SUMMARY
Defining conditioning regimen intensity has become a critical issue for the hemopoietic stem cell transplant community. In the present report we propose to define conditioning regimens in three categories: (a) myeloablative conditioning (MA) , (b) reduced intensity conditioning (RIC) and (c) non myeloablative conditioning (NMA). Assignment to these categories is based on the duration of cytopenia and on the requirement for stem cell (SC) support: MA regimens cause irreversible cytopenia and SC support is mandatory. NMA regimens cause minimal cytopenia and can be given also without SC support. RIC regimens do not fit criteria for MA or NMA regimens : they cause cytopenia of variable duration and should be given with stem cell support, although cytopenia may not be irreversible. This report also assigns commonly used regimens to one of these categories. based upon the agents, dose or combinations. Standardized classification of conditioning regimen intensities will allow comparison across studies and interpretation of study results.
INTRODUCTION
Patients undergoing an allogeneic hemopoietic stem cell transplant (HSCT), are prepared with chemotherapy alone or chemotherapy combined with radiotherapy , the so called conditioning regimen, with two aims: reduce the tumor burden -when the disease is neoplastic- and suppress the recipient’s immune system, in order to allow engraftment of stem cells (1). Exceptions to this rule are infants with combined immune deficiency (SCID) (2) and patients with severe aplastic anemia (SAA) with an identical twin donor, who may be grafted without conditioning.
The intensity of the conditioning regimen can vary significantly. The conventional conditioning for most young patients with leukemia/lymphoma is either cyclophosphamide (CY) 120 mg/kg and total body irradiation (TBI) (10–15 Gy) (referred to as CY-TBI) (3) or busulfan (BU) 16 mg/kg p.o. and CY 120 mg/kg, (referred to as BU-CY) (4). Several attempts have been made in the past 30 years to limit early transplant toxicity, by reducing the intensity of the conditioning regimen: John Hobbs used half the dose of BU (8 mg/kg) in children with inborn errors (5); Peter Tutchka reduced the dose of CY from 200 to 120 in the classic BU-CY regimen (6), and Guido Lucarelli reduced the dose of busulfan from 16 mg/kg to 14 mg/kg for his thalassemia conditioning regimen (7). In contrast some regimens were intensified with the aim of reducing leukemia relapse: the Seattle team delivered 15.75 Gy rather than 12 Gy in patients with leukemia (8). Other investigators introduced the use of etoposide in combination with TBI (9). Very few regimens have been prospectively compared head to head, with the exception of the Seattle TBI regimens (8), and we have no evidence that intensified conditioning improves survival: the reason being that any decrease of leukemia recurrence with a higher dose of TBI is achieved at the expense of increased toxicity (8).
Within the past 15 years two changes have occurred in the conditioning regimens: the introduction of fludarabine (10–13) and further dose reduction of the alkylating agents (14–16) or TBI (17). These regimens, were specifically designed for patients ineligible for conventional conditioning, either because of age (usually above 50 years) or because of the presence of co-morbidities (18). By reducing the intensity of the conditioning regimen, the benefit of allogeneic transplantation would come from a graft-versus-malignancy effect, rather than from the upfront cyto-reductive effect of the conditioning regimen (12). These modified regimens have rapidly become popular, such that by 2001 almost 30% of transplants were performed with reduced intensity regimens (19) .
Regimens using fludarabine and/or reduced doses of chemo/radiotherapy have been referred to as non myeloablative stem cell transplants (NMA), reduced intensity conditioning transplants (RIC) or mini transplants. Several workshops have been convened on this issue: a panel of transplant physicians on behalf of the European Group for Blood and Marrow Transplantation (EBMT) considered the term mini-transplant inappropriate , because it was misleading for patients , care providers, physicians and insurance companies (18). A workshop convened by the CIBMTR addressed the dose spectrum which defines a reduced intensity conditioning regimen (20) . The interest on defining conditioning regimens, is justified by the need of a common language in the scientific community, and also pertains transplant registration and documentation requirements , which are now mandatory in several national and international regulatory agencies.
In the present report we will discuss three categories of conditioning regimens: myeloablative, reduced intensity and non myeloablative. The terminology reflects the early regimen related toxicity towards host marrow cells , and not the biologic effect of the transplant. The latter component is complex, involving engraftment of donor lymphohematopoietic cells, followed by “displacement” of host lympho-hematopoietic cells , through an immune-mediated myeloablation (1).
MYELOABLATIVE CONDITIONING REGIMENS (MA)
The term myeloablation refers to the administration of total body irradiation (TBI) and/or alkylating agents , at doses which will not allow autologous hematologic recovery. Over 50 years ago Lorenz and coworkers showed that mice exposed to 10 Gy of TBI would succumb to pancytopenia (21) . Work of Lorenz and other outstanding investigators, confirmed that animals could be rescued by intravenous administration of a bone marrow cell suspension (21–22): bone marrow transplantation (BMT) was born and human studies were initiated (23). Initial attempts to apply BMT in humans were hampered by the lack of appropriate donor-recipient matching procedures: the discovery of the human leukocyte antigen system (HLA) initiated the widespread clinical use of allogeneic transplants (3, 4) The agents chosen to prepare humans for BMT were TBI, CY and BU , at the dose used in animals: TBI 10 Gy, CY 200 mg/kg (3) and BU 16 mg/kg (4) . The combinations of BU-CY or CY-TBI are considered to be a myeloablative conditioning regimen (MA). Other agents have been introduced in the conditioning regimen at high doses, and in different combinations with CY or TBI, usually with the intention of further intensification : these include melphalan (MEL) (24), thiotepa (THIO) (10) , etoposide (VP16) (25) , and dimethylbusulfan (26) .
MA regimens usually produce rapid engraftment of donor cells, which may be followed . in a proportion of patients , by graft versus host disease (GvHD). MA regimens are associated with toxicity and mortality –referred to as transplant related mortality (TRM)- depending on variables such as patient age, donor age, donor/recipient HLA matching , gender matching, phase of the underlying disease (early or advanced) , and year of transplant. The risk of TRM after a MA regimen has decreased over time, although the exact reason for improvement is not entirely clear (27) . It most probably relates to improved HLA-matching technology, in the unrelated donor setting, and better supportive care. Patients with early leukemia seem to have most benefited of this improvement (27).
Cure of the underlying disease depends in part on the intensity of the MA regimen (1): this was proven in a prospective randomized trial showing that TBI 15.75 Gy was associated with a lower risk of relapse compared to TBI 12 Gy (8). However, patients receiving the higher dose of TBI also had a higher incidence of GvHD ,thus, making unclear the relative contribution of the TBI to the lower risk of relapse. Other retrospective studies have confirmed the impact of conditioning intensity on relapse (9). Unfortunately the higher TBI dose was also associated with a higher risk of TRM, so that survival was comparable in the two groups (8). The anti-leukemia effect of MA regimens can be further enhanced with the use of targeted radio-immunoconjugates (28), which would in theory not increase regimen related toxicity . However since the use of these agents in the context of conditioning regimens remains investigational, their exact place in the intensity spectrum is unknown.
It should be noted that it is probably impossible to “myelo-ablate” completely an animal or an individual (29), and indeed cases of autologous reconstitution have been reported after high dose chemotherapy or accidental exposure to radiation (30–31). Therefore the term myeloablation should be considered an operational definition, indicating a regimen causing irreversible pancytopenia in almost all patients : autologous recovery (at best following prolonged life-threatening cytopenia) would be the exception.
Definition of MA regimen: A combination of agents expected to produce profound pancytopenia and myeloablation within 1–3 weeks from administration; pancytopenia is long lasting, usually irreversible and in most instances fatal, unless hematopoiesis is restored by hemopoietic stem cell infusion.
Examples of Myeloablative regimens (MA) are shown in Table 1.
Table 1.
Example of myeloablative, and nonmyeloablative regimens according to commonly used agents and combinations. Reduced intensity regimens (RIC) are regimens which do not fit these two categories; examples of these regimens in the text-
A workshop was convened at a CIBMTR/ASBMT meeting to assess whether expert transplanters would agree on what is considered a MA regimen (20). The regimens listed in Table 1 obtained a general consensus.
NON MYELOABLATIVE CONDITIONING REGIMENS (NMA)
Transplant related mortality (TRM) after MA regimens increases with increasing patient age, and 50 years used to be considered an upper age limit (25). With the aim of reducing toxicity, thus making transplantation available in the older patient population , so called non-myeloablative (NMA) conditioning regimens were developed. Examples of NMA regimens include: fludarabine and cyclophosphamide (FLU-CY) (32) , TBI 2 Gy (17), TBI 1 Gy (33), total lymphoid radiation (TLI) and anti-thymocyte globulin (ATG) (34). NMA typically cause minimal cytopenia, and little early toxicity, but are immunosuppressive to the extent that, when followed by G-CSF mobilized peripheral blood stem cells (PBSC) , they usually result in full engraftment of donor lympho-hemopoietic stem cells. A good example is the FLU-CY combination developed in Houston for the treatment of chronic lymphocytic leukemia (CLL): the same regimen followed by mobilized allogeneic PBSC, results in donor engraftment (30). In addition to myelo-ablation , we introduce here the concept of immuno-ablation. NMA are immuno-ablative regimens , and this is why donor cells engraft . However NMA also require a large number of donor T lymphocytes and donor CD34+ cells, to facilitate donor engraftment. It is therefore the combination of immuno-ablation and large numbers of donor cells that constitute the essence of NMA programs (33). These transplants are followed by low early toxicity, despite older patient age and greater number of patients with co-morbidity (35). TRM is lower after NMA as compared to MA regimens (35).
Acute GvHD after NMA is delayed ,and may develop after day +100, at a time when chronic GvHD is usually diagnosed after a MA regimen (36). GvHD remains a significant cause of morbidity and mortality also after NMA (36). NMA have been explored in patients with leukemia (37) lymphoma (32), myeloma (38) and solid tumours (39).
Definition of NMA regimen: a regimen which will cause minimal cytopenia and does not require stem cell support.
This is an operational definition: indeed only some of the regimens classified as NMA are truly non ablative, such as the FLU-CY or TLI-ATG: on the other hand TBI also at low doses causes some degree of ablation of the stem cell reservoir. But because TBI 1 or 2 Gy do not cause cytopenia and can be given without SC support, they can be defined as NMA. In addition NMA refers only to the conditioning regimen: in fact the transplant, as a procedure, is myelo-ablative, because engrafted donor T cells will eventually eliminate host hemopoietic cells, allowing the establishment of donor hematopoiesis.
Examples of Non-myeloablative (NMA) regimens are shown in Table 1.
REDUCED INTENSITY CONDITIONING REGIMENS (RIC)
Reduced intensity conditioning regimens (RIC) is an intermediate category of regimens which do not fit the definition for MA or NMA. RIC regimens differ from NMA : they cause cytopenia, which may be prolonged, and do require stem cell support. It is possible that autologous recovery would eventually occur, although pancytopenia would be of such duration to cause significant morbidity and mortality .
RIC regimens differ from MA conditioning, because the dose of alkylating agents or TBI is reduced by at least 30%. Most often these regimens combine fludarabine with an alkylating agent, melphalan (40), busulfan (14), thiotepa (15) in reduced doses, or fludarabine with reduced dose TBI (41)., Transplant mortality is reduced after RIC regimens, as shown by several registry based studies comparing RIC and MA regimens , (43–46). RIC programs require stem cell support to be practical in the clinic: RIC regimens have used a wide selection of agents, given at a wide range of doses . RIC regimens have been explored in patients with acute and chronic leukemia, lymphoma, myeloma and patients with myelodisplastic syndromes , as shown by large Registry based studies (42,43).
For some conditioning regimens ,classification may be not straightforward . One example is cyclophosphamide 200 mg/kg (CY 200) with or without thymic radiation (47,48) . This is a truly non-ablative regimen, since it does not kill stem cells, but it does cause profound cytopenia, especially when given over 4 days (50 mg/kg/day x4) in patients with severe aplastic anemia (SAA), and is followed by allogeneic hemopoietic stem cells. Some of these patients may recover an autologous hematopoies, and CY 200 has also been given in aplastic anemia , without stem cells support (49) , although the rate of lethal infections , due to prolonged cytopenia, was very high (50) . Therefore CY 200 does not fit our working definition of a MA conditioning nor of a NMA conditioning , and falls in the category of RIC conditioning.
Definition of a RIC regimen: a regimen which can not be classified as NMA or MA
CONCLUSION
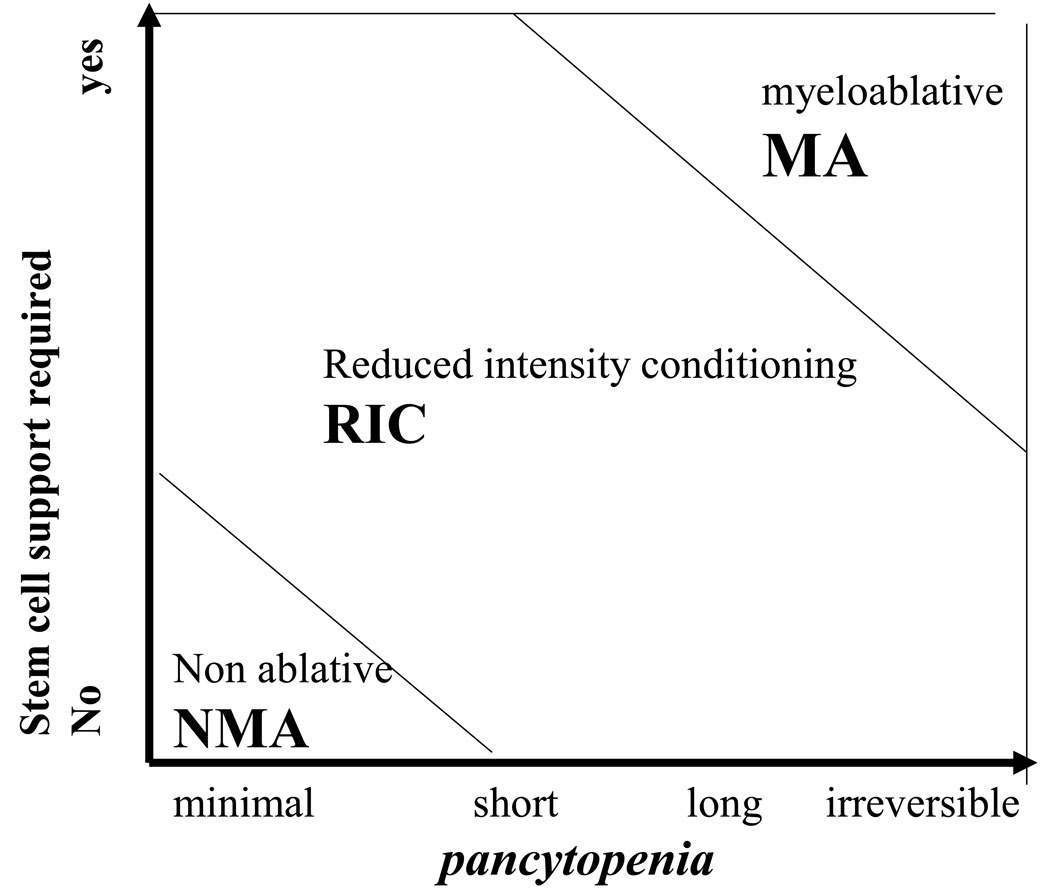
We propose to define the intensity of the conditioning regimen on the basis of the duration of pancytopenia induced and on the requirement for stem cell support, as shown in Figure 1.
Fig.1.
A myeloablative conditioning regimen (MA) will cause irreversible (or close to irreversible) pancytopenia. Stem cell support is required to rescue marrow function, and prevent aplasia related death.
A non myeloablative conditioning regimen (NMA) is a regimen which will produce minimal cytopenia, and there is no need for stem cell support.
A conditioning regimen which does not fulfill MA or NMA is defined as reduced intensity conditioning regim (RIC)
These definitions should be regarded as a starting point, which may be re-discussed in the near future. The notion of the two conventional MA regimens (CY TBI and BU CY) is well established. The concept of NMA regimens, is also clear, and based on agents, or combination of agents, producing minimal cytopenia. All other regimens should be called RIC, not because they are fludarabine based, but rather because they do not fit criteria for MA or NMA regimens.
Adoption of a classification for preparative regimens in three different categories would be important for cross referencing in the scientific literature. The inclusion of new agents in conditioning regimens, such as disease specific drugs or targeted therapies with monoclonal antibodies, will need to be incorporated in the intensity spectrum. This classification and terminology, if adopted by the transplant community, will serve as a starting point to standardize these transplant modalities, and facilitate interpretation of retrospective studies and development of prospective trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vriesendorp HM. Aims of the conditioning regimen. Exp. Hematol. 2003;31:844–854. doi: 10.1016/s0301-472x(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 2.Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B, Notarangelo LD, Roifman CM. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006 Feb 1;295(5):508–518. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 3.Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, Goodell BW, Hickman RO, Lerner KG, Neiman PE, Sale GE, Sanders JE, Singer J, Stevens M, Storb R, Weiden PL. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977 Apr;49(4):511–533. [PubMed] [Google Scholar]
- 4.Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, Braine HG, Burns WH, Elfenbein GJ, Kaizer H, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983 Dec 1;309(22):1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs JR, Barrett AJ, Chambers D, James DCO, Hugh-Jones K, Byrom N, Henry -K, Lucas CF. Reversal of clinical features of hurler’s disease and biochemical improvement after treatment by bone marrow transplantation. Lancet. 1981;3:709–712. doi: 10.1016/s0140-6736(81)91046-1. [DOI] [PubMed] [Google Scholar]
- 6.Kahl C, Leisenring W, Deeg HJ, Chauncey TR, Flowers ME, Martin PJ, Sanders JE, Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70(5):1382–1388. 26. [PubMed] [Google Scholar]
- 7.Lucarelli G, Polchi P, Izzi T, Manna M, Delfini C, Galimberti M, Porcellini A, Moretti L, Manna A, Sparaventi G. Marrow transplantation for thalassemia after treatment with busulfan and cyclophosphamide. Ann N Y Acad Sci. 1985;445:428–431. doi: 10.1111/j.1749-6632.1985.tb17212.x. [DOI] [PubMed] [Google Scholar]
- 8.Clift RA, Buckner CD, Appelbaum FR, Sullivan KM, Storb R, Thomas ED. Long-term follow-Up of a randomized trial of two irradiation regimens for patients receiving allogeneic marrow transplants during first remission of acute myeloid leukemia. Blood. 1998 Aug 15;92(4):1455–1456. No abstract available. [PubMed] [Google Scholar]
- 9.Marks DI, Forman SJ, Blume KG, Pérez WS, Weisdorf DJ, Keating A, Gale RP, Cairo MS, Copelan EA, Horan JT, Lazarus HM, Litzow MR, McCarthy PL, Schultz KR, Smith DD, Trigg ME, Zhang MJ, Horowitz MM. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant. 2006;12:438–453. doi: 10.1016/j.bbmt.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Terenzi A, Aristei C, Aversa F, Perruccio K, Chionne F, Raymondi C, Latini P, Martelli MF. Efficacy of fludarabine as an immunosuppressor for bone marrow transplantation conditioning: preliminary results. Transplant Proc. 1996 Dec;28(6):3101. No abstract available. [PubMed] [Google Scholar]
- 11.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, Reisner Y, Martelli MF. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998 Oct 22;339(17):1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 12.Giralt S, Estey E, Albitar M, van Besien K, Rondón G, Anderlini P, O’Brien S, Khouri I, Gajewski J, Mehra R, Claxton D, Andersson B, Beran M, Przepiorka D, Koller C, Korblau S, Körbling M, Keating M, Kantarjian H, Champlin R. Engraftment of allogeneic hematopoietic progenitor cells with purine analog containing chemotherapy: Harnessing graft-versus-leukemia without myelablative therapy. Blood. 1997;89(12):4531–4536. [PubMed] [Google Scholar]
- 13.Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, Claxon D, Donato M, Bruton J, Cohen A, Davis M, Andersson BA, Anderlini P, Gajewski J, Kornblau S, Andreeff M, Prezpiorka D, Ueno NT, Molldrem J, Champlin R. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97(3):631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 14.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R. Non-myeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998 Feb 1;91(3):756–763. [PubMed] [Google Scholar]
- 15.Corradini P, Zallio F, Mariotti J, Farina L, Bregni M, Valagussa P, Ciceri F, Bacigalupo A, Dodero A, Lucesole M, Patriarca F, Rambaldi A, Scime R, Locasciulli A, Bandini G, Gianni AM, Tarella C, Olivieri A. Effect of age and previous autologous transplantation on nonrelapse mortality and survival in patients treated with reduced-intensity conditioning and allografting for advanced hematologic malignancies. J Clin Oncol. 2005;23:6690–6698. doi: 10.1200/JCO.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 16.Kottaridis PD, Chakraverty R, Milligan DW, Chakrabarti S, Robinson S, Chopra R, Pettengell R, Marsh J, Mahendra P, Schey S, Morgan G, Williams C, Hale G, Waldmann H, Linch DC, Devereux S, Glodstone AH, Mackinnon S. A nonmyeloablative regimen for allogeneic stem cell transplantation with a low incidence of GVHD. Bone Marrow Transplant. 2000;25:S26. [Google Scholar]
- 17.Storb R. Nonmyeloablative preparative regimens: how relevant for acute myelogenous leukemia? Leukemia. 2001 Apr;15(4):662–663. doi: 10.1038/sj.leu.2402034. Review. No abstract available. [DOI] [PubMed] [Google Scholar]
- 18.Bacigalupo A. Third EBMT/AMGEN Workshop on reduced-intensity conditioning allogeneic haemopoietic stem cell transplants (RIC-HSCT), and panel consensus. Bone Marrow Transplant. 2004 Apr;33(7):691–696. doi: 10.1038/sj.bmt.1704416. [DOI] [PubMed] [Google Scholar]
- 19.Gratwohl A, Baldomero H, Passweg J, Urbano-Ispizua A European Group for Blood and Marrow Transplantation (EBMT) Accreditation Committee. Increasing use of reduced intensity conditioning transplants: report of the 2001 EBMT activity survey. Bone Marrow Transplant. 2002 Dec;30(12):813–831. doi: 10.1038/sj.bmt.1703819. [DOI] [PubMed] [Google Scholar]
- 20.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, Sandmaier B. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009 Mar;15(3):367. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz E, Congdon C, Uphoff D. Modification of acute irradiation injury in mice and guinea-pigs by bone marrow injections. Radiology. 1952 Jun;58(6):863–877. doi: 10.1148/58.6.863. [DOI] [PubMed] [Google Scholar]
- 22.Vos O, Davids JA, Weyzen WW, Van Bekkum DW. Evidence for the cellular hypothesis in radiation protection by bone marrow cells. Acta Physiol Pharmacol Neerl. 1956 Mar;4(4):482–486. [PubMed] [Google Scholar]
- 23.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957 Sep 12;257(11):491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 24.Helenglass G, Powles RL, McElwain TJ, Lakhani A, Milan S, Gore M, Nandi A, Zuiable A, Perren T, Forgeson G, et al. Melphalan and total body irradiation (TBI) versus cyclophosphamide and TBI as conditioning for allogeneic matched sibling bone marrow transplants for acute myeloblastic leukaemia in first remission. Bone Marrow Transplant. 1988 Jan;3(1):21–29. [PubMed] [Google Scholar]
- 25.Jamieson CH, Amylon MD, Wong RM, Blume KG. Allogeneic hematopoietic cell transplantation for patients with high-risk acute lymphoblastic leukemia in first or second complete remission using fractionated total-body irradiation and high-dose etoposide: a 15-year experience. Exp Hematol. 2003;(10):981–986. doi: 10.1016/s0301-472x(03)00231-5. [DOI] [PubMed] [Google Scholar]
- 26.Kanfer EJ, Buckner CD, Fefer A, Storb R, Appelbaum FR, Hill RS, Amos D, Doney KC, Clift RA, Shulman HM. Allogeneic and syngeneic marrow transplantation following high dose dimethylbusulfan, cyclophosphamide and total body irradiation. Bone Marrow Transplant. 1987;1(4):339–346. [PubMed] [Google Scholar]
- 27.Bacigalupo A, Sormani MP, Lamparelli T, Gualandi F, Occhini D, Bregante S, Raiola AM, di Grazia C, Dominietto A, Tedone E, Piaggio G, Podesta M, Bruno B, Oneto R, Lombardi A, Frassoni F, Rolla D, Rollandi G, Viscoli C, Ferro C, Garbarino L, VanLint MT. Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004 Oct;89(10):1238–1247. [PubMed] [Google Scholar]
- 28.Pagel JM, Matthews DC, Appelbaum FR, BerNMAein ID, Press OW. The use of radioimmunoconjugates in stem cell transplantation. Bone Marrow Transplant. 2002 May;29(10):807–816. doi: 10.1038/sj.bmt.1703524. [DOI] [PubMed] [Google Scholar]
- 29.Fox BW, Lajtha LG. Radiation damage and repair phenomena. Br Med Bull. 1973 Jan;29(1):16–22. doi: 10.1093/oxfordjournals.bmb.a070949. [DOI] [PubMed] [Google Scholar]
- 30.Brown RA, Herzig RH, Wolff SN, Frei-Lahr D, Pineiro L, Bolwell BJ, Lowder JN, Harden EA, Hande KR, Herzig GP. High-dose etoposide and cyclophosphamide without bone marrow transplantation for resistant hematologic malignancy. Blood. 1990;76:473–479. [PubMed] [Google Scholar]
- 31.Jammet H, Mathé G, Pendic B, et al. Etude de six cas d’irradiation totale aigue accidentelle. Rev Franc Etudes Clin Biol. 1959;4:210–225. [PubMed] [Google Scholar]
- 32.Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, Obrien S, Giralt S, Ippoliti C, von Wolff B, Gajewski J, Donato M, Claxton D, Ueno N, Andersson B, Gee A, Champlin R. Transplant lite: induction of graft versus malignancy using fludarabine based non ablative chemotherapy and allogeneic blood progenitor cell transplantation as treatment for lymphoid malignances. J.Clin.Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 33.Ballen KK, Colvin G, Porter D, Quesenberry PJ. Low dose total body irradiation followed by allogeneic lymphocyte infusion for refractory hematologic malignancy--an updated review. Leuk Lymphoma. 2004 May;45(5):905–910. doi: 10.1080/10428190310001628167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, Laport GG, Stockerl-Goldstein KELJ, Hoppe RT, Bloch DA, Blume KG, Negrin RS, Strober S. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005 Sep 29;353(13):1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 35.Sorror ML, Maris MB, Storer B, Sandmaier BM, Diaconescu R, Flowers C, Maloney DG, Storb R. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004 Aug 15;104(4):961–968. doi: 10.1182/blood-2004-02-0545. [DOI] [PubMed] [Google Scholar]
- 36.Mielcarek M, Martin PJ, Leisenring W, Flowers ME, Maloney DG, Sandmaier BM, Maris MB, Storb R. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003 Jul 15;102(2):756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 37.Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H, Cordonnier C, Rio B, Gratwohl A, Lange T, Al-Ali H, Storer B, Maloney D, McSweeney P, Chauncey T, Agura E, Bruno B, Maziarz RT, Petersen F, Storb R. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiationbased conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006 Jan 20;24(3):444–453. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 38.Maloney DG, Molina AJ, Sahebi F, Stockerl-Goldstein KE, Sandmaier BM, Bensinger W, Storer B, Hegenbart U, Somlo G, Chauncey T, Bruno B, Appelbaum FR, Blume KG, Forman SJ, McSweeney P, Storb R. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003 Nov 1;102(9):3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 39.Childs RW, Clave E, Tisdale J, Plante M, Hensel N, Barrett J. Successful treatment of metastatic renal cell carcinoma with a nonmyeloablative allogeneic peripheral-blood progenitor-cell transplant: evidence for a graft-versus-tumor effect. J Clin Oncol. 1999 Jul;17(7):2044–2049. doi: 10.1200/JCO.1999.17.7.2044. [DOI] [PubMed] [Google Scholar]
- 40.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, Marsh J, Milligan D, Goldstone A, Hunter A, Khwaja A, Chopra R, Littlewood T, Peniket A, Parker A, Jackson G, Hale G, Cook M, Russell N, Mackinnon S. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005 Dec 20;23(36):9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 41.Schmid C, Schleuning M, Schwerdtfeger R, HerteNMAein B, Mischak-Weissinger E, Bunjes D, Harsdorf SV, Scheid C, Holtick U, Greinix H, Keil F, Schneider B, Sandherr M, Bug G, Tischer J, Ledderose G, Hallek M, Hiddemann W, Kolb HJ. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006 Aug 1;108(3):1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 42.Crawley C, Szydlo R, Lalancette M, Bacigalupo A, Lange A, Brune M, Juliusson G, Nagler A, Gratwohl A, Passweg J, Komarnicki M, Vitek A, Mayer J, Zander A, Sierra J, Rambaldi A, Ringden O, Niederwieser D, Apperley JF Chronic Leukemia Working Party of the EBMT. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EEBMT. Blood. 2005 Nov 1;106(9):2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 43.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, Frassoni F, Boiron JM, Yin JL, Finke J, Shouten H, Blaise D, Falda M, Fauser AA, Esteve J, Polge E, Slavin S, Niederwieser D, Nagler A, Rocha V. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005 Dec;19(12):2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 44.Crawley C, Lalancette M, Szydlo R, Gilleece M, Peggs K, Mackinnon S, Juliusson G, Ahlberg L, Nagler A, Shimoni A, Sureda A, Boiron JM, Einsele H, Chopra R, Carella A, Cavenagh J, Gratwohl A, Garban F, Zander A, Bjorkstrand B, Niederwieser D, Gahrton G, Apperley JF Chromic Leukaemia Working Party of the EBMT. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005 Jun 1;105(11):4532–4539. doi: 10.1182/blood-2004-06-2387. [DOI] [PubMed] [Google Scholar]
- 45.Robinson SP, Goldstone AH, Mackinnon S, Carella A, Russell N, de Elvira CR, Taghipour G, Schmitz N Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002 Dec 15;100(13):4310–4316. doi: 10.1182/blood-2001-11-0107. [DOI] [PubMed] [Google Scholar]
- 46.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, Bacigalupo A, Beelen D, Reiffers J, Devergie A, Alessandrino E, Mufti GJ, Barge R, Sierra J, Ruutu T, Boogaerts M, Falda M, Jouet JP, Niederwieser D, de Witte T Myelodysplastic Syndrome subcommittee of the Chronic Leukemia Working Party of the European Blood and Marrow Transplantation Group. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006 Aug 1;108(3):836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 47.Storb R. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long-term follow-up. Br J Haematol. 2005 Sep;130(5):747–751. doi: 10.1111/j.1365-2141.2005.05667.x. [DOI] [PubMed] [Google Scholar]
- 48.Mapara MY, Pelot M, Zhao G, Swenson K, Pearson D, Sykes M. Induction of stable long-term mixed hematopoietic chimerism following nonmyeloablative conditioning with T cell-depleting antibodies, cyclophosphamide, and thymic irradiation leads to donor-specific in vitro and in vivo tolerance. Biol Blood Marrow Transplant. 2001;7(12):646–655. doi: 10.1053/bbmt.2001.v7.pm11787527. [DOI] [PubMed] [Google Scholar]
- 49.Brodsky RA, Chen AR, Brodsky I, Jones RJ. High dose cyclophosdphamide as salvage therapy for severe aplastic anemia. Exp HEmatol. 2004;125:408–409. doi: 10.1016/j.exphem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Tisdale JF, Dunn DE, Geller N, Plante M, Nunez O, Dunbar CE, Barrett AJ, Walsh TJ, Rosenfeld SJ, Young NS. High-dose cyclophosphamide in severe aplastic anaemia: a randomised trial. Lancet. 2000 Nov 4;356(9241):1554–1559. doi: 10.1016/S0140-6736(00)03126-3. [DOI] [PubMed] [Google Scholar]