Pituitary adenylate cyclase-activating polypeptide (PACAP) promotes neuronal differentiation, in part via a Rit GTPase signaling cascade. Here we show that PACAP-mediated Rit activation involves Src kinase-dependent TrkA receptor transactivation and identify TrkA-Rit signaling as a key contributor to PACAP-dependent neuronal differentiation.
Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a potent neuropeptide that possesses both neurotrophic and neurodevelopmental effects. Recently, the Rit GTPase was found to be activated by a novel Gα/cAMP/exchange protein activated by cyclic AMP (Epac)-dependent signaling pathway and required for PACAP-dependent cAMP response element-binding protein activation and neuronal differentiation. However, Epac did not function as a Rit guanine nucleotide exchange factor (GEF), and the nature of the PACAP regulatory cascade remained unclear. Here, we show that PACAP-mediated Rit activation involves Src family kinase-dependent TrkA receptor transactivation. PACAP receptor (PACR1) stimulation triggered both Giα and Gsα/cAMP/Epac regulatory cascades resulting in Src kinase activity, which in turn induced TrkA kinase tyrosine phosphorylation. Importantly, Src inhibition, or the lack of functional Trk receptors, was found to inhibit PACAP-mediated Rit activation, whereas constitutively active Src alone was sufficient to stimulate Rit-guanosine triphosphate levels. A single tyrosine (Y499) phosphorylation event was identified as critical to both PACAP-mediated transactivation and TrkA-dependent Rit activation. Accordingly, PACAP stimulation resulted in TrkA-dependent phosphorylation of both the Shc adaptor and son of sevenless (SOS)1/2 GEFs, and Rit activation was inhibited by RNA interference silencing of SOS1/2, implicating a TrkA/Shc/SOS signaling complex in Rit regulation. Together, these observations expand upon the nature of PACR1-mediated transactivation and identify TrkA-Rit signaling as a key contributor to PACAP-dependent neuronal differentiation.
INTRODUCTION
The pituitary adenylate cyclase-activating polypeptide (PACAP) is widely expressed in the nervous system and regulates several physiological functions, including neuronal and pheochromocytoma cell differentiation (Deutsch and Sun, 1992; Ravni et al., 2006), axonal and dendritic growth, and neuronal survival (Waschek, 2002). These actions are mediated by the activation of seven transmembrane receptors, including both the high-specificity type I PACAP receptor (PACR1) and the type II receptors (VPAC1 and VPAC2) (Vaudry et al., 2000; Somogyvari-Vigh and Reglodi, 2004). Although a comprehensive understanding of the signal transduction network mobilized by these receptors is lacking, activation of adenyl cyclase and regulation of mitogen-activated protein (MAP) kinase signaling pathways play prominent roles in the biological actions of PACAP (Kiermayer et al., 2005; Ravni et al., 2006; Shi et al., 2006; Gerdin and Eiden, 2007). Although cAMP can induce PC12 cell differentiation (Hansen et al., 2000), and cAMP signaling has been classically associated with activation of protein kinase A (PKA), the neurotrophic effects of PACAP do not rely solely upon PKA signaling. Instead, recent studies have identified the exchange protein activated by cyclic AMP (Epac) guanine nucleotide exchange factors (GEFs) as crucial mediators of PKA-independent cAMP signaling (Bos, 2003; Seino and Shibasaki, 2005). Indeed, Epac proteins are required for PACAP-mediated differentiation signaling (Kiermayer et al., 2005; Shi et al., 2006), acting to link Gsα-coupled receptor signaling to MAP kinase cascade activation in neurons (Lin et al., 2003; Kiermayer et al., 2005).
Recent studies have identified the Rit small guanosine triphosphate (GTP)-binding protein as regulating cellular signaling pathways critical to neuronal differentiation (Andres et al., 2005; Shi and Andres, 2005; Shi et al., 2006). Rit is expressed in the majority of adult and embryonic tissues, including a variety of primary neurons and the developing brain (Lee et al., 1996; Wes et al., 1996; Spencer et al., 2002a; Lein et al., 2007), and it shares a unique effector domain with the closely related Rin and Drosophila Ric proteins (Wes et al., 1996; Shao et al., 1999; Harrison et al., 2005). Rit is activated after nerve growth factor (NGF) and PACAP stimulation, and RNA interference (RNAi)-mediated silencing of endogenous Rit inhibits NGF- and PACAP-mediated neurite outgrowth in pheochromocytoma cells (Spencer et al., 2002b; Shi and Andres, 2005; Shi et al., 2006). Rit signaling also seems to control neuronal morphogenesis, by both inhibiting dendritic and promoting axonal growth (Lein et al., 2007; Andres et al., 2008). Additional studies indicate that Rit signaling plays a central role in morphological events as diverse as BMP7-mediated dendritic growth (Lein et al., 2007) and interferon-γ–mediated dendritic retraction (Andres et al., 2008). Despite the importance of understanding the regulation of Rit signaling, to date no known Rit regulatory factors (GEFs or GTPase activation proteins [GAPs]) have been characterized. Thus, although modulation of the activation status of Rit may represent a generalized mechanism for regulating axonal versus dendritic growth modes (Kaech et al., 2007; Lein et al., 2007; Andres et al., 2008), as well as neuronal survival (Spencer et al., 2002a), the underlying regulatory machinery remain uncharacterized.
G protein-coupled receptors (GPCRs) are known to use two general signaling mechanisms to exert their biological effects (Marinissen and Gutkind, 2001). Classical signaling involves the activation of canonical G protein-activated effector pathways. For PACR1, these include stimulation of adenyl cyclase, phospholipase C, phosphatidylinositol 3-kinase, and MAP kinase pathways (Ravni et al., 2006; Gerdin and Eiden, 2007). Recent work has established a second general signaling cascade that involves transactivation of a subset of receptor tyrosine kinases (RTKs) (Lee et al., 2002a; Werry et al., 2005; Delcourt et al., 2007a). For example, the TrkA receptor tyrosine kinase is activated directly by nerve growth factor (NGF) binding, but it is also involved in the activation of MAP kinase and Akt pathways by the PACR1 receptor (Lee et al., 2002a,b). On ligand activation, several intracellular tyrosine residues become phosphorylated on TrkA (Huang and Reichardt, 2003). In turn, Trk receptors phosphorylate and activate signaling proteins through the recruitment of proteins to specific phosphotyrosine residues (Huang and Reichardt, 2003). PACAP induces tyrosine phosphorylation of Trk receptors in a Src family kinase-dependent but neurotrophin-independent manner (Lee et al., 2002a,b). Thus, PACAP-mediated neuronal signaling involves both RTK-independent and RTK-dependent pathways. However, a comprehensive understanding of PACAP-mediated transactivation signaling is lacking, and therefore the physiological importance of signal integration between GPCR- and RTK-dependent pathways awaits further characterization.
We have previously demonstrated that Rit signaling plays a critical role in the neurotrophic effects of PACAP38 (Shi et al., 2006) and that PACAP-induced Rit activation involves a novel cAMP-Epac signaling cascade (Shi et al., 2006). However, Epac was not a direct Rit GEF, suggesting that an additional regulatory protein(s) was necessary to couple Epac signaling to Rit activation. In the present study, we report that PACAP-mediated Rit activation involves Epac-Src kinase-dependent transactivation of the Trk RTK. A single tyrosine (Y499) phosphorylation event within TrkA was identified as critical to Rit activation. Together with our previous observations, these results suggest that transactivation of a novel Trk-Rit signaling cascade plays a critical role in PACAP-mediated neuronal differentiation signaling.
MATERIALS AND METHODS
Plasmids and Reagents
Rit (Shi et al., 2006) and Gα subunit expression vectors (Shi et al., 2008) have been described previously. Constitutively active Epac2 (Li et al., 2006) and constitutively active SOS1 (Quilliam et al., 1999) were provided by Dr. L. A. Quilliam (Indiana University School of Medicine, Indianapolis, IN), whereas hemagglutinin (HA)-tagged wild-type rat TrkA and its mutants (TrkA [Y499F] and [Y794F]) were provided by Dr. S. Meakin (Roberts Research Institute, London, ON, Canada). Human Shc1 and Grb2 were purchased from Origene (Rockville, MD). Dominant-negative mutants of Shc1 (Shc1-FFF [Y239F/Y240F/Y317F]; Ravichandran, 2001; McFarland et al., 2006) and Grb2 (Grb2-R86K) (Skolnik et al., 1993b; Holt et al., 1996) were generated using the QuikChange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). Antibodies against the following targets were purchased: FLAG (Sigma-Aldrich, St. Louis, MO); TrkA and anti-phosphotyrosine (Millipore, Billerica, MA); and SOS1, SOS2, C3G guanine-nucleotide exchange factor (C3G), c-Src, and actin (Santa Cruz Biotechnology, Santa Cruz, CA). The Src kinase inhibitor PP2 and its inactive analogue PP3, 2′,5′-dideoxyadenosine (ddA), adenosine 3′,5′-cyclic monophosphorothioate, adenosine 3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-cAMP), 8-(p-chlorophenylthio)-2′-O-methyl-adenosine-3′,5′-cyclic monophosphate (8-CPT-2-Me-cAMP), and cholera toxin (CTX) were purchased from Calbiochem (San Diego, CA), whereas pertussis toxin (PTX) was from Sigma-Aldrich.
Cell Lines, Cell Culture, and Transfection
PC6 cells (provided by Dr. T. Vanaman, University of Kentucky, Lexington, KY) were maintained as described previously (Spencer et al., 2002a; Shi and Andres, 2005). Nnr5 cells are a subline of PC12 cells that fail to express TrkA receptor (a kind gift from Dr. C. Wu, Stanford University, Palo Alto, CA; Green et al., 1986; Clary and Reichardt, 1994). Nnr5 cells were cultured in DMEM supplemented with 10% (vol/vol) horse serum and 5% fetal bovine serum (FBS) (vol/vol), 100 μg/ml streptomycin, and 100 U/ml penicillin at 37°C in a humidified atmosphere of 5% CO2. NIH-3T3 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in DMEM supplemented with 10% (vol/vol) FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin at 37°C in a humidified atmosphere of 5% CO2. PC6 cells and nnr5 cells were transfected with Effectene (QIAGEN, Valencia, CA) as described previously (Shi and Andres, 2005) or with DharmaFectDuo reagents (Dharmacon RNA Technologies, Lafayette, CO), whereas NIH-3T3 cells were transfected using SuperFect (QIAGEN) (Shi and Andres, 2005) or DharmaFectDuo reagents. Although both nnr5 and NIH-3T3 cells lack TrkA receptor expression, NIH-3T3 cells display higher transfection efficiency and were used to examine TrkA signaling.
RNA Interference
The mammalian expression vector pSUPER-GFP/Neo (Oligoengine, Seattle, WA) was used for expression of small interfering RNA (siRNA) as described previously (Shi and Andres, 2005; Shi et al., 2008). The gene-specific insert sequence of rat SOS2-3434 (AGTTCCTCCTCCGCTTCCC [target sense]) was synthesized and then subcloned to generate pSUPER-shSOS2-3434 (shSOS2-3434). Other rat target sense sequences used in this study include shSOS1-4316, CCTTTACACCGCCACCTCC; shPACR1-384, CTACTTCGATGCTTGTGGG; shC3G-128, AGCCCTCTCCTCTTGCTAT; shC3G-2739, GAACCTCCATGTATCCCAT; and a siRNA with no predicted target site in the rat genome (Scramber, shCTR; Shi and Andres, 2005) and a shEpac1-1501 that silences Epac1 expression (Shi et al., 2006). The resulting constructs were verified by DNA sequencing. To silence SOS1 expression, an On-Target plus SMARTpool of siRNA duplexes directed to rat SOS1 (siSOS1) was purchased from Dharmacon RNA Technologies, whereas an ON-TARGETplus nontargeting siRNA (siCTR) was used as negative control. To determine the effects of shSOS or shC3G on the expression of endogenous SOS or C3G proteins, PC6 cells were transfected with of shSOS1-4316, shSOS2-3434, shC3G-128, shC3G-2739, or shCTR as control (1.5 μg), and then they were subjected to G418 selection (400 μg/ml) for 60 h to enrich for transfected cells. To determine the efficiency of SOS1 silencing or double knockdown of both SOS1 and SOS2 proteins mediated by siRNA, PC6 cells were transfected with either siCTR or siSOS1 (20 nmol of siRNA duplex final; Dharmacon RNA Technologies), together with either shCTR or shSOS2-3434 (1 μg) by using DharmaFectDuo transfection reagents, and the transfected cells enriched by G418 selection (400 μg/ml; 60 h). Total cell lysates were prepared and subjected to immunoblotting to determine the expression level of endogenous proteins. To determine the effect of shPACR1-384 on PACR1 expression, PC6 cells were transfected with shCTR or shPACR1-384 (1.5 μg), and subjected to total RNA isolation using the RNeasy Mini kit (QIAGEN). Total RNA (2 μg) was used for reverse transcription using the Omniscript Reverse Transcription kit (QIAGEN) and rat PACR1 and β-actin levels determined by reverse transcription-polymerase chain reaction (RT-PCR) as described previously (Shi et al., 2008).
Rit-GTP Precipitation Assays
Glutathione transferase (GST) fusion proteins containing the Rit binding domain (RBD) of RGL3 (residues 610-709) were expressed in bacterial and purified, and Rit activation was assessed as described previously (Shi and Andres, 2005). Bound GTP-Rit was detected by immunoblot analysis using anti-FLAG monoclonal antibody as described previously (Shi and Andres, 2005; Shi et al., 2006, 2008). It should be noted that that interexperimental differences in basal Rit-GTP levels make it difficult to compare absolute GTP-Rit levels in separate studies.
SOS GEF Activity Assay
The fluoresce base GEF assay was performed as described previously for Epac and Rit (Shi et al., 2006). The catalytic fragment of human Sos1 (residue 564-1049) was expressed as a His-tagged fusion protein in BL21DE3 and purified by nickel affinity chromatography and gel filtration.
Immunoprecipitation and Phosphorylation Analysis
The activation state of TrkA, Src, Shc1, and SOS was monitored by phosphotyrosine-specific immunoblotting after immunoprecipitation (Shi and Andres, 2005). In brief, transiently transfected PC6 cells were harvested, and whole cell lysates were prepared using kinase lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 50 mM KF, 50 mM β-glycerolphosphate, 2 mM EGTA, pH 8.0, 1 mM Na3VO4, 1% Triton X-100, 10% glycerol, and 1× protease cocktail) and subsequently incubated with the appropriate antibody and 30 μl of protein G-Sepharose resin (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) at 4°C for 1–2 h with end-to-end rotation. After incubation, the resin was collected by centrifugation (10,000 rpm for 5 min at 4°C), washed four times with kinase lysis buffer, and the bound proteins were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and subjected to immunoblot analysis with appropriate antibodies as described previously (Shi and Andres, 2005).
Neurite Outgrowth
To examine the contribution of Shc1-Grb2-SOS signaling to PACAP38-mediated neuronal differentiation, neurite elongation analysis was performed as described previously (Shi and Andres, 2005). Neurite outgrowth was analyzed by counting 200 cells in 9 to 12 random fields at day 7 after initiation, and the percentage of neurite-bearing cells and neurite number per cell are presented as mean ± SD from three independent experiments performed in triplicate.
RESULTS
Several Gα Pathways Activate Rit
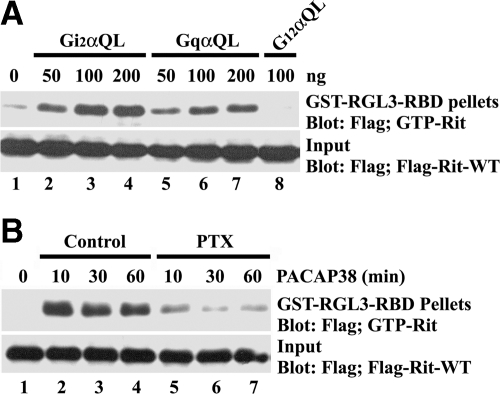
We recently demonstrated that PACAP38-mediated neuronal differentiation and cAMP response element-binding protein (CREB)-dependent transcription rely on activation of the Rit GTPase, which is regulated downstream of a novel Gsα/cAMP/Epac-dependent signaling cascade (Shi et al., 2006; Gerdin and Eiden, 2007). However, although endogenous Epac proteins were required for PACAP-mediated Rit activation, Epac1/2 did not directly activate Rit, suggesting that an additional factor(s) is involved in this regulatory cascade. Although PACAP-GPCR signaling is thought to primarily operate through a Gsα-cAMP cascade, studies have reported PACAP38-mediated activation of other Gα family members (McCulloch et al., 2002). To determine whether these alternative pathways were involved in Rit signaling, PC6 cells were cotransfected with 3xFLAG-Rit-wild type (WT) and constitutively active Giα, Gqα, and G12α. Coexpression of GTPase-deficient Gi2αQ204L and GqαQ209L, but not G12αQ229L, resulted in Rit activation (Figure 1A). Moreover, treatment of PC6 cells with PTX, an inhibitor of Giα signaling (Moss, 1987), potently suppressed PACAP38-mediated Rit activation (Figure 1B). These data indicate that PACAP38-dependent Rit activation relies upon both Giα and Gsα (Shi et al., 2006) signaling cascades.
Figure 1.
Giα and Gqα signaling activates Rit. (A) PC6 cells transiently cotransfected with 3xFLAG-Rit-WT and either Gi2αQ204L, GqαQ209L, or G12αQ229L, were serum starved (5 h) before the preparation of whole cell lysates. GTP-bound Rit levels were examined after GST-RGL3-RBD precipitation as described in Materials and Methods. (B) PC6 cells expressing 3xFLAG-Rit-WT were starved in serum-free DMEM for 5 h and subsequently pretreated with PTX (100 ng/ml for 30 min) or dimethyl sulfoxide before PACAP38 (10 nM) stimulation. Rit-GTP levels were assayed as described in A. The data are representative of the three individual experiments.
Src Is Required for PACAP38-mediated Rit Activation
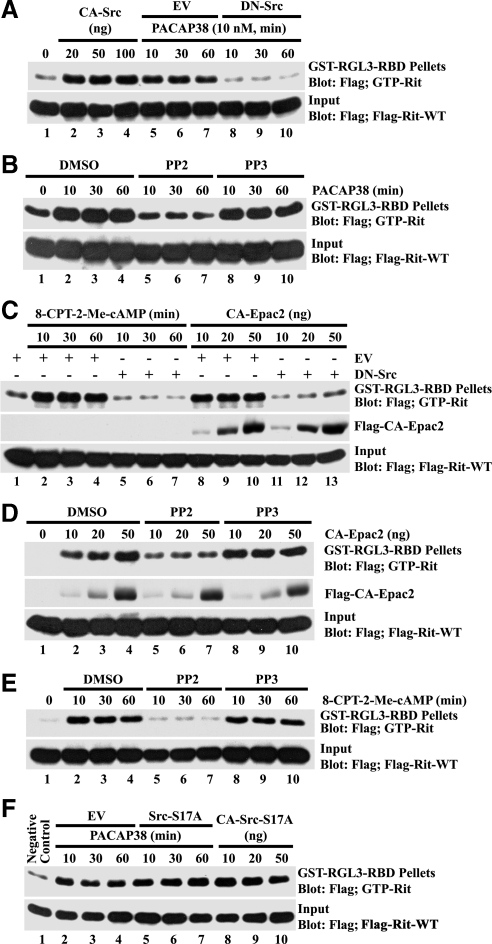
Recent studies indicate that both Giα and Gsα are stimulated in PACAP38-treated PC12 cells (Lazarovici et al., 1998; Lee et al., 2002b) and activate a Src-dependent signaling cascade to promote neurite outgrowth (Obara et al., 2004). To determine whether PACAP38-mediated Rit activation involves a similar pathway, we next examined whether Rit was regulated in a Src-dependent manner. Consistent with a role for Src in this cascade, expression of a dominant inhibitory Src mutant (DN-Src [SrcK296R/Y528F]) suppressed PACAP38-mediated Rit activation, whereas expression of an activated Src mutant (CA-Src [SrcY529F]) alone resulted in strongly elevated Rit-GTP levels (Figure 2A). Moreover, treatment with the Src inhibitor PP2 (10 μM), but not the inactive PP3 isomer (10 μM), potently inhibited PACAP38-mediated Rit activation (Figure 2B).
Figure 2.
Src is required for PACAP38-cAMP-Epac-mediated Rit activation. (A) PC6 cells were transiently cotransfected with 3xFLAG-Rit-WT and CA-Src or DN-Src, allowed to recover for 36 h, serum starved for 5 h, and then stimulated with PACAP38 (10 nM). GTP-Rit levels were determined by GST-RGL3-RBD pull-down assay as described in Materials and Methods. (B) PC6 cells expressing FLAG-tagged Rit were serum starved for 5 h and pretreated with PP2 (10 μM), PP3 (10 μM), or dimethyl sulfoxide (DMSO) (vehicle) for 30 min before PACAP38 stimulation (10 nM). GTP-Rit levels were monitored by GST-RGL3-RBD pull-down. (C) PC6 cells were cotransfected with 3xFLAG-Rit-WT and DN-Src or empty vector in the presence or absence of CA-Epac2, or they were stimulated with 8-CPT-2-Me-cAMP (25 μM) as indicated. GTP-bound Rit levels were analyzed as described above. (D) PC6 cells were coexpressed with 3xFLAG-Rit-WT and CA-Epac2 after pretreatment with PP2 (10 μM), PP3 (10 μM), or vehicle DMSO, and GTP-Rit levels were analyzed as described above. (E) Rit-WT–transfected cells were serum starved (5 h) and pretreated with PP2 (10 μM), PP3 (10 μM), or DMSO for 30 min before stimulation with 8-CPT-2-Me-cAMP (25 μM). GTP-Rit levels were measured from whole cell lysates as described above. (F) Serum-starved PC6 cells cotransfected with 3xFLAG-Rit-WT and either Src-S17A, the indicated amount of CA-Src-S17A, or empty vector (EV), were stimulated with PACAP38 (10 nM). GTP-Rit levels were analyzed as described above. The data in each panel are representative of the results from a minimum of three independent experiments.
PACAP38-mediated Rit activation involves a cAMP-Epac signaling cascade (Shi et al., 2006). Consistent with a role for Src downstream of Epac in this pathway, expression of DN-Src was found to potently inhibit Rit activation after stimulation of PC6 cells with the Epac-selective cAMP analog 8-CPT-2′-O-Me-cAMP or coexpression with a constitutively active Epac2 mutant (CA-Epac2; Figure 2C). Moreover, treatment with PP2, but not PP3, inhibited both CA-Epac2- (Figure 2D) and 8-CPT-2′-O-Me-cAMP (Figure 2E)-mediated Rit activation. Together, these data suggest that Src plays a central role in PACAP38-dependent Rit activation.
PKA-dependent phosphorylation of Src at Ser17 contributes to the activation of Rap1 GTPases in response to both elevated cAMP and NGF stimulation in PC12 cells (Obara et al., 2004). As seen in Figure 2F, expression of SrcS17A (a Src mutant incapable of being phosphorylated by PKA) had no effect on PACAP38-mediated Rit activation. Furthermore, expression of an activated SrcS17A mutant (CA-SrcY529F/S17A) resulted in potent Rit activation (Figure 2F). Thus, although Src signaling is critical in PACAP-mediated Rit activation, PKA-mediated Src phosphorylation is not required.
Involvement of TrkA in PACAP38-mediated Rit Activation
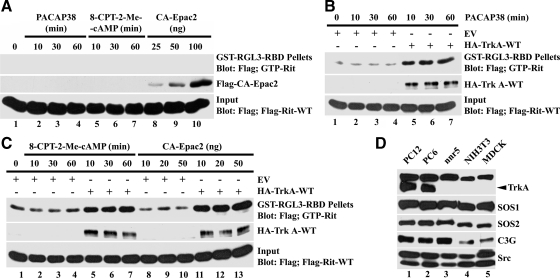
PACAP has been found to stimulate Trk receptor tyrosine kinase activity in a Src-dependent cross-talk cascade to promote neuronal survival (Lee et al., 2002a,b). To investigate whether the Trk receptor was required for PACAP38-mediated Rit activation, we made use of nnr5 cells, a PC12 cell variant that fails to express TrkA (Figure 3D; Green et al., 1986). As seen in Figure 3A, treatment with 8-CPT-2′-O-Me-cAMP, PACAP38, or overexpression of CA-Epac2 failed to promote elevated GTP-Rit levels of nnr5 cells. The deficiency in Rit activation can be traced to the lack of TrkA receptor, because expression of the wild-type receptor in nnr5 cells restored PACAP38- (Figure 3B), 8-CPT-2′-O-Me-cAMP-, and CA-Epac2 (Figure 3C)-mediated Rit activation. Thus, PACAP38-dependent Rit activation requires Trk receptor.
Figure 3.
TrkA receptor activity is required for PACAP38- and Epac-mediated Rit activation. (A) nnr5 cells expressing 3xFLAG-Rit-WT were cotransfected with or without CA-Epac2 and serum starved before exposure to PACAP38 (10 nM) or 8-CPT-2-Me-cAMP (25 μM). GTP-Rit levels were analyzed as described in Materials and Methods. (B) nnr5 cells were transfected with 3xFLAG-Rit-WT in the presence or absence of TrkA receptor (WT-TrkA), subjected to serum starvation (5 h) and stimulated with PACAP38 (10 nM). Cellular GTP-Rit levels were analyzed using GST-RGL3-RBD pull-down as described above. Note that TrkA expression restored PACAP-mediated Rit activation. (C) nnr5 cells expressing 3xFLAG-Rit-WT were transfected with CA-Epac2 and/or WT-TrkA as indicated, and stimulated with 8-CPT-2-Me-cAMP (25 μM) after serum starvation (5 h). GTP-bound Rit levels were determined by RGL3-RBD pull-down. The results in A–C are representative of three to five independent experiments. (D) Total cell lysates (100 μg) were prepared from PC12, PC6, nnr5, NIH-3T3 and Madin-Darby canine kidney (MDCK) cells, subjected to fractionation by SDS-PAGE, and examined by immunoblotting. Note that nnr5, NIH-3T3, and MDCK cells fail to express detectable TrkA.
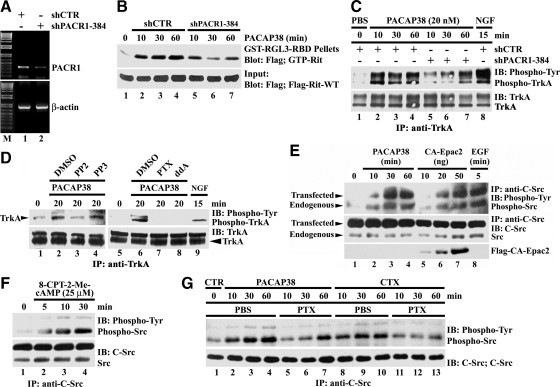
PACAP38-mediated TrkA Activation Requires Src
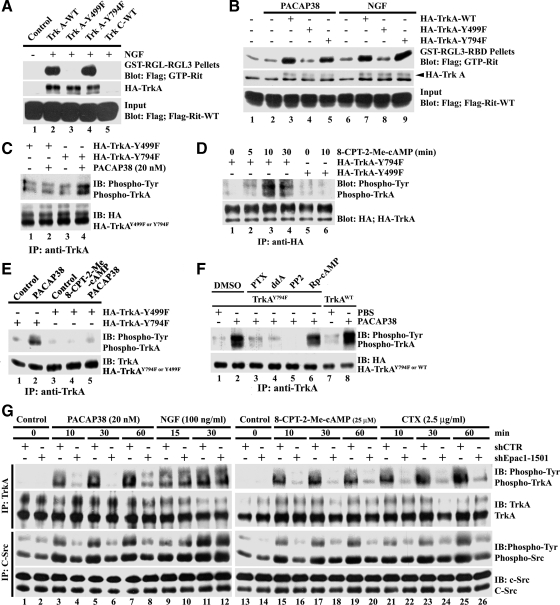
PACAP38 effects are mediated by PACR1 receptors expressed in PC12 cells, including PACR1-mediated transactivation of Trk neurotrophin receptors (Lee et al., 2002b; Ravni et al., 2006). To confirm that PACAP38-dependent Rit activation requires PACR1 in PC6 cells, the endogenous receptor was silenced by transfection with a selective short hairpin (sh)RNA vector (shPACR1-384; Figure 4A). Partial receptor loss impaired PACAP38-mediated Rit activation (Figure 4B). To examine whether PACAP38-dependent Rit signaling involves GPCR-mediated transactivation, anti-phosphotyrosine Western blotting was used to analyze immunoprecipitated TrkA receptors. Activated TrkA receptors were observed within 10 min of PACAP38 stimulation and remained active for at least 1 h (Figure 4C). PACAP-mediated TrkA activation was partially inhibited by PACR1 receptor silencing, supporting a pathway in which PACAP-mediated Trk transactivation contributes to Rit signaling (Figure 4C). Application of the Src inhibitor PP2, but not PP3, resulted in a marked decrease in the level of tyrosine phosphorylated TrkA receptors elicited by PACAP38 (Figure 4D). Phosphorylated TrkA levels were also inhibited by pretreatment with either the Giα inhibitor PTX or the direct adenyl cyclase inhibitor ddA, before PACAP38 stimulation (Figure 4D). Cumulatively, these data suggest that regulation of TrkA by PACAP38/cAMP/Epac signaling may be mediated by a Src family kinase.
Figure 4.
PACAP38-mediated Rit activation involves PACR1-dependent TrkA transactivation. (A) PC6 cells were transfected with either shCTR or shPACR1-384 and then subjected to G418 (400 μg/ml) selection for 60 h. Total RNA was isolated and RT-PCR used to monitor PACR1 silencing. (B) PC6 cells cotransfected with 3xFLAG-Rit-WT and either shCTR or shPACR1-384 were starved in serum-free DMEM (5 h) and stimulated with PACAP38 (20 nM). Cell lysates were prepared and subjected to GST-RGL3-RBD pull-down. (C) PC6 cells transfected with either shCTR or shPACR1-384 were enriched with G418 (400 μg/ml; 60 h) and serum starved (5 h) before stimulation with either PACAP38 (20 nM) or NGF (100 ng/ml). Total cell lysates (1 mg) were prepared and subjected to anti-TrkA immunoprecipitation. Anti-phosphotyrosine specific immunoblotting was used to detect activate TrkA, and the stripped membrane was reprobed with anti-TrkA antibody to demonstrate equal loading. (D) PC6 cells were pretreated with PP2 (10 μM), PP3 (10 μM), PTX (100 ng/ml), ddA (50 μM), or dimethyl sulfoxide (DMSO); stimulated with PACAP38 (20 nM); and total cell lysates were prepared. NGF stimulation (100 ng/ml) served as positive control. Endogenous TrkA was immunoprecipitated (1 mg of total cell lysate) and activated receptor identified by anti-phosphotyrosine immunoblotting. (E) PC6 cells were transfected with c-Src-WT in the presence or absence of CA-Epac2, serum starved, and then stimulated with PACAP38 (10 nM), EGF (100 ng/ml), or not treated (phosphate-buffered saline [PBS]). Total lysates (500 μg) were subjected to anti-c-Src immunoprecipitation, and active Src levels were examined by phosphotyrosine-specific immunoblotting. Note that both PACAP38 and CA-Epac2 result in c-Src activation. (F) PC6 cells expressing c-Src-WT were starved for 5 h before stimulation with 8-CPT-2-Me-cAMP (25 μM) for 5, 10, or 30 min, and the phosphotyrosine levels of Src determined as described above. (G) PC6 cells expressing c-Src-WT were starved (5 h) and pretreated with or without PTX (100 ng/ml; 30 min) before stimulation with either PACAP38 (20 nM; 10, 30, or 60 min) or CTX (2.5 μg/ml; 10, 30, or 60 min). Total cell lysates were prepared and analyzed for Src tyrosine phosphorylation analysis as described above. The data in each panel are representative of the results from a minimum of three independent experiments.
Src family kinases have been implicated as mediators of receptor tyrosine kinase transactivation by several G protein-coupled agonists, including PACAP38 (Luttrell et al., 1999; Lee et al., 2002a; Delcourt et al., 2007a). If a Src family kinase was involved in the activation of Trk receptors by PACAP38, we reasoned that Src should not only be activated after PACAP38 stimulation of PC6 cells but also operate downstream of activated Epac. Treatment of Src expressing PC6 cells with PACAP38 resulted in a marked and time-dependent increase in the level of tyrosine phosphorylated Src, as measured by anti-phosphotyrosine immunoblotting of precipitated Src kinases (Figure 4E). The level of tyrosine phosphorylated Src was also increased in a dose-dependent manner by cotransfected CA-Epac2 (Figure 4E) or by treatment with 8-CPT-2′-O-Me-cAMP (Figure 4F), PACAP38- (Figure 4, E and G) and CTX (a known Gsα activator; Figure 4G), whereas both PACAP38- and CTX-mediated Src activation was inhibited by PTX (Figure 4G), implicating Src activation in PACAP transactivation signaling.
Tyrosine 499 of TrkA Is Required for PACAP-mediated Rit Activation
On activation Trk receptors become phosphorylated on five tyrosine residues within the intracellular domain and use these residues to recruit, phosphorylate, and activate a variety of intracellular signaling proteins (Huang and Reichardt, 2003). To identify the site(s) required for TrkA-mediated Rit activation, we tested two TrkA point mutants defective in binding to specific signaling proteins. As seen in Figure 5A, NGF stimulation of NIH-3T3 cells coexpressing FLAG-tagged wild-type Rit and either wild-type TrkA or TrkAY794F resulted in potent Rit activation as monitored by GST-RGL3 pull-down analysis (Figure 5A, lanes 2 and 4). However, the TrkAY499A mutant failed to promote Rit activation, indicating that this residue is required the regulation of downstream Rit signaling. NIH-3T3 cells lack endogenous TrkA receptor (Figure 3D), and NGF-dependent Rit activation required a functional receptor, because coexpressed TrkC, which is not activated by NGF (Reichardt, 2006), failed to restore Rit activation (Figure 5A, lane 5). Similar results were seen following PACAP38 stimulation, in which expression of either WT-TrkA or TrkAY794F, but not TrkAY499F, restored Rit activation (Figure 5B). Thus, tyrosine 499 of TrkA is critical for both NGF- and PACAP-mediated Rit activation.
Figure 5.
PACAP/Epac-dependent Rit activation and TrkA transactivation require TrkAY499. (A and B) NIH-3T3 cells were transfected with 3xFLAG-Rit-WT in the presence or absence of TrkA-WT, TrkA-Y499F, TrkA-Y794F, or TrkC-WT as control (A) and then serum starved (5 h) before stimulation with NGF (100 ng/ml; 15 min) or PACAP38 (10 nM; 20 min). GTP-bound Rit levels were determined as described in Materials and Methods. NIH-3T3 (C and E) or PC6 (D) cells expressing either HA-TrkA-Y499F or -Y794F were stimulated with PACAP38 (20 nM; 20 min) or 8-CPT-2-Me-cAMP (25 μM; 30 min [E] or as indicated [D]) after starvation with serum-free DMEM (5 h). Total cell lysates were subjected to anti-TrkA or anti-HA immunoprecipitation and TrkA activation examined by anti-phosphotyrosine immunoblotting. (F) NIH-3T3 cells expressing HA-TrkA-WT or HA-TrkA-Y794F were starved for 5 h and subsequently pretreated with PTX (100 ng/ml), ddA (50 μM), PP2 (10 μM), or Rp-cAMP (50 μM) for 30 min before stimulation with PACAP38 (20 nM) for 20 min. NIH-3T3 cells expressing HA-TrkA-WT served as control. TrkA activation was analyzed as described in A. (G) Epac1 silencing attenuates PACAP38-Epac signaling but not NGF-mediated Src and TrkA activation. PC6 cells were transfected with HA-TrkA-WT and C-Src-WT in presence of either shCTR or shEpac1–1501 and subjected to G418 (400 μg/ml) selection to enrich for transfected cells. Note that PC6 cells express Epac1 but not Epac2 (Shi et al., 2006). Cells were then starved with serum-free DMEM for 5 h before be stimulated with PACAP38 (20 nM; 10, 30, or 60 min), 8-CPT-2-Me-cAMP (25 μM; 10, 30, or 60 min), CTX (2.5 μg/ml; 10, 30, or 60 min), or NGF (100 ng/ml; 15 or 30 min). Total lysates were subsequently prepared, subjected to anti-TrkA (2 μg) and anti-C-Src (4 μg) immunoprecipitation, and resolved by SDS-PAGE, and then the phosphotyrosine levels of TrkA and Src were examined by phosphotyrosine-specific immunoblotting. The results are representative of three or four independent experiments.
To explore whether these same specific tyrosine residues are required for PACAP38-mediated TrkA transactivation, NIH-3T3 cells expressing either TrkAY499F or TrkAY794F were subject to PACAP38 stimulation and TrkA activity was monitored by anti-phosphotyrosine immunoblotting of the precipitated receptor. As seen in Figure 5, C and E, PACAP38 signaling resulted in robust phosphorylation of TrkY794F but failed to stimulate the TrkAY499F mutant receptor. As expected, 8-CPT-2′-O-Me-cAMP–mediated Epac activation also failed to promote phosphorylation of the TrkAY499F mutant but promoted robust TrkAY794F phosphorylation (Figure 5, D and E). Together, these data suggest that in addition to being critical for Rit activation, PACAP-dependent TrkA transactivation requires an intact Tyr499 phosphotyrosine docking site.
PACAP-mediated TrkA activation was inhibited by pharmacological blockade of Giα (PTX), adenyl cyclase (ddA), or Src (PP2) but not protein kinase A (Rp-cAMP), supporting a role for cAMP/Src signaling in transactivation (Figure 5F). In addition, PACAP-, 8-CPT-2′-O-Me-cAMP-, and CTX-dependent TrkA stimulation, but importantly not NGF-mediated receptor activation, was inhibited by Epac1 silencing (Figure 5G). In keeping with a PACR1-dependent transactivation cascade in which Epac functions upstream of Src family kinases, Epac1 silencing resulted in a dramatic inhibition of PACAP-, 8-CPT-2′-O-Me-cAMP-, and CTX-mediated Src activation, as measured by anti-phosphotyrosine immunoblotting of precipitated Src kinases (Figure 5F). Together, these data suggest that PACAP-dependent TrkA transactivation involves a cAMP/Epac/Src signaling cascade.
PACAP-mediated Rit Activation Involves SOS
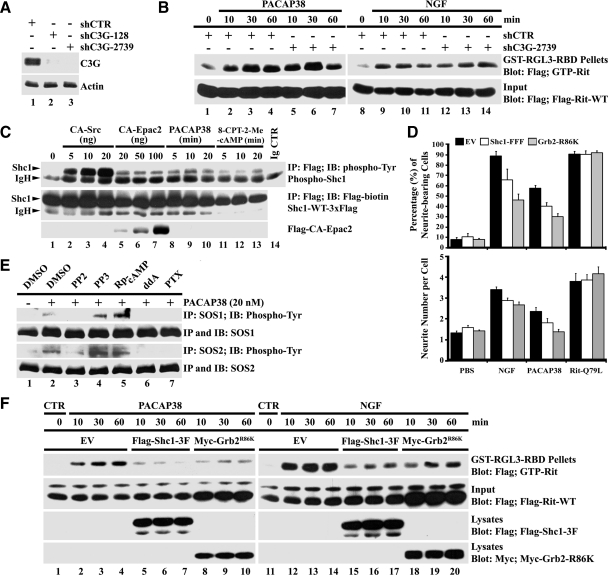
On TrkA activation phosphorylated Tyr499 provides a recruitment site for both the Shc/Grb2/SOS complex to promote Ras activation, and FRS2, which associates with the adapter protein Crk/RapGEF C3G complex to stimulate Rap GTPase signaling (Huang and Reichardt, 2003; Reichardt, 2006). To directly assess the contribution of these GEFs to Rit activation, we used shRNAi-mediated silencing (Elbashir et al., 2002) to selectively knock down endogenous C3G and SOS1/2 in PC6 cells. As shown in Figure 6A, both shC3G-128 and shC3G-2739, but not shCTR (control shRNA with no predicted target in the rat genome; Shi et al., 2005), silenced C3G in PC6 cells. However, C3G knockdown had no obvious effect on either PACAP38- or NGF-mediated Rit activation (Figure 6B), suggesting that another GEF is required.
Figure 6.
C3G is not required for Rit activation, but the Shc1/Grb2 adaptors contribute to PACAP38-dependent neuronal differentiation. (A) Anti-C3G immunoblotting demonstrates silencing of endogenous C3G after transfection with shC3G-128 and shC3G-2739 but not shCTR, in PC6 cells. (B) PC6 cells expressing 3xFLAG-Rit-WT were cotransfected with either shCTR or shC3G-2739 and then stimulated with PACAP38 (20 nM) or NGF (100 ng/ml). GTP-bound Rit levels were determined by GST-RGL3-RBD pull-down. (C) PC6 cells expressing Shc1-WT-3xFLAG were either cotransfected with CA-Src, CA-Epac2, or stimulated with PACAP38 (20 nM) or 8-CPT-2-Me-cAMP (25 μM) after serum starvation. Total cell lysates were prepared, subjected to anti-FLAG immunoprecipitation (500 μg), and the levels of phosphorylated Shc1 determined by anti-phosphotyrosine immunoblotting. (D) Neurite outgrowth was initiated by cotransfection of PC6 cells with 3xFLAG-Rit-Q79L and either empty-3xFLAG vector, Shc1-FFF-3xFLAG, or Grb2-R86K-3xFLAG, or by the addition of NGF (100 ng/ml) or PACAP38 (20 nM). Cells were replated (1:4 dilution) after transfection and enriched by G418 (400 μg/ml) selection. Neurite outgrowth was analyzed at day 7, and the percentage of neurite-bearing cells (top) and neurite number per cell (bottom) calculated as described in Materials and Methods. The results were represented as mean ± SD from three independent experiments performed in triplicate. (E) PC6 cells were starved in serum-free DMEM (10 h) and then pretreated with PP2 (10 μM), PP3 (10 μM), Rp-cAMP (50 μM), ddA (50 μM), PTX (100 ng/ml), or dimethyl sulfoxide (DMSO, vehicle control) for 30 min before stimulation with or without PACAP38 (20 nM) for 20 min. Total cell lysates (500 μg) were prepared and subjected to either anti-SOS1 or anti-SOS2 immunoprecipitation, and active SOS levels were determined by anti-phosphotyrosine immunoblotting. (F) PC6 cells were transfected with 3xFLAG-Rit-WT in the presence or absence of either FLAG-Shc1–3F (FFF) or Myc-Grb2-R86K and starved for 5 h before stimulation with either PACAP38 (20 nM) or NGF (100 ng/ml) as indicated. GTP-Rit levels were examined as described in Materials and Methods. The results are representative of three independent experiments.
The activation of downstream signaling pathways by TrkA is complex, but recruitment to the active receptor often requires the Shc1 and Grb2 adaptor proteins and results in tyrosine phosphorylation of Shc1 and both SOS1 and SOS2 GEFs (Liu and Meakin, 2002; Reichardt, 2006). As seen in Figure 6C, PACAP38 stimulation resulted in rapid Shc1 phosphorylation. Furthermore, treatment with 8-CPT-2′-O-Me-cAMP, expression of CA-Src, or CA-Epac2, also elevated the levels of tyrosine phosphorylated Shc1 in PC6 cells (Figure 6C). Expression of dominant-negative adaptor proteins has been shown to inhibit both downstream TrkA signaling and NGF-dependent neuritogenesis in PC12 cells, by disrupting the recruitment and activation of a variety of intracellular signaling proteins to the active receptor (Skolnik et al., 1993a,b; McFarland et al., 2006). Consistent with these earlier studies (Thomas and Bradshaw, 1997), expression of dominant-negative Shc1 (Shc1-FFF), or a Grb2 (Grb2-R86K) mutant that disrupts formation of the Shc-Grb2 complex, was found to significantly inhibit both neurite outgrowth and neurite number per cell body in NGF-stimulated PC6 cells (Figure 6D). Despite the fact that PACR1 signaling does not rely upon adaptor recruitment, expression of these mutants also inhibited PACAP38-mediated PC6 cell neurite outgrowth and neurite number, but importantly it had no effect on neuronal differentiation induced by expression of activated Rit (Figure 6D). As expected, expression of these adapter mutants inhibited NGF-dependent Rit stimulation, but importantly, it also disrupted PACAP38-mediated Rit activation (Figure 6F).
PACAP38 stimulation also resulted in the generation of tyrosine phosphorylated SOS1 and SOS2 (Figure 6E). Moreover, pretreatment of PC6 cells with PP2, but not PP3, resulted in a marked decrease in the levels of tyrosine phosphorylated SOS1 and SOS2 after PACAP38 stimulation. Phosphorylated SOS1/2 levels were also inhibited by pretreatment with either the Giα inhibitor PTX, or the direct adenylate cyclase inhibitor ddA, but not the PKA inhibitor Rp-cAMP (Figure 6E). Together, these studies suggest that both NGF- and PACAP38-mediated Rit activation involves the adaptor-dependent recruitment of SOS by TrkA.
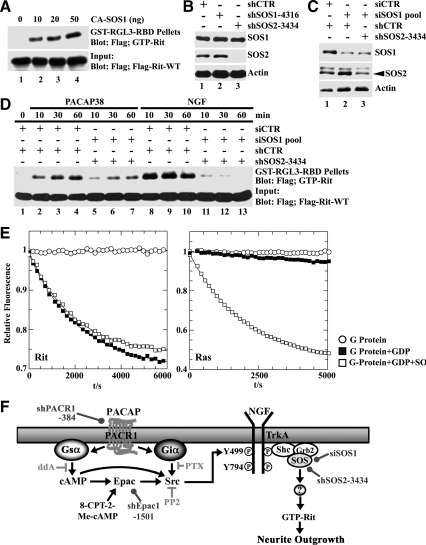
We next examined whether SOS1/2 contributed to PACAP38-dependent Rit activation. As expected, expression of constitutively active SOS1 resulted in a dose-dependent stimulation of Rit-GTP levels (Figure 7A). To extend this analysis, two approaches were used to silence the SOS1 and SOS2 GEFs, which are both endogenously expressed in PC6 cells (Figure 3D). Although shSOS2-3434 proved effective at knocking down endogenous SOS2, shRNA vectors proved ineffective at silencing SOS1 (Figure 7B). Instead, transfection with a synthetic siRNA pool was used to selectively reduce SOS1 expression and the combined introduction of the siSOS1 pool and shSOS2-3434 to PC6 cells resulted in the knockdown of both SOS family members (Figure 7C). After silencing of either SOS1 or SOS2 alone failed to inhibit PACAP38-mediated Rit activation (data not shown), the simultaneous knockdown of both SOS1/2 GEFs potently inhibited PACAP38- and NGF-mediated Rit activation (Figure 7D), suggesting that SOS1/2 GEFs are involved in TrkA-mediated Rit activation.
Figure 7.
SOS1/2 silencing inhibits PACAP38- and NGF-mediated Rit activation in PC6 cells, but SOS1 does not function as an in vitro RitGEF. (A) PC6 cells were cotransfected with 3xFLAG-Rit-WT and CA-SOS1, and cell lysates prepared after starvation in serum-free DMEM (5 h). GTP-Rit levels were determined as described in Materials and Methods. (B) PC6 cells expressing shSOS1-4316, shSOS2-3434, or shCTR were enriched by G418 selection (400 μg/ml), and the expression of SOS1 and SOS2 determined by immunoblotting. Note that shSOS2-3434 silences SOS2. (C) PC6 cells were cotransfected with shCTR or siSOS1 (20 nmol) together with shCTR or shSOS2-3434, and enriched by G418 (400 μg/ml; 60 h) selection. Cell lysates were prepared and subjected anti-SOS1 or anti-SOS2 immunoblotting. (D) PC6 cells expressing 3xFLAG-Rit-WT were cotransfected with either siSOS1/shSOS2–3434 or shCTR/shCTR as control and stimulated with PACAP38 (20 nM) or NGF (100 ng/ml). Total cell lysates were subjected to GST-RGL3-RBD precipitation and levels of GTP-Rit determined as described in Materials and Methods. (E) 200 nM Rit (left) or H-Ras (right) loaded with the fluorescent GDP analogue mGDP were incubated alone (open circles), in the presence of 200-fold excess unlabeled GDP (closed squares) or in the presence of unlabeled GDP and 500 nM Sos (open squares). The time-dependent decrease in fluorescence intensity monitors the exchange of protein bound mGDP for GDP. Note that in the absence of excess GDP the G protein nucleotide complex is stable. (F) Schematic diagram of the putative PACAP-Epac-Src-TrkA-SOS-Rit signal transduction cascade. The sites of action of various pharmacological inhibitors (⊣), selective activators (→), and the targets of shRNA silencing reagents (•) are indicated.
To determine whether SOS functions as a direct RitGEF, the catalytic domain of human SOS1 was incubated with fluorescent mGDP-loaded Rit or H-Ras in the presence of excess unlabeled guanosine diphosphate (GDP), and the exchange of guanine nucleotides was followed in real time as a decrease in fluorescence (Rehmann, 2005). As shown in Figure 7E, when these rates were compared with the intrinsic exchange rate for mGDP-Rit measured in the same experiment, no difference was seen. As expected, SOS1 exhibited in vitro catalytic activity toward H-Ras (Quilliam et al., 2002), demonstrating the utility of the assay (Figure 7E). Thus, although RNAi methods indicate that SOS proteins are required for Rit activation, the ability of SOS to function as an in vitro Rit GEF could not be proven.
DISCUSSION
Neurotrophic signaling cascades regulate a wide range of nerve cell functions, including differentiation, axonal and dendritic morphology, and survival, and they contribute to aspects of learning and memory. Although signaling through the Trk receptor tyrosine kinase family plays crucial roles in these regulatory processes (Reichardt, 2006), it is clear that additional cellular factors contribute prominently to these diverse biological effects. PACAP is widely expressed in the nervous system, and although it has been shown to promote neurotrophic signaling in many neuronal populations, the specific molecular pathways directing these actions remain incompletely characterized. Here we describe a novel signaling mechanism which contributes to the ability of PACAP38 to promote neurotrophic effects. Through cross-talk with Trk receptor tyrosine kinases, PACAP is capable of activating the Rit GTPase, to promote differentiation. This mechanism explains a gap in our understanding of Rit activation in addition to identifying a novel transactivation-dependent neuronal signaling cascade (Figure 7F).
A conserved characteristic of GPCR-dependent RTK transactivation is the requirement for tyrosine phosphorylation of the targeted growth factor receptor (Luttrell et al., 1999; Waters et al., 2004; Delcourt et al., 2007a). Previous work found that PACAP stimulation results in phosphorylation of tyrosine signaling sites on TrkA but that the specific phosphotyrosine docking sites and activation of downstream signaling pathways had not been characterized (Lee et al., 2002a,b; El Zein et al., 2007). Using site-directed TrkA mutants (Figure 5), RNAi silencing methods (Figures 4–7), and the expression of dominant-negative adaptor mutants (Figure 6), we implicate the recruitment and activation of a SOS/Shc-Grb2 adaptor complex to the Y499 phosphotyrosine docking site of TrkA in Rit activation after either NGF stimulation or in PACAP-directed transactivation (Figure 7F). However, in vitro biochemical analysis failed to demonstrate that SOS1 functions as a direct RitGEF (Figure 7E). These studies are complicated by the high intrinsic rate of guanine nucleotide exchange of recombinant Rit1-211 (18 residue C-terminal truncation), which rivals that seen in SOS catalyzed H-Ras nucleotide exchange reactions (Figure 7E). Analysis of in vivo Rit activation is inconsistent with endogenous protein displaying rapid uncatalyzed nucleotide exchange. Basal GTP-bound Rit levels are low and ligand stimulation results in a rapid increase in GTP-Rit levels in a variety of cell systems (Andres et al., 2005, 2008; Shi and Andres, 2005; Shi et al., 2006; Lein et al., 2007). Thus, it is possible that removal of the Rit C terminus, which is required for bacterial protein expression (Shao et al., 1999), destabilizes nucleotide binding. Addressing this issue is an important future goal and will require the generation of stable full-length Rit in sufficient quantities to repeat these nucleotide exchange assays.
Assembly of a phosphotyrosine-specific signaling complex at TrkAY499 is required for NGF-dependent neuronal differentiation (Nimnual et al., 1998; Reichardt, 2006). Importantly, previous studies have shown that Rit signaling is necessary for both NGF- and PACAP-dependent neurite elongation in pheochromocytoma cells (Shi and Andres, 2005; Shi et al., 2006) and must be factored into models for neurotrophin-mediated neuronal differentiation (Ravni et al., 2006; Gerdin and Eiden, 2007). However, the cellular role of Rit downstream of these two receptors seems to differ. Rit has been shown to regulate both extracellular signal-regulated kinase and p38 MAP kinase signaling after NGF stimulation (Shi and Andres, 2005), but only PACAP-mediated p38 kinase activation (Shi et al., 2006). This difference is particularly striking because data presented here indicates that a Trk receptor signaling complex directs Rit activation after exposure to either ligand (Figures 3–7) and that the kinetics of Rit activation are similar after either PCAP38 or NGF stimulation (Shi and Andres, 2005; Shi et al., 2006). What might account for these differences? A growing literature indicates that the organization of higher order signaling complexes is a common mechanism to confer specificity to cellular signaling. For example, Ras and Rap1 GTPase signaling pathways are selectively activated by the recruitment of distinct regulatory proteins to Trk receptors (Stork, 2005; Reichardt, 2006). The nature of such a signaling complex may be controlled not only by the selective activation of phosphotyrosine docking sites on TrkA but also by interaction of TrkA with accessory binding partners, such as the p75 receptor (Reichardt, 2006), human tumorous imaginal disk 1 (Liu et al., 2005), ARMS/Kidins220 (Arevalo et al., 2004), or perhaps through direct interactions with the PACR1 as recently described for transactivation by the extracellular signal-regulated kinase-1 receptor (Arevalo et al., 2004; Delcourt et al., 2007a,b). Ongoing studies seek to determine whether Trk coreceptor binding contributes to the specificity of Rit-mediated downstream signaling.
Previous work established a role for Src-family kinases in Trk receptor transactivation signaling. Consistent with a role for a Src kinase in PACAP-mediated Rit activation, expression of activated Src was sufficient to induce Rit activation (Figure 2A). Furthermore, expression of dominant-negative Src or pharmacological blockade of Src kinase signaling inhibits both PACAP- and Epac-mediated Rit activation (Figure 2) and PACAP-stimulated TrkA tyrosine phosphorylation (Figure 4D). The molecular mechanisms by which GPCR signaling promotes Src activation remain poorly understood but both Giα and Gsα have been found to associate with and stimulate Src activity (Ma et al., 2000). Interestingly, Kim et al. have identified a direct interaction between Rit and both Goα and Gsα (Kim et al., 2008), suggesting that a higher order signaling complex containing Gα, Src, and Rit might contribute to transactivation. Similar to Rit activation, Epac-mediated c-Jun NH2-terminal kinase kinase activation requires a conserved Ras exchange motif (REM) domain but does not involve the RapGEF domain (Hochbaum et al., 2003). It will be important to determine whether REM, or another domain within Epac, directs Src activation. Direct phosphorylation of TrkA by Src family kinases has been demonstrated in vitro (Tsuruda et al., 2004; Rajagopal and Chao, 2006), and our studies suggest that Src may lead to selective activation of TrkA. Although the activation status of individual tyrosine residues in TrkA was not directly examined in this study, we found a critical role for Y499 in both PACAP38-mediated Rit activation (Figure 5B) and to overall TrkA tyrosine phosphorylation (Figure 5, C and D). The importance of Y499 to the recruitment and activation of the Shc/Grb2/SOS1/2 regulatory complex is well established (Reichardt, 2006); however, its significance to PACAP-mediated TrkA activation is less clear. Several mechanisms for Src family kinase-mediated regulation of Ras GTPase signaling have been described previously. These include direct phosphorylation and activation of either individual GEFs or their adaptor proteins and modulation of GAP protein activity (Giglione et al., 2001; Wolf et al., 2001; Bernards and Settleman, 2004; He et al., 2005; Kawakatsu et al., 2005; Mitin et al., 2005). In addition, the docking of Src family members to a TrkA receptor signaling complex might allow these kinases to regulate Trk catalytic activity, or to direct Trk receptor trafficking. Further studies are needed to fully characterize the role for Src kinases in both PACAP-mediated Rit activation and TrkA transactivation.
In conclusion, our results demonstrate that PACAP38-dependent Rit activation involves transactivation of the TrkA receptor. In this cascade, cAMP/Epac signaling regulates Src kinase-dependent phosphorylation of TrkAY499 that leads to Rit activation, probably via the recruitment and activation of a cellular GEF, perhaps SOS (Figure 7F). These studies further highlight the importance of transactivation in GPCR signaling. This is particularly true in the case of PACAP/Trk signaling, with a variety of studies indicating that transactivation plays a critical role in PACAP-mediated neuronal differentiation and survival signaling (Lee et al., 2002a; Delcourt et al., 2007a). PACAP and PACR1 have established signaling roles in the regulation of complex behavioral and neurological effects (Vaudry et al., 2000; Somogyvari-Vigh and Reglodi, 2004; Hashimoto et al., 2006). Moving forward, it will be necessary to consider the role of PACAP-mediated Trk transactivation and the contribution of Rit signaling to these physiological processes.
ACKNOWLEDGMENTS
We thank Dr. H. Rehmann (Department of Physiological Chemistry and Center for Biomedical Genetics, University Medical Center Utrecht, Utrecht, The Netherlands) for performing the in vitro SOS nucleotide exchange assays and Drs. L. A. Quilliam, S. Meakin, and C. Wu for reagents. This work was supported by U.S. Public Health Service grant NS045103 (to D.A.A.) from the National Institute of Neurological Disorders and Stroke and by grant P20RR20171 from the Centers of Biomedical Research Excellence program of the National Center for Research Resources, a component of the National Institutes of Health.
Abbreviations used:
- 8-CPT-2-Me-cAMP
8-(p-chlorophenylthio)-2′-O-methyl-adenosine-3′,5′-cyclic monophosphate
- C3G
C3G guanine-nucleotide exchange factor
- CTX
cholera toxin
- ddA
2′,5′-dideoxyadenosine
- Epac
exchange protein activated by cyclic AMP
- GPCR
G protein-coupled receptor
- NGF
nerve growth factor
- PTX
pertussis toxin
- PACAP
pituitary adenylyl cyclase-activating peptide
- PACR1
pituitary adenylyl cyclase-activating peptide receptor type I
- PKA
protein kinase A
- Rp-cAMP
adenosine 3′,5′-cyclic monophosphorothioate, Rp-isomer
- RTK
receptor tyrosine kinase
- SOS
son of sevenless.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-12-1033) on March 10, 2010.
REFERENCES
- Andres D. A., Rudolph J. L., Sengoku T., Shi G. X. Analysis of rit signaling and biological activity. Methods Enzymol. 2005;407:499–512. doi: 10.1016/S0076-6879(05)07040-0. [DOI] [PubMed] [Google Scholar]
- Andres D. A., Shi G. X., Bruun D., Barnhart C., Lein P. J. Rit signaling contributes to interferon-gamma-induced dendritic retraction via p38 mitogen-activated protein kinase activation. J. Neurochem. 2008;107:1436–1447. doi: 10.1111/j.1471-4159.2008.05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo J. C., Yano H., Teng K. K., Chao M. V. A unique pathway for sustained neurotrophin signaling through an ankyrin-rich membrane-spanning protein. EMBO J. 2004;23:2358–2368. doi: 10.1038/sj.emboj.7600253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards A., Settleman J. GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 2004;14:377–385. doi: 10.1016/j.tcb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Bos J. L. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Reichardt L. F. An alternatively spliced form of the nerve growth factor receptor TrkA confers an enhanced response to neurotrophin 3. Proc. Natl. Acad. Sci. USA. 1994;91:11133–11137. doi: 10.1073/pnas.91.23.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcourt N., Bockaert J., Marin P. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol. Sci. 2007a;28:602–607. doi: 10.1016/j.tips.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Delcourt N., Thouvenot E., Chanrion B., Galeotti N., Jouin P., Bockaert J., Marin P. PACAP type I receptor transactivation is essential for IGF-1 receptor signalling and antiapoptotic activity in neurons. EMBO J. 2007b;26:1542–1551. doi: 10.1038/sj.emboj.7601608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Sun Y. The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J. Biol. Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- El Zein N., Badran B. M., Sariban E. The neuropeptide pituitary adenylate cyclase activating protein stimulates human monocytes by transactivation of the Trk/NGF pathway. Cell. Signal. 2007;19:152–162. doi: 10.1016/j.cellsig.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Elbashir S. M., Harborth J., Weber K., Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- Gerdin M. J., Eiden L. E. Regulation of PC12 cell differentiation by cAMP signaling to ERK independent of PKA: do all the connections add up? Sci STKE. 2007;2007:pe15. doi: 10.1126/stke.3822007pe15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglione C., Gonfloni S., Parmeggiani A. Differential actions of p60c-Src and Lck kinases on the Ras regulators p120-GAP and GDP/GTP exchange factor CDC25Mm. Eur. J. Biochem. 2001;268:3275–3283. doi: 10.1046/j.1432-1327.2001.02230.x. [DOI] [PubMed] [Google Scholar]
- Green S. H., Rydel R. E., Connolly J. L., Greene L. A. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J. Cell Biol. 1986;102:830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. O., Rehfeld J. F., Nielsen F. C. Cyclic AMP-induced neuronal differentiation via activation of p38 mitogen-activated protein kinase. J. Neurochem. 2000;75:1870–1877. doi: 10.1046/j.1471-4159.2000.0751870.x. [DOI] [PubMed] [Google Scholar]
- Harrison S. M., Rudolph J. L., Spencer M. L., Wes P. D., Montell C., Andres D. A., Harrison D. A. Activated RIC, a small GTPase, genetically interacts with the Ras pathway and calmodulin during Drosophila development. Dev. Dyn. 2005;232:817–826. doi: 10.1002/dvdy.20346. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Shintani N., Baba A. New insights into the central PACAPergic system from the phenotypes in PACAP- and PACAP receptor-knockout mice. Ann. N.Y. Acad. Sci. 2006;1070:75–89. doi: 10.1196/annals.1317.038. [DOI] [PubMed] [Google Scholar]
- He J. C., Gomes I., Nguyen T., Jayaram G., Ram P. T., Devi L. A., Iyengar R. The G alpha(o/i)-coupled cannabinoid receptor-mediated neurite outgrowth involves Rap regulation of Src and Stat3. J. Biol. Chem. 2005;280:33426–33434. doi: 10.1074/jbc.M502812200. [DOI] [PubMed] [Google Scholar]
- Hochbaum D., Tanos T., Ribeiro-Neto F., Altschuler D., Coso O. A. Activation of JNK by Epac is independent of its activity as a Rap guanine nucleotide exchanger. J. Biol. Chem. 2003;278:33738–33746. doi: 10.1074/jbc.M305208200. [DOI] [PubMed] [Google Scholar]
- Holt K. H., Waters S. B., Okada S., Yamauchi K., Decker S. J., Saltiel A. R., Motto D. G., Koretzky G. A., Pessin J. E. Epidermal growth factor receptor targeting prevents uncoupling of the Grb2-SOS complex. J. Biol. Chem. 1996;271:8300–8306. doi: 10.1074/jbc.271.14.8300. [DOI] [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kaech S., Banker G., Stork P. Putting on the RITz. Sci STKE. 2007;2007:pe71. doi: 10.1126/stke.4162007pe71. [DOI] [PubMed] [Google Scholar]
- Kawakatsu T., Ogita H., Fukuhara T., Fukuyama T., Minami Y., Shimizu K., Takai Y. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J. Biol. Chem. 2005;280:4940–4947. doi: 10.1074/jbc.M408710200. [DOI] [PubMed] [Google Scholar]
- Kiermayer S., Biondi R. M., Imig J., Plotz G., Haupenthal J., Zeuzem S., Piiper A. Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol. Biol. Cell. 2005;16:5639–5648. doi: 10.1091/mbc.E05-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Kim S., Ghil S. H. Rit contributes to neurite outgrowth triggered by the alpha subunit of Go. Neuroreport. 2008;19:521–525. doi: 10.1097/WNR.0b013e3282f9e473. [DOI] [PubMed] [Google Scholar]
- Lazarovici P., Jiang H., Fink D., Jr The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase. Mol. Pharmacol. 1998;54:547–558. doi: 10.1124/mol.54.3.547. [DOI] [PubMed] [Google Scholar]
- Lee C.H.J., Della N. G., Chew C. E., Zack D. J. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. J. Neurosci. 1996;16:6784–6794. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. S., Rajagopal R., Chao M. V. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002a;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- Lee F. S., Rajagopal R., Kim A. H., Chang P. C., Chao M. V. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J. Biol. Chem. 2002b;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- Lein P. J., Guo X., Shi G. X., Moholt-Siebert M., Bruun D., Andres D. A. The novel GTPase Rit differentially regulates axonal and dendritic growth. J. Neurosci. 2007;27:4725–4736. doi: 10.1523/JNEUROSCI.5633-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Asuri S., Rebhun J. F., Castro A. F., Paranavitana N. C., Quilliam L. A. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J. Biol. Chem. 2006;281:2506–2514. doi: 10.1074/jbc.M508165200. [DOI] [PubMed] [Google Scholar]
- Lin S. L., Johnson-Farley N. N., Lubinsky D. R., Cowen D. S. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J. Neurochem. 2003;87:1076–1085. doi: 10.1046/j.1471-4159.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- Liu H. Y., MacDonald J. I., Hryciw T., Li C., Meakin S. O. Human tumorous imaginal disc 1 (TID1) associates with Trk receptor tyrosine kinases and regulates neurite outgrowth in nnr5-TrkA cells. J. Biol. Chem. 2005;280:19461–19471. doi: 10.1074/jbc.M500313200. [DOI] [PubMed] [Google Scholar]
- Liu H. Y., Meakin S. O. ShcB and ShcC activation by the Trk family of receptor tyrosine kinases. J. Biol. Chem. 2002;277:26046–26056. doi: 10.1074/jbc.M111659200. [DOI] [PubMed] [Google Scholar]
- Luttrell L. M., Daaka Y., Lefkowitz R. J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- Ma Y. C., Huang J., Ali S., Lowry W., Huang X. Y. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Marinissen M. J., Gutkind J. S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol. Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- McCulloch D. A., MacKenzie C. J., Johnson M. S., Robertson D. N., Holland P. J., Ronaldson E., Lutz E. M., Mitchell R. Additional signals from VPAC/PAC family receptors. Biochem. Soc. Trans. 2002;30:441–446. doi: 10.1042/bst0300441. [DOI] [PubMed] [Google Scholar]
- McFarland K. N., Wilkes S. R., Koss S. E., Ravichandran K. S., Mandell J. W. Neural-specific inactivation of ShcA results in increased embryonic neural progenitor apoptosis and microencephaly. J. Neurosci. 2006;26:7885–7897. doi: 10.1523/JNEUROSCI.3524-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitin N., Rossman K. L., Der C. J. Signaling interplay in Ras superfamily function. Curr. Biol. 2005;15:R563–R574. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Moss J. Signal transduction by receptor-responsive guanyl nucleotide-binding proteins: modulation by bacterial toxin-catalyzed ADP-ribosylation. Clin. Res. 1987;35:451–458. [PubMed] [Google Scholar]
- Nimnual A. S., Yatsula B. A., Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- Obara Y., Labudda K., Dillon T. J., Stork P. J. PKA phosphorylation of Src mediates Rap1 activation in NGF and cAMP signaling in PC12 cells. J. Cell Sci. 2004;117:6085–6094. doi: 10.1242/jcs.01527. [DOI] [PubMed] [Google Scholar]
- Quilliam L. A., Castro A. F., Rogers-Graham K. S., Martin C. B., Der C. J., Bi C. M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J. Biol. Chem. 1999;274:23850–23857. doi: 10.1074/jbc.274.34.23850. [DOI] [PubMed] [Google Scholar]
- Quilliam L. A., Rebhun J. F., Castro A. F. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog. Nucleic Acid Res. Mol. Biol. 2002;71:391–444. doi: 10.1016/s0079-6603(02)71047-7. [DOI] [PubMed] [Google Scholar]
- Rajagopal R., Chao M. V. A role for Fyn in Trk receptor transactivation by G-protein-coupled receptor signaling. Mol. Cell. Neurosci. 2006;33:36–46. doi: 10.1016/j.mcn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Ravichandran K. S. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- Ravni A., et al. The neurotrophic effects of PACAP in PC12 cells: control by multiple transduction pathways. J. Neurochem. 2006;98:321–329. doi: 10.1111/j.1471-4159.2006.03884.x. [DOI] [PubMed] [Google Scholar]
- Rehmann H. Characterization of the activation of the rap-specific exchange factor epac by cyclic nucleotides. Methods Enzymol. 2005;407:159–173. doi: 10.1016/S0076-6879(05)07014-X. [DOI] [PubMed] [Google Scholar]
- Reichardt L. F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S., Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Shao H., Kadono-Okuda K., Finlin B. S., Andres D. A. Biochemical Characterization of the Ras-Related GTPases Rit and Rin. Arhc. Biochem. Biophys. 1999;371:207–219. doi: 10.1006/abbi.1999.1448. [DOI] [PubMed] [Google Scholar]
- Shi G. X., Andres D. A. Rit contributes to nerve growth factor-induced neuronal differentiation via activation of B-Raf-extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades. Mol. Cell. Biol. 2005;25:830–846. doi: 10.1128/MCB.25.2.830-846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G. X., Han J., Andres D. A. Rin GTPase couples nerve growth factor signaling to p38 and b-Raf/ERK pathways to promote neuronal differentiation. J. Biol. Chem. 2005;280:37599–37609. doi: 10.1074/jbc.M507364200. [DOI] [PubMed] [Google Scholar]
- Shi G. X., Jin L., Andres D. A. Pituitary adenylate cyclase-activating polypeptide 38-mediated Rin activation requires Src and contributes to the regulation of HSP27 signaling during neuronal differentiation. Mol. Cell. Biol. 2008;28:4940–4951. doi: 10.1128/MCB.02193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G. X., Rehmann H., Andres D. A. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol. Cell. Biol. 2006;26:9136–9147. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Batzer A., Li N., Lee C. H., Lowenstein E., Mohammadi M., Margolis B., Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993a;260:1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993b;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyvari-Vigh A., Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr. Pharm. Des. 2004;10:2861–2889. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- Spencer M. L., Shao H., Andres D. A. Induction of neurite extension and survival in pheochromocytoma cells by the Rit GTPase. J. Boil. Chem. 2002a;277:20160–20168. doi: 10.1074/jbc.M201092200. [DOI] [PubMed] [Google Scholar]
- Spencer M. L., Shao H., Tucker H. M., Andres D. A. Nerve Growth Factor-dependent Activation of the Small GTPase Rin. J. Biol. Chem. 2002b;277:17605–17615. doi: 10.1074/jbc.M111400200. [DOI] [PubMed] [Google Scholar]
- Stork P. J. Directing NGF's actions: it's a Rap. Nat. Cell Biol. 2005;7:338–339. doi: 10.1038/ncb0405-338. [DOI] [PubMed] [Google Scholar]
- Thomas D., Bradshaw R. A. Differential utilization of ShcA tyrosine residues and functional domains in the transduction of epidermal growth factor-induced mitogen-activated protein kinase activation in 293T cells and nerve growth factor-induced neurite outgrowth in PC12 cells. Identification of a new Grb2.Sos1 binding site. J. Biol. Chem. 1997;272:22293–22299. doi: 10.1074/jbc.272.35.22293. [DOI] [PubMed] [Google Scholar]
- Tsuruda A., Suzuki S., Maekawa T., Oka S. Constitutively active Src facilitates NGF-induced phosphorylation of TrkA and causes enhancement of the MAPK signaling in SK-N-MC cells. FEBS Lett. 2004;560:215–220. doi: 10.1016/S0014-5793(04)00115-2. [DOI] [PubMed] [Google Scholar]
- Vaudry D., Gonzalez B. J., Basille M., Yon L., Fournier A., Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Waschek J. A. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2002;24:14–23. doi: 10.1159/000064942. [DOI] [PubMed] [Google Scholar]
- Waters C., Pyne S., Pyne N. J. The role of G-protein coupled receptors and associated proteins in receptor tyrosine kinase signal transduction. Semin. Cell. Dev. Biol. 2004;15:309–323. doi: 10.1016/j.semcdb.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Werry T. D., Sexton P. M., Christopoulos A. “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol. Metab. 2005;16:26–33. doi: 10.1016/j.tem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Wes P. D., Yu M., Montell C. RIC, a calmodulin-binding Ras-like GTPase. EMBO J. 1996;15:5839–5848. [PMC free article] [PubMed] [Google Scholar]
- Wolf R. M., Wilkes J. J., Chao M. V., Resh M. D. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J. Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]