The cancer drug geldanamycin, an HSP90 inhibitor, decreases the stability of key components of the miRNA regulatory pathway, the efficacy of siRNAs, and the formation of P-bodies without affecting endogenous miRNA function.
Abstract
Key components of the miRNA-mediated gene regulation pathway are localized in cytoplasmic processing bodies (P-bodies). Mounting evidence suggests that the presence of microscopic P-bodies are not always required for miRNA-mediated gene regulation. Here we have shown that geldanamycin, a well-characterized HSP90 inhibitor, abolishes P-bodies and significantly reduces Argonaute and GW182 protein levels but does not affect the miRNA level and the efficiency of miRNA-mediated gene repression; however, it significantly impairs siRNA loading and the efficacy of exogenous siRNA. Our data suggests that HSP90 protein chaperones Argonautes before binding RNA and may facilitate efficient loading of small RNA.
INTRODUCTION
Small regulatory RNAs such as small interfering RNAs (siRNAs) and micro RNAs (miRNAs) regulate gene expression mainly at the posttranscriptional level in eukaryotes. Argonautes, small RNAs, and miRNA-targeted mRNAs have all been shown to be localized in cytoplasmic processing bodies (P-bodies; Ding et al., 2005; Jakymiw et al., 2005; Liu et al., 2005a; Pillai et al., 2005; Sen and Blau, 2005; Bhattacharyya et al., 2006). This cytoplasmic structure is involved in various mRNA decay mechanisms including poly(A)-dependent decapping, AU-rich element–mediated mRNA destabilization, and nonsense-mediated mRNA degradation (NMD; Jakymiw et al., 2007; Eulalio et al., 2007a).
Metazoan Argonautes interact with the GW182 protein family, which were first identified as a P-body marker (Eystathioy et al., 2002; Ding et al., 2005; Liu et al., 2005a; Meister et al., 2005; Rehwinkel et al., 2005; Landthaler et al., 2008). Depletion of the GW182 family abolishes visible P-bodies; this also leads to a loss of miRNA-mediated repression in reporter constructs because the Argonautes' interaction with GW182 is required for miRNA-mediated gene repression (Liu et al., 2005a; Meister et al., 2005; Behm-Ansmant et al., 2006; Eulalio et al., 2008, 2009; Lian et al., 2009). Many studies have shown that the function of GW182 proteins in translational repression can be uncoupled from Argonautes, suggesting that miRNA-loaded Argonautes recruit GW182 proteins to the targeted mRNA (Li et al., 2008; Chekulaeva et al., 2009; Lazzaretti et al., 2009; Lian et al., 2009; Zipprich et al., 2009). Other proteins, such as Mov10 and RCK/p54 that interact with Argonautes and have an essential role in miRNA-mediated gene repression also colocalize with Argonautes in the P-bodies (Meister et al., 2005; Chu, 2006). Depletion of RCK/p54 also disrupts the formation of visible P-bodies (Chu and Rana, 2006).
The presence of microscopic P-bodies does not seem to be a prerequisite for miRNA-mediated gene repression. For instance, depletion of Lsm1, a P-body component, disrupts P-body formation in mammalian cells but does not significantly affect miRNA-mediated gene repression (Chu, 2006). It has also been suggested that P-body formation is the consequence of RNA silencing (Eulalio et al., 2007b). In addition, recent studies have shown that GW182 function can be uncoupled from its localization in P-bodies (Eulalio et al., 2009).
Interestingly, formation of P-bodies requires the presence of small regulatory RNAs. Depletion of Drosha leads to the loss of visible P-bodies that could be reconstituted by the introduction of synthetic siRNAs (Pauley et al., 2006). Introduction of functional siRNAs into mammalian cells induce up-regulation of Ago2 and GW182 and produce more microscopic P-bodies (Lian et al., 2007; Jagannath and Wood, 2009).
Biochemical purifications revealed that Argonaute proteins frequently copurify with heat shock proteins (Hock et al., 2007; Landthaler et al., 2008). It was shown that heat shock protein 90 (HSP90) binds directly to the N-terminus of overexpressed mammalian Ago2 and was proposed to be required for stabilizing Dicer interactions with Ago2 (Tahbaz et al., 2001, 2004). However, very recently a study suggested that HSP90 function is required for RISC function (Pare et al., 2009), and genetic studies in Arabidopsis revealed that a known binding partner of HSP90, Cyclophilin 40, is required for miRNA activity (Smith et al., 2009). HSP90 is part of a multiprotein chaperone complex that is dependent on ATP activity. Unlike other molecular chaperones, HSP90 seems to only act upon a specific subset of about 200 proteins (Picard, 2002). The majority of the set are made up of signaling proteins, cell cycle regulators, and apoptotic factors (Workman et al., 2007). HSP90 may regulate the function or turnover of its client proteins. In the presence of HSP90 inhibitors, such as geldanamycin, which mimics ATP binding (Smith et al., 1995), the client proteins become unstable and are quickly degraded. Because of the vast majority of HSP90 client proteins being mutated in cancer, HSP90 is a promising drug target. Geldanamycin analogues such as 17AAG [17-(allylamino)-17-demethoxygeldanamycin] are currently in clinical trials (Banerji et al., 2005).
In this study, we have found that visible P-bodies were abolished when cells were treated with geldanamycin or 17AAG. HSP90 inhibitors were shown to significantly reduce the levels of human Argonautes and GW182 proteins. Our data also indicates that loss of P-bodies and the geldanamycin-dependent decrease in expression of key components of the miRNA regulatory pathway did not result in alteration of miRNA level or miRNA-mediated gene repression. However, the efficacy of exogenous siRNAs was significantly reduced. Therefore, we propose that geldanamycin inhibits programming of RISC by facilitating the depletion of Argonautes that are not bound to small RNAs.
MATERIALS AND METHODS
Reagents
The following HSP90 inhibitors were used: 10 mM stock solutions of geldanamycin and 17AAG (LC Laboratories, Woburn, MA) were dissolved in dimethylsulfoxide and stored at −80°C. MG132 (Calbiochem, La Jolla, CA) was dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich, Dorset, United Kingdom).
Antibodies
Antibodies and dilutions used for Western blotting were as follows: monoclonal mouse anti-α-tubulin (Sigma-Aldrich), 1:10,000; monoclonal mouse anti-β-actin (Oncogene Research Products, Boston, MA), 1:250,000; mouse anti-Dicer (Abcam, Cambridge, United Kingdom), 1:250; monoclonal mouse anti-FLAG (Sigma-Aldrich), 1:1000 and 1:100 for immunofluorescence; rat monoclonal anti-Ago2 (11A9; Rudel et al., 2008), 1:100; rat monoclonal to Ago1 (Rudel et al., 2008), 1:50; rabbit polyclonal anti-drosha (Abcam), 1:1000; monoclonal mouse anti-HSP90 (Assay Designs, Ann Arbor, MI), 1:6000; monoclonal rabbit anti-Ras (Abcam), 1:50,000; human anti-GW182 (gift from Marvin J. Fritzler, University of Calgary), 1:2000 and 1:5000 for immunofluorescence; HRP-conjugated goat anti-mouse IgG, 1:10,000; HRP-conjugated donkey anti-rabbit IgG, 1:10,000; HRP-conjugated donkey anti-rat IgG, 1:10,000; HRP-conjugated donkey anti-Human IgG, 1:10,000; FITC-conjugated goat anti-mouse IgG, 1:100; and FITC-conjugated goat anti-Human IgG, 1:100 (all from Jackson ImmunoResearch, West Grove, PA).
Oligonucleotides
The following oligonucleotides were used: RNA oligonucleotides (MWG Eurofins, Ebersberg, Germany) for Northern hybridization to detect mir-21 (5′-UCAACAUCAGUCUGAUAAGCUA-3′) and to detect let-7 (5′-UAUACAACC UACUACCUCAUU-3′); DNA oligonucleotides (Sigma) for Northern hybridization to detect U6: U6-fwd (5′-GGAACGATACAGAGAAGATTAGCATGGCCCCTGCGCAAGG-3′) and U6-rev (5′-CCTTGCGCAG-3′); siRNAs (MWG) to knock down pGL3: sense 5′-CUUACGCUGAGUACUUCGAdTdT-3′; nontargeting siRNA duplex (MWG): sense 5′-AGGUAGUGUAAUCGCCUUGdTdT-3′; 2′-O-methyl oligonucleotides (Dharmacon, Boulder, CO): let-7 complementary (5′-Bio-UCUUCACUAUACAACCU CUA CCU CAACCUU-3′) and control 2′-O-Methyl-oligonucleotide (5′-Bio-CAUCACGUACGCGGAAUCUUCGAAAUGUC-3′).
Plasmids
pRL-TK, pGL2, and pGL3 (Invitrogen, Paisley, United Kingdom) and psiCHECK2-let-7X8 and psiCHECK2-let-7X0 (Iwasaki et al., 2009; gifts from Yukihide Tomari, University of Tokyo) were used in this study. Reporters that contain the 3′ untranslated regions (UTRs) of nRAS and kRAS were pGL3-NRASlong and PGL3-KRAS (Johnson et al., 2005). To produce let-7X3 (renilla luciferase that contains three perfect complementary let-7a sites) an oligo containing three target sites for human let-7a (5′-GTTGCGGCCGCTGAGGTAGTAGGTTGTATAGTTTCGACTGAGGTAGTAGGTTGTATAGTTTCGACTGAGGTAGTAGGTTGTATAGTTCTCGAGTTG-3′) and let-7X3m (which carries three point mutations in the let-7a seeds and one at the cleavage sites) an oligo with mutated target sites (5′-GTTGCGGCCGCTGTCCTAGTAGCTTGTATAGTTTCGACTGTCCTAGTAGCTTGTATAGTTTCGACTGTCCTAGTAGCTTGTATAGTTCTCGAGTTG-3′) were made double-stranded using Klenow's reagent, digested with NotI and XhoI, and ligated into psiCheck-2 (Promega, Southampton, United Kingdom) that had been linearized with the same restriction enzymes. To produce let-7X1 (renilla luciferase which contains one perfect complementary let-7a sites) oligos containing one target site for human let7a sense 5′-GATCGCTCGAGAACTATACAACCTACTACCTCAGCGGCCGCTG-3′ and antisense 5′-CAGCGGCCGCTGAGGTAGTAGGTTGTATAGTTCTCGAGCGATC-3′ were annealed and let-7X1m (which carries three point mutations in the let-7a seed and one at the cleavage site) oligos containing mutated target site sense 5′ GATCGCTCGAGAACTATACAAGCTACTAGGACAGCGGCCGCTG-3′ and anti-sense 5′-CAGCGGCCGCTGTCCTAGTAGCTTGTATAGTTCTCGAGCGATC-3′ were annealed, digested with NotI and XhoI, and ligated into psiCheck-2 (Promega) that had been linearized with the same restriction enzymes. For production of FLAG::Ago2(PAZ10), DNA encoding part of Ago2 that includes the PAZ domain was obtained by a double restriction digest of Ago2(PAZ10)-Myc (Liu et al., 2005b) using EcoRV and PasI. This fragment was used to replace the corresponding part of wild-type Ago2 in a pCMV5 vector expressing FLAG-tagged Ago2 (CLS Cloning Service, University of Dundee). pcDNA5 FRT/TO FLAG-1 TNRC6C and pCDNA5 FRT/TO FLAG-1 Ago2 plasmids (CLS Cloning Service, University of Dundee) were used for generating stable inducible cells.
Cell Culture
HeLa and U2OS cells were maintained at 37°C, 5% CO2, in DMEM (GlutaMax, Invitrogen, Paisley, United Kingdom) supplemented with 10% FCS and 60 U/ml penicillin and streptomycin. Stably expressing FLAG-Ago2 and FLAG-TNRC6C HEK-293 Flp-In (T-Rex) cells under the control of a tetracycline responsive promoter were generated by cotransfecting each plasmid, either pcDNA5 FRT/TO FLAG-1 Ago2 or pcDNA5 FRT/TO FLAG-1 TNRC6C, with pOG44 using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions, followed by selection with hygromycin B (100 μg/ml) and blasticidin (15 μg/ml). After selection cells were cultured in DMEM supplemented with 10% FCS (tetracycline free) and 60 U/ml penicillin and streptomycin and 100 μg/ml Hygromycin B and 15 μg/ml blasticidin. Cells were induced overnight with tetracycline (100 ng/ml).
Transfections
For dual luciferase assays, DMSO or geldanamycin (10 μM 16 h) pretreated HeLa cells were transfected in triplicate with luciferase constructs (0.16 μg/well). Transfections were performed in 24-well plates using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. After 6 h the media were replaced with full media containing either DMSO or geldanamycin (10 μM) for another 16 h.
For transfection and cotransfection with siRNAs, DMSO or geldanamycin (10 μM; 16 h) pretreated HeLa cells were cotransfected in triplicate with increasing amounts of siRNA against pGL3 and DNA plasmids, pGL3 (0.2 μg/well) and pRL-TK (0.1 μg/well), using Lipofectamine 2000 (Invitrogen). Six hours after transfection, the media were replaced with full media containing either DMSO or geldanamycin (10 μM) for another 16 h. Also, HeLa cells were cotransfected with increasing amounts of siRNA against pGL3 and either cognate-targeted plasmids pGL3 (1 μg/well) and nontargeted pRL-TK (0.5 μg/well) or nontargeted pRL-TK plasmid (1.5 μg/well). Transfections were performed in six-well plates with 10 μl of Lipofectamine 2000 (Invitrogen). After 6 h, the media were replaced with full media containing either DMSO or geldanamycin (10 μM) for another 16 h.
HeLa cells were cotransfected for 24 h with plasmids expressing either FLAG::Ago2 (2.5 μg) or FLAG::Ago2(PAZ10) (4 μg) or Ago2 and siRNA against pGL3 (10 nM) or FLAG::Ago2(PAZ10) and siRNA against pGL3 (10 nM) with Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions to generate equal expression of the constructs. Transfected cells were then treated with either DMSO or geldanamycin (10 μM; 16 h).
Dual Luciferase Assays
All cells were harvested with 100 μl 1× passive lysis buffer (Promega) per well of a 24-well plate. Luciferase activity was quantified using a dual luciferase reporter assay system (Promega), and the luminescence was measured on a Microlumat Plus LB96V microplate luminometer (EG&G Berthold, Natick, MA).
Cell Lysis, Immunoprecipitation, and Immunoblotting
HeLa cells were treated with either DMSO or geldanamycin for 16 h. Lysates were prepared by lysing cells in NP40 lysis buffer (50 mM Tris/HCl, pH 7.5, 150 mM sodium chloride, 1% [vol/vol] NP40 (alternative), 10 μM DTT, and one complete protease inhibitor cocktail tablet [Roche, Lewes, East Sussex, United Kingdom] per 10 ml of buffer). Immunoprecipitation (IP) of Ago2 was performed using rat monoclonal anti-Ago2 [11A9] for 2 h at 4°C on a carousel mixer. rec-protein G Sepharose beads (Invitrogen) were washed three times with 1 ml Tris-buffered saline and 0.1% Tween (TBST) and resuspended in NP40 lysis buffer and then added to the IPs for an additional hour. Stably expressing FLAG-Ago2 HEK-293s Flp-In (T-Rex) cells under the control of a tetracycline-responsive promoter were lysed in NP40 buffer with the addition of RNAse inhibitor 40 U/ml (New England Biolabs, Hitchin, Herts, United Kingdom). IP of FLAG-Ago2 was performed using ANTI-FLAG M2 affinity gel. The bound fractions were washed three times for 5 min with NP40 lysis buffer. Ago2-associated proteins were eluted by boiling in SDS loading dye and were subjected to analysis by SDS-PAGE and Western blotting.
The protein concentrations of cell lysates were determined by BCA assay (Thermo Scientific, Waltham, MA). Samples in 1× SDS loading dye were heated to 95°C for 10 min before being resolved in 10% SDS-polyacrylamide gels and transferred to PVDF membranes (Millipore, Watford, Herts, United Kingdom). Membranes were blocked in 5% dry milk (wt/vol)/TBST for 30 min, then incubated with primary antibody diluted in 1× TBS, 2% BSA (wt/vol), 0.1% Tween-20, and 0.1% NaN3, and incubated with the membrane overnight at 4°C. HRP-conjugated secondary antibodies diluted in 5% dry milk [wt/vol]/TBST were incubated with the membrane for 1 h at room temperature. Membranes were visualized with ECL (Supersignal West Pico Chemiluminescent Substrate [Thermo Scientific] or Immoblion Western [Millipore]).
RNA Isolation and Northern Blotting
RNA was isolated with Trizol reagent (Invitrogen). Detection of small RNAs was performed using a sensitive Northern blot method described by Pall et al. (2007) and were probed with the RNA oligonucleotides described above after 5′ end labeling with polynucleotide kinase (New England Biolabs). Hybridization was done at 37°C overnight, and the blots were washed twice for at least 1 h at 37°C in 2× SSC, 0.1% (wt/vol) SDS. For U6, a DNA probe was synthesized from a single-stranded template using Klenow reagent (Stratagene, La Jolla, CA) on the oligonucleotides described above in the presence of labeled dATP. After synthesis, the duplex was denatured at 95°C, and hybridization and washing were performed at 55°C. Northern membranes were stripped by boiling for 5 min in 0.1% SDS. Imaging was performed with FLA-5100 phosphoimager (Fujifilm, Tokyo, Japan) using Fujifilm screens and visualized and quantified with ImageGauge 4.1 (Fujifilm).
Immobilized 2′-O-methyl Oligonucleotide Capture of miRNA Complexes
Stably expressing FLAG-Ago2 HEK-293s Flp-In (T-Rex) cells under the control of a tetracycline-responsive promoter were lysed in NP40 buffer with added RNAse inhibitor (40 U/ml; New England Biolabs) and half of the the lysate was used to perform the FLAG IP (described above). The remaining lysate was incubated overnight at 4°C with 2′-O-methyl oligonucleotides (100 pmol) that were bound to 25 μl of Dynabeads M-270 Magnetic Streptavidin (Dynal Biotech, Lake Success, NY) per reaction. The bound fraction was washed with lysis buffer three times, collected with a magnetic stand, and assayed by Western blotting.
HeLa cells were pretreated with either DMSO or geldanamycin for 16 h. Cell were lysed in NP40 buffer with added RNAse inhibitor (40 U/ml; New England Biolabs) and incubated overnight at 4°C with 2′-O-methyl oligonucleotides (100 pmol) that were bound to 25 μl of Dynabeads M-270 Magnetic Streptavidin (Dynal Biotech) per reaction. The bound fraction was washed with lysis buffer three times, collected with a magnetic stand, and assayed by Western blotting.
Immunofluorescence
Cells were grown on coverslips before treatment as indicated and fixed for 5 min in 4% PFA/PBS. Cells were permeabilized for 10 min with 0.1% Triton X-100/PBS. Cells were then blocked in 5% normal donkey serum and TBST for 30 min before washing three times for 5 min in TBST. Cells were stained with primary antibody diluted in blocking solution for 1 h at room temperature before washing three times for 5 min in TBST and were incubated with secondary antibodies for a further hour and washed three times for 5 min in TBST. Cells were then stained with 0.1 mg/ml DAPI and mounted for imaging with Vectashield mounting medium (Vector Laboratories, Peterborough, United Kingdom). Images were collected using a Deltavision DV3 widefield microscope and processed using Softworx (Applied Precision, Issaquah, WA) and OME software. Images are presented as maximal intensity projections.
Quantification of Western Blots and Northern Blots
Densitometery quantification was performed using ImageGuage (Fujifilm).
RESULTS
Geldanamycin Decreases the Levels of Mammalian Argonaute and GW182 Proteins
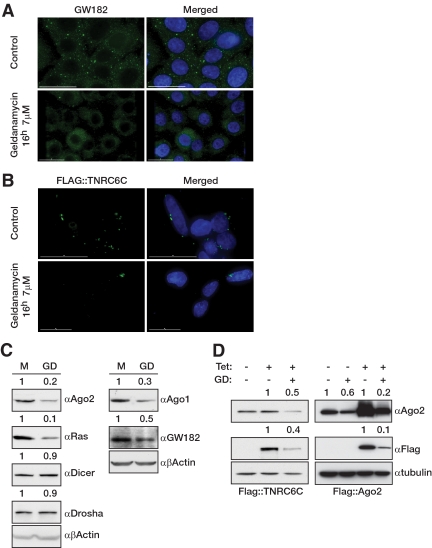
By testing several factors that may influence the microscopic appearance of diverse cytoplasmic and nuclear structures, we have found that geldanamycin, a potent inhibitor of HSP90, significantly decreases the number of visible P-bodies in mammalian cells. Immunofluorescence studies showed that in the presence of geldanamycin, the P-body marker GW182 became dispersed, and the number and size of visible P-bodies were decreased compared with the mock-treated cells (Figure 1A). Similar results were obtained when we followed the expression and localization of cells that stably expressed FLAG-tagged TNRC6C, under the control of a tetracycline-responsive promoter (Figure 1, B and D). It has been shown that geldanamycin dampens the expression of mammalian Ago2 in vitro (Tahbaz et al., 2001); however, the depletion of Ago2 does not significantly alter the phenotype of visible P-bodies (Lian et al., 2007; Jagannath and Wood, 2009). Therefore we tested the expression levels of key components of the miRNA pathway, and we confirmed that endogenous and overexpressed Ago2 and endogenous Ago1 were sensitive to geldanamycin (Figures 1, C and D, and 2A) and 17AAG (Supplementary Figure 1A). Overexpressed human Ago3 also failed to accumulate in HeLa cells treated with geldanamycin (Supplementary Figure 1B). In addition, we have shown that the expression of endogenous GW182 and stably overexpressed TNRC6C also decreased after geldanamycin treatment (Figures 1, C and D, and 2A). In contrast Drosha and Dicer, two key components of miRNA maturation, did not seem to respond to geldanamycin treatment (Figure 1C). We could alleviate the effects of geldanamycin upon Ago1 and Ago2 by the addition of the proteasome inhibitor MG132, suggesting that HSP90 is required for protecting Ago1 and Ago2 from degradation by the proteasome (Supplementary Figure 1C).
Figure 1.
Geldanamycin treatments disperse microscopic P-bodies and decrease the level of Argonautes and GW182 proteins. (A and B) Geldanamycin decreases the size and number of P-bodies in human cells. HeLa (A) and T-Rex 293 (B) cells were treated with geldanamycin (7 μM for 16 h), and P-bodies were visualized with immunostaining for endogenous GW182 (A) or overexpressed FLAG-tagged TNRC6C (B). DAPI staining was used to visualize cell nuclei. (C) Geldanamycin dampens endogenous Argonautes and GW182 expression. HeLa cells were treated with geldanamycin (10 μM for 16 h), and the expression level of proteins involved in the miRNA pathway as well as the let-7 target Ras were followed by Western blotting. β-Actin was used as a loading control. The numbers on the top of each panel indicate the relative abundance of the respective proteins. (D) Overexpressed Ago2 and TNRC6C are sensitive to HSP90 inhibition. 293 T-Rex cells stably expressing tetracycline-inducible (Tet) FLAG-tagged hAgo2, and FLAG-tagged TNRC6C were treated with geldanamycin (GD; 10 μM for 16 h), and the expressions of the transgenes were followed by Western blot using a FLAG antibody. The efficiencies of the geldanamycin treatments were confirmed by Western blotting of endogenous hAgo2. α-Tubulin was used as the loading control. The numbers on the top of each panel indicate the relative abundance of the respective proteins.
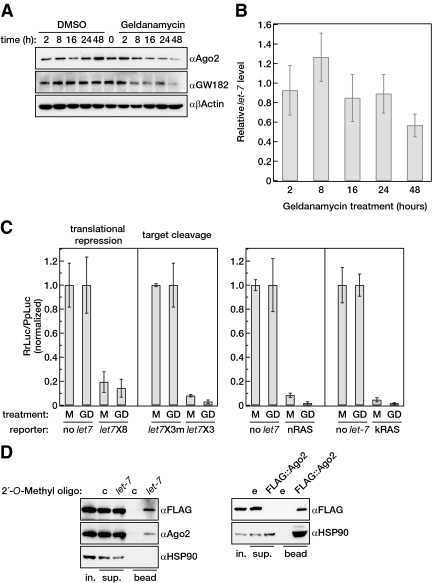
Figure 2.
Geldanamycin treatment does not alter miRNA level and miRNA function in human cells. (A) Geldanamycin decreases Ago2 and GW182 levels after 8 h treatment. HeLa cells were treated with DMSO or with geldanamycin (10 μM) for the indicated times. The protein level of endogenous hAgo2 and GW182 were followed by Western blotting. β-Actin was used as loading control for Western blotting. (B) miRNA level is unaltered after up to 24 h of geldanamycin treatment. HeLa cells were treated with DMSO or geldanamycin (10 μM) for the indicated times shown in A. The relative let-7 level between the DMSO and geldanamycin-treated cells was calculated at each time point using U6 as a loading control. The graph shows the mean of three independent experiments; error bars, ±SE. (C) Inhibition of HSP90 activity does not impair miRNA functions. HeLa cells were pretreated with geldanamycin (10 μM for 16 h) and transfected with luciferase reporters that measure translational repression and RNAi-mediated by endogenous let-7 (left panel) and reporters that contain 3′UTRs of known let-7–targeted mRNAs (right panel). The dual luciferase data obtained from the geldanamycin-treated cells (GD) were normalized to the luciferase reading of the mock-treated cells (M). Error bars, ±SE of three experiments. Left, no let-7, Renilla luciferase containing no let-7 sites; let-7X8, Renilla luciferase containing eight let-7 sites that mediate translational repression; let-7X3, Renilla luciferase contains three perfect complementary let-7a sites; and let-7X3m, similar to let-7X3 but carrying three point mutations in the let-7a seeds and one at the cleavage sites. Right, nRAS, Firefly luciferase carrying the 3′UTR of nRAS; kRAS, Firefly luciferase carrying the 3′UTR of kRAS. (D) HSP90 association with complexes containing mature miRNAs is not stoichiometric. Affinity purification was carried out with control (c) and 2′-O-methyl oligo complementary to human let-7a (let-7) in T-Rex cells inducible expressing FLAG::Ago2. The bound fractions were assayed for the presence of FLAG::Ago2, hAgo2, and HSP90 with Western blotting (left panel); 10% of total lysate was loaded and half of the bound fraction. FLAG IP was carried out in the same lysate using identical conditions. The bound fraction was assayed for FLAG::Ago2, and HSP90 with Western blotting (right panel). Empty bead (e) was used as a negative control in the IP experiment. Ten percent of the total lysate was loaded, and half of the IP for the HSP90 panel and 10% of the total IP for the Flag::Ago2 panel was loaded.
Geldanamycin Treatment Does Not Affect miRNA-mediated Gene Regulation
Interestingly, when we examined the miRNA levels in the geldanamycin-treated cells, we found no decrease in the steady-state levels of let-7 and miR-21, two abundant miRNAs in HeLa cells, up to and beyond 24 h after treatment (Figure 2B and Supplementary Figure 2, B and C), despite Ago2 and GW182 protein levels significantly decreasing by 16 h after treatment (Figure 2A, Supplementary Figure 2A).
Next we investigated whether these miRNAs remained functional in the presence of geldanamycin. Mock and geldanamycin (10 μM for 16 h) pretreated HeLa cells were cotransfected with luciferase reporters that are regulated by endogenous let-7. One miRNA sensor contained eight let-7 sites in the 3′UTR that regulate the reporter expression through miRNA-mediated translational repression (Iwasaki et al., 2009). The second construct contained three perfect complementary sites to let-7a; thus it is regulated by sequence-specific RNA cleavage by endogenous let-7 miRNAs. Because the construct containing three perfect complementary sites could be deemed a strong repressor, we also used a third reporter construct containing only one perfect complementary site to let-7a. For controls we used similar reporters that contained either no let-7 target sites, or the let-7 target sites were mutated to make it insensitive to let-7. We have also used previously published reporters that contain the 3′UTRs of nRAS and kRAS, which have been shown to be regulated by let-7 in human cells (Johnson et al., 2005). Figure 2C and Supplementary Figure 3A show that geldanamycin treatment had no effect on the expression of the reporter plasmids. Endogenous let-7 was able to regulate through translational repression as well as RNA interference (RNAi) despite the significant loss of endogenous Ago2 in the geldanamycin-treated cells (Supplementary Figure 3, B and C). In addition, we observed a remarkable drop rather than increase in the endogenous RAS protein level in the geldanamycin-treated cells (Figure 1C), the opposite effect of that which would be expected if miRNA regulation had been impaired. These results suggest that HSP90 inhibition has no effect on the activity of the functional miRISC.
Having established that HSP90 activity is not required for the activity of endogenous miRNA, we tested if HSP90 associates with Argonautes bound to miRNAs. For this, we used a biotinylated let-7 complementary 2′-O-methyl oligo that was shown to effectively inhibit miRNA-programmed RISCs in vitro and in vivo and is able to pull down proteins that associate with single-stranded miRNAs (Hutvagner et al., 2004; Meister et al., 2004; Robb and Rana, 2007). Using this approach, we showed that HSP90 was absent from the bound fraction of the affinity purification carried out with the biotinylated let-7 complementary 2′-O-methyl oligo in spite of the fact that Ago2 and transfected FLAG::Ago2 was retained (Figure 2D and Supplementary Figure 3D). This is unlikely to be a result of the conditions of the pull down because using an identical experimental set up, we could successfully coimmunoprecipitate HSP90 with overexpressed FLAG::Ago2 (Figure 2D). This suggests that HSP90 does not bind Argonaute complexes that contain unwound miRNAs stoichiometrically; therefore, HSP90 probably functions upstream from RISC action.
HSP90 Inhibition Affects miRNA Loading
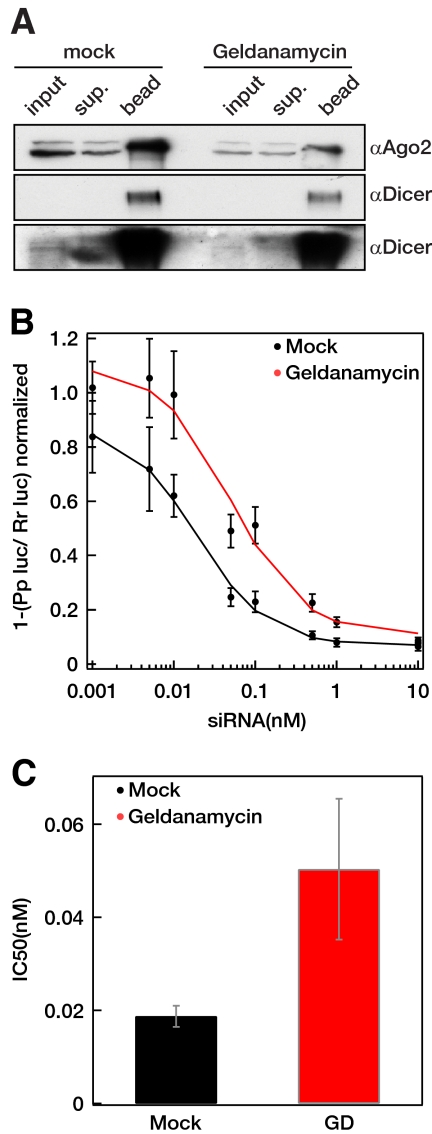
Because geldanamycin has no affect on RISC function, we tested if it influences small RNA loading. It has already been published that geldanamycin prevents the direct binding of Dicer to Argonaute in vitro and was suggested to disrupt the RISC loading complex (RLC) formation in human cells (Tahbaz et al., 2004). Very recently, it has been suggested that the mechanism responsible for small RNA loading in human cells is analogous to fly Ago1, which does not require the RLC (Yoda et al., 2010). In spite of this, we have tested if geldanamycin affects the interaction of Dicer and Ago2 in vivo. We immunoprecipitated endogenous Ago2 from mock- and geldanamycin-treated cells and assayed the level of endogenous Dicer that coimmunoprecipitated (Figure 3A and Supplementary Figure 4A). Dicer levels were comparable in the bound fraction of both Ago2 immunoprecipitates, despite the lower levels of Ago2 detected in geldanamycin cells.
Figure 3.
Inhibition of HSP90 does not affect Argonaute–Dicer interaction but impairs siRNA efficiency (A) Geldanamycin treatment does not change the amount of Dicer associated with hAgo2. hAgo2 was immunoprecipitated from mock- and geldanamycin-treated (10 μM for 16 h) Hela cells, and the bound fractions were assayed for the presence of hAgo2 and Dicer with Western blotting. Sup, supernatant. Ten percent of the total lysate and 25% of the total IP were loaded. A longer exposure of the Dicer Western blot is presented to show input Dicer levels. (B and C) Geldanamycin treatment decreases siRNA efficiency. Mock- and geldanamycin-treated (10 μM for 16 h) HeLa cells were cotransfected with renilla and firefly luciferase plasmids and with increasing concentrations of siRNA targeting the firefly luciferase. Dual luciferase data at each concentration were normalized to data obtained with a nontargeting siRNA (10 nM) and fitted to a sigmoid curve using a Hill coefficient of 1. The concentration of siRNA required for half-maximal inhibition (IC50) was calculated and separately plotted (C).
Next we tested if the loading of exogenous small RNA is affected by HSP90 inhibition. Mock-treated and geldanamycin-pretreated HeLa cells were transfected with increasing amounts of firefly luciferase siRNAs and plasmids expressing firefly and renilla luciferases. Figure 3, B and C, and Supplementary Figure 4, B–D, show that geldanamycin treatment significantly reduced the level of endogenous Ago2 and the efficacy of the luciferase siRNA by decreasing the IC50 value three- to sevenfold.
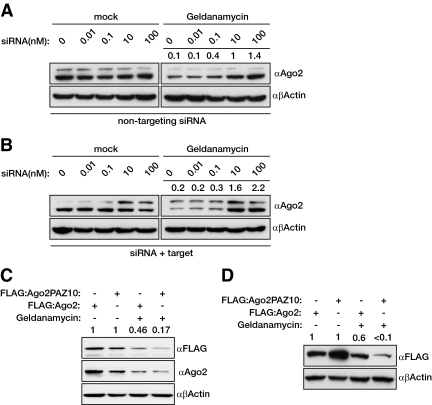
Unloaded Argonautes Are Sensitive to Geldanamycin
Our previous experiment suggests that the inhibition of HSP90 impairs siRNA function in mammalian cells; however, this experiment cannot distinguish between either the impairment of siRNA loading or the activity of siRNA programmed RISC. However, we already have shown that geldanamycin does not influence endogenous RISC activity; therefore, we hypothesized that HSP90 activity is required for the stability of RNA-free Argonautes. If HSP90 activity is required to stabilize RNA-free Argonautes, then preloading siRNA into Argonautes should alleviate geldanamycin inhibition. We transfected increasing amounts of luciferase siRNA into HeLa cells with and without providing the cognate target luciferase expression plasmid and then subsequently treated the cells with geldanamycin. We observed that transfection of increasing amounts of siRNA prevented geldanamycin having an effect on Ago2 levels (Figure 4A). The increased stability of Ago2 was concentration dependent but the presence or absence of the target mRNA had no effect (Figure 4B). We also predicted that Ago2 that is impaired in small RNA binding would be more sensitive to geldanamycin treatment than wild-type Ago2. To test this we used a characterized Ago2 mutant containing 10 mutations in the PAZ domain that was shown to bind small RNAs less effectively (Liu et al., 2005b). Figure 4C shows that the transiently expressed PAZ mutant Ago2 was more sensitive to geldanamycin treatment than the wild-type Ago2 construct. Next, we repeated this experiment while cotransfecting 10 nM of a nontargetting siRNA together with the FLAG-tagged Ago plasmids. As Figure 4D shows, the cotransfected siRNA has a more pronounced effect, alleviating the effects of HSP90 inhibition on wild-type Ago2 but not the PAZ mutant. These data together strengthen our hypothesis that HSP90 is mainly required for the stability of unloaded Argonautes; however, we cannot rule out the possibility that the conformational changes that may be introduced by the mutations influence the stability of the Ago2 PAZ mutant.
Figure 4.
HSP90 stabilizes unloaded Argonautes. (A and B) Increasing the level of loaded hAgo2 makes it more resistant to geldanamycin treatment. HeLa cells were transfected with increasing amount of siRNA targeting the Firefly luciferase without (A) and with (B) the cognate target plasmid followed by treatment of either DMSO or geldanamycin (10 μM for 16 h). The level of endogenous hAgo2 was assayed with Western blotting. β-Actin was used as a loading control. (C and D) Argonaute that is impaired in small RNA binding is more sensitive to HSP90 inhibition. (C) HeLa cells were transiently transfected (24 h) with FLAG-tagged hAgo2 (FLAG:Ago2) or FLAG-tagged hAgo2 with a series of mutations within its PAZ domain (FLAG:Ago2PAZ10) and were subsequently treated with either DMSO or geldanamycin (10 μM for 16 h). Expressions of the overexpressed plasmids and endogenous hAgo2 were assayed with Western blotting. β-Actin was used as a loading control. (D) A similar experiment to that desribed in C, only the FLAG-tagged Ago2s were cotransfected with 10 nM nontargeting siRNA.
DISCUSSION
HSP90 together with other cochaperones are the major component of the proteome that copurifies with Argonaute and Piwi proteins (Hock et al., 2007; Landthaler et al., 2008; Vagin et al., 2009). Here we have shown that the inhibition of HSP90 activity by well-described inhibitors such as geldanamycin and 17AAG resulted in the decrease of microscopic P-bodies and the destabilization of key components of the miRNA-mediated regulatory pathway such as Argonautes and GW182 proteins. While we were revising this manuscript, the destabilization of human Ago2 by the inhibition of HSP90 has been independently reported (Suzuki et al., 2009). We identified that HSP90 inhibition reduces siRNA efficacy, and we are proposing that HSP90 is required for the stabilization of RNA free Argonautes.
Originally HSP90 function was suggested to stabilize the interaction between Dicer and human Ago2, implicating it in the facilitation of loading of miRNAs to RISC (Tahbaz et al., 2004). This role of HSP90 has been recently challenged, and it was suggested that HSP90 modulates Argonaute function at the effector step of miRNA-mediated gene regulation and miRNA-mediated sequence-specific cleavage (Pare et al., 2009). Our finding is partially consistent with the latest reports because we also have found that geldanamycin does not disrupt the interaction between Dicer and Ago2 in vivo. We also confirmed that geldanamycin treatment either abolishes or reduces the size of microscopic P-bodies (Pare et al., 2009; Suzuki et al., 2009). However, Pare and colleagues have found that inhibition of HSP90 activity impairs both miRNA function in translational repression and sequence specific RNA cleavage. In contrast, we presented several lines of evidence that show that HSP90 is not required for miRNA-mediated gene repression and RNAi at the regulatory step in human cells. First, we have shown that mature miRNA levels were not affected and that HSP90 is not a stoichiometric component of the human RISC. Second, we have explicitly demonstrated, using five let-7 regulated reporters, that HSP90 activity did not impair the activity of endogenous let-7 even though Ago2 levels were significantly decreased upon HSP90 inhibition. Furthermore, the expression of an endogenous let-7 target, Ras, declined rather than increased after geldanamycin treatment, suggesting that let-7 function was not altered by the drug. This is consistent with previous findings that Ras directly interacts with Raf and upon HSP 90 inhibition the interaction dissociates and Raf is degraded (Schulte et al., 1995).
Our data suggest that HSP90 activity is required for the stability of Ago2 when it is not loaded with small RNA. We have shown that HSP90 was not associated with Ago2 when it was affinity purified with the let-7 complementary biotinylated 2′-O-methyl oligo. We then identified that Ago2 sensitivity to geldanamycin could be attenuated by the transfection of siRNA into the cells, presumably by generating excess siRNA-associated Argonautes. Finally, we demonstrated that Ago2 defective in small RNA binding was more sensitive to HSP90 inhibition. In our interpretation, the in vitro experiment conducted by Pare et al. (2009) in fly ovary lysates also supports our findings that HSP90 inhibition impairs small RNA loading, because they have shown that geldanamycin-treated lysate could be poorly programmed with exogenously siRNAs to cleave the complementary target.
The simplest hypothesis for the role of HSP90 in miRNA-mediated gene regulation is that HSP90 chaperones RNA-free Argonautes, allowing them to adopt a conformation that facilitates efficient loading of an RNA substrate. After Argonaute binds to the RNA substrate, HSP90 dissociates. This may be analogous to the role that HSP90 has in stabilizing steroid receptors with an exposed hydrophobic cleft to facilitate ligand binding (Pratt et al., 2008). Indeed, structural and biochemical studies of the PAZ domain of Argonautes showed that it binds to the 3′ overhang of a RNA molecule with a fairly closed hydrophobic binding pocket (Lingel et al., 2003, 2004; Song et al., 2003; Yan et al., 2003). This interaction was shown to be relatively weak in vitro, suggesting that it may need such a chaperone to be effective in binding in vivo (Lingel et al., 2003).
Interestingly, while we were revising this manuscript, a study proposed that small RNAs in human cells do not form the canonical RLCs that has been described to be necessary for loading Drosophila Ago2. It is suggested that loading of human Argonautes is analogous to Drosophila Ago1 where loading is uncoupled from Dicer (Yoda et al., 2010). Also, it was shown that the loading of small RNAs in human cells is stimulated by ATP (Yoda et al., 2010). Our findings may suggest that small RNAs are directly loaded into Argonautes, and the ATP dependence of this loading event may reflect the chaperoning activity of HSP90, which protects unloaded Argonautes from degradation by the proteosome.
We have also shown that inhibition of HSP90 reduced the number of microscopic P-bodies. This is probably a consequence of the destabilization of the GW182 protein family, a key component of the P-bodies, rather than Ago2 because the presence of Ago2 is not required to form this cytoplasmic structure (Lian et al., 2007; Jagannath and Wood, 2009). Also, when we rescued Ago2 from HSP90 inhibition by transfecting increasing amounts of siRNA into the cells before geldanamycin treatment (Figure 4A), we did not observe a significant increase in the number or size of P-bodies in the geldanamycin-treated cells (Supplementary Figure 5, A and B), suggesting that the depletion of P-bodies is independent of the effect of HSP90 on Argonautes. Interestingly, the almost total depletion of such cytoplasmic foci did not result in impairment of miRNA-mediated gene regulation, supporting the recent hypothesis that visible P-body formation is not a prerequisite for normal function of miRNAs (Eulalio et al., 2009). The drastic drop in the number of visible P-bodies also coincides with a marked decrease of Argonautes and GW182 proteins, two key proteins in miRNA-mediated gene regulation, without any detectable defect in the function of endogenous miRNAs. This may be because the bulk of P-bodies contain GW182 proteins that are not active in miRNA-mediated gene regulation.
In our studies we applied geldanamycin for a limited time, usually up to 16 h, which caused an alteration in the protein levels of Argonaute and GW182. In this timescale we observed a significant reduction of exogenous siRNA efficacy without the impairment of endogenous miRNA functions. Our findings also suggest that endogenous miRNA complexes are very stable with a turnover exceeding 16 h. However, longer exposure of geldanamycin resulted in a decline of endogenous miRNA levels (Figure 2B and Supplementary Figure 2B), suggesting that in the long-term, HSP90 inactivation would inhibit miRNA-mediated gene repression by preventing loading of miRNAs because of the loss of Argonautes.
The antitumor effects of HSP90 inhibitors have been quite astounding: geldanamycin analogues that are currently in clinical trials have been shown to slow tumor growth by 50% (Goetz et al., 2003). HSP90 inhibitors potentially benefit, by inhibiting the functions of overexpressed miRNAs that promote oncogenesis (Sotiropoulou et al., 2009). It would be interesting to see how such treatments influence miRNA-mediated gene regulation in tumors.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for Gregory Hannon (Cold Spring Harbor Laboratory) for providing the PAZ mutant Ago2 clone, Gunter Meister (Max Planck Institute of Biochemistry) for the Ago2 antibody, Frank Slack (Yale University) for the nRas and kRas Luciferase constructs, and Marvin J. Fritzler for the GW182 antibody. We also thank Adel Ibrahim for the help in cloning the FLAG::TNRC6C and FLAG::Ago2 construct. We are grateful for Martin Simard for useful discussion of the manuscript. The work has been funded by the Wellcome Trust and European Framework 6 SIROCCO consortium fund (G.H.). G.H. is a Wellcome Trust CD fellow. M.J. is funded by the Medical Research Council Ph.D. training program. M.C.G. is supported by a fellowship from the RUBICON European Union FP6 Network of Excellence. A.S. is funded by a BBSRC Doctoral Training Grant.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-10-0885) on March 17, 2010.
REFERENCES
- Banerji U., Walton M., Raynaud F., Grimshaw R., Kelland L., Valenti M., Judson I., Workman P. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin. Cancer Res. 2005;11:7023–7032. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4, NOT deadenylase and DCP1, DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S. N., Habermacher R., Martine U., Closs E. I., Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Chekulaeva M., Filipowicz W., Parker R. Multiple independent domains of dGW182 function in miRNA-mediated repression in Drosophila. RNA. 2009;15:794–803. doi: 10.1261/rna.1364909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Rana T. M. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4(7):e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Spencer A., Morita K., Han M. The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell. 2005;19:437–447. doi: 10.1016/j.molcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007a;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 2007b;27:3970–3981. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Helms S., Fritzsch C., Fauser M., Izaurralde E. A C-terminal silencing domain in GW182 is essential for miRNA function. RNA. 2009;15:1067–1077. doi: 10.1261/rna.1605509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Huntzinger E., Izaurralde E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat. Struct. Mol. Biol. 2008;15:346–353. doi: 10.1038/nsmb.1405. [DOI] [PubMed] [Google Scholar]
- Eystathioy T., Chan E. K., Tenenbaum S. A., Keene J. D., Griffith K., Fritzler M. J. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell. 2002;13:1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M. P., Toft D. O., Ames M. M., Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann. Oncol. 2003;14:1169–1176. doi: 10.1093/annonc/mdg316. [DOI] [PubMed] [Google Scholar]
- Hock J., Weinmann L., Ender C., Rudel S., Kremmer E., Raabe M., Urlaub H., Meister G. Proteomic and functional analysis of Argonaute-containing mRNA-protein complexes in human cells. EMBO Rep. 2007;8:1052–1060. doi: 10.1038/sj.embor.7401088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G., Simard M. J., Mello C. C., Zamore P. D. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004;2:E98. doi: 10.1371/journal.pbio.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S., Kawamata T., Tomari Y. Drosophila argonaute1 and argonaute2 employ distinct mechanisms for translational repression. Mol. Cell. 2009;34:58–67. doi: 10.1016/j.molcel.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Jagannath A., Wood M. J. Localization of double-stranded small interfering RNA to cytoplasmic processing bodies is Ago2 dependent and results in up-regulation of GW182 and Argonaute-2. Mol. Biol. Cell. 2009;20:521–529. doi: 10.1091/mbc.E08-08-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakymiw A., Lian S., Eystathioy T., Li S., Satoh M., Hamel J. C., Fritzler M. J., Chan E. K. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 2005;7:1267–1274. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- Jakymiw A., Pauley K. M., Li S., Ikeda K., Lian S., Eystathioy T., Satoh M., Fritzler M. J., Chan E. K. The role of GW/P-bodies in RNA processing and silencing. J. Cell Sci. 2007;120:1317–1323. doi: 10.1242/jcs.03429. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K. L., Brown D., Slack F. J. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Landthaler M., Gaidatzis D., Rothballer A., Chen P. Y., Soll S. J., Dinic L., Ojo T., Hafner M., Zavolan M., Tuschl T. Molecular characterization of human Argonaute-containing ribonucleoprotein complexes and their bound target mRNAs. RNA. 2008;14:2580–2596. doi: 10.1261/rna.1351608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaretti D., Tournier I., Izaurralde E. The C-terminal domains of human TNRC6A, TNRC6B, and TNRC6C silence bound transcripts independently of Argonaute proteins. RNA. 2009;15:1059–1066. doi: 10.1261/rna.1606309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lian S. L., Moser J. J., Fritzler M. L., Fritzler M. J., Satoh M., Chan E. K. Identification of GW182 and its novel isoform TNGW1 as translational repressors in Ago2-mediated silencing. J. Cell Sci. 2008;121:4134–4144. doi: 10.1242/jcs.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian S., Fritzler M. J., Katz J., Hamazaki T., Terada N., Satoh M., Chan E. K. Small interfering RNA-mediated silencing induces target-dependent assembly of GW/P bodies. Mol. Biol. Cell. 2007;18:3375–3387. doi: 10.1091/mbc.E07-01-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian S. L., Li S., Abadal G. X., Pauley B. A., Fritzler M. J., Chan E. K. The C-terminal half of human Ago2 binds to multiple GW-rich regions of GW182 and requires GW182 to mediate silencing. RNA. 2009;15:804–813. doi: 10.1261/rna.1229409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingel A., Simon B., Izaurralde E., Sattler M. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature. 2003;426:465–469. doi: 10.1038/nature02123. [DOI] [PubMed] [Google Scholar]
- Lingel A., Simon B., Izaurralde E., Sattler M. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nat. Struct. Mol. Biol. 2004;11:576–577. doi: 10.1038/nsmb777. [DOI] [PubMed] [Google Scholar]
- Liu J., Rivas F. V., Wohlschlegel J., Yates J. R., Parker R., Hannon G. J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005a;7:1161–1166. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Valencia-Sanchez M. A., Hannon G. J., Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 2005b;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G., Landthaler M., Dorsett Y., Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–550. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G., Landthaler M., Peters L., Chen P. Y., Urlaub H., Luhrmann R., Tuschl T. Identification of novel Argonaute-associated proteins. Curr. Biol. 2005;15:2149–2155. doi: 10.1016/j.cub.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Pall G. S., Codony-Servat C., Byrne J., Ritchie L., Hamilton A. Carbodiimide-mediated cross-linking of RNA to nylon membranes improves the detection of siRNA, miRNA and piRNA by northern blot. Nucleic Acids Res. 2007;35:e60. doi: 10.1093/nar/gkm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare J. M., Tahbaz N., Lopez-Orozco J., LaPointe P., Lasko P., Hobman T. C. Hsp90 regulates the function of argonaute 2 and its recruitment to stress granules and P-bodies. Mol. Biol. Cell. 2009;20:3273–3284. doi: 10.1091/mbc.E09-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley K. M., Eystathioy T., Jakymiw A., Hamel J. C., Fritzler M. J., Chan E. K. Formation of GW bodies is a consequence of microRNA genesis. EMBO Rep. 2006;7:904–910. doi: 10.1038/sj.embor.7400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol. Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R. S., Bhattacharyya S. N., Artus C. G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Morishima Y., Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J. Biol. Chem. 2008;283:22885–22889. doi: 10.1074/jbc.R800023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J., Behm-Ansmant I., Gatfield D., Izaurralde E. A crucial role for GW182 and the DCP 1, DCP2 decapping complex in miRNA-mediated gene silencing. RNA. 2005;11:1640–1647. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb G. B., Rana T. M. RNA helicase A interacts with RISC in human cells and functions in RISC loading. Mol. Cell. 2007;26:523–537. doi: 10.1016/j.molcel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Rudel S., Flatley A., Weinmann L., Kremmer E., Meister G. A multifunctional human Argonaute2-specific monoclonal antibody. RNA. 2008;14:1244–1253. doi: 10.1261/rna.973808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T. W., Blagosklonny M. V., Ingui C., Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J. Biol. Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- Sen G. L., Blau H. M. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 2005;7:633–636. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Whitesell L., Nair S. C., Chen S., Prapapanich V., Rimerman R. A. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol. Cell Biol. 1995;15:6804–6812. doi: 10.1128/mcb.15.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Willmann M. R., Wu G., Berardini T. Z., Moller B., Weijers D., Poethig R. S. Cyclophilin 40 is required for microRNA activity in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2009;106:5424–5429. doi: 10.1073/pnas.0812729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. J., Liu J., Tolia N. H., Schneiderman J., Smith S. K., Martienssen R. A., Hannon G. J., Joshua-Tor L. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat. Struct. Biol. 2003;10:1026–1032. doi: 10.1038/nsb1016. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G., Pampalakis G., Lianidou E., Mourelatos Z. Emerging roles of microRNAs as molecular switches in the integrated circuit of the cancer cell. RNA. 2009;15:1443–1461. doi: 10.1261/rna.1534709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Minami M., Suzuki M., Abe K., Zenno S., Tsujimoto M., Matsumoto K., Minami Y. The Hsp90 inhibitor geldanamycin abrogates colocalization of eIF4E and eIF4E-transporter into stress granules and association of eIF4E with eIF4G. J. Biol. Chem. 2009;284:35597–35604. doi: 10.1074/jbc.M109.036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahbaz N., Carmichael J. B., Hobman T. C. GERp95 belongs to a family of signal-transducing proteins and requires Hsp90 activity for stability and Golgi localization. J. Biol. Chem. 2001;276:43294–43299. doi: 10.1074/jbc.M107808200. [DOI] [PubMed] [Google Scholar]
- Tahbaz N., Kolb F. A., Zhang H., Jaronczyk K., Filipowicz W., Hobman T. C. Characterization of the interactions between mammalian PAZ PIWI domain proteins and Dicer. EMBO Rep. 2004;5:189–194. doi: 10.1038/sj.embor.7400070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin V. V., Wohlschlegel J., Qu J., Jonsson Z., Huang X., Chuma S., Girard A., Sachidanandam R., Hannon G. J., Aravin A. A. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009;23:1749–1762. doi: 10.1101/gad.1814809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P., Burrows F., Neckers L., Rosen N. Drugging the cancer chaperone HSP 90, combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann. NY Acad. Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- Yan K. S., Yan S., Farooq A., Han A., Zeng L., Zhou M. M. Structure and conserved RNA binding of the PAZ domain. Nature. 2003;426:468–474. doi: 10.1038/nature02129. [DOI] [PubMed] [Google Scholar]
- Yoda M., Kawamata T., Paroo Z., Ye X., Iwasaki S., Liu Q., Tomari Y. ATP-dependent human RISC assembly pathways. Nat. Struct. Mol. Biol. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipprich J. T., Bhattacharyya S., Mathys H., Filipowicz W. Importance of the C-terminal domain of the human GW182 protein TNRC6C for translational repression. RNA. 2009;15:781–793. doi: 10.1261/rna.1448009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.