Abstract
The authors have previously demonstrated a neuroprotective mechanism of facial motoneuron (FMN) survival after facial nerve transection that is dependent on CD4+T helper 2 (Th2) cell interactions with peripheral antigen presenting cells, as well as central nervous system (CNS) resident microglia. Pituitary adenylyl cyclase activating polypeptide is expressed by injured FMN and increases Th2-associated chemokine expression in cultured murine microglia. Collectively, these data suggest a model involving CD4+ Th2 cell migration to the facial motor nucleus after injury via microglial expression of Th2-associated chemokines. In this study, the authors tested the hypothesis that Th2-associated chemokine expression occurs in the facial motor nucleus after facial nerve axotomy at the stylomastoid foramen. Initial microarray analysis of Th2-associated and Th1-associated chemokine mRNA levels was accomplished after facial nerve axotomy in wild type (WT) and presymptomatic mutant superoxide dismutase 1 (mSOD1) [model of familial amyotrophic lateral sclerosis (ALS)] mice. Based on that initial microarray analysis, the Th2-associated chemokine, CCL11, and Th1-associated chemokine, CXCL11, were further analyzed by RT-PCR. The results indicate that facial nerve injury predominantly increases Th2-associated chemokine, but not Th1-associated chemokine mRNA levels in the mouse facial motor nucleus. Interestingly, no differences were detected between WT and mSOD1 mice for CCL11 and CXCL11 after injury. These data provide a basis for further investigation into Th2-associated chemokine expression in the facial motor nucleus after FMN injury, which may lead to more specifically targeted therapeutics in motoneuron diseases, such as ALS.
Keywords: CCL11, CXCL11, Neuroprotection, Chemokine
INTRODUCTION
Our laboratory has previously demonstrated a positive role for CD4+ T cells in facial motoneuron (FMN) survival after peripheral facial nerve axotomy at the stylomastoid foramen in the mouse.1,2 Raivich et al.3 have found a maximum increase in T-cell infiltration of the axotomized mouse facial motor nucleus between 7 and 21 days after injury. In agreement with those findings by Raivich et al.,3 our laboratory has shown that CNS resident microglia are necessary to reactivate CD4+ T cells centrally,4 suggesting the existence of a mechanism for peripheral CD4+ T cell recruitment into the brainstem from the draining cervical lymph nodes.
Once activated, naïve CD4+ T cells differentiate into interleukin 4 positive (IL-4+) T helper 2 (Th2) or interferon gamma (IFN-γ)+ Th1 effector cells.5 From recent work in our laboratory we found that both Th2 and Th1 subsets develop in draining lymph nodes after facial nerve axotomy,6 but that the Th2, not the Th1, effector subset is necessary for immune mediated-neuroprotection.7 Additionally, Armstrong et al.8 demonstrated increased mRNA levels for pituitary adenylyl cyclase activating polypeptide (PACAP) mRNA levels in FMNs after facial nerve axotomy. As cultured murine microglia exposed to PACAP increase Th2-associated chemokine expression,9 the data suggest that, following a peripheral nerve injury outside the blood–brain-barrier (BBB), microglia, which are capable of antigen presentation centrally,10 interact with neuroprotective Th2 cells through a mechanism of recruitment dependent on Th2-associated chemokine expression.
Chemokines, otherwise known as chemotactic cytokines, are named because of their ability to recruit cells that express the corresponding chemokine receptor. Unlike T cell homing in the lymph nodes and gut, chemokines responsible for T cell recruitment of normal CNS have not been identified.11 In models of peripheral nerve injury in which the BBB remains intact, however, chemokines have been shown to be expressed by FMN,12,13 dorsal root ganglion sensory, and spinal neurons,14 as well as glia.15 Furthermore, antibody-mediated chemokine neutralization after peripheral nerve injury decreases leukocyte homing of the CNS,16 confirming the hypothesis that CNS-expression of chemokines is capable of facilitating peripheral to central compartment recruitment. However, to date, few chemokines have been studied in the context of motoneuron injury or as a result of neurodegenerative disease progression.
Dysregulated expression of the macrophage-recruiting chemokine, CCL2, has been demonstrated in spinal cord and cerebrospinal fluid (CSF) of patients with the degenerative motoneuron disease, amyotrophic lateral sclerosis (ALS).17,18 Mutant superoxide dismutase 1 (mSOD1) mice with familial-like ALS pathology have also been shown to exhibit aberrant CCL2 expression.19 However, data are lacking regarding the expression of other chemokines in the mSOD1 mouse model. Based on the neuroprotection of injured FMN by CD4+Th2 cells7 and requirement of CD4+ T cell interaction with microglia,4 as well as the accelerated motoneuron death in mSOD1 mice during CD4+ T cell deficiency,20 we hypothesized that Th2-associated chemokine expression increases in the facial motor nucleus after peripheral facial nerve injury in wild type (WT) and mSOD1 mice.
In this report, we used microarray analysis to investigate Th2-associated and Th1-associated chemokine (Figure 1) mRNA levels in the facial motor nucleus at 7 days post-axotomy (dpa); a time point consistent with significant T cell infiltration.3 Based on microarray analysis, we focused our investigation on the Th2-associated chemokine, CCL11, and Th1-associated chemokine, CXCL11.
Figure 1.
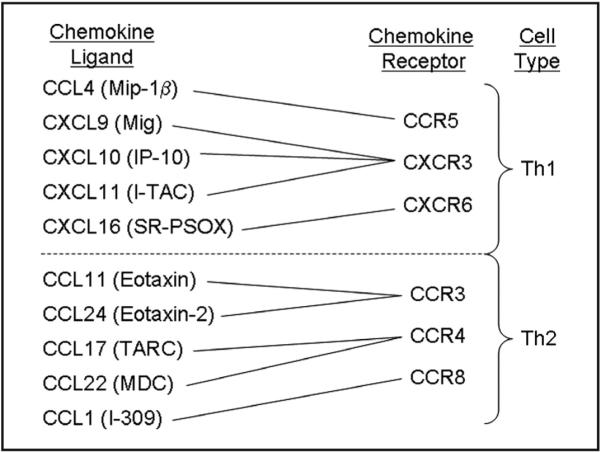
Diagram of chemokines and chemokine receptors associated with the recruitment of Th2 and Th1 cells. T-helper cell recruitment is dependent on chemokine receptor expression and tissue expression of chemokines. Chemokines that recruit Th2 cells (Th2-associated chemokines) and Th1 cells (Th1-associated chemokines) and the receptor that they act on are shown. Chemokine abbreviations shown include: Mip-1β, macrophage inflammatory protein; Mig, monokine induced by gamma interferon; IP-10, interferon inducible protein; I-TAC, interferon inducible T cell α chemoattractant; SR-PSOX, scavenger receptor that binds phosphatidylserine and oxidized lipoprotein; TARC, thymus and activation-related chemokine; and MDC, macrophage-derived chemokine.21-23
MATERIALS AND METHODS
Animals and surgical procedures
Seven-week-old female C57BL/6 and transgenic mice harboring the G93A mutant of human superoxide dismutase 1 (mSOD1) (B6.Cg-Tg(SOD1*G93A)1Gur) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were provided autoclaved pellets and water ad libitum. Mice were permitted 1 week to acclimate to their environment before being manipulated and used at 8 weeks of age in all experiments. All experimental manipulations were performed approximately 4 hours into the light cycle under aseptic conditions. All surgical procedures were completed in accordance with National Institutes of Health guidelines on the care and use of laboratory animals for research purposes. Mice were anesthetized with 3 percent isoflurane for all surgical procedures. Using aseptic techniques, the right facial nerve of each animal was exposed and transected at its exit from the stylomastoid foramen.24 The distal nerve stump was pushed away from the proximal nerve stump, thereby preventing reconnection of the two transected ends of the facial nerve. Behavioral observations were used to ensure that reconnection of the facial nerve was prevented. None of the animals in the present study showed any signs of recovering from unilateral facial paralysis after complete transection of the facial nerve.
Chemokine gene array
Seven days after right facial nerve transection, C57BL/6 mice (n = 4) were euthanized with CO2 and the brains rapidly removed. Coronal sections of brain stem including both facial motor nuclei were collected and tissue punches of the left (uninjured control) and right (axotomized) facial motor nuclei were obtained from these sections and pooled (right axotomy vs left control). The tissue punches were placed into 0.65 mL microfuge tubes with 20 × 1.4 mm2 Lysing Matrix D (Q-Biogene, Morgan Irvine, CA) and 80 μL working solution D (1 mL solution D and 7.2 μL β-mercaptoethanol). RNA was isolated from tissue punches and cells were harvested by guanidinium-thiocyanate extraction.25 Samples were processed at Superarray Bioscience Corporation (Frederick, MD) using the Mouse Chemokines and Receptors Microarray, OMM-022. Fold-changes in gene expression (right axotomy/left control) were calculated for pair-wise comparison using the GEArray Expression Analysis suite.
Laser microdissection
Mouse brains were removed uninjured or at 7, 14, and 30 dpa (n = 3-8 per timepoint) and flash frozen [in 62.5 percent n-butyl bromide (Fisher Scientific, Pittsburgh, PA) + 37.5 percent 2-methylbutane (Fisher Scientific) surrounded by crushed dry ice]. Frozen brains were sectioned on the Leica CM3000 cryostat (Leica, Bannockburn, IL) with a temperature of −24°C and immersed into Tissue Teck O.C.T. Compound (Sakura Finetek USA, Inc., Torrance, CA) at 25 μm intervals and thaw-mounted onto membrane-coated glass slides (Leica Microsystems Inc., Bannockburn, IL). Slides were stained for Nissl substance to visualize the facial motor nucleus and dehydrated sequentially: 100 percent EtOH (30 seconds), diethyl pyrocarbonate (DEPC)-treated H2O × 2 (30 seconds), working thionin (30 seconds), DEPC-treated H2O × 2 (30 seconds), 70 percent EtOH (30 seconds), 95 percent EtOH (30 seconds), and 100 percent EtOH (30 seconds). Slides were dried in a closed container for 3 minutes. Laser microdissection of left (control) and right (axotomized) facial motor nuclei using the Leica AS LMD (Leica Microsystems Inc.) was accomplished, capturing tissue into 0.5 mL microcentrifuge tube caps with 65 μL Extraction Buffer (PicoPure RNA Isolation kit, Arcturus, Mountain View, CA). After microdissection of each animal, microfuge tubes were spun at 800g for 2 minutes to collect cell extract and stored at −80°C.
RNA isolation and real–time PCR
A PicoPure RNA Isolation kit (Arcturus) was used to extract RNA and followed the manufacturer’s instructions. Complementary DNA was generated and used in real-time PCR reactions and amplification was detected with SYBR green fluorescent dye (Applied Biosystems, Carlsbad, CA). The following primers were obtained from Superarray Bioscience Corporation: CCL11 (NM 011330; PPM02967E-200), CXCL11 (NM 019494; PPM03192B-200), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (NM 008084; PPM02946E-200). GAPDH served as the reference gene. Amplification was performed using the iCycler iQ Detection System (Bio-Rad Laboratories, Hercules, CA) under the following conditions: 10 minutes at 95°C, followed by 40 cycles of 30 seconds at 95°C, 30 seconds at 54°C, and 30 seconds at 65°C. For each sample, percent change in CCL11 and CXCL11 mRNA levels were calculated using the formula (axotomy/control × 100) − 100 percent.
Statistical analysis
Data were analyzed using the analysis of variance method, followed by post hoc comparisons using the Newman-Keuls test.
RESULTS
Microarray analysis
At 7 dpa, mRNA levels were increased for three of the examined Th2-associated chemokines, CCL11, CCL22, and CCL24 (9.5-fold, 3.9-fold, and 2.8-fold, respectively), but not for two of the Th2-associated chemokines, CCL1 and CCL17, in wild-type mice (Figure 2). In contrast, none of the mRNA levels for the Th1-associated chemokines, CCL4, CXCL9, CXCL10, CXCL11, and CXCL16, were altered at 7 dpa in WT mice (n = 1). Thus, facial nerve axotomy appears to predominantly increase Th2-associated chemokine, but not Th1-associated chemokine mRNA levels in WT mouse facial motor nucleus.
Figure 2.
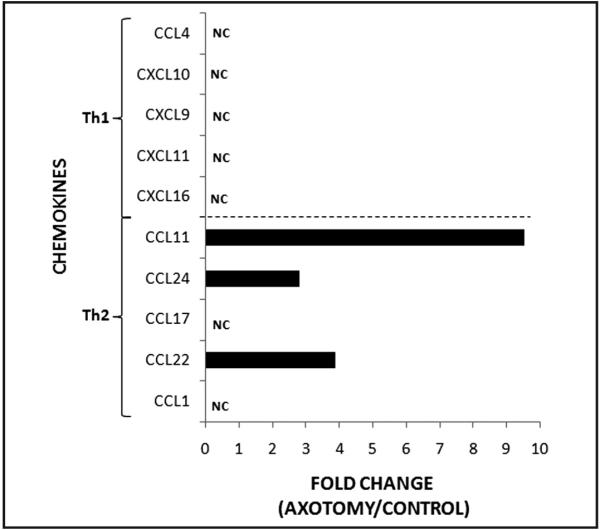
Th2-associated and Th1-associated chemokine mRNA levels in mouse facial motor nucleus 7 days after facial nerve axotomy. Chemokine mRNA levels are displayed as the fold change between the axotomized side and the uninjured control side. NC, no change; not greater than 2.5-fold change.
RT–PPCR analysis
Based on the microarray analysis, CCL11 was further analyzed with RT-PCR (Figure 3). At 7 and 14 dpa, WT mouse CCL11 mRNA levels increased to 245 ± 32 percent (n = 5) and 306 ± 47 percent (n = 8), respectively, above the uninjured (contralateral) control side and were significantly above baseline values (10 ± 21 percent; p < 0.01; n = 5). By 30 dpa, WT mouse CCL11 mRNA levels were significantly reduced to 90 ± 45 percent (n = 5) above the uninjured side. At 7 and 14 dpa, mSOD1 mouse CCL11 mRNA levels increased to 244 ± 38 percent (n = 5) and 368 ± 42 percent (n = 6), respectively, above the uninjured (contralateral) control side and were significantly above baseline values (10 ± 21 percent; p < 0.01; n = 5). By 30 dpa, mSOD1 mouse CCL11 mRNA levels were significantly reduced to 101 ± 39 percent (n = 5) above the uninjured side. No significant differences were found between WT and mSOD1 mouse at any of the time points. Thus, facial nerve axotomy increases CCL11 mRNA levels in WT and mSOD1 mouse facial motor nucleus within the first week after injury and is sustained for several weeks until beginning to decline.
Figure 3.
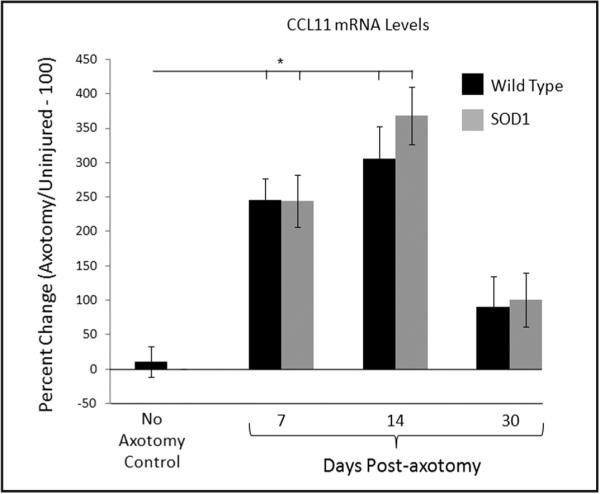
CCL11 mRNA levels in control or axotomized WT (black bars) and presymptomatic mSOD1 (grey bars) mouse facial motor nucleus at 7, 14, and 30 days after facial nerve axotomy. Control CCL11 mRNA levels are displayed as the percent change between the right (axotomized) side and the left (uninjured) control side within each animal. At 7, 14, and 30 days post-axotomy, CCL11 mRNA levels are displayed as the percent change between the right (axotomized) side and the left (uninjured) control side within each animal. Bar heights represent means (±SEM). *Denotes significant differences at p ≤ 0.01.
Based on the previous work indicating the presence of IFN-γ in facial motor nucleus by 14 dpa3 and the ability of IFN-γ to increase Th1-associated chemokine expression, CXCL11 was further analyzed with RT-PCR (Figure 4). WT mouse CXCL11 mRNA levels were not affected by axotomy at any of the time points examined [−2.5 ± 18 percent (n = 4), −36 ± 14 percent (n = 5), and −23 ± 11 percent (n = 4)], respectively, relative to the uninjured (contralateral) control side and when statistically compared to baseline values (3 ± 7 percent; n = 4). mSOD1 mouse CXCL11 mRNA levels were not affected by axotomy at any of the time points examined [−12 ± 12 percent (n = 3), 14 ± 20 percent (n = 3), and 16 ± 11 percent (n = 4)], respectively, relative to the uninjured (contralateral) control side and when statistically compared with baseline values (3 ± 7 percent; n = 4). No significant differences were found between WT and mSOD1 mouse at any of the time points. These results confirm the microarray data and indicate that facial nerve axotomy does not affect CXCL11 mRNA levels in WT and mSOD1 mouse facial motor nucleus.
Figure 4.
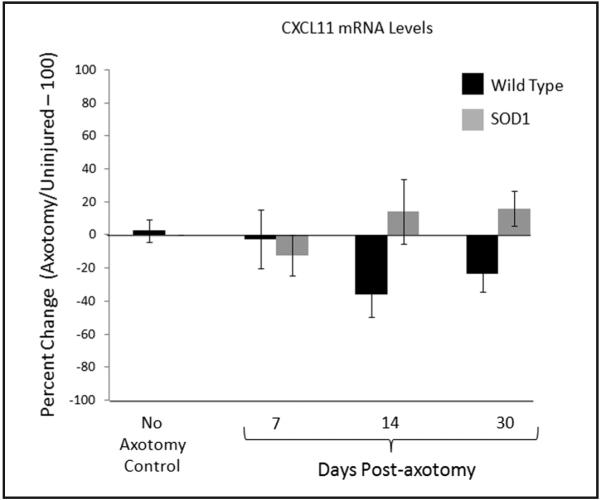
CXCL11 mRNA levels in control or axotomized WT (black bars) and presymptomatic mSOD1 (grey bars) mouse facial motor nucleus at 7, 14, and 30 days after facial nerve axotomy. Control CXCL11 mRNA levels are displayed as the percent change between the right (axotomized) side and the left (uninjured) control side within each animal. At 7, 14, and 30 days post-axotomy, CXCL11 mRNA levels are displayed as the percent change between the right (axotomized) side and the left (uninjured) control side within each animal. Bar heights represent means (±SEM).
DISCUSSION
Previous work from our laboratory has established that mouse FMN survival after facial nerve axotomy depends on both peripheral APCs for initial CD4+ T cell activation and centrally located microglia for CD4+ T cell reactivation.4 Furthermore, we discovered that, after facial nerve axotomy, CD4+Th2 cells develop6 and are responsible for mediating FMN survival.7 These data combined with evidence that PACAP mRNA is expressed by injured mouse FMN after facial nerve axotomy8 and PACAP increases Th2-associated chemokine expression in cultured murine microglia9 led to the current investigation. Using microarray and RT-PCR analyses, we identified an increase in Th2-associated, but not Th1-associated, chemokine mRNA levels in the WT mouse facial motor nucleus after facial nerve axotomy. Additionally, we compared the mRNA levels for select Th2-associated and Th1-associated chemokines between WT and mSOD1 mice, given the disparity in FMN survival of WT and presymptomatic mSOD1 mice after facial nerve injury.
Mariotti et al.26 first identified a significant decrease in FMN survival in presymptomatic mSOD1 mice compared with WT littermates after facial nerve axotomy. Interestingly, Serpe et al.1,2 had already demonstrated a positive role for the immune system in FMN survival suggesting a potential defect in the immune response of mSOD1 mice after facial nerve axotomy. Recent studies have validated this hypothesis and have shown a positive role for CD4+ T cells in slowing mSOD1-mediated disease progression,20 as well as a profound and progressive immunodeficiency that is linked to T cell dysfunction.27 Furthermore, mSOD1 mice have altered levels of the macrophage-recruiting chemokine, CCL2.19 However, no previous studies have investigated chemokines capable of attracting neuroprotective Th2 cells to the facial motor nucleus after facial nerve axotomy in mSOD1 mice.
In the present study, the microarray preliminary results led to the focus on the Th2-associated chemokine, CCL11. The data indicate a transient, but large, increase in CCL11 mRNA levels in WT mice coincident with peripheral immune activation. When WT and presymptomatic mSOD1 mice were compared for CCL11 mRNA levels, no difference was found. The Th1-associated chemokine, CXCL11, mRNA levels did not change throughout the 30-day time course for WT and mSOD1 mice. Furthermore, the lack of difference between WT and mSOD1 mice for CCL11 and CXCL11 mRNA levels suggests similar compensatory mechanisms after motoneuron trauma in normal and diseased animals. Collectively, these data support the hypothesis of selective Th2 cell recruitment to the facial motor nucleus after facial nerve axotomy.
The microarray and real-time PCR analyses provide a firm foundation for proceeding with additional techniques for investigating Th2-associated and Th1-associated chemokine expression in the facial motor nucleus after facial nerve axotomy. However, the limitations of this study include the lack of cell specific localization at the mRNA and protein level for the Th2-associated chemokine, CCL11, and Th1-associated chemokine, CXCL11. Additional techniques that would complement the analyses used in this study include in situ hybridization (ISH) and immunohistochemistry (IHC). Although ISH would determine the cellular localization of the synthesized mRNA, IHC would determine the cellular localization of the synthesized protein, number of cells that are positive for that protein, as well as provide information as to where the translated product is expressed within positive cells. In the future, protein analysis is particularly important for the Th2-associated chemokine, CCL11, and Th1-associated chemokine, CXCL11, as previous reports have demonstrated differences between mRNA and protein levels for chemokines in the facial motor nucleus after facial nerve axotomy.12,13
In summary, this in vivo investigation has identified a Th2-associated, but not Th1-associated, chemokine profile in the facial motor nucleus after facial nerve injury at the mRNA level. Furthermore, as no differences were detected between WT and mSOD1 facial motor nucleus for CCL11 mRNA levels, additional Th2-associated chemokines that were identified by microarray analysis will be characterized in the future. Given the important role of Th2 cells after facial nerve axotomy7 and the profound immunodeficiency in mSOD1-mediated disease,27 understanding the role of injury-induced CNS expression of chemokines may eventually lead to more specifically targeted therapeutics in motoneuron diseases, such as ALS.
ACKNOWLEDGMENTS
This work was supported by the Les Turner ALS Foundation (D.A.W. and K.J.J.) and NIH grant NS40433 (K.J.J. and V.M.S.).
REFERENCES
- 1.Serpe CJ, Coers S, Sanders VM, et al. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 2.Serpe CJ, Kohm AP, Huppenbauer CB, et al. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raivich G, Jones LL, Kloss CU, et al. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byram SC, Carson MJ, DeBoy CA, et al. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stout RD, Bottomly K. Antigen-specific activation of effector macrophages by IFN-γ producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–765. [PubMed] [Google Scholar]
- 6.Xin J, Wainwright DA, Serpe CJ, et al. Phenotype of CD4(+) T cell subsets that develop following mouse facial nerve axotomy. Brain Behav Immun. 2007;22:528–537. doi: 10.1016/j.bbi.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deboy CA, Xin J, Byram SC, et al. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exp Neurol. 2006;201:212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong BD, Hu Z, Abad C, et al. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- 9.Wainwright DA, Xin J, Sanders VM, et al. Differential actions of pituitary adenylyl cyclase-activating polypeptide and interferon gamma on Th2- and Th1-associated chemokine expression in cultured murine microglia. J Neurodegener Regen. 2008;1:31–34. [PMC free article] [PubMed] [Google Scholar]
- 10.Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naïve T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55:127–134. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 12.Flügel A, Hager G, Horvat A, et al. Neuronal MCP-1 expression in response to remote nerve injury. J Cereb Blood Flow Metab. 2001;21:69–76. doi: 10.1097/00004647-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Harrison JK, Jiang Y, Chen S, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 15.Babcock AA, Kuziel WA, Rivest S, et al. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Shi XQ, Echeverry S, et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci. 2007;27:12396–12406. doi: 10.1523/JNEUROSCI.3016-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata T, Nagano I, Shiote M, et al. Elevation of MCP-1 and MCP-1/VEGF ratio in cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neurol Res. 2007;29:772–776. doi: 10.1179/016164107X229795. [DOI] [PubMed] [Google Scholar]
- 18.Henkel JS, Engelhardt JI, Siklós L, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 19.Henkel JS, Beers DR, Siklós L, et al. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci. 2006;31:427–437. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Beers DR, Henkel JS, Zhao W, et al. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CH, Kunkel EJ, Boisvert J, et al. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest. 2001;107:595–601. doi: 10.1172/JCI11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantovani A. The chemokine system: Redundancy for robust outputs. Immunol Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 24.Jones KJ, LaVelle A. Changes in nuclear envelope invaginations in axotomized immature and mature hamster facial motoneurons. Brain Res. 1985;353:241–249. doi: 10.1016/0165-3806(85)90212-3. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Mariotti R, Cristino L, Bressan C, et al. Altered reaction of facial motoneurons to axonal damage in the presymptomatic phase of a murine model of familial amyotrophic lateral sclerosis. Neuroscience. 2002;115:331–335. doi: 10.1016/s0306-4522(02)00448-7. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee R, Mosley RL, Reynolds AD, et al. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]


