Mitotic induction tightly associates with increased reactivity to mitotic phosphoprotein antibody MPM-2. Here we report that phosphorylation of TP motifs surrounded by hydrophobic residues at the −1 and +1 positions plays a dominant role in M phase–associated MPM-2 reactivity. The majority of these motifs are not phosphorylated by mitotic Cdk or MAPK.
Abstract
M phase induction in eukaryotic cell cycles is associated with a burst of protein phosphorylation, primarily at serine or threonine followed by proline (S/TP motif). The mitotic phosphoprotein antibody MPM-2 recognizes a significant subset of mitotically phosphorylated S/TP motifs; however, the required surrounding sequences of and the key kinases that phosphorylate these S/TP motifs remain to be determined. By mapping the mitotic MPM-2 epitopes in Xenopus Cdc25C and characterizing the mitotic MPM-2 epitope kinases in Xenopus oocytes and egg extracts, we have determined that phosphorylation of TP motifs that are surrounded by hydrophobic residues at both −1 and +1 positions plays a dominant role in M phase–associated burst of MPM-2 reactivity. Although mitotic Cdk and MAPK may phosphorylate subsets of these motifs that have a basic residue at the +2 position and a proline residue at the −2 position, respectively, the majority of these motifs that are preferentially phosphorylated in mitosis do not have these features. The M phase–associated burst of MPM-2 reactivity can be induced in Xenopus oocytes and egg extracts in the absence of MAPK or Cdc2 activity. These findings indicate that the M phase–associated burst of MPM-2 reactivity represents a novel type of protein phosphorylation in mitotic regulation.
INTRODUCTION
Induction of mitosis and meiosis in the eukaryotic cell cycle is tightly associated with a burst of protein phosphorylation. Among mitotic phosphoproteins, a large subset is recognized by the mitotic phosphoprotein mAb 2 (MPM-2), which preferentially stains mitotic cells across species (Davis et al., 1983; Davis and Rao, 1987). Because an mAb presumably recognizes one specific epitope, the M phase–associated burst of MPM-2 reactivity implies that a master protein kinase(s) phosphorylates a widely shared sequence motif during M phase induction. Because MPM-2–reactive proteins include multiple mitotic regulators and structural proteins in mitotic apparatus (Taagepera et al., 1993; Kuang et al., 1994; King et al., 1995; Mueller et al., 1995; Stukenberg et al., 1997; Wells et al., 1999; Tomashevski et al., 2001), phosphorylation of the MPM-2 epitope (sequence motif that becomes recognized by MPM-2 upon phosphorylation) is likely to be a critical event in M phase induction. However, despite a wide use of MPM-2 as a mitotic marker, the chemical basis of and responsible kinases for the M phase–associated burst of MPM-2 reactivity remain obscure.
Two systematic approaches have been utilized to deduce the MPM-2 epitope consensus sequence. Westendorf et al. (1994) phosphorylated a 15-aa peptide library displayed on phage particles with fractions of mitotic HeLa cell lysates enriched in histone H1 kinase activity (indicative of Cdc2 kinase activity) and identified the phosphopeptides that were immunoprecipitated by MPM-2. From 56 independent isolations, 16 peptide sequences were identified, and each of them contained one or two serine or threonine residues followed by proline (S/TP) motifs. When the surrounding sequences were analyzed, all of them appeared to be in a string of five amino acids, and the sequence reflecting the most frequent amino acid at each position was LTPLK, meeting the Cdc2 phosphorylation consensus sequence S/T-P-X-K/R (Langan et al., 1979; Nigg, 1991, 1993; Songyang et al., 1994; Holmes and Solomon, 1996). However, the actual MPM-2–reactive S/T-P–containing motifs in these peptides were not mapped and characterized. In the identified MPM-2–reactive proteins MPP1 and MPP2, most of the potential MPM-2 epitope sequences were significantly different from LTPLK. Moreover, phosphorylated peptide FTPLQ showed a significantly higher affinity for MPM-2 than phosphorylated peptide LTPLK, suggesting that a basic residue at the +2 position was selected by Cdc2 being the kinase rather than by MPM-2 recognition. Thus the only concrete conclusion from this pioneer work is that MPM-2 recognizes a subset of phosphorylated S/T-P motifs that contains at least four residues. To further define this subset of phosphorylated S/T-P motifs, Yaffe et al. (1997) screened degenerate peptide libraries that centered on phosphorylated S or SP by MPM-2 immunoprecipitation. Their results showed that MPM-2 preferentially recognizes phosphorylated SP motif that is surrounded by aromatic or hydrophobic residues at the −1, −2, and −3 and the +1 positions, supporting the concept that MPM-2 recognizes a subset of phosphorylated S/T-P motifs. However, whether this longer consensus sequence is required for or maximizes the ability of SP phosphorylation to generate MPM-2 reactivity was not determined. Neither was the deduced sequence verified in MPM-2–reactive proteins.
Cdc2/cyclin B is a master proline-directed protein kinase that phosphorylates one or multiple S/TP motifs in a large number of proteins involved in mitosis and meiosis (Holmes and Solomon, 1996; Ubersax et al., 2003; Loog and Morgan, 2005; Miller et al., 2008; Holt et al., 2009; Errico et al., 2010), making it possible that Cdc2-catalyzed phosphorylation is one of the major reasons for the M phase–associated burst of MPM-2 reactivity. Consistent with this possibility, in vitro phosphorylations of MPP1, MP68, MP110, MP48, Sox3, Xbr1, MP30, and Oct91 by Cdc2 kinase generated MPM-2 reactivity (Westendorf et al., 1994; Stukenberg et al., 1997). ERK-MAP kinases (MAPK) also phosphorylate S/TP motifs in a large family of substrates (Alvarez et al., 1991; Clark-Lewis et al., 1991; Gonzalez et al., 1991). Although ERK activations are not M phase specific, phosphorylation of human Cdc25C (hCdc25C) by ERKs has been demonstrated to be M phase specific in somatic cells (Wang et al., 2007). Thus, MAPK-catalyzed phosphorylation of S/TP motifs may also contribute to the M phase–associated burst of MPM-2 reactivity. Nevertheless, whether Cdc2/cyclin B and/or MAPK indeed play major roles in the M phase–associated burst of MPM-2 reactivity has not been determined.
Recent profiling of mitotic phosphorylation events in human cell lines by comprehensive and quantitative mass spectrometry identified >1000 proteins with increased phosphorylations in mitosis, and the data clearly demonstrate that the majority of M phase–specific phosphorylations occur at S/TP motifs (Dephoure et al., 2008; Malik et al., 2009; Olsen et al., 2010). Because Cdc2 kinase is the only proline-directed kinase identified that is specifically activated during M phase induction, the M phase–specific phosphorylation of S/TP motifs has been generally attributed to Cdc2 kinase activity. Since MPM-2 recognizes a significant portion of mitotically phosphorylated S/TP motifs, characterization of the mitotic MPM-2 epitopes and responsible kinases may provide insights on whether MAPK or other kinases also play important parts in the mitotic phosphorylation of S/TP motifs. In this study, we mapped the mitotic MPM-2 epitopes in Xenopus Cdc25C (xCdc25C) and determined the role of MAPK and Cdc2 kinase in the phosphorylation of the MPM-2 epitopes in xCdc25C and other MPM-2–reactive proteins in Xenopus oocytes and egg extracts. Our results provide strong evidence that phosphorylation of TP motifs that are surrounded by hydrophobic residues at both −1 and +1 positions plays a dominant role in the M phase–associated burst of MPM-2 reactivity and that neither Cdc2/cyclin B nor MAPK is the major kinase that produces the M phase–associated burst of MPM-2 reactivity.
MATERIALS AND METHODS
Preparation of M Phase– and Interphase-arrested Xenopus Egg Extracts
M phase–stabilizing egg extraction buffer (EB) consists of 80 mM β-glycerophosphate, 20 mM EGTA, and 15 mM MgCl2, pH 7.4 (Wu and Gerhart, 1980). M phase/interphase neutral egg extraction buffer (XB) consists of 100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 50 mM sucrose, pH 7.7 (Murray and Kirschner, 1989). M phase–arrested Xenopus egg extracts (MEE) were prepared in EB supplemented with 20 mM NaF, 5 mM DTT, 1 mM ATP-γ-S (Roche, Indianapolis, IN), 1 μM okadaic acid (OA; Calbiochem, La Jolla, CA), and 10 μg/ml each of leupeptin, chymostatin, and pepstatin (Roche; Kuang and Ashorn, 1993; Wang et al., 2007). Interphase-arrested Xenopus egg extracts depleted mitotic cyclins (IE) were prepared by treating cytostatic factor (CSF)-arrested Xenopus egg extracts prepared in XB with 0.4 mM CaCl2 and 100 μg/ml cycloheximide (Solomon et al., 1990; Kuang et al., 1994). IE was diluted with an equal volume of EB or XB and incubated at room temperature with 1 μM OA or 3 μM microcystin (MC; Sigma, St. Louis, MO).
Immunoblotting and Kinase Assays
MPM-2 ascites was produced in our previous studies (Kuang et al., 1989; Kuang et al., 1994). Polyclonal antibodies used to immunoblot xCdc25C were either a generous gift from Dr. William G. Dunphy (California Institute of Technology) (Kumagai and Dunphy, 1992) or generated in our previous studies (Wang et al., 2007). mAb used to immunoblot Cdc2 and polyclonal antibodies used to immunoblot p42 MAPK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies used to immunoblot phosphorylated/activated p42-MAPK and Y15-phosphorylated/inactivated Cdc2 were obtained from Cell Signaling (Beverly, MA). Proteins were separated by 12.5% SDS-PAGE and immunoblotted with primary antibodies and horseradish peroxidase–conjugated secondary antibodies (GE Healthcare, Piscataway, NJ) and developed with enhanced chemiluminescence (ECL) Western blotting detecting reagents (GE Healthcare). Histone H1 and myelin basic protein (MBP) kinase activities were assayed by 32P incorporation as previously described (Kuang and Ashorn, 1993; Wang et al., 2007).
Production and Phosphorylation of Cdc25 Recombinant Proteins
The pGEX4T3-based expression plasmids for glutathione S-transferase (GST)-tagged 9-550 (xCdc25C), 9-205 (N), 205-550 (C), 9-129 (F1), 128-213 (F2), and 251-353 (F3) fragments of xCdc25C, T48V-F1, and hCdc25C were described previously (Wang et al., 2007). The pGEX4T3-based expression plasmids for GST-tagged P46A-F1, L47R-F1, T48S-F1, T48D-F1, T48ST67V-F1, T48ST112V-F1, and T48DT112V were generated by site-directed mutagenesis of the expression construct for GST-F1 using ExSite PCR-based site-directed mutagenesis kit (Stratagene, La Jolla, CA). All GST-tagged proteins were expressed in Escherichia coli BL-21 strain and affinity-absorbed onto glutathione Sepharose (GE Healthcare; Wang et al., 2007). The immobilized proteins were used as substrates in phosphorylation reactions. Activated recombinant MAPK and semirecombinant Cdc2/cyclin B were prepared as previously described (Wang et al., 2007).
Phosphorylation buffer was EB freshly supplemented with 20 mM Tris-HCl, pH 7.4, 1 mM ATP, 0.5 μM OA, and 5 mM DTT. Kinase samples were diluted with phosphorylation buffer to desired concentrations, and supplemented with 60 μM roscovitine (LC Laboratories, Woburn, MA) or 300 nM RO-3306 (Calbiochem) whenever indicated. Diluted kinase samples of 50 μl were incubated with 1 μg/5 μl immobilized recombinant proteins at room temperature for indicated lengths of time. Phosphorylation reactions were stopped by adding 100 μl of ice-cold phosphorylation buffer without OA, and immobilized substrates were washed three times with the same buffer. Bound proteins were eluted by heating washed beads in 30 μl of SDS-PAGE sample buffer at 90°C for 10 min and immunoblotted with MPM-2.
Microinjection and Maturation of Xenopus Oocytes
Isolation, microinjection, maturation, UO126/UO124 treatment, and extraction of Xenopus oocytes were performed as previously described (Che et al., 1999; Wang et al., 2007). The pCS2+MT-based expression constructs for myc-tagged xCdc25C, T48V-xCdc25C, and CA-MEK were generated in our previous studies (Wang et al., 2007). The pCS2+MT-based expression construct for myc-tagged T48D-xCdc25C was generated from the expression construct for myc-xCdc25C with ExSite PCR-based site-directed mutagenesis kit (Stratagene). The pCS2+MT-based expression construct for myc-tagged sea urchin cyclin B Δ-90 (Murray et al., 1989) was constructed by subcloning the cDNA fragment encoding the sea urchin cyclin B Δ-90 into pCS2+MT vector. In vitro transcription of RNA and RNA purification were performed using mMESSAGE mMACHINE High Yield Capped RNA Transcription kit (Ambion, Austin, TX).
RESULTS
The MAPK Phosphorylation Site T48 Resides in a Strong MPM-2 Epitope
Our previous studies demonstrated that xCdc25C undergoes a phosphorylation-dependent dramatic gel mobility shift and becomes recognized by MPM-2 during progesterone-induced meiotic maturation of Xenopus oocytes and cyclin B–induced activation of Cdc2 in interphase-arrested Xenopus egg extracts (Kuang et al., 1994; Wang et al., 2007). Both the gel mobility shift and generation of the MPM-2 reactivity can be recapitulated by incubation of GST-tagged xCdc25C (GST-xCdc25C) with MEE (Che et al., 1998). Among the M phase–specific proline-directed phosphorylation sites in xCdc25C, T48, and S205 are specifically phosphorylated by MAPK; S285 and T308 are specifically phosphorylated by Cdc2/cyclin B, and T138 is phosphorylated by both MAPK and Cdc2/cyclin B (Wang et al., 2007). These previous studies make xCdc25C an excellent platform molecule to characterize the mitotic MPM-2 epitopes and responsible kinases.
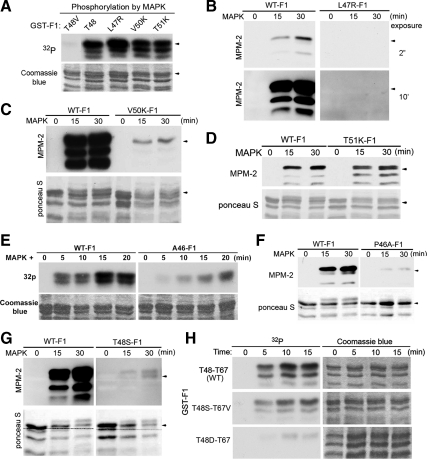
To identify the mitotic MPM-2 epitopes in xCdc25C, we first phosphorylated different GST-tagged fragments of xCdc25C illustrated in Figure 1A with 1:5-diluted MEE for 30 min and immunoblotted the products with MPM-2. Strikingly, phosphorylation of the 9-205 (N) fragment of xCdc25C generated strong MPM-2 reactivity, whereas a parallel treatment of the 205-550 (C) fragment of xCdc25C generated no detectable MPM-2 reactivity (Figure 1B). When GST-tagged F1 (9-129), F2 (128-213), and F3 (251-353) fragments of xCdc25C were phosphorylated, F1 became readily detectable by MPM-2 at 5-s exposure, whereas F2 and F3 showed weak or no MPM-2 reactivity even at 5-min exposure (Figure 1C). Because F1 only contains the MAPK site T48, these results indicate that only this phosphorylation site may reside in an MPM-2 epitope. To test this possibility, we phosphorylated the wild type and the T48V mutant form of GST-F1 with activated recombinant MAPK and immunoblotted the products with MPM-2. Although phosphorylation of the wild-type protein generated robust MPM-2 reactivity, the residual phosphorylation of the mutant protein, which mostly occurred at T67 (data not shown), did not generate MPM-2 reactivity (Figure 1D). We also phosphorylated the wild type and the T48V mutant form of GST-xCdc25C with activated MAPK or Cdc2/cyclin B and immunoblotted the products with MPM-2. We should note that MAPK activity was adjusted to be two-fold higher than that in 1:5-diluted MEE (data not shown) and that the Cdc2/cyclin B activity was adjusted to match the MAPK activity toward the MAPK preferred substrate MBP (Figure 1E). Because Cdc2/cyclin B is known to phosphorylate MBP less efficiently than MAPK, Cdc2/cyclin B was used at a higher molar concentration than MAPK in this experiment. As shown in Figure 1F, the two enzymes phosphorylated the wild type and the mutant GST-xCdc25C to similar extents by 32P incorporation; however, only MAPK-catalyzed phosphorylation of the wild-type GST-xCdc25C generated MPM-2 reactivity. These results demonstrate that of the five identified Cdc2/MAPK phosphorylation sites in xCdc25C, only the MAPK phosphorylation site T48 resides in an MPM-2 epitope.
Figure 1.
The MAPK phosphorylation site T48 resides in a strong MPM-2 epitope. (A) Diagrams of GST-tagged xCdc25C fragments used as substrates in phosphorylation. (B) GST-tagged N and C fragments of xCdc25C were simultaneously phosphorylated with 1:5-diluted MEE for 30 min, washed, gel-separated, and immunoblotted with MPM-2. Total proteins were stained by Ponceau S. (C) GST-tagged F1, F2, and F3 fragments of xCdc25C were treated as described for B. (D) The wild type (WT) and the T48V mutant form of GST-F1 were phosphorylated with activated recombinant MAPK in the presence of [γ-32P]ATP, and washed substrates were gel-separated and then subjected to autoradiography or immunoblotted with MPM-2 and anti-GST antibodies. (E) The activated recombinant MAPK and semirecombinant Cdc2/cyclin B were adjusted to similar levels of MBP-phosphorylating activity as determined by 32P incorporation. (F) The wild type (WT) and the T48V mutant form of GST-xCdc25C were phosphorylated with activated recombinant MAPK and semirecombinant Cdc2/cyclin B used in E in the presence of [γ-32P]ATP, and washed substrates were gel-separated and the subjected to autoradiography or immunoblotted with MPM-2.
A Phosphorylated TP Motif That Is Surrounded by Hydrophobic Residues at Both −1 and +1 Positions Generates Strong MPM-2 Reactivity
Compared with the other four MAPK/Cdc2 phosphorylation sites in xCdc25C, the T48-containing sequence 46PLTPVT51 is unique in that 1) the proline-directed phosphorylation site is surrounded by hydrophobic residues at both −1 and +1 positions; and 2) the residue at the −2 position of the S/T-P motif is proline (Figure S1A). To determine whether the first feature is critical for the T48 phosphorylation to generate MPM-2 reactivity, we individually mutated L47 at the −1 position to R, V50 at the +1 position to K, and T51 at the +2 position to K (as a negative control) and phosphorylated the wild type and the mutant GST-F1 with activated recombinant MAPK. None of the mutations affected the phosphorylation of GST-F1 by MAPK, as determined by 32P incorporation (Figure 2A). However, when the products were immunoblotted with MPM-2, the L47R mutation eliminated the MPM-2 reactivity (Figure 2B), and the V50K mutation dramatically reduced the MPM-2 reactivity (Figure 2C). In contrast, the T51K mutation did not have inhibitory effects on the MPM-2 reactivity (Figure 2D). These results demonstrate that having a hydrophobic residue at the −1 position is required for the T48 phosphorylation to generate detectable MPM-2 reactivity and that having a hydrophobic residue at both −1 and +1 positions is required for the T48 phosphorylation to generate strong MPM-2 reactivity. Consistent with this conclusion, MAPK-mediated phosphorylation of T48 in hCdc25C, which naturally has R at the −1 position (46PRTPVG51) did not generate detectable MPM-2 reactivity (Figure S1B).
Figure 2.
A phosphorylated TP motif that is surrounded by hydrophobic residues at both −1 and +1 positions generates strong MPM-2 reactivity. (A) The wild type (WT) and indicated mutant forms of GST-F1 were phosphorylated with activated recombinant MAPK for 20 min in the presence of [γ-32P]ATP, and washed substrates were gel-separated, stained with Coomassie blue, and subjected to autoradiography. (B) The wild type (WT) and the L47R mutant form of GST-F1 were phosphorylated with activated recombinant MAPK for indicated minutes, and washed substrates were gel-separated and immunoblotted with MPM-2. (C) The wild type (WT) and the V50K mutant form of GST-F1 were treated as described for B. Total proteins were stained by Ponceau S. (D) The wild type (WT) and the T51K mutant form of GST-F1 were treated as described for C. (E) The wild type and the P46A mutant forms of GST-F1 were phosphorylated with activated recombinant MAPK in the presence of [γ-32P]ATP, and washed substrates were gel-separated, stained with Coomassie blue and subjected to autoradiography. (F) The wild type (WT) and the P46A mutant forms of GST-F1 were treated as described for C. (G) The wild type (WT) and the T48S mutant form of GST-F1 were treated as described for C. (H) The wild type (WT) and indicated mutant forms of GST-F1 were treated as described for E.
Previous studies by others suggested that a proline at the −2 position enhances MAPK-catalyzed phosphorylation of certain S/T-P motifs (Sanghera et al., 1990; Alvarez et al., 1991; Clark-Lewis et al., 1991). To determine whether proline at the −2 position is also important for the T48 phosphorylation to generate strong MPM-2 reactivity, we mutated P46 in F1 to A46 and phosphorylated the wild type and the mutant GST-F1 with activated recombinant MAPK. The P46A mutation greatly reduced the MAPK-catalyzed phosphorylation of F1 (Figure 2E), consistent with the previous results. However, the low levels of MAPK-phosphorylated F1 were detectable by MPM-2 (Figure 2F), indicating that having a proline at the −2 position is not important for MPM-2 recognition.
Although MPM-2 is thought to recognize a subset(s) of phosphorylated S/T-P motifs, only phospho-threonine was detected in the MPM-2 immunoprecipitates from mitotic HeLa cells lysates (Zhao et al., 1989), suggesting that TP phosphorylation is more efficient than SP phosphorylation in generating MPM-2 reactivity. To test this possibility, we mutated T48 to S48 and phosphorylated the wild type and the T48S mutant form of GST-F1 with activated recombinant MAPK. Although the T48S mutation did not affect the ability of MAPK to cause a slight gel mobility shift of GST-F1, it dramatically reduced the phosphorylation-generated MPM-2 reactivity (Figure 2G). When MAPK-catalyzed phosphorylation of the wild type and the T48S-T67V or the T48D mutant form of GST-F1 was determined by 32P incorporation, the phosphorylation efficiency of the double mutant protein was only slightly lower than that of the wild-type GST-F1 but much higher than that of the T48D mutant GST-F1 (Figure 2H), indicating that MAPK phosphorylates T48 and S48 with similar efficiencies. These results demonstrate that having T as the phospho-acceptor site is an important determinant for the T48 phosphorylation to generate strong MPM-2 reactivity.
In summary, our characterization of the T48-containing MPM-2 epitope indicates that a phosphorylated TP motif that is surrounded by hydrophobic residues at both −1 and +1 positions generates strong MPM-2 reactivity.
The T48-containing MPM-2 Epitope LTPV Represents a Common Mitotic MPM-2 Epitope
To determine whether the T48-containing MPM-2 epitope LTPV represents a common mitotic MPM-2 epitope, we first analyzed mitotically phosphorylated TP motifs in a recently published database (Dephoure et al., 2008) and determined the frequency of the LTPV-like motifs. Among 230 mitotically phosphorylated TP motifs in the A–F categories, 24 had hydrophobic residues at both −1 and +1 positions (Table S1A), reaching >10% frequency. We then analyzed the MPM-2–reactive peptides previously identified from the phage peptide display library phosphorylated by Cdc2 kinase–enriched fractions of mitotic cell lysates (Westendorf et al., 1994). Among the 13 high-affinity MPM-2–reactive peptides isolated at 0.1 nM MPM-2, 11 peptides contain a TP motif surrounded by hydrophobic residues at both −1 and +1 positions (Table S2), reaching 85% frequency. The remaining two peptides contained the SP motifs IFSPP and IFSPL, and both have an aromatic hydrophobic residue at −1 position and hydrophobic residues at the −2 and +1 positions, meeting the previously determined SP-containing consensus sequence that generates MPM-2 reactivity (Yaffe et al., 1997). Together, these results demonstrate that LTPV represents a common high-affinity mitotic MPM-2 epitope. Strikingly, 10 of the 11 LTPV-like motifs in Table S2 have a basic residue at the +2 position of the TP motif, which is the most influential determinant for Cdc2-catalyzed phosphorylation (Holmes and Solomon, 1996). Such a strong preference indicates that Cdc2/cyclin B specifically phosphorylates the subset of LTPV-like motifs that have a basic residue at the +2 position.
The Novel Phosphorylation site T112 Also Resides in an MPM-2 Epitope
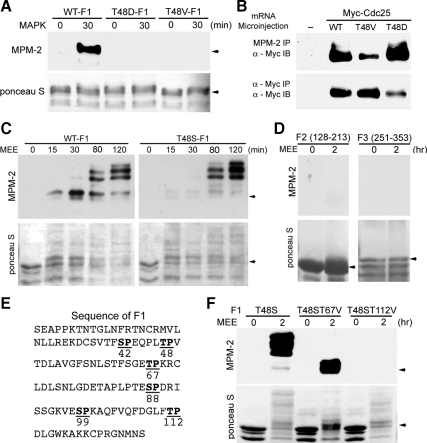
Because phosphorylation of GST-xCdc25C by both MAPK and Cdc2 is insufficient to reconstitute the dramatic gel mobility shift and full activation of GST-xCdc25C induced by MEE (unpublished data), full phosphorylation and activation of xCdc25C during M phase induction may involve phosphorylation at additional sites, and some of the additional phosphorylations may also generate MPM-2 reactivity. To explore this possibility, we first mutated T48 in F1 to D48 and determined that this phospho-mimetic mutation did not mimic the MAPK-catalyzed phosphorylation of T48 to generate MPM-2 reactivity (Figure 3A). We then ectopically expressed the wild type and the T48V or T48D mutant form of myc-xCdc25C in Xenopus oocytes and immunoprecipitated mature oocyte extracts with MPM-2 or anti-myc antibodies. MPM-2 immunoprecipitation followed by myc immunoblotting showed that neither the T48V nor the T48D mutation abolished the immunoprecipitation of myc-xCdc25C by MPM-2 (Figure 3B). The T48V mutation reduced the MPM-2 bound myc-xCdc25C to ∼20% of the wild-type level, predicting that xCdc25C in mature Xenopus oocytes is phosphorylated at additional S/TP motifs besides the five defined Cdc2/MAPK phosphorylation sites and that at least one of the additional phosphorylation sites resides in an MPM-2 epitope. In contrast to the T48V mutation, the phospho-mimetic T48D mutation increased the MPM-2 bound myc-xCdc25C by two- to three-fold, indicating that the phosphorylation of the additional MPM-2 epitope may be facilitated by the T48 phosphorylation.
Figure 3.
The novel phosphorylation site T112 also resides in a strong MPM-2 epitope. (A) The wild type (WT) and indicated mutant forms of GST-F1 were phosphorylated with activated recombinant MAPK for indicated minutes, and washed substrates were gel-separated and immunoblotted with MPM-2. Total proteins were stained by Ponceau S. (B) Xenopus oocytes were injected with mRNAs for the wild type (WT) or the T48D or T48V mutant form of myc-xCdc25C. Extracts of mature oocytes were immunoprecipitated with MPM-2 or anti-myc antibodies, and the immunocomplexes were immunoblotted with anti-myc antibodies. (C) The wild type (WT) and T48S mutant form of GST-F1 were phosphorylated with 1:5-diluted MEE for indicated minutes, and the washed and gel-separated substrates were immunoblotted with MPM-2 after protein staining with Ponceau S. (D) GST-F2 and GST-F3 were phosphorylated with 1:5-diluted MEE for 2 h or were mock-treated and washed substrates were gel-separated and immunoblotted with MPM-2 after protein staining with Ponceau S. (E) F1 contains two additional TP motifs in addition to the T48-containing TP motif. (F) The wild type (WT) and T67V or T112V mutant form of T48S-F1 were treated as described for D.
To locate the additional MPM-2 epitope(s) in xCdc25C, we phosphorylated the wild type and the T48S mutant form of GST-F1 in parallel with GST-F2 and GST-F3 with 1:5-diluted MEE for up to 2 h and immunoblotted the products with MPM-2. As shown in Figure 3C, MPM-2 recognized a slightly shifted band at 15 and 30 min (presumably phosphorylated at T48) and dramatically shifted bands at 80 and 120 min in the wild-type GST-F1. The T48S mutant form of GST-F1 showed dramatically reduced MPM-2 reactivity in the slightly shifted band at early time points as expected but showed comparable MPM-2 reactivity in the dramatically shifted bands at 80 and 120 min. Figure 3D shows that F2 and F3 neither became reactive to MPM-2 nor underwent gel mobility shifts at the end of the phosphorylation. These results indicate that F1 contains at least one additional MPM-2 epitope that is phosphorylated after MAPK-catalyzed phosphorylation of T48.
To identify the additional MPM-2 epitope in F1, we analyzed the F1 protein sequence for an additional TP-containing motif that might be a strong MPM-2 epitope. Although both T67 and T112 are followed by proline, neither is surrounded by hydrophobic residues at both −1 and +1 positions. However, the T112-containing sequence 110LFTPD114 contains F at the −1 position and an additional hydrophobic residue at the −2 position (Figure 3E), similar to the two SP-containing high-affinity MPM-2 epitopes in Table S2. Because previous studies demonstrated that phosphorylated FTPLQ has higher affinity than phosphorylated LTPLK for MPM-2 (Westendorf et al., 1994) and that aromatic or hydrophobic residues at −1, −2, and −3 positions of phosphorylated SP all favor MPM-2 reactivity (Yaffe et al., 1997), it is conceivable that these enhancing features compensate for the lack of a hydrophobic residue at the +1 position, allowing the T112 phosphorylation to generate strong MPM-2 reactivity. To test this possibility, we produced the T67V and the T112V mutant forms of T48S-F1 and examined their abilities to become MPM-2 reactive upon 2-h phosphorylation with 1:5-diluted MEE. As shown in Figure 3F, the T67V mutation reduced the gel mobility shift but did not eliminate the MPM-2 reactivity of T48S-F1. In contrast, the T112V mutation eliminated the MPM-2 reactivity but not the gel mobility shift of T48S-F1. These results demonstrate that T112 resides in the additional MPM-2 epitope. To determine whether the T112-containing MPM-2 epitope LFTP represents a common mitotic MPM-2 epitope, we determined the frequency of LFTP-like motifs in Table S1A. In contrast to LTPV-like motifs, no LFTP-like motifs are found in this dataset, indicating that LFTP represents one of the rare MPM-2 epitopes.
The robust phosphorylation of T112 by MEE predicts that T112 is phosphorylated in mature Xenopus oocytes. To test this prediction, we ectopically expressed the wild type and the T112V or T112D mutant form of myc-tagged D48-F1 (myc-D48-F1) in Xenopus oocytes and immunoprecipitated extracts of progesterone-matured oocytes with MPM-2. Immunoblotting of the immunocomplexes with anti-myc antibodies showed that both the T112V and T112D mutations dramatically reduced the ability of myc-D48-F1 to be immunoprecipitated by MPM-2 (Figure S2A), indicating that the T112-containing MPM-2 epitope is phosphorylated in mature oocytes. We also ectopically expressed the wild type and the T112V mutant form of myc-xCdc25C in Xenopus oocytes at suboptimal levels and compared their abilities to induce Xenopus oocyte maturation. At the same low expression levels, the wild-type myc-xCdc25C induced ∼20% of oocyte maturation at 5.5 h, whereas the T112V mutant form of myc-xCdc25C only induced ∼10% of oocyte maturation. Doubling the expression level of the mutant protein reduced the difference (Figure S2B). These results indicate that the T112 phosphorylation has positive effects on xCdc25C activation.
Phosphorylation of the T112-containing MPM-2 Epitope Is Facilitated by the Prior Phosphorylation at T48
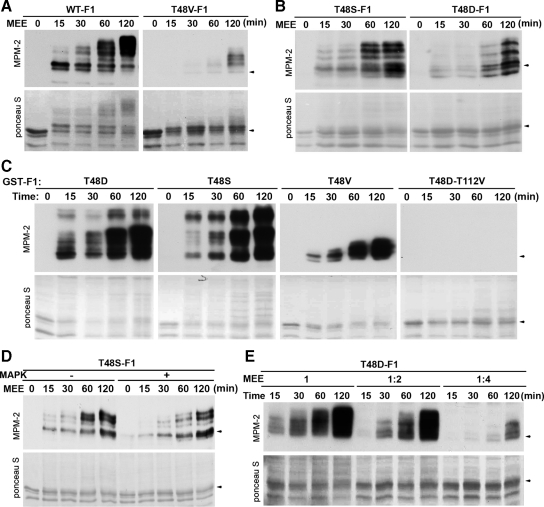
The significant delay of the T112 phosphorylation compared with the T48 phosphorylation predicts that the efficient T112 phosphorylation involves a preparation process. To characterize this process, we first tested the hypothesis that the prior phosphorylation at T48 facilitates the T112 phosphorylation. For this objective, we phosphorylated the wild type and the T48V mutant form of GST-F1 with 1:5-dilued MEE and immunoblotted the products with MPM-2. As shown in Figure 4A, the phospho-defective T48V mutation not only eliminated the MPM-2 reactivity at early time points but also dramatically reduced the dramatically shifted MPM-2–reactive bands at later time points. However, when we performed a similar experiment with the T48S and T48D mutant forms of GST-F1, the phospho-mimetic T48D mutation only moderately delayed the appearance of the shifted MPM-2–reactive bands (Figure 4B). Moreover, when we simultaneously phosphorylated the T48D, T48S, T48V, and T48D-T112V mutant forms of GST-F1 with undiluted MEE, the phospho-defective T48V mutation greatly reduced the MPM-2 reactivity, whereas the phospho-mimetic T48D mutation had little effect on the MPM-2 reactivity. The T48D-T112V double mutations completely eliminated the MPM-2 reactivity as expected (Figure 4C). Together, these results demonstrate that the prior phosphorylation at T48 facilitates the T112 phosphorylation and that the phospho-mimetic T48D mutation partially mimics the T48 phosphorylation in this capacity.
Figure 4.
Phosphorylation of the T112-containing MPM-2 epitope is facilitated by the prior phosphorylation at T48. (A) The wild type (WT) and the T48V mutant form of GST-F1 were phosphorylated with 1:5-diluted MEE for indicated minutes, and washed substrates were gel-separated and immunoblotted with MPM-2 after protein staining with Ponceau S. (B) The T48S and the T48D mutant forms of GST-F1 were treated as described for A. (C) Indicated mutant forms of GST-F1 were phosphorylated with undiluted MEE for indicated minutes, and washed and gel-separated substrates were immunoblotted with MPM-2 after protein staining with Ponceau S. (D) The T48S mutant form of GST-F1 was first phosphorylated with recombinant MAPK or mock-treated, and then phosphorylated with 1:4-diluted MEE for indicated minutes. The washed and gel-separated substrates were immunoblotted with MPM-2 after protein staining with Ponceau S. (E) The T48D mutant form of GST-F1 was phosphorylated with indicated dilutions of MEE for indicated minutes, and washed substrates were gel-separated and immunoblotted with MPM-2 after protein staining with Ponceau S.
The T112 Phosphorylation Involves an Additional Preparation Step
To determine whether the T48 phosphorylation is the only reason for the delayed nature of the T112 phosphorylation, we phosphorylated the T48S mutant form of GST-F1 that is with or without prior phosphorylation by recombinant MAPK (Figure S3A) and examined the kinetics of the appearance of the MPM-2–reactive bands. As shown in Figure 4D, the prior phosphorylation by MAPK did not accelerate the appearance of MPM-2–reactive bands, indicating that the T112 phosphorylation involves an additional preparation step(s). To ascertain whether the additional preparation step for the T112 phosphorylation is likely to involve one or multiple factors, we first examined the relationship between MEE concentration and the T112 phosphorylation. If the additional preparation step involves only one factor, the MEE concentration should correlate with the T112 phosphorylation without significantly affecting the lag period. However, when the T48D mutant form of GST-F1 was phosphorylated with undiluted, 1:2-diluted, or 1:4-diluted MEE, increasing the MEE concentration from 25 to 50% strength reduced the lag period by fourfold and increased the T112 phosphorylation by more than fourfold (Figure 4E). When the wild type and the T48S mutant form of GST-F1 were phosphorylated with 1:10-, 1:20-, and 1:40-diluted MEE, only 1:10-diluted MEE generated readily detectable levels of dramatically shifted MPM-2–reactive bands. In contrast, MEE at all three dilutions generated strong MPM-2 reactivity in the slightly shifted band in the wild-type GST-F1, indicative of the T48 phosphorylation by MAPK (Figure S3B). These results demonstrate that phosphorylation of the T112-containing MPM-2 epitope has a nonlinear relationship with MEE concentration. Next, we fractionated MEE and followed the T112 phosphorylating activity that makes T48D-F1 MPM-2 reactive. If the additional preparation step for the T112 phosphorylation involves multiple factors, fractionation of MEE is likely to result in great or complete loss of the T112 phosphorylating activity. As shown in Figure S3D, only 25% of the start activity was recovered from Superose 6 gel filtration of the 40% ammonium sulfate precipitates of MEE. Further fractionation of the pooled positive fractions by Q-Sepharose chromatography resulted in a near complete loss of the T112 phosphorylating activity (data not shown). Together, these results support the hypothesis that the additional preparation step for the T112 phosphorylation involves multiple factors in MEE.
Neither MAPK Nor Cdc2 Is Responsible for the Phosphorylation of the T112-containing MPM-2 Epitope
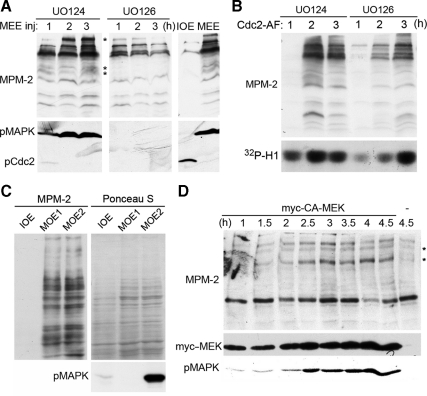
Because the T112 phosphorylation is likely to be a complex process that involves multiple factors, the inability of MAPK or Cdc2 kinase alone to phosphorylate T112 does not exclude the possibility that MAPK or Cdc2 kinase is the ultimate kinase factor for the process. To determine the involvement of MAPK in the T112 phosphorylation, we injected Xenopus oocytes with a nondegradable cyclin B, which arrests M phase even in the absence of MAPK activity (Murray et al., 1989), in the continued presence or absence of the MEK (MAPK/ERK kinase) inhibitor UO126 and stimulated the oocytes with progesterone. Mature oocyte extracts (MOE) prepared from these oocytes were then used to phosphorylate the T48D mutant form of GST-F1, which does not require the MAPK-mediated T48 phosphorylation for the T112 phosphorylation. As shown in Figure 5A, UO126 completely inhibited MAPK activation but did not inhibit Cdc2 activation in the cyclin B–injected and progesterone-stimulated oocytes. Figure 5B shows that MOE without MAPK activation (MOE1) and MOE with MAPK activation (MOE2) made T48D-F1 MPM-2 reactive with similar kinetics. These results demonstrate that MAPK is not the kinase factor for the T112 phosphorylation.
Figure 5.
Neither MAPK nor Cdc2 is responsible for the phosphorylation of the T112-containing MPM-2 epitope. (A) Xenopus oocytes cultured in the absence or continued presence of UO126 were first injected with a nondegradable cyclin B and then stimulated by progesterone. Extracts of oocytes collected at the indicated hours after progesterone stimulation were immunoblotted with antibodies for activated-MAPK (pMAPK), total MAPK, and inactive Cdc2 (pCdc2). UO126-treated mature oocyte extracts (MOE1) and non-UO126-treated mature oocyte extracts (MOE2) were used for phosphorylation in B. (B) The T48D mutant form of GST-F1 was phosphorylated with MOE1 and MOE2 described in A for the indicated minutes, and the washed substrates were gel-separated and immunoblotted with MPM-2 after protein staining with Ponceau S. (C) Undiluted MEE was incubated with DMSO, 300 nM RO-3306, or 60 μM roscovitine (ROSCO) for indicated minutes, and histone H1 kinase activity was determined by 32P incorporation. (D) The T48D mutant form of GST-F1 was phosphorylated with undiluted MEE supplemented with DMSO, 300 nM RO-3306 or 60 μM roscovitine (ROSCO) for indicated minutes. The washed substrates were gel-separated and immunoblotted with MPM-2 after protein staining with Ponceau S.
To determine the involvement of Cdc2 kinase in the T112 phosphorylation, we inhibited Cdk activity in MEE by 60 μM roscovitine or 300 nM RO-3306 (Vassilev et al., 2006) and phosphorylated the T48D mutant form of GST-F1 with either mock-treated or Cdk inhibitor-treated MEE. Because we had previously determined that >80% of the histone H1 kinase activity in MEE is due to Cdc2 kinase (Kuang et al., 1991), H1 kinase activity assay was used to monitor Cdc2 inhibition. As shown in Figure 5C, both roscovitine and RO-3306 inhibited Cdc2 kinase activity in MEE. Figure 5D shows that neither of these drugs significantly affected the kinetics of the T112 phosphorylation as determined by MPM-2 immunoblotting. Consistent with these results, depletion of Cdc2 kinase from MEE by affinity absorption with immobilized p13suc1 protein did not remove the T112-phosphorylating activity (Figure S4). Together, these results demonstrate that Cdc2 kinase is not the kinase factor for the T112 phosphorylation.
MAPK or Cdc2 Does Not Play a Major Role in the M Phase–associated Burst of MPM-2 Reactivity
Our characterization of the MPM-2 epitopes in xCdc25C and analysis of the previously identified high-affinity MPM-2–reactive peptides led us to divide the mitotic MPM-2 epitopes into three main subtypes according to their phosphorylating kinases. One subtype has a proline at the −2 position and is phosphorylated by MAPK (MAPK subtype). Another subtype has a basic residue at the +2 position and is phosphorylated by Cdc2 kinase (Cdc2 subtype). The third subtype does not have either of these features and is phosphorylated by a yet-to-be identified kinase (kinase X subtype). To estimate the contribution of mitotic Cdk and MAPK to the M phase–associated burst of MPM-2 reactivity, we first determined the relative abundance of the three subtypes of the LTPV-like motifs in mitotically phosphorylated peptides listed in Table S1A. Among 24 phosphorylated LTPV-like motifs in this dataset, three (12.5%) belongs to the MAPK subtype that has a proline residue at the −2 position, three (12.5%) belongs to the Cdc2 subtype that has a basic residue at the +2 position, and the remaining 18 (75%) belongs to the kinase X-subtype that has neither of these features (Table S1B). Similar results were obtained when the entire database (A–Z) was analyzed by custom-designed computer programs (data not shown). These results predicted that Cdc2 or MAPK does not play a major role in the M phase–associated burst of MPM-2 reactivity.
To determine the role of MAPK in the M phase associated burst of MPM-2 reactivity, we induced Xenopus oocyte maturation by injection of MEE or mRNA for inhibitory phosphorylation-insensitive Cdc2 (Cdc2-AF) in the continued presence or absence of the MEK inhibitor UO126 and examined appearance of MPM-2–reactive proteins. In MEE-injected oocytes, complete inhibition of MAPK activation by UO126 only prevented appearance of a very small subset of MPM-2–reactive proteins (Figure 6A). Although UO126 caused a moderate reduction in the overall MPM-2 reactivity at later time points, this could be explained by a compromised M phase arrest in the absence of MAPK-dependent CSF activity (Gross et al., 1999; Maller et al., 2001, 2002; Tunquist et al., 2002; Nishiyama et al., 2007; Wu and Kornbluth, 2008). In Cdc2-AF expressed oocytes, UO126 delayed but did not prevent the appearance of MPM-2–reactive proteins. The delay could be explained by delayed M phase entry, as judged by H1 kinase activity (Figure 6B). These results indicate that MAPK-catalyzed phosphorylation of the MPM-2 epitope does not play a major role in the M phase–associated burst of MPM-2 reactivity. To further test this hypothesis, we induced Xenopus oocyte maturation by progesterone stimulation of nondegradable cyclin B–injected oocytes in the continued presence or absence of UO126 and examined the induction of MPM-2–reactive proteins. As shown in Figure 6C, although UO126 treatment completely inhibited MAPK activation, it did not affect the level or pattern of the induced MPM-2–reactive proteins. In addition, we ectopically expressed below M phase–inducing levels of a constitutively active MEK (CA-MEK) in Xenopus oocytes and examined appearance of MPM-2–reactive proteins. As shown in Figure 6D, forced activation of MAPK in the absence of M phase induction only induced low levels of very few MPM-2–reactive proteins. Consistent with each other, these results demonstrate that MAPK-catalyzed phosphorylation of the MPM-2 epitope does not play a major role in the M phase–associated burst of MPM-2 reactivity.
Figure 6.
MAPK-catalyzed phosphorylation of the MPM-2 epitope does not play a major role in the M phase–associated burst of MPM-2 reactivity. (A) Xenopus oocytes cultured in the continued presence of UO126 or UO124 were injected with MEE, and extracts of oocytes collected at the indicated hours after the injection were immunoblotted with MPM-2 and antibodies that recognize phosphorylated/activated MAPK (pMAPK) or phosphorylated/inactivated Cdc2 (pCdc2). Asterisk indicates the MPM-2–reactive proteins inhibited by UO126. (B) Xenopus oocytes cultured in the continued presence of UO126 or UO124 were injected with mRNA for Cdc2-AF, and extracts of oocytes collected at the indicated hours after the injection were immunoblotted with MPM-2 and assayed for H1 kinase activity by 32P incorporation. (C) Xenopus oocytes were treated as described for Figure 5A, and IOE, MOE1, and MOE2 were immunoblotted with MPM-2 and antibodies that recognize phosphorylated/activated MAPK (pMAPK) after total proteins were stained with Ponceau S. (D) Extracts of oocytes collected at the indicated hours after injection of below M phase–inducing levels of CA-MEK were immunoblotted with MPM-2 and antibodies that recognize phosphorylated/activated MAPK (pMAPK) or myc-epitope tag. Asterisk, the MPM-2–reactive proteins induced by activated MAPK.
To determine the role of Cdc2 in the M-phase associated burst of MPM-2 reactivity, we first stimulated control or Wee1-injected Xenopus oocytes with progesterone and examined the appearance of MPM-2–reactive proteins. Although complete inhibition of activation of Cdc2 and xCdc25C by Wee1 prevented the burst of the MPM-2–reactive proteins (data not shown), initially incomplete inhibition of xCdc25C and Cdc2 did not prevent the appearance of most of the MPM-2–reactive proteins even when xCdc25C and Cdc2 activations were completely inhibited in the end (Figure 7A). These results suggested that Cdc2 does not play a major role in the M-phase–associated burst of MPM-2 reactivity. To test this hypothesis, we induced an M phase-like state in IE that was depleted of mitotic cyclins by combinational treatment with the phosphatase inhibitor OA and a classical MPF extraction buffer (EB). These treatments were previously shown to both cause the dramatic gel mobility shift of xCdc25C and to induce nuclear envelope breakdown and chromosome condensation in the absence of Cdc2 and Cdk2 proteins (Izumi and Maller, 1995). Immunoblotting of samples collected at different time points with MPM-2 and anti-xCdc25C antibodies showed that EB plus OA induced high levels of MPM-2–reactive proteins similar to those in MEE and the dramatic gel mobility shift of xCdc25C, whereas treatment with OA alone had much smaller effects (Figure 7B). The induction of MPM-2–reactive proteins was not sensitive to the above-mentioned Cdk inhibitors roscovitine and RO-3306 (Figure D), which inhibited H1 kinase activity in MEE in parallel assays (Figure 7C). In addition to containing high levels of MPM-2–reactive proteins, IE treated with OA plus EB was also able to make the T48D mutant form of GST-F1 MPM-2 reactive (Figure 7E), indicating that the T112-phosphorylating system was activated. In contrast to these positive effects, OA plus EB did not increase the histone H1 kinase activity in IE (Figure 7F), confirming its lack of mitotic cyclins. Together, these results demonstrate that Cdc2 does not play a major role in the M phase–associated burst of MPM-2 reactivity. Consistent with this conclusion, adding high levels of biochemically purified Cdc2/cyclin B (Figure S5) to IE did not induce MPM-2–reactive proteins although the added Cdc2/cyclin B was able to enhance the ability of OA (but not EB) to induce MPM-2–reactive proteins (Figure 7G). However, the enhancing effect of Cdc2/cyclin B could be explained by positive roles of Cdc2/cyclin B in one of the pathways that activate the key MPM-2 epitope kinase(s).
Figure 7.
The Cdc2-catalyzed phosphorylation of the MPM-2 epitope does not play a major role in the M phase–associated burst of MPM-2 reactivity. (A) Extracts of oocytes collected at indicated hours after injection of Xenopus Wee1 or Xp95 (control) RNA were immunoblotted with MPM-2, anti-xCdc25C antibodies or antibodies that recognize phosphorylated/inactivated Cdc2 (pCdc2). Asterisk indicates an MPM-2–reactive band inhibited by Wee1. (B) After IE was diluted with an equal volume of EB or XB and incubated with OA for indicated minutes, samples were immunoblotted along with MEE by MPM-2 and anti-xCdc25C antibodies. Arrow, the position of interphase xCdc25C; asterisk, gel mobility–shifted xCdc25; and ×, a nonspecific band recognized by anti-xCdc25C antibodies. (C) After MEE was mixed with DMSO, RO-3306, or roscovitine (ROSCO), histone H1 kinase activity was determined by 32P incorporation. (D) After IE was mixed with DMSO, RO-3306, or roscovitine (ROSCO), it was diluted with EB and incubated with OA for the indicated minutes. Samples were gel-separated and immunoblotted with MPM-2 after protein staining with Ponceau S. (E) After the T48D mutant form of GST-F1 was incubated with control buffer, MEE, or IE treated with EB plus OA, washed substrates were gel-separated and immunoblotted with MPM-2 after protein staining with Ponceau S. (F) After IE was incubated with XB or EB plus OA for 30 min, samples were immunoblotted with MPM-2 and determined for phosphorylation of histone H1 by 32P incorporation. (G) Indicated reagents were added to XB-diluted IE, and samples collected at 30 min were immunoblotted with MPM-2 in parallel with MEE.
DISCUSSION
A burst of MPM-2 reactivity on a large family of mitotic phosphoproteins is a conserved phenomenon that is tightly associated with M phase induction. Because phosphorylation of the MPM-2 epitope is likely to be a critical event for M phase induction, defining the mitotic MPM-2 epitopes and identifying the key kinases that phosphorylate these epitopes are likely to be important steps toward a complete understanding of mitotic regulation. Historical efforts to deduce the MPM-2 epitope consensus sequence with peptide libraries generated a concept that the M phase–associated burst of MPM-2 reactivity is mainly due to phosphorylation of certain S/TP motifs. However, the surrounding sequences that make phosphorylated S/T-P motifs MPM-2 reactive were ill defined, and thus there was not a solid foundation for identifying the key MPM-2 epitope kinases. In this study, we demonstrate that recognition of phosphorylated S/T-P motifs by MPM-2 is highly selective. Among seven phosphorylated S/T-P motifs identified in xCdc25C, only two are recognized by MPM-2. One of them is 47LTPV50, and its strong MPM-2 reactivity depends on having T as the phosphorylation site and surrounding phosphorylated TP by hydrophobic residues at both −1 and +1 positions. Because LTPV-like motifs account for >10% of mitotically phosphorylated TP motifs in a current phosphorylation database (Table S1A) and are present in most of the previously identified high-affinity MPM-2–reactive peptides (Table S2), this sequence motif represents a common mitotic MPM-2 epitope. The other one is 110LFTP113, which in the N-terminus resembles the previously determined SP-containing motifs that generate strong MPM-2 reactivity (Table S2). In contrast to LTPV-like motifs, LFTP-like motifs are rare in the mitotic phosphorylation database. Together, these findings strongly suggest that phosphorylation of LTPV-like motifs plays a dominant role in the M phase–associated burst of MPM-2 reactivity. Further studies are required to define the full-spectrum of TP- and SP-containing motifs that generate strong and weak MPM-2 reactivity.
After the historical finding that MPM-2 recognizes a subset(s) of phosphorylated S/TP motifs in 1994 (Westendorf et al., 1994), key questions in studies on the MPM-2 epitope kinases were whether Cdc2/cyclin B and MAPK are able to phosphorylate the proteins that become MPM-2 reactive in mitosis and make them MPM-2 reactive in vitro. Although positive results were obtained for both Cdc2/cyclin B and MAPK in some of the substrates examined (Kuang and Ashorn, 1993; Westendorf et al., 1994; Stukenberg et al., 1997), these occasional examples did not resolve the issue of whether one or both of these two kinases are the key kinases that produce the M phase–associated burst of MPM-2 reactivity. In this study, we made the first attempt to address this issue in a systematic manner. Our results show that having proline residue at the −2 position is required for MAPK to efficiently phosphorylate the T48-containing MPM-2 epitope in xCdc25C even though the other two MAPK sites T138 and S205 do not have a proline at the −2 position. Our analysis of previously identified MPM-2–reactive peptides, resulting from Cdc2-catalyzed phosphorylation made us realize that almost all of the LTPV-like motifs have a basic residue at the +2 position even though the Cdc2 phosphorylation sites T138 and S285 in xCdc25C do not have this feature. However, the majority of mitotically phosphorylated LTPV-like motifs in currently available databases do not have these features that support the MAPK- or Cdc2-catalyzed phosphorylation of LTPV-like motifs. More importantly, we further showed that the M phase–associated burst of MPM-2 reactivity can be induced in Xenopus oocytes and egg extracts in the absence of MAPK and Cdc2 activities. Together, these findings eliminate MAPK and Cdc2 kinase from being the key kinases that produce the M phase–associated burst of MPM-2 reactivity.
Because neither MAPK nor Cdc2 kinase is the key MPM-2 epitope kinase in mitosis, there are currently no good candidates for the key kinases that produce the M phase–associated burst of MPM-2 reactivity. Although Polo-like kinases, Aurora kinases, and the NIMA-related kinases (Nrk) are activated in mitosis, and each phosphorylates many substrates (Nigg, 2001; O'Connell et al., 2003; Brittle and Ohkura, 2005; Marumoto et al., 2005; Petronczki et al., 2008; Kelly and Funabiki, 2009; Lindqvist et al., 2009), none of these kinases prefers proline-directed and hydrophobic residue–surrounded phosphorylation sites (Songyang et al., 1996; Nakajima et al., 2003; Ferrari et al., 2005; Gadea and Ruderman, 2006; Zhang et al., 2007; Miller et al., 2008). There are other proline-directed kinases, including other members of Cdk family (Pines and Hunter, 1991; Holmes and Solomon, 1996; Songyang et al., 1996; Miller et al., 2008), other members of the MAPK family (Karin, 1994; Cano and Mahadevan, 1995; Treisman, 1996; Miller et al., 2008), RCKs (Fu et al., 2006; Miller et al., 2008), GSK3 (Yang et al., 1993; Ye et al., 1995; Miller et al., 2008), mTOR (LoPiccolo et al., 2008; Miller et al., 2008), and DYRK1A (Himpel et al., 2000; Campbell and Proud, 2002). However, these kinases are neither preferentially activated in mitosis nor critically involved in M phase induction. Thus, the widespread phosphorylation of the MPM-2 epitope is likely to represent a novel mechanism in mitotic regulation, and identification of the key players in this mechanism and defining its unique functional impact will advance our understanding of mitotic regulation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH), National Cancer Institute (NCI) Grant R01 CA93941 (J.K.); Department of Defense Congressionally Directed Medical Research Program Grants W81XWH-09-1-0274 (J.K.) and W81XWH-09-1-0272 (S.H.L.); and National Science Foundation of China Grant 30971470 (Q.L.). DNA sequencing was performed by the DNA Analysis Facility of the University of Texas M.D. Anderson Cancer Center supported by NCI Grant CA-16672.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-06-0486) on March 10, 2010.
REFERENCES
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J. Biol. Chem. 1991;266:15277–15285. [PubMed] [Google Scholar]
- Brittle A. L., Ohkura H. Centrosome maturation: Aurora lights the way to the poles. Curr. Biol. 2005;15:R880–R882. doi: 10.1016/j.cub.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Campbell L. E., Proud C. G. Differing substrate specificities of members of the DYRK family of arginine-directed protein kinases. FEBS Lett. 2002;510:31–36. doi: 10.1016/s0014-5793(01)03221-5. [DOI] [PubMed] [Google Scholar]
- Cano E., Mahadevan L. C. Parallel signal processing among mammalian MAPKs. Trends Biochem. Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Che S., El-Hodiri H. M., Wu C. F., Nelman-Gonzalez M., Weil M. M., Etkin L. D., Clark R. B., Kuang J. Identification and cloning of xp95, a putative signal transduction protein in Xenopus oocytes. J. Biol. Chem. 1999;274:5522–5531. doi: 10.1074/jbc.274.9.5522. [DOI] [PubMed] [Google Scholar]
- Che S., Wu W., Nelman-Gonzalez M., Stukenberg T., Clark R., Kuang J. A phosphatase activity in Xenopus oocyte extracts preferentially dephosphorylates the MPM-2 epitope. FEBS Lett. 1998;424:225–233. doi: 10.1016/s0014-5793(98)00158-6. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J. Biol. Chem. 1991;266:15180–15184. [PubMed] [Google Scholar]
- Davis F. M., Rao P. N. Antibodies to mitosis-specific phosphoproteins. In: Potu N. Rao., editor. Molecular Regulation of Nuclear Events in Mitosis and Mieosis. San Diego: Academic Press; 1987. pp. 259–293. [Google Scholar]
- Davis F. M., Tsao T. Y., Fowler S. K., Rao P. N. Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N., Zhou C., Villen J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico A., Deshmukh K., Tanaka Y., Pozniakovsky A., Hunt T. Identification of substrates for cyclin dependent kinases. Adv. Enzyme Regul. 2010 doi: 10.1016/j.advenzreg.2009.12.001. in press. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Marin O., Pagano M. A., Meggio F., Hess D., El-Shemerly M., Krystyniak A., Pinna L. A. Aurora-A site specificity: a study with synthetic peptide substrates. Biochem. J. 2005;390:293–302. doi: 10.1042/BJ20050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., et al. Identification of yin-yang regulators and a phosphorylation consensus for male germ cell-associated kinase (MAK)-related kinase. Mol. Cell. Biol. 2006;26:8639–8654. doi: 10.1128/MCB.00816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadea B. B., Ruderman J. V. Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc. Natl. Acad. Sci. USA. 2006;103:4493–4498. doi: 10.1073/pnas.0600702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. A., Raden D. L., Davis R. J. Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J. Biol. Chem. 1991;266:22159–22163. [PubMed] [Google Scholar]
- Gross S. D., Schwab M. S., Lewellyn A. L., Maller J. L. Induction of metaphase arrest in cleaving Xenopus embryos by the protein kinase p90Rsk. Science. 1999;286:1365–1367. doi: 10.1126/science.286.5443.1365. [DOI] [PubMed] [Google Scholar]
- Himpel S., Tegge W., Frank R., Leder S., Joost H. G., Becker W. Specificity determinants of substrate recognition by the protein kinase DYRK1A. J. Biol. Chem. 2000;275:2431–2438. doi: 10.1074/jbc.275.4.2431. [DOI] [PubMed] [Google Scholar]
- Holmes J. K., Solomon M. J. A predictive scale for evaluating cyclin-dependent kinase substrates. A comparison of p34cdc2 and p33cdk2. J. Biol. Chem. 1996;271:25240–25246. doi: 10.1074/jbc.271.41.25240. [DOI] [PubMed] [Google Scholar]
- Holt L. J., Tuch B. B., Villen J., Johnson A. D., Gygi S. P., Morgan D. O. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325:1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T., Maller J. L. Phosphorylation and activation of the Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol. Biol. Cell. 1995;6:215–226. doi: 10.1091/mbc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Signal transduction from the cell surface to the nucleus through the phosphorylation of transcription factors. Curr. Opin. Cell Biol. 1994;6:415–424. doi: 10.1016/0955-0674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Kelly A. E., Funabiki H. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr. Opin. Cell Biol. 2009;21:51–58. doi: 10.1016/j.ceb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Peters J. M., Tugendreich S., Rolfe M., Hieter P., Kirschner M. W. A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell. 1995;81:279–288. doi: 10.1016/0092-8674(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Kuang J., Ashorn C. L. At least two kinases phosphorylate the MPM-2 epitope during Xenopus oocyte maturation. J. Cell Biol. 1993;123:859–868. doi: 10.1083/jcb.123.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J., Ashorn C. L., Gonzalez-Kuyvenhoven M., Penkala J. E. cdc25 is one of the MPM-2 antigens involved in the activation of maturation-promoting factor. Mol. Biol. Cell. 1994;5:135–145. doi: 10.1091/mbc.5.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J., Penkala J. E., Wright D. A., Saunders G. F., Rao P. N. A novel M phase-specific H1 kinase recognized by the mitosis-specific monoclonal antibody MPM-2. Dev. Biol. 1991;144:54–64. doi: 10.1016/0012-1606(91)90478-l. [DOI] [PubMed] [Google Scholar]
- Kuang J., Zhao J., Wright D. A., Saunders G. F., Rao P. N. Mitosis-specific monoclonal antibody MPM-2 inhibits Xenopus oocyte maturation and depletes maturation-promoting activity. Proc. Natl. Acad. Sci. USA. 1989;86:4982–4986. doi: 10.1073/pnas.86.13.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Langan T. A., Zeilig C. E., Leichtling B. Analysis of multiple site phosphorylation of H1 Histone. Protein phosphorylation and bio-regulation. FMI-EMBO Workshop, Basel. 1979;1979:70–82. [Google Scholar]
- Lindqvist A., Rodriguez-Bravo V., Medema R. H. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loog M., Morgan D. O. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- LoPiccolo J., Blumenthal G. M., Bernstein W. B., Dennis P. A. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist. Update. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R., Lenobel R., Santamaria A., Ries A., Nigg E. A., Korner R. Quantitative analysis of the human spindle phosphoproteome at distinct mitotic stages. J Proteome Res. 2009;8:4553–4563. doi: 10.1021/pr9003773. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Schwab M. S., Gross S. D., Taieb F. E., Roberts B. T., Tunquist B. J. The mechanism of CSF arrest in vertebrate oocytes. Mol. Cell. Endocrinol. 2002;187:173–178. doi: 10.1016/s0303-7207(01)00695-5. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Schwab M. S., Roberts B. T., Gross S. D., Taieb F. E., Tunquist B. J. The pathway of MAP kinase mediation of CSF arrest in Xenopus oocytes. Biol. Cell. 2001;93:27–33. doi: 10.1016/s0248-4900(01)01127-3. [DOI] [PubMed] [Google Scholar]
- Marumoto T., Zhang D., Saya H. Aurora-A—a guardian of poles. Nat. Rev. Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- Miller M. L., et al. Linear motif atlas for phosphorylation-dependent signaling. Sci. Signal. 2008;1:ra2. doi: 10.1126/scisignal.1159433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. R., Coleman T. R., Dunphy W. G. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol. Biol. Cell. 1995;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Toyoshima-Morimoto F., Taniguchi E., Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. The substrates of the cdc2 kinase. Semin. Cell Biol. 1991;2:261–270. [PubMed] [Google Scholar]
- Nigg E. A. Cellular substrates of p34(cdc2) and its companion cyclin-dependent kinases. Trends Cell Biol. 1993;3:296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Ohsumi K., Kishimoto T. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature. 2007;446:1096–1099. doi: 10.1038/nature05696. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Krien M. J., Hunter T. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol. 2003;13:221–228. doi: 10.1016/s0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Olsen J. V., et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- Petronczki M., Lenart P., Peters J. M. Polo on the rise—from mitotic entry to cytokinesis with Plk1. Dev. Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Cyclin-dependent kinases: a new cell cycle motif? Trends Cell Biol. 1991;1:117–121. doi: 10.1016/0962-8924(91)90116-q. [DOI] [PubMed] [Google Scholar]
- Sanghera J. S., Aebersold R., Morrison H. D., Bures E. J., Pelech S. L. Identification of the sites in myelin basic protein that are phosphorylated by meiosis-activated protein kinase p44mpk. FEBS Lett. 1990;273:223–226. doi: 10.1016/0014-5793(90)81090-b. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990;63:1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Blechner S., Hoagland N., Hoekstra M. F., Piwnica-Worms H., Cantley L. C. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Songyang Z., et al. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol. Cell. Biol. 1996;16:6486–6493. doi: 10.1128/mcb.16.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenberg P. T., Lustig K. D., McGarry T. J., King R. W., Kuang J., Kirschner M. W. Systematic identification of mitotic phosphoproteins. Curr. Biol. 1997;7:338–348. doi: 10.1016/s0960-9822(06)00157-6. [DOI] [PubMed] [Google Scholar]
- Taagepera S., Rao P. N., Drake F. H., Gorbsky G. J. DNA topoisomerase II alpha is the major chromosome protein recognized by the mitotic phosphoprotein antibody MPM-2. Proc. Natl. Acad Sci. USA. 1993;90:8407–8411. doi: 10.1073/pnas.90.18.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashevski A., Husseman J., Jin L. W., Nochlin D., Vincent I. Constitutive Wee1 activity in adult brain neurons with M phase-type alterations in Alzheimer neurodegeneration. J. Alzheimer's Dis. 2001;3:195–207. doi: 10.3233/jad-2001-3205. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Tunquist B. J., Schwab M. S., Chen L. G., Maller J. L. The spindle checkpoint kinase bub1 and cyclin e/cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr. Biol. 2002;12:1027–1033. doi: 10.1016/s0960-9822(02)00894-1. [DOI] [PubMed] [Google Scholar]
- Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., Shah K., Shokat K. M., Morgan D. O. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- Vassilev L. T., Tovar C., Chen S., Knezevic D., Zhao X., Sun H., Heimbrook D. C., Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl Acad. Sci. USA. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., He G., Nelman-Gonzalez M., Ashorn C. L., Gallick G. E., Stukenberg P. T., Kirschner M. W., Kuang J. Regulation of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell. 2007;128:1119–1132. doi: 10.1016/j.cell.2006.11.053. [DOI] [PubMed] [Google Scholar]
- Wells N. J., Watanabe N., Tokusumi T., Jiang W., Verdecia M. A., Hunter T. The C-terminal domain of the Cdc2 inhibitory kinase Myt1 interacts with Cdc2 complexes and is required for inhibition of G(2)/M progression. J. Cell Sci. 1999;112(Pt 19):3361–3371. doi: 10.1242/jcs.112.19.3361. [DOI] [PubMed] [Google Scholar]
- Westendorf J. M., Rao P. N., Gerace L. Cloning of cDNAs for M-phase phosphoproteins recognized by the MPM2 monoclonal antibody and determination of the phosphorylated epitope. Proc. Natl. Acad. Sci. USA. 1994;91:714–718. doi: 10.1073/pnas.91.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Q., Kornbluth S. Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era. J. Cell Sci. 2008;121:3509–3514. doi: 10.1242/jcs.036855. [DOI] [PubMed] [Google Scholar]
- Wu M., Gerhart J. C. Partial purification and characterization of the maturation-promoting factor from eggs of Xenopus laevis. Dev. Biol. 1980;79:465–477. doi: 10.1016/0012-1606(80)90131-1. [DOI] [PubMed] [Google Scholar]
- Yaffe M. B., et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278:1957–1960. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- Yang S. D., Song J. S., Yu J. S., Shiah S. G. Protein kinase FA/GSK-3 phosphorylates tau on Ser235-Pro and Ser404-Pro that are abnormally phosphorylated in Alzheimer's disease brain. J. Neurochem. 1993;61:1742–1747. doi: 10.1111/j.1471-4159.1993.tb09811.x. [DOI] [PubMed] [Google Scholar]
- Ye X. S., Xu G., Pu R. T., Fincher R. R., McGuire S. L., Osmani A. H., Osmani S. A. The NIMA protein kinase is hyperphosphorylated and activated downstream of p34cdc2/cyclin B: coordination of two mitosis promoting kinases. EMBO J. 1995;14:986–994. doi: 10.1002/j.1460-2075.1995.tb07079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Lan W., Ems-McClung S. C., Stukenberg P. T., Walczak C. E. Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol. Biol. Cell. 2007;18:3264–3276. doi: 10.1091/mbc.E07-01-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. Y., Kuang J., Adlakha R. C., Rao P. N. Threonine phosphorylation is associated with mitosis in HeLa cells. FEBS Lett. 1989;249:389–395. doi: 10.1016/0014-5793(89)80665-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.