Abstract
Receptors for the Fc domain of IgG mediate target recognition, signal transduction, and effector functions including antibody-dependent cytolysis, phagocytosis, and phagolysosome formation. To better understand FcγR-mediated functions and to identify potential therapeutic strategies, we employed cell-penetrating (“Trojan”) peptides to deliver “wild-type” (LTL) or modified (AAA) FcγRIIA tail sequences to the neutrophil’s cytoplasm. The Trojan-LTL peptide appeared to label the endoplasmic reticulum whereas the Trojan-AAA peptide distributed throughout the cytoplasm. The Trojan-LTL peptide, but not the Trojan-AAA peptide, decreased Ca2+ signaling at the phagosome and reduced phagolysosome formation. These studies suggest that FcγRIIA’s tail can act as a peptide decoy thereby blunting FcγRIIA-mediated processes, which, in turn, suggests a possible route in managing inflammatory tissue damage.
Keywords: human, neutrophil, phagocytosis, rheumatoid arthritis, inflammation, Fc receptor
1. Introduction
Three classes of FcγRs for IgG (I, II, and III) are expressed by human leukocytes [1, 2]. As FcγRI (CD64) binds to monomeric IgG, it is usually saturated in the presence of serum. FcγRII (CD32) and III (CD16) bind to aggregated IgG, typically in the form of immune complexes (ICs) or IgG-opsonized targets. FcγRI, IIA, IIIA and IIIB activate cellular responses, whereas FcγRIIB inhibits cell responses. FcγRIIA is the most highly expressed FcγR. It is also the only receptor for human IgG2, the most common autoantibody isotype [3]. Hence, FcγRIIA is a potential contributor to IC-related diseases, such as arthritis.
Several lines of evidence indicate that FcγRIIA plays an important role in rheumatoid arthritis (RA). For example, FcγRIIA polymorphisms correlate with several chronic inflammatory diseases, including RA [3]. Disease severity correlates with FcγRIIA expression levels [4, 5]. Importantly, blockade of FcγRIIA, but not FcγRIII, inhibits TNF-α production by monocytes in response to precipitated human ICs [6, 7]. Animal studies also support the role of FcγRIIA in arthritis. However, as rodents lack an ortholog of human FcγRIIA, it is difficult to directly compare murine and human studies. Importantly, Hogarth and colleagues have used human FcγRIIA-transgenic mice to demonstrate an important role of FcγRIIA in a humanized animal model of collagen-induced arthritis [8]. FcγRIIA-transgenic mice are exhibit spontaneous autoimmune disease and are hyper-responsive to pathogenic antibodies [3, 8]. Also in the murine system, gene knock-out experiments have shown that activating FcγRs directly mediate cartilage destruction [9] and that the development of arthritis does not require the presence of the murine orthologs of the activating FcγRI and III [10]. Hence, FcγRIIA has emerged as a leading target for new drug candidates, including therapeutics aimed at the extracellular region of FcγRIIA [3, 11].
While FcγRIIA’s extracellular region mediates IC recognition, its intracellular C-terminal domain plays a key role in signal transduction [12]. The cytoplasmic domain contains the ITAM (immunoreceptor tyrosine-based activation motif) consensus sequence YxxLx(5–12)Yx(2–3)L/I. Phosphorylated tyrosine residues of ITAMs are high affinity binding sites for Syk, a tyrosine kinase that participates in phagocytosis. In addition to phagocytosis, FcγRIIA’s cytoplasmic tail also mediates phagolysome formation, the delivery of lysosomal contents to the phagosome or, in the context of frustrated phagocytosis, the extracellular release of lysosomal contents. We have discovered that the tripeptide LTL sequence is a key mediator of phagolysosomal fusion [12, 13]. Modification of the LTL sequence to an AAA sequence dramatically reduces phagolysosome formation. AAA was chosen due to the residues having similar properties of hydropathy and linearity, and a three residue sequence being too short to scramble. Although the LTL sequence bears some resemblance to the dileucine lysosomal signal sequence found in other pathways [14], the LTL sequence is highly specific for FcγRIIA.
To further examine the mechanism by which FcγRIIA’s cytoplasmic LTL sequence affects cell physiology and to test the potential therapeutic role of LTL-containing peptides in cells, it is necessary to use cell-penetrating peptides. Recently, several cell-penetrating peptides, which are cationic in nature, have been developed [15–20]. Penetratin is a typical cell-penetrating Trojan peptide; it is a 16 amino acid peptide derived from the third helix of the Drosophila transcription factor Antennapedia, corresponding to residues 43–58 of the homeodomain [17]. This peptide has the ability to translocate across cell membranes in a concentration-, energy- and endocyosis-independent manner [17–20]. Importantly, penetratin is able to transport covalently attached molecules, such as oligonucleotides and oligopeptides, into cells [17–20]. These “Trojan” peptide constructs may become useful as delivery vehicles for therapeutic compounds. We now show in live human neutrophils that it is possible to reduce Ca2+ signaling and phagolysosome fusion by treatment with a Trojan peptide construct.
2. Materials and Methods
2.1. Materials
Indo-1-AM, pluronic-127, cell media, phosphate buffered saline (PBS), LysoTracker Red DND-99 and LysoTracker Green DND-26 were obtained from Invitrogen Corp. (Carlsbad, CA). Cover-glass bottom dishes were purchased from MatTek Corporation (Ashland, MA). Unless otherwise noted, chemicals were obtained from Sigma Chemical Company (St. Louis, MO).
2.2. Trojan Peptide Synthesis
Trojan peptides contained the penetratin sequence in tandem with a portion of the cytoplasmic domain of the FcγRIIA receptor (Fig. 1). For use as a control, a second peptide was constructed in which the LTL motif was substituted with AAA sequence. To visualize these peptides, the Trojan-LTL and Trojan-AAA peptides were conjugated to tetramethylrhodamine and fluorescein, respectively. Custom peptides were prepared by Bio-Synthesis, Inc. (Lewiston, TX). Peptides were purified by HPLC and confirmed by mass spectroscopy. Peptides were stored in sterile PBS at −20° C.
Fig. 1.

Trojan peptide constructs. Trojan peptides contained the cell-permeable penetratin sequence of Antennapedia in tandem with the C-terminal end of FcγRIIA’s cytoplasmic domain. The penetratin sequence is underlined, and the Fc receptor sequence is in bold type with its critical motif highlighted. As a control, a peptide was constructed in which the LTL motif was substituted with AAA. The peptide containing the LTL motif (Trojan-LTL peptide) was conjugated to tetramethylrhodamine whereas the AAA substituted (Trojan-AAA) peptide was labeled with fluorescein. Prior to use, the lyophilized peptides were dissolved in a stock solution with sterile PBS at a concentration of 900 μM, and stored at −20° C. Prior to use peptides were diluted to a concentration of 45 μM.
2.3. Isolation of Human Neutrophils
Peripheral blood was collected from healthy human donors in compliance with the guidelines of the University of Michigan Institutional Review Board for Human Subject Research. Neutrophils were isolated from peripheral blood using Ficoll-Histopaque density gradient centrifugation (Sigma Chemical Co., St. Louis, MO). Samples were re-suspended and then washed in PBS by centrifugation.
2.4. Fluorescence Microscopy of Peptides
Poly-D-lysine coated cover slips for Trojan peptide fluorescence microscopy experiments were prepared by incubating cover slips with 0.01% (w/v) poly-D-lysine for 1 hour at 37° C followed by washing with PBS. Neutrophils were allowed to adhere to poly-lysine-coated cover slips for 15 minutes at 37° C. Adherent neutrophils were labeled at 37° C for one hour with the Trojan-LTL peptide (45 μM), control peptide (45 μM), or ER-Tracker (Invitrogen; 50 μM) in PBS. ER-Tracker, a fluorescent dye, has been shown to label the endoplasmic reticulum [21]. Cover slips were washed with PBS and imaged on a Ziess Axiovert 135 inverted microscope coupled to an intensified charge coupled device camera (QImaging, Barnaby, BC, Canada). Cover slips were observed using bright field and fluorescence microscopy. To detect fluorescein, excitation and emission filters at 482 nm and 530 nm were used. For ER Tracker blue-white DPX, optical filters with excitation at 364 nm and emission at 520 nm were used. To image tetramethylrhodamine, excitation and emission filters at 535 nm and 590 nm, respectively, were employed. Images were obtained using Volumescan (Vaytek, Inc., Fairfield, IA) and were processed using Image-Pro Plus (Media Cybernetics, Silver Spring, MD) and MicroTome Deconvolution software (Vaytek, Inc.).
2.5. Phagocytosis of Targets
Neutrophils were labeled with Trojan-LTL or -AAA peptide at 45 μM. Sheep red blood cells (SRBCs) were opsonized with rabbit anti-sheep erythrocyte IgG antibody by using the highest sub-agglutinating dilution of antibody. IgG-coated erythrocytes (EAs) or untreated latex beads (Sigma) were added to cover slips with adherent neutrophils at a ratio of 10:1 (target/effector) and incubated for 45 minutes at 37° C in PBS. Peptide-treated neutrophils were labeled with LysoTracker Red DND-99 (for fluorescein-Trojan-AAA peptide) or LysoTracker Green DND-26 (for tetramethylrhodamine-Trojan-LTL peptide) at 20 μM in PBS for 5 minutes at 37°C (Invitrogen). The LysoTracker dyes have been shown to localize in lysosomes and phagolysosomes [22]. In experiments involving EAs, the coverslips were moved to 4° C to stop phagocytosis, and external EAs were distinguished from internal EAs by labeling with a secondary fluorescent anti-IgG (internalized EAs are not labeled).
2.6. Imaging of Phagosome-Associated Ca2+
For Ca2+ experiments, neutrophils were loaded with Indo-1-AM (14 μM) for 45 minutes at 37°C. Cells were washed and then labeled with Trojan-LTL or -AAA peptides (45 μM) for 15 minutes at 37°C. EA were added to samples at a 10:1 ratio followed by a further incubation for 20 minutes at 37°C. Cells were washed by centrifugation, re-suspended in imaging buffer [23] and then allowed to adhere to cover slips for 20 minutes at 37°C. Afterwards, the cover slips were washed to remove unbound EA, then incubated for a further one hour and 40 minutes. Imaging was performed using a 37°C heated microscope stage. Fluorescence excitation was provided by a 100 W mercury lamp. Samples were imaged using a Princeton Instruments PI-Max II intensified charge coupled device detector coupled to a Dual-View emission-splitting device (Optical Insights, Tuscon, AZ) attached to a side port of a Nikon TE-2000-U microscope. Indo-1-labeled samples were excited using a D350/50x filter and a 380 nm dichroic beamsplitter (Chroma). Emission light was directed into the Dual-View apparatus, which produces simultaneous images at two emission wavelengths. This apparatus utilized a D405/30m filter for the high Ca2+ wavelength emission of Indo-1, a D485/25m for the low Ca2+ emission and a 440 nm dichroic reflector (Chroma). The result is an image stack wherein each frame is a dual image. WinSpec (dual-image) files were imported into MetaMorph (Molecular Devices) where they were split and re-formatted. The files were opened in MetaFluor (Molecular Devices), where a ratio (405/485) image is produced. A threshold was applied to limit ratio calculations to within cell boundaries. For quantitative purposes, phagosmes were evaluated for Ca2+. High Ca2+ phagosomes were scored as those that had a higher Ca2+ ratio in the phagosome than in the cell body; whereas those with equal or lower Ca2+ ratio were scored as low.
3. Results
3.1. Trojan Peptides Containing FcγRIIA’s Tail Bind of to an ER-Like Structure of Human Neutrophils in an LTL-Dependent Fashion
In this paper we test the hypothesis that the tail of FcγRIIA can interfere with physiological processes in living neutrophils. To address this hypothesis, it is necessary to first ascertain if Trojan peptides containing FcγRIIA tail sequences are capable of entering neutrophils. To observe the intracellular accumulation and distribution of Trojan peptides, neutrophils were incubated with labeled peptides and then imaged by fluorescence microscopy. The Trojan-LTL peptide accumulated within the cytoplasm of cells (Fig. 2B). The intracellular distribution of the Trojan-LTL peptide appeared to resemble that of the endoplasmic reticulum (Fig. 2B), as suggested by the intracellular labeling pattern of the dye ER Tracker (Fig. 2D). In contrast, cells labeled with the Trojan-AAA peptide did not show specific sub-cellular accumulation, only a diffuse intracellular labeling pattern was observed throughout the cells (Fig. 2F); this suggests that the LTL sequence of the Trojan-LTL peptide promoted specific delivery to a cell compartment resembling the ER. As expected, these results demonstrate that peptides containing the penetratin leader sequence cross the neutrophil’s plasma membrane, as has been previously demonstrated in many cell types [17–20]. Furthermore, sub-cellular accumulation of Trojan-LTL peptides suggests that FcγRIIA’s tail may have a binding site associated with the ER, and the LTL motif may be involved in this interaction.
Fig. 2.
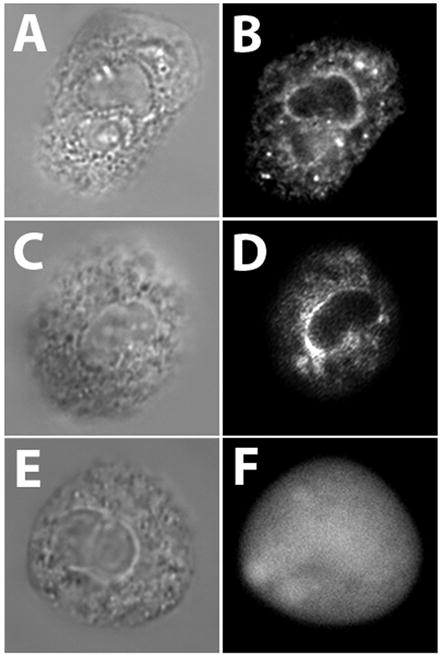
The Trojan-LTL peptide labels an ER-like cellular compartment. Adherent neutrophils were labeled with Trojan-LTL peptide, the control Trojan-AAA peptide, or ER-Tracker for 1 hour at 37°C and imaged as described in Materials and Methods. The Trojan-LTL peptide accumulated within cells (A and B). This pattern of labeling was very similar to the labeling pattern of cells stained with ER Tracker (C and D), suggesting that Trojan-LTL peptides may interact with the ER. In cells labeled with the Trojan-AAA peptide only a diffuse non-specific labeling pattern was seen throughout the cell (E and F), suggesting that Trojan-AAA peptides enter cells, but do not selectively bind to organelles. (x1800) (A and B, n=6; C and D, n=5; E and F, n=3)
3.2. LTL Labeling Patterns During Phagocytosis
The fluorescent tag on the Trojan-LTL peptide enabled us to visualize its distribution during phagocytosis. The Trojan-LTL peptide accumulated at sites of EA binding prior to internalization (Fig. 3A and B). Interestingly, the Trojan-LTL peptide was found about the perimeter of EAs after internalization (Fig. 3C and D). On the other hand, cells stained with the Trojan-AAA peptide did not display preferential localization during phagocytosis (Fig. 3E and F). To determine if Trojan-LTL peptide accumulation near targets was specific for antibody-dependent events, experiments were performed with non-opsonized erthrocytes and latex bead targets. In the absence of IgG, no binding or internalization of erythrocyte targets was observed. However, phagocytosis of latex beads was observed in the absence of IgG (data not shown). In these experiments, there was no detectable shift in Trojan-LTL peptide localization to targets. As in the antibody-dependent experiments described above, there was no detectable change in the Trojan-AAA peptide accumulation using a latex bead target (Fig. 4). Thus, Trojan-LTL peptide redistribution is not a general feature of phagocytosis.
Fig. 3.
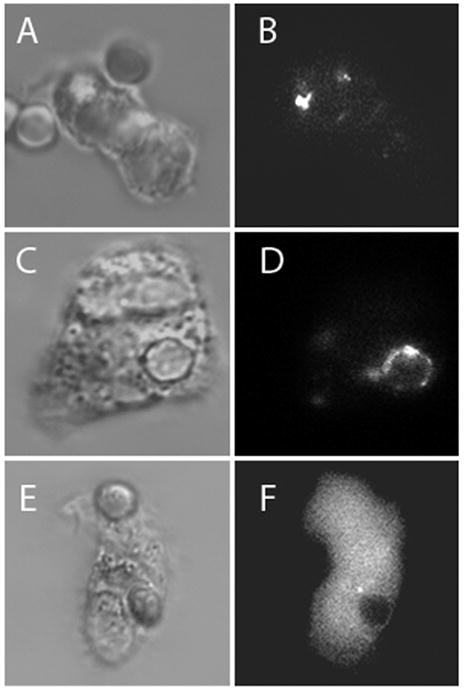
The Trojan-LTL peptide can be found near targets during IgG-dependent phagocytosis. Adherent neutrophils with internalized EA targets were labeled with the Trojan-LTL peptide and -AAA peptide. External EA were labeled with a secondary fluorescent anti-IgG (internalized EA are not labeled). The Trojan-LTL peptide accumulated at the site of EA binding before internalization (A and B); after internalization, it localized around the edge of EA-containing phagosomes (C and D). Cells stained with the control peptide exhibited no change in localization after phagocytosis (E and F). (x1800) (A–F, n=4; G and H, n=3)
Fig. 4.
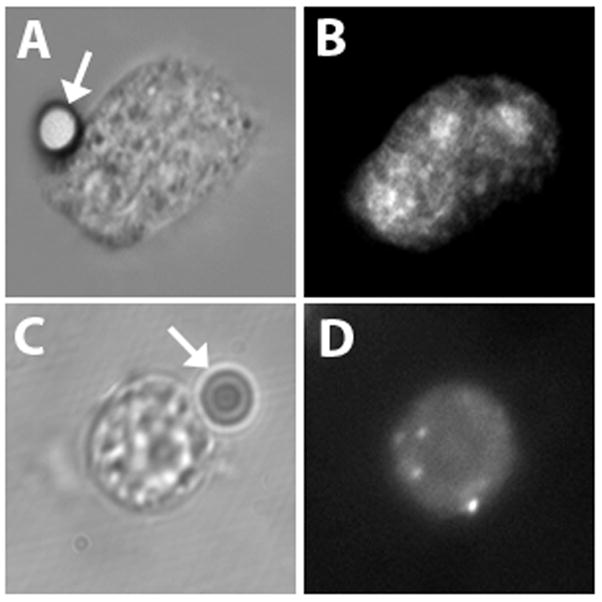
Latex beads do not influence cell labeling with Trojan peptides. Neutrophils were incubated with latex beads targets. Adherent neutrophils with bound latex bead targets were labeled with the Trojan-LTL peptide or -AAA peptide. No changes in the labeling patterns of Trojan-LTL peptide (A and B) and Trojan-AAA peptide (C and D) were observed upon interaction with the beads. Latex beads are indicated by arrows. (x1800) (A and B, n=4; C and D, n=3)
3.3. Incubation with the Trojan-LTL Peptide Reduces Ca2+ Signaling at the Phagosome
To assess the ability of Trojan peptides to influence signal transduction pathways, Ca2+ experiments were performed. Ratiometric Ca2+ imaging experiments were performed to evaluate Ca2+ signaling in the vicinity of EA phagosomes. Neutrophils were labeled with Indo-1, then treated with Trojan-AAA or -LTL peptide. After peptide treatment, IgG-coated EAs were added, cells were washed by centrifugation, re-suspended and plated on coverslips, followed by a further incubation for 2 hours and then imaged. Ratio images were prepared as described in the Materials and Methods; the relative Ca2+ ratios in the cell cytoplasm and phagosome were evaluated. Fig. 5A shows the quantification of the percentage of phagosomes with high Ca2+ after 2 hours. Untreated cells exhibited high phagosomal Ca2+ 36 ± 5.8% of the time; Trojan-AAA treated cells showed a similar rate of 38 ± 8.7%. When neutrophils were treated with the Trojan-LTL peptide, the percentage of high phagosomal Ca2+ signals was dramatically reduced to 17 ± 5.7% (Fig. 5A). Representative images of Trojan-AAA (Fig. 5B and C) and -LTL (Fig 5D and E) treated cells are shown. These data were further quantified using line profile analysis, as shown in Fig. 5F. As these data show, the calcium ratio is higher in the region of the phagosome for Trojan-AAA peptide-treated cells. Thus, cells treated with Trojan peptides incorporating the tail region of FcγRIIA demonstrate reduced Ca2+ signaling in the vicinity of phagosomes.
Fig. 5.
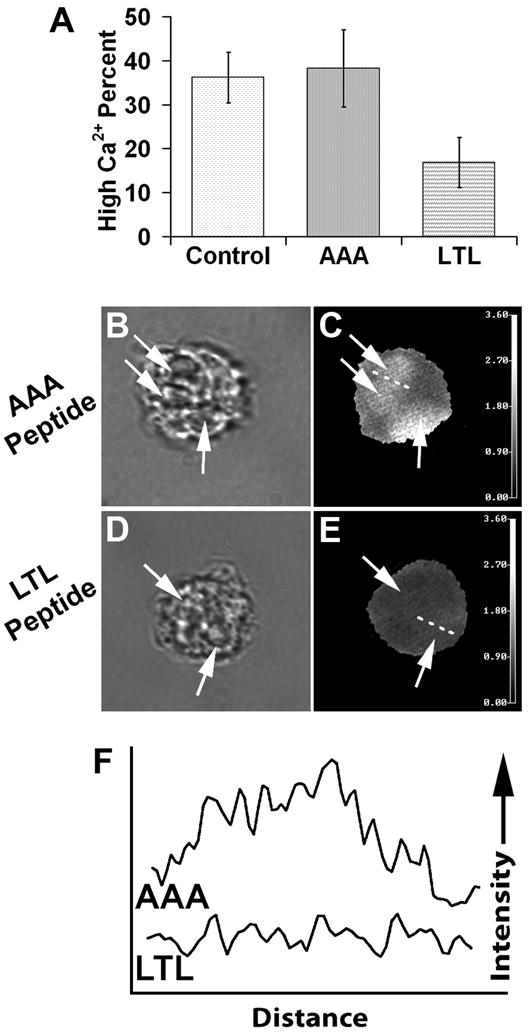
Trojan-LTL peptide treatment reduces Ca2+ signal intensity at the phagosome. Neutrophils were labeled with Indo-1 then treated with Trojan-AAA or -LTL peptides. Adherent cells were allowed to internalize EA, incubated an additional 2 hours, and then imaged as described in the Materials and Methods. Using ratio images, the Ca2+ signals of the cellular cytoplasm and phagosomes were evaluated for their relative Ca2+ ratios. For quantitative purposes, high Ca2+ ratio phagosomes were defined as those with a higher Ca2+ ratio in the phagosome than in the cell body, whereas phagosomes with equal or lower Ca2+ ratio than the cell body were considered to be low intensity. Panel A shows a quantification of the percentage of phagosomes (arrows) with high Ca2+ signals. Untreated control cells had high phagosome Ca2+ levels, similar to those in Trojan-AAA peptide-treated cells (A). When cells were treated with the Trojan-LTL peptide, a greatly reduced percentage of phagosomes exhibited high Ca2+ levels, in comparison to Trojan-AAA peptide-treated cells (p<0.0001). Panels B–E show examples of ratiometric Ca2+ images of cells treated with Trojan-AAA (B and C) and Trojan-LTL peptides (D and E). Phagosomes are denoted with arrows. High ratio phagosomes in C are evident in comparison to the low Ca2+ ratio phagosomes in E. Dashed lines through phagosomes in C and E show the line used for line profile analyses (F, traces AAA and LTL, respectively). The line profiles illustrate the higher Ca2+ ratios of the phagosome. (A, error bars representing standard deviation; B–E, x1000; B and C, n=5; D and E, n=4)
3.4. The Trojan-LTL Peptide Reduces Phagosome-Lysosome Fusion
To test the hypothesis that Trojan-LTL peptide treatment influences phagolysosome formation, neutrophils were treated with Trojan-AAA or -LTL peptides then incubated with EA. To assess phagolysosome formation, cells were labeled with LysoTracker. Untreated cells and cells treated with the Trojan-AAA peptide display fusion of lysosomes with phagosomes at indistinguishable rates of 74 ± 4.0% and 73 ± 4.9%, respectively (Fig. 6A). Representative micrographs illustrating phagolysosome formation in Trojan-AAA-treated cells are shown in Fig. 6B–C. In contrast, cells treated with the Trojan-LTL peptide display a greatly reduced rate of phagolysosome formation of 25 ± 1.7% (Fig. 6A, D–E). This suggests that the Trojan-LTL peptide blocks the downstream function of phagolysosome formation. Phagolysosome formation was also quantitatively confirmed using line profile analysis, which is illustrated in Fig. 6F. In contrast to the Trojan-LTL-treated cells, a bright rim of fluorescence is observed about the perimeter of the target EA in Trojan-AAA-treated cells indicating that phagolysosome formation has taken place.
Fig. 6.
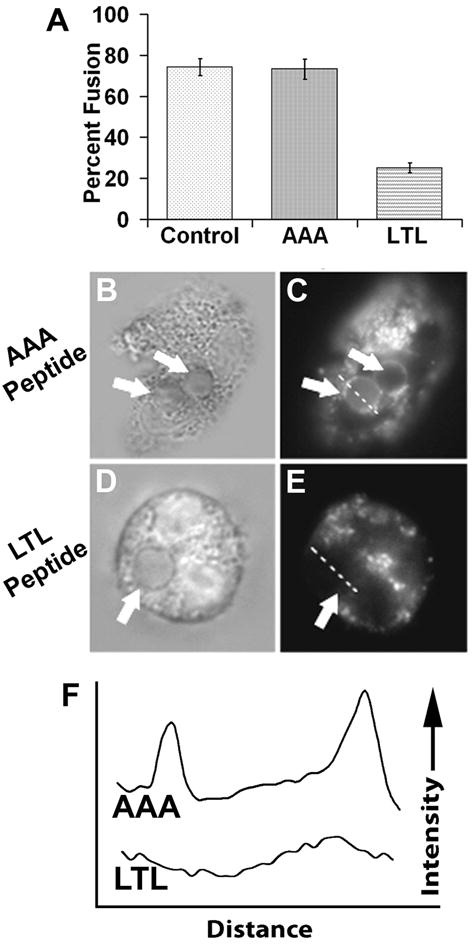
Trojan-LTL peptide treatment blocks phagosome-lysosome fusion. Adherent neutrophils were treated with the Trojan-AAA or Trojan-LTL peptides then allowed to internalize EA. After a two hour incubation, cells were labeled with LysoTracker and imaged as described in the Materials and Methods. Phagosomes were scored for phagosome-lysosome fusion. The percentage of labeled phagosomes (phagolysosomes) was tallied for the various cell treatments (A). Untreated cells (control) and cells treated with the Trojan-AAA peptide show indistinguishable fusion percentages, while LTL treated cells show a much lower percentage of phagolysosome formation (p<0.0001). Panels B–E show examples of lysosome labeling during Trojan-AAA (B and C) and Trojan-LTL (D and E) treatment. Phagosomes are denoted with arrows. Lysosome-fused phagosmes in C have a distinctive bright ring around the edge, whereas the unfused phagosome in E has no apparent labeling. Dashed lines through phagosomes in C and E show the line used for line profile analyses (F, traces AAA and LTL, respectively). The line profiles show plots of LysoTracker intensity across the distance of the line shown in the corresponding micrograph. (n=4) (A, error bars represent standard deviation; B–E, x1400)
4. Discussion
In this study, we have developed a novel Trojan peptide containing the cytoplasmic tail sequence of FcγRIIA that affects both antibody-dependent signal transduction and phagolysosome formation. These results are in accordance with previous molecular biological findings showing that the tail of FcγRIIA plays an important role in promoting phagolysosome formation [12] and that the LTL motif within the tail is crucial in regulating this activity [13]. Specifically, CHO cells expressing the wild-type FcγRIIA were capable of mediating antibody-dependent phagolysosome formation whereas tranfectants expressing a mutant form in which the LTL motif had been replaced with a AAA sequence were competent to phagocytose, but not to form phagolysosomes. Our present study using human neutrophils demonstrates that the addition of exogenous FcγRIIA tail domains to a cell suppresses phagolysosome formation, perhaps by acting as a molecular decoy for effector molecules.
Previous studies have illustrated the value of cell membrane-permeable peptides [for reviews see ref. 17–20], including their role as a delivery system to affect biological signal transduction. For example, a cell-permeable peptide containing the growth factor receptor binding protein 2 (Grb2) binding site of the epidermal growth factor (EGF) receptor selectively inhibited growth factor-stimulated mitogenesis [24, 25]. Moreover, a cationic peptide containing the phosphatidylinositol 4,5-bisphosphate binding region of gelsolin interfered with actin assembly and neutrophil motility [26]. This study extends the Trojan peptide field by showing that Trojan peptides can influence phagosome-associated signals and the ability of cells to carry out antibody-dependent phagolysosome formation.
Using fluorescent Trojan peptides, we have shown that LTL-containing peptides, but not AAA-containing peptides, accumulate at intracellular sites resembling the ER. As microtubules interact with the ER and help determine its shape [27], some similarity in distribution to these structures may also be observed. In addition, fluorescent Trojan-LTL peptides, but not AAA-containing peptides, are selectively enriched at sites of phagocytosis. Although the ER’s role in regulating phagolysosome formation is highly speculative, recent studies have demonstrated the accumulation of ER-related proteins at phagosomes or phagocytotic cups [28]. One possible interpretation of our findings is that a binding site for FcγRIIA’s cytoplasmic domain may exist in the ER. We speculate that the Trojan-LTL peptide may compete with the cell surface FcγRIIA for this receptor site, thus interfering with normal cell function.
A well-known precedent for plasma membrane-to-ER communication during immunological events is capacitative Ca2+ signaling, which has been linked with antibody-dependent effector functions [29]. Previous studies have shown enhanced intercellular free Ca2+ in the periphagosomal area of human neutrophils [30]. Under control conditions, we have also observed enhanced Ca2+ signaling in the vicinity of phagosomes, which is dramatically reduced or eliminated in the presence of Trojan-LTL peptides. This reduction in Ca2+ signaling could be mediated by perturbed ER-to-plasma membrane cooperation. Although Ca2+ signaling is not a requirement for phagocytosis, it plays a central role in phagolysosome fusion and degranulation. Hence, we suggest that the Trojan-LTL peptide-mediated disruption of Ca2+ signaling is an important contributor to the reduction of phagolysosome formation.
Our long-range goal is to develop therapeutic peptides that diminish the capacity of FcγRIIA to mediate tissue damage in arthritis [3–11]; the present study is an important step in this direction. Therapeutic peptides are the most rapidly growing segment of the pharmaceutical market. Currently, there are 40 therapeutic peptides marketed and prescribed worldwide including vancomycin, cyclosporine, fuzeon, integrillin, oxytocin, lypressin, sandostatin, glatiramer, and desmopressin. Currently marketed peptides are used to treat many different clinical indications. Another 270 peptides are in clinical tests while 400 are in advanced pre-clinical studies [31]. We speculate that it may be possible to construct therapeutic LTL-containing peptides to reduce FcγRIIA-mediated phagolysosome formation and degranulation by human leukocytes [1, 2] and synovial lining cells [32].
In summary, we have treated living cells with a peptide that selectively blocks phagosome-associated Ca2+ signaling and diminishes phagolysosome formation in response to IgG-opsonized targets. The specificity of the Ca2+ inhibition suggests that this approach might be useful in drug development. Therapeutic peptides containing the LTL motif may reduce phagolysosome formation and the release of degradative lysosomal enzymes in vivo, thereby diminishing tissue damage in arthritis. As these peptides are too costly for animal trials, we are presently engineering more economical peptide-based compounds for future in vivo studies.
Acknowledgments
This work was supported by NIH grant CA74120 and the Michigan Arthritis Foundation to H.R.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ravetch JV, Kinet JP. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 2.Ivan E, Colovai AI. Human Fc receptors: critical targets in the treatment of autoimmune diseases and transplant rejections. Hum Immunol. 2006;67:479–491. doi: 10.1016/j.humimm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Sardjono CT, Mottram PL, Hogarth PM. The role of FcγRIIa as an inflammatory mediator in rheumatoid arthritis and systemic lupus erythematosus. Immunol Cell Biol. 2003;81:374–381. doi: 10.1046/j.1440-1711.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- 4.Wijngaarden S, van Roon JA, van de Winkel JG, Bijlsma JW, Lafeber FP. Down-regulation of activating Fcγ receptors on monocytes of patients with rheumatoid arthritis upon methotrexate treatment. Rheumatol. 2005;44:729–734. doi: 10.1093/rheumatology/keh583. [DOI] [PubMed] [Google Scholar]
- 5.Wijngaarden S, van Roon JA, Bijlsma JW, van de Winkel JG, Lafeber FP. Fc receptor expression levels on monocytes are elevated in rheumatoid arthritis patients with high erythrocyte sedimentation rate who do not use anti-rheumatic drugs. Rheumatol. 2003;42:681–688. doi: 10.1093/rheumatology/keg174. [DOI] [PubMed] [Google Scholar]
- 6.Mathsson L, Lampa J, Mullazehi M, Ronnelid J. Immune complexes from rheumatoid arthritis synovial fluid induce FcγRlla dependent and rheumatoid factor correlated production of tumor necrosis factor-α by peripheral blood mononuclear cells. Arthritis Res Therapy. 2006;8:R64. doi: 10.1186/ar1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullazehi M, Mathsson L, Lampa J, Ronnelid J. Surface-bound anti-type II collagen-containing immune complexes induce production of tumor necrosis factor α, interleukin-1β, and interleukin-8 from peripheral blood monocytes via Fcγ receptor IIa. Arthritis Rheum. 2006;54:1759–1771. doi: 10.1002/art.21892. [DOI] [PubMed] [Google Scholar]
- 8.Sardjono CT, Mottram PL, ven de Velde NC, Powell MS, Power D, Slocombe RF, Wicks IP, Campbell IK, McKenzie SE, Brooks M, Stevenson AW, Hogarth PM. Development of spontaneous multisystem autoimmune disease and hypersensitivity to antibody-induced inflammation in Fcγ receptor IIa-transgenic mice. Arthritis Rheum. 2005;52:3220–3229. doi: 10.1002/art.21344. [DOI] [PubMed] [Google Scholar]
- 9.van Lent PL, Grevers L, Lubberts E, de Vries TJ, Nabbe KC, Verbeek S, Oppers B, Sloetjes A, Blom AB, van den Berg WB. Fcγ receptors directly mediate cartilage, but not bone, destruction in murine antigen-induced arthritis: uncoupling of cartilage damage from bone erosion and joint inflammation. Arthritis Rheum. 2006;54:3868–3877. doi: 10.1002/art.22253. [DOI] [PubMed] [Google Scholar]
- 10.Boross P, van Lent PL, Martin-Ramirez J, van der Kaa J, Mulder MH, Claassens JW, van den Berg WB, Arandhara VL, Verbeek JS. Destructive arthritis in the absence of both FcγRI and FcγRIII. J Immunol. 2008;180:5083–5091. doi: 10.4049/jimmunol.180.7.5083. [DOI] [PubMed] [Google Scholar]
- 11.Pietersz GA, Mottram PL, van de Velde NC, Sardjono CT, Esparon S, Ramsland PA, Moloney G, Baell JB, McCarthy TD, Matthews BR, Powell MS, Hogarth PM. Inhibition of destructive autoimmune arthritis in FcγRIIa transgenic mice by small chemical entities. Immunol Cell Biol. 2009;87:3–12. doi: 10.1038/icb.2008.82. [DOI] [PubMed] [Google Scholar]
- 12.Worth RG, Mayo-Bond L, van de Winkel JG, Todd RF, III, Petty HR, Schreiber AD. The cytoplasmic domain of FcγRIIA (CD32) participates in phagolysosome formation. Blood. 2001;98:3429–3434. doi: 10.1182/blood.v98.12.3429. [DOI] [PubMed] [Google Scholar]
- 13.Worth RG, Kim MK, Kindzelskii AL, Petty HR, Schreiber AD. Signal sequence within FcγRIIA controls calcium wave propagation patterns: Apparent role in phagolysosome fusion. Proc Natl Acad Sci, USA. 2003;100:4533–4538. doi: 10.1073/pnas.0836650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 15.Oehlke J, Scheller A, Wiesner B, Krause E, Beyermann M, Klauschenz E, Melzig M, Bienert M. Cellular uptake of an alpha-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim Biophys Acta. 1998;1414:127–139. doi: 10.1016/s0005-2736(98)00161-8. [DOI] [PubMed] [Google Scholar]
- 16.Scheller A, Oehlke J, Wiesner B, Dathe M, Krause E, Beyermann M, Melzig M, Bienert M. Structural requirements for cellular uptake of alpha-helical amphipathic peptides. J Pept Sci. 1999;5:185–194. doi: 10.1002/(SICI)1099-1387(199904)5:4<185::AID-PSC184>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 18.Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- 19.Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci. 2000;21:45–48. doi: 10.1016/s0165-6147(99)01429-7. [DOI] [PubMed] [Google Scholar]
- 20.Hallbrink M, Floren A, Elmquist A, Pooga M, Bartfai T, Langel U. Cargo delivery kinetics of cell-penetrating peptides. Biochim Biophys Acta. 2001;1515:101–109. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 21.Cole L, Davies D, Hyde GJ, Ashford AE. ER-Tracker dye and BODIPY-brefeldin A differentiate the endoplasmic reticulum and golgi bodies from the tubular-vacuole system in living hyphae of Pisolithus tinctorius. J Microsc. 2000;197(Pt 3):239–49. doi: 10.1046/j.1365-2818.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- 22.Via LE, Fratti RA, McFalone M, Pagan-Ramos E, Deretic D, Deretic V. Effects of cytokines on mycobacterial phagosome maturation. J Cell Sci. 1998;111(Pt 7):897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 23.Clark AJ, Petty HR. Observation of calcium microdomains at the uropod of living morphologically polarized human neutrophils using flash lamp-based fluorescence microscopy. Cytometry A. 2008;73:673–678. doi: 10.1002/cyto.a.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall H, Williams EJ, Moore SE, Walsh FS, Prochiantz A, Doherty P. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr Biol. 1996;6:580–587. doi: 10.1016/s0960-9822(02)00544-4. [DOI] [PubMed] [Google Scholar]
- 25.Williams EJ, Dunican DJ, Green PJ, Howell FV, Derossi D, Walsh FS, Doherty P. Selective inhibition of growth factor-stimulated mitogenesis by a cell-permeable Grb2-binding peptide. J Biol Chem. 1997;272:22349–22354. doi: 10.1074/jbc.272.35.22349. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham CC, Vegners R, Bucki R, Funaki M, Korde N, Hartwig JH, Stossel TP, Janmey PA. Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J Biol Chem. 2001;276:43390–43399. doi: 10.1074/jbc.M105289200. [DOI] [PubMed] [Google Scholar]
- 27.Klopfenstein DR, Kappeler F, Hauri HP. A novel direct interaction of endoplasmic reticulum with microtubules. EMBO J. 1998;17:6168–77. doi: 10.1093/emboj/17.21.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller-Taubenberger A, Lupas AN, Li H, Ecke M, Simmeth E, Gerisch G. Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. Embo J. 2001;20:6772–6782. doi: 10.1093/emboj/20.23.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 30.Jaconi ME, Lew DP, Carpentier JL, Magnusson KE, Sjogren M, Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990;110:1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marx V. Watching peptide drugs grow up. Chem Engin News. 2005;83:17–24. [Google Scholar]
- 32.Athanasou NA, Quinn J. Immunocytochemical analysis of human synovial lining cells: phenotypic relation to other marrow derived cells. Ann Rheum Dis. 1991;50:311–315. doi: 10.1136/ard.50.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]


