Abstract
Introduction
Microgravity animal models have demonstrated corticospinal plasticity; however, little is understood of its functional significance. In this pilot study, we explored corticospinal plasticity in a bed rest model. We hypothesized that the lack of weight bearing would induce cortical reorganization correlating with performance.
Methods
Four subjects underwent functional MRI (fMRI), transcranial magnetic stimulation (TMS), and functional mobility testing (FMT) before and after 90 d of bed rest. Recruitment curves (RC) were created by measuring motor evoked potentials over a range of TMS intensities with changes in the slope of the RC reflecting changes in corticospinal excitability.
Results
Significant leg RC slope decreases were observed on post-bed rest day 1 (P1) (t(2805) = −4.14, P < 0.0001), P2 (t(2805) = −6.59, P < 0.0001), P3 (t(2805) = −6.15, P < 0.0001), P5 (t(2805) = −7.93, P < 0.0001), P8 (t(2805) = −3.30, P = 0.001), and P12 (t(2805)= −3.33, P = 0.0009), suggesting a group decrease in corticospinal excitability in the immediate post-bed rest period with recovery approaching baseline over the following 2 wk. Significant effects were observed for hand RC slopes only for P2 (t(2916) = 1.97, P = 0.049), P3 (t(2916) = −2.12, P = 0.034), and P12 (t(2916) = −2.19, P = 0.029); no significant effects were observed for days P0 (t(2916) = −1.32, ns), P1 (t(2916) = 1.00, ns), P5 (t(2916) = −0.21, ns), or P8 (t(2916) = −0.27, ns). fMRI showed no change in activation for the hand but an increase in activation post-bed rest for the leg. On an individual basis, a more heterogeneous response was found which showed a potential association with performance on FMT.
Discussion
Results of this research include a better understanding of the cortical plasticity associated with leg disuse and may lead to applications in patient and astronaut rehabilitation.
Keywords: functional MRI (fMRI), transcranial magnetic stimulation (TMS), motor, performance
Research on the effects of microgravity on thecerebral cortex using animal models (2, 10, 13) has demonstrated changes in neurotransmitter concentrations and neural architecture plasticity, however, little is understood of the functional significance of such neural plasticity. In the sensorimotor system, alterations in motor cortex excitability have been shown to occur in a model of disuse that may be relevant to microgravity (lower extremity immobilization) (31). Alterations in corticospinal excitability have also been demonstrated in humans undergoing parabolic flight (6). It is likely that cortical motor reorganization occurs as an adaptive response to the microgravity environment. Functional cortical reorganization can be seen as a form of motor learning following, for example, the practicing of a complex motor task or during periods of adjustment to altered sensory inputs (5, 35). Even though normal movement clearly involves vestibular systems along with cerebellar control, motor cortical areas are the final common pathway for control of movement and therefore play a pivotal role in adaptation to microgravity. Although this adaptation may be useful in a microgravity environment, it may not be optimal for crew returning to Earth, operations on a planetary surface, or in the case of an emergency landing where optimal motor performance is required for safe crew egress. The identification and proper understanding of cortical plasticity in microgravity and its relationship to astronaut performance is a critical step in preparing for future long-term human space habitation. Additional applications include the rehabilitation of patients suffering from brain injury or prolonged confinement to bed.
In this pilot study, we used functional magnetic resonance imaging (fMRI) and transcranial magnetic stimulation (TMS) to explore corticospinal plasticity in a long-term bed rest model. TMS allows noninvasive, painless stimulation and evaluation of the cortex through the intact human skull using time-varying magnetic fields. TMS has been shown to be a useful method for motor cortex identification (30) and its safety has been established in both animal and human studies (39). We hypothesized that alterations in afferent signaling, as a result of lack of lower extremity weight bearing, would induce cortical reorganization by altering corticospinal excitability. This would be reflected in changes in the neurophysiological properties of the lower extremity motor cortex as measured by TMS-derived recruitment curves and by fMRI motor activation. We further hypothesized that the degree of change in corticospinal excitability would correlate with performance on a test of functional mobility.
METHODS
In order to evaluate corticospinal plasticity induced by long-term bed rest, four experimental subjects (two men and two women, all right-handed, mean age 35.5 yr, range 27 to 44 yr) underwent TMS to assess motor cortex excitability before and after 90 d of bed rest. Subjects also underwent functional mobility testing (FMT) as a NASA standard measure of motor performance. Additionally, subjects underwent functional brain imaging, fMRI, to further assess motor cortex activation changes induced by long-term bed rest. This research was performed as part of a multi-investigator study and therefore all subjects underwent additional experimental procedures reported elsewhere. The bed rest study was carried out within the University of Texas Medical Branch GCRC and the subjects were monitored continuously throughout to ensure compliance. Diet was strictly controlled. Written signed informed consent was obtained from all subjects and the study was approved by the Medical University of South Carolina Institutional Review Board, the University of Texas Medical Branch Institutional Review Board, and the Johnson Space Center Committee for the Protection of Human Subjects.
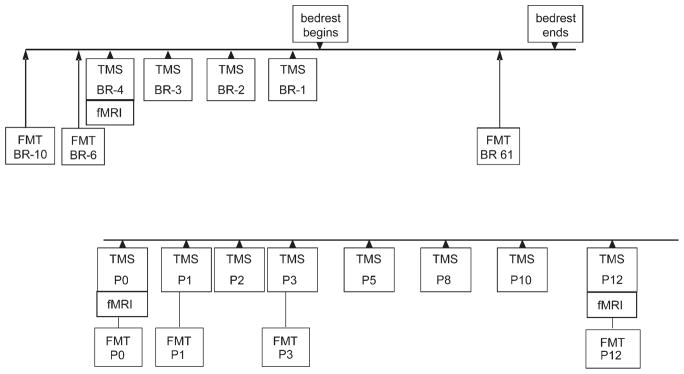
In order to establish baseline measurements of corticospinal excitability and motor performance, TMS, fMRI, and FMT were performed on the subjects before bed rest (Fig. 1). TMS was performed on all subjects for 4 d immediately before bed rest (BR − 4, BR − 3, BR − 2, BR − 1) and fMRI on day BR − 4. FMT was performed on BR − 10 and BR − 6. All subjects were then placed at bed rest in a 6° head-down position. FMT was also performed on day 61 of bed rest. Following 90 d of bed rest (post bed rest), on day P0, fMRI and TMS were again performed. Before and during the TMS session on day P0, the subjects were kept in the head down position without lower extremity weight bearing. Immediately following this TMS session, the subjects underwent FMT testing (also day P0) and then were allowed to return to normal activity, but remained at the GCRC for an additional 2 wk. During these 2 wk, TMS sessions were repeated on post bed rest days P1, P2, P3, P5, P8, P10, and P12. fMRI was performed on day P12. FMT was performed on P1, P3, and P12.
Fig. 1.
Timeline of study showing the data collection points for transcranial magnetic stimulation (TMS), functional MRI (fMRI), and functional mobility testing (FMT).
TMS
All TMS sessions included resting motor threshold (rMT) determination followed by the acquisition of recruitment curves (RC) for the right hand and leg. The rMT is defined as the minimal magnetic pulse causing a contraction of the target muscle as detected by electromyography (EMG) recordings of the motor evoked potential (MEP). The rMT value was then used to set TMS intensities for obtaining recruitment curves. Recruitment curves are created by measuring the MEP amplitudes in a target muscle over a range of TMS intensities. As the TMS output is increased, MEPs of increasing amplitude are obtained until a plateau level is reached (7). Changes in the slope of the RC reflect changes in corticospinal excitability and have been used to detect changes in motor cortex output induced for example by ischemia or amputation (28).
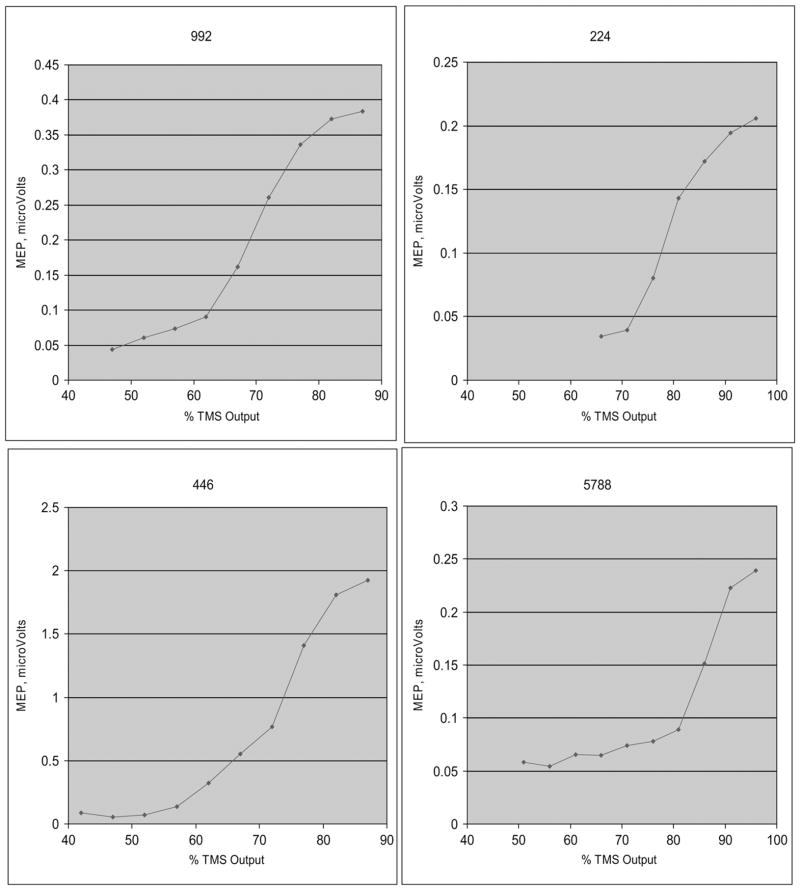
All TMS sessions were performed using MRI guidance. For TMS, the subjects were positioned supine with their hands and legs fully relaxed as verified by EMG recordings. Surface electrodes were placed along the right abductor pollicis brevis and the right medial gastrocnemius muscle bellies. In all subjects for each session, the leg was tested first followed by testing of the hand. The peak-to-peak amplitudes of the MEPs were recorded using equipment and software from Cambridge Electronic Design, the signal was converted from analog to digital using the Micro 1401 MK II and conditioned using the CED1902 signal conditioner (Cambridge Electronic Design, Cambridge, UK). For cortical stimulation, a TMS device with a figure-of-eight coil (hand) or a double coned coil (leg) was used (Magstim 200 2, The Magstim Company Limited, Whitland, South West Wales, UK). For the first TMS session, the optimal location for stimulus induction (the location that gave the maximum MEP amplitude) for each muscle was identified and the coil was secured in place by a holder. For hand activation using the figure-of-eight coil, the coil was oriented such that the handle was pointed occipitally (monophasic, posteriorly directed current). During subsequent sessions, the TMS coil was positioned using a neuronavigational guidance system (Brainsight™, Rogue Research Inc., Montreal, PQ, Canada). The stereotactic guidance system consisted of trackers attached to the TMS coil and to the subject’s head which could be detected by an optical position sensor. Following a calibration procedure, these trackers were monitored by the software that displayed the position and orientation of the coil on the subject’s anatomical MRI scan. This technique facilitated repeated positioning of the TMS coil over the subject’s brain at the same location identified during the first session. At this location, the rMT was determined as the intensity needed to evoke a MEP in the relaxed muscle of more than 50 μV in 5 out of 10 consecutive trials. RCs were then obtained. Ten stimuli were delivered at each of eight intensities ranging from five points below the rMT and increasing by five increments (% maximum machine output: −5, 0, + 5, + 10, + 15, + 20, + 25, + 30, + 35, + 40) or until 100% of stimulator output was reached. The stimulus intensities were randomized throughout the RC acquisition. This procedure was performed during rest which was assured by continuous monitoring of the EMG by software control such that no TMS pulse was administered if the EMG amplitude was above 10 μV (Fig. 2).
Fig. 2.
Examples of the leg recruitment curves. These curves were acquired during the pre-bed rest period on day BR −2.
Compound Motor Action Potential (CMAP)
For the hand, MEP amplitudes following supramaximal stimulation of the median nerve at the wrist were acquired. Next, for the leg, MEP amplitudes following supramaximal stimulation of the tibial nerve within the popliteal fossa were also acquired. These were acquired both before and after bed rest.
FMT
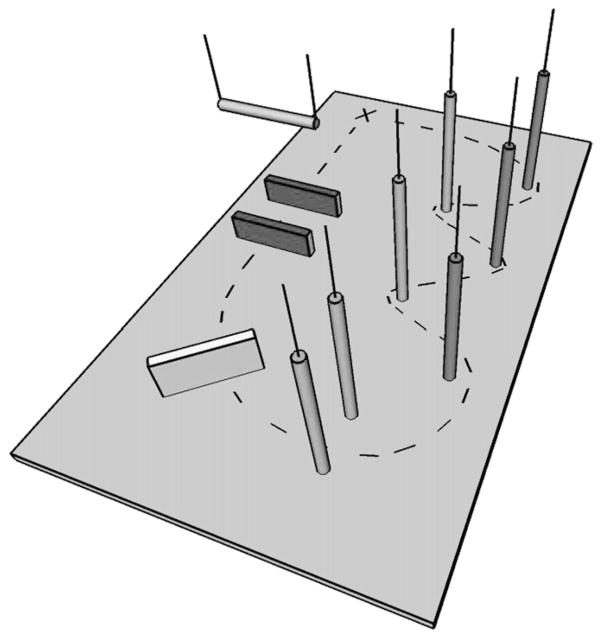
The FMT is part of the NASA Standard Measures and was conducted by NASA. The FMT is an obstacle course set up on a base of 10-cm thick medium density foam (Sunmate Foam, Dynamic Systems, Inc., Leicester, NC). The foam base introduces a proprioceptive challenge into the otherwise normal walking task. The 6.0 m × 4.0 m course consisted of five vertical foam pylons arranged in a slalom fashion, a 46-cm foam hurdle, a “portal”, and a “gate” (Fig. 3). The portal obstacle was comprised of two successive 31-cm foam hurdles with a horizontal foam bar suspended between them. The horizontal foam bar was adjusted to the height of each subject’s shoulders. The portal required subjects to step over hurdles while bending at the waist to duck under the horizontal foam bar. The gate obstacle was comprised of two vertical foam pylons adjusted to each subject’s shoulder width, requiring the subject to “ squeeze ” through the pylons without touching them. Subjects walked in bare feet or socks at a preferred pace through the course, beginning and ending each trial at a start/finish line marked on the foam floor. They were instructed to complete the course as quickly as possible without running and without touching any of the obstacles. This task was performed three times in the clockwise direction and three times in the counterclockwise direction in a randomized order for a total of six trials during any given session. The dependent measure for each trial is time to complete the course measured in seconds.
Fig. 3.
Diagram of the functional mobility testing (FMT) obstacle course.
fMRI
Also, changes in motor brain activity were explored using functional brain imaging of these four subjects before and after bed rest. Cortical control of finger movement and of leg movement was assessed using fMRI performed pre-bed rest (BR − 4), immediately post-bed rest on the day when subjects returned to normal activity (P0), and at the end of the 2-wk bed rest recovery period (P12) (15). A standard alternating block design was employed (30 s rest, 30 s task, 6 min total). Participants performed a random-pattern finger tapping task in one scanning trial and a random-pattern foot movement in another trial. Functional images were acquired on a 1.5T scanner (GE Medical Systems, Milwaukee, WI) using the following parameters: TR = 3000 ms, TE = 60 ms, 120 volumes, 8 transverse slices per volume with 5 mm thickness and 7 mm gap, slice dimension of 64 × 64 with 3.44 × 3.44 mm resolution. The top slice was positioned at the brain vertex to provide coverage of the motor cortex.
Data analysis was completed using FEAT (FMRI Expert Analysis Tool) Version 5.63, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Prestatistics processing included motion correction, spatial smoothing using a Gaussian kernel of FWHM 9 mm, intensity normalization, and highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 30.0 s). A standard time-series statistical analysis was specified based on the block design, with statistical threshold set using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05. A percent signal change (PSC) was calculated from rest intensity to task intensity, using values extracted at the voxel of maximum Z statistic.
Data Analysis
For statistical analysis of the group recruitment curve data, we used Hierarchical Linear Modeling (HLM) to assess the effects of time (pre-bed rest versus post-bed rest) on recruitment curves. HLM has been shown to appropriately handle nested models with serially dependent data points (3, 4) and it allows for modeling of variables at the individual subject-level (e.g., each subject’s individual recruitment curves), at the broader organizational level to which each individual is assigned, and with respect to effects nested within individuals (pre-bed rest versus post-bed rest).
MEP values that comprised the recruitment curves were log-transformed to correct for nonlinearity and non-normality of the curves for statistical analyses. The linearity of the transformed recruitment curves and the normality of the residuals were verified by fitting an ordinary least squares model and examining normal probability plots in SPSS. For HLM analysis, “ proc mixed ” was used in SAS (37), and the estimation method of the model was Restricted Maximum Likelihood (REML). The covariance structure was “ Unstructured. ” Intercepts at the individual subject level were entered into the model as effects at level-1.
HLM is a powerful method for evaluating changes in recruitment curves over time. Its power to detect significant changes is affected by a number of factors but the primary factors of interest are: 1) the number of participants, 2) the number of data points collected per participant, and 3) the effect size. The power (1-β) for this study was 0.84.
RESULTS
All of the subjects tolerated the study well without immediate or delayed adverse side effects from TMS. One subject became diaphoretic during his first TMS session, however, vital signs remained stable and the subject quickly recovered to baseline. The subject attributed this reaction to anxiety and did not become diaphoretic on subsequent sessions. Following 90 d of bed rest, subject 5788 demonstrated delayed functional recovery and at 2 wk post-bed rest had not returned to the subject’s pre-bed rest baseline as evidenced by performance on the functional mobility task. One of the subjects, subject 224, reported performing leg contraction exercises during the bed rest period. This was permitted as the protocol allowed the subjects to move the legs freely (while maintaining the head down position without weight bear) during bed rest.
CMAP amplitude was not found to have changed significantly in either the hand (9.0 ± 2.6 pre-bed rest versus 11.1 ± 2.1 post-bed rest) or leg (18.9 ± 4.8 pre-bed rest versus 19.6 ± 5.1 post-bed rest) following bed rest.
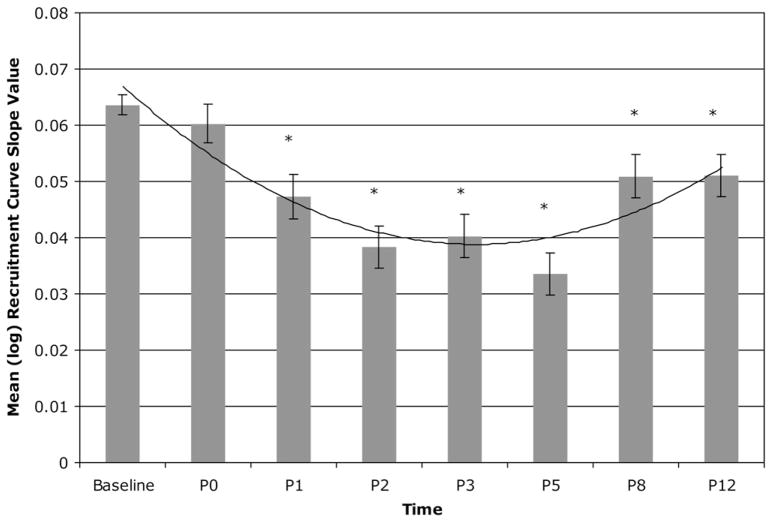
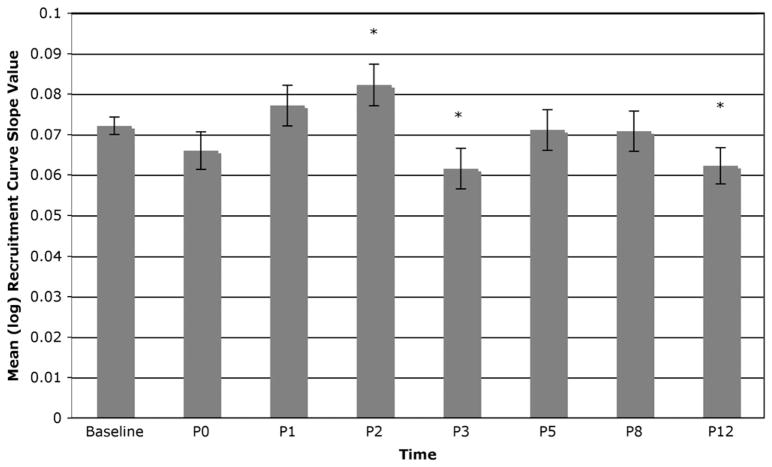
Significant effects were observed on recruitment curve slopes for the leg [F(7,2805) = 15.95, P < 0.0001]. Significant leg RC curve decreases were observed on days P1 [t(2805) = − 4.14, P < 0.0001], P2 [t(2805) = − 6.59, P < 0.0001], P3 [t(2805) = − 6.15, P < 0.0001], P5 [t(2805) = − 7.93, P < 0.0001], P8 [t(2805) = − 3.30, P = 0.001], and P12 [t(2805) = − 3.33, P = 0.0009] post-bed rest but not P0 [t(2805) = − 0.96, n.s.), suggesting a decrease in corticospinal excitability in the immediate post-bed rest period with recovery approaching baseline over the 2 wk following bed rest. Fig. 4 shows the means (and SE) of the log MEP slope values across all study measurement periods, highlighting the significant effects described above.
Fig. 4.
Means (and SE) of the log recruitment curve slope values across all study measurement periods for the leg.
Fig. 5 shows that a similar finding was not observed on hand RC slopes. Although there was a significant main effect observed for hand RC slopes over time [F(7,2916) = 2.66, P = 0.0097] only for days P2 [t(2916) = 1.97, P = 0.049], P3 [t(2916)= −2.12, P = 0.034] and P12 [t(2916) = − 2.19, P = 0.029], no significant effects were observed for days P0 [t(2916)= −1.32, n.s.], P1 [t(2916) = 1.00, n.s.], P5 [t(2916) = − 0.21, n.s.], or P8 [t(2916) = − 0.27, n.s.].
Fig. 5.
Means (and SE) of the log recruitment curve slope values across all study measurement periods for the hand.
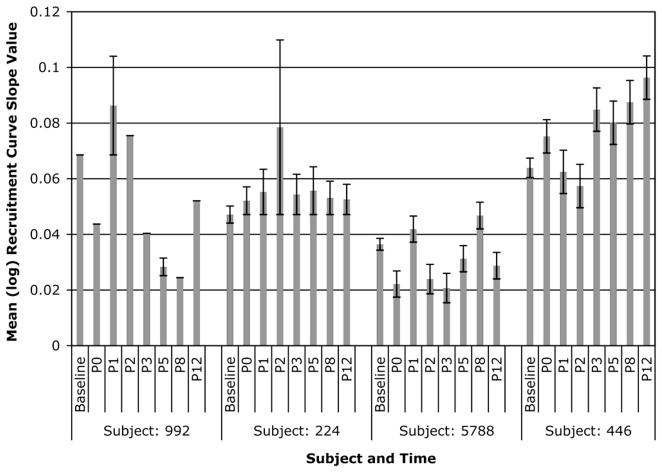
Individual RC slopes for each of the four subjects post-bed rest are shown in Fig. 6. In this small group, a heterogeneous response was noted. Subject 992 demonstrated an increase in RC slopes on days P1 and P2 with decreased slopes for other days. Subject 224 demonstrated increased RC slopes throughout the post-bed rest period with a spike in slope on day P2. Subject 5788 demonstrated no discernable pattern in post-bed rest RC slopes from baseline. Subject 446 demonstrated a delayed response with an increase in RC slopes occurring toward the end of the 2-wk post-bed rest recovery period.
Fig. 6.
Means (and SE) of individual log recruitment curve slope values across all study measurement periods for the leg.
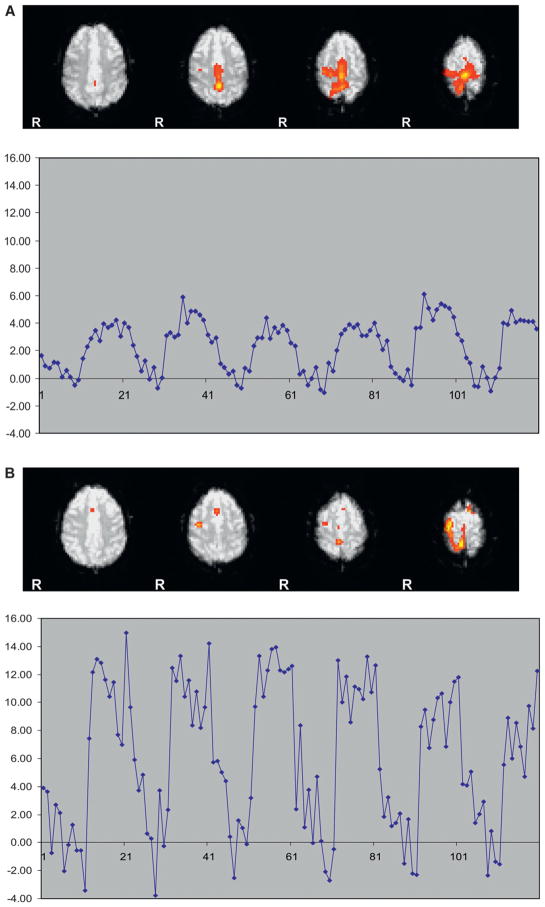
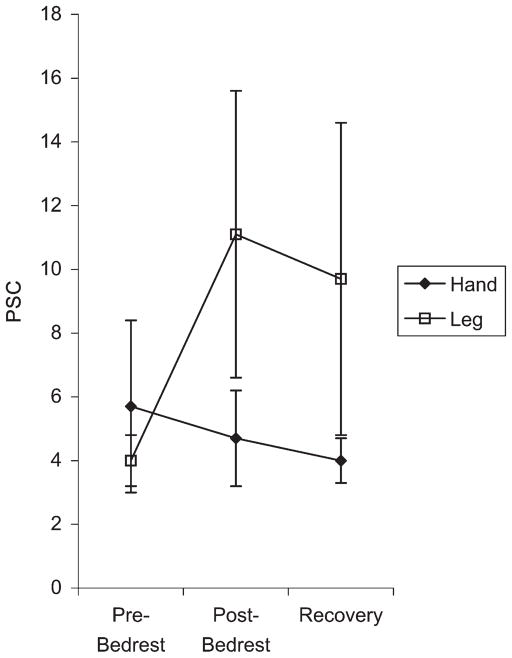
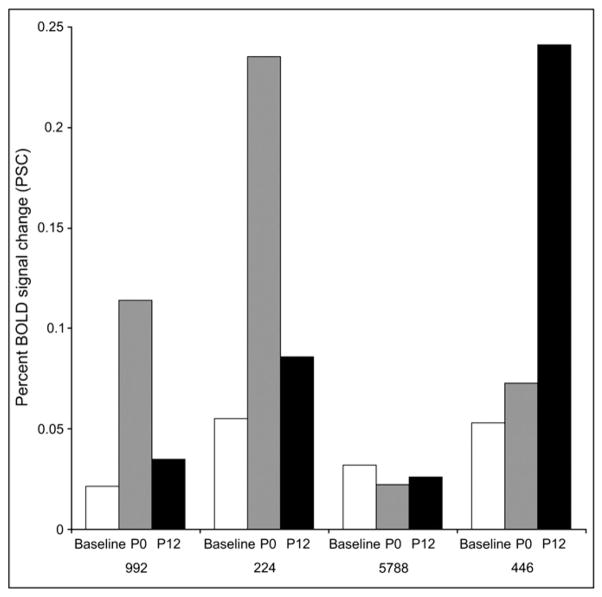
fMRI motor activation was achieved for all subjects (Fig. 7A and B). fMRI showed similar results with no change in PSC for the hand but with an increase in PSC post-bed rest for the leg on both day P0 and on day P12. For the hand, mean pre-bed rest PSC was 5.7 (SE = 2.7), mean post-bed rest day P0 PSC was 4.7 (SE = 1.5), and mean-bed rest day P12 PSC was 4.0 (SE = 0.7). For the leg, mean pre-bed rest PSC was 4.0 (se = 0.8), mean postbed rest day P0 PSC was 11.1 (SE = 4.5), and mean bed-rest day P12 PSC was 9.7 (SE = 4.9) (Fig. 8). Statistical significance was not determined due to the small number of subjects. Individual fMRI data for each of the four subjects is shown in Fig. 9. Again a heterogeneous individual response was seen. Subjects 992 and 224 demonstrated increased fMRI activation immediately post-bed rest on day P0 compared with baseline. Subject 5788 showed no such increase. Subject 446 again demonstrated a delayed response with increased fMRI activation seen on day P12.
Fig. 7.
Fig. 7A. fMRI activation map in a subject for the leg movement task obtained before bed rest. The plot shows the time course of activation. The y-axis is percent signal change.
Fig. 7B. fMRI activation map in a subject for the leg movement task obtained after bed rest. The plot shows the time course of activation. The y-axis is percent signal change.
Fig. 8.
fMRI percent signal change (PSC) calculated from rest intensity to task intensity, using values extracted at the voxel of maximum Z statistic. The y-axis is fMRI PSC. The x-axis represents three discrete time points: baseline pre-bed rest scan (performed on day BR −4), immediate post-bed rest performed on day P0, and scan performed on day P12. Error bars are SE. Note that the hand percent signal change progressively decreases with scans, while the leg PSC increases dramatically with bed rest and then begins to decline during the recovery period.
Fig. 9.
fMRI percent signal change (PSC) for individual subjects.
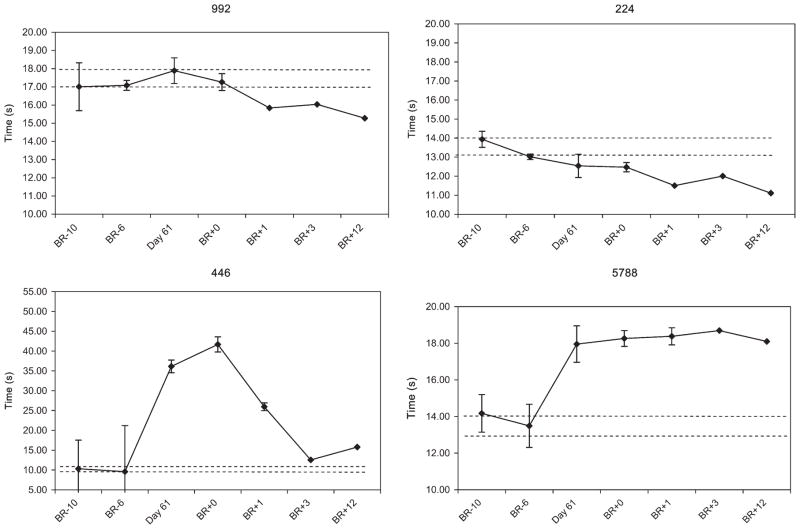
FMT provided a measure of balance and locomotor performance before and following bed rest. The time to complete the course is shown for each subject (Fig. 10). Subjects 992 and 224 did not show a change in performance post-bed rest and, in fact, subject 224 had improved performance over baseline immediately post-bed rest with continued improvement throughout the post-bed rest period. Subject 5788 demonstrated a decrement in post-bed rest performance without recovery during the observed post-bed rest period. Subject 446 demonstrated a post-bed rest decrement in performance with delayed recovery. The number of obstacles touched by each subject was also documented. Compared with the two pre-bed rest measures (average 1.8 obstacles touched), at the first post-bed rest period, there was an increase in the number of obstacles touched by 3 of the 4 subjects (average 7.5 obstacles touched), however, this was not statistically significant.
Fig. 10.
Functional mobility testing (FMT) course completion times for each subject.
DISCUSSION
The aims of this study were: 1) to evaluate leg corticospinal plasticity in response to long-term bed rest; and 2) to correlate alterations in corticospinal excitability with a measure of functional mobility.
Taking the group as a whole, in comparison with pre-bed rest measurements, we observed the development of decreased group leg corticospinal excitability in the immediate post-bed rest period as evidenced by a statistically significant decrease in RC slopes on days P1, P2, P3, P5, P8, and P12 following return to normal activity. No similar finding was observed in hand corticospinal excitability consistent with the expectation that no change in upper extremity use would occur during bed rest. The finding of overall decreased leg corticospinal excitability in these subjects might reflect reduced motor output drive during prolonged bed rest.
To our knowledge, no study has previously examined corticospinal plasticity in a bed rest model. In an immobilization model of lower extremity disuse, Liepert et al. (18) studied patients with leg/ankle fractures undergoing casting and found decreased motor excitability with a decrease in the area of lower extremity TMS-derived cortical maps. The mean duration of immobilization was 16 wk with changes becoming more prominent with a longer duration of casting up to 60 wk. In contrast, Zanette et al. reported increased motor excitability in patients undergoing upper extremity immobilization also for fracture fixation (42, 43). The investigators found increased upper extremity recruitment curves in these patients immediately after cast removal which returned to baseline on follow-up approximately 1 mo later. Comparison with these studies is difficult because confounds such as pain and inflammation would be expected to be present. Increased excitability of the motor cortex has also been observed in other conditions of disuse such as experimentally induced deafferentation (40, 44) or prolonged changes in limb position (33, 34). However, bed rest as a form of disuse is unique in that subjects, such as in this study, freely move the lower extremities. Our subjects were only required to maintain the head-down position, with the main limitation being no weight-bearing. This bed rest model, therefore, more closely reflects patients undergoing long-term confinement to bed rest as well as astronauts in microgravity.
Individually, however, our study group showed a heterogeneous response in both the time course of corticospinal changes and performance on FMT. For example, subject 446 demonstrated a decrement in performance on FMT and recovery was delayed, approaching but not attaining baseline performance by post-bed rest days P3 and P12. This subject also demonstrated a delayed change in corticospinal excitability with an increase occurring toward the end of the 2-wk post-bed rest recovery period. fMRI activation in this subject was also not increased on day P0 but was increased on day P12.
Subject 5788 experienced a decrement in performance post-bed rest but without improvement during the 2-wk post-bed rest recovery period as course completion time remained increased on day P12. Interestingly, this subject demonstrated no increase in corticospinal excitability with no significant change in RC slopes from baseline and no increased fMRI activation over the baseline measurement throughout the recovery period.
Subjects 992 and 224 experienced no significant decrement in performance. Subject 992 demonstrated post-bed rest increased corticospinal excitability with increased RC slopes on days P1 and P2 following bed rest, which was followed by decreased RC slopes on other post-bed rest days. Similarly, subject 224 showed an increase in corticospinal excitability with increased RC slopes throughout the post-bed rest period. In both of these subjects, fMRI activation was increased on day P0 but had returned to baseline by day P12.
These findings suggest that in the post bed rest period, increased corticospinal excitability was associated with improvement in individual performance. RC slopes were increased in subjects who also demonstrated improved performance on the functional mobility task. The increased excitability of the primary motor cortex may therefore reflect learning, or in our case “ relearning, ” of leg control during ambulation as a result of long-term disuse. Our results are in line with previous studies which have demonstrated increased cortical excitability during periods of plasticity and learning. There is evidence for the critical role of the primary motor cortex in early motor consolidation (21) and increased excitability has been shown to occur during acquisition of new motor skills (9, 20, 23).
Training regimes have been shown by TMS to induce motor cortex plasticity as evidenced by increased MEP values (14, 24, 25), increased RC slopes (14), and altered short-interval intracortical inhibition (24). Jensen et al. (14) found that while 4 wk of visuo-motor skill training lead to increased RC slopes, 4 wk of strength training lead to decreased RC slopes, implying that increased cortical excitability is induced by highly skilled training and not strength training alone. Similar results were found by Perez et al. and Beck et al. in investigating lower extremity training (1, 24). Perez and coworkers (24) reported that 32 min of motor skill training, but not passive or nonskill training, increases motor cortex excitability of the leg motor area as indexed by higher recruitment curves and decreased intracortical inhibition. This is also in line with prior animal studies in which cortical representational changes occurred in squirrel monkeys following motor skill acquisition but was not seen following repetitive movements alone (26). Therefore TMS derived measures may be markers for training regimes that are more efficient at driving cortical plasticity and might prove useful to space mission planners in assessing prescribed astronaut exercise routines.
While numerous studies have demonstrated cortical plasticity in response to various environmental alterations, such as disuse as in our study, the functional implications are not yet completely understood. Although further work is necessary to clarify the functional relevance of changes in corticospinal excitability in response to limb nonuse, investigators have recently shown that altering corticospinal excitability can improve measures of functionality in different patient populations, including chronic stroke (16, 41), dystonia (22, 36), and Parkinson disease (11, 17). Changes in excitability of corticomotor circuits can be induced by peripheral nerve stimulation (PNS) (12, 29), by repetitive TMS (8, 27), and by a technique of combined PNS and TMS known as paired associative stimulation (PAS) (32, 38). It is interesting to speculate that subject 5788, who did not demonstrate increased corticospinal excitability with poor performance through out recovery, might have benefited from such a regime designed to enhance corticospinal excitability.
Limitations of the current pilot study include a very small sample size and the lack of complete immobilization of the lower extremities. In fact, subject 224 reported daily leg exercises although he remained in a head down position throughout the study. While three of the subjects reported clumsiness in the first days following return to ambulation, this subject reported no such difficulties, which was also documented on FMT. Limitations of fMRI, including unequal/partial brain coverage and the possibility of movement confound, may have contributed to the marked PSC obtained from the motor cortex following bed rest, as well as previously unstudied effects of bed rest on the fMRI signal. In our study, the contribution to plasticity following immobilization of other levels along the corticospinal system was not assessed. However, none of the previous immobilization studies, which also investigated other levels of the nervous system, found any significant relationship between changes of TMS measures related to immobilization and measures of spinal excitability and/or neuromuscular transmission (18, 42, 43). Results from upper limb immobilization studies performed on patients (42, 43) and from studies on motor learning (19, 24) indicate that increased MEP recruitment curves following limb disuse or motor learning are mainly associated with an imbalance between intracortical inhibition and facilitation resulting in motor cortical hyperexcitation (as shown by the paired pulse technique). These data would strongly suggest that the observed effects are likely to mainly rely on cortical mechanisms, even though contribution from other structures along the corticospinal pathway can not be excluded.
In this pilot study of four subjects, we have identified alterations in motor cortex excitability following 90 d of head down bed rest. Additionally, we have demonstrated a potential association between increased corticospinal excitability and performance on a functional mobility task during the 2-wk recovery period. Results of this research include a better understanding of the basic neurophysiology underlying cortical plasticity occurring during lower extremity disuse. Following future testing of many additional subjects, these data may lead to applications in modulating corticospinal pathways furthering the rehabilitation of patients who have undergone prolonged confinement to bed. Also, as TMS is an easily portable unit, there is the potential for future onboard use as part of a countermeasure regime by astronauts on long-term space missions to remediate lower extremity dysfunction prior to operations on a planetary surface.
Acknowledgments
We thank Dr. Janice V. Meck and the NASA and UTMB bed rest teams for their invaluable assistance. Supported by NASA grant number NNJ04HF70G and NIH grant number NNJ06HB811.
Contributor Information
Donna R. Roberts, Department of Radiology and Radiological Sciences, Medical University of South Carolina, Charleston, SC; Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC
David Ramsey, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC; South Carolina Research Authority, Charleston, SC
Kevin Johnson, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC
Jejo Kola, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC
Raffaella Ricci, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC; Department of Psychology, University of Turin, Turin, Italy
Christian Hicks, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC.
Jeffrey J. Borckardt, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC; Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC
Jacob J. Bloomberg, Human Adaptation and Countermeasures Office, NASA-Johnson Space Center, Houston, TX
Charles Epstein, Department of Neurology, Emory University, Atlanta, GA
Mark S. George, Center for Advanced Imaging Research, Medical University of South Carolina, Charleston, SC; Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC
References
- 1.Beck S, Taube W, Gruber M, Amtage F, Gollhofer A, Schubert M. Task-specific changes in motor evoked potentials of lower limb muscles after different training interventions. Brain Res. 2007;1179:51–60. doi: 10.1016/j.brainres.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Belichenko PV, Krasnov IB. The dendritic spines of the pyramidal neurons in layer V of the rat sensorimotor cortex following a 14-day space flight. Biull Eksp Biol Med. 1991;112 (11):541–2. [PubMed] [Google Scholar]
- 3.Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychol Bull. 1987;101:147–58. [Google Scholar]
- 4.Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- 5.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 6.Davey NJ, Rawlinson SR, Nowicky AV, McGregor AH, Dubois K, et al. Human corticospinal excitability in microgravity and hypergravity during parabolic flight. Aviat Space Environ Med. 2004;75:359–63. [PubMed] [Google Scholar]
- 7.Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–38. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- 8.Di Lazzaro V, Dileone M, Profice P, Pilato F, Cioni B, et al. Direct demonstration that repetitive transcranial magnetic stimulation can enhance corticospinal excitability in stroke. Stroke. 2006;37:2850–3. doi: 10.1161/01.STR.0000244824.53873.2c. [DOI] [PubMed] [Google Scholar]
- 9.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–7. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Dyachkova LN. Ultrastructural characteristics of plastic changes in the brain cortex of rats exposed to space flight. Physiologist. 1991;34 (1, Suppl):S185–6. [PubMed] [Google Scholar]
- 11.Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for Parkinson’s disease: a systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry. 2005;76:1614–23. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–8. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- 13.Hyde TM, Wu LC, Krasnov IB, Sigworth SK, Daunton NG, D’Amelio F. Quantitative autoradiographic analysis of muscarinic cholinergic and GABAA (benzodiazepine) receptors in the forebrain of rats flown on the Soviet Biosatellite COSMOS 2044. Brain Res. 1992;593:291–4. doi: 10.1016/0006-8993(92)91321-5. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol. 2005;99:1558–68. doi: 10.1152/japplphysiol.01408.2004. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KA, Kola J, Roberts DR, Ramsey D, Epstein CM, George MS. Mapping cortical plasticity in the bedrest analogue of space flight using TMS and fMRI. Neuroimage; 12th Annual Meeting of the Organization for Human Brain Mapping; 2006 June 11–15; Florence, Italy. 2006. (Abstract; Poster 107 TH-AM) [Google Scholar]
- 16.Kim YH, You SH, Ko MH, Park JW, Lee KH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–6. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 17.Lefaucheur JP, Drouot X, Von Raison F, Menard-Lefaucheur I, Cesaro P, Nguyen JP. Improvement of motor performance and modulation of cortical excitability by repetitive transcranial magnetic stimulation of the motor cortex in Parkinson’s disease. Clin Neurophysiol. 2004;115:2530–41. doi: 10.1016/j.clinph.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalogr Clin Neurophysiol. 1995;97:382–6. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- 19.Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126:866–72. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- 20.Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–8. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- 21.Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, et al. Early consolidation in human primary motor cortex. Nature. 2002;415 (6872):640–4. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 22.Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, et al. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer’s cramp. Brain. 2005;128:104–15. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- 23.Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–9. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- 24.Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- 25.Perez MA, Lundbye-Jensen J, Nielsen JB. Task-specific depression of the soleus H-reflex after cocontraction training of antagonistic ankle muscles. J Neurophysiol. 2007;98:3677–87. doi: 10.1152/jn.00988.2007. [DOI] [PubMed] [Google Scholar]
- 26.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- 27.Ricci R, Ramsey D, Johnson K, Borckardt JJ, Vallejo M, et al. A pilot feasibility study of daily rTMS to modify corticospinal excitability during lower limb immobilization. Ther Clin Risk Manag. 2008;4:1127–34. doi: 10.2147/tcrm.s2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–4. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 29.Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res. 1999;126:536–44. doi: 10.1007/s002210050762. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DR, Vincent DJ, Speer AM, Bohning DE, Cure J, et al. Multi-modality mapping of motor cortex: comparing echoplanar BOLD fMRI and transcranial magnetic stimulation. J Neural Transm. 1997;104:833–43. doi: 10.1007/BF01285552. Short communication. [DOI] [PubMed] [Google Scholar]
- 31.Roberts DR, Ricci R, Funke FW, Ramsey P, Kelley W, et al. Lower limb immobilization is associated with increased corticospinal excitability. Exp Brain Res. 2007;181:213–20. doi: 10.1007/s00221-007-0920-5. [DOI] [PubMed] [Google Scholar]
- 32.Roy FD, Norton JA, Gorassini MA. Role of sustained excitability of the leg motor cortex after transcranial magnetic stimulation in associative plasticity. J Neurophysiol. 2007;98:657–67. doi: 10.1152/jn.00197.2007. [DOI] [PubMed] [Google Scholar]
- 33.Sanes JN, Wang J, Donoghue JP. Immediate and delayed changes of rat motor cortical output representation with new forelimb configurations. Cereb Cortex. 1992;2:141–52. doi: 10.1093/cercor/2.2.141. [DOI] [PubMed] [Google Scholar]
- 34.Sanes JN, Donoghue JP. Static and dynamic organization of motor cortex. Adv Neurol. 1997;73:277–96. [PubMed] [Google Scholar]
- 35.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 36.Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurology. 1999;52:529–37. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- 37.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–55. [Google Scholar]
- 38.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 39.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 40.Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain. 2002;125:1402–13. doi: 10.1093/brain/awf140. [DOI] [PubMed] [Google Scholar]
- 41.Yozbatiran N, Alonso-Alonso M, See J, Demirtas-Tatlidede A, Luu D, et al. Safety and behavioral effects of high-frequency repetitive transcranial magnetic stimulation in stroke. Stroke. 2009;40:309–12. doi: 10.1161/STROKEAHA.108.522144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, et al. Reversible changes of motor cortical outputs following immobi lization of the upper limb. Electroencephalogr Clin Neurophysiol. 1997;105:269–79. doi: 10.1016/s0924-980x(97)00024-6. [DOI] [PubMed] [Google Scholar]
- 43.Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol. 2004;115:1264–75. doi: 10.1016/j.clinph.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 44.Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci. 1998;18:7000–7. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]