Abstract
Androgen Receptor (AR) mediated oncogenic pathways have not been fully elucidated. In this study we utilized high throughput microarray analysis on two AR-positive Prostate Cancer (CaP) cell lines to identify 16 AR-responsive microRNAs (miRNAs). We focused on miR-21 because of its previously reported oncogenic activity in other cancers. We demonstrate androgen-induced AR binding to the defined miR-21 promoter, miPPR-21, suggesting direct transcriptional regulation. Moreover, inhibition of miR-21 diminished androgen-induced CaP cell proliferation, providing new evidence that microRNAs can contribute to androgen-driven cell growth. Elevated expression of miR-21 enhanced CaP tumor growth in vivo and, surprisingly, was sufficient for androgen-dependent tumors to overcome castration-mediated growth arrest. Thus, elevated miR-21 expression alone is sufficient to impart castration resistance. Moreover, quantitative RT-PCR analysis revealed elevated miR-21 expression in CaP when compared to adjacent normal tissue. These results suggest that miR-21 may contribute to CaP pathogenesis.
Keywords: Prostate cancer, microRNA, Androgen Receptor
Introduction
AR is a ligand-dependent transcription factor and member of the hormone nuclear receptor superfamily (1). In adult prostate, activated AR triggers epithelial cell growth arrest and differentiation. However, in CaP the AR-pathway is modified to facilitate cell survival, proliferation and androgen-independent growth (2, 3). To date, the downstream proliferative pathways of AR have not been fully elucidated. miRNAs are 18–24 nucleotide RNA polymerase II transcribed RNAs that regulate the translation of specific mRNAs. Deregulated 3 miRNA expression has been reported in many human cancers, including prostate (4–11). More recently, oncogenic and tumor suppressor transcription factors, such as Myc and p53, have been shown to directly regulate miRNA transcription (12) (13). Further, miRNAs themselves have been shown to have oncogenic and tumor suppressor properties (14). In light of this, we hypothesized that activated AR may directly regulate the transcription of certain miRNAs, and that these miRNAs may contribute to the CaP phenotype.
In the current study, we identified a set of androgen-induced miRNAs in androgen-responsive CaP cells. Among these was the oncogenic miRNA, miR-21. We have completed a series of experiments to investigate the mechanism behind AR-mediated miR-21 gene regulation and to further evaluate the contribution of miR-21 to androgen-dependent and castration-resistant CaP growth. These studies establish a link between clinical tumor expression, AR interaction with a known microRNA promoter, microRNA induction, and subsequent androgen-dependent and independent proliferation.
Materials and Methods
Cell lines and chemicals
LNCaP and LAPC-4 were kindly provided by John Isaacs. LNCaP, C4-2 and CWR22Rv1 were grown in RPMI 1640 (Cellgro). LAPC-4 were maintained in Iscove’s media (InVitrogen) with 1nM R1881 (Perkin-Elmer). All media contained 10 µg/ml Ciprofloxacin Hydrochloride (US Biological) and 10% FBS. For androgen deprivation, cells were plated in phenol-red free media supplemented with 10 % charcoal-stripped serum (Hyclone) 24 hours prior to treatment with R1881 (Perkin-Elmer). Vehicle was ethanol.
Cell proliferation/viability
For transient transfection assays, one million cells were oligofectamine-transfected with 100 nM synthetic pre-miR-21 or FAM™-labeled Pre-miR™Negative-control#1 (Ambion). For stable expression studies, 90,000 retrovirally-transduced LNCaP cells were grown in androgen-depleted media (refreshed at 0.5 and 3 days, 24-well plates). Viability was determined on day 6 by Trypan blue exclusion (InVitrogen). For androgen inhibition, 5,000 cells were seeded in complete media in a 96-multiwell plate and treated with 10 µM Casodex (LKT Laboratories Inc.) or vehicle. After 6 days, MTS (Promega) was performed according to manufacturer’s instructions. For miR-21 inhibition experiments, LNCaP vector or miR-21 sublines were infected with 10 MOI of either Ad-Sponge-miR-21 or Ad-Sponge (Supplementary Material and Methods). After 24 hours cells were harvested and grown in 0 or 0.1 nM R1881. MTS (Promega) was performed at 6 days.
miRNA Microarrays and Northern Blotting
3.7 million cells were seeded in 75-cm flasks. 24 hours after growth in androgen-free media, cells were treated with 0, 0.1, 1 or 10 nM R1881 and RNA isolated with Trizol reagent (InVitrogen) for microarray analysis. Androgen-depleted C4-2 and CWR22Rv1 were treated with 10 nM R1881 or vehicle for 72h. miRNA Northern blotting was performed as reported (13). Custom microarrays (Combimatrix) were applied. A detailed explanation of this section is found in the supplementary material and methods and Geo database (GSE16225).
Chromatin Immunoprecipitation
Charcoal-stripped LNCaP were stimulated for 7 hours with androgens or vehicle. ChIP was performed as reported (15). Briefly, 1% formaldehyde crosslinked-chromatin cells were lysed and sonicated (DNA fragments ~ 750bp). Lysates were diluted in buffer supplemented with 20 µg/ml sheared salmon sperm DNA (Stratagene) and precleared with Protein-G-Dynabeads (InVitrogen) for 1h at 4°. Supernatants were incubated overnight with anti-AR (Santa Cruz Biotechnology, clone N20) or control IgG (Santa Cruz Biotechnology) with Protein-G-Dynabeads. Precipitated complexes were washed with increasing stringency and eluted. Recovered DNA was purified with PCR Purification Kit (Qiagen) and analyzed by qPCR (Supplementary Table S2).
Patient samples
Prostate tissue specimens were fresh frozen surgical specimens harvested from 10 patients undergoing radical prostatectomy at the Johns Hopkins Hospital (1993 to 2000). Frozen tissue blocks were trimmed to enrich for tumor and normal tissues matched for each case. The use of these specimens for molecular studies was approved by the Institutional Review Board at Johns Hopkins Medical Institutions.
Tumor xenografts
20 male 4–6 week old athymic Nu/Nu mice (Charles River Laboratories) were injected subcutaneously with two million LNCaP-control or LNCaP-miR-21 cells suspended in equal volumes with Matrigel (BD). Castration was performed at average tumor volume of ~375 mm3. Data points represent average tumor volumes. Studies were performed according protocols approved by the Animal Care and Use Committee at Johns Hopkins University.
Results and Discussion
Identification of AR-induced miRNAs
To identify androgen-responsive miRNAs, we employed two androgen-responsive CaP cell lines, LNCaP and LAPC-4. Cells were plated in androgen-free media and treated with 0.1–10 nM of synthetic androgen (R1881). Expected androgen induction of growth and PSA was confirmed in all samples (results not shown). Total RNA from these cells was analyzed using a previously developed custom microarray specific for the mature form of the miRNA (13). The criteria for an androgen-responsive miRNA was defined as those displaying a signal above background in 0.1–10 nM R1881, plus exhibiting at least a 1.5-fold androgen-induced change in both cell lines. A total of 16 miRNAs fit these criteria (Table 1), and 10 of the androgen-responsive miRNAs are embedded in miRNA clusters with multiple members of the cluster exhibiting coordinated upregulation. Many of these were previously reported to be upregulated in CaP (6, 11). No miRNAs were found to be down-regulated by more than 1.5 fold in both cell lines under these conditions. In previous studies, miR-338 and miR-125b have also been reported as androgen-induced in LNCaP cells (4, 9). These miRNAs were not upregulated by more than 1.5 fold in our arrays. Northern blotting was performed to more directly evaluate the androgen responsiveness of these miRNAs in our models. Following 72 hours of androgen treatment, miR-125b appeared to be mildly suppressed by androgens (Supplementary Fig 1). miR-338, on the other hand, was undetectable by both northern and microarray analysis.
Table 1.
miRNAs induced ≥ 1.5 Fold by androgens in both LNCaP and LAPC-4.
| miRNA | Transcription unit† | Maximum Fold Change | |
|---|---|---|---|
| LAPC-4 | LACaP | ||
| miR-594 | miR-594 | 3.34 | 2.67 |
| miR-16 | (miR-16-1/miR 15a);(miR-16-2/miR-15b) | 2.40 | 2.68 |
| miR-21 | miR-21 | 2.36 | 2.05 |
| miR-29b | (miR-29b-2/miR-29c);(miR-29b-1/miR-29a) | 2.28 | 2.08 |
| miR-148a | miR-148a | 2.25 | 3.34 |
| miR-29c | (miR-29b-2/miR-29c) | 2.24 | 3.56 |
| miR-106a | (miR-106a/miR-18b/miR-20b/miR-19b-2/miR-92a-2/miR-363) | 2.23 | 1.86 |
| miR-17-5p | (miR-17/miR-18a/miR-19a/miR-20a/miR-19b-1/miR-92a-1) | 2.10 | 1.76 |
| miR-20a | (miR-17/miR-18a/miR-19a/miR-20a/miR-19b-1/miR-92a-1) | 2.09 | 1.76 |
| miR-20b | (miR-106a/miR-18b/miR-20b/miR-19b-2/miR-92a-2/miR-363) | 2.08 | 2.65 |
| miR-29a | (miR-29b-1/miR-29a) | 1.95 | 2.88 |
| miR-19b | (miR-106a/miR-18b/miR-20b/miR-19b-2/miR-92a-2/miR-363);(miR-17/ miR-18a/miR-19a/miR-20a/miR-19b-1/miR-92a-1) |
1.94 | 1.51 |
| miR-93 | (miR-106b/miR-93/miR-25) | 1.90 | 2.07 |
| let-7g | let-7g | 1.68 | 1.80 |
| miR-15b | (miR-16-2/miR-15b) | 1.67 | 1.56 |
| let-7d | (let-7a-1/let-7f-1/let-7d) | 1.63 | 1.67 |
Individual transcription units are separated by semicolons; clustered miRNAs are indicated in parentheses
Androgen-induced miR-21 in Prostate Cancer
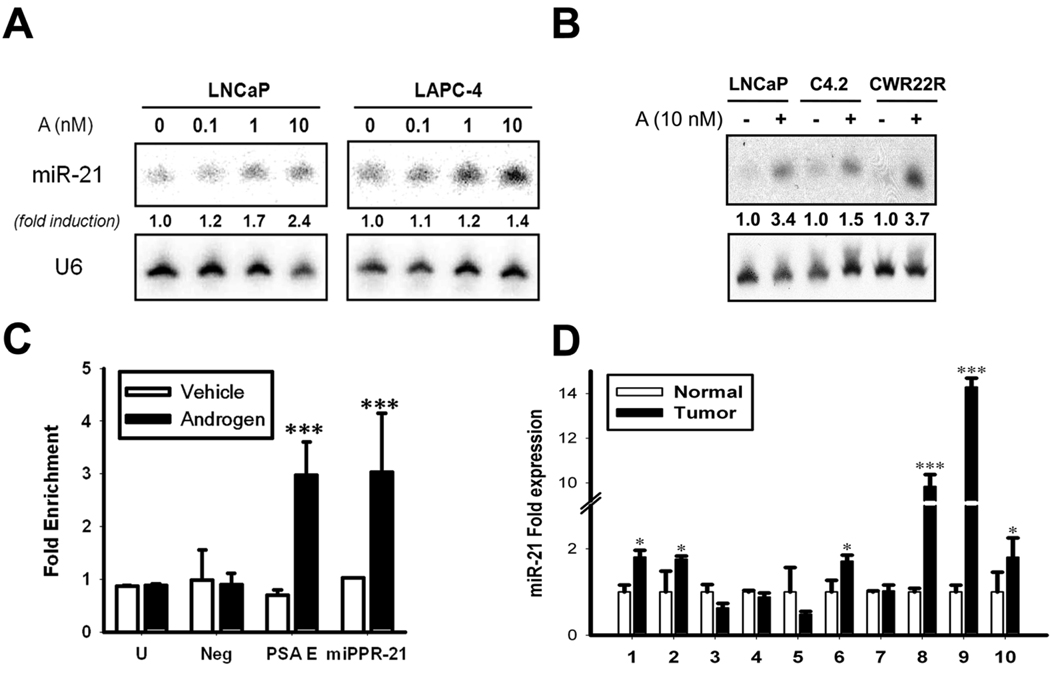
Northern blot analysis confirmed androgen-induced miR-21 expression in LNCaP and LAPC-4 cells (Fig. 1A). miR-21 was also induced in the AR-positive C4-2 and CWR22Rv1 CaP cells (Fig. 1B). To evaluate if miR-21 was directly regulated by AR, we analyzed the reported promoter, miPPR-21 (16). A highly conserved Androgen Response Element (ARE) was identified inside of miPPR-21 and tested for androgen-induced AR occupancy by Chromatin Immunoprecipitation (ChIP). For negative control regions, we tested a non-conserved ARE located upstream of miPPR-21 (Fig. 1C, Neg) and an unlinked locus (Fig. 1C, U). The PSA enhancer served as a positive control. Results proved androgen-induced AR binding of miPPR-21 to a similar extent as the positive control (Fig. 1C, PSA E). Although miR-21 is clearly androgen-responsive, its expression does not exclusively rely on AR. miR-21 expression has been reported in several AR-negative CaP cells and other non-prostatic cells. Also, it has been reported that IL-6 and phorbol esters can induce miR-21, while estradiol can inhibit its expression (16–18). Therefore, the transcriptional regulation of miR-21 appears to be a complex network in which AR can participate. Nonetheless, this is the first evidence that an annotated microRNA transcript and promoter can be directly regulated by AR.
Fig. 1. miR-21 expression and regulation in CaP.
A, Androgen-stimulated miR-21 expression. Northern blots of miR-21 dose-response (A, R1881). U6 used as a loading control. ’Fold-induction’ represents miR-21 relative to the non-stimulated state. B, miR-21 induction in additional AR-positive CaP cell lines. C, miPPR-21 Chromatin Immunoprecipitation. White = non-treated, Black = androgen-stimulated. Fold-enrichment represents AR immunoprecipitation relative to control antibody. ‘PSA E’: PSA Enhancer; ‘U’ control amplicon; ‘Neg’: non-conserved ARE. Mean ± SE from three independent measurements, *P<0.05, ***P<0.001 (t-student test). D, miR-21 in human CaP tumors. Fold-expression normalized to U6 by qRT-PCR. Mean ± SE, *P<0.05, ***P<0.001 (Two way ANOVA).
To evaluate if miR-21 expression was elevated in human tumors, we quantified expression in 10 tumor-normal matched samples from early grade radical prostatectomy specimens. In total, 6 of 10 tumors showed a statistically significant increase in miR-21 expression (Fig. 1D). On average, miR-21 was upregulated 3.52-fold in CaP. In this small sample set, miR-21 expression did not correlate with stage, grade, or PSA [Supplementary Table S1]. Nonetheless, this data suggests that elevated miR-21 expression may be an early event in CaP. In our own analysis of a separate data set published by Porkka and colleagues (8), miR-21 was also found to be more highly expressed in CaP. The androgen-responsive miRNAs, miR-125b and miR-338, have also been included in published clinical studies. miR-125b has been reported as upregulated in CaP (9); however, other studies have also found miR-125b to be repressed (7). Ambs and colleagues, who identified miR-338 as androgen-responsive in vitro, did not find miR-338, miR-125b, or miR-21 to be significantly upregulated in human tumor samples (4). Additional studies are therefore required to determine the degree these androgen-responsive miRNAs are expressed in CaP and if their expression correlates with disease progression.
miR-21 induces androgen-dependent and independent proliferation
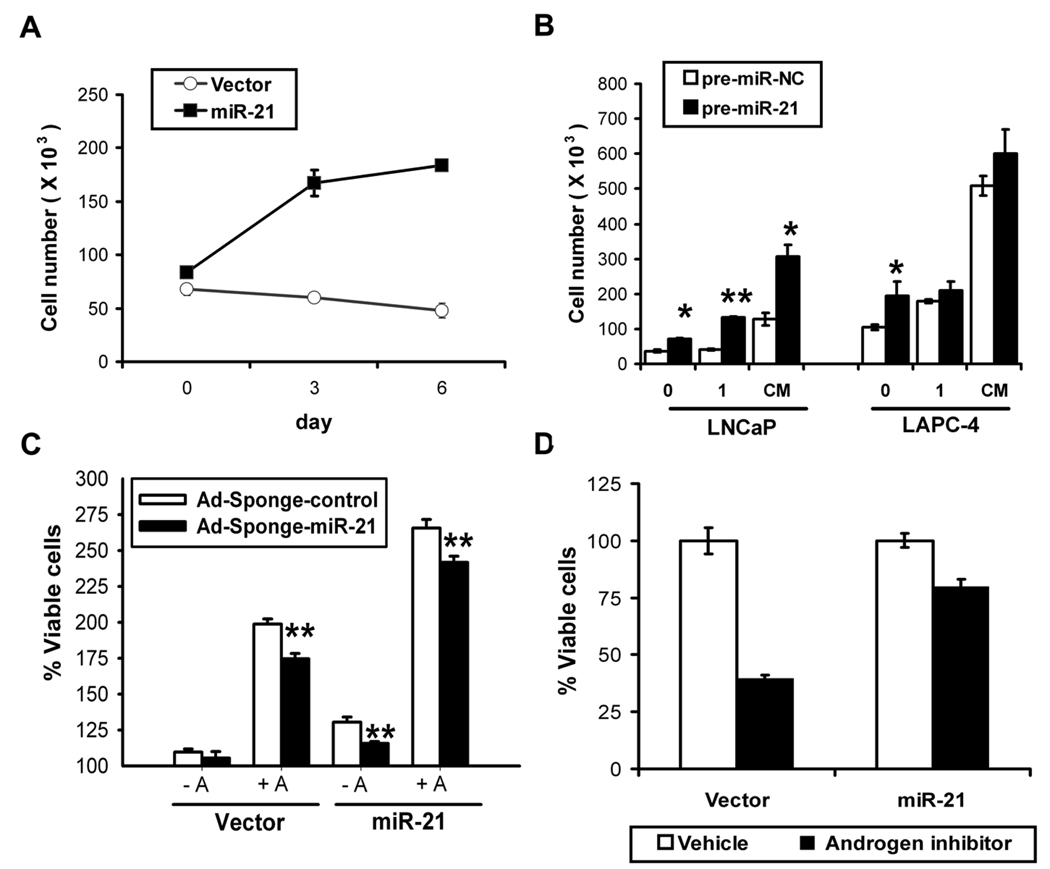
We hypothesized that elevated miR-21 may participate in androgen-induced cell growth or impart androgen-independent growth. To investigate the later, we infected LNCaP with a control or miR-21 expressing retrovirus and selected stably-infected populations. The miR-21 infected LNCaP cells expressed 5-fold more miR-21 than controls (Supplementary Fig. S2A), which is physiologically relevant when compared to levels in human tumors. Elevated miR-21 alone was sufficient to rescue LNCaP from androgen-ablated growth arrest and drive androgen-independent growth (Fig. 2A). Parallel experiments with transiently transfected synthetic miR-21 (pre-miR-21) or negative control (pre-miR-NC) confirmed miR-21-mediated androgen-independent growth in LNCaP and LAPC-4 cells (Fig. 2B, 0 nM). In view of these results, we evaluated if miR-21 also contributed to androgen-independent growth in AR-negative CaP cells. As positive control, we included MCF-7 cells where a reduction of miR-21 by 50% is known to reduce proliferation (19). Results showed that partial inhibition of miR-21 was sufficient to moderately impair the growth of PC-3 and DU145, cells suggesting that miR-21 may also contribute to AR-independent CaP growth (Supplementary Fig. S3).
Fig. 2. miR-21-induced proliferation.
A, miR-21 mediated androgen-independent growth. LNCaP retrovirally transduced to stably express miR-21 (black square) or empty vector (white circle) grown in androgen-depleted media. Mean ± S.E. B, Effects of synthetic miR-21 on growth. Transient transfectantions with synthetic pre-miR-21 (black) or pre-miR-Negative Control (white) after 6 days in androgen-depleted (0), 1 nM R1881 (1) or complete media (CM). Viable cells quantified by trypan blue (Mean ± S.E. from 3 different wells), *P<0.05, **P<0.01 (t-student test). C, Effects of miR-21 inhibition. LNCaP miR-21 or vector control cells infected with 10 MOI of Ad-Sponge-control (white) or anti-miR-21 Ad-Sponge-miR-21 (black) after 6 days in charcoal-stripped (−A) or R1881 supplemented (+A) media. Percent growth calculated by MTS. Mean ± S.E of 12 independent measurements, **P<0.01 (t-student test). D, Elevated miR-21 partially overcomes AR blockade. LNCaP miR-21 or control vector in complete media treated with vehicle (white) or 10 µM Casodex (black). Cell proliferation calculated by MTS (relative to vehicle, 6 days). Mean ± S.E of 6 independent measurements.
We also assessed the effect of elevated miR-21 in AR-driven proliferation. Enhanced growth was found in androgen-supplemented or complete media when cells were transfected with synthetic miR-21 (Fig. 2B) or virally-transduced with miR-21 expression vectors (Supplementary Fig. S4). To estimate the contribution of miR-21 to AR-driven proliferation, we treated LNCaP-miR-21 cells with a nonsteroidal anti-androgen. Casodex treatment inhibited 60% of LNCaP-control cell proliferation, whereas LNCaP-miR-21 proliferation was only inhibited by 20% (Fig. 2D). Blockade of miR-21 after androgen induction imparted a moderate but statistically significant inhibition of androgen-induced growth (Fig. 2C, vector −A vs. +A). These studies only partially account for the contribution of miR-21 to androgen-stimulated growth due to limited inhibition of miR-21 by only 40% (data not shown). Inhibition of miR-21 in the absence of androgens had no effect. As a positive control LNCaP-miR-21 cells were included, where miR-21 inhibition partially reversed the androgen-independent growth imparted by elevated miR-21 (Fig. 2C, miR-21 −A) and diminished androgen-mediated CaP growth (Fig. 2C, miR-21 +A). Taken together, these data suggest that miR-21 participates in AR-driven proliferation.
CaP growth can be attributed to increased growth rates, a decrease in apoptosis, or both. We believe that miR-21 is contributing to cell proliferation in our models. Death rates after androgen deprival were unaffected by miR-21 expression. Moreover, exogenous expression of miR-21 was unable to confer resistance against a variety of chemotherapeutics (Supplementary Fig. S5).
miR-21 promotes enhanced tumor growth and castration resistance in vivo
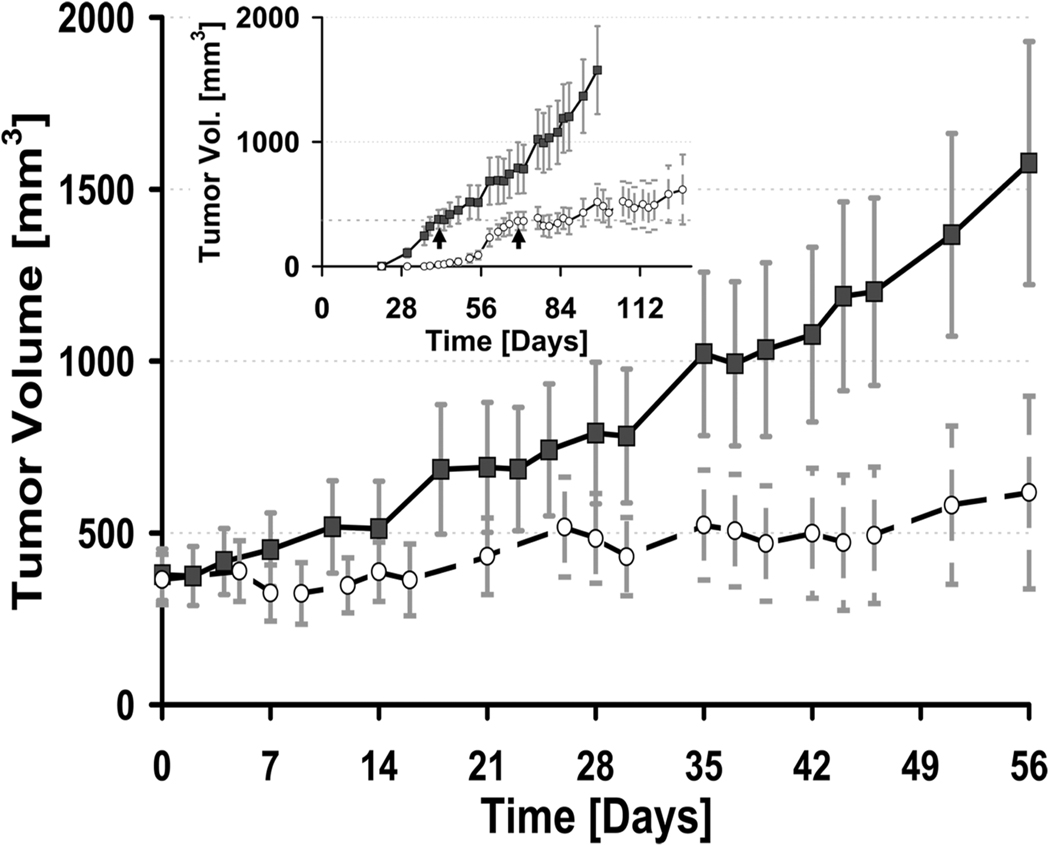
To assess the contribution of elevated miR-21 to CaP growth in vivo we generated subcutaneous tumors with retrovirally transduced LNCaP-miR-21 or LNCaP-control cells. LNCaP-miR-21 cells had a greater tumor take of 100% when compared to LNCaP-control cells (80%). Moreover, palpable tumor onset of the LNCaP-miR-21 population was ~17 days earlier than controls (Fig. 3 inset). LNCaP-miR-21 tumors also grew more quickly (slope of 19.2) when compared with controls (slope of 12.9) (Fig. 3). Therefore, elevated miR-21 enhances androgen-dependent growth in vivo. To evaluate androgen-dependence, bilateral orchiectomy was performed on both subpopulations when tumors reached an average volume of 375 mm3. Castration markedly reduced tumor growth rates for control tumors (slope from 12.9 to 4.5), whereas LNCaP-miR-21 tumors were unaffected (slope from 19.2 to 20.1). These results demonstrate that elevated miR-21 expression alone is sufficient for androgen-dependent tumors to overcome castration and become androgen-independent. In support of this, our analysis of data from Porkka and colleagues suggests that miR-21 is elevated in androgen-independent CaP when compared to hormone naïve cases (8). This could be explained by ligand-independent AR activation or by AR-independent pathways. For example, IL-6 has been shown to up-regulate miR-21 in CaP cells (17). Of note, accumulating evidence exists that IL-6 may contribute to CaP progression and metastasis (20).
Fig. 3. Elevated expression of miR-21 promotes enhanced tumor growth and castration-resistance in vivo.
LNCaP miR-21 (black square) or a control vector (white circle) subcutaneous xenograft growth before and after castration. Castration was performed at ~ 375 mm3 tumor volume for each group. Tumor growth is normalized to the time of castration (day 0). Inset: Time course of tumor growth from the time of injection. Castration indicated by arrows. Mean ± S.E of at least 10 independent values. *P<0.05 (t-student test).
In summary, these studies identify miR-21 as an AR-regulated microRNA which enhances androgen-dependent CaP growth and is sufficient to mediate castration resistance.
Supplementary Material
Acknowledgments
We thank Tom Dunn, John and William Isaacs for their help and guidance. This work was supported by the Patrick C. Walsh Prostate Cancer Research Fund and the DOD Prostate Cancer Research Fund, W81XWH-08-1-0156. JR has a Beatriu de Pinos fellowship from “Departament d’ Universitats, Recerca i Societat de la Informacio de la Generalitat de Catalunya” (Catalunya, Spain).
Abbreviations
- AR
Androgen Receptor
- CaP
Prostate Cancer
- miRNA
microRNA
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dehm SM, Tindall DJ. Androgen receptor structural and functional elements: role and regulation in prostate cancer. Mol Endocrinol. 2007;21:2855–2863. doi: 10.1210/me.2007-0223. [DOI] [PubMed] [Google Scholar]
- 3.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–6170. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. Rna. 2008;14:417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 7.Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 8.Porkka KP, Pfeiffer MJ, Waltering KK, et al. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 9.Shi XB, Xue L, Yang J, et al. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci U S A. 2007;104:19983–19988. doi: 10.1073/pnas.0706641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun T, Wang Q, Balk S, et al. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009;69:3356–3363. doi: 10.1158/0008-5472.CAN-08-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 13.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–6196. doi: 10.1038/sj.onc.1209913. [DOI] [PubMed] [Google Scholar]
- 15.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 16.Fujita S, Ito T, Mizutani T, et al. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol. 2008;378:492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Loffler D, Brocke-Heidrich K, Pfeifer G, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 18.Wickramasinghe NS, Manavalan TT, Dougherty SM, et al. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 20.Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001;12:33–40. doi: 10.1016/s1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.