Abstract
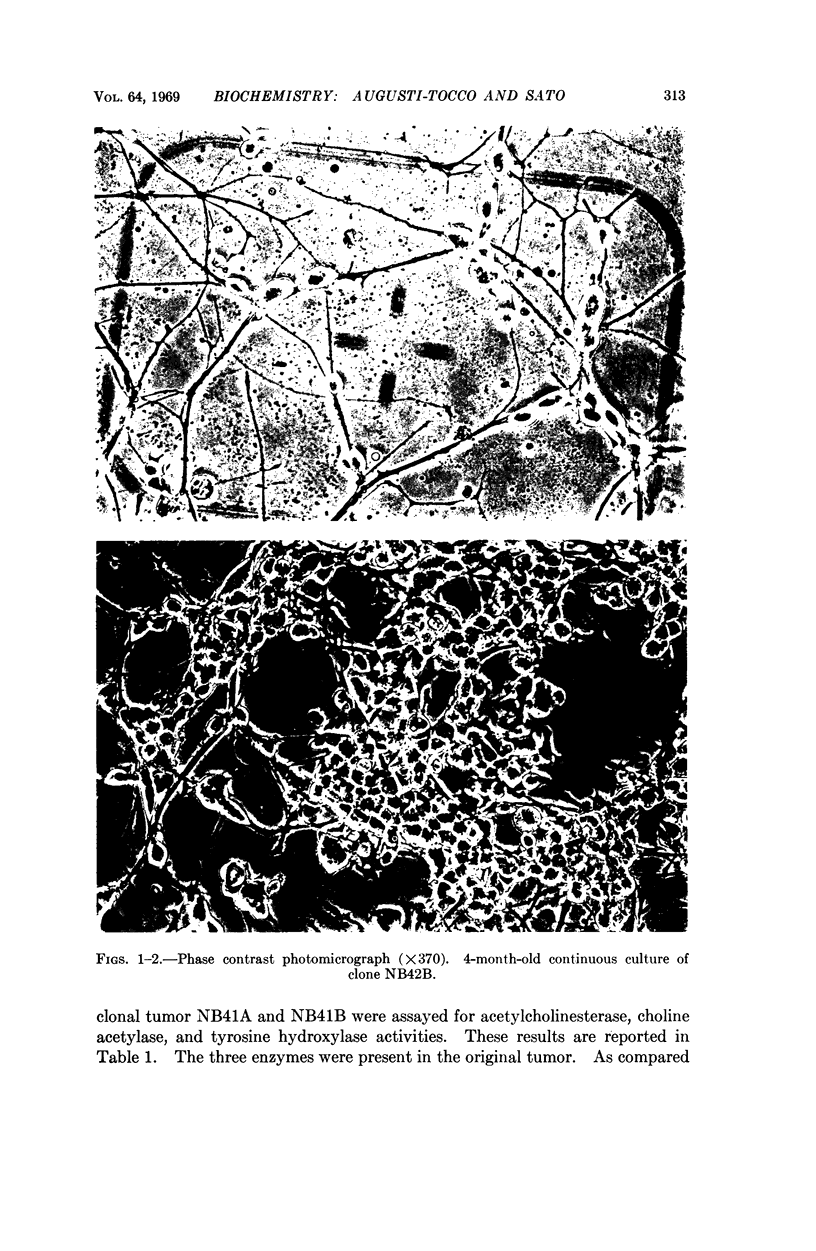
Clonal lines of neurons were obtained in culture from a mouse neuroblastoma. The neuroblastoma cells were adapted to culture growth by the animal-culture alternate passage technique and cloned after single-cell plating. The clonal lines retained the ability to form tumors when injected back into mice. A striking morphological change was observed in the cells adapted to culture growth; they appeared as mature neurons, while the cells of the tumor appeared as immature neuroblasts.
Acetylcholinesterase and the enzymes for the synthesis of neurotransmitters, cholineacetylase and tyrosine hydroxylase were assayed in the tumor and compared with brain levels; tyrosine hydroxylase was found to be particularly high, as described previously in human neuroblastomas. The three enzymes were found in the clonal cultures at levels comparable to those found in the tumors. Similarly, there were no remarkable differences between the three clones examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUONASSISI V., SATO G., COHEN A. I. Hormone-producing cultures of adrenal and pituitary tumor origin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1184–1190. doi: 10.1073/pnas.48.7.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. ACETYLCHOLINE IN ADRENERGIC TRANSMISSION. Annu Rev Pharmacol. 1965;5:163–182. doi: 10.1146/annurev.pa.05.040165.001115. [DOI] [PubMed] [Google Scholar]
- Benda P., Lightbody J., Sato G., Levine L., Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968 Jul 26;161(3839):370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- Cremer J. E., Johnston P. V., Roots B. I., Trevor A. J. Heterogeneity of brain fractions containing neuronals and glial cells. J Neurochem. 1968 Nov;15(11):1361–1370. doi: 10.1111/j.1471-4159.1968.tb05915.x. [DOI] [PubMed] [Google Scholar]
- De Jong R. H., Robles R., Morikawa K. I. Gallamine (Flaxedil) and synaptic transmission in the spinal cord. Science. 1968 May 17;160(3829):768–769. doi: 10.1126/science.160.3829.768. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN M. N., BURDMAN J. A., JOURNEY L. J. LONG-TERM TISSUE CULTURE OF NEUROBLASTOMAS. II. MORPHOLOGIC EVIDENCE FOR DIFFERENTIATION AND MATURATION. J Natl Cancer Inst. 1964 Jan;32:165–199. [PubMed] [Google Scholar]
- GOLDSTEIN M. N., PINKEL D. Longterm tissue culture of neuroblastomas. J Natl Cancer Inst. 1958 Apr;20(4):675–689. [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- HYDEN H. Quantitative assay of compounds in isolated, fresh nerve cells and glial cells from control and stimulated animals. Nature. 1959 Aug 8;184:433–435. doi: 10.1038/184433a0. [DOI] [PubMed] [Google Scholar]
- LABROSSE E. H., BELEHRADEK J., BARSKI G., BOHUON C., SCHWEISGUTH O. METABOLIC ACTIVITY OF NEURAL CREST TUMOURS IN TISSUE CULTURE. Nature. 1964 Jul 11;203:195–196. doi: 10.1038/203195b0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOTS B. I., JOHNSTON P. V. NEURONS OF OX BRAIN NUCLEI: THEIR ISOLATION AND APPEARANCE BY LIGHT AND ELECTRON MICROSCOPY. J Ultrastruct Res. 1964 Apr;10:350–361. doi: 10.1016/s0022-5320(64)80014-9. [DOI] [PubMed] [Google Scholar]
- Rose S. P. Preparation of enriched fractions from cerebral cortex containing isolated, metabolically active neuronal and glial cells. Biochem J. 1967 Jan;102(1):33–43. doi: 10.1042/bj1020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Abe S. Preparation and characterization of nerve cell perikaryon from rat cerebral cortex. J Biochem. 1966 Jan;59(1):72–75. doi: 10.1093/oxfordjournals.jbchem.a128261. [DOI] [PubMed] [Google Scholar]