Abstract
To examine the long-term effects of stress experienced early in gestation on the programming of offspring feeding behaviors and energy balance, pregnant mice were exposed to stress during early pregnancy (days 1–7) and adult offspring examined on chow and high fat diets for long-term outcomes. Placental 11 β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) and insulin-like growth factor 2 (IGF-2) expression was measured to determine the possible sex-specific contribution of prenatal stress (PNS) on fetal programming of embryo growth and development during early pregnancy. PNS mice showed a basal hyperphagia when on chow diet. Prenatal treatment differences were ameliorated when adult mice were on a high fat diet. Interestingly, PNS male mice also had significantly reduced body weights compared to control males on both chow and high fat diets. Body composition analyses revealed reduced body fat and increased lean mass in PNS mice on the high fat diet, but no differences were detected in plasma leptin or insulin-like growth factor 1 (IGF-1) levels. Mechanistic examination of gene expression in embryonic day 12 placentas found that early PNS was associated with increased IGF-2 expression and sex-dependent effects of stress on 11 β-HSD2, supporting specific aspects of early pregnancy. These studies suggest that the long-term effects of stress during pregnancy on programming of feeding behavior and energy homeostasis begin much earlier in development than previously thought.
Keywords: early pregnancy, stress, high fat diet, metabolic programming
As the Barker hypothesis suggests, a mismatch between early developmental programming and postnatal environment may contribute to inappropriate responses to specific challenges [1–4]. Studies examining effects of prenatal stress have predominantly focused on late gestation when the embryo was undergoing the greatest period of growth to determine long-term offspring outcomes [5, 6]. Stress in the form of daily restraint during the last week of pregnancy produced decreased birth weights, glucose intolerance, and hyperglycemia [7, 8]. Further, repeated handling stress during the postnatal period resulted in increased adult food intake and body weights [9]. Together, these studies indicate late pregnancy or early postnatal life exposure to stress may be associated with an increased risk for obesity and type-2 diabetes development [10, 11]. While the outcomes of these perturbations are similar, the mechanism by which prenatal and postnatal events impact later-life obesity development may be different [12].
We have previously shown that mild prenatal stress produces temporally specific effects on offspring body weights and energy homeostasis dependent on the timing of the stress, either early, mid- or late in gestation, without altering maternal food intake, weight gain during pregnancy, offspring litter weights, litter size or maternal behavior [13]. These results support that developmental programming of pathways involved in regulation of energy balance may begin much earlier than previously thought [7–9, 14, 15]. Therefore, to investigate the effect of early pregnancy stress on long-term food intake and energy balance outcomes on low and high fat diets, we examined male and female offspring exposed to stress during days 1 – 7 of pregnancy. Further, mechanistic studies were conducted to identify potential targets of prenatal stress that may be influencing this early gestational programming. Elucidation of fetal antecedents contributing to long-term changes in energy homeostasis may provide insight into how individual differences in behavior and physiology may occur.
Methods
Animals
Mice of a mixed C57Bl/6:129 background were used in these studies. Mice were housed under a 12 hr light/day (L/D) photoperiod with a standard chow diet (Purina Rodent Chow, St. Louis, MO; 28.1% protein, 59.8% carbohydrate, 12.1% fat) and water available ad libitum except where noted. All studies were done according to experimental protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Breeding
Twelve male and 26 virgin female mice (1 litter/female) were mated as adults to produce offspring (n = 68) used in these experiments. For experimental testing, one litter is n = 1. Three females and one male were placed together 3 hrs prior to lights out. At the start of the next light cycle, each female was inspected for the presence of a copulation plug. Evidence of a copulatory plug denoted day 1 of gestation and the female was immediately individually housed and randomly distributed to a stress or control treatment group.
Prenatal Stress Paradigm
Pregnant mice were randomly assigned to receive chronic, variable stress during the first 7 days of pregnancy (PNS) or to control non-stressed groups (Ctrl). Prenatal stress entailed exposure of mice once per day for 7 days to a different stressor and included: 36 h of constant light, novel noise overnight, 5 min restraint stress, 15 min predator odor exposure, novel object (marbles) overnight, multiple cage changes, and damp bedding overnight [13]. These mild stressors were designed to produce a stress response but not induce pain or directly influence maternal food intake, weight gain or behavior as previously described [13]. In addition, litter size and the sex ratio of female:male pups were not altered with the prenatal stress.
High Fat Diet Exposure
Offspring within prenatal stress treatment were randomly group housed 2–3 per cage at weaning. When mice reached 6–8 weeks of age, all mice were randomly assigned to receive ad libitum access to either a high fat diet (Research Diets, New Brunswick, NJ; 15.8% protein, 44.2% carbohydrates, and 45.0% fat; 4.73 kcal/g) or remained on standard chow (Purina Rodent Chow, St. Louis, MO; 28.1% protein, 59.8% carbohydrate, 12.1% fat; 4.00 kcal/g) for 17 weeks (Male, Ctrl: chow n = 9, high fat n = 6; PNS: chow n = 11, high fat n = 11; Female, Ctrl: chow n = 6, high fat n = 7; PNS: chow n = 7, high fat n = 8). All mice were given food pellets in the food hopper and on the cage floor.
Food Intake
Twenty-four hour food intake was measured for each cage once per week to calculate caloric intake and total caloric efficiency (n = 3–4 cages). Caloric efficiency was calculated as weight gained divided by total calories consumed. Within each sex, a 2-way ANOVA followed by Fisher’s PLSD comparison was used to assess the effect of prenatal stress and diet on caloric intake and efficiency. All data are reported as mean ± SEM.
Body Weights
Mice were weighed daily 4 hrs prior to lights off until sacrifice (n = 6–11 per group). A 2-way repeated measures ANOVA followed by Fisher’s PLSD comparisons was used to assess the effect of prenatal stress and diet on body weight.
Glucose Levels
Fasting glucose levels following an overnight (12 hr) fast were determined monthly during diet exposure (n = 6–11 per group). Samples were measured using a Lifescan One Touch Glucometer from tail blood (Johnson and Johnson, Milpitas, CA). Within each sex, a 2-way ANOVA was used to assess the effect of prenatal stress and diet on fasting glucose levels.
nMRI
Offspring body lean and fat mass percentages were compared by nMRI (EchoMRI 3-in-1 from Echo Medical Systems, LLC, Houston, TX) 24 hrs prior to sacrifice at the University of Pennsylvania Metabolic Core, Institute of Diabetes, Obesity and Metabolism. Briefly, mice were placed for 1 min into the nMRI machine and scanned (n = 6–11 per group). The total weight of both lean and fat mass was measured and used to calculate percentage of lean and fat mass based on body weight. Within each sex, a 2-way ANOVA was used to assess the effect of prenatal stress and diet on fat and lean mass.
Leptin and IGF-I
To determine the impact of prenatal stress and high fat diet exposure on basal leptin and insulin-like growth factor 1 (IGF-1) levels, a blood sample was collected immediately following decapitation following 4 mo of diet exposure. Blood samples were centrifuged, plasma collected and frozen at −80 C until the assays were performed. Plasma levels were measured by radioimmunoassay kit for leptin (LINCO Research, Inc., St. Louis, MO) or IGF-1 (Diagnostic Systems Laboratories, Webster, TX) on a subset of mice (n = 5–6 per group). The protocol was modified to use 50 μl of plasma for each assay. The intra-assay coefficient of variation was less than 6% for leptin, and less than 7% for IGF-1.
BAT Biochemistry
Western blot analysis was used to compare brown adipose tissue (BAT) uncoupling protein-1 (UCP-1, Calbiochem) levels between control and PNS mice on either a high fat diet or chow diet (n = 4) [16]. BAT was removed from male and female mice at sacrifice. Densitometric analysis was performed using IPLab (BD Biosciences/Scanalytics, San Jose, CA) and comparisons were made within each blot. Blots were stripped and re-analyzed with β-actin for normalization. Student’s t-tests within each blot were conducted to determine differences in UCP-1 levels between treatment groups.
Placental Real-Time PCR
To determine gene expression profiles following prenatal stress, 1 male and 1 female placentas (n = 16) from a second set of 8 dams were taken at embryonic day 12 (E12), when placenta formation is complete (Male: Ctrl: n = 5, PNS n = 4; Female: Ctrl: n = 3, PNS n = 4). In addition, waiting 5 days after the end of prenatal stress allows measurement of gene expression changes that are the result of accumulated prenatal stress and not an indicator of the most recent stressor experienced. To determine the sex of the embryo associated with each placenta, genomic DNA was isolated from embryos and PCR was performed to check for the presence or absence of the Y-chromosome specific Sry gene (15,869: 5′ CAG CCC TAC AGC CAC ATG AT 3′; 15,870: 5′ GAG TAC AGG TGT GCA GCT CTA 3′) [17]. Placentas were added to TRIzol reagent (Invitrogen, Calsbad, CA) and briefly sonicated prior to total RNA isolation with chloroform and precipitation with isopropanol. RNA was resuspended in RNAse free water and the concentration measured on a spectrophotometer (Eppendorf AG, Hamburg, Germany). cDNA was transcribed using the SuperScript First-Strand Synthesis System for RT-PCR with random primers (Invitrogen, Carlsbad, CA). Changes in 11-beta-hydroxysteroid dehydrogenase-2 (11β-HSD2) and insulin-like growth factor-2 (IGF-2) gene expression were assessed using TaqMan Gene Expression Assay (Applied Biosystems, Forster City, CA). Each assay provided specific primers for the gene of interest and the manufacture’s protocol was followed. β-actin was measured for each sample as an endogenous control, and the cycle threshold subtracted from the target threshold value. All samples and controls were run in duplicate using the Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems). Raw ΔCT values were converted to fold change calculating 2(−ΔCT) for each sample.
Results
Food Intake
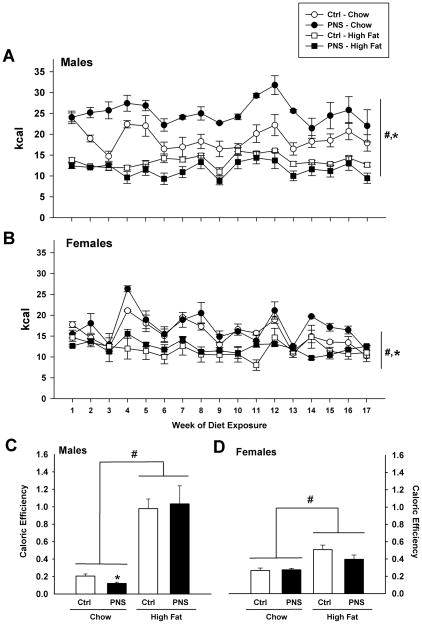
Caloric intake and total caloric efficiency values were calculated from 24 hr food intake measurements. Caloric intake was calculated per cage (n = 3–4). Male and female mice, regardless of prenatal stress treatment, had significantly greater caloric intake on a chow diet than mice on the high fat diet as previously reported (Males: F(1, 35) = 52.562, p < 0.001; Females: F(1, 28) = 44.603, p < 0.001) (Fig. 1A&1B) [18]. PNS mice on a chow diet had significantly greater caloric intake than controls on the same diet (F(1, 27) = 5.318, p = 0.01, main effect of prenatal treatment) (Fig. 1A&1B). PNS males also showed a significant reduction in caloric efficiency on a chow diet compared to control males (F(1,13) = 7.209, p < 0.05) (Fig. 1C). No differences in caloric efficiency were found for PNS females compared to control females on chow diet (F(1,11) = 1.411) (Fig. 1D). High fat diet increased caloric efficiency for both sexes regardless of treatment group (F(1,24) = 50.593, p < 0.0001) (Fig. 1C&D).
Fig. 1. PNS mice are hyperphagic on a chow diet.
(A, B) PNS mice on a chow diet show significantly greater caloric intake than control mice over 4 months (*, p < 0.05, main effect of PNS treatment). Mice consume significantly fewer total calories while on the high fat diet (45% fat) compared to chow intake over 4 months (#, p < 0.001, main effect of diet). (C) PNS males on a chow diet show a significantly reduced caloric efficiency compared to control males (*, p < 0.05). (C) Male and (D) female mice on a high fat diet show greater caloric efficiency compared to chow diet (#, p < 0.0001, main effect of diet).
Body Weight
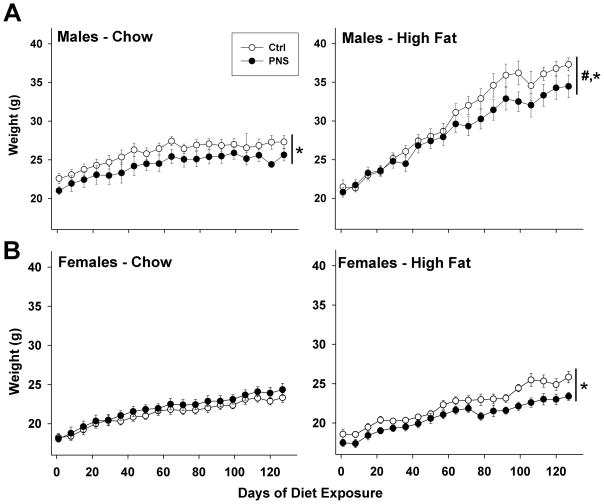
There were no effects of PNS treatment on start body weights (Male: F(1,34) = 0.305; Female: F(1,27) = 0.253) (Male Control: 20.8 ± 0.5 g, Male PNS: 20.6 ± 0.5 g; Female Control: 18.0 ± 0.4 g, Female PNS: 17.5 ± 0.5 g). PNS male mice on chow weighed significantly less than control males (F(1,4694) = 95.140, p < 0.0001) (Fig. 2A, left panel). Male mice on a high fat diet gained significantly more weight than males on chow (F(1,4694) = 814.442, p < 0.0001) (Fig. 2A, right panel). PNS females weighed significantly less than control females on a high fat diet (F (1, 3473) = 7.735, p < 0.01) (Fig. 2B, right panel).
Fig. 2. PNS attenuates weight gain on a high fat diet.
Body weights were measured during 4 months of high fat diet exposure compared to chow diet in male and female PNS and control mice. (A) PNS male mice weighed significantly less than control males on both chow and high fat diets (*, p < 0.0001, main effect of PNS treatment). All males on a high fat diet gained more weight than males on a chow diet (#, p < 0.0001, main effect of diet). No differences were found between PNS and control male start weights at 6–8 wks of age. End body weights were significantly increased for males on a high fat diet compared to chow diet males (#, p < 0.0001, main effect of diet). (B) Female mice on a high fat diet weighed significantly more than females on a chow diet (#, p < 0.0001, main effect of diet). No main effect of PNS treatment was detected over 4 months of diet exposure. However, examination of the last 60 days of diet exposure detected a significant reduction in weight gain for PNS females on a high fat diet compared to control females (*, p < 0.001, main effect of PNS treatment for high fat diet).
Glucose Levels
Fasting glucose levels were measured to assess the impact of prenatal stress and high fat diet on glucose maintenance (Table 1). Following one and two months of high fat diet exposure in male mice, there were no significant differences in fasting glucose levels (Month 1: F(1,33) = 2.476; Month 2: F(1,33) = 0.484). Male mice exposed to three and four months of a high fat diet had significantly elevated glucose levels (Month 3: F(1,33) = 41.176, p < 0.0001; Month 4: F(1,33) = 19.515, p < 0.0001). There was no impact of PNS in male mice on glucose levels (Month 1: F(1,33) = 1.288; Month 2: F(1,33) = 1.853; Month 3: F(1,33) = 0.152; Month 4: F(1,33) = 0.048). Following one, two and three months of high fat diet exposure, female mice had significantly elevated glucose levels (Month 1: F(1,25) = 25.103, p < 0.05; Month 2: F(1,25) = 24.042, p < 0.0001; Month 3: F(1,25) = 9.913, p < 0.01).
Table 1.
Monthly fasting glucose levels in mice
| MONTH |
||||||
|---|---|---|---|---|---|---|
| ONE | TWO | THREE | FOUR | |||
| Male | Chow | Ctrl | 104.6 ± 7.5 | 105.3 ± 7.7 | 82.3 ± 3.7 | 106.2 ± 8.9 |
| PNS | 114.7 ± 8.6 | 126.7 ± 2.9 | 86.6 ± 6.2 | 100.3 ± 6.2 | ||
| High Fat | Ctrl | 130.6 ± 6.8 | 120.4 ± 6.0 | 133.5 ± 6.3* | 132.6 ± 5.3* | |
| PNS | 107.8 ± 6.3 | 118.6 ± 5.6 | 133.4 ± 9.7* | 137.9 ± 7.8* | ||
| Female | Chow | Ctrl | 101.6 ± 6.6 | 106.1 ± 8.5 | 86.6 ± 6.6 | 98.3 ± 5.3 |
| PNS | 93.1 ± 7.5 | 90.0 ± 7.0 | 81.1 ± 5.5 | 101.0 ± 11.6 | ||
| High Fat | Ctrl | 108.9 ± 6.8* | 147.7 ± 9.8* | 111.1 ± 7.5* | 119.6 ± 5.4 | |
| PNS | 116.8 ± 6.0* | 124.1 ± 4.4* | 98.5 ± 6.3* | 103.1 ± 6.2 | ||
All results are shown as mg/dl.
Main effect of high fat diet (Males: p < 0.0001; Females: p < 0.01).
Male mice exposed to a high fat diet for three and four months show significantly greater fasting glucose levels (*, p < 0.0001). No effect of PNS treatment was detected for male mice on either chow or high fat diet. Female mice show an early impact of high fat diet on fasting glucose levels with significantly greater levels at one, two and three months (*, p < 0.01). These differences were not detected at four months of diet exposure. No effect of PNS treatment was observed.
nMRI
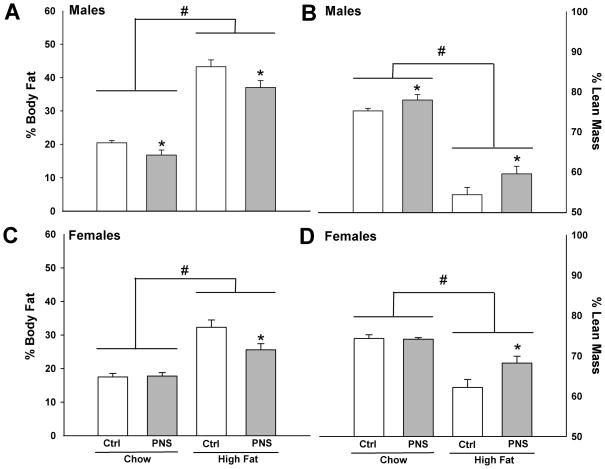
Percent body fat and lean mass was measured to determine the impact of PNS and high fat diet exposure on body composition. Following 4 mo on chow or high fat diets, PNS male mice had significantly lower percentages of body fat and higher percentages of lean mass (Fat mass: F(1,32) = 5.438, p < 0.05; Lean mass: F(1,32) = 4.327, p < 0.05) (Fig. 3A&B). PNS female mice showed a decreased percentage of body fat and increased lean mass on a high fat diet compared to control females (Fat mass: F(1,13) = 5.475, p < 0.05; Lean mass: F(1,13) = 4.3931, p < 0.05) (Fig. 3C&D). No differences were found for PNS females on chow diet (Fat mass: F(1,11) = 0.676; Lean mass: F(1,11) = 0.226).
Fig. 3. PNS mice have reduced body fat and increased lean mass.
PNS males had a significantly (A) lower percentage of body fat and (B) increased lean mass compared to control males on either chow or high fat diets (*, p < 0.01). PNS females had a significantly (C) lower percentage of body fat and a (D) higher percentage of lean mass than controls on a high fat diet (*, p < 0.05). High fat diet increased the percent body fat and reduced the percent lean mass in all groups (#, p < 0.0001, main effect of diet).
Leptin and IGF-1
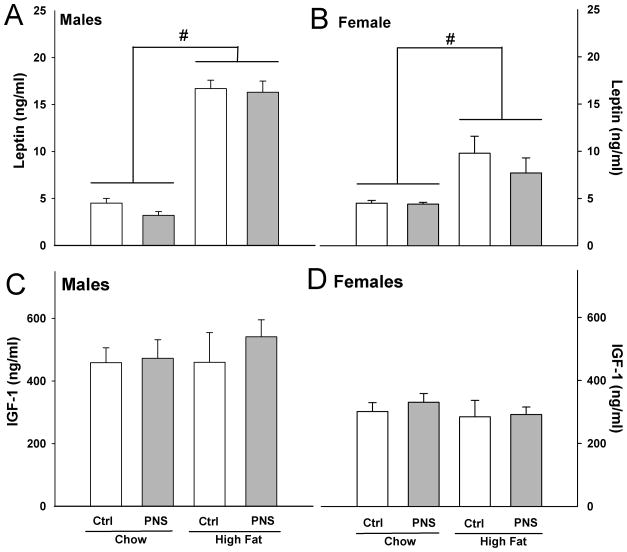
The impact of PNS and high fat diet on plasma leptin and IGF-1 levels was measured. High fat diet exposure increased leptin levels in male and female mice compared to mice on chow diet (Male: F(1,18) = 208.792, p < 0.0001; Female: F(1,16) = 12.001, p < 0.01) (Fig. 4A&B). No differences were found for leptin levels between treatment groups (Male: F (1,18) = 0.043; Female: F(1,18) = 0.482). No significant effects of treatment group or diet were found for IGF-1 levels in male or female mice (Male treatment group: F(1,15) = 0.389, Male diet: F(1,15) = 0.294; Female treatment group: F(1,16) = 0.185, Female diet: F(1.16) = 0.526) (Fig. 4C&D).
Fig. 4. Leptin and IGF-1 levels were not affected by PNS treatment.
(A) Male and (B) female mice, regardless of PNS treatment, had increased plasma leptin levels following 4 mo on a high fat diet (#, p < 0.0001, main effect of diet). No differences were found in IGF-1 levels on either chow or high fat diets for (C) male or (D) female mice.
BAT Biochemistry
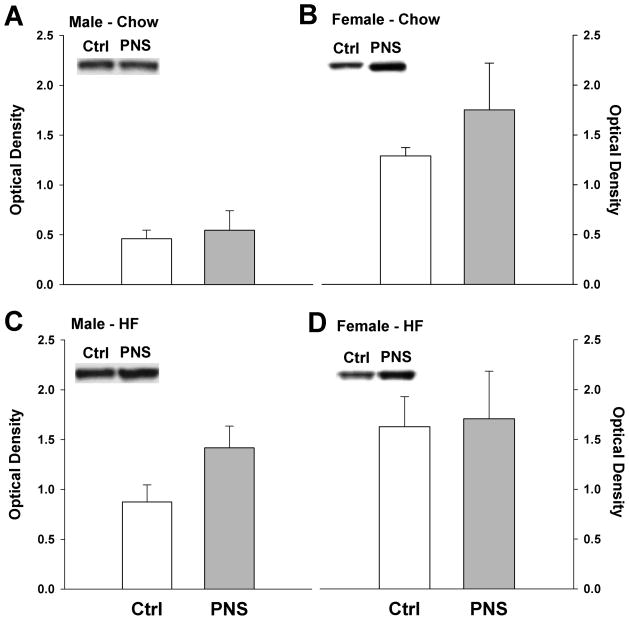
As PNS mice showed increased caloric intake but reduced weight gain, UCP-1 levels in BAT were examined using western blot analysis as a marker of thermogenesis. PNS male mice showed a trend for increased UCP-1 on a high fat diet compared to control males (p = 0.08) (Fig. 5A&C). No differences were detected for males on a chow diet (p = 0.358). In PNS females, there was a nonsignificant trend for PNS females to have increased UCP-1 levels on a chow diet (p = 0.07), but no differences were detected between groups on a high fat diet (p = 0.28) (Fig. 5B&D).
Fig 5. UCP-1 levels in brown adipose tissue in control and PNS mice.
(A) Male PNS mice on a chow diet showed no differences in BAT UCP-1 levels. (B) PNS female mice showed a trend for increased UCP-1 on a chow diet compared to control females (p = 0.07). (C) Male PNS mice on a high fat diet showed a trend for increased UCP-1 compared to control males (p = 0.08). (D) No differences were found for UCP-1 levels in females on a high fat diet.
Placental Real-Time PCR
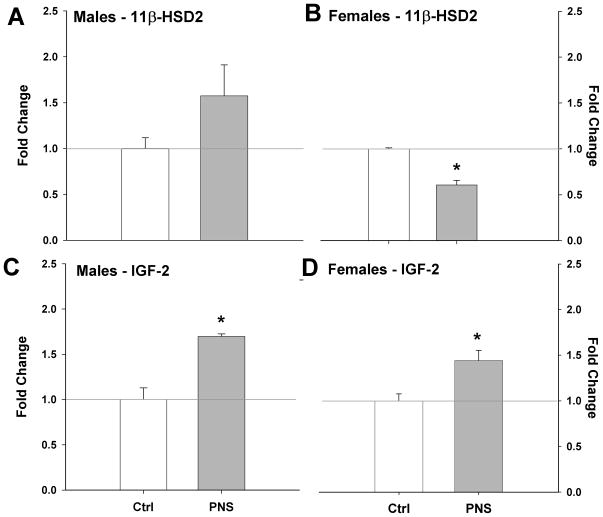
Expression of 11β-HSD2 and IGF-2 in PNS male and female E12 placentas was examined as a possible contributing mechanism of early gestational stress on long-term offspring outcome. PNS male placentas showed a trend for increased expression of 11β-HSD2 (p = 0.09) (Fig. 6A). PNS female placentas showed the opposite effect with a significant reduction in expression of 11β-HSD2 (p < 0.005) (Fig. 6B). PNS significantly increased IGF-2 expression in male (p < 0.01) and female (p < 0.05) placentas compared to controls (Fig. 6C&D).
Fig. 6. Altered placental expression of 11 β-HSD2 and IGF-2 at E12 in PNS mice.
(A) Male 11 β-HSD2 levels showed a trend to be elevated with PNS (p = 0.09). (B) PNS significantly reduced 11 β-HSD2 in female placentas (*, p < 0.05). IGF-2 levels were significantly increased in PNS (C) male and (D) female placentas compared to controls (**, p < 0.01).
Discussion
Fetal antecedents including influences from maternal stress and nutritional state have been associated with altered programming of offspring feeding and metabolic pathways [11, 14, 19, 20]. This programming has predominantly been examined across the late prenatal and early postnatal developmental window. As such, the impact related to early gestational experience has not been well characterized. We examined the long-term outcome of stress during the first seven days of pregnancy on multiple indices of feeding and metabolic function in male and female offspring, and mechanistically examined gene expression changes in placental tissue from these mice as predictors of effects of this stress on embryo growth and development [21].
In examination of caloric intake in adult offspring exposed to early prenatal stress (PNS), both male and female PNS mice were hyperphagic compared to controls on a chow diet. These results are consistent with previous work examining late pregnancy stress that has been associated with intra-uterine growth restriction and long-term obesity risk [7, 22]. Offspring hyperphagia in intra-uterine growth restriction is ascribed to a compensation occurring for a poor nutritional state during the portion of pregnancy where there is the greatest embryo growth and brain development [1, 23]. However, as we have previously reported no significant differences in birth or growth weights or maternal behavior in our early PNS model, the hyperphagia detected in these mice as adults may be the result of a distinct mechanism related to this early period in development [5, 13, 24]. To further determine feeding responses during the challenge of a high caloric environment, mice were also examined during exposure to a high fat diet for 4 months. Interestingly, the high fat diet ameliorated the hyperphagia in both male and female PNS mice. The increased fat content in the high fat diet (45%) may induce a greater satiation than the chow diet (12%), resulting in the reduced intake of PNS mice.
In examination of the diet and treatment group differences in body weights during diet exposure, we detected reduced weights in PNS males compared to control mice on the chow and high fat diets. These results were surprising in light of the hyperphagia detected in this group on the chow diet. Subsequently, PNS male mice also showed a significant reduction in caloric efficiency on the chow diet. However, on the high fat diet, PNS males normalized their caloric efficiency suggesting that the increased fat intake provided adequate energy storage ability in these mice. PNS female mice also weighed less than control females, but this was only significant on the high fat diet. We have previously reported that PNS mice do not show alterations in locomotor activity, for either total distance traveled or speed of travel, suggesting that the observed weight differences were likely not due to treatment effects on activity levels [25]. The disparity between food intake and weight gain in PNS mice suggests possible basal alterations in energy storage or utilization in these mice.
Following four months of high fat diet exposure, both PNS and control male mice had significantly elevated fasting glucose levels compared to chow groups. Control females showed an earlier detectable rise in fasting glucose following 2 months of the high fat diet, but no effect of diet was found in fasting glucose in PNS females. Increased adiposity is thought to be a contributing factor to diet-induced insulin insensitivity. Body composition for adiposity and lean body mass was analyzed in these mice following 4 months of diet exposure. Regardless of diet, PNS males had significantly reduced body fat and higher lean mass than control males. Female PNS mice also showed reduced body fat and increased lean mass when exposed to a high fat diet. In examination of plasma leptin levels no differences in leptin were found between PNS and controls, despite group differences in adiposity following 4 months of high fat diet exposure. As different adipose depots can differentially release leptin, these data show discordance between changes in adiposity and circulating leptin may support depot-specific adipose storage in PNS mice [26, 27]. Future studies will examine specific fat pads in these mice, results that were not obtainable from our nMRI analyses. As previous studies examining intrauterine perturbations have established an important role for insulin-like growth factors in development, growth and offspring long-term regulation of energy balance, we also measured IGF-1 levels in adult PNS mice on chow and high fat diets [28, 29]. No differences were detected in IGF-1 for treatment or diet groups for male or female mice.
Uncoupling protein 1 (UCP-1) levels in brown adipose tissue were measured as an indicator of non-shivering thermogenesis to determine if PNS mice may be increasing their energy utilization [30]. Surprisingly, there were no significant differences between treatment groups on either a chow or a high fat diet. However, following four months of high fat diet PNS males showed a non-significant trend for increased UCP-1 compared to control mice suggesting a potential increase in energy expenditure that would account for the decreased body weights and lower adiposity in these mice while on the high fat diet. As we have previously reported that there are no treatment differences in locomotor activity in these mice, possible effects of PNS on programming of indices directly affecting metabolic rate should be explored in future studies [31].
Mechanistically, how perturbations during early pregnancy influence fetal programming of long-term caloric intake and energy balance is not understood. Alterations in the maternal hormonal milieu resulting from maternal stress likely impinge on the development and function of the placenta which forms during this critical period [31]. As placental transport is the essential mechanism whereby maternal information gains access to the fetal compartment, effects on local hormone and growth factor production can directly impact growth and development and have been associated with a predisposition for metabolic syndrome, growth restriction and hypertension in offspring [32, 33]. To determine possible contributing factors that may have influenced the programming of offspring exposed to stress during early gestation, we examined expression levels of the glucocorticoid-inactivating enzyme 11 β-hydroxysteroid dehydrogenase type II (11β-HSD2) and the insulin-like growth factor -2 (IGF-2) in placentas at embryonic day 12, a point at which the placenta is fully developed and is 5 days following the last stressor experienced. As 11β-HSD2 is highly enriched in the placenta through late pregnancy to protect the fetus from exposure to maternal glucocorticoids, changes in levels of this enzyme would be predictive of a possible influence of maternal stress on fetal development [34, 35]. We detected a surprising sex-dependent effect of stress on placental 11β-HSD2 expression. Males showed a non-significant trend to increase expression, while female placentas had a significant reduction in this enzyme, supporting a possible mechanism contributing to sex differences in long-term outcome following early PNS exposure. Recent studies examining gene expression patterns in human placenta have also reported a correlation of fetal sex with genes related to growth and development that may influence programming of sex difference outcomes [36].
The insulin-like growth fact system, including IGF-2, is critical in regulation of placental and embryonic growth and development [29, 37, 38]. As an imprinted gene, IGF-2 is also positioned to be sensitive to developmental perturbations and to exert long-term influences on fetal programming and outcome. Decreased IGF-2 expression has been linked to both IUGR and long-term obesity development [14, 29, 39]. Further, glucocorticoids are a major mechanism for regulating IGF-2 during development and in relation to intrauterine perturbations [40]. Following early PNS, we found a significant increase in placental IGF-2 in both male and female placentas. Placenta IGF-2 regulates expression of key transporters, including those for glucose and amino acids, supporting a possible mechanism whereby elevated placental IGF-2 following PNS exposure could influence metabolic programming via alterations in nutrient delivery to the growing fetus [29, 41–43]. We have previously reported that mice exposed to our early PNS paradigm do not show differences in gestational length, litter size, or birth weight suggesting that the possible impact of elevated IGF-2 is not via a global effect on absolute growth, but rather may be acting to re-program circuitry regulating energy balance, as suggested by the Barker hypothesis [1, 13]. We have recently reported that early prenatal stress increased male placental expression of glucose transporter 4 (GLUT4) and insulin-like growth factor binding protein 1 (IGFBP-1), further supporting effects of maternal stress on nutrient and growth support of the developing fetus that are sex specific even at the level of the placenta [44].
These results indicate that stress early in pregnancy has a long-term impact on feeding behavior and energy metabolism in offspring that mechanistically may result from altered placental gene expression important in long-term fetal programming. While previous studies have highlighted the effects of late gestational and early postnatal stress on offspring outcome, our results illustrate that sensitivity to such fetal antecedents may begin far earlier in pregnancy than previously thought, essentially re-programming the offspring for increased food intake and energy utilization [16, 19, 45–47]. While this overall phenotype suggests a beneficial effect of reduced weight gain, suggestive of a predictive adaptive response, under certain environmental conditions such as famine this phenotype would likely result in a disadvantaged state for the organism.
Acknowledgments
This work was supported by a joint collaborative grant from AstraZeneca (Wilmington, DE).
References
- 1.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–7. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 5.Symonds ME, Stephenson T, Gardner DS, Budge H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev. 2007;19:53–63. doi: 10.1071/rd06130. [DOI] [PubMed] [Google Scholar]
- 6.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond) 2007;113:1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 7.Lesage J, Del-Favero F, Leonhardt M, Louvart H, Maccari S, Vieau D, Darnaudery M. Prenatal stress induces intrauterine growth restriction and programmes glucose intolerance and feeding behaviour disturbances in the aged rat. J Endocrinol. 2004;181:291–6. doi: 10.1677/joe.0.1810291. [DOI] [PubMed] [Google Scholar]
- 8.Barlow SM, Knight AF, Sullivan FM. Delay in postnatal growth and development of offspring produced by maternal restraint stress during pregnancy in the rat. Teratology. 1978;18:211–8. doi: 10.1002/tera.1420180206. [DOI] [PubMed] [Google Scholar]
- 9.Vallee M, Mayo W, Maccari S, Le Moal M, Simon H. Long-term effects of prenatal stress and handling on metabolic parameters: relationship to corticosterone secretion response. Brain Res. 1996;712:287–92. doi: 10.1016/0006-8993(95)01459-4. [DOI] [PubMed] [Google Scholar]
- 10.Jones RH, Ozanne SE. Intra-uterine origins of type 2 diabetes. Arch Physiol Biochem. 2007;113:25–9. doi: 10.1080/13813450701318484. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–98. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- 12.Thompson NM, Norman AM, Donkin SS, Shankar RR, Vickers MH, Miles JL, Breier BH. Prenatal and postnatal pathways to obesity: different underlying mechanisms, different metabolic outcomes. Endocrinology. 2007;148:2345–54. doi: 10.1210/en.2006-1641. [DOI] [PubMed] [Google Scholar]
- 13.Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav. 2006;88:605–14. doi: 10.1016/j.physbeh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–88. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 15.Nyirenda MJ, Seckl JR. Intrauterine events and the programming of adulthood disease: the role of fetal glucocorticoid exposure (Review) Int J Mol Med. 1998;2:607–14. doi: 10.3892/ijmm.2.5.607. [DOI] [PubMed] [Google Scholar]
- 16.Carlin KM, Vale WW, Bale TL. Vital functions of corticotropin-releasing factor (CRF) pathways in maintenance and regulation of energy homeostasis. Proc Natl Acad Sci U S A. 2006;103:3462–7. doi: 10.1073/pnas.0511320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–77. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Teegarden SL, Bale TL. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol Behav. 2008:713–23. doi: 10.1016/j.physbeh.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–33. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- 20.Vickers MH, Krechowec SO, Breier BH. Is later obesity programmed in utero? Curr Drug Targets. 2007;8:923–34. doi: 10.2174/138945007781386857. [DOI] [PubMed] [Google Scholar]
- 21.De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrinol Metab. 2007;293:E75–82. doi: 10.1152/ajpendo.00689.2006. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. J Intern Med. 2007;261:437–52. doi: 10.1111/j.1365-2796.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- 23.Desai M, Gayle D, Han G, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci. 2007;14:329–37. doi: 10.1177/1933719107303983. [DOI] [PubMed] [Google Scholar]
- 24.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol Endocrinol Metab. 2007;293:E1012–20. doi: 10.1152/ajpendo.00649.2006. [DOI] [PubMed] [Google Scholar]
- 27.Montague CT, Prins JB, Sanders L, Digby JE, O’Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–7. doi: 10.2337/diab.46.3.342. [DOI] [PubMed] [Google Scholar]
- 28.Vickers MH, Ikenasio BA, Breier BH. IGF-I treatment reduces hyperphagia, obesity, and hypertension in metabolic disorders induced by fetal programming. Endocrinology. 2001;142:3964–73. doi: 10.1210/endo.142.9.8390. [DOI] [PubMed] [Google Scholar]
- 29.Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm Res. 2006;65 (Suppl 3):50–8. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- 30.Bale TL, Anderson KR, Roberts AJ, Lee KF, Nagy TR, Vale WW. Corticotropinreleasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology. 2003;144:2580–7. doi: 10.1210/en.2002-0091. [DOI] [PubMed] [Google Scholar]
- 31.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–52. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav. 2006;88:234–43. doi: 10.1016/j.physbeh.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 33.Moritz KM, Boon WM, Wintour EM. Glucocorticoid programming of adult disease. Cell Tissue Res. 2005;322:81–8. doi: 10.1007/s00441-005-1096-6. [DOI] [PubMed] [Google Scholar]
- 34.Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- 35.Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151 (Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 36.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103:5478–83. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–12. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 38.Forbes K, Westwood M. The IGF axis and placental function. a mini review. Horm Res. 2008;69:129–37. doi: 10.1159/000112585. [DOI] [PubMed] [Google Scholar]
- 39.Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab. 2005;86:84–90. doi: 10.1016/j.ymgme.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–76. [PubMed] [Google Scholar]
- 41.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–8. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 42.Constancia M, Angiolini E, Sandovici I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley CP, Reik W, Fowden A. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102:19219–24. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GJ, Fowden AL, Constancia M. Placental-specific insulin-like growth factor 2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proc Natl Acad Sci U S A. 2004;101:8204–8. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–65. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinsley C, Svare B. Prenatal stress effects: are they mediated by reductions in maternal food and water intake and body weight gain? Physiol Behav. 1986;37:191–3. doi: 10.1016/0031-9384(86)90405-1. [DOI] [PubMed] [Google Scholar]
- 46.Grove KL, Grayson BE, Glavas MM, Xiao XQ, Smith MS. Development of metabolic systems. Physiol Behav. 2005;86:646–60. doi: 10.1016/j.physbeh.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 47.Dulloo AG, Seydoux J, Jacquet J. Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiol Behav. 2004;83:587–602. doi: 10.1016/j.physbeh.2004.07.028. [DOI] [PubMed] [Google Scholar]