Abstract
The consistently observed inverse relationship of allergic conditions with glioma risk and our previous demonstration that IgE levels also were lower in glioma patients than controls suggest that atopic allergy may be related to a mechanism that inhibits or prevents glioma. We sought to extend these results with a new and larger series of patients (n=535 with questionnaire data; 393 with IgE measures) and controls (n=532 with questionnaire data; 470 with IgE measures). As expected, glioma cases were less likely than controls to report history of allergies (among self-reported cases, OR = 0.59, 95% CI: 0.41–0.85). IgE levels also were lower in glioma cases versus controls (OR per unit log IgE=0.89, 95% CI (0.82–0.98). However, this inverse relationship was only apparent among cases receiving temozolomide, a treatment which became part of the “standard of care” for glioblastoma patients during the study period. Among patients receiving temozolomide, IgE levels in cases whose blood samples were obtained within 30 days of diagnosis were slightly higher than controls, while IgE levels in cases whose blood sample was obtained >60 days after diagnosis were significantly lower than controls (OR = 0.80; 95% CI: 0.71–0.89). Thus, while our results robustly confirm the inverse association between allergy and glioma, the results for IgE are affected by temozolomide treatments which may have influenced IgE levels. These results have implications for the study of immunologic factors in glioma as well as for immunotherapy protocols for treating glioma.
INTRODUCTION
Approximately 14,000 patients are diagnosed with a glioma each year in the United States. The etiology of adult glioma is largely unknown and is thought to be multi-factorial; various genetic, infectious and immunological factors have been implicated1. Recent epidemiological studies have reported that adults with glioma are 1.5 to 4-fold less likely than controls to report a variety of allergies2–6, which ranks the lack of allergies among the most consistent risk factors for glioma reported to date. In addition, we previously reported an inverse relationship between immunoglobulin E (IgE), a biomarker for atopic allergy, and glioma risk7. We found poor concordance between self-reported allergy and IgE levels, and the strongest IgE-glioma association was observed among the least prevalent allergen – food IgE7. In addition, glioma patients who had elevated levels of IgE had approximately 8 months longer survival than individuals with lower or undetectable levels8, demonstrating potential clinical significance.
The goal of this current study was to utilize new population-based cases and controls to confirm our previous reports of an inverse relationship between self-reported allergy, IgE levels and glioma risk. However, during the new subject recruitment period, the standard of care for glioblastoma changed to include treatment with temozolomide (Temodar)9, a chemotherapy agent. Therefore, in addition to replicating our previous studies, the current analysis provides the first opportunity to evaluate the relationship between self-reported allergies, IgE levels and glioma risk in patients treated with temozolomide.
MATERIALS AND METHODS
Subjects, interviews, and specimen collection
Histologically confirmed gliomas (International Classification of Diseases for Oncology, morphology codes 9380–9481) diagnosed from November 2001 to September 2004 were identified using the Northern California Rapid Case Ascertainment program and included in this study. Eligible cases were aged 20 or older, had pathologically confirmed glioma, and resided in the 6 county San Francisco Bay Area, (Alameda, Contra Costa, Marin, San Mateo, San Francisco, and Santa Clara). Controls aged 20 years or older from the same residential area as cases were identified using random digit dialing and were frequency matched to cases based on age, gender and ethnicity. The University of California San Francisco Committee on Human Research approved the methods for this study (IRB approval H6539-04956-21A). We call this ascertainment series, “Series 3” to distinguish it from the previous recruitments, Series 1 (1992–4) and Series 2 (1997–2000).
In-person interviews with cases (or their proxies) and controls lasted approximately two hours and used a structured questionnaire and show cards. Subjects were offered a brief telephone interview if they declined the full in-person interview. The allergy history assessment questions were very similar to the ones we used for our Series 2 glioma study5.
Detailed information regarding history of allergies was collected in tabular form on 8 questionnaire pages. Data were collected by asking “Have you ever had reactions to” the following allergens: house dust, mold or mildew, pollens, poison oak/ivy, stinging or biting insects, eggs, dairy products, shellfish, wheat, peanuts or peanut butter, other nuts, soy, alcohol, coffee, other foods, toiletry items (soaps/detergents), cosmetics, deodorant, (perfumes/colognes/aftershave), cats, dogs, other animals, prescription and non-prescription drugs, tobacco smoke, and wool. Additional spaces were included in the questionnaire for “other” items that the patient identified as allergens but were not specifically asked for by name by the interviewer. Interviewers prompted subjects with show cards for each of the general allergen categories. For a “yes” response, the interviewer then asked whether the allergens produced any of the following symptoms also listed on show cards (runny nose, burning/watery eyes, sneezing/congestion, wheezing/asthma, rash/hives, itching, swelling/inflammation, nausea/vomiting, diarrhea, headaches, anaphylactic shock, other: specify).
The questionnaire also asked extensive information about family and personal medical history including asthma and eczema, demography, drugs used, and other personal information including smoking and diet.
Blood and sera were collected either at the time of interview or at a later time. Participants were asked on a separate blood draw questionnaire at that time about currently used medications and chemo- and radiation therapy. For analytical purposes, medications were classified into seventeen categories (Supplementary Table 1).
Treatment information (such as, temozolomide, other chemotherapy, biopsy versus resection, radiation therapy) for the brain tumor was obtained through medical record abstraction and SEER registry data. Additional information on drug treatments was obtained with a short questionnaire asking for a list of current medications that were being given at the time of blood draw.
We attempted to obtain pathological specimens for all glioma cases which were then reviewed and classified by an academic neuropathologist. To date, 484/535 cases have been reviewed, including 365 of 396 with IgE measurements. Kenneth Aldape (MD Anderson, n=318) or Tarik Tihan (UCSF, n=43), or both (n=4) reviewed these cases.
IgE measurements
IgE levels were assessed using a standardized clinical instrument designed for this purpose: Pharmacia Diagnostics UniCAP fluorescent “sandwich” assay10. Total, food, and respiratory allergens were measured. We compare some of the IgE measurements from an earlier series to the current series in the results section of this paper to help to clarify the basis of differences observed; please see the earlier papers5, 7 for details about Series 2 subjects and methods. IgE levels were determined at only a single time point for all SF Bay Area patients.
Longitudinal (Repeated) IgE Measurements
We examined the stability of IgE measurements in a single patient at six time points over one month. This patient was not a part of the SF Bay Area Glioma Study. This patient who has been previously reported by Heimberger, et al.11, had completed a 6-week daily temozolomide course with radiation therapy, a six-week EGFRvIII vaccine course without temozolomide, and three prior 1-month cycles with 5 days on temozolomide followed by 23 days off. On the 23rd day (when blood cell counts recovered) the EGFRvIII vaccine was administered. Blood for the current study was drawn during the 16th treatment cycle of sequential temozolomide and EGFRvIII vaccine. The patient did not have a history of atopic allergy prior to surgery.
Statistical methods
Odds ratios (OR) for cases versus controls reporting a history of allergy were estimated with logistic regression, controlling for age, gender, and ethnicity (white/non-white), education (college education) and smoking history. Odds ratios were estimated for all cases versus controls, self-reporting cases versus controls, and proxy-reported cases versus controls. Odds ratios also were computed for having any versus no history of allergy, by numbers of allergies reported (none, 1–3 and 4 or more), and by route/source of exposure, respiratory or food. Descriptive statistics and odds ratios were computed with SAS12.
IgE quantities were compared in cases versus controls based on the following categories:. 1) For total IgE, IgE levels > 100 kU/L are clinically “elevated”, 25–100 kU/L “borderline,” and < 25 kU/L “normal”; 2) for food and respiratory IgE, < 0.35 kU/L are termed “non-elevated,” and ≥ 0.35 kU/L, “elevated.” Continuous measures were determined by measuring fluorescence against the standard curve with known quantity inputs; these data also were log transformed to improve normality. Measures of IgE that fell under the lower limit were adjusted to 0.35 kU/L. Quartiles of IgE levels based on those of controls also were created.
Control for potential bias and confounders
A common difficulty for retrospective interview studies is the potential for reporting bias; that is, patients with disease might be more or less likely to recall, to fabricate, or to be prompted by interviewers to supply information that might have contributed to the etiology of their disease. This seems unlikely to happen for history of allergies which would not be commonly thought to affect glioma, or if they were, might be thought to be positively associated rather than negatively. Another bias could come from an estimate of lifetime incidence of allergy that may be biased by the age of the individual; that is, older individuals might provide less accurate information. Also, proxies might be less likely to know of and report temporally distant or minor allergies. To help control for these potential biases, odds ratios were adjusted for age, gender, and ethnicity, and results emphasize associations for self-reported cases. Odds ratios were also adjusted by education level attained (college degree/no college degree). Finally, to control for potential confounders, odds ratios were computed stratifying by tumor histopathology (glioblastoma or other glioma histologies), by self/proxy status, and by temozolomide treatment status, where indicated.
RESULTS
Of the 745 eligible cases, full interviews were obtained for 535 cases (72%) and abbreviated interviews for 27 (4%). Of the remaining eligible cases, there were language problems (n=3), the subject’s physician refused contact with the subject (n=4), the subject or a proxy could not be located (n=15), the subject or proxy refused (n=97), or they were not reported through Rapid Case Ascertainment (n=64). Of the 10,952 phone numbers dialed to obtain controls, 6% (n=600) identified an eligible control, 2,477 refused information before or after study introduction (23%) and the remaining numbers (71%) either had no response after 10 calls (25.7%) or did not identify an eligible subject due to language barrier (5.8%), business/fax/modem line (9.1%), line out of service (12.2%), etc. Of eligible controls, 92% (n=565) agreed to participate either in the full (n=532) or an abbreviated (n=33) interview. For the current analysis only subjects completing the full interview were included leaving 535 cases along with 532 controls (Table 1). For 129 of the 535 enrolled cases (24.2%), questionnaire data was reported by a proxy. Additional information on the distribution of ethnic groups, sex, age, education, income, smoking status, proxy status and histopathological diagnosis for cases and controls are presented in Table 1.
Table 1.
Description of Participants: Age, Gender, Ethnicity, Education, Income, Smoking History and Histology, San Francisco Bay Area Adult Glioma Study (2001–2005)
| Glioma Cases | Controls | Chi-square/t-test | |||
|---|---|---|---|---|---|
| All (n=535) | Self-reporting (n=406) | Proxy-reporting (n=129) | All (n=532) | p-value* | |
| Mean Age ± SE | 54.6 ± 0.7 | 50.9 ± 0.7 | 66.2 ± 1.1 | 53.8 ± 0.7 | 0.421 |
|
| |||||
| % White | 76 | 76 | 75 | 75 | 0.683 |
|
| |||||
| % Male | 56 | 58 | 52 | 52 | 0.151 |
|
| |||||
| % College Graduate | 51 | 54 | 38 | 59 | 0.007 |
|
| |||||
| Mean Education (yrs) ± SE | 14.9 ± 0.2 | 15.3 ± 0.2 | 13.7 ± 0.3 | 15.7 ± 0.1 | <0.001 |
|
| |||||
| Household Income (USD/yr) (%) | 0.537 | ||||
| <=$29,999 | 20 | 17 | 30 | 18 | |
| $30–49,999 | 16 | 13 | 24 | 15 | |
| $50–69,999 | 13 | 14 | 10 | 17 | |
| $70–99,999 | 16 | 18 | 12 | 18 | |
| $100,000+ | 31 | 35 | 19 | 32 | |
|
| |||||
| Smoking History (%) | 0.699 | ||||
| Never Smoked | 47 | 50 | 37 | 48 | |
| Past Smoker | 39 | 38 | 44 | 40 | |
| Current Smoker | 13 | 12 | 19 | 12 | |
|
| |||||
| Histology (%) | |||||
| Glioblastoma | 62 | 55 | 85 | NA | |
| Anaplastic Astrocytoma | 12 | 14 | 8 | NA | |
| Astrocytoma | 7 | 9 | 1 | NA | |
| Anaplastic Oligodendroglioma | 4 | 4 | 2 | NA | |
| Oligodendroglioma | 5 | 6 | 2 | NA | |
| Oligoastrocytoma | 1 | 2 | 0 | NA | |
| Ependymoma | 1 | 1 | 0 | NA | |
| Juvenile Pilocytic Astrocytoma | 2 | 2 | 0 | NA | |
| Medulloblastoma | 2 | 2 | 2 | NA | |
| Other | 4 | 5 | 1 | NA | |
| Astrocytoma NOS | 1 | 1 | 1 | NA | |
testing for difference between cases and controls. Chisquare used for categorical, and T-test for continuous variables.
Cases and controls did not differ significantly by age, percent white, sex, total years of education, or smoking history. Controls were more likely to have a college degree, (p=0.007, χ2). Within the cases, the proxy-reporting cases were significantly older than self-reporting cases (66 vs. 51 years, respectively p < 0.001), had a lower percentage of college graduates (38% vs. 54%, respectively p = 0.001), lower reported income (p < 0.001), significantly different smoking status (p = 0.019), and a higher percentage of glioblastoma multiforme diagnoses (p < 0.001). Proxy reporting and self-reporting cases did not differ by ethnicity or sex.
Allergy and Glioma
Glioma patients reported significantly fewer allergies than controls when pooled (OR = 0.50, 95% CI: 0.36–0.70, Table 2) or separated by proxy or self-reporting patients (OR = 0.28, 95% CI: 0.17–0.46, and OR =0.59, 95% CI: 0.41–0.85, respectively, Table 2). Adjustments were made for age, gender and ethnicity (white/non-white), college education and smoking history, which did not substantively change any allergy or IgE odds ratios. ORs were 0.58 for subjects reporting 1–3 allergies and 0.39 for subjects reporting 4 or more allergies, suggestive of a dose-response. There was a significant deficit of history of respiratory but not food allergies in cases compared to controls (Table 2).
Table 2.
Case-Control Odds Ratios for History of Allergy, San Francisco Bay Area Adult Glioma Study (2001–2005)
| Controls |
All cases |
Self-report cases |
Proxy Report cases |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR* (95% CI) | p-value | n | % | OR* (95% CI) | p- value |
n | % | OR* (95% CI) | p- value |
|
| Number of Allergies | ||||||||||||||
| 1 | 12 | 2 | ||||||||||||
| No allergies reported | 67 | 3 | 3 | 3 | 1.00 | 80 | 20 | 1.00 | 43 | 33 | 1.00 | |||
| 46 | 8 | 40 | 7 | 32 | <0.00 | |||||||||
| >= 1 allergy | 5 | 7 | 8 | 7 | 0.50 (0.36–0.70) | <0.001 | 2 | 80 | 0.59 (0.41–0.85) | 1 | 86 | 67 | 0.28 (0.17–0.46) | <0.001 |
|
| ||||||||||||||
| Number of Allergies | ||||||||||||||
| 1 | 12 | 2 | 19. | 33. | ||||||||||
| No allergies reported | 67 | 3 | 3 | 3 | 1.00 | 80 | 9 | 1.00 | 43 | 3 | 1.00 | |||
| 26 | 5 | 27 | 5 | 20 | 51. | 51. | ||||||||
| 1–3 allergies | 6 | 0 | 3 | 1 | 0.58 (0.41–0.82) | 0.002 | 6 | 2 | 0.66 (0.45–0.96) | 0.029 | 67 | 9 | 0.36 (0.22–0.61) | <0.001 |
| 19 | 3 | 13 | 2 | 11 | 28. | 14. | ||||||||
| 4+ allergies | 9 | 7 | 5 | 5 | 0.39 (0.27–0.57) | <0.001 | 6 | 9 | 0.50 (0.33–0.75) | 0.001 | 19 | 7 | 0.15 (0.08–0.29) | <0.001 |
|
| ||||||||||||||
| Respiratory Allergies | ||||||||||||||
| 19 | 3 | 26 | 5 | 18 | 44. | 65. | ||||||||
| No respiratory allergies | 9 | 8 | 5 | 1 | 1.00 | 0 | 8 | 1.00 | 85 | 9 | 1.00 | |||
| 32 | 6 | 25 | 4 | 21 | 53. | 33. | ||||||||
| >=1 respiratory allergy | 3 | 2 | 8 | 9 | 0.63 (0.49–0.81) | <0.001 | 5 | 5 | 0.73 (0.56–0.96) | 0.025 | 43 | 3 | 0.35 (0.23–0.54) | <0.001 |
|
| ||||||||||||||
| Food Allergies | ||||||||||||||
| 40 | 7 | 42 | 8 | 31 | 78. | 10 | 82. | |||||||
| No food allergies | 7 | 7 | 4 | 0 | 1.00 | 7 | 9 | 1.00 | 7 | 9 | 1.00 | |||
| 12 | 2 | 10 | 2 | 20. | 17. | |||||||||
| >= 1 food allergy | 1 | 3 | 6 | 0 | 0.88 (0.65–1.18) | 0.40 | 84 | 9 | 0.90 (0.66–1.24) | 0.538 | 22 | 1 | 0.77 (0.45–1.31) | 0.333 |
Models were adjusted for age, gender, ethnicity, college education and smoking history
Serum IgE and Glioma
Total IgE levels were available for 393 cases and 470 controls. The mean log transformed serum IgE levels were lower for cases (3.26 ± 0.08) than for controls (3.49 ± 0.07). The case-control OR was 0.89 (95% CI: 0.82–0.98) for each unit of increase in log IgE. When clinical categorical IgE levels were considered, IgE levels were lower among cases than controls, but failed to reveal a dose response. Categorical food and respiratory IgE yielded odds ratios lower than 1 (OR = 0.58 and 0.80, respectively), but in either case the 95% confidence intervals included 1 and were not significant (Table 3).
Table 3.
Case-Control Odds Ratios for IgE Levels, San Francisco Bay Area Adult Glioma Study (2001–2005)
| Controls |
All cases |
|||||
|---|---|---|---|---|---|---|
| n | mean ± SE | n | mean ± SE | OR* (95% CI) | p-value | |
| Ln (Total IgE)** | 470 | 3.49 ± 0.69 | 393 | 3.26 ± 0.079 | 0.89 (0.82–0.98) | 0.01 |
|
| ||||||
| n | % | n | % | OR* (95% CI) | p-value | |
|
| ||||||
| Total IgE | ||||||
| Normal, <25 | 199 | 42 | 197 | 50 | 1.00 | |
| Boderline, 25 to 100 | 165 | 35 | 111 | 28 | 0.66 (0.48–0.90) | 0.05 |
| Elevated, >100 | 106 | 23 | 85 | 22 | 0.79 (0.55–1.12) | 0.18 |
|
| ||||||
| Respiratory IgE | ||||||
| Nonelevated | 273 | 58 | 244 | 62 | 1.00 | |
| Elevated | 197 | 42 | 148 | 38 | 0.80 (0.60–1.06) | 0.12 |
|
| ||||||
| Food IgE | ||||||
| Nonelevated | 431 | 92 | 372 | 95 | 1.00 | |
| Elevated | 39 | 8 | 19 | 5 | 0.58 (0.33–1.03) | 0.06 |
|
| ||||||
| IgE Quartiles | ||||||
| Quartile 1 | 117 | 25 | 123 | 31 | 1.00 | 0.07*** |
| Quartile 2 | 118 | 25 | 90 | 23 | 0.69 (0.47–1.01) | 0.05 |
| Quartile 3 | 118 | 25 | 89 | 23 | 0.68 (0.47–1.00) | 0.05 |
| Quartile 4 | 117 | 25 | 91 | 23 | 0.70 (0.48–1.03) | 0.07 |
Models were adjusted for age, gender, ethnicity, college education and smoking history
Ln(IgE) was expressed as a continuous variable.
P-value for overall trend test.
We noted that in the current series, time from blood draw to laboratory processing was on average one day faster than the prior series (median 1 day between draw and freezing, as opposed to 2 day in prior series). This time is not different between cases and controls, but we tested whether it would have any difference to measured IgE levels. Sera from 3 healthy volunteers were drawn into multiple red-top tubes and stored at room temperature for 0, 1, 2, 3, and 4 days, then processed and frozen. IgEs were measured from the frozen sera; in all cases measurements did not decrease over time and IgE levels scored within 5% of the original “0 time” measurement, indicating that there is no detectable degradation of IgE in whole coagulated blood over the variable time frames that samples are processed. One of the three volunteers displayed “elevated” IgE, one high “borderline,” and the third, “normal.”
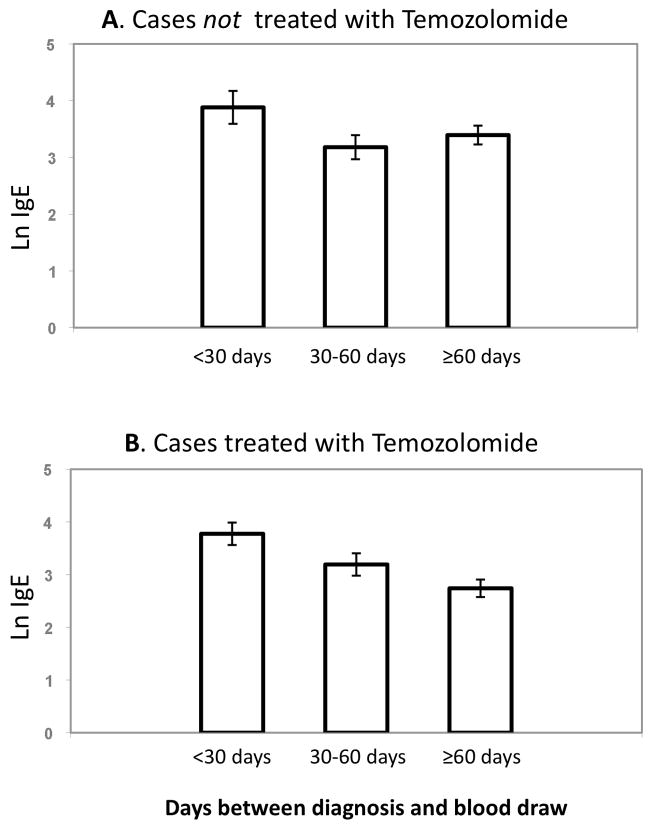
Since we were able to ascertain and interview cases in the current series an average of 43 days faster than the previous series (the average time from diagnosis to blood draw was 69 days in Series 3 vs. 112 days in Series 27), we also considered whether bloods drawn closer to the time of diagnosis had higher IgE levels, more similar to control levels. For patients taking temozolomide, there was an inverse relationship between IgE levels and time between diagnosis and blood draw; for cases not treated with temozolomide, the IgE levels were highest within 30 days of diagnosis but there was no additional decrease in levels >60 days from diagnosis (Figure 1).
Figure 1.
Relationship of IgE level in blood among cases and the length of time between diagnosis and blood draw. A. Series 3 patients, no temozolomide treatment. B: Series 3 patients who are on temozolomide therapy. Mean and standard error are shown. Using a general linear model, the trend of IgE was not significantly different among the three categories in no temozolomide treatment, (A, p = 0.15), but significant among the temozolomide-treated (B, p = 0.001).
Glioma medications
Most medications were prescribed to too few patients to substantially bias the case-control odds ratios (see Supplementary Table 1). However, large numbers of cases took temozolomide and dexamethasone. Those that were prescribed temozolomide (63% of our patient population) demonstrated significantly lower IgE levels; least square mean (LS mean) of IgE for temozolomide patients was 3.14 (SE = 0.13) compared to those not taking temozolomide (LS mean = 3.61, P = 0.006). Only the temozolomide-treated cases had lower IgE levels compared to controls, and this relationship held true for both glioblastoma and lower grade gliomas (Table 4). Patients taking temozolomide were more likely to be self-reporting and younger, but were not significantly different with regards to gender, ethnicity, or income (Table 5). Our study ascertainment period intersected closely with the introduction of temozolomide, as only 14% of glioblastoma cases were on the drug early in this case series, and 76% near the end (Table 5). IgE levels of people taking this drug were substantially lower with increasing time since diagnosis (Figure 1). Cases taking dexamethosome at the time of blood draw had a ln IgE mean of 3.32, and when temozolomide treated patients were removed, 3.47. For temozolomide-treated patients not on dexamethosone (only 33 patients), the mean ln IgE was 3.08. We did not detect significant effects of other medications, or other therapies on IgE levels (including surgery and radiation) when statistically adjusted for temozolomide treatment (data not shown).
Table 4.
Case-control odds ratios for IgE levels by Temodar Use and GBM status, San Francisco Bay Area Adult Glioma Study (2001–2005)
| Controls |
All Cases |
GBM |
Non-GBM |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR* (95% CI) | p- value |
n | % | OR* (95% CI) | p- value |
n | % | OR* (95% CI) | p- value |
|
| All Cases** | ||||||||||||||
| Total IgE | ||||||||||||||
| 19 | 5 | 11 | 5 | 8 | 4 | |||||||||
| Normal, <25 | 199 | 42 | 7 | 0 | 1.00 | 6 | 2 | 1.00 | 1 | 8 | 1.00 | |||
| 11 | 2 | 0.66 (0.48–0.90) | 2 | 0.56 (0.38–0.83) | 5 | 3 | 0.82 (0.54–1.25) | |||||||
| Borderline, 25 to 100 | 165 | 35 | 1 | 8 | 0.01 | 56 | 5 | 0.00 | 5 | 3 | 0.36 | |||
| 2 | 0.79 (0.55–1.12) | 2 | 0.81 (0.54–1.23) | 3 | 2 | 0.70 (0.43–1.16) | ||||||||
| Elevated, >100 | 106 | 23 | 85 | 2 | 0.18 | 52 | 3 | 0.33 | 3 | 0 | 0.17 | |||
|
| ||||||||||||||
| No Temodar Use | ||||||||||||||
| Total IgE | ||||||||||||||
| 4 | 4 | 4 | 4 | |||||||||||
| Normal, <25 | 199 | 42 | 62 | 6 | 1.00 | 21 | 5 | 1.00 | 1 | 7 | 1.00 | |||
| 3 | 0.77 (0.49–1.22) | 3 | 0.81 (0.39–1.67) | 2 | 3 | 0.77 (0.45–1.34) | ||||||||
| Borderline, 25 to 100 | 165 | 35 | 40 | 0 | 0.27 | 14 | 0 | 0.57 | 6 | 0 | 0.36 | |||
| 2 | 0.93 (0.57–1.53) | 2 | 1.11 (0.51–2.38) | 2 | 2 | 0.83 (0.45–1.53) | ||||||||
| Elevated, >100 | 106 | 23 | 32 | 4 | 0.78 | 12 | 6 | 0.80 | 0 | 3 | 0.55 | |||
|
| ||||||||||||||
| Used Temodar | ||||||||||||||
| Total IgE | ||||||||||||||
| 12 | 5 | 5 | 3 | 4 | ||||||||||
| Normal, <25 | 199 | 42 | 1 | 4 | 1.00 | 88 | 6 | 1.00 | 3 | 9 | 1.00 | |||
| 2 | 0.57 (0.39–0.83) | 2 | 0.46 (0.30–0.72) | 2 | 3 | 0.90 (0.50–1.61) | ||||||||
| Borderline, 25 to 100 | 165 | 35 | 60 | 7 | 0.00 | 35 | 2 | 0.00 | 5 | 7 | 0.72 | |||
| 1 | 0.62 (0.40–0.96) | 2 | 0.69 (0.43–1.11) | 1 | 0.45 (0.20–1.00) | |||||||||
| Elevated, >100 | 106 | 23 | 43 | 9 | 0.03 | 34 | 2 | 0.13 | 9 | 3 | 0.05 | |||
Models were adjusted for age, gender, ethnicity, college education, and smoking history.
Includes cases with unknown Temodar status (35 total).
Table 5.
Temodar use by various characteristics and by histologic type (excluding those with unknown temodar use) N=479, San Francisco Bay Area Adult Glioma Study (2001–2005)
| GBM |
Non-GBM |
|||||||
|---|---|---|---|---|---|---|---|---|
| Used Temodar | No Temodar | Used Temodar | No Temodar | |||||
| n | row % | n | row % | n | row % | n | row % | |
| Ethnicity | ||||||||
| Non-White | 41 | 63 | 24 | 37 | 21 | 41 | 30 | 59 |
| White | 145 | 62 | 88 | 38 | 53 | 41 | 77 | 59 |
|
| ||||||||
| Age | ||||||||
| <50 | 52 | 76 | 16 | 24 | 48 | 41 | 70 | 59 |
| 50–59 | 46 | 68 | 22 | 32 | 14 | 47 | 16 | 53 |
| 60–69 | 58 | 69 | 26 | 31 | 9 | 47 | 10 | 53 |
| 70+ | 30 | 38 | 48 | 62 | 3 | 21 | 11 | 79 |
|
| ||||||||
| Gender | ||||||||
| Female | 71 | 55 | 58 | 45 | 28 | 35 | 53 | 65 |
| Male | 115 | 68 | 54 | 32 | 46 | 46 | 54 | 54 |
|
| ||||||||
| Proxy Status | ||||||||
| Self report | 154 | 76 | 48 | 24 | 71 | 43 | 95 | 57 |
| Proxy report | 32 | 33 | 64 | 67 | 3 | 20 | 12 | 80 |
|
| ||||||||
| Blood collection status | ||||||||
| No blood collected | 27 | 29 | 65 | 71 | 7 | 26 | 20 | 74 |
| Blood collected | 159 | 77 | 47 | 23 | 67 | 44 | 87 | 56 |
|
| ||||||||
| Total IgE | ||||||||
| IgE missing | 29 | 31 | 65 | 69 | 7 | 26 | 20 | 74 |
| Normal IgE | 88 | 81 | 21 | 19 | 33 | 45 | 41 | 55 |
| Borderline IgE | 35 | 71 | 14 | 29 | 25 | 49 | 26 | 51 |
| Elevated IgE | 34 | 74 | 12 | 26 | 9 | 31 | 20 | 69 |
|
| ||||||||
| Diagnosis Year | ||||||||
| Dx Year 2001 | 2 | 14 | 12 | 86 | 2 | 29 | 5 | 71 |
| Dx Year 2002 | 49 | 60 | 32 | 40 | 22 | 31 | 49 | 69 |
| Dx Year 2003 | 67 | 59 | 46 | 41 | 32 | 48 | 35 | 52 |
| Dx Year 2004 | 68 | 76 | 22 | 24 | 18 | 50 | 18 | 50 |
|
| ||||||||
| Allergy History | ||||||||
| No Reported Allergy History | 48 | 59 | 33 | 41 | 11 | 42 | 15 | 58 |
| Reported Allergy History | 138 | 64 | 79 | 36 | 63 | 41 | 92 | 59 |
|
| ||||||||
| Education | ||||||||
| Not a College Graduate | 89 | 57 | 67 | 43 | 33 | 40 | 50 | 60 |
| College Graduate | 97 | 68 | 45 | 32 | 41 | 42 | 57 | 58 |
bold percentages indicate that the chi-square p-value is less than 0.05.
Cohort effects
Besides the introduction of temozolomide, another difference between our two series pertains to the birth cohort of participants. We note that the greatest difference between cases and controls in Series 2 is the age 53–65 central age tertile, OR = 0.58 (95% CI: 0.45–0.76, Supplementary Table 2). The median age of these controls is age 59, which corresponds to a median birthdate of 1939. For Series 3, the corresponding cohort tertile shifts 9 years forward with a median birthdate of 1948. Individuals within this same age tertile in Series 3, who were not treated with temozolomide, did not demonstrate reduced IgE (OR = 0.94, 95% CI:0.70–1.26, Supplementary Table 2).
Seasonality
We analyzed whether there were differences in seasonality of blood draws. Due to recruiting schedules, slightly more controls were ascertained in January-April time frame. Pollen levels are highest in our study area (San Francisco Bay Area) in the months of March-June. However, when we included the season of blood draw variable in the case-control model, ORs were not changed (data not shown). We also considered the question, “Have you had an allergic reaction in the past month?” An answer “yes” to this question was not predictive for high IgE levels among controls (data not shown).
Repeated measurements
We performed repeat measurements for a single patient who was not part of the SF Bay Area Glioma Study. IgE levels in this patient remained nearly constant at 30 kUnits/liter, which is a clinical “low borderline” level for allergy. This patient did not have any history of allergy, and the IgE were not reactive to respiratory (Phadiotop) or food (Fx5) allergens (data not shown). Given that IgE half-life in serum is only 36 hours, this indicated that IgE production is not sensitive to the hematotoxic or hemato-recovery cycles induced by the temozolomide treatments. The steady state of IgE is a contrast to varied T-regulatory cell, CD8+ cell counts, and other immune cell counts measured with the same blood samples (Supplementary Table 4, and reference 11).
DISCUSSION
This manuscript describes a case-control replication study in which, due to a change in the standard of care, the majority of cases received an immune-modulating factor: temozolomide. The inverse association of self-reported allergy and glioma (including a dose-response) here was nearly exactly the same as our previous series, indicating a robust association5, 7. IgE levels were also inversely related to glioma although far more weakly than we previously observed and most of the inverse relationship was confined to analyses including only cases who had received temozolomide as a treatment. This inverse association may also be impacted by length of temozolomide treatment (Figure 1). Despite this introduction of temozolomide as the standard of care, the prevalence of elevated IgE levels among cases was paradoxically increased in the current series (Series 3), reducing IgE case control differences observed previously7. Self-reported allergies were more frequent in the current series among cases (20% in Series 3 vs. 13% in Series 2), suggesting some potential cohort differences between the Series, which may also impact IgE levels. Both new treatments and a potential new cohort of individuals in Series 3 have then complicated this replication study with regards to IgE as a variable impacting glioma status.
During the early stages of glioma treatment, temozolomide is given daily, typically over the same time course as radiation treatments. Following initial treatment, temozolomide is prescribed in 5-day “on” and 23 day “off” recovery periods. It appears to be during this subsequent period that IgE levels were lower, and case-control odds ratios become significant (Figure 1, Supplementary Table 3). As these were cross-sectional data, the possibility remains that the lower levels were due to other unmeasured factors. A similar loss of immune function parameters (CD4 T-helper cells) was found in a study of melanoma patients treated with temozolomide over a longer period13. CD4 cells were not significantly lost after the first treatment cycle but became more severe with subsequent cycles13, mirroring the lymphopenia effect noticed in glioblastoma patients9 and the fall in IgE shown here (Figure 1). Whether temozolomide may actively suppress IgE levels cannot be answered with the current data series and requires a study of repeat measurements during therapy. The one patient with repeat measurements here indicated that IgE levels are stable over a one month period during later stages of treatment, and are not sensitive to changes in T-regulatory cells among other cell types (Supplementary Table 4). This patient does not inform what happens to IgE during initial therapy, which is a question that will require additional studies.
We did not detect case-control differences in IgE levels with cases not treated with temozolomide, who comprised 37% of the patient cohort. No significant differences in gender, income, ethnicity, or histopathological diagnosis were found among patients who did not take temozolomide compared to those who did (Table 5). However, there was a strong inverse association of temozolomide therapy and age – older individuals were less likely to be prescribed temozolomide. In our previous series the relationship between IgE and case-control status was weakest in the oldest tertile (Supplementary Table 2). Assuming a similar profile for the current Series 3, we would expect less of an IgE difference between older cases and controls, which constitute the bulk of the patients not treated with temozolomide. This factor, combined with unknown clinical considerations directing the decision not to treat with temozolomide, make it difficult to compare the temozolomide treated and untreated patients. Like our previous study, we did not detect significant effects from dexamethosone on IgE levels, nor other medications outside of the cytotoxic chemotherapeutics.
A remaining possible explanation on IgE differences between the two cohorts may be that the patients themselves are fundamentally different in Series 3, having grown up in the post-World War II era. Interestingly, the prevalence of childhood allergies is quite different between these groups, reported at 13% in Series 2 and 20% in Series 3 (P = 0.02). This corresponds to the rise of suburbanization in the United States with concomitant rise in the prevalence of allergies14–16. Indeed, the average of 50 years of age for those with IgE measurements in Series 3 corresponds to birthdates between 1950 and 1954, which puts the bulk of participants at a post-WWII birth when modern trends including reduced exposure to microbes and endotoxins, intestinal parasites, and increased use in antibiotics, affected the prevalence of allergies and potentially affected the allergic axis in the interaction with brain tumors. The increases in allergies are attributed to the “hygiene hypothesis” which posits that the immune system has not had a normal early modulation from frequent infections, making it vulnerable to overreact to other environmental antigens17. This enhanced predisposition to overreact is sometimes referred to as the “missing immune deviation” hypothesis, since humans are born with a strong Th2-allergic-phenotype which is poised to develop balanced immunity in response to frequent infections early in life18. If the immune deviation to a balanced Th1/Th2 immune system does not occur early in life, then lifetime risk of allergic disease is increased. Our control population had the same IgE distribution in the current series as the prior series7, indicating that profound population shifts have not taken place; however, our cases had higher IgE levels in the current series. It may be possible that these cases are reacting to their tumor or to temozolomide treatment (initially) with higher IgE levels due to an enhanced predisposition to allergies due to their birth in the modern era. Further studies on a possible cohort effect are warranted.
For the current series we obtained sera from a higher proportion of cases than for our prior series – and ascertained them somewhat closer to diagnosis. We were also able to transport the blood on average one day faster (one vs. two days) from the field to the laboratory. We assessed whether time since diagnosis and transit time for blood was related to IgE levels, with no significant relationship found (when excluding patients who took temozolomide). We also performed a reconstruction experiment in the laboratory to assess whether IgE levels are affected by storing whole blood at room temperature. IgE levels did not perceptibly change during a four day period that blood was stored at room temperature. We also note that the improvement in transport time affected both cases and controls equally, so is unlikely to play a role in case-control differences. It is therefore highly unlikely that our improvement of sampling techniques would explain any differences between Series 2 and 3.
While we have emphasized the differences between the series, it is also important to note that many results were similar. Respiratory IgE had similar odds ratios in both series and reported respiratory allergy odds ratios were also similar and significant. In both series we observed much stronger odds ratios among proxy-compared to self-reported individuals; in both Series this is likely the result of reporting bias. Proxies may not know about allergies, amplifying the odds ratios; therefore self-report odds ratios are likely to be closer to the truth (Table 2). Case-control ORs for self-reported food allergies also did not differ between the series; however, one key difference was found in the relationship of food IgE between the two series (OR = 0.12 vs 0.88, respectively, Series 2 and 3). The Series 2 result may be a false positive finding due to small numbers. The relationship of reported respiratory allergens is more robust; it exhibited virtually the same relationship in both reports. This finding should refocus attention on allergens and allergic responses that enter via the nasal route, and away from the digestive route, which was not confirmed in the current analysis (Series 3). Interestingly, cytokines and other peptides administered intranasally can enter the brain directly19, presenting a possible direct effect on intracranial immune responses from respiratory allergy pathophysiology.
In sum, our current results on reported allergy and glioma are quite similar to our and others previous reports. Although we still observed overall that cases had lower levels of IgE than controls, the effect was only apparent in the temozolomide treated patients, and our cross-sectional analysis suggests that the lower levels of IgE in the patients could possibly be due to temozolomide treatment. To address differences between the series to the extent possible, we considered recruitment and sampling changes and differences in the population ascertained, including the later birth cohort of the Series 3 population. Our efforts do not fully explain the differences between the series, and cast some uncertainty on the capacity of IgE as an adequate biomarker to illuminate an immunologic mechanism that suppresses glioma, at least in the current temozolomide era. Less clear is the reason for an overall higher IgE levels in glioma patients compared to the previous series, which occurred despite the temozolomide IgE suppression that may take place over the course of treatment. This IgE suppression mirrors the loss of T-helper cell function as studied in a temozolomide-treated melanoma cohort13 and serves as a warning to those developing immune-mediated glioma treatments. Definitive evaluation of the role of IgE as a biomarker of glioma risk awaits results from a large cohort study that collects serum before diagnosis. Additionally, the complete effects of temozolomide and other therapies on IgE await the analysis of serial samples obtained before and throughout glioma therapy.
Supplementary Material
Acknowledgments
Thanks to the Northern California Cancer Center for glioma patient case finding and to the pathology departments of Alexian Hospital, Alta Bates Medical Center, Brookside, California Pacific Medical Center, DR Pinole, Eden Hospital, El Camino Hospital, Good Samaritan, Highland Hospital, John Muir, Kaiser Redwood City, Kaiser San Francisco, Kaiser Santa Teresa, Los Gatos Hospital, Los Medanos Hospital, Marin General, Merrithew, Mills Peninsula Hospital, Mt. Diablo Hospital, Mt. Zion Medical Center, Naval Hospital, O’Connor Hospital, Ralph K Davies Medical Center, Saint Louise, San Francisco General, San Jose, San Leandro, San Mateo County, San Ramon Valley, Santa Clara Valley, Sequoia, Seton Medical Center, St. Francis, St. Lukes, St. Rose, Stanford, Summit, UC San Francisco, Valley Livermore, Veterans Palo Alto, Veterans SF, and Washington Hospital for providing tumor specimens for review. NIH grants CA52689, CA097257, CA89032, ES06717, and ES04705. JLW is a Scholar of the Leukemia and Lymphoma Society of America.
Supported by NIH grants, R01CA52689 and P50CA097257. JLW is a Scholar of the Leukemia and Lymphoma Society of America.
References
- 1.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-oncol. 2002;4:278–99. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner AV, Linet MS, Fine HA, Shapiro WR, Selker RG, Black PM, Inskip PD. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int J Cancer. 2002;99:252–9. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 3.Schlehofer B, Blettner M, Preston-Martin S, Niehoff D, Wahrendorf J, Arslan A, Ahlbom A, Choi WN, Giles GG, Howe GR, Little J, Menegoz F, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int J Cancer. 1999;82:155–60. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Schwartzbaum J, Jonsson F, Ahlbom A, Preston-Martin S, Lonn S, Soderberg KC, Feychting M. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. Int J Cancer. 2003;106:423–8. doi: 10.1002/ijc.11230. [DOI] [PubMed] [Google Scholar]
- 5.Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609–15. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 6.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99:1544–50. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 7.Wiemels JL, Wiencke JK, Patoka J, Moghadassi M, Chew T, McMillan A, Miike R, Barger G, Wrensch M. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64:8468–73. doi: 10.1158/0008-5472.CAN-04-1706. [DOI] [PubMed] [Google Scholar]
- 8.Wrensch M, Wiencke J, Wiemels J, Miike R, Patoka J, Moghadassi M, McMillan A, Kelsey K, Aldape K, Lamborn KR, Parsa A, Sison JD, et al. Serum IgE, tumor EGFR expression and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66:4531–41. doi: 10.1158/0008-5472.CAN-05-4032. [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 10.Szeinbach SL, Barnes JH, Sullivan TJ, Williams PB. Precision and accuracy of commercial laboratories’ ability to classify positive and/or negative allergen-specific IgE results. Ann Allergy Asthma Immunol. 2001;86:373–81. doi: 10.1016/S1081-1206(10)62481-7. [DOI] [PubMed] [Google Scholar]
- 11.Heimberger AB, Sun W, Hussain SF, Dey M, Crutcher L, Aldape K, Gilbert M, Hassenbusch SJ, Sawaya R, Schmittling B, Archer GE, Mitchell DA, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SAS/STAT User’s Guide. 6. Cary, NC: SAS Institute, Inc; 1990. [Google Scholar]
- 13.Rietschel P, Wolchok JD, Krown S, Gerst S, Jungbluth AA, Busam K, Smith K, Orlow I, Panageas K, Chapman PB. Phase II study of extended-dose temozolomide in patients with melanoma. J Clin Oncol. 2008;26:2299–304. doi: 10.1200/JCO.2007.14.5292. [DOI] [PubMed] [Google Scholar]
- 14.Anderson HR, Gupta R, Strachan DP, Limb ES. 50 years of asthma: UK trends from 1955 to 2004. Thorax. 2007;62:85–90. doi: 10.1136/thx.2006.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isolauri E, Huurre A, Salminen S, Impivaara O. The allergy epidemic extends beyond the past few decades. Clin Exp Allergy. 2004;34:1007–10. doi: 10.1111/j.1365-2222.2004.01999.x. [DOI] [PubMed] [Google Scholar]
- 16.Von Hertzen LC, Haahtela T. Asthma and atopy-the price of affluence? Allergy. 2004;59:124–37. doi: 10.1046/j.1398-9995.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 17.Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–77. doi: 10.1016/j.jaci.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Romagnani S. Coming back to a missing immune deviation as the main explanatory mechanism for the hygiene hypothesis. J Allergy Clin Immunol. 2007;119:1511–3. doi: 10.1016/j.jaci.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH., 2nd Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience. 2008;152:785–97. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.