We characterize a ternary complex of PDZK1, EBP50, and ezrin that is regulated by their individual inter- and intramolecular interactions. PDZK1 is shown to undergo cell confluence-dependent nucleocytoplasmic shuttling that regulates the formation of this complex. A functional redundancy between PDZK1 and EBP50 in microvilli maintenance is shown.
Abstract
PDZK1 and ezrin, radixin, moesin binding phosphoprotein 50 kDa (EBP50) are postsynaptic density 95/disc-large/zona occludens (PDZ)-domain–containing scaffolding proteins found in the apical microvilli of polarized epithelial cells. Binary interactions have been shown between the tail of PDZK1 and the PDZ domains of EBP50, as well as between EBP50 and the membrane–cytoskeletal linking protein ezrin. Here, we show that these molecules form a regulated ternary complex in vitro and in vivo. Complex formation is cooperative because ezrin positively influences the PDZK1/EBP50 interaction. Moreover, the interaction of PDZK1 with EBP50 is enhanced by the occupancy of EBP50's adjacent PDZ domain. The complex is further regulated by location, because PDZK1 shuttles from the nucleus in low confluence cells to microvilli in high confluence cells, and this regulates the formation of the PDZK1/EBP50/ezrin complex in vivo. Knockdown of EBP50 decreases the presence of microvilli, a phenotype that can be rescued by EBP50 re-expression or expression of a PDZK1 chimera that is directly targeted to ezrin. Thus, when appropriately located, PDZK1 can provide a function necessary for microvilli formation normally provided by EBP50. By entering into the ternary complex, PDZK1 can both enhance the scaffolding at the apical membrane as well as augment EBP50's role in microvilli formation.
INTRODUCTION
Cell polarity requires regulated mechanisms to localize the appropriate plasma membrane lipids and proteins at the right time and place and in the correct abundance. A classic example is provided by polarized epithelial cells, in which the apical aspect is generally studded with microvilli and has a different lipid and protein composition from the basolateral surface. Microvilli are filamentous-actin (F-actin)–based protrusions of the plasma membrane whose primary function in epithelia is thought to be to provide an enhanced surface area for absorption in cell types such as the kidney proximal tubules and intestine. Microvilli are dynamic structures, and the regulation of their formation and turnover is poorly understood. We are attempting to address how cells regulate the presence of microvilli on their apical surface, and how these structures modulate the presence of appropriate membrane proteins.
Ezrin is a critical component of epithelial cell microvilli that provides a regulated membrane-cytoskeletal linking function (Bretscher et al., 2002; Saotome et al., 2004). Ezrin is composed of an amino-terminal protein 4.1, ezrin, radixin, moesin (FERM) domain and a carboxy-terminal ezrin, radixin, moesin association domain (C-ERMAD) that are linked by a long α-helical region. The FERM and C-ERMAD domains interact in a regulated manner to create either an inactive “closed” or active “open” structure. Significantly, when ezrin is closed, the intramolecular association masks binding sites for ezrin, radixin, moesin (ERM) binding phosphoprotein 50 kDa (EBP50; also known as NHERF1) and F-actin in the amino- and carboxy-terminal domains, respectively (Gary and Bretscher, 1995; Reczek and Bretscher, 1998). Like other members of the ERM protein family, ezrin is known to be activated by phosphorylation (at threonine 567 in ezrin) and binding of the membrane phospholipid phosphatidylinositol bisphosphate (Bretscher et al., 2002; Fievet et al., 2004). Structurally, ezrin's intermediate α-helical region is suggested to undergo a major change from a coiled-coil configuration via a “switch-blade”-type reorganization into an extended helix when the molecule opens (Li et al., 2007).
The release of ezrin's intramolecular association unmasks the binding site on its FERM domain for the scaffolding protein EBP50, a microvillar protein consisting of two postsynaptic density 95/disc-large/zona occludens (PDZ) domains and a carboxy-terminal tail responsible for its ability to bind ezrin (Reczek et al., 1997; Reczek and Bretscher, 1998). Interestingly, EBP50 is also capable of an inhibitory intramolecular interaction. In this case, the second PDZ domain binds the tail, and this serves to reduce binding of ligands to both PDZ domains (Morales et al., 2007). Regulation of EBP50 is likely to be physiologically important because this scaffold protein interacts with many signaling molecules at the plasma membrane, including the cystic fibrosis transmembrane conductance regulator (CFTR), NaPi IIa cotransporter, and β2-adrenergic receptor (Short et al., 1998; Cao et al., 1999; Hernando et al., 2002). EBP50 also regulates the structure of microvilli (Morales et al., 2004; Hanono et al., 2006).
Another apically localized scaffolding protein is PDZK1 (also known as NHERF3) that consists primarily of four PDZ domains. We have found that the tail of PDZK1 binds to the PDZ domains of EBP50 (LaLonde and Bretscher, 2009). In addition, we have demonstrated that PDZK1 also undergoes an intramolecular interaction through its C-terminal DTEM motif (the final four residues of PDZK1) and its first PDZ domain, although the overall regulatory consequences of this interaction are unknown (LaLonde and Bretscher, 2009). However, elimination of the interaction by deletion of the first PDZ domain releases the PDZK1 tail to allow more efficient interaction with the PDZ domains of EBP50 (LaLonde and Bretscher, 2009). Similar to EBP50, PDZK1 is well characterized for its regulation of transmembrane proteins (Lamprecht and Seidler, 2006; Kocher and Krieger, 2009). In particular, PDZK1 maintains the abundance of scavenger receptor β1 in liver and subsequently regulates plasma levels of high-density lipoprotein (Silver, 2002; Kocher et al., 2003).
Here, we examine the interactions of ezrin, EBP50, and PDZK1 to reveal that the three proteins form a complex regulated by intramolecular associations. We also show that PDZK1 cycles between the nucleus and microvilli in a manner that is dependent on cell confluence and that this regulates the formation of the ternary complex in vivo. Finally, we show that either EBP50 or PDZK1 targeted to ezrin can provide a function necessary for microvillar maintenance, implying that PDZK1 acts as a modular adaptor protein to augment the functioning of EBP50 in microvilli.
MATERIALS AND METHODS
Antibodies and Reagents
Monoclonal antibody (mAb) versus EEA1 was purchased from BD Biosciences Transduction Laboratories (Lexington, KY). Infrared-labeled secondary antibodies and IRDye blue protein stain were purchased from LI-COR Biosciences (Lincoln, NE). Polyclonal antibodies against full-length green fluorescent protein (GFP) and human PDZK1 (amino acids 293-519), both of which were purified as glutathione transferase (GST)-tagged proteins, were generated in rabbits by Abraham Hanono (Cornell University) using standard protocols. The polyclonal antibodies versus EBP50 and E3KARP/NHERF2 were as described previously (Reczek et al., 1997; Ingraffea et al., 2002). The mAb against ezrin developed at the National Cancer Institute (Bethesda, MD) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by Department of Biology, The University of Iowa, Iowa City, IA. The mAb HA.11 versus the hemagglutinin (HA) epitope was purchased from Covance Research Products (Princeton, NJ). Streptavidin-Sepharose was obtained from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). Calf intestinal alkaline phosphatase (CIAP) was purchased from Invitrogen (Carlsbad, CA).
Cell Culture
JEG3 and LLC-PK1 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in a 5% CO2 humidified atmosphere at 37°C in minimal essential medium or DMEM (Invitrogen), respectively, with glutamine, penicillin, streptomycin, and 10% fetal bovine serum. LLC-PK1 cells constitutively expressing pGlue-PDZK1 were selected and maintained in DMEM containing 1 mg/ml puromycin. Transfections were performed with polyethylenimine (PEI) as described previously (Hanono et al., 2006) or with FuGENE HD according to the manufacturer's protocols.
DNA Constructs
All GST-tagged PDZK1 constructs were as described previously (LaLonde and Bretscher, 2009). All point-mutated constructs were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The PDZK1/EBP50 chimera was generated via polymerase chain reaction (PCR) by generating both fragments with overlapping ends then combining them for a third PCR reaction to generate the full-length chimeric construct. All tagged constructs were generated using standard methodologies and subcloned into the vectors pGEX6P1, pEGFPC2, pESumo, or pGlue (pGlue vector provided by Stephane Angers, University of Toronto, Toronto, ON, Canada).
Preparation of Recombinant Proteins
GST-tagged fusion proteins were prepared according to standard protocols. The ezrin FERM domain was purified over a hydroxyapatite column followed by a Mono-S Sepharose column as described previously (Reczek and Bretscher, 1998) using an ÄKTA FPLC system (GE Healthcare). The FERM domain was linked to cyanogen bromide-activated Sepharose (Sigma-Aldrich, St. Louis, MO) by standard protocols.
For purification of SUMO-tagged constructs, bacterial pellets were first resuspended in binding buffer (20 mM sodium phosphate, 500 mM NaCl, 20 mM imidazole, pH 7.4, and 0.1% Triton X-100) and then sonicated and centrifuged to remove debris. The supernatant was then bound to a His GraviTrap column (GE Healthcare), washed, and eluted with elution buffer (similar to binding buffer but with imidazole at 500 mM). The eluted proteins were then dialyzed overnight in binding buffer followed by incubation with 6His-tagged Ulp1 for 40 min at 30°C to cleave off the SUMO tag and leave untagged protein. The resulting solution was then applied to a second nickel column to remove the 6His-tagged Ulp1 and the nontagged purified EBP50 was collected in the flowthrough. 6His-tagged EBP50 PDZ interactor of 64 kDa (EPI64) was purified from insect cells according to standard protocols.
Binding Experiments
Precipitations of GST-tagged proteins from cellular lysates were performed as described previously (LaLonde and Bretscher, 2009). For CIAP treatment, lysates were incubated with 1 U of CIAP per 5 μl of lysate for 45 min at 30°C in the absence of phosphatase inhibitors before the precipitation step. Streptavidin-Sepharose (GE Healthcare) was used to precipitate pGlue-tagged PDZK1, which contains a streptavidin-binding tag, followed by biotin elution and analysis by Western blotting, similarly to what has been described previously, but without the second binding step using the calmodulin-binding tag (Angers et al., 2006). In vitro binding assays between recombinant proteins were performed as described previously (LaLonde and Bretscher, 2009). In experiments with soluble proteins, they were preincubated together in a small volume of binding buffer before the addition of the resin bound probe. All Western blot or stained SDS-polyacrylamide gel electrophoresis (PAGE) gels were imaged using an Odyssey infrared imaging system (LI-COR Biosciences) with either infrared-labeled secondary antibodies or IRDye blue protein stain, respectively.
Immunofluorescence, Microvillar Rescue Experiments, and Cell Fractionation
LLC-PK1 or JEG3 cells were grown on glass coverslips, fixed in 3.7% formaldehyde, permeabilized in 0.2% Triton X-100, and then washed in phosphate-buffered saline (PBS). Images were acquired either on a spinning disk microscope (PerkinElmer Life and Analytical Sciences, Boston, MA) with 100× 1.4 numerical aperture (NA) objective and Orca-ER charge-coupled device (CCD) camera (Hamamatsu, Bridgewater, NJ) or with a CSU-X spinning disk microscope (Intelligent Imaging Innovations, Denver, CO) with 63× 1.4 NA objective and HQ2 CCD camera (Photometrics, Tucson, AZ). Acquired images were processed with either ImageJ (http://rsb.info.nih.gov/ij/) or SlideBook 5.0 (Intelligent Imaging Innovations, Denver, CO). For microvillar rescue assays, cells were transfected with appropriate cDNAs and then treated with small interfering RNA (siRNA) the following day and allowed to grow for a further 48 h before processing for microscopy. siRNA treatments and microvillar counting were performed as described previously (Hanono et al., 2006). Nuclear fractionation was performed as described previously (Michaelson et al., 2008).
RESULTS
The Microvillar Protein PDZK1 Forms a Complex with EBP50 and Ezrin
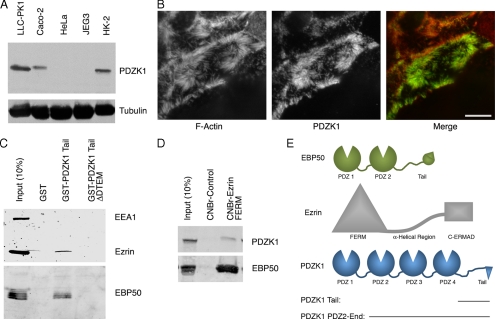
To facilitate analysis of endogenous PDZK1, we generated a polyclonal antibody and used it to show that the protein is present in cells derived from polarized epithelia, including LLC-PK1 and HK-2 renal tubular epithelial cells and Caco-2 intestinal cells but that it is absent from cervical HeLa or placentally derived JEG3 cells (Figure 1A). The specificity of the PDZK1 antibody was confirmed in Supplemental Figure S1A. These same cell lines were also immunoblotted for the presence of EBP50 and E3KARP/NHERF2, which revealed that JEG3 cells are rich in EBP50 but lack detectable E3KARP (Supplemental Figure S1B). Immunostaining shows that endogenous PDZK1 is located in microvilli in LLC-PK1 cells, as has been reported previously (Figure 1B) (Gisler et al., 2001). We have demonstrated previously that the tail of PDZK1 interacts with the PDZ domains of EBP50 (LaLonde and Bretscher, 2009), so we investigated whether a ternary complex with PDZK1, EBP50, and ezrin might exist in vivo (schematics of these proteins are presented in Figure 1E). The tail of PDZK1 fused to GST was used to precipitate EBP50 from lysates prepared from JEG3 cells, which are rich in EBP50 and ezrin but do not contain endogenous PDZK1 (Figure 1A). Ezrin and EBP50 coprecipitated with the GST-PDZK1 tail, and this is a specific interaction as it requires the C-terminal DTEM sequence of the GST-PDZK1-tail construct that is needed to interact with the EBP50 PDZ domains (Figure 1C; LaLonde and Bretscher, 2009). As an alternative way to probe for the existence of the complex, we made use of the finding that immobilized ezrin FERM domain binds tightly to the tail of EBP50 (Reczek et al., 1997). Ezrin FERM domain immobilized on Sepharose recovered both EBP50 and a fraction of the PDZK1 from extracts of LLC-PK1 cells (Figure 1D). Together, these results suggest that PDZK1 binds a pool of EBP50 that is also bound to ezrin and that the ezrin FERM domain binds a pool of EBP50 that is also bound to PDZK1.
Figure 1.
PDZK1 is found in microvilli and forms a complex with EBP50 and ezrin. (A) Immunoblot with PDZK1 antibody of lysates from the indicated cell lines, with tubulin used as a loading control. (B) Immunofluorescence localization of PDZK1 to microvilli in LLC-PK1 cells. F-actin was labeled with rhodamine phalloidin. The image represents a maximum projection of the apical aspect of the cell. Bar, 10 μm. (C) The GST constructs indicated were used for precipitations from JEG3 cell lysates. Retained and eluted material was blotted for ezrin and EBP50, with EEA1 serving as a negative control. (D) The ezrin FERM domain coupled to Sepharose or control Sepharose beads were used to precipitate proteins from LLC-PK1 cells. Both EBP50 and PDZK1 were coprecipitated with the FERM domain. (E) Schematics of PDZK1, EBP50 and ezrin are shown with the relevant domains and truncation constructs indicated.
EBP50 Serves a Linker between PDZK1 and Ezrin
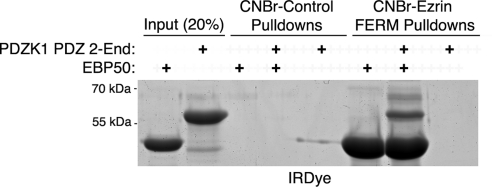
Our results with cell extracts could be explained by EBP50 and PDZK1 each binding individually to ezrin, or PDZK1 binding through EBP50 to ezrin. We therefore sought to distinguish these possibilities in vitro. Beads on which ezrin FERM had been immobilized, or control beads, were incubated with recombinant EBP50 and/or recombinant PDZK1-PDZ2-End. PDZK1-PDZ2-End was used for this assay as removal of PDZ1 frees the PDZK1 tail from its intramolecular interaction to allow more effective binding to EBP50 (LaLonde and Bretscher, 2009). The results reveal that PDZK1 does not bind the ezrin FERM domain directly but is coprecipitated in the presence of EBP50, illustrating that EBP50 serves as a linker between PDZK1 and ezrin (Figure 2). To recapitulate this finding in cells, EBP50 knockdown was performed in JEG3 cells, followed by GST-PDZK1 tail pull-downs to determine whether ezrin coprecipitation would be lost. This approach was not fully successful, as the GST-PDZK1 tail construct was able to efficiently precipitate the residual EBP50. Nevertheless, quantitative Western blotting revealed a reduction in the recovery of ezrin that was proportional to the reduction in precipitated EBP50 (there is a 34 ± 10% loss of precipitated EBP50 and a 39 ± 7% loss of precipitated ezrin).
Figure 2.
EBP50 serves as a linker between PDZK1 and ezrin. In vitro binding assays were performed between the indicated CNBr resin-bound proteins and soluble proteins. Although the FERM domain strongly precipitates EBP50, it only precipitates PDZK1 PDZ 2-End when EBP50 is also present. These results indicate the existence of a PDZK1–EBP50–ezrin ternary complex with EBP50 as the intermediate component.
EBP50 Phosphorylation Does Not Impact Its Ability to Link PDZK1 to Ezrin
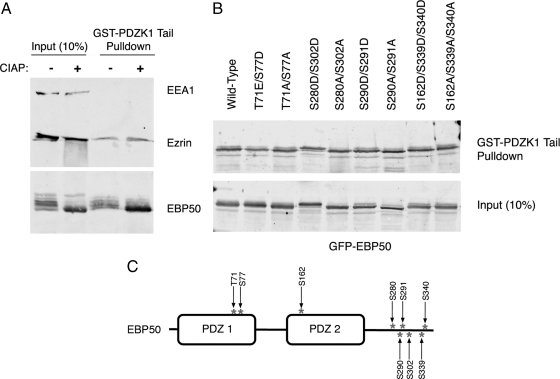
EBP50 is a substrate for a large number of kinases, and these have been suggested to regulate the ability of this protein to oligomerize or interact with PDZ domain-binding partners (Lau and Hall, 2001; Voltz et al., 2007). We therefore wanted to explore whether EBP50 phosphorylation might impact its interaction with PDZK1. We first tested the ability of the GST-PDZK1 tail to recover EBP50 and ezrin from JEG3 cell lysates treated with CIAP to dephosphorylate all the proteins. EBP50 migrates in SDS gels as a series of bands that can be collapsed into a single faster-migrating species upon CIAP treatment, and so the multiple species therefore represent differentially phosphorylated forms (Figure 3A). When the extracts were subjected to GST-PDZK1 tail pull-downs, EBP50 and ezrin were recovered equally from control and phosphatase-treated extracts (Figure 3A). We conclude that phosphorylation does not have a major positive or negative effect on the binding of EBP50 to the PDZK1 tail. Ezrin was also efficiently coprecipitated, showing that there is a pool of EBP50 bound to ezrin that is resistant to serine/threonine dephosphorylation. To test specific phosphorylation sites in EBP50, we generated phosphomimetic (S/T->D) or phosphodeficient (S/T->A) mutations of several of the reported phosphorylation sites (Figure 3C) and expressed tagged versions of each in JEG3 cells (Weinman et al., 1998; He et al., 2001; Fouassier et al., 2005; Voltz et al., 2007). Each of the eight variants tested was found to be competent to bind the PDZK1 tail (Figure 3B), suggesting that none of these sites significantly impacts the PDZK1–EBP50 interaction. It is also notable that the GST-PDZK1 tail is able to precipitate all of the phospho-bands of EBP50 that are apparent via Western blotting (Figures 1C and 3A), further reinforcing our findings that EBP50 phosphorylation does not significantly impact its ability to complex with PDZK1 and ezrin.
Figure 3.
EBP50 phosphorylation does not significantly impact its association with PDZK1. (A) JEG3 cell extracts were treated with or without CIAP then subjected to pull-downs with the resin-bound GST-PDZK1 tail. Dephosphorylation of EBP50, as indicated by the loss of bands of different mobility by SDS-PAGE, did not alter the association with the GST-PDZK1 tail. (B) The indicated phosphomimetic and phosphodeficient EBP50 mutants were expressed in JEG3 cells. Lysates were prepared and subjected to pull-downs with the resin-bound GST-PDZK1 tail. All constructs bound the PDZK1 tail comparably. (C) Schematic of EBP50 showing the location of phosphorylation sites that were mutated.
Ezrin Binding to EBP50 Positively Regulates the EBP50/PDZK1 Association
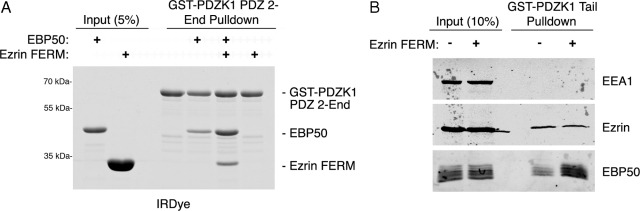
It has been found that ezrin binding to the tail domain of EBP50 releases an intramolecular inhibitory interaction of EBP50, thus allowing EBP50's PDZ domains to bind ligand more effectively, although this has only been demonstrated for a few substrates (Li et al., 2005, 2009; Morales et al., 2007). Using recombinant proteins, we explored whether an association between the ezrin FERM domain and EBP50 influences the binding of PDZK1 to EBP50. We again used the GST-PDZK1-PDZ2-End protein as the lack of the first PDZ domain allows PDZK1 to be open and competent to bind EBP50, with binding equivalent to the PDZK1 tail alone (LaLonde and Bretscher, 2009). The presence of the ezrin FERM domain significantly enhanced the binding of EBP50 to PDZK1 (Figure 4A). Also, the FERM domain coprecipitates with EBP50 and PDZK1, consistent with the in vitro results in Figure 2. Notably, the increase in EBP50 binding is proportional to the amount of FERM coprecipitating, illustrating how FERM binding increases the amount of EBP50 available to interact with PDZK1, without being required for the baseline amount (Figure 4A). Similar results were seen using the GST-PDZK1 tail and are included in Supplemental Figure S2A but are less clear as the FERM domain runs at the same size on SDS-PAGE as the GST-PDZK1 tail. Also, in vitro binding assays were performed between resin-bound GST-PDZK1 tail and soluble EBP50 with increasing amounts of soluble ezrin FERM and illustrate a dose-dependence of this effect (Supplemental Figure S2B). A similar experiment was performed examining the ability of GST-PDZK1 tail to recover EBP50 from JEG3 cell extracts, to which either the ezrin FERM domain or bovine serum albumin had been added. Consistent with the results in Figure 4A, there is a significant increase in the amount of EBP50 bound to the PDZK1 fusion when the ezrin FERM domain was added (Figure 4B). Interestingly, the amount of endogenous ezrin coprecipitated is unchanged, indicating that the pool of EBP50 bound to endogenous ezrin is very stably associated and is not immediately competed off by the added excess ezrin FERM domain (Figure 4B).
Figure 4.
The ezrin FERM domain positively regulates the association between EBP50 and PDZK1. (A) In vitro binding assays were performed between resin-bound GST-PDZK1 PDZ 2-End versus soluble EBP50 or ezrin FERM in the indicated combinations. The FERM domain both coprecipitates with PDZK1 when EBP50 is present and increases the affinity of EBP50 for PDZK1. (B) Resin-bound GST-PDZK1 tail was used to precipitate proteins from JEG3 cell extracts with either BSA or soluble ezrin FERM domain added to the lysates. EBP50 binds the GST-PDZK1 tail more efficiently in the presence of excess ezrin FERM domain.
Allosteric Interactions between EBP50's Two PDZ Domains
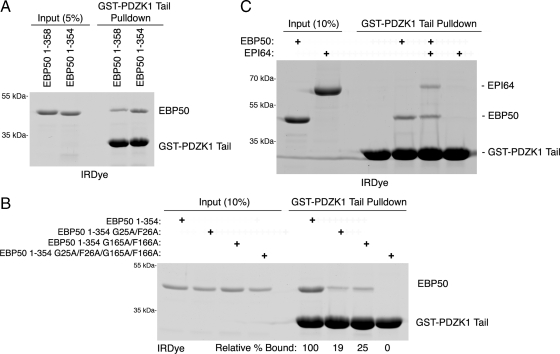
Because PDZK1's tail binds to EBP50, we wanted to determine which of EBP50's PDZ domains are involved. Although we have shown that the PDZK1 tail can bind to either of EBP50's separated PDZ domains (LaLonde and Bretscher, 2009), we wanted to explore which domain might be more relevant in the context of the full-length molecule and how the presence of another ligand that binds specifically to just one of the PDZ domains might affect the interaction. We first compared the association between the PDZK1 tail and either full-length EBP50 (1-358) or a mutant lacking its final four residues (1-354). In vitro binding assays between the GST-PDZK1 tail and the recombinant EBP50 constructs show that the truncated EBP50 construct binds significantly better than the wild-type construct, with 2.4 times as much precipitated in this experiment (Figure 5A). In our initial report detailing PDZK1's interaction with EBP50, recombinant PDZK1 bound equally well to either isolated GST-EBP50 PDZ domain (LaLonde and Bretscher, 2009). We repeated this experiment using EBP50 (1-354) with wild-type PDZ domains, or with an inactive PDZ1 mutant (G25A/F26A), or an inactive PDZ2 (G165A/F166A) mutant, or a version with both PDZ domains mutated. These mutations are point mutations in the carboxylate-binding loop of the PDZ domain that abolish binding to tail ligands (Doyle et al., 1996; LaLonde and Bretscher, 2009). Surprisingly, mutation of either of the PDZ domains greatly reduced binding, whereas mutation of both abolished it (Figure 5B). These results indicate that both PDZ domains need to be functional for optimal binding to the PDZK1 tail, perhaps suggestive of allosteric cooperative binding, similar to what has been shown for some other multi-PDZ domain-containing proteins (Grootjans et al., 2000; van den Berk et al., 2007). We next wanted to explore how the binding would be affected when one of the PDZ domains was occupied by a different ligand. The microvillar protein EPI64 ends in -DTYL and binds strongly and selectively to EBP50's PDZ1 (Reczek and Bretscher, 2001). To evaluate how binding of EPI64 to EBP50 might influence association with the PDZK1 tail, we performed in vitro binding assays between full-length EBP50 and the GST-PDZK1 tail either with or without full-length recombinant EPI64 (Figure 5C). The amount of EBP50 recovered was only slightly reduced by the presence of EPI64. Moreover, the amount of EPI64 recovered is essentially identical to EBP50, suggesting that the PDZ1 domain is saturated with EPI64 and a majority of the EBP50 binding to PDZK1 is occurring through the EBP50 PDZ2 domain. Increasing the amount of EPI64 does not further inhibit EBP50 binding to the GST-PDZK1 tail, indicating that PDZ1 is saturated with EPI64 and EBP50 binding to the GST-PDZK1 tail can occur predominantly through PDZ2 when PDZ1 is bound (Supplemental Figure S3). Several interesting points can be drawn from these results. First, EPI64 is able to enter into a multimolecular complex with EBP50 and PDZK1 in vitro, because it coprecipitates with the PDZK1 tail only when EBP50 is present (Figure 5C). Second, the PDZK1 tail is able to bind PDZ2 of EBP50 efficiently when PDZ1 is occupied by EPI64. Because the PDZK1 tail binds PDZ2 weakly when PDZ1 is mutated to be nonfunctional and therefore empty, it follows that PDZK1 binds the second PDZ domain of EBP50 more efficiently when the first PDZ domain is occupied (Figure 5, B and C). Overall, these results indicate that the PDZ domains of EBP50 interact with some ligands in a cooperative, allosterically regulated manner, with PDZK1 binding to the second PDZ domain being positively regulated by ligation of the first PDZ domain.
Figure 5.
Characterization of the interaction between PDZK1 and the EBP50 PDZ domains. (A) In vitro binding assays were performed between resin-bound GST-PDZK1 tail and the indicated EBP50 constructs. EBP50 lacking its final four residues (1-354) binds PDZK1 more efficiently than full-length EBP50 (1–358). (B) In vitro binding assays were performed between resin-bound GST-PDZK1 tail and soluble EBP50 with mutated, inactive PDZ domains (G25A/F26A has an inactive PDZ1, whereas G165A/F166A has an inactive PDZ2). The relative amounts of PDZ mutants bound compared with the nonmutated EBP50 construct are shown below the gel. Mutation of either PDZ domain reduces the association between PDZK1 and EBP50. (C) In vitro binding assays were performed between resin-bound GST-PDZK1 tail and the indicated constructs. EPI64, a strong binder of EBP50 PDZ1, is able to precipitate with the GST-PDZK1 tail in the presence of EBP50. The results of B and C indicate that PDZK1 can bind PDZ2 of EBP50 more efficiently when PDZ1 is occupied.
PDZK1 Cycles between the Nucleus and Microvilli Based on Cell Confluence
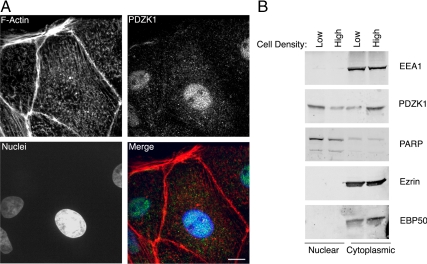
Our data indicate that a three-way complex occurs with PDZK1, EBP50, and ezrin and that this complex is regulated by their individual intramolecular interactions. In addition, examination of PDZK1 localization under different conditions provides another level of in vivo regulation. PDZK1 is found primarily in microvilli in high-confluence LLC-PK1 cells (Figure 1B), whereas at low confluence, when the cells are still in islands but have not yet begun to significantly pack together, it is predominantly nuclear (Figure 6A). This confluence-dependent shuttling is also observed in Caco-2 cells (our unpublished observations) and is not unique, because other proteins such as the tight junction protein zona occludens-2 has been reported to undergo similar cell density-dependent localization changes (Islas et al., 2002). To validate the localization differences, we fractionated LLC-PK1 cells into nuclear and cytoplasmic fractions after growth to low or high confluence (Figure 6B). PDZK1 is more abundant in the nuclear fraction of low confluence cells and is enriched in the cytoplasmic fraction of high confluence cells. However, the shift is not complete as there is a nuclear pool of PDZK1 in high confluence cells and a cytosolic pool in low confluence cells, probably indicative of changes in confluence that occur over the entire population of cells that are in a tissue culture plate. Quantification of comparative amounts of nuclear to cytosolic PDZK1 indicates ∼25% is cytosolic in low confluence cells and 75% cytosolic in high confluence cells. Notably, neither ezrin nor EBP50 showed any nuclear localization under any of these conditions (Figure 6B). PARP served as a nuclear control protein, whereas EEA1 served as a cytosolic control protein (Figure 6B). The apical localization of PDZK1 may be dependent on the presence of fully mature cell–cell junctions as PDZK1 exits the nucleus in both interior and border cells within a patch of LLC-PK1 cells that have been seeded for some time (our unpublished observations).
Figure 6.
PDZK1 cycles into and out of the nucleus based on cell confluence. (A) Immunofluorescence localization of endogenous PDZK1 in the nucleus in low confluence LLC-PK1 cells costained to show F-actin and cell nuclei. The image represents a maximum projection through the cell. Bar, 10 μm. (B) LLC-PK1 cells at either high or low confluence were fractionated into nuclear and cytoplasmic fractions then blotted for the indicated proteins. PDZK1 becomes less enriched in the nucleus in high confluence cells, whereas EBP50 and ezrin are not found in the nuclear fraction.
PDZK1 Nuclear Shuttling Regulates Its Association with EBP50 and Ezrin
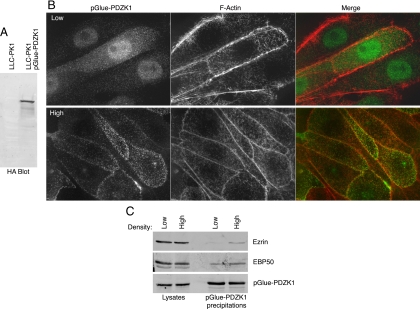
To explore whether the localization of PDZK1 affected its ability to associate with EBP50 and ezrin, we generated an LLC-PK1 cell line stably expressing pGlue-PDZK1, which is a tagged version containing an amino-terminal streptavidin-binding domain, a tobacco etch virus cleavage site, an HA tag, and a calmodulin binding domain that can be used for tandem affinity precipitation (Figure 7A; Angers et al., 2006). Like the endogenous protein, the stably expressed tagged version of PDZK1 was found in the nucleus in low confluence cells and in the surface microvilli in highly confluent, packed cell layers (Figure 7B). Precipitations of pGlue-PDZK1 using the streptavidin-binding domain recovered about twice as much EBP50 and ezrin in cells grown under high confluence compared with those grown under low confluence (Figure 7C). This result is in line with the approximate threefold increased level of PDZK1 in the cytoplasm in cells of higher density.
Figure 7.
Cell confluence regulates the association between PDZK1 and ezrin/EBP50. (A) Lysates from cells stably expressing pGlue-PDZK1 and parental cells were blotted with monoclonal anti-HA antibody to show expression of the pGlue construct. (B) Immunofluorescence localization of stably expressed pGlue-tagged PDZK1 in LLC-PK1 cells at high and low confluence costained with rhodamine phalloidin to show F-actin. The tagged version of PDZK1 exhibits similar nuclear shuttling to the endogenous protein. (C) pGlue-PDZK1 was precipitated out of the stably expressing cell line at both high and low confluence and analyzed by SDS-PAGE. Twice as much eluate was analyzed for the ezrin and EBP50 blots as was used for the HA blot for PDZK1. EBP50 and ezrin are both found to coprecipitate with PDZK1 more efficiently under higher cell confluence.
PDZK1 Exhibits Functional Redundancy with EBP50 in Microvilli Maintenance
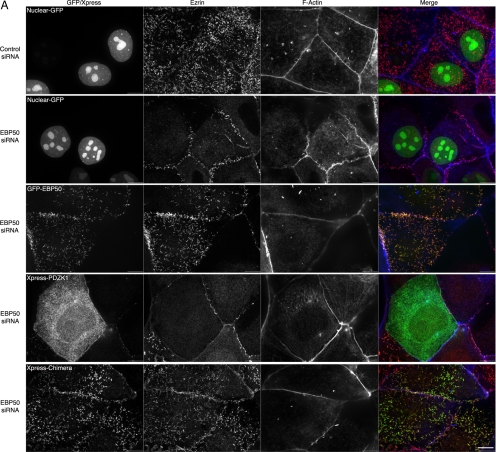
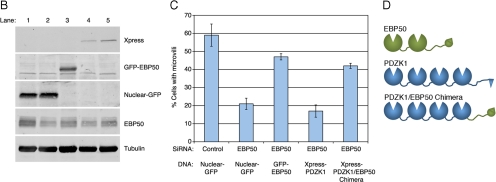
Based on the close primary sequence similarity of the PDZ domains of EBP50 and PDZK1, it has been suggested that PDZK1 evolved by duplication and fusion of the EBP50 gene (Donowitz et al., 2005). In support of this idea, they share some ligands in common, such as NHE3 and CFTR (Lamprecht and Seidler, 2006). We considered how the two scaffolding proteins might function together at the apical membrane. One possibility is that they have similar functions, with PDZK1 having evolved to bind EBP50 to enhance the recruitment of certain ligands. We therefore wanted to test whether EBP50 and PDZK1 might have some functional overlap. We have shown that siRNA depletion of EBP50 from JEG3 cells reduces the abundance of microvilli on their apical surface (Hanono et al., 2006). We therefore wanted to deplete cells of EBP50 and recruit PDZK1 to the apical surface in an EBP50-independent manner to see whether microvilli were restored. To do this, we generated a chimera consisting of the PDZ domains of PDZK1 fused to the tail of EBP50, which contains the ezrin binding site, thereby providing a direct linkage between PDZK1 and ezrin (Figure 8D). Our strategy was to treat cells with siRNA to EBP50 and then express either a control GFP construct that is targeted to the nucleus (Nuclear-GFP), or an siRNA-resistant GFP-EBP50 construct, or tagged PDZK1 (Xpress-PDZK1), or the tagged PDZK1-EBP50-tail chimera (Xpress-Chimera), and then examine the cells for the presence of microvilli. Supplemental Figure S4A illustrates enhanced binding between resin-bound ezrin FERM domain and the Xpress-Chimera compared with Xpress-PDZK1, indicative of the expected direct interaction. There is a dramatic loss of microvilli in EBP50 knockdown cells, with a small number of microvilli remaining at the cell borders. This loss of microvilli can be rescued by the expression of RNA interference-resistant GFP-EBP50 but not by expression of either the nuclear-GFP or tagged wild-type PDZK1 constructs (Figure 8, A–C). However, expression of the PDZK1-EBP50-tail chimera in the EBP50 siRNA cells restores microvilli nearly as efficiently as expression of GFP-EBP50 does (Figure 8, A and C). This implies a measure of functional redundancy between PDZK1 and EBP50. Moreover, expression of tagged wild-type PDZK1 in EBP50 knockdown cells mildly further inhibited microvilli, indicating a dominant negative effect by this protein, possibly by mislocalizing some crucial binding partner of the EBP50 PDZ domains (Figure 8C). Notably, the Xpress-PDZK1 construct is capable of localizing to microvilli in JEG3 cells that have not been depleted of EBP50 (Supplemental Figure S4B). Thus, PDZK1 seems to be able to recruit some factor necessary for microvilli, and this function requires its recruitment by EBP50.
Figure 8.
PDZK1 exhibits functional redundancy with EBP50 in microvilli formation. (A) JEG3 cells were transfected with the indicated constructs, treated with siRNA to EBP50 or a control luciferase oligonucleotide one day later, allowed to grow for 2 d and then processed for immunofluorescence. Bar, 10 μm. (B) Lysates of the conditions listed in A and C were blotted as indicated. The lane numbers correspond to the five conditions represented in A moving from top to bottom or on the graph in C moving from left to right. (C) Percentage of cells exhibiting microvilli under the indicated conditions was quantified. EBP50 knockdown inhibits microvilli formation and this is significantly (p < 0.01) rescued by both EBP50 and a PDZK1/EBP50 chimera but not by wild-type PDZK1. (D) Schematic of the domains present in the PDZK1/EBP50 chimera versus the wild-type proteins.
DISCUSSION
One mechanism that cells use to regulate the structure and composition of the plasma membrane is through regulated scaffolding proteins. A well-characterized scaffolding protein found at the apical aspect of epithelial cells is EBP50, which is localized there by its association with ezrin. Several reasons prompted us to investigate PDZK1. First, this scaffolding protein is also localized to the apical aspect of polarized epithelial cells (Gisler et al., 2001); second, the tail of PDZK1 binds to EBP50 (LaLonde and Bretscher, 2009); and third, because PDZK1 and EBP50 seem to share a close evolutionary origin (Donowitz et al., 2005), we wondered whether they might have similar functions.
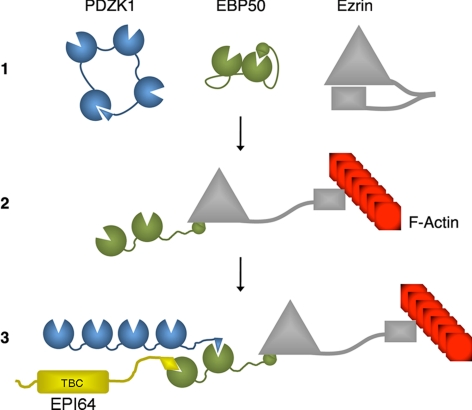
We find that the microvillar proteins PDZK1, EBP50, and ezrin form a complex and that this complex is regulated by their individual intramolecular interactions (Figure 9). Specifically, each of them is known to undergo a negative regulatory intramolecular association [the first PDZ domain of PDZK1 interacts with its tail; LaLonde and Bretscher, 2009); the second PDZ domain of EBP50 interacts with its tail (Morales et al., 2007); the FERM domain of ezrin interacts with the C-ERMAD (Gary and Bretscher, 1995)] that regulates binding to their neighboring molecules. We show that an EBP50 mutant in which the negative intramolecular interaction is abolished by truncating the C terminus binds better than wild-type EBP50 to PDZK1, and this same effect can be achieved with wild-type EBP50 if activated by the addition of the ezrin FERM domain. Thus, there is secondhand cooperative binding that is associated with ezrin binding to EBP50 to release the EBP50 intramolecular interaction and increase the PDZK1/EBP50 association, as has been indicated for a few other EBP50 binding partners in previous reports (Li et al., 2005; Morales et al., 2007). It is difficult to assess whether the reverse is true, that is, whether PDZK1 binding increases the association between EBP50 and ezrin, as the latter interaction is already so tight in vitro, with nearly complete, stoichiometric binding. An intriguing aspect of this study is that using the PDZK1 tail we were readily able to isolate an EBP50–ezrin complex from extracts of cells, yet our previous unpublished experiments have not convincingly revealed an EBP50–ezrin complex when we simply immunoprecipitated ezrin or EBP50. Thus, it is possible that binding of PDZK1 to EBP50 in some manner stabilizes the EBP50–ezrin interaction. Therefore, cooperative interactions may occur both ways within this ternary complex allowing a “domino-effect” of interaction reinforcement that can happen either from the PDZK1 or ezrin side of the complex.
Figure 9.
Schematic of the PDZK1–EBP50–ezrin complex. 1. The microvillar scaffold proteins PDZK1, EBP50, and ezrin exist in intramolecularly associated conformations before entering into a complex. These conformations reduce their associations with one another. 2. Ezrin becomes “activated” or “open” through a combination of membrane binding and phosphorylation. This allows its amino-terminal FERM domain to interact with EBP50 and its carboxy-terminal C-ERMAD to interact with F-actin. 3. The association with ezrin locks the EBP50 in an open conformation, thereby enhancing the association between EBP50 and the tail of PDZK1. PDZK1, EBP50 and ezrin are then able to form a complex. Notably, the tail of PDZK1 must be released from its intramolecular interaction with its own first PDZ domain to interact with EBP50. Additional EBP50 binding partners, such as EPI64 (labeled with its amino-terminal TBC domain), may also enter into this macromolecular complex.
This description of a regulated ternary complex is just the beginning of our understanding of the scaffolding mechanisms that regulate the proteins at the apical membrane. There are a myriad of regulatory possibilities given the wide array of proteins that have been shown to interact with either EBP50, or PDZK1, or both. For example, proteins that bind to the first PDZ domain of PDZK1 might displace its tail and make it more accessible to EBP50. Alternatively, ligands that bind to other PDZ domains might have positive or negative regulatory consequences. Although such studies are planned for the future, we did examine how the PDZ domains of EBP50 participate in formation of the ternary complex, which revealed another level of regulation.
In our initial study, we found that each of the PDZ domains of EBP50, when expressed in isolation as recombinant proteins, were able to bind PDZK1 equally well (LaLonde and Bretscher, 2009). Herein, we examined this in the context of the full-length EBP50 protein in the expectation that this would more closely reflect in vivo binding, and we saw similar results. Thus, loss of function of either PDZ domain in the context of full-length EBP50 substantially decreases the association with PDZK1. One possibility is that independent occupation of both PDZ domains by two PDZK1 tails greatly enhances the affinity, or occupation of one PDZ domain enhances the affinity of the other, or both mechanisms. To test the second possibility, we set up a binding experiment in which the first PDZ domain of EBP50 was saturated with EPI64, and asked whether the PDZK1 tail bound to PDZ2 better than free EBP50 with a nonfunctional and therefore empty first PDZ domain (Figure 5, B and C). The results show that the PDZK1 tail binds EBP50 PDZ2 more efficiently when PDZ1 is occupied by EPI64 than when PDZ1 is empty, thus illustrating an allosteric mechanism whereby ligand binding to the first PDZ domain positively regulates PDZK1 binding to the second PDZ domain of EBP50. It is notable that the isolated PDZ2 of EBP50 has been found to have few binding partners, perhaps because it may require PDZ1 ligation for optimal binding. A similar scenario has been found with other PDZ domain-containing proteins (Grootjans et al., 2000; van den Berk et al., 2007).
Although PDZK1 has been found previously in microvilli, this is the first report that it may function in an aspect of microvillar formation or retention. We have shown that the presence of microvilli on JEG3 cells requires EBP50, because they are lost in cells when the EBP50 level is lowered by siRNA (Hanono et al., 2006). Using this system, we show that microvilli can be restored by PDZK1 expression, but only when we fuse a sequence to link it to ezrin, a linkage that is normally provided through EBP50. This suggests a functional redundancy between the PDZ domains of PDZK1 and EBP50, suggesting that the two scaffolding proteins might normally functional to reinforce each other. However, we show that cells lacking PDZK1, such as JEG3 cells, are able to form robust microvilli. As such, we propose that PDZK1 plays a role as an accessory scaffolding protein that serves to enhance the functioning of EBP50 in microvilli both due to their multiple common binding partners and the ability of our PDZK1/EBP50 chimera to rescue microvilli in EBP50-depleted cells (Figure 8). Our future studies will be aimed at identifying a common ligand for EBP50 and PDZK1 that may be necessary to generate microvilli in these cells.
The finding that PDZK1 is enriched in either the nucleus or microvilli depending on cell confluence may provide valuable insight into the regulation of microvilli under different cellular conditions. How this shuttling is regulated and what motifs serve to target PDZK1 to its different subcellular destinations needs to be elucidated. There is no obvious nuclear localization sequence in PDZK1, so perhaps it piggy-backs on another protein when it is enriched in the nucleus. An important additional question is whether PDZK1 serves some nuclear function or if the nucleus is simply used to sequester it until needed at the apical aspect of cells. The most straightforward model is that PDZK1 exits from the nucleus to microvilli in high confluence cells to assist EBP50 in some aspect of microvilli functioning that is qualitatively or quantitatively different in high and low confluence cells. Interestingly, microvilli of LLC-PK1 cells vary based on confluence, with sparser microvilli under low-density conditions and bushier microvilli under high-density conditions (our unpublished observations).
In summary, our results indicate that the microvillar proteins PDZK1, EBP50, and ezrin form a ternary complex and this is regulated by their individual intramolecular interactions. This complex is clearly regulated by additional PDZ interactions, such as with EPI64, and contributes to the presence of microvilli on cells. Although we did not see any significant effect of EBP50 phosphorylation on ternary complex formation, it seems likely that in the context of additional ligands or unknown factors it will be functionally relevant. The characterization of this ternary complex provides a foundation toward understanding how microvilli are generated and contribute to apical plasma membrane protein composition.
Supplementary Material
ACKNOWLEDGMENTS
We thank Janet Ingraffea for purifying 6His-tagged EPI64 from insect cells and Abraham Hanono for generating polyclonal antibodies versus PDZK1 and green fluorescent protein. Stephane Angers (University of Toronto) and W. Lee Kraus (Cornell University, Ithaca, NY) are gratefully acknowledged for reagents. This work was supported by National Institutes of Health grant GM-036652 (to A. B.) and National Research Service Award Postdoctoral Fellowship GM-080847 (to D.P.L.).
Abbreviations used:
- CFTR
cystic fibrosis transmembrane conductance regulator
- EBP50
ezrin, radixin, moesin binding phosphoprotein 50 kDa
- EPI64
EBP50 PDZ interactor of 64 kDa
- F-actin
filamentous actin
- FERM
protein 4.1, ezrin, radixin, moesin
- GFP
green fluorescent protein
- PDZ
postsynaptic density 95/disc-large/zona occludens.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-01-0008) on March 17, 2010.
REFERENCES
- Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-beta-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 2006;8:348–357. doi: 10.1038/ncb1381. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Edwards K., Fehon R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Cha B., Zachos N. C., Brett C. L., Sharma A., Tse C. M., Li X. NHERF family and NHE3 regulation. J. Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D. A., Lee A., Lewis J., Kim E., Sheng M., MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- Fievet B. T., Gautreau A., Roy C., Del Maestro L., Mangeat P., Louvard D., Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 2004;164:653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouassier L., Nichols M. T., Gidey E., McWilliams R. R., Robin H., Finnigan C., Howell K. E., Housset C., Doctor R. B. Protein kinase C regulates the phosphorylation and oligomerization of ERM binding phosphoprotein 50. Exp. Cell Res. 2005;306:264–273. doi: 10.1016/j.yexcr.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Gary R., Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol. Biol. Cell. 1995;6:1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisler S. M., Stagljar I., Traebert M., Bacic D., Biber J., Murer H. Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J. Biol. Chem. 2001;276:9206–9213. doi: 10.1074/jbc.M008745200. [DOI] [PubMed] [Google Scholar]
- Grootjans J. J., Reekmans G., Ceulemans H., David G. Syntenin-syndecan binding requires syndecan-synteny and the co-operation of both PDZ domains of syntenin. J. Biol. Chem. 2000;275:19933–19941. doi: 10.1074/jbc.M002459200. [DOI] [PubMed] [Google Scholar]
- Hanono A., Garbett D., Reczek D., Chambers D. N., Bretscher A. EPI64 regulates microvillar subdomains and structure. J. Cell Biol. 2006;175:803–813. doi: 10.1083/jcb.200604046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Lau A. G., Yaffe M. B., Hall R. A. Phosphorylation and cell cycle-dependent regulation of Na+/H+ exchanger regulatory factor-1 by Cdc2 kinase. J. Biol. Chem. 2001;276:41559–41565. doi: 10.1074/jbc.M106859200. [DOI] [PubMed] [Google Scholar]
- Hernando N., Deliot N., Gisler S. M., Lederer E., Weinman E. J., Biber J., Murer H. PDZ-domain interactions and apical expression of type IIa Na/P(i) cotransporters. Proc. Natl. Acad. Sci. USA. 2002;99:11957–11962. doi: 10.1073/pnas.182412699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraffea J., Reczek D., Bretscher A. Distinct cell type-specific expression of scaffolding proteins EBP50 and E3KARP: EBP50 is generally expressed with ezrin in specific epithelia, whereas E3KARP is not. Eur. J. Cell Biol. 2002;81:61–68. doi: 10.1078/0171-9335-00218. [DOI] [PubMed] [Google Scholar]
- Islas S., Vega J., Ponce L., Gonzalez-Mariscal L. Nuclear localization of the tight junction protein ZO-2 in epithelial cells. Exp. Cell Res. 2002;274:138–148. doi: 10.1006/excr.2001.5457. [DOI] [PubMed] [Google Scholar]
- Kocher O., Krieger M. Role of the adaptor protein PDZK1 in controlling the HDL receptor SR-BI. Curr. Opin. Lipidol. 2009;20:236–241. doi: 10.1097/MOL.0b013e32832aee82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Yesilaltay A., Cirovic C., Pal R., Rigotti A., Krieger M. Targeted disruption of the PDZK1 gene in mice causes tissue-specific depletion of the high density lipoprotein receptor scavenger receptor class B type I and altered lipoprotein metabolism. J. Biol. Chem. 2003;278:52820–52825. doi: 10.1074/jbc.M310482200. [DOI] [PubMed] [Google Scholar]
- LaLonde D. P., Bretscher A. The scaffold protein PDZK1 undergoes a head-to-tail intramolecular association that negatively regulates its interaction with EBP50. Biochemistry. 2009;48:2261–2271. doi: 10.1021/bi802089k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht G., Seidler U. The emerging role of PDZ adapter proteins for regulation of intestinal ion transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G766–G777. doi: 10.1152/ajpgi.00135.2006. [DOI] [PubMed] [Google Scholar]
- Lau A. G., Hall R. A. Oligomerization of NHERF-1 and NHERF-2 PDZ domains: differential regulation by association with receptor carboxyl-termini and by phosphorylation. Biochemistry. 2001;40:8572–8580. doi: 10.1021/bi0103516. [DOI] [PubMed] [Google Scholar]
- Li J., Callaway D. J., Bu Z. Ezrin induces long-range interdomain allostery in the scaffolding protein NHERF1. J. Mol. Biol. 2009;392:166–180. doi: 10.1016/j.jmb.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Dai Z., Jana D., Callaway D. J., Bu Z. Ezrin controls the macromolecular complexes formed between an adapter protein Na+/H+ exchanger regulatory factor and the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2005;280:37634–37643. doi: 10.1074/jbc.M502305200. [DOI] [PubMed] [Google Scholar]
- Li Q., Nance M. R., Kulikauskas R., Nyberg K., Fehon R., Karplus P. A., Bretscher A., Tesmer J. J. Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. J. Mol. Biol. 2007;365:1446–1459. doi: 10.1016/j.jmb.2006.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D., Abidi W., Guardavaccaro D., Zhou M., Ahearn I., Pagano M., Philips M. R. Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J. Cell Biol. 2008;181:485–496. doi: 10.1083/jcb.200801047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales F. C., Takahashi Y., Kreimann E. L., Georgescu M. M. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl. Acad. Sci. USA. 2004;101:17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales F. C., Takahashi Y., Momin S., Adams H., Chen X., Georgescu M. M. NHERF1/EBP50 head-to-tail intramolecular interaction masks association with PDZ domain ligands. Mol. Cell. Biol. 2007;27:2527–2537. doi: 10.1128/MCB.01372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D., Berryman M., Bretscher A. Identification of EBP 50, A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D., Bretscher A. The carboxyl-terminal region of EBP50 binds to a site in the amino-terminal domain of ezrin that is masked in the dormant molecule. J. Biol. Chem. 1998;273:18452–18458. doi: 10.1074/jbc.273.29.18452. [DOI] [PubMed] [Google Scholar]
- Reczek D., Bretscher A. Identification of EPI64, a TBC/rabGAP domain-containing microvillar protein that binds to the first PDZ domain of EBP50 and E3KARP. J. Cell Biol. 2001;153:191–206. doi: 10.1083/jcb.153.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome I., Curto M., McClatchey A. I. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Short D. B., Trotter K. W., Reczek D., Kreda S. M., Bretscher A., Boucher R. C., Stutts M. J., Milgram S. L. An apical PDZ protein anchors the cystic fibrosis transmembrane conductance regulator to the cytoskeleton. J. Biol. Chem. 1998;273:19797–19801. doi: 10.1074/jbc.273.31.19797. [DOI] [PubMed] [Google Scholar]
- Silver D. L. A carboxyl-terminal PDZ-interacting domain of scavenger receptor B, type I is essential for cell surface expression in liver. J. Biol. Chem. 2002;277:34042–34047. doi: 10.1074/jbc.M206584200. [DOI] [PubMed] [Google Scholar]
- van den Berk L. C., Landi E., Walma T., Vuister G. W., Dente L., Hendriks W. J. An allosteric intramolecular PDZ-PDZ interaction modulates PTP-BL PDZ2 binding specificity. Biochemistry. 2007;46:13629–13637. doi: 10.1021/bi700954e. [DOI] [PubMed] [Google Scholar]
- Voltz J. W., Brush M., Sikes S., Steplock D., Weinman E. J., Shenolikar S. Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J. Biol. Chem. 2007;282:33879–33887. doi: 10.1074/jbc.M703481200. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Steplock D., Tate K., Hall R. A., Spurney R. F., Shenolikar S. Structure-function of recombinant Na/H exchanger regulatory factor (NHE-RF) J. Clin. Invest. 1998;101:2199–2206. doi: 10.1172/JCI204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.