoblot; e, 22Na+ uptake in brush-border membrane vesicles; f, Na+-dependent Acridine Orange fluorescence recovery after quenching in brush-border membrane vesicles; g, fluorimetric assay of Na+-dependent pHi recovery in suspended proximal tubules; h, fluorimetric assay of Na+-dependent pHi recovery in primary culture of proximal tubular cells; i, 22Na+ uptake in primary culture of proximal tubule cells. ND, not determined; NHE3, Na+/H+ exchanger isoform 3.
Although there is no definitive evidence documenting an NHE defect in human hypertension, both NHE1 and NHE3 remain plausible candidates and more studies are needed to clarify their involvement, as well as the possible involvement of other NHE isoforms.
Na+/H+ exchangers in ischemia/reperfusion injury
Ischemia/reperfusion injury is an extremely complex, incompletely understood phenomenon. A simplified working paradigm is that ischemia deprives energy required to maintain ionic gradients with reperfusion triggering an inflammatory response which further exacerbates injury. Mechanisms of ischemic injury are common to all solid organs, but there are specific characteristics for each.
One proposed model of ischemia/reperfusion-induced ionic imbalance poises NHE in an important role during both ischemia and reperfusion. NHE is activated by intracellular acidosis upon ischemia (Fig. 4a), but transport is repressed by the low pH of the interstitium. Restoration of flow during reperfusion (Fig. 4b) normalizes first extracellular pH, resulting in maximal kinetic stimulation of NHE. Restoration of intracellular pH by NHE comes at the price of rising intracellular Na+ concentration. Normally, the Na+/K+-ATPase would extrude excess Na+, but ATP depletion hampers this process. At high intracellular Na+ concentrations, Ca2+ extrusion via NCX is inhibited, and NCX can operate in a reverse mode, loading the cell with Ca2+ resulting in cellular injury by multiple mechanisms [62]. While activation of NHE restores intracellular pH, the concomitant increase in intracellular Na+ can lead to Ca2+ overload and exacerbation of tissue injury, a phenomenon termed the pH paradox.
Figure 4. Theoretical model of Na+/H+ exchange in ischemia/reperfusion.
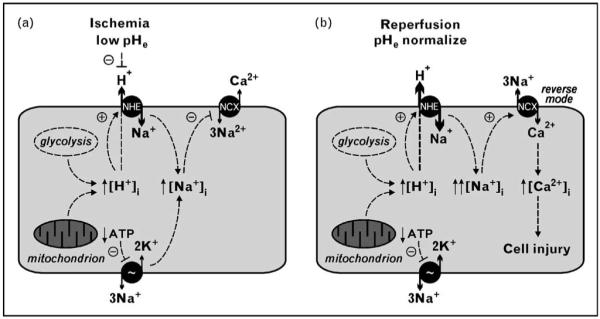
(a) Ischemia leads to intracellular acidosis by anaerobic glycolysis and mitochondrial dysfunction. Na+/H+ exchange is driven by the intracellular/extracellular H+ gradient and allosterically activated by intracellular protons (dashed curved arrow). Acidification of the interstitium (low pHe) partially suppresses the gradient-driven Na+/H+ exchange. At the same time, cellular ATP depletion leads to Na+/K+-ATPase dysfunction. The net result is intracellular sodium loading. Rising [Na+]i slows down the Na+/Ca2+ exchanger (NCX), impairing calcium extrusion from the cell. (b) During reperfusion pHe normalizes and Na+/H+ exchange is fully activated by allosteric regulation and ionic gradients. With cellular ATP and Na+/K+-ATPase activity still insufficient, intracellular Na+ rises to yet higher levels. At some point during this process, rising [Na+]i leads to operation of the Na+/Ca2+ exchanger in reverse mode. NCX extrudes Na+ at the price of raising [Ca2+]i, which can achieve deleterious levels and lead to cell injury and apoptosis. NHE, Na+/H+ exchanger.
Cardiac ischemia
NHE1 is the predominant isoform expressed in cardiomyocytes, where it functions to rectify intracellular acidification. There is a steep relationship between pHi and NHE1 activity in cardiac cells, with pKi of about 7.4 [63]. The relative resistance to cardiac ischemia/reperfusion injury in NHE1−/− mice is comparable with the effect of cariporide inhibition of NHE1 in wild-type mice [22], supporting the significance of NHE1 action in cardiac ischemia/reperfusion injury in the rodent model.
There are two major groups of NHE1 inhibitors: pyrazine derivatives (amiloride, dimethyl amiloride, ethylisopropyl amiloride) and benzoylguanidine derivatives (HOE-694, cariporide, eniporide) [64]. Treatment with both pyrazine and benzoylguanidine derivatives has protective effects in cardiac ischemia/reperfusion injury in animal models – especially if used prior to, rather than during or after ischemia [65-69,70•].
As a warning remark, it has to be pointed out that although pharmacological inhibition is a powerful and valuable tool, its results have to be interpreted and translated to the molecular level with some caution. Amiloride has different inhibitory potencies on different NHEs, but also inhibits epithelial Na+ channels, acidsensing ion channels and Na+/Ca2+ exchangers [64,71]. Other NHE inhibitors have relatively higher specificities, but are still far from mechanistically inhibiting a single isoform [64]. The same applies for NCX inhibitors [72].
In humans, the GUARDIAN [73] and ESCAMI [74] trials, using cariporide and eniporide respectively, failed to demonstrate a significant benefit in patients with various cardiac ischemic syndromes. However, in patients undergoing coronary artery bypass graft surgery, a subgroup of the GUARDIAN trial, cariporide offered a 25% relative risk reduction of all-cause death or myocardial infarction throughout the 6-month trial [75]. The results of cariporide and eniporide in human trials are surprising and somewhat disappointing considering the wealth of animal data heralding the efficacy of these agents. One possible explanation could be the timing of the treatment. NHE inhibition has been mostly beneficial in animal models if applied before the onset of ischemia, and has only offered protection in humans undergoing coronary artery bypass graft. Another plausible explanation is that in humans with cardiac ischemia, the effect of NHE inhibitors may be masked by parallel events such as diseased vessels, which are inherently not present in animal models. Studies have started to address this issue by using senescent or atherosclerotic animals, but with inconclusive results as yet [76,77]. While it is difficult to provide treatment preceding ischemia in patients, inhibiting NHE exchange could be beneficial in improving the outcome of ischemia/reperfusion in humans when used in conjunction with other cardioprotective therapies.
Another potential target in the model depicted in Fig. 4 is NCX. In theory, inhibiting NCX should prevent intracellular accumulation of Ca2+ while still permitting the myocyte to defend against low pH. In isolated rabbit hearts cariporide infusion before ischemia reduced infarct size, but its infusion upon reperfusion failed to offer protection. In contrast, infusion of the NCX inhibitor KB-R7943 similarly reduced infarct size when infused before and after ischemia, suggesting that ischemia/reperfusion injury is more efficiently suppressed by blockade of NCX than NHE [78]. Similarly, the NCX inhibitor SEA0400 had cardioprotective effects in canine and rodent cardiac ischemia/reperfusion models [79,80]. To date, human trials with NCX inhibitors are not available.
Cerebral ischemia
In neurons, pHi can affect neuronal excitability by ionchannel gating and, conversely, neuronal activity can alter pHi. Neuronal activity can vary enormously from one neuron to another; hence production of metabolic acids can vary greatly. Efficient acid-extrusion mechanisms are critical for homeostasis. Because of their high metabolic rate, neurons are susceptible to injury if exposed to excessive activity (seizure) or ischemia (stroke). pH regulation is likely to be crucial in both physiological and pathophysiological conditions. NHE1 plays an important role in the pH regulation of both neurons [81] and astrocytes [82•].
A salient characteristic of cerebral ischemia is the failure of ATP-dependent glutamate transporters to remove glutamate, the major excitatory neurotransmitter, from the synaptic cleft, leading to overactivation of glutamate receptors and a rise in cytosolic Ca2+ concentration – a phenomenon termed excitotoxicity [83]. The fact that glutamate receptor antagonists have failed to offer neuroprotection in clinical trials [84] is likely due to the coexistence of multiple mechanisms contributing to ion imbalance in cerebral ischemia. These mechanisms may include opening of Ca2+- and Na+-permeable acidsensing ion channels (ASIC1a) [85,86], activation of the Na+/K+/Cl− cotransporter NKCC1 [87], excitotoxicity-induced proteolytic cleavage of NCX1 and NCX3 [88], as well as activation of NHE1 [81,82•].
The NHE1 inhibitor SM-20220 significantly reduced the extent of cerebral edema, Na+ content and infarcted area in a transient focal ischemia rat model [89]. In primary culture of rat cortical neurons, SM-20220 was protective against glutamate-induced excitotoxicity [90•]. Treatment with cariporide before hypothermic circulatory arrest in pig dramatically improved early neurologic recovery [91]. In primary cultured astrocytes from NHE1+/+ mice, oxygen and glucose deprivation led to a 5-fold increase in intracellular Na+ and cell swelling. In both NHE1−/− astrocytes and NHE1+/+ astrocytes treated with cariporide, the oxygen- and glucose-deprivation-induced Na+ rise was less than 2-fold, and swelling was significantly reduced [82•].
Renal ischemia
Five NHE isoforms (NHE1–4 and NHE8) are expressed in the plasma membrane of different renal cells (Fig. 2), rendering the understanding of their individual roles in response to ischemia/reperfusion injury more complex. Rats subjected to renal ischemia/reperfusion exhibited drastic reduction in kidney NHE3 mRNA, mild decrease in NHE2 and NHE4 mRNA, and mild increase in NHE1 mRNA [92,93]. At the protein level a different study found decreased NHE3, type II Na+/Pi cotransporter and Na+/K+-ATPase [94]. A pathophysiologic interpretation of these findings could ascribe the observed renal Na+ wasting to loss of Na+ transporters due to ischemia damage [92-94]. An alternative and not exclusive view is that this is not a consequence of damage but rather an adaptive mechanism to cope with low ATP supply. If the primary insult is energy limitation, turning off NHE in the proximal tubule can achieve protective effects by blocking apical Na+ entry and thus reducing Na+/K+-ATPase ATP consumption. The increased distal delivery will repress glomerular filtration rate to avoid massive volume loss as per the ‘acute renal success’ theory of Thurau and Boylan [95]. Reducing intracellular Na+ by blocking apical entry may also reduce Ca2+ loading by NCX. In one study, the NHE3 inhibitor S3226 improved renal function after experimental ischemia/reperfusion [96]. The NCX inhibitor SEA0400 was beneficial in a rat renal ischemia/reperfusion model [97], and ischemia/reperfusion-induced renal dysfunction was attenuated in NCX1+/− heterozygous mice (having NCX1 protein expression in the kidney decreased to about half) compared to NCX1+/+ mice [98].
Conclusion
Vast amounts of published data have started to shed light on the elaborate works of the proteins capable of exchanging Na+ for H+ across lipid bilayers. Piecing together this massive body of literature is a formidable task, but even more challenging is the vastness of the areas that remain to be explored. From solving protein structures and explaining transport machineries in molecular detail, to establishing relevance for human disease and therapeutic intervention, Na+/H+-exchange research has yet to reach its full potential.
Acknowledgements
The authors are supported by the National Institutes of Health (R01-48481, P01-DK20543, M01-RR00633 to O.W.M.) and the National Kidney Foundation (to I.A.B.). We thank Dr Olivier Bonny for useful discussion and critical reading of the manuscript.
Abbreviations
- NCX
Na+/Ca2+ exchanger
- NHE
Na+/H+ exchanger
- VSMC
vascular smooth muscle cell
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Moyle J. Proton translocation coupled to ATP hydrolysis in rat liver mitochondria. Eur J Biochem. 1968;4:530–539. doi: 10.1111/j.1432-1033.1968.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P, Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969;9:149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 4••.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. An excellent review and comprehensive phylogenetic analysis of NHEs. The classification of NHEs based on their evolutionary development can be correlated with NHE expression, structure and function, and may be used to identify the most appropriate model organisms for the study of individual NHE isoforms.
- 5••.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. An excellent recent review on mammalian NHEs in general, including distribution, physiological roles and regulation.
- 6••.Nakamura N, Tanaka S, Teko Y, et al. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. The less known NHE isoforms 6, 7, 8 and 9 are shown to have distinct, yet partly overlapping distributions in organellar membranes. NHE8 and NHE9 in heterologous systems are in recycling endosomes. Assays with organelle-specific pH-sensitive fluorophores suggest a role of these NHEs in organellar pH regulation. The paper also investigates the transport kinetics of NHE8 expressed in yeast and reconstituted in liposomes.
- 7•.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol. 2005;288:F530–F538. doi: 10.1152/ajprenal.00229.2004. This paper describes the renal localization of NHE8, investigated by generation of isoform-specific NHE8 antibodies. Renal NHE8 expression is also studied in NHE3−/− mice.
- 8.Chambrey R, Warnock DG, Podevin RA, et al. Immunolocalization of the Na+/H+ exchanger isoform NHE2 in rat kidney. Am J Physiol Renal Physiol. 1998;275:F379–F386. doi: 10.1152/ajprenal.1998.275.3.F379. [DOI] [PubMed] [Google Scholar]
- 9.Chambrey R, St John PL, Eladari D, et al. Localization and functional characterization of Na+/H+ exchanger isoform NHE4 in rat thick ascending limbs. Am J Physiol Renal Physiol. 2001;281:F707–F717. doi: 10.1152/ajprenal.2001.281.4.F707. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Chen R, Ghishan FK. Subcloning, localization and expression of the rat intestinal sodium-hydrogen exchanger isoform 8 (NHE-8) Am J Physiol Gastrointest Liver Physiol. 2005;24:24. doi: 10.1152/ajpgi.00552.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–280. doi: 10.1016/0092-8674(89)90901-x. [DOI] [PubMed] [Google Scholar]
- 12.Putney LK, Barber DL. Na+/H+ exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem. 2003;278:44645–44649. doi: 10.1074/jbc.M308099200. [DOI] [PubMed] [Google Scholar]
- 13•.Wu KL, Khan S, Lakhe-Reddy S, et al. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. This paper investigates the role of NHE1 binding to ezrin, radixin and moesin (ERM) in cellular resistance to apoptosis. Ectopic overexpression of NHE1, but not NHE3, rescued NHE1-deficient cells from experimentally induced apoptosis, arguing for a role of NHE1 in promoting cell survival independent of its transport function.
- 14.Park K, Evans RL, Watson GE, et al. Defective fluid secretion and NaCl absorption in the parotid glands of Na+/H+ exchanger-deficient mice. J Biol Chem. 2001;276:27042–27050. doi: 10.1074/jbc.M102901200. [DOI] [PubMed] [Google Scholar]
- 15.Denker SP, Huang DC, Orlowski J, et al. Direct binding of the Na+/H+ exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H+ translocation. Mol Cell. 2000;6:1425–1436. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 16•.Baumgartner M, Patel H, Barber DL. Na+/H+ exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol. 2004;287:C844–C850. doi: 10.1152/ajpcell.00094.2004. A review of non-transport function of NHE1 as a membrane anchor and scaffold through interactions with signaling molecules and discussion of the potential roles of NHE1 in signaling.
- 17.Good DW, George T, Watts BA., 3rd Basolateral membrane Na+/H+ exchange enhances HCO3− absorption in rat medullary thick ascending limb: evidence for functional coupling between basolateral and apical membrane Na+/H+ exchangers. Proc Natl Acad Sci USA. 1995;92:12525–12529. doi: 10.1073/pnas.92.26.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Good DW, Watts BA, 3rd, George T, et al. Transepithelial HCO3− absorption is defective in renal thick ascending limbs from Na+/H+ exchanger NHE1 null mutant mice. Am J Physiol Renal Physiol. 2004;287:F1244–F1249. doi: 10.1152/ajprenal.00176.2004. This paper demonstrates a HCO3− reabsorption defect in NHE1−/− mice and argues that the mechanism for this defect is a decrease of apical NHE3 activity, secondary to lack of positive regulation by NHE1.
- 19•.Watts BA, 3rd, George T, Good DW. The basolateral NHE1 Na+/H+ ex-changer regulates transepithelial HCO3− absorption through actin cytoskeleton remodeling in renal thick ascending limb. J Biol Chem. 2005;280:11439–11447. doi: 10.1074/jbc.M410719200. This paper proposes that a functional crosstalk between basolateral NHE1 and apical NHE3 may occur via actin cytoskeleton remodeling. HCO3− absorption was measured in suspended rat medullary thick ascending limbs, with the separate or concomitant use of NHE1 inhibitors and actin modifiers.
- 20.Cox GA, Lutz CM, Yang CL, et al. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell. 1997;91:139–148. doi: 10.1016/s0092-8674(01)80016-7. [DOI] [PubMed] [Google Scholar]
- 21.Bell SM, Schreiner CM, Schultheis PJ, et al. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol. 1999;276:C788–C795. doi: 10.1152/ajpcell.1999.276.4.C788. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Meyer JW, Ashraf M, Shull GE. Mice with a null mutation in the NHE1 Na+/H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ Res. 2003;93:776–782. doi: 10.1161/01.RES.0000094746.24774.DC. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Xue J, Gavrialov O, Haddad GG. Na+/H+ exchanger 1 deficiency alters gene expression in mouse brain. Physiol Genomics. 2004;18:331–339. doi: 10.1152/physiolgenomics.00076.2004. [DOI] [PubMed] [Google Scholar]
- 24.Schultheis PJ, Clarke LL, Meneton P, et al. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest. 1998;101:1243–1253. doi: 10.1172/JCI1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultheis PJ, Clarke LL, Meneton P, et al. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet. 1998;19:282–285. doi: 10.1038/969. [DOI] [PubMed] [Google Scholar]
- 26.Schnermann J. Micropuncture analysis of tubuloglomerular feedback regulation in transgenic mice. J Am Soc Nephrol. 1999;10:2614–2619. doi: 10.1681/ASN.V10122614. [DOI] [PubMed] [Google Scholar]
- 27.Du Z, Duan Y, Yan Q, et al. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci USA. 2004;101:13068–13073. doi: 10.1073/pnas.0405179101. This paper proposes a mechanosensory function for the proximal tubule microvilli. The flow-dependent increase in fluid absorption was found to be disrupted in tubules from NHE3−/− mice or after treatment with cytochalasin D. These findings advance the hypothesis that the actin cytoskeleton relays flow information from the microvilli to modulate transport by NHE3.
- 28.Woo AL, Noonan WT, Schultheis PJ, et al. Renal function in NHE3-deficient mice with transgenic rescue of small intestinal absorptive defect. Am J Physiol Renal Physiol. 2003;284:F1190–F1198. doi: 10.1152/ajprenal.00418.2002. [DOI] [PubMed] [Google Scholar]
- 29•.Noonan WT, Woo AL, Nieman ML, et al. Blood pressure maintenance in NHE3-deficient mice with transgenic expression of NHE3 in small intestine. Am J Physiol Regul Integr Comp Physiol. 2005;288:R685–R691. doi: 10.1152/ajpregu.00209.2004. This paper underscores the importance of renal NHE3 in NHE3−/− mice with genetic NHE3 rescue in the gut. These mice were able to maintain blood pressure only if given a high-salt diet, but were still hypotensive on a normal-salt diet.
- 30.Ledoussal C, Woo AL, Miller ML, Shull GE. Loss of the NHE2 Na+/H+ exchanger has no apparent effect on diarrheal state of NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1385–G1396. doi: 10.1152/ajpgi.2001.281.6.G1385. [DOI] [PubMed] [Google Scholar]
- 31.Choi JY, Shah M, Lee MG, et al. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest. 2000;105:1141–1146. doi: 10.1172/JCI9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gawenis LR, Greeb JM, Prasad V, et al. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem. 2005;280:12781–12789. doi: 10.1074/jbc.M414118200. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, King SM, Quill TA, et al. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–1122. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 34.Komlosi P, Fintha A, Bell PD. Current mechanisms of macula densa cell signalling. Acta Physiol Scand. 2004;181:463–469. doi: 10.1111/j.1365-201X.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 35.D’Souza S, Garcia-Cabado A, Yu F, et al. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- 36.Moe OW. Acute regulation of proximal tubule apical membrane Na+/H+ exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol. 1999;10:2412–2425. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 37.Collazo R, Fan L, Hu MC, et al. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem. 2000;275:31601–31608. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- 38.Yang X, Amemiya M, Peng Y, et al. Acid incubation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Cell Physiol. 2000;279:C410–C419. doi: 10.1152/ajpcell.2000.279.2.C410. [DOI] [PubMed] [Google Scholar]
- 39.Hu MC, Fan L, Crowder LA, et al. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 40.Peng Y, Amemiya M, Yang X, et al. ET(B) receptor activation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Renal Physiol. 2001;280:F34–F42. doi: 10.1152/ajprenal.2001.280.1.F34. [DOI] [PubMed] [Google Scholar]
- 41.du Cheyron D, Chalumeau C, Defontaine N, et al. Angiotensin II stimulates NHE3 activity by exocytic insertion of the transporter: role of PI 3-kinase. Kidney Int. 2003;64:939–949. doi: 10.1046/j.1523-1755.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 42.McDonough AA, Biemesderfer D. Does membrane trafficking play a role in regulating the sodium/hydrogen exchanger isoform 3 in the proximal tubule? Curr Opin Nephrol Hypertens. 2003;12:533–541. doi: 10.1097/00041552-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Gekle M, Volker K, Mildenberger S, et al. NHE3 Na+/H+ exchanger supports proximal tubular protein reabsorption in vivo. Am J Physiol Renal Physiol. 2004;287:F469–F473. doi: 10.1152/ajprenal.00059.2004. [DOI] [PubMed] [Google Scholar]
- 44.Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol. 2003;284:F467–F473. doi: 10.1152/ajprenal.00352.2002. [DOI] [PubMed] [Google Scholar]
- 45.Numata M, Petrecca K, Lake N, Orlowski J. Identification of a mitochondrial Na+/H+ exchanger. J Biol Chem. 1998;273:6951–6959. doi: 10.1074/jbc.273.12.6951. [DOI] [PubMed] [Google Scholar]
- 46.Brett CL, Wei Y, Donowitz M, Rao R. Human Na+/H+ exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282:C1031–C1041. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- 47.Kuro-o M, Hanaoka K, Hiroi Y, et al. Salt-sensitive hypertension in transgenic mice overexpressing Na+-proton exchanger. Circ Res. 1995;76:148–153. doi: 10.1161/01.res.76.1.148. [DOI] [PubMed] [Google Scholar]
- 48.Orlov SN, Adragna NC, Adarichev VA, Hamet P. Genetic and biochemical determinants of abnormal monovalent ion transport in primary hypertension. Am J Physiol Cell Physiol. 1999;276:C511–C536. doi: 10.1152/ajpcell.1999.276.3.C511. [DOI] [PubMed] [Google Scholar]
- 49.Lifton RP, Hunt SC, Williams RR, et al. Exclusion of the Na+-H+ antiporter as a candidate gene in human essential hypertension. Hypertension. 1991;17:8–14. doi: 10.1161/01.hyp.17.1.8. [DOI] [PubMed] [Google Scholar]
- 50•.Iwamoto T, Kita S, Zhang J, et al. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med. 2004;10:1193–1199. doi: 10.1038/nm1118. Several rodent models were used to provide multiple lines of evidence suggesting that the vascular smooth muscle Na+/Ca2+ exchanger NCX1 in reverse mode is involved in the pathogenesis of hypertension.
- 51.Morduchowicz GA, Sheikh-Hamad D, Jo OD, et al. Increased Na+/H+ antiport activity in the renal brush border membrane of SHR. Kidney Int. 1989;36:576–581. doi: 10.1038/ki.1989.233. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi M, Yoshida T, Monkawa T, et al. Na+/H+-exchanger 3 activity and its gene in the spontaneously hypertensive rat kidney. J Hypertens. 1997;15:43–48. [PubMed] [Google Scholar]
- 53.Kelly MP, Quinn PA, Davies JE, Ng LL. Activity and expression of Na+/H+ exchanger isoforms 1 and 3 in kidney proximal tubules of hypertensive rats. Circ Res. 1997;80:853–860. doi: 10.1161/01.res.80.6.853. [DOI] [PubMed] [Google Scholar]
- 54.LaPointe MS, Sodhi C, Sahai A, Batlle D. Na+/H+ exchange activity and NHE-3 expression in renal tubules from the spontaneously hypertensive rat. Kidney Int. 2002;62:157–165. doi: 10.1046/j.1523-1755.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi K, Monkawa T, Hayashi M, Saruta T. Expression of the Na+/H+ exchanger regulatory protein family in genetically hypertensive rats. J Hypertens. 2004;22:1723–1730. doi: 10.1097/00004872-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Orlov SN, Pokudin NI, Postnov YV. 86Rb and 22Na transport in primary cultured renal cells from spontaneously hypertensive rats. J Hypertens Suppl. 1991;9:S290–S291. [PubMed] [Google Scholar]
- 57•.Parenti P, Ferrari P, Ferrandi M, et al. Effect of amiloride analogues on sodium transport in renal brush border membrane vesicles from Milan hypertensive rats. Biochem Biophys Res Commun. 1992;183:55–61. doi: 10.1016/0006-291x(92)91608-s. [DOI] [PubMed] [Google Scholar]
- 58.Capasso G, Rizzo M, Evangelista C, et al. Altered expression of the renal apical plasma membrane Na+ transporters in the early phase of genetic hypertension. Am J Physiol Renal Physiol. 2005;288:F1173–F1182. doi: 10.1152/ajprenal.00228.2004. The Milan strain of hypertensive rats was used to investigate early changes in protein and mRNA levels of the Na+ transporters NHE3, Na+/K+/Cl− cotransporter 2 (NKCC2), Na+/Cl− cotransporter (NCC) and epithelial sodium channel (ENaC). NKCC2 was found to be upregulated, NCC and ENaC downregulated, and NHE3 not altered.
- 59.Hoagland KM, Flasch AK, Dahly-Vernon AJ, et al. Elevated BSC-1 and ROMK expression in Dahl salt-sensitive rat kidneys. Hypertension. 2004;43:860–865. doi: 10.1161/01.HYP.0000120123.44945.47. [DOI] [PubMed] [Google Scholar]
- 60.Yang LE, Zhong H, Leong PK, et al. Chronic renal injury-induced hypertension alters renal NHE3 distribution and abundance. Am J Physiol Renal Physiol. 2003;284:F1056–F1065. doi: 10.1152/ajprenal.00317.2002. [DOI] [PubMed] [Google Scholar]
- 61.Zhu H, Sagnella GA, Dong Y, et al. Molecular variants of the sodium/hydrogen exchanger type 3 gene and essential hypertension. J Hypertens. 2004;22:1269–1275. doi: 10.1097/01.hjh.0000125428.28861.11. [DOI] [PubMed] [Google Scholar]
- 62.Brookes PS, Yoon Y, Robotham JL, et al. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 63.Frelin C, Vigne P, Ladoux A, Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988;174:3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- 64.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur J Med Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 65.Karmazyn M. Amiloride enhances postischemic ventricular recovery: possible role of Na+/H+ exchange. Am J Physiol Heart Circ Physiol. 1988;255:H608–H615. doi: 10.1152/ajpheart.1988.255.3.H608. [DOI] [PubMed] [Google Scholar]
- 66.Karmazyn M, Moffat MP. Role of Na+/H+ exchange in cardiac physiology and pathophysiology: mediation of myocardial reperfusion injury by the pH paradox. Cardiovasc Res. 1993;27:915–924. doi: 10.1093/cvr/27.6.915. [DOI] [PubMed] [Google Scholar]
- 67.Symons JD, Correa SD, Schaefer S. Na+/H+ exchange inhibition with cariporide limits functional impairment caused by repetitive ischemia. J Cardiovasc Pharmacol. 1998;32:853–862. doi: 10.1097/00005344-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 68.Hale SL, Kloner RA. Effect of combined K(ATP) channel activation and Na+/H+ exchange inhibition on infarct size in rabbits. Am J Physiol Heart Circ Physiol. 2000;279:H2673–H2677. doi: 10.1152/ajpheart.2000.279.6.H2673. [DOI] [PubMed] [Google Scholar]
- 69.Gumina RJ, Moore J, Schelling P, et al. Na+/H+ exchange inhibition prevents endothelial dysfunction after I/R injury. Am J Physiol Heart Circ Physiol. 2001;281:H1260–H1266. doi: 10.1152/ajpheart.2001.281.3.H1260. [DOI] [PubMed] [Google Scholar]
- 70•.Chen L, Chen CX, Gan XT, et al. Inhibition and reversal of myocardial infarction-induced hypertrophy and heart failure by NHE-1 inhibition. Am J Physiol Heart Circ Physiol. 2004;286:381–387. doi: 10.1152/ajpheart.00602.2003. The NHE1 inhibitor EMD-87580 reduced myocardial hypertrophy, ventricular dilatation, and heart failure, even when administered 4 weeks after cardiac ischemia produced by coronary artery ligation in Sprague–Dawley rats.
- 71.Waldmann R, Champigny G, Bassilana F, et al. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 72.Reuter H, Henderson SA, Han T, et al. Knockout mice for pharmacological screening: testing the specificity of Na+/Ca2+ exchange inhibitors. Circ Res. 2002;91:90–92. doi: 10.1161/01.res.0000027529.37429.38. [DOI] [PubMed] [Google Scholar]
- 73.Theroux P, Chaitman BR, Danchin N, et al. Guard during ischemia against necrosis (GUARDIAN) Investigators Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Circulation. 2000;102:3032–3038. doi: 10.1161/01.cir.102.25.3032. [DOI] [PubMed] [Google Scholar]
- 74.Zeymer U, Suryapranata H, Monassier JP, et al. The Na+/H+ exchange inhibitor eniporide as an adjunct to early reperfusion therapy for acute myocardial infarction. Results of the evaluation of the safety and cardioprotective effects of eniporide in acute myocardial infarction (ESCAMI) trial. J Am Coll Cardiol. 2001;38:1644–1650. doi: 10.1016/s0735-1097(01)01608-4. [DOI] [PubMed] [Google Scholar]
- 75.Boyce SW, Bartels C, Bolli R, et al. Impact of sodium-hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk CABG surgery patients: results of the CABG surgery cohort of the GUARDIAN study. J Thorac Cardiovasc Surg. 2003;126:420–427. doi: 10.1016/s0022-5223(03)00209-5. [DOI] [PubMed] [Google Scholar]
- 76.Besse S, Tanguy S, Boucher F, et al. Cardioprotection with cariporide, a sodium-proton exchanger inhibitor, after prolonged ischemia and reperfusion in senescent rats. Exp Gerontol. 2004;39:1307–1314. doi: 10.1016/j.exger.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Jung O, Albus U, Lang HJ, et al. Effects of acute and chronic treatment with the sodium hydrogen exchanger 1 (NHE-1) inhibitor cariporide on myocardial infarct mass in rabbits with hypercholesterolaemia. Basic Clin Pharmacol Toxicol. 2004;95:24–30. doi: 10.1111/j.1742-7843.2004.t01-1-pto950105.x. [DOI] [PubMed] [Google Scholar]
- 78.Matsumoto T, Miura T, Miki T, et al. Blockade of the Na+/Ca2+ exchanger is more efficient than blockade of the Na+/H+ exchanger for protection of the myocardium from lethal reperfusion injury. Cardiovasc Drugs Ther. 2002;16:295–301. doi: 10.1023/a:1021725808547. [DOI] [PubMed] [Google Scholar]
- 79.Takahashi T, Takahashi K, Onishi M, et al. Effects of SEA0400, a novel inhibitor of the Na+/Ca2+ exchanger, on myocardial stunning in anesthetized dogs. Eur J Pharmacol. 2004;505:163–168. doi: 10.1016/j.ejphar.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 80.Yoshiyama M, Nakamura Y, Omura T, et al. Cardioprotective effect of SEA0400, a selective inhibitor of the Na+/Ca2+ exchanger, on myocardial ischemia-reperfusion injury in rats. J Pharmacol Sci. 2004;95:196–202. doi: 10.1254/jphs.fpj03101x. [DOI] [PubMed] [Google Scholar]
- 81.Yao H, Ma E, Gu XQ, Haddad GG. Intracellular pH regulation of CA1 neurons in Na+/H+ isoform 1 mutant mice. J Clin Invest. 1999;104:637–645. doi: 10.1172/JCI6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Kintner DB, Su G, Lenart B, et al. Increased tolerance to oxygen and glucose deprivation in astrocytes from Na+/H+ exchanger isoform 1 null mice. Am J Physiol Cell Physiol. 2004;287:C12–C21. doi: 10.1152/ajpcell.00560.2003. Resting intracellular pH was found to be reduced, and pH recovery after an acid load was found to be impaired, in primary cultured astrocytes from NHE1−/− mice compared to NHE1+/+ controls. However, NHE1−/− astrocytes are more resistant to oxygen and glucose deprivation. Similarly, NHE1 inhibition improves resistance to oxygen and glucose deprivation in NHE1+/+ astrocytes.
- 83.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxicischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 84.Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies – the need for new approaches. Cerebrovasc Dis. 2004;17(suppl 1):153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- 85.Yermolaieva O, Leonard AS, Schnizler MK, et al. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiong ZG, Zhu XM, Chu XP, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 87.Chen H, Luo J, Kintner DB, et al. Na+-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- 88.Bano D, Young KW, Guerin CJ, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 89.Kuribayashi Y, Itoh N, Kitano M, Ohashi N. Cerebroprotective properties of SM-20220, a potent Na+/H+ exchange inhibitor, in transient cerebral ischemia in rats. Eur J Pharmacol. 1999;383:163–168. doi: 10.1016/s0014-2999(99)00645-7. [DOI] [PubMed] [Google Scholar]
- 90•.Matsumoto Y, Yamamoto S, Suzuki Y, et al. Na+/H+ exchanger inhibitor, SM-20220, is protective against excitotoxicity in cultured cortical neurons. Stroke. 2004;35:185–190. doi: 10.1161/01.STR.0000106910.42815.C2. This paper shows that NHE inhibition attenuates glutamate-induced excitotoxicity in cultured neurons.
- 91.Castella M, Buckberg GD, Tan Z. Neurologic preservation by Na+/H+ exchange inhibition prior to 90 minutes of hypothermic circulatory arrest. Ann Thorac Surg. 2005;79:646–654. doi: 10.1016/j.athoracsur.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Wang Z, Rabb H, Craig T, et al. Ischemic-reperfusion injury in the kidney: overexpression of colonic H+/K+-ATPase and suppression of NHE-3. Kidney Int. 1997;51:1106–1115. doi: 10.1038/ki.1997.153. [DOI] [PubMed] [Google Scholar]
- 93.Wang Z, Rabb H, Haq M, et al. A possible molecular basis of natriuresis during ischemic-reperfusion injury in the kidney. J Am Soc Nephrol. 1998;9:605–613. doi: 10.1681/ASN.V94605. [DOI] [PubMed] [Google Scholar]
- 94.Kwon TH, Frokiaer J, Han JS, et al. Decreased abundance of major Na+ transporters in kidneys of rats with ischemia-induced acute renal failure. Am J Physiol Renal Physiol. 2000;278:F925–F939. doi: 10.1152/ajprenal.2000.278.6.F925. [DOI] [PubMed] [Google Scholar]
- 95.Thurau K, Boylan JW. Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med. 1976;61:308–315. doi: 10.1016/0002-9343(76)90365-x. [DOI] [PubMed] [Google Scholar]
- 96.Hropot M, Juretschke HP, Langer KH, Schwark JR. S3226, a novel NHE3 inhibitor, attenuates ischemia-induced acute renal failure in rats. Kidney Int. 2001;60:2283–2289. doi: 10.1046/j.1523-1755.2001.00058.x. [DOI] [PubMed] [Google Scholar]
- 97.Ogata M, Iwamoto T, Tazawa N, et al. A novel and selective Na+/Ca2+ exchange inhibitor, SEA0400, improves ischemia/reperfusion-induced renal injury. Eur J Pharmacol. 2003;478:187–198. doi: 10.1016/j.ejphar.2003.08.082. [DOI] [PubMed] [Google Scholar]
- 98.Yamashita J, Kita S, Iwamoto T, et al. Attenuation of ischemia/reperfusion-induced renal injury in mice deficient in Na+/Ca2+ exchanger. J Pharmacol Exp Ther. 2003;304:284–293. doi: 10.1124/jpet.102.039024. [DOI] [PubMed] [Google Scholar]