In this article it is shown that EGF stimulation leads to rapid activation of RhoG through Vav GEFs and the GEF PLEKHG6. Importantly, different cellular responses induced by EGF are determined by the available GEFs. Furthermore, this article presents results showing that EGF-stimulated cell migration and EGFR internalization are regulated by RhoG.
Abstract
RhoG is a member of the Rac-like subgroup of Rho GTPases and has been linked to a variety of different cellular functions. Nevertheless, many aspects of RhoG upstream and downstream signaling remain unclear; in particular, few extracellular stimuli that modulate RhoG activity have been identified. Here, we describe that stimulation of epithelial cells with epidermal growth factor leads to strong and rapid activation of RhoG. Importantly, this rapid activation was not observed with other growth factors tested. The kinetics of RhoG activation after epidermal growth factor (EGF) stimulation parallel the previously described Rac1 activation. However, we show that both GTPases are activated independently of one another. Kinase inhibition studies indicate that the rapid activation of RhoG and Rac1 after EGF treatment requires the activity of the EGF receptor kinase, but neither phosphatidylinositol 3-kinase nor Src kinases. By using nucleotide-free RhoG pull-down assays and small interfering RNA-mediated knockdown studies, we further show that guanine-nucleotide exchange factors (GEFs) of the Vav family mediate EGF-induced rapid activation of RhoG. In addition, we found that in certain cell types the recently described RhoG GEF PLEKHG6 can also contribute to the rapid activation of RhoG after EGF stimulation. Finally, we present results that show that RhoG has functions in EGF-stimulated cell migration and in regulating EGF receptor internalization.
INTRODUCTION
The family of Rho GTPases comprises 22 members that are divided into five subgroups based on amino acid sequence similarity (Wherlock and Mellor, 2002; Burridge and Wennerberg, 2004). The discovery of their role in regulating actin organization and focal adhesion dynamics was a hallmark in understanding the importance of these molecules (Ridley and Hall, 1992; Ridley et al., 1992). Rho GTPases are now known to be involved in controlling a diverse set of cellular functions, including cell migration, gene expression, endocytosis, cell cycle progression, and differentiation (Jaffe and Hall, 2005).
Rho GTPases are molecular switches, alternating between an active guanosine triphosphate (GTP)-bound form and an inactive guanosine diphosphate (GDP)-bound form. Downstream signaling from Rho GTPases occurs through effector molecules which can specifically bind to the GTP-bound form of the GTPase (Bishop and Hall, 2000). The activity state of Rho GTPases is regulated by three classes of upstream regulatory proteins. Guanine-nucleotide exchange factors (GEFs) promote the exchange of GDP for GTP, thereby activating the Rho protein (Rossman et al., 2005). Conversely, GTPase-activating proteins (GAPs) stimulate the intrinsic GTPase activity of Rho GTPases promoting GTP hydrolysis, thereby rendering the Rho proteins unable to interact with downstream effectors (Moon and Zheng, 2003). Guanine-nucleotide dissociation inhibitors (GDIs) are the third class of regulators and they bind and sequester Rho GTPases in the inactive form (Dovas and Couchman, 2005). The number of different GEFs and GAPs greatly exceeds the number of Rho GTPases (∼70 GEFs, ∼50 GAPs), and they each have individual binding specificities for different Rho GTPases. The mechanisms of how most Rho GEFs and GAPs are regulated remain unknown.
RhoG is an evolutionarily conserved member of the Rac-like subgroup of Rho GTPases, with homologues identified in mammals, Drosophila, and Caenorhabditis elegans (Hakeda-Suzuki et al., 2002; Vigorito et al., 2004). Several cellular functions have been associated with RhoG, including neurite outgrowth, gene expression, apoptosis, macropinocytosis, and uptake of apoptotic cells (Katoh et al., 2000; Murga et al., 2002; Vigorito et al., 2003; deBakker et al., 2004; Ellerbroek et al., 2004; Yamaki et al., 2007), and also several pathogenic bacteria exploit RhoG function of the host cells during their infection cycle (Patel and Galan, 2006; Roppenser et al., 2009). However, knowledge of RhoG regulation remains obscure, because only a few GEFs for RhoG have been described and no cellular RhoG GAPs are currently known. Until now, intercellular adhesion molecule (ICAM-1) and syndecan-4 are the only cell surface receptors that were shown to modulate RhoG activity after their engagement (van Buul et al., 2007; Elfenbein et al., 2009). However, given the high degree of phylogenetic conservation of RhoG, its broad expression pattern, and the diversity of RhoG functions, it has to be assumed that other receptors are also modulating RhoG activation.
Epidermal growth factor (EGF) receptor signaling involves a complex network of pathways (Oda et al., 2005). On ligand binding, the dimerized EGF receptor (EGFR) undergoes a conformational shift that stimulates its intrinsic kinase activity (Ferguson, 2008; Landau and Ben-Tal, 2008). Autophosphorylation is a primary result and facilitates binding of numerous proteins to phosphorylated residues on the receptor, thereby initiating various downstream signaling pathways. In addition, direct phosphorylation of target proteins by the EGFR also contributes to signaling. Major signaling targets known to be initiated by EGFR activation are Src-family kinases (Osherov and Levitzki, 1994), phosphatidylinositol 3-kinase (PI3K) (Rodrigues et al., 2000), mitogen-activated protein kinase (MAPK) (Sasaoka et al., 1994; Jones et al., 1999), and several Rho GTPases. For example, different mechanisms have been described for the activation of Rac1 and cdc42 after EGF stimulation (Scita et al., 1999; Marcoux and Vuori, 2003; Ray et al., 2007; Itoh et al., 2008).
In the present study, we looked for extracellular factors that stimulate RhoG activity. We identified EGF as a strong and rapid activator of RhoG, whereas other growth factors were not. We focused our studies on the immediate activation of RhoG and its close homologue Rac1 within 30 s after EGF stimulation. Surprisingly, at this early time point RhoG and Rac1 activation occur independently of Src and PI3K signaling, suggesting direct signaling from the activated EGFR to the RhoG GEF(s). We found different GEFs to be involved in EGF-induced RhoG activation in a cell type-specific manner, including members of the Vav family, and PLEKHG6, and that the composition of GEFs in a given cell type dictates the morphological change induced by EGF stimulation (dorsal ruffling vs. cell spreading). Furthermore, we found that RhoG regulates EGF induced cell migration and early EGF receptor internalization processes.
MATERIALS AND METHODS
RhoG and Rac1 Activity Assays
To measure endogenous levels of GTP-loaded RhoG and Rac1, we used pull-down assays as described previously (van Buul et al., 2007). In brief, cells were washed twice with ice-cold Tris-buffered saline (50 mM Tris, pH 7.4, 5 mM MgCl2, 150 mM NaCl) and then lysed in lysis buffer (50 mM Tris, pH 7.4, 10 mM MgCl2, 150 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 10 μg/ml each of aprotinin and leupeptin). After clearing the lysates by centrifugation at 14,000 × g for 5 min, protein concentrations of the supernatants were determined, and equal amounts of total protein were used to measure RhoG.GTP and Rac1.GTP. Therefore, the supernatants were rotated for 30 min with glutathione-Sepharose beads (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), which were loaded with 60–90 μg of either glutathione transferase (GST)-ELMO (GST fusion protein containing the full-length RhoG effector ELMO) or GST-Pak1-binding domain (PBD). Subsequently the beads were washed four times in lysis buffer. Pull-downs and lysates were then immunoblotted for RhoG or Rac1, respectively.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
For semiquantitative RT-PCR analysis of knockdown efficiency or expression of PLEKHG6 and Src homology 3 domain-containing guanine nucleotide exchange factor (SGEF), total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) reagent and reverse transcribed using the First-Strand cDNA synthesis kit (Roche Diagnostics, Indianapolis, IN). PCR was done using the following primers: SGEF: forward, 5′-ATGGACGGCGAAAGCGAGGTG-3′ and reverse, 5′-CCATCAAATGTTCCTTGTCCT-3′; PLEKHG6: forward, 5′-CCACCCTGGACCTGACGTCC-3′ and reverse, 5′-TCAGTGGCCAGCTTTCAGGAACAGAG-3′; and β2-microglobulin: forward, 5′-CTCGCGCTACTCTCTCTTTCTGG-3′ and reverse, 5′-GCTTACATCTCTCAATCCCACTTAA-3′.
RhoG-Nucleotide-free Pull-Down Assays and Immunoprecipitations (IPs)
To pull down cellular GEFs acting on RhoG, GST-RhoG-15A protein was bacterially expressed and bound to glutathione-Sepharose beads. Cells were lysed in nucleotide-free lysis buffer (150 mM NaCl, 20 mM HEPES, pH 7.6, 5 mM MgCl2, 1% Triton X-100, 1 mM PMSF, 1 mM ortho-vanadate, and 10 μg/ml each of aprotinin and leupeptin). After clearing the lysates by centrifugation at 14,000 × g for 5 min, protein concentrations of the supernatants were determined, and equal amounts of total protein were used for the pull down (45 min at 4°C). Subsequently, the beads were washed three times in nucleotide-free lysis buffer. To immunoprecipitate Vav2 or Vav3, cell lysates were prepared as described above, but using IP lysis buffer (50 mM Tris, pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, 1 mM PMSF, 1 mM ortho-vanadate, and 10 μg/ml each of aprotinin and leupeptin). Antibody binding was performed overnight at 4°C, followed by protein A-Sepharose beads binding for 1 h and three wash steps.
Cells, Media, Transfection, and Growth Factor Stimulation
HeLa, A431, and NIH-3T3 cells were obtained from the LCCC tissue culture facility (University of North Carolina at Chapel Hill, Chapel Hill, NC). Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza Walkersville (Walkersville, MD). HeLa, A431, and NIH-3T3 cells were cultured in DMEM (high glucose, +glutamine; Invitrogen) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and antibiotics (Invitrogen). HUVECs were cultured in EBM-2 (Lonza Walkerville). For transfection of plasmid DNA, FuGENE6 (Roche Diagnostics) was used according to the manufacturer's protocol. For transfection of small interfering RNAs (siRNAs) into HeLa cells siQuest reagent (Mirus Bio, Madison, WI) was used according to the manufacturer's instructions. In brief, 1.5 ml of serum-free media was mixed with 18 μl of siQuest reagent and 11.25 μl of a 20 μM siRNA stock solution. After 20 min incubation at room temperature, the complexes were added to a 10 cm tissue culture dish (cell confluence, ∼50%), with 7.5 ml of fresh media containing serum. Twenty-four hours after transfection, the media were changed, and 48 h after transfection, the cells were split 1:2. The cells were used for subsequent experiments between 72 and 96 h after transfection.
For growth factor stimulation experiments, cells were initially starved in DMEM/0.5% bovine serum albumin (BSA) (delipidated BSA; Sigma-Aldrich) for 4 h. Subsequently, the media were replaced by DMEM/0.5% BSA supplemented with the corresponding growth factor at the indicated concentration. Human recombinant EGF and VEGF were obtained from R&D Systems (Minneapolis, MN), human recombinant platelet-derived growth factor (PDGF) was obtained from Sigma-Aldrich. All growth factors were used at a final concentration of 20 ng/ml unless otherwise indicated.
Antibodies, Reagents, Expression Plasmids, and siRNAs
The following antibodies were used: mouse anti-Rac1 (BD Biosciences, San Jose, CA), mouse anti-tubulin (Sigma-Aldrich), rabbit anti-phospho-473-Akt, rabbit anti-Akt, rabbit anti-phospho-418-Src, mouse anti-phospho-extracellular signal-regulated kinase (ERK)1/2, rabbit phospho-serine protein kinase C (PKC) substrate (Cell Signaling Technology, Danvers, MA), mouse anti-green fluorescent protein (GFP) (Roche Diagnostics), mouse anti-myc (clone 9E12), rabbit anti-Vav2 (clone C64H2; Cell Signaling Technology), rabbit anti-Vav3 (Cell Signaling Technology), and mouse anti-phospho-tyrosine (clone 4G10). The anti-RhoG antibody was described previously (Meller et al., 2008) and was kindly provided by M. Schwartz (University of Virginia). The pEGFP-Trio construct was a kind gift of Betty Eipper (University of Connecticut). To express myc-PLEKHG6, the full-length sequence of human PLEKHG6 (clone 40035014; Open Biosystems, Huntsville, AL) was cloned into the pCMV-myc vector. The following pharmacological inhibitors were used at the indicated final concentrations, if not mentioned otherwise: LY294002 (Calbiochem, San Diego, CA), 30 μM; PP2 (Sigma-Aldrich), 10 μM; SU6656 (Sigma-Aldrich), 2.5 μM; AG1478 (Sigma-Aldrich), 10 μM; Gö6983 (Calbiochem), 1.32 μM; and U0126 (Sigma-Aldrich), 10 μM. All used siRNA duplexes targeting mRNAs or nontargeting control siRNAs were synthesized by Sigma-Aldrich or Dharmacon RNA Technologies (Lafayette, CO) and sequences are indicated in Table 1.
Table 1.
siRNA sequences
| Protein | Antisense | Sense |
|---|---|---|
| PLEKHG6 (#1) | UAGCAGAUCAGUCAUGAUCdTdT | GAUCAUGACUGAUCUGCUAdTdT |
| PLEKHG6 (#2) | UUAGUUUCUUGCUGUUCUCdTdT | GAGAACAGCAAGAAACUAAdTdT |
| Rac1 | AAACUCGCUAUGAAAUCACUU | GUGAUUUCAUAGCGAGUUUUU |
| RhoG | GCAACAGGAUGGUGUCAAGUU | CUUGACACCAUCCUGUUGCUU |
| SGEF | UUAGCGGCAAGGCCAUUUGUU | CAAAUGGCCUUGCCGCUAAUU |
| Vav2 | UCACAGAGGCCAAGAAAUUdTdT | AAUUUCUUGGCCUCUGUGAdTdT |
| Vav3 | UUUCAGAACUUAAUGCUCCTGdTdT | GGAGCAUUAAGUUCUGAAAdTdT |
| Control | UCACUCGUGCCGCAUUUCCdTdT | GGAAAUGCGGCACGAGUGAdTdT |
EGFR Internalization Assays
HeLa cells were plated in DMEM/10% fetal bovine serum on coverslips coated with fibronectin (20 μg/ml fibronectin in phosphate-buffered saline [PBS]; 3 × 104 cells/well). After cell attachment, cells were starved for 4 h in DMEM/0.5% BSA. The coverslips were then incubated for 1 h at 4°C in cold PBS/Alexa-488-EGF (1 μg/ml) and then transferred to 37°C with DMEM/0.5% BSA for the indicated times (2–20 min). After washing twice with cold PBS, noninternalized EGF-Alexa-488 was removed by washing once for 2 min and once for 10 s with PBS/HCl, pH 2.1, before fixing the cells with 3.7% formaldehyde. To quantify the magnitude of internalized EGF-Alexa-488 at a certain time point, fluorescent micrographs were taken with a 63× objective at multiple random positions of a coverslip. By using MetaMorph software (Molecular Devices, Sunnyvale, CA), the integrated fluorescence of at least 20 cells per condition was measured, background subtracted, and averaged.
Wound Healing Assays
For wound healing assays, confluent HeLa cell cultures were starved for 4 h in DMEM before scratching the monolayer with a micropipette tip. The cells were subsequently incubated in media with or without EGF for 18 h. Images were taken at 0 and 18 h, and the migration speed of each cell front was calculated: [ (width 18 h) − (width at 0 h) ]/2.
RESULTS
EGF Induces Rapid Activation of RhoG
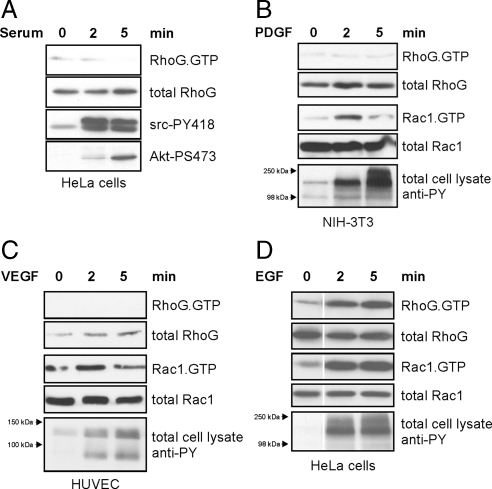
To identify physiological stimuli that regulate RhoG signaling, we tested a panel of growth factors and measured the activity status of RhoG. In agreement with observations by others (Meller et al., 2008), we did not observe RhoG activation in starved HeLa cells after serum treatment (Figure 1A). We next treated NIH-3T3 fibroblasts with PDGF and HUVECs with vascular endothelial growth factor (VEGF) for 2 and 5 min, but neither of these stimuli resulted in activation of RhoG (Figure 1, B and C). Both PDGF and VEGF treatment did however activate Rac1 as observed previously (Pandey et al., 2000; Stockton et al., 2004; Gavard and Gutkind, 2006; Garrett et al., 2007). Interestingly, when we treated starved HeLa cells with media containing physiological concentrations of EGF (20 ng/ml) (Sigismund et al., 2005), we found a strong increase in active RhoG levels compared with cells treated with media lacking EGF (Figure 1D). Levels of active Rac1 also increased after EGF treatment, as described previously (Dise et al., 2008).
Figure 1.
RhoG is activated after EGF stimulation but not by other growth factors or serum. Serum-starved cells were treated with the indicated growth factors or serum for 0, 2, and 5 min. Endogenous RhoG and Rac1 activities were measured from total cell lysates by pull-down assays by using GST-ELMO and GST-PBD, respectively. (A) HeLa cells were treated with 20% serum. Confirmation that Src and PI3K signaling occurred in responses to the serum treatment was done by blotting total cell lysates for Src-PY418 and Akt-PS473. (B) NIH-3T3 fibroblasts were treated with 20 ng/ml PDGF. (C) HUVECs were treated with 20 ng/ml VEGF. (D) HeLa cells were treated with EGF (20 ng/ml). Cellular responses in B, C, and D to stimulation by the specific growth factors were measured by blotting total cell lysates for phospho-tyrosine at the molecular weights corresponding to the relevant receptor tyrosine kinases.
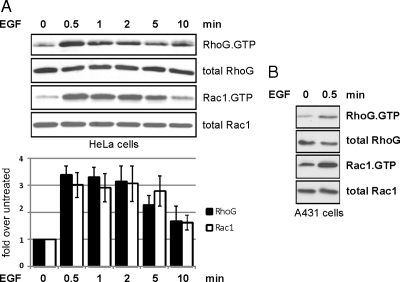
To understand the kinetics of RhoG activation after EGF stimulation, we measured levels of active RhoG in HeLa cells at time points from 30 s to 10 min after EGF (20 ng/ml) exposure. Interestingly, RhoG peak activation was observed as early as 30 s after EGF treatment, whereupon its activity began to decrease between 5 and 10 min. Rac1 activity followed the same kinetics (Figure 2A) as also reported by others in a different cell type (Patel et al., 2007). Equivalent activation kinetics of RhoG and Rac1 occurred in experiments using different concentrations of EGF (100 and 300 ng/ml; data not shown). We also observed RhoG activation after 30 s of EGF treatment in a different cell line (A431; Figure 2B), which showed that EGF-induced RhoG activation at 30 s is not limited to HeLa cells.
Figure 2.
EGF induces rapid activation of RhoG and Rac1 in different cell types within seconds. Endogenous levels of RhoG.GTP and Rac1.GTP were measured in serum-starved HeLa cells (A) or A431 cells (B) after EGF treatment (20 ng/ml) for the indicated times. The bar graph summarizes multiple independent experiments with HeLa cells (n ≥ 3; error bars represent SEM).
Because of the fast activation of RhoG and Rac1 by EGF treatment (30 s) and to separate these events from later (potentially secondary) activation of these GTPases, we refer from here on to their activation at 30 s as the “rapid activation.”
Rapid Activation of RhoG and Rac1 by EGF Is Not Interdependent
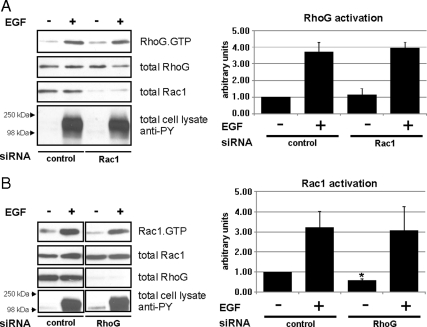
Next, we sought to determine whether the rapid activation of RhoG and Rac1 by EGF treatment depend on each other, because cross-talk between different Rho GTPases has been described previously, particularly in the context of Rac1 activation by RhoG (Hall, 1998; Katoh and Negishi, 2003). First, we examined whether RhoG activation by EGF depends on Rac1. HeLa cells were depleted of Rac1 by siRNA transfection and active RhoG was measured after EGF stimulation. As expected, we found that Rac1 depletion did not alter the rapid activation of RhoG within 30 s of EGF treatment (Figure 3A).
Figure 3.
Rapid activation of RhoG and Rac1 after EGF treatment is not interdependent. (A and B) HeLa cells were transfected with the indicated siRNAs: Rac1-specific siRNA (A), RhoG-specific siRNA (B), and control siRNA (A and B). Rapid activation of RhoG or Rac1 after EGF treatment was measured using GST-ELMO and GST-PBD pull-down assays, respectively. The bar graphs summarize the results of multiple independent experiments (n ≥ 3; error bars represent SEM). The asterisk indicates a significant difference (p < 0.05) in basal Rac1 activity between nonstimulated control siRNA and nonstimulated RhoG siRNA-transfected cells.
Rac1 can be regulated downstream of RhoG (Katoh and Negishi, 2003). Indeed, we observed that the basal activity level of Rac1 was significantly decreased when RhoG was knocked down in unstimulated HeLa cells, compared with cells that were transfected with control siRNA (Figure 3B). However, when RhoG knockdown cells were treated with EGF for 30 s, they still responded with rapid Rac1 activation to the same extent as control transfected cells (Figure 3B). This demonstrates that basal activity of Rac1 is influenced by RhoG, whereas rapid activation of Rac1 after EGF stimulation is not. These data indicate that the rapid activation of both RhoG and Rac1 occur independently and do not depend on signaling cross-talk between each other.
Signaling Pathways Involved in EGF-mediated Activation of RhoG
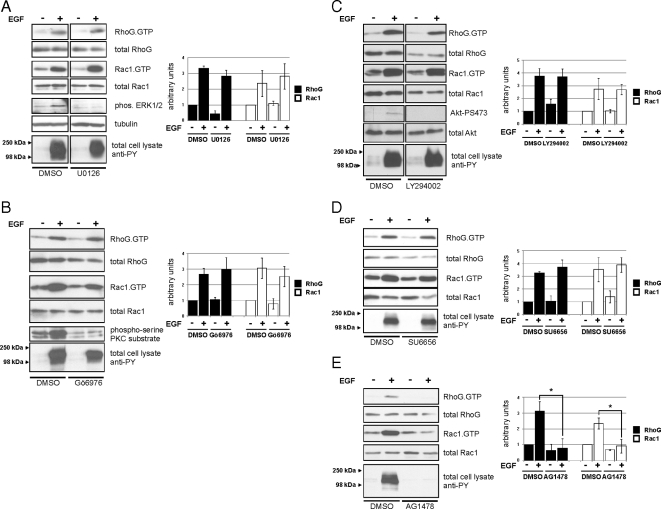
RhoG activation by EGF has not been described previously; thus, we were interested in understanding the underlying signaling pathways. To this end, we tested whether inhibition of key EGF-activated signaling pathways affected the rapid activation of RhoG. By using the pharmacological inhibitors U0126 and Gö6976, we tested the involvement of mitogen-activated protein kinase kinase (MEK)1/2 and PKCα, respectively. EGF-stimulated rapid activation of RhoG and Rac1 was not affected by these inhibitors when compared with dimethyl sulfoxide-treated control cells (Figure 4, A and B). Both Src and PI3K activity were described previously to be involved in the activation of Rac1 in colon epithelial cells after 3 min of EGF stimulation (Dise et al., 2008). Therefore, we also measured how the inhibition of these kinases affected the rapid activation of RhoG and Rac1. We found that inhibition of PI3K activity by LY294002 did not significantly affect rapid RhoG and Rac1 activation 30 s after EGF treatment (Figure 4C). Furthermore, we found that inhibition of Src-family kinases by the inhibitor SU6656 also did not interfere with the rapid activation of both RhoG and Rac1 (Figure 4D). An additional Src-inhibitor (PP2) yielded the same result (Supplemental Figure S1). Finally, we tested whether the activation of RhoG depends on the kinase activity of the EGF-receptor (EGFR) itself. As expected, inhibition of the kinase activity of the EGFR by the pharmacological inhibitor AG1478 abolished activation of both RhoG and Rac1 after 30 s (Figure 4E). In summary, the only kinase activity we found to be necessary for the rapid activation of RhoG and Rac1 upon EGF stimulation was the intrinsic activity of the EGFR itself.
Figure 4.
Effects of different kinase inhibitors on rapid activation of RhoG and Rac1 after EGF treatment. Serum-starved HeLa cells were stimulated with EGF for 30 s in the presence of different pharmacological inhibitors as indicated (pretreatment for 1 h). (A) MEK1/2 inhibitor, U0126 (10 nM). (B) PKCα inhibitor, Gö6976 (1.32 μM). (C) PI3K inhibitor, LY294002 (30 nM). (D) Src-family kinase inhibitor, SU6656 (2.5 nM). (E) EGFR kinase inhibitor, AG1478 (10 μM). RhoG.GTP and Rac1.GTP were measured by pull-down assays. The bar graphs summarize multiple independent experiments (n ≥ 3; error bars represent SEM). The asterisks indicate significant differences (p < 0.05).
Identification of GEFs Mediating the Rapid Activation of RhoG
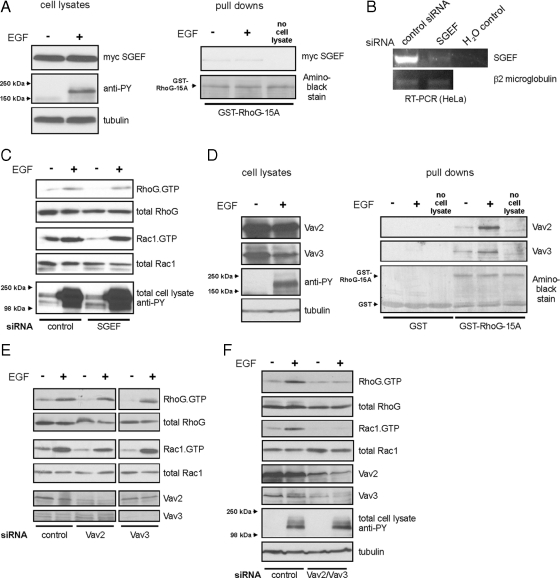
Next, we aimed to identify GEFs involved in the rapid activation of RhoG after EGF stimulation. Only a limited number of GEFs have been described that stimulate nucleotide exchange of RhoG. We first sought to test the involvement of the RhoG GEF SGEF (Ellerbroek et al., 2004) in this pathway by using a nucleotide-free RhoG pull-down assay for detection of active GEFs (Garcia-Mata et al., 2006). RhoG-15A is a nucleotide-free mutant with high affinity for activated RhoG GEFs (Wennerberg et al., 2002). We expressed myc-SGEF in HeLa cells and performed a pull-down assay with bacterial GST-RhoG-15A protein on lysates from untreated cells and from cells treated for 30 s with EGF. However, we did not detect any change in the affinity of myc-SGEF for RhoG-15A after 30 s of EGF treatment (Figure 5A), indicating that SGEF is not contributing to the rapid RhoG activation. We further confirmed this result by knocking down endogenous SGEF expression in HeLa cells. RT-PCR analysis confirmed that endogenous expression of SGEF mRNA was strongly decreased after transfection of SGEF siRNAs (Figure 5B). In agreement with the nucleotide-free pull-down assay, we still observed rapid activation of RhoG when we transfected HeLa cells with siRNA targeting SGEF (Figure 5C). Together, these results suggest that SGEF does not play a role in the rapid activation of RhoG after EGF treatment.
Figure 5.
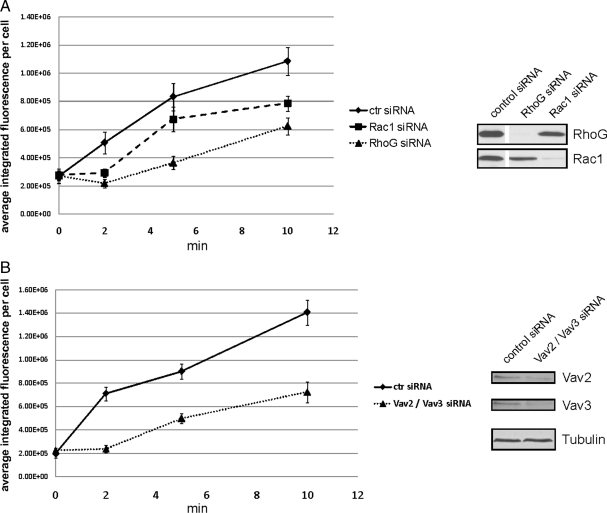
GEFs of the Vav family mediate rapid activation of RhoG and Rac1. (A) HeLa cells overexpressing myc-SGEF were lysed without stimulation or after 30 s of EGF treatment (20 ng/ml). The fraction of active SGEF was precipitated using GST-RhoG-15A protein and revealed by Western blotting. (B) Knockdown efficiency of SGEF siRNA was tested by SGEF-specific RT-PCR from control- and SGEF-siRNA–transfected HeLa cells. RT-PCR of β2-microglobulin RNA served as a control. (C) HeLa cells were transfected 72 h before EGF stimulation with control or SGEF-specific siRNA. Rapid activation of RhoG or Rac1 after 30 s of EGF treatment was measured using ELMO- and PBD-pull-down assays, respectively. (D) Serum-starved HeLa cells were lysed without any stimulus or after 30 s of EGF treatment (20 ng/ml) and activated RhoG-specific GEFs were precipitated using GST-RhoG-15A protein. Immunoblots of the precipitates revealed that both Vav2 and Vav3 are rapidly activated in HeLa cells after EGF stimulation (right). (E and F) Experimental conditions as in B; siRNAs: control, Vav2, and Vav3 (E); control, Vav2/Vav3 simultaneously (F).
GEFs of the Vav family seemed to be other likely candidates for the rapid activation, because they have been implicated downstream of EGFR (Pandey et al., 2000; Zeng et al., 2000). Vav2 and Vav3 are expressed in many different cell types (Turner and Billadeau, 2002), and they are known to activate Rac1 after EGF stimulation. To test whether endogenous Vav2 or Vav3 become activated as RhoG-specific GEFs after EGF stimulation, we again performed RhoG-15A pull-down assays from untreated cells or from cells after 30 s of EGF treatment. Interestingly, we found that binding of both Vav2 and Vav3 to GST-RhoG-15A strongly increased after 30 s of EGF stimulation, whereas binding to GST only did not change (Figure 5D). This result strongly suggested that Vav2 and Vav3 are mediating the rapid activation of RhoG. To test this further, we knocked down endogenous expression of Vav2 and Vav3. Even though we observed only minor effects on rapid activation of RhoG when Vav2 or Vav3 were knocked down individually (Figure 5E), the simultaneous knockdown of both Vav2 and Vav3 abolished the rapid activation of RhoG after EGF treatment (Figure 5F). Rapid activation of Rac1 showed a similar decrease in activity as RhoG after Vav2/Vav3 single and double knockdowns (Figure 5, E and F).
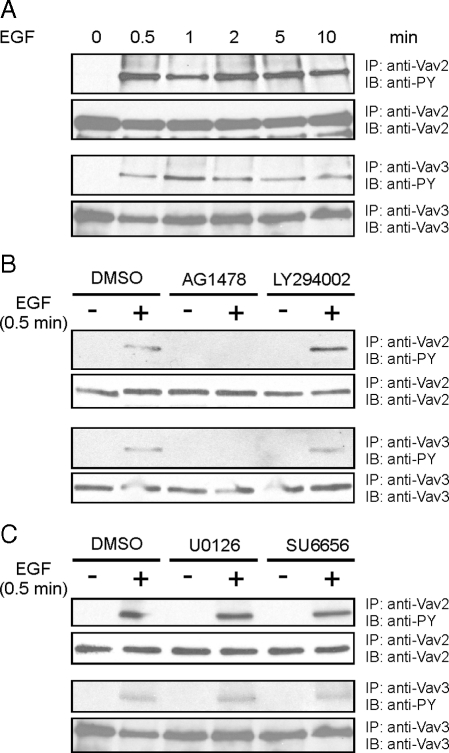
Vav2 and Vav3 were shown before to be activated by tyrosine phosphorylation after EGF stimulation (Patel et al., 2007). However, it had not been tested whether the fast kinetics we observe for RhoG activation also can be observed for Vav2/Vav3 phosphorylation. Different mechanisms for Vav2 and Vav3 activation have been proposed, some of these suggesting mechanisms other than tyrosine phosphorylation directly via the EGFR (Marignani and Carpenter, 2001; Tamas et al., 2003; Dise et al., 2008). Therefore, we tested whether Vav2 and Vav3 are phosphorylated with the same kinetics as we observed for RhoG activation. Indeed, by immunoprecipitating Vav2 and Vav3 with specific antibodies and blotting for phospho-tyrosine, we observed phosphorylation of both proteins after 30 s of EGF stimulation (Figure 6A), recapitulating the kinetics of RhoG and Rac1 activation. Inhibition of the EGFR kinase abolished Vav2/Vav3 phosphorylation, but this phosphorylation was not prevented by inhibiting any other kinase activity we tested (PI3K, src, MEK; Figure 6, B and C). Therefore, we conclude that Vav2 and Vav3 jointly mediate the rapid activation of both RhoG and Rac1 through their direct phosphorylation by the EGFR kinase.
Figure 6.
Rapid phosphorylation of Vav2 and Vav3 after EGF stimulation. HeLa cells were stimulated with EGF (20 ng/ml) for the indicated times. Immunoprecipitated Vav2 and Vav3 were blotted for phospho-tryosine and Vav2 or Vav3, respectively. (A) Kinetics of Vav2 and Vav3 phosphorylation after different times of EGF stimulation. (B and C) Preceding EGF stimulation for 30 s, the cells were treated for 1 h with pharmacological inhibitors for different kinases: EGFR kinase inhibitor: AG1478 (10 nM); PI3K inhibitor, LY294002 (30 nM); MEK1/2 inhibitor, U0126 (10 nM); Src-family kinase inhibitor, SU6656 (2.5 nM).
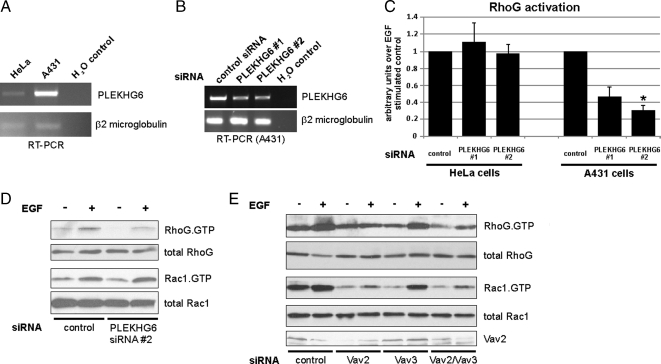
Involvement of PLEKHG6 in Rapid Activation of RhoG in A431 Cells
Recently, another GEF (PLEKHG6) was found to activate RhoG and that EGF-induced macropinocytosis in A431 cells depends on PLEKHG6 (D'Angelo et al., 2007). Because macropinocytosis is a known RhoG-mediated function, we wondered whether PLEKHG6 also contributes to rapid RhoG activation in A431 cells in a cell type-specific manner. First, we wanted to confirm that PLEKHG6 has in vivo GEF specificity for RhoG. In agreement with results from D'Angelo and coworkers, endogenous RhoG activity was increased when myc-PLEKHG6 was expressed in HeLa cells (Supplemental Figure S2). PLEKHG6 does not seem to be ubiquitously expressed in all tissues and cell lines (D'Angelo et al., 2007). RT-PCR analysis revealed that the amount of endogenous PLEKHG6 mRNA in HeLa cells is low and close to the detection threshold level, whereas mRNA for PLEKHG6 is relatively abundant in A431 cells (Figure 7A). We therefore considered that PLEKHG6 might be an additional GEF mediating the rapid activation of RhoG after EGF stimulation in those cells in which it is expressed. siRNAs targeting PLEKHG6 were transfected into HeLa and A431 cells. The PLEKHG6 siRNAs were shown to target PLEKHG6 RNA based on RT-PCR analysis of A431 cells transfected with these siRNAs (Figure 7B). Strikingly, we observed decreased RhoG activity after EGF stimulation in PLKHG6-depleted A431 cells but not in HeLa cells (Figure 7C). Interestingly, knockdown of PLEKHG6 in A431 cells only affected rapid RhoG activation after EGF stimulation but did not change rapid Rac1 activation (Figure 7D). This result is in agreement with the previously described specificity of PLEKHG6, which preferentially activates RhoG (D'Angelo et al., 2007), and further implies that RhoG and Rac1 are differentially regulated in A431 cells. We also tested the influence of knockdown of the Vav family GEFs in A431 cells (Figure 7E). Transfection of siRNA directed against Vav2/Vav3 decreased the levels of rapidly activated RhoG in A431 cells. Interestingly, and again different from HeLa cells, we could not detect Vav3 in A431 cells by Western blotting (data not shown). Accordingly, when we performed single knockdowns of Vav2 and Vav3 in A431 cells, we found that Vav2 knockdown led to the same decrease in the rapid activation of RhoG and Rac1 as the double knockdown of Vav2/Vav3 in HeLa cells (Figure 7E). In summary, we found that the composition of GEFs in A431 cells is different from HeLa cells (PLEKHG6 and Vav2 vs. Vav2/Vav3) with regard to the regulation of rapid RhoG and Rac1 activation after EGF stimulation.
Figure 7.
PLEKHG6 and Vav-family GEFs are involved in EGF stimulated RhoG activation in A431 cells. (A) RT-PCR analysis with primers specific for PLEKHG6 was performed with total RNA samples from HeLa and A431 cells. RT-PCR of β2-microglobulin RNA served as a control. (B) To confirm knockdown with the PLEKHG6 siRNAs, RT-PCR analysis with primers specific for PLEKHG6 was performed with total RNA samples from A431 cells that have been transfected with control or one of two different PLEKHG6-specific siRNAs (PLEKHG6 #1, PLEKHG6 #2). RT-PCR of β2-microglobulin RNA served as a control. (C) A431 cells and HeLa cells were transfected with control siRNA or one of two different PLEKHG6-specific siRNAs (PLEKHG6 #1, PLEKHG6 #2). All cells were stimulated for 30 s with EGF before GST-ELMO pull-down assays were performed. The bar graphs represent RhoG activity 30 s after EGF treatment in PLEKHG6 knockdown conditions compared with siRNA controls (n = 3; error bars represent SEM). The asterisk indicates significant differences compared with control siRNA-transfected cells (p < 0.05). (D and E) A431 cells were transfected with the indicated siRNAs: control, PLEKHG6 #2 (D); control, Vav2, Vav3, and Vav2/Vav3 (E). Rapid activation of RhoG or Rac1 after 30 s EGF treatment was measured using GST-ELMO and GST-PBD pull-down assays, respectively.
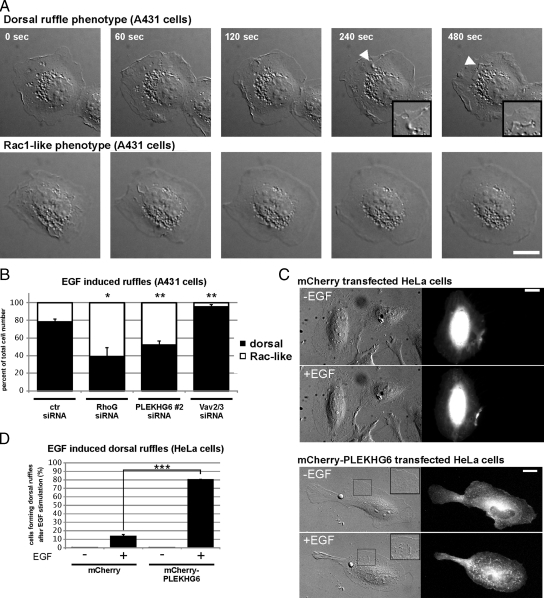
A431 cells are well known for the rapid formation of dorsal ruffles after EGF treatment (Araki et al., 2007). Furthermore, PLEKHG6 was shown previously to induce dorsal ruffle formation when overexpressed in different mammalian cells (D'Angelo et al., 2007). We therefore tested whether RhoG and PLEKHG6 regulate EGF-induced dorsal ruffle formation in A431 cells. siRNA-transfected A431 cells were plated at subconfluent densities and serum-starved before being stimulated with 20 ng/ml EGF. Differential interference contrast (DIC) microscopy movies of single cells were taken and later categorized for cells that form dorsal ruffles (dorsal-ruffle phenotype; Figure 8A, top row) versus cells that spread a large lamellipodium in multiple directions (Rac-like phenotype; Figure 8A, bottom row). We found that the majority of control siRNA-transfected cells (78 ± 3.5%; mean ± SEM) responded to EGF treatment with the formation of dorsal ruffles (Figure 8B). Interestingly, when we knocked down RhoG or PLEKHG6, the fraction of dorsal ruffling cells significantly decreased, and increased numbers of cells showed the Rac-like phenotype (RhoG siRNA, 39% dorsal ruffling and 61% Rac-like phenotype, each ±10.2% SEM; PLEKHG6 siRNA, 52% dorsal ruffling and 48% Rac-like phenotype, each ±4.7% SEM). These results show that RhoG and PLEKHG6 are necessary for dorsal ruffle formation in A431 cells after EGF stimulation. Furthermore, when we analyzed A431 cells transfected with siRNA targeting Vav2/Vav3, we observed significantly fewer cells with a Rac-like phenotype, and more cells formed dorsal ruffles (95% dorsal ruffling and 5% Rac-like phenotype, each ±2.7% SEM). Of note, the dorsal ruffles formed by cells transfected with Vav2/Vav3 siRNA had an overall different morphology compared with control cells, in that the dorsal protrusions seemed to extend directly from the dorsal surface of the cell rather than from extended lamellipodia (data not shown). Overall, these results indicate that PLEKHG6 is the critical component that dictates the different EGF-induced responses of HeLa and A431 cells. To test whether PLEKHG6 is indeed a component that controls EGF-induced dorsal ruffle formation, we attempted to change EGF-induced ruffling behavior of HeLa cells, which usually do not form prominent dorsal ruffles. Therefore, we expressed PLEKHG6 protein at very low levels in HeLa cells. At this low expression level, we did not observe formation of spontaneous dorsal ruffles (Figure 8, C and D). Interestingly, after stimulating the cells with EGF, we observed formation of dorsal ruffles of such PLEKHG6 expressing HeLa cells, which were barely observed in cells transfected with mCherry empty vector alone (Figure 8, C and D).
Figure 8.
RhoG and PLEKHG6 are required for dorsal ruffle formation in A431 cells after EGF stimulation. A431 cells were transfected with control, RhoG-, or PLEKHG6-specific siRNAs. After 72 h, the cells were plated on fibronectin-coated coverslips (5 μg/ml). After 4 h of serum starvation, the cells were treated with 20 ng/ml EGF and DIC microscopy movies of individual cells were taken (10 s/frame; 480 s total). Cellular ruffling responses showed either a “dorsal ruffling phenotype” (A, top row, arrowheads indicate dorsal ruffles, also shown enlarged in the inset) or a “Rac-like ruffling phenotype” (A, bottom row). Bar, 20 μm. (B) Quantification of the occurrence of dorsal-ruffle phenotype versus Rac-like phenotype after transfecting the different siRNAs. Shown are averaged results from three independently performed experiments. Asterisks indicate significant difference compared with control siRNA transfected cells (Student's t test, p < 0.05 and p < 0.01, respectively). (C) HeLa cells were transfected with low amounts of expression constructs for mCherry or mCherry-PLEKHG6 and analyzed for dorsal ruffle formation after EGF treatment. Bars, 20 μm. (D) Quantification of the frequency of EGF induced dorsal ruffle formation of HeLa cells transfected with the indicated expression constructs. Shown are averaged results from three independently performed experiments. Asterisks indicate significant differences (Student's t test, p < 0.001).
In conclusion, multiple GEFs (Vav-GEFs and PLEKHG6) are involved in EGF-induced RhoG activation in a cell type-specific manner and dictate the resultant cellular phenotype.
RhoG Regulates EGF-induced Cell Migration and EGFR Internalization
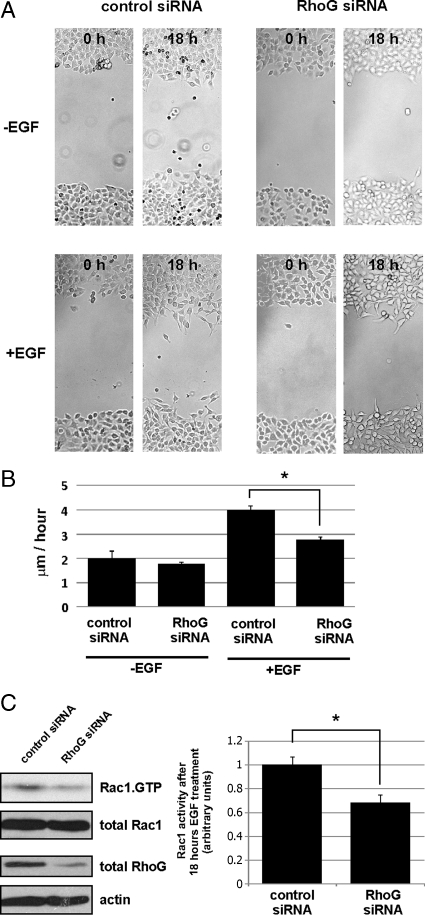
Recently, it was found that fibroblast growth factor (FGF)-induced cell migration is regulated by RhoG, whereas RhoG does not influence cell migration under nonstimulated conditions (Meller et al., 2008; Elfenbein et al., 2009). Therefore, we next tested whether EGF induced cell migration also requires RhoG. Interestingly, in wound healing assays we found that HeLa cells transfected with RhoG targeting siRNAs migrated significantly slower compared with control transfected cells. In contrast, under nonstimulated conditions, migration speed was not significantly changed (Figure 9, A and B). Importantly, the decrease in wound closure in RhoG knocked down cells was not due to decreased cell proliferation (Supplemental Figure S3). We showed earlier that rapid activation of RhoG and Rac1 after EGF treatment occurs independently of each other but that under nonstimulated conditions baseline Rac1 activity is increased by RhoG (Figure 3B). Because the wound healing assays extended over a long time (18 h), we wondered whether in contrast to the rapid activation mechanism, Rac1 activity after 18 h of EGF treatment is again influenced downstream from RhoG, similar to the nonstimulated conditions. Interestingly, we indeed found that after EGF treatment for 18 h, Rac1 activity was significantly reduced in cells that have been knocked down for RhoG (Figure 9C). This result suggests that the observed RhoG knockdown-dependent decrease in EGF-induced cell migration occurs as a result of decreased Rac1 activity.
Figure 9.
EGF-induced cell migration is regulated by RhoG. HeLa cells were transfected with the indicated siRNAs. The cell monolayers were starved before wounding and cultured with or without EGF. (A) Representative images of wounds immediately after wounding (0 h) or after 18 h. (B) Averaged migration speed of the cell fronts calculated from 36 image sets per condition (0 h/18 h) as described in Materials and Methods. Asterisks indicate significant differences between control and RhoG siRNA-transfected cells (Student's t test, p < 0.05). (C) Rac1 activity was determined in control or RhoG siRNA-transfected HeLa cells that were exposed to EGF containing media for 18 h. The bar graph on the right summarizes three different experiments. Asterisks indicate significant differences between control and RhoG siRNA-transfected cells (Student's t test, p < 0.05).
Cell migration is a process occurring in the range of minutes to hours. Because we focused in this study mainly on the rapid activation of RhoG within 30 s, we wondered whether other cellular processes with faster kinetics are influenced by the rapid activation of RhoG. Therefore, we tested whether short-term EGF signaling to the nucleus by MAPK signaling is affected by RhoG, Rac1, or their Vav regulators. However, we could not detect differences in pERK signaling after knockdown of RhoG, Rac1, or Vav2/3 (Supplemental Figure S4).
The process of EGF receptor internalization occurs very rapidly after EGF stimulation and its regulation is important for the precise control of EGF signaling (Carpenter, 2000). Rho GTPases have been implicated in modulating the efficiency of EGFR internalization (Lamaze et al., 1996; Kaneko et al., 2005). Therefore, we sought to determine whether EGFR internalization after EGF stimulation is regulated by RhoG and Rac1. EGFR internalization assays were performed using HeLa cells and Alexa488-conjugated EGF. Cells transfected with control siRNAs started to internalize EGFR within the first 2 min after shifting the cells to 37°C, and the amount of internalized EGFR continued to increase over 10 min (Figure 10A). Interestingly, cells transfected with siRNA against Rac1 or RhoG displayed a significantly decreased amount of internalized EGFR at all times. Building on our previous results showing that Vav2 and Vav3 are required for rapid activation of both RhoG and Rac1 in HeLa cells, we asked whether EGFR internalization was also reduced when Vav2 and Vav3 were simultaneously depleted. Knocking down Vav2 and Vav3 expression in HeLa cells resulted in a delayed and reduced amount of internalized EGFR (Figure 10B). We conclude that the activation of RhoG and Rac1 after EGF stimulation by the GEFs Vav2 and Vav3 regulates internalization of the EGFR.
Figure 10.
EGFR internalization is modulated by RhoG and Rac1. HeLa cells were transfected with the indicated siRNAs 72 h before the experiment (A: RhoG or Rac1; B: Vav2/Vav3). After binding of EGF-Alexa488 (1 μg/ml in PBS) for 1 h at 4°C to HeLa cells, EGFR internalization was induced by shifting the temperature to 37°C for the indicated times. The integrated fluorescence intensities of multiple cells from each transfection were measured and averaged. Error bars represent SEM. All measured time points were significantly different from the control (p < 0.001), except at 0 min.
DISCUSSION
RhoG has been implicated in various cellular activities, including phagocytosis, macropinocytosis, neurite outgrowth, apoptosis, and regulation of gene expression (Katoh et al., 2000; Murga et al., 2002; Vigorito et al., 2003; deBakker et al., 2004; Ellerbroek et al., 2004). Due to the broad diversity of these processes, which occur in a cell-type and tissue-specific manner, one would predict that multiple pathways regulate RhoG activity. However, RhoG upstream and downstream signaling pathways are to date poorly characterized. Only a few RhoG downstream effectors have been identified, such as the ELMO protein family, PI3K, phospholipase D1, and kinectin (Katoh et al., 2000; Vignal et al., 2001; Wennerberg et al., 2002; Yamaki et al., 2007). Regarding upstream regulation, a small number of RhoG GEFs are known (Vav proteins, SGEF, PLEKHG6, Trio, and Kalirin), but not a single cellular GAP has been described (Schuebel et al., 1998; Movilla and Bustelo, 1999; Blangy et al., 2000; May et al., 2002; Ellerbroek et al., 2004).
Compared with other Rho GTPases, only a few extracellular stimuli have been found to regulate RhoG activity. These include the cross-linking of ICAM-1 receptors on endothelial cells (van Buul et al., 2007) and syndecan-4 engagement by FGF (Elfenbein et al., 2009). Our results presented here reveal that EGF strongly activates RhoG (Figure 2A). EGF is a prominent growth factor influencing the behavior of many different tissues and cell types, including epithelial cells, smooth muscle cells, endothelial cells, and neural progenitor cells (Igura et al., 1996; Major and Keiser, 1997; Beier et al., 2008; Bertrand-Duchesne et al., 2009; Schwindt et al., 2009). Recently, FGF treatment of endothelial cells also was described to result in RhoG activation (Elfenbein et al., 2009). However, the kinetics of this differ strikingly from EGF-induced RhoG activation: In response to FGF treatment, RhoG activity peaks after 10 min, whereas the peak activation of RhoG in response to EGF is reached by 30 s (Figure 2A) and has returned almost to baseline levels by 10 min. EGF-induced RhoG activation also differs mechanistically from FGF-induced RhoG activation. Although EGF stimulation leads to rapid RhoG activation by activating GEFs in a PKC-independent manner (Figure 4B), syndecan-4 engagement by FGF results in a release of RhoG from GDI-1 by PKCα-mediated phosphorylation of GDI-1 (Elfenbein et al., 2009).
The observation that PDGF and VEGF did not stimulate rapid RhoG activation was striking given that, like EGF, both PDGF and VEGF induce Rac1 activation (Figure 1). Previous work has shown that the tyrosine kinase receptors for each of these growth factors activate the Vav family of GEFs (Pandey et al., 2000; Garrett et al., 2007; Takahashi et al., 2008). It was not expected that one growth factor would stimulate the Vav GEFs to activate both Rac1 and RhoG but that other growth factors would stimulate the same GEFs to activate only Rac1. A possible explanation for the EGF-specific activation of RhoG is the involvement of a receptor-specific scaffold, which promotes coupling of Vav GEFs to RhoG. Interestingly, after EGF and PDGF stimulation, 90% of the activated signaling proteins are identical and only 10% are unique to each growth factor (Kratchmarova et al., 2005). It will be interesting to look for the critical component(s) mediating EGF-induced, but not PDGF- or VEGF-induced, activation of RhoG. Similarly, it will be important to identify other tyrosine kinase receptors that activate RhoG.
Activation of Rac1 in response to EGF stimulation has been studied previously (Liu and Burridge, 2000; Beier et al., 2008; Dise et al., 2008; Itoh et al., 2008), and multiple different GEFs have been shown to be involved, including members of the Vav family, Tiam, Sos, and Asef (Scita et al., 1999; Marcoux and Vuori, 2003; Ray et al., 2007; Itoh et al., 2008). Even though no study has attempted to analyze comprehensively all these GEFs simultaneously and their interplay, it seems likely that Rac1 activation by EGF is controlled in a cell type- and cell function-dependent manner through these different GEFs. Our studies on RhoG activation after EGF stimulation support this concept, because we found different combinations of GEFs being involved in different epithelial cell types. Certainly, due to different GTPase specificities, this set of EGF-responsive RhoG GEFs (Vav2/Vav3 and PLEKHG6) has to be different from those found for Rac1.
We did not observe any influence of PI3K and Src-family kinase inhibition on either RhoG or Rac1 activation, and we have identified only the kinase activity of the EGFR itself as necessary for the rapid activation of RhoG and Rac1. This suggests that the rapid activation of both RhoG and Rac1 results from direct phosphorylation of the Vav GEFs by the EGFR kinase. Interestingly, the various studies implicating PI3K, Src, or both, have examined Rac1 activation at later times after EGF stimulation (Marignani and Carpenter, 2001; Tamas et al., 2003; Dise et al., 2008).
At the outset of this work, we did not anticipate finding a role for the GEF PLEKHG6 in the rapid activation of RhoG in response to EGF stimulation. PLEKHG6 was described previously as a GEF for RhoG, and to a lesser extent also for Rac1 (D'Angelo et al., 2007). We showed that in A431 cells PLEKHG6 together with Vav-family GEFs is involved in the rapid activation of RhoG after EGF stimulation (Figure 7, D and E), whereas EGF induced Rac1 activity is not influenced by this GEF. Furthermore, PLEKHG6 seems to be a critical factor that determines the cellular response after EGF treatment. We could convert nondorsal-ruffling HeLa cells to dorsally ruffling cells by expressing low levels of exogenous PLEKHG6 and stimulating with EGF. D'Angelo and coworkers found that PLEKHG6 acts as a scaffold, forming a ternary complex consisting of Ezrin, PLEKHG6, and RhoG (D'Angelo et al., 2007). Thus, it is possible that in addition to its function as a RhoG GEF, PLEKHG6 might also serve to localize RhoG to the plasma membrane, placing it in proximity with the EGFR. Experiments with cells expressing PLEKHG6 mutants that do not bind to Ezrin, but which retain GEF activity, could further clarify the role of PLEKHG6 in the rapid activation of RhoG in response to EGF stimulation.
EGF signaling affects many major cellular processes, including proliferation, survival and migration. RhoG has similarly been shown to contribute to the regulation of these activities (Katoh et al., 2000; Murga et al., 2002; Katoh et al., 2006; Yamaki et al., 2007; Elfenbein et al., 2009). Our results demonstrate that initial RhoG activation after EGF stimulation is followed by a rapid decline in activity (Figure 2A). For the regulation of most of the above-mentioned functions, this time course of RhoG activation seems to be too fast. Even though we found differences in the migration behavior of HeLa cells when RhoG was knocked down, this seemed to be more due to reduced Rac1 effects resulting from cross-talk between RhoG and Rac1. However, processes like dorsal ruffle formation and internalization of the EGFR occur within the time frame of rapid activation. EGFR internalization is an endocytic process and functional roles for Rho GTPases in endocytic processes are well accepted (Qualmann and Mellor, 2003). We have found that knocking down the expression of either RhoG or Rac1 decreases the internalization of the EGFR after EGF stimulation. Simultaneous knockdown of Vav2 and Vav3 also decreases EGFR endocytosis. Even though clathrin-mediated endocytosis seems to be the major pathway of EGFR internalization, clathrin-independent endocytosis also has been reported (Osherov and Levitzki, 1994; Sigismund et al., 2005; Zhu et al., 2005). Our experiments did not address which endocytic entry route of EGFR is affected by knocking down RhoG or Rac1, or their regulators Vav2/Vav3. Both these GTPases and the Vav GEFs have been implicated in different endocytotic pathways previously. For example, RhoG is known to stimulate macropinocytosis and caveolar endocytosis (Prieto-Sanchez et al., 2006), whereas clathrin-independent interleukin-2 receptor internalization was shown to be stimulated by Rac1 (Grassart et al., 2008). While this work was in revision, B cell receptor internalization after agonist stimulation was found to depend on Vav GEFs and Rac GTPases (Malhotra et al., 2009). All these examples argue for clathrin-independent mechanisms by which RhoG and Rac1 may affect EGFR internalization. One possible link between RhoG and Rac1 and multiple different endocytic mechanisms is the RhoG and Rac1 effector phospholipase D1 (Wennerberg et al., 2002), which was recently shown to stimulate EGFR internalization by activating dynamin (Lee et al., 2006). Determining how RhoG and Rac1 regulate the internalization route will be important, because it was recently suggested that the fate of internalized EGFR (degradation vs. recycling) is determined by the route of entry, more precisely, if it is mediated via a clathrin-dependent or independent pathway (Sigismund et al., 2008).
In summary, we demonstrate here that RhoG is rapidly activated by EGF through GEFs of the Vav family and, depending on the cell type, through the GEF PLEKHG6. Importantly, the specific combination of available GEFs in a given cell type determines the cellular response (i.e., spreading vs. dorsal ruffling). Specifically, we observe that the EGF-induced ruffling behavior changes when particular GEFs are depleted or added. Functionally, we found that RhoG contributes to EGF-stimulated cell migration in wound healing assays and furthermore that both RhoG and Rac1 and their regulators of the Vav family regulate early dynamics of EGFR endocytosis. Future work is required to clarify whether RhoG and Rac1 influence specific endocytotic entry routes of the EGFR (clathrin dependent or independent).
Supplementary Material
ACKNOWLEDGMENTS
We thank Anja Samson, Erika Wittchen, and John Brumell for comments and critical reading of the manuscript and Lisa Sharek for great technical assistance. We thank M. Schwartz for providing the anti-RhoG antibody. This work was supported by National Institute of Health grants HL-080166, GM-029860, and GM-057464. T. S. was supported by a fellowship from the American Heart Association (0825379E). Fellowships from the National Institute of Health support C. W. (F30HL094020-02 and T32-GM-80079) and from the American Cancer Society support E. M.-B. (PF-09-119-01-CSM).
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-09-0809) on March 17, 2010.
REFERENCES
- Araki N., Egami Y., Watanabe Y., Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp. Cell Res. 2007;313:1496–1507. doi: 10.1016/j.yexcr.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Beier I., Dusing R., Vetter H., Schmitz U. Epidermal growth factor stimulates Rac1 and p21-activated kinase in vascular smooth muscle cells. Atherosclerosis. 2008;196:92–97. doi: 10.1016/j.atherosclerosis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bertrand-Duchesne M. P., Grenier D., Gagnon G. Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferation in vitro. J. Periodontal Res. 2010;45:87–93. doi: 10.1111/j.1600-0765.2009.01205.x. [DOI] [PubMed] [Google Scholar]
- Bishop A. L., Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Blangy A., Vignal E., Schmidt S., Debant A., Gauthier-Rouviere C., Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J. Cell Sci. 2000;113:729–739. doi: 10.1242/jcs.113.4.729. [DOI] [PubMed] [Google Scholar]
- Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- D'Angelo R., Aresta S., Blangy A., Del Maestro L., Louvard D., Arpin M. Interaction of ezrin with the novel guanine nucleotide exchange factor PLEKHG6 promotes RhoG-dependent apical cytoskeleton rearrangements in epithelial cells. Mol. Biol. Cell. 2007;18:4780–4793. doi: 10.1091/mbc.E06-12-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBakker C. D., et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr. Biol. 2004;14:2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Dise R. S., Frey M. R., Whitehead R. H., Polk D. B. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G276–G285. doi: 10.1152/ajpgi.00340.2007. [DOI] [PubMed] [Google Scholar]
- Dovas A., Couchman J. R. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfenbein A., Rhodes J. M., Meller J., Schwartz M. A., Matsuda M., Simons M. Suppression of RhoG activity is mediated by a syndecan 4-synectin-RhoGDI1 complex and is reversed by PKCalpha in a Rac1 activation pathway. J. Cell Biol. 2009;186:75–83. doi: 10.1083/jcb.200810179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbroek S. M., Wennerberg K., Arthur W. T., Dunty J. M., Bowman D. R., DeMali K. A., Der C., Burridge K. SGEF, a RhoG guanine nucleotide exchange factor that stimulates macropinocytosis. Mol. Biol. Cell. 2004;15:3309–3319. doi: 10.1091/mbc.E04-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. M. Structure-based view of epidermal growth factor receptor regulation. Annu. Rev. Biophys. 2008;37:353–373. doi: 10.1146/annurev.biophys.37.032807.125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R., Wennerberg K., Arthur W. T., Noren N. K., Ellerbroek S. M., Burridge K. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- Garrett T. A., Van Buul J. D., Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp. Cell Res. 2007;313:3285–3297. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J., Gutkind J. S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Grassart A., Dujeancourt A., Lazarow P. B., Dautry-Varsat A., Sauvonnet N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 2008;9:356–362. doi: 10.1038/embor.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakeda-Suzuki S., Ng J., Tzu J., Dietzl G., Sun Y., Harms M., Nardine T., Luo L., Dickson B. J. Rac function and regulation during Drosophila development. Nature. 2002;416:438–442. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Igura T., et al. Expression of heparin-binding epidermal growth factor-like growth factor in neointimal cells induced by balloon injury in rat carotid arteries. Arterioscler. Thromb. Vasc. Biol. 1996;16:1524–1531. doi: 10.1161/01.atv.16.12.1524. [DOI] [PubMed] [Google Scholar]
- Itoh R. E., Kiyokawa E., Aoki K., Nishioka T., Akiyama T., Matsuda M. Phosphorylation and activation of the Rac1 and Cdc42 GEF Asef in A431 cells stimulated by EGF. J. Cell Sci. 2008;121:2635–2642. doi: 10.1242/jcs.028647. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jones J. T., Akita R. W., Sliwkowski M. X. Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett. 1999;447:227–231. doi: 10.1016/s0014-5793(99)00283-5. [DOI] [PubMed] [Google Scholar]
- Kaneko T., et al. Rho mediates endocytosis of epidermal growth factor receptor through phosphorylation of endophilin A1 by Rho-kinase. Genes Cells. 2005;10:973–987. doi: 10.1111/j.1365-2443.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- Katoh H., Hiramoto K., Negishi M. Activation of Rac1 by RhoG regulates cell migration. J. Cell Sci. 2006;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- Katoh H., Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein Elmo. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- Katoh H., Yasui H., Yamaguchi Y., Aoki J., Fujita H., Mori K., Negishi M. Small GTPase RhoG is a key regulator for neurite outgrowth in PC12 cells. Mol. Cell Biol. 2000;20:7378–7387. doi: 10.1128/mcb.20.19.7378-7387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratchmarova I., Blagoev B., Haack-Sorensen M., Kassem M., Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- Lamaze C., Chuang T. H., Terlecky L. J., Bokoch G. M., Schmid S. L. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Landau M., Ben-Tal N. Dynamic equilibrium between multiple active and inactive conformations explains regulation and oncogenic mutations in ErbB receptors. Biochim. Biophys. Acta. 2008;1785:12–31. doi: 10.1016/j.bbcan.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Kim I. S., Park J. B., Lee M. N., Lee H. Y., Suh P. G., Ryu S. H. The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat. Cell Biol. 2006;8:477–484. doi: 10.1038/ncb1401. [DOI] [PubMed] [Google Scholar]
- Liu B. P., Burridge K. Vav2 activates Rac1, Cdc42, and RhoA downstream from growth factor receptors but not beta1 integrins. Mol. Cell Biol. 2000;20:7160–7169. doi: 10.1128/mcb.20.19.7160-7169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major T. C., Keiser J. A. Inhibition of cell growth: effects of the tyrosine kinase inhibitor CGP 53716. J. Pharmacol. Exp. Ther. 1997;283:402–410. [PubMed] [Google Scholar]
- Malhotra S., Kovats S., Zhang W., Coggeshall K. M. Vav and Rac activation in B cell antigen receptor endocytosis involves Vav recruitment to the adapter protein LAB. J. Biol. Chem. 2009;284:36202–36212. doi: 10.1074/jbc.M109.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux N., Vuori K. EGF receptor mediates adhesion-dependent activation of the Rac GTPase: a role for phosphatidylinositol 3-kinase and Vav2. Oncogene. 2003;22:6100–6106. doi: 10.1038/sj.onc.1206712. [DOI] [PubMed] [Google Scholar]
- Marignani P. A., Carpenter C. L. Vav2 is required for cell spreading. J. Cell Biol. 2001;154:177–186. doi: 10.1083/jcb.200103134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V., Schiller M. R., Eipper B. A., Mains R. E. Kalirin Dbl-homology guanine nucleotide exchange factor 1 domain initiates new axon outgrowths via RhoG-mediated mechanisms. J. Neurosci. 2002;22:6980–6990. doi: 10.1523/JNEUROSCI.22-16-06980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller J., Vidali L., Schwartz M. A. Endogenous RhoG is dispensable for integrin-mediated cell spreading but contributes to Rac-independent migration. J. Cell Sci. 2008;121:1981–1989. doi: 10.1242/jcs.025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S. Y., Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Movilla N., Bustelo X. R. Biological and regulatory properties of Vav-3, a new member of the Vav family of oncoproteins. Mol. Cell Biol. 1999;19:7870–7885. doi: 10.1128/mcb.19.11.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murga C., Zohar M., Teramoto H., Gutkind J. S. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene. 2002;21:207–216. doi: 10.1038/sj.onc.1205036. [DOI] [PubMed] [Google Scholar]
- Oda K., Matsuoka Y., Funahashi A., Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005;1 doi: 10.1038/msb4100014. 2005 0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherov N., Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur. J. Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- Pandey A., Podtelejnikov A. V., Blagoev B., Bustelo X. R., Mann M., Lodish H. F. Analysis of receptor signaling pathways by mass spectrometry: identification of vav-2 as a substrate of the epidermal and platelet-derived growth factor receptors. Proc. Natl. Acad. Sci. USA. 2000;97:179–184. doi: 10.1073/pnas.97.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. C., Galan J. E. Differential activation and function of Rho GTPases during Salmonella-host cell interactions. J. Cell Biol. 2006;175:453–463. doi: 10.1083/jcb.200605144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Rosenfeldt H. M., Lyons R., Servitja J. M., Bustelo X. R., Siroff M., Gutkind J. S. Persistent activation of Rac1 in squamous carcinomas of the head and neck: evidence for an EGFR/Vav2 signaling axis involved in cell invasion. Carcinogenesis. 2007;28:1145–1152. doi: 10.1093/carcin/bgm008. [DOI] [PubMed] [Google Scholar]
- Prieto-Sanchez R. M., Berenjeno I. M., Bustelo X. R. Involvement of the Rho/Rac family member RhoG in caveolar endocytosis. Oncogene. 2006;25:2961–2973. doi: 10.1038/sj.onc.1209333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B., Mellor H. Regulation of endocytic traffic by Rho GTPases. Biochem. J. 2003;371:233–241. doi: 10.1042/BJ20030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R. M., Vaidya R. J., Johnson L. R. MEK/ERK regulates adherens junctions and migration through Rac1. Cell Motil. Cytoskeleton. 2007;64:143–156. doi: 10.1002/cm.20172. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues G. A., Falasca M., Zhang Z., Ong S. H., Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell Biol. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roppenser B., Roder A., Hentschke M., Ruckdeschel K., Aepfelbacher M. Yersinia enterocolitica differentially modulates RhoG activity in host cells. J. Cell Sci. 2009;122:696–705. doi: 10.1242/jcs.040345. [DOI] [PubMed] [Google Scholar]
- Rossman K. L., Der C. J., Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Sasaoka T., Langlois W. J., Leitner J. W., Draznin B., Olefsky J. M. The signaling pathway coupling epidermal growth factor receptors to activation of p21ras. J. Biol. Chem. 1994;269:32621–32625. [PubMed] [Google Scholar]
- Schuebel K. E., Movilla N., Rosa J. L., Bustelo X. R. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt T. T., Motta F. L., Gabriela F. B., Cristina G. M., Guimaraes A. O., Calcagnotto M. E., Pesquero J. B., Mello L. E. Effects of FGF-2 and EGF removal on the differentiation of mouse neural precursor cells. An. Acad. Bras. Cienc. 2009;81:443–452. doi: 10.1590/s0001-37652009000300009. [DOI] [PubMed] [Google Scholar]
- Scita G., Nordstrom J., Carbone R., Tenca P., Giardina G., Gutkind S., Bjarnegard M., Betsholtz C., Di Fiore P. P. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- Sigismund S., Argenzio E., Tosoni D., Cavallaro E., Polo S., Di Fiore P. P. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev. Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton R. A., Schaefer E., Schwartz M. A. p21-activated kinase regulates endothelial permeability through modulation of contractility. J. Biol. Chem. 2004;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Rikitake Y., Nagamatsu Y., Hara T., Ikeda W., Hirata K., Takai Y. Sequential activation of Rap1 and Rac1 small G proteins by PDGF locally at leading edges of NIH3T3 cells. Genes Cells. 2008;13:549–569. doi: 10.1111/j.1365-2443.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- Tamas P., Solti Z., Bauer P., Illes A., Sipeki S., Bauer A., Farago A., Downward J., Buday L. Mechanism of epidermal growth factor regulation of Vav2, a guanine nucleotide exchange factor for Rac. J. Biol. Chem. 2003;278:5163–5171. doi: 10.1074/jbc.M207555200. [DOI] [PubMed] [Google Scholar]
- Turner M., Billadeau D. D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- van Buul J. D., Allingham M. J., Samson T., Meller J., Boulter E., Garcia-Mata R., Burridge K. RhoG regulates endothelial apical cup assembly downstream from ICAM1 engagement and is involved in leukocyte trans-endothelial migration. J. Cell Biol. 2007;178:1279–1293. doi: 10.1083/jcb.200612053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignal E., Blangy A., Martin M., Gauthier-Rouviere C., Fort P. Kinectin is a key effector of RhoG microtubule-dependent cellular activity. Mol. Cell Biol. 2001;21:8022–8034. doi: 10.1128/MCB.21.23.8022-8034.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito E., Bell S., Hebeis B. J., Reynolds H., McAdam S., Emson P. C., McKenzie A., Turner M. Immunological function in mice lacking the Rac-related GTPase RhoG. Mol. Cell Biol. 2004;24:719–729. doi: 10.1128/MCB.24.2.719-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito E., Billadeu D. D., Savoy D., McAdam S., Doody G., Fort P., Turner M. RhoG regulates gene expression and the actin cytoskeleton in lymphocytes. Oncogene. 2003;22:330–342. doi: 10.1038/sj.onc.1206116. [DOI] [PubMed] [Google Scholar]
- Wennerberg K., Ellerbroek S. M., Liu R. Y., Karnoub A. E., Burridge K., Der C. J. RhoG signals in parallel with Rac1 and Cdc42. J. Biol. Chem. 2002;277:47810–47817. doi: 10.1074/jbc.M203816200. [DOI] [PubMed] [Google Scholar]
- Wherlock M., Mellor H. The Rho GTPase family: a Racs to Wrchs story. J. Cell Sci. 2002;115:239–240. doi: 10.1242/jcs.115.2.239. [DOI] [PubMed] [Google Scholar]
- Yamaki N., Negishi M., Katoh H. RhoG regulates anoikis through a phosphatidylinositol 3-kinase-dependent mechanism. Exp. Cell Res. 2007;313:2821–2832. doi: 10.1016/j.yexcr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Zeng L., Sachdev P., Yan L., Chan J. L., Trenkle T., McClelland M., Welsh J., Wang L. H. Vav3 mediates receptor protein tyrosine kinase signaling, regulates GTPase activity, modulates cell morphology, and induces cell transformation. Mol. Cell Biol. 2000;20:9212–9224. doi: 10.1128/mcb.20.24.9212-9224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. X., Goldoni S., Bix G., Owens R. T., McQuillan D. J., Reed C. C., Iozzo R. V. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J. Biol. Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.