Abstract
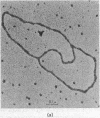
Intermediates involved in the replication of double-stranded ϕX174 RF DNA have been identified and partially characterized. Analysis of pulselabeled RF DNA suggests that the synthesis of progeny RF molecules involves, in part, the addition of nucleotides to linear complementary strands on a circular parental strand as template, so as to produce intermediate DNA strands of greater than viral length. Electron microscopy reveals DNA rings with “tails” and “double rings,” which could be the intermediate structures. A model is postulated for the replication process.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Kidson C. Deoxyribonucleic acid secondary structure in the region of the replication point. J Mol Biol. 1966 May;17(1):1–9. doi: 10.1016/s0022-2836(66)80089-x. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. Vegetative lambda DNA. IV. Fractionation of replicating lambda DNA on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1969 Mar 28;40(3):467–490. doi: 10.1016/0022-2836(69)90166-1. [DOI] [PubMed] [Google Scholar]
- Knippers R., Komano T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XXI. Replication and fate of the replicative form. Proc Natl Acad Sci U S A. 1968 Feb;59(2):577–581. doi: 10.1073/pnas.59.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Salivar W. O., Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXVI. Transfer of the parental DNA of bacteriophage phiX174 into progeny bacteriophage particles. J Mol Biol. 1969 Feb 14;39(3):641–654. doi: 10.1016/0022-2836(69)90150-8. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phi X174. 23. DNA synthesis in cells infected by a coat protein mutant. J Mol Biol. 1968 Aug 14;35(3):591–605. doi: 10.1016/s0022-2836(68)80016-6. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- Smith M. G., Burton K. Fractionation of deoxyribonucleic acid from phage-infected bacteria. Biochem J. 1966 Jan;98(1):229–241. doi: 10.1042/bj0980229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]
- Yarus M. J., Sinsheimer R. L. The process of infection with bacteriophage phiX174. 8. Evidence for an essential bacterial "site". J Virol. 1967 Feb;1(1):135–144. doi: 10.1128/jvi.1.1.135-144.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]