Abstract
Background
Machado-Joseph disease (MJD), caused by a CAG repeat expansion located in exon10 of the ATXN3 gene, is now regarded as one of the most common spinocerebellar ataxia (SCA) in the world. The relative frequency of MJD among SCA has previously been estimated at about 50% in the Chinese population and has been reported to be related to the frequency of large normal alleles in some populations. Taq polymerase has been used for PCR in nearly all studies reported previously.
Methods
Normal and expanded alleles of ATXN3 were detected via PCR using LA Taq DNA polymerase (better for GC-rich sequences) and denaturing polyacrylamide gel electrophoresis in 150 normal individuals and 138 unrelated probands from autosomal dominant SCA families. To compare reaction efficiency, 12 MJD patients' expanded alleles were amplified with La Taq and Taq polymerase respectively in the same amplifying systems and reaction conditions.
Results
Normal alleles ranged from 12 to 42 CAG repeats. The most common allele contained 14 repeats with a frequency of 23.3%, which corroborates previous reports. The frequency of large normal alleles (>27 repeats) was 0.28, which was very high relative to previous reports. The frequency of MJD in SCA patients was 72.5%, which was significantly higher than those in previous reports about the Chinese and other Asian populations. This frequency was one of the highest reported worldwide, with only Portuguese and Brazilian populations exhibiting higher proportions. All 12 expanded alleles were amplified in PCR with La Taq polymerase, whereas only 2 expanded alleles were amplified with Taq polymerase.
Conclusion
We have first reported the highest relative frequency of MJD in Asia, and we attribute this high frequency to a more efficient PCR using LA Taq polymerase and hypothesized that large ANs may act as a reservoir for expanded alleles in the Southeastern Chinese population.
Background
Machado-Joseph disease (MJD), also called spinocerebellar ataxia type 3 (SCA3), is associated with a variety of clinical manifestations, including progressive ataxia, ophthalmoplegia, a variable degree of pyramidal signs, extrapyramidal signs and facial myokymia [1]. MJD was initially reported in Portuguese-Azorean descendants and is now regarded as one of the most common spinocerebellar ataxia (SCA) in the world [2,3]. It is caused by a CAG repeat expansion located in exon10 of the ATXN3 gene on chromosome 14q32.1 [4]. The number of CAG repeats was first described as 13-36 in normal alleles (ANs) and 68-79 in expanded alleles. With continued research and publication of related data, the range of ANs has broadened to 12-44, and now MJD is usually molecularly diagnosed when the CAG repeat crosses a threshold of 52 without regard for whether some patients carry "intermediate alleles" or "reduced penetrance alleles", such as 45-51[5].
The most common method used to study the expanded alleles of ATXN3 is to amplify expanded alleles with MJD52/MJD25 or MJD52/MJD70 primers [4]. Although amplifying systems and ion conditions vary across studies, Taq polymerase was used for PCR in nearly all previous reports. The efficiency of amplification is affected by long length and high GC content in the amplified sequence when using Taq polymerase [6] and it is difficult to amplify expanded alleles having large CAG repeats [7]. To alleviate this, here we use the LA Taq polymerase which is better for amplifying expanded alleles with GC-rich sequences.
The frequency of MJD in SCA patients differs in different population, and some studies [8-15] reported that the relative frequency of dominant SCA (including MJD) was related to the frequency of large normal alleles in some populations. In the present study, we have recruited 150 unrelated healthy individuals and 138 probands from autosomal dominant SCA families of Southeastern Chinese origin to analyze the distributions and characteristics of CAG repeats of ATXN3, and have found a much higher frequency (72.5%) of MJD in SCA patients compared to previous reports. In fact, this relative frequency is only slightly less than that reported in the Portuguese and Brazilian populations (84.2%) [16,17]. We have attributed this high relative frequency to better detection of expanded alleles via more efficient PCR using LA Taq polymerase and hypothesized that large ANs may act as a reservoir for expanded alleles in the Southeastern Chinese population.
Methods
Subjects
One hundred and fifty unrelated healthy individuals and 138 unrelated probands from autosomal dominant SCA families were recruited from the Southeastern Chinese population (figure 1) between July 9, 2003 and June 31, 2009. Patients were clinically diagnosed in accordance with the previously published standard [18]. Each patient was given detailed clinical and neurological examinations by two experienced neurologists and a clinical history was obtained. Informed consent was obtained from each subject (if <18 years of age, consent was obtained from their legal guardians) and the protocol was approved by the ethical committee. Genomic DNA was extracted from peripheral EDTA blood via the salt precipitation method [19] or with a QIAamp DNA Blood Minikit (QIAGEN, Hilden, Germany).
Figure 1.
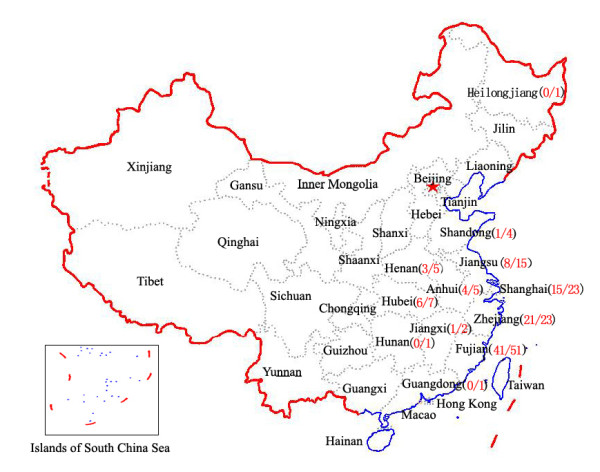
Distribution of the SCA families in the present study. The numbers in parentheses indicate numbers of MJD families/numbers of SCA families. (Reproduced with permission from http://nfgis.nsdi.gov.cn)
Molecular analysis
The CAG repeat expansion located in exon 10 of ATXN3 was amplified using MJD52/MJD25 primers as described in the previous report [4]. The PCR amplification was performed in a total volume of 25 μL containing 0.10 μg of genomic DNA, 0.10 μmol/L of each primer, 50 μmol/L of each dNTP and 1.25 units of LA Taq polymerase with 12.5 μL 2× GC buffer I (TaKaRa, Chiba, Japan). PCR products were generated using a Mini-Cycler PCR system (Applied Biosystems, Foster City, CA, USA). After an initial denaturation for 2 minutes at 94°C, the PCR reaction included 30 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 45 seconds and extension at 72°C for 1 minute, followed by a final extension at 72°C for 5 minutes. PCR products were separated by an 8% polyacrylamide gel that was run at 27 V/cm at 55°C for 3 hours. The pGEM-3Zf (+) DNA-Hae III marker was used as a DNA size marker and the gel was silver stained to visualize the bands. Based on the marker size (bp) and the transport ratio of the DNA marker in the gel, we used a statistical package (version 11.0, SPSS, Chicago, IL) to generate a curve with the equation Y = aXb (Y indicated the size of fragments of PCR products, X indicated the transport ratio of fragments of PCR products, a and b indicated coefficient and exponent that all generated by the statistical package, respectively) to estimate size of PCR products. The estimated number of CAG repeats = (size of fragments of PCR products- 161bp)/3. The numbers of CAG repeats of all subjects were estimated in this way at first. To verify accuracy of CAG numbers estimated, 10 normal alleles which were estimated to contain 14 CAG repeats and all expanded alleles were further confirmed by sequencing. The PCR products were electrophoresed on a 2.5% agarose gel and separated bands were excised. The DNA contained in excised bands was purified from the gel using the Geneclean II Kit (Qbiogene, Carlsbad, CA, USA). The purified product was sequenced using the procedure which has been described elsewhere [20].
To compare the efficiency of amplification using La Taq and Taq polymerase, 12 MJD patients' expanded alleles which were confirmed by PCR using La Taq polymerase were amplified with Taq polymerase in the same amplifying systems and reaction conditions mentioned above except that LA Taq polymerase with 12.5 μL 2× GC buffer I was displaced by Taq polymerase with 2.5 μL 10× buffer (Dichuan Inc, Shanghai, China). The PCR products amplified with Taq and La Taq polymerase were all electrophoresed on 2.5% agarose gel. The DL2000 (Tiangen, Bejing, China) was used as a DNA size marker.
Statistical analysis
All statistical analyses were performed using SPSS software version 11.0 (SPSS, Chicago, IL). The mean, median, variance and skewness were determined for the distributions of ANs. In accordance with the previous report[9], the alleles carrying more than 27 CAG repeats (>27 repeats) were defined as large ANs. Chi-square tests were used to analyse the difference between present study and other studies both in the frequency of the large ANs and the relative prevalence of MJD. The results were considered statistically significant at p < 0.05.
Results
Analysis of CAG repeats in normal individuals
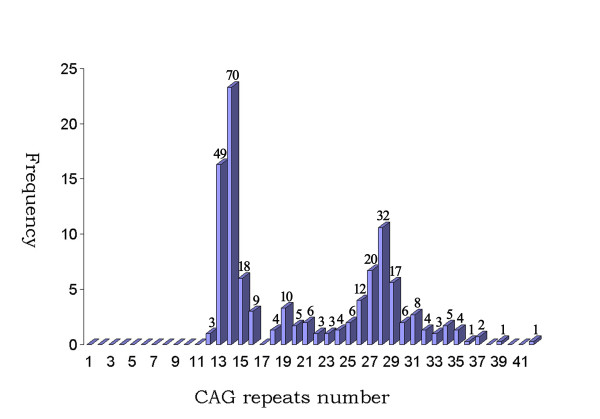
Twenty-seven alleles with the heterozygosity of 0.78 were identified in 150 normal individuals. The distribution of the 27 alleles is shown in figure 2. The number of CAG repeats ranged from 12 to 42. The three most frequent alleles had 14 (23.3%), 13 (16.3%) and 28 (10.6%) CAG repeats. The mean, median, variance and skewness were 20.8, 19.0, 56.0 and 0.42, respectively. The polyacrylamide gel electrophoresis analysis is shown in figure 3A and the results for the individual who was carrying homozygous for an allele which was estimated to contain 14 CAG repeats was confirmed by sequencing (figure. 4A). The difference in the frequency of large ANs between the present study and other studies involving Japanese [9], Indian [10], Czech [11] populations and a combined population comprised of Acadian, Black, Caucasian, Inuit and Thai [21] is shown in table 1.
Figure 2.
Distribution of the CAG repeats in 300 ANs from 150 healthy Chinese individuals. The numbers upon the column indicate the number of allele.
Figure 3.
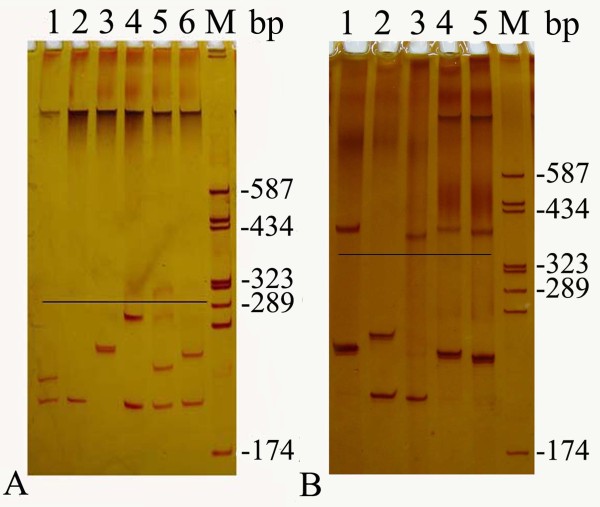
The polyacrylamide gel electrophoresis analysis of ANs (A) and alleles of SCA patients (B). In figure 2A, lanes 1-5 were normal individuals. The black line indicates the upper limit of ANs. In figure 2B, lanes 1, 3, 4, and 5 were MJD patients; lane 2 was an SCA patient, but not a MJD patient. The black line indicates the place of the lower limit of expanded alleles. M: pGEM-3Zf (+) DNA-Hae III marker.
Figure 4.
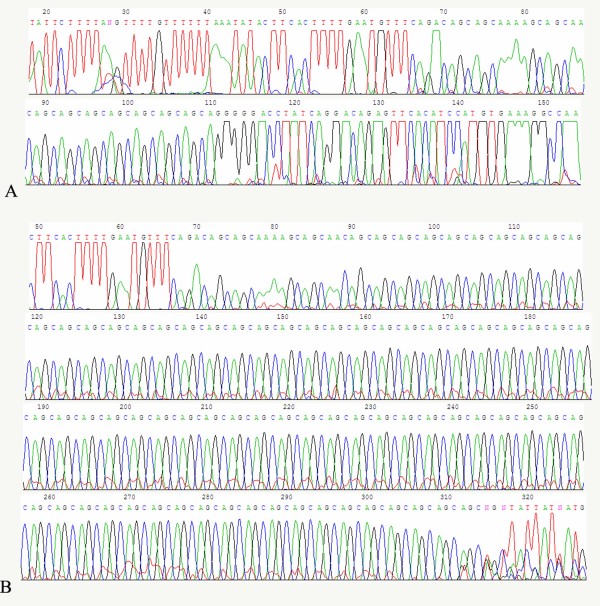
Chromatograms of the AN with 14 CAG (A) and the expanded allele with 81 CAG (B).
Table 1.
The frequencies of large normal alleles of ATXN3 gene
| Number of CAG repeats | Present study | Japanese | Indian | Czech | Combined Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Frequency | χ2 | P | Frequency | χ2 | P | Frequency | χ2 | P | Frequency | χ2 | P | |
| >27 | 0.28 | 0.21 | 3.683 | 0.055 | 0.12 | 42.045 | 0.000 | 0.09 | 27.663 | 0.000 | 0.17 | 8.616 | 0.003 |
| >28 | 0.19 | 0.11 | 6.788 | 0.009 | 0.08 | 29.060 | 0.000 | 0.04 | 21.956 | 0.000 | 0.11 | 10.341 | 0.001 |
| >29 | 0.13 | 0.07 | 5.869 | 0.015 | 0.04 | 28.517 | 0.000 | 0.04 | 11.836 | 0.001 | 0.08 | 5.264 | 0.002 |
| >30 | 0.10 | 0.05 | 4.342 | 0.037 | 0.03 | 22.329 | 0.000 | 0.03 | 8.499 | 0.004 | 0.07 | 3.192 | 0.074 |
| >31 | 0.07 | 0.05 | 1.332 | 0.248 | 0.02 | 14.120 | 0.000 | 0.01 | 8.186 | 0.004 | 0.05 | 2.190 | 0.139 |
Analysis of MJD expanded alleles
One hundred out of 138 probands were identified as having one expanded allele (>60 repeats), with the numbers of repeats varying from 68 to 84. Thus, the frequency of MJD in 138 SCA families was 72.5% (100/138), which is significantly higher than those reported in other Asian populations (table 2) and is quite high worldwide, being lower only than the Portuguese and Brazilian populations (table 3). In the other 38 probands with no expanded allele, 6 of them were homozygous for normal alleles and need to be further studied by other techniques. The polyacrylamide gel electrophoresis analysis is shown in figure 3B and the chromatogram of an expanded allele with 81 CAG repeats is shown in figure 4B.
Table 2.
Frequencies of MJD in SCA patients from different Asia populations
| Number of MJD | Number of SCA | Frequency (%) | P | Reference | |
|---|---|---|---|---|---|
| Present study | 100 | 138 | 72.5 | ||
| Chinese mainland | 59 | 120 | 49.2 | <0.001 | 23 |
| 41 | 85 | 48.2 | <0.001 | 24 | |
| 26 | 75 | 34.7 | <0.001 | 25 | |
| 1 | 8 | 12.5 | <0.001 | 22 | |
| Chinese Taiwan | 35 | 74 | 47.3 | <0.001 | 26 |
| 26 | 81 | 32.1 | <0.001 | 27 | |
| Singapore | 15 | 36 | 41.7 | 0.001 | 28 |
| Japan | 87 | 202 | 43.1 | <0.001 | 9 |
| 12 | 29 | 41.4 | 0.001 | 29 | |
| 91 | 330 | 27.6 | <0.001 | 30 | |
| 30 | 113 | 26.5 | <0.001 | 31 | |
| 35 | 143 | 24.5 | <0.001 | 32 | |
| 28 | 117 | 23.9 | <0.001 | 33 | |
| 8 | 46 | 17.4 | <0.001 | 34 | |
| 3 | 86 | 3.5 | <0.001 | 35 | |
| Thailand | 35 | 182# | 19.2 | <0.001 | 36 |
| Korea | 4 | 40 | 10.0 | <0.001 | 37 |
| 13 | 237 | 5.5 | <0.001 | 38 | |
| India | 5 | 14 | 35.7 | 0.005 | 39 |
| 15 | 105 | 14.3 | <0.001 | 40 | |
| 2 | 39 | 5.1 | <0.001 | 12 | |
| 7 | 143 | 4.9 | <0.001 | 10 | |
| 1 | 32 | 3.1 | <0.001 | 41 | |
| 2 | 77 | 2.6 | <0.001 | 42 | |
| 0 | 28 | 0 | <0.001 | 43 | |
# composed of patients with dominant SCA and sporadic ataxia
Table 3.
Frequencies of MJD in SCA patients from various countries
| Countries | Number of MJD | Number of SCA | Frequency (%) | Reference |
|---|---|---|---|---|
| Portugal | 32 | 38 | 84.2 | 16 |
| Brazil | 96 | 114 | 84.2 | 17 |
| Present study | 100 | 138 | 72.5 | |
| Portugal/Brazil | 67 | 106 | 63.2 | 44 |
| Germany | 32 | 77 | 41.6 | 45 |
| France | 25 | 87 | 28.7 | 46 |
| Holland | 64 | 227 | 28.2 | 47 |
| America* | 31 | 149 | 20.8 | 48 |
| Spain | 11 | 72 | 15.3 | 49 |
| Australia | 11 | 88 | 12.5 | 13 |
| Mexico | 13 | 108 | 12.0 | 50 |
| South Africa | 2 | 54 | 3.7 | 51 |
| Italy | 2 | 183 | 1.1 | 52 |
| UK | 0 | 22 | 0 | 53 |
| Serbia | 0 | 38 | 0 | 54 |
| Finland | 0 | 49 | 0 | 55 |
| Norway | 0 | 19 | 0 | 56 |
| Czech | 0 | 118# | 0 | 11 |
| Combined | 486 | 1687 | 28.8 |
*The patient subjects mixed with African American, Caucasian American, Cambodian, Japanese, Pakistani, Lebanese and Peruvian; # composed of patients with dominant SCA and sporadic ataxia
The agarose gel electrophoresis analysis of alleles of 12 MJD patients is shown in figure 5. All normal alleles were able to be amplified in PCR with La Taq or Taq polymerase. However, reaction efficiency was different for expanded alleles. All 12 and only 2 expaneded alleles were able to be amplified in PCR with La Taq polymerase (figure. 5B) and Taq polymerase (figure. 5A), respectively, for the same 12 MJD patients.
Figure 5.
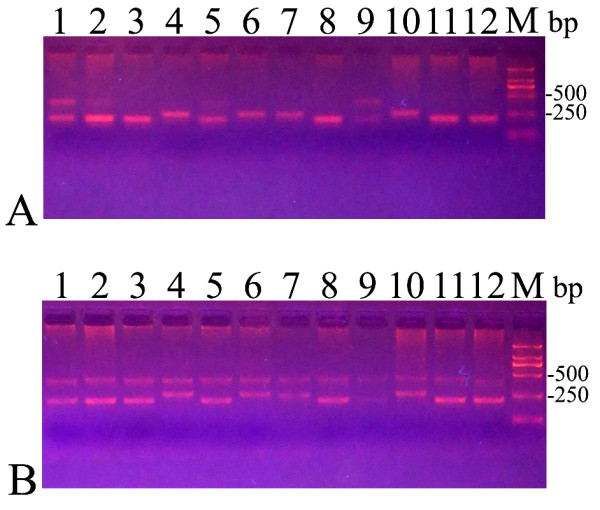
The agarose gel electrophoresis analysis of alleles of MJD patients. Lanes 1-12 were all MJD patients and were the same patients in A and B. A: The alleles were amplified with Taq polymerase. B: The alleles were amplified with LA Taq polymerase. M: DL2000 marker.
Discussion
In the present study, we have analyzed the characteristics of CAG repeats of ATXN3 in 300 chromosomes of healthy Chinese individuals and found that the CAG repeat number ranged from 12 to 42. The most common allele contained 14 CAG repeats, which was also the most common allele found in the studies of Limprasert et al [21] and Takano et al [9]. The frequency of large ANs (>27 repeats) was 0.28, which is higher than the frequency in previously studied populations [9-11,21].
The frequency of MJD in the 138 SCA families involved in our study was 72.5%, which is, as far as we know, the highest rate in Asia based on studies of the Chinese mainland individuals [22-25], Chinese Taiwanese [26,27], Singaporean [28], Japanese [9,29-35], Thais[36] Korean [37,38] and Indian [10,12,39-43]. Moreover, it is one of the highest worldwide, with only Portuguese and Brazilian populations exhibiting higher proportions [11,13,16,17,44-56]. We presume that there are two possibilities why the relative prevalence of MJD is so high in the present study.
The first reason is that the frequency of large ANs in the present study is very high. Some studies [8-15] suggest that the frequency of normal ANs with a relatively large number of CAG repeats is related to the prevalence of the dominant SCA (including MJD), since the large ANs share the same haplotypes as those of expanded alleles and therefore might act as a reservoir for expanded alleles. However, some other studies reported the prevalence of MJD is not an indirect reflection of the frequency of large normal alleles in Portuguese [57,58]. Here, the frequencies of large ANs are significantly higher than in related data reported by Limprasert et al [21] in >27, >28, and >29 repeats, Takano et al [9] in >28, >29, and >30 repeats, Chattopadhyay et al [10] and Bauer et al [11] in >27, >28, >29, >30, and >31 repeats. In fact, the relative frequency of MJD in the present study was the highest of any published data other than those from Portugal and Brazil, and was significantly higher than in the study of Takano et al [9], Chattopadhyay et al [10] and Bauer et al [11]. As such, the high frequency of large ANs associated with such a high relative frequency of MJD suggests that in this population large ANs may constitute a reservoir from which the expanded alleles may be emerging.
Our use of LA Taq polymerase to amplify alleles of SCA patients is another possible reason for the result. Arezi et al [6] reported that the efficiency of amplification using Taq polymerase was decreased with an increase in amplicon length and GC content, and the reason for the decrease may be related to the enzyme's lack of proofreading activity. Thus, it is difficult to amplify expanded alleles (GC-rich) and sometimes it is impossible to amplify expanded alleles when expanded alleles have a large quantity of CAG repeats [7]. We amplified expanded alleles of the same 12 MJD patients with Taq polymerase and LA Taq polymerase respectively and found most expanded alleles were non-amplified in PCR with Taq polymerase whereas all expanded alleles were amplified in PCR with LA Taq polymerase (figure. 5). For all the normal alleles that were amplified in PCR with Taq polymerase, it was possible that there were false negative results in molecular testing of MJD. The relative frequency of MJD in the present study is significantly higher than in the previous studies that also investigated Chinese SCA patients [22-28]. Notably, in these previous studies, the expanded alleles were amplified using Taq polymerase rather than LA Taq polymerase. Therefore, it may be better to use DNA polymerases that are better at amplifying GC-rich sequences, such as LA Taq, when amplifying the alleles of SCA patients. Additionally, 6 out of the 38 probands with no expanded allele were homozygous for normal alleles. It is possible that these results are false negative results because of either extremely large repeat size or the presence of polymorphisms in the primer-annealing regions [7]. Therefore we need to apply to other techniques such as Southern blot to exclude this possibility of false negative results in further study. However, no matter whatever the results of this further study show, the high relative frequency of MJD in the present study is not affected.
In addition, Martins et al [59] concluded that MJD might first occur in Asia and extend to Europe later, reaching its high prevalence in Portuguese due to founder effect. The high prevalence of MJD (19.2 per 100,000 inhabitants) restricted to the Gosei area of Toyama in Japan [60] may support this conclusion [59]. Therefore we suppose that possible Asian origin of MJD and founder effect may also contribute to the high relative frequency of MJD in the present study.
Conclusion
We have reported the characteristics of CAG repeats in ATXN3 in a normal Chinese population and in patients with SCA. We found the highest relative frequency of MJD observed in Asian SCA patients. The results support the hypothesis that in this population large ANs may constitute a reservoir from which the expanded alleles may be emerging. Furthermore, LA Taq polymerase was proven to be more efficient than Taq polymerase in the amplification of the expanded alleles, facilitating and improving the molecular diagnosis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SRG carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. JJW carried out the molecular genetic studies and participated in the sequence alignment. NW, SXM and CZL participated in analysis and interpretation of data. SSS and STW participated in the sequence alignment. ZYW designed and supervised the study, and critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Shi-Rui Gan, Email: ganshirui1981@163.com.
Sheng-Sheng Shi, Email: shishengsheng@gmail.com.
Jian-Jun Wu, Email: jungliw@gmail.com.
Ning Wang, Email: nwang63@yahoo.com.cn.
Gui-Xian Zhao, Email: zhaogx5639@yahoo.com.cn.
Sheng-Tong Weng, Email: wst3344@126.com.
Shen-Xing Murong, Email: zhiyingwucn@yahoo.com.cn.
Chuan-Zhen Lu, Email: luchuanzhen@yahoo.com.cn.
Zhi-Ying Wu, Email: zhiyingwu67@yahoo.com.
Acknowledgements
This work was supported by grant from Huashan Hospital for special professorship of Fudan University, Shanghai, grant FMU-RT002 of program for Innovative Research Team in Science and Technology in Fujian Province University, Fuzhou, China. We sincerely thank the participants for their help and willingness to participate in this study, and also thank the reviewers for improving this manuscript.
References
- Takiyama Y, Oyanagi S, Kawashima S, Sakamoto H, Saito K, Yoshida M, Tsuji S, Mizuno Y, Nishizawa M. A clinical and pathologic study of a large Japanese family with Machado-Joseph disease tightly linked to the DNA markers on chromosome 14q. Neurology. 1994;44:1302–1308. doi: 10.1212/wnl.44.7.1302. [DOI] [PubMed] [Google Scholar]
- Sequeiros J, Coutinho P. Epidemiology and clinical aspects of Machado-Joseph disease. Adv Neurol. 1993;61:139–153. [PubMed] [Google Scholar]
- Schöls L, Bauer P, Schmidt T, Schulte T, Riess O. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3:291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, Kawakami H, Nakamura S, Nishimura M, Akiguchi I, Kimura J, Narumiya S, Kakizuka A. CAG repeat expansions in a novel gene for Machado-Joseeph disease at chromosome 14q32.1. Nature Genet. 1994;8:221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- NCBI Bookshelf-GeneReviews-Spinocerebellar Ataxia Type 3. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=sca3
- Arezi B, Xing W, Sorge JA, Hogrefe HH. Amplification efficiency of thermostable DNA polymerases. Anal Biochem. 2003;321:226–235. doi: 10.1016/S0003-2697(03)00465-2. [DOI] [PubMed] [Google Scholar]
- Maciel P, Costa MC, Ferro A, Rousseau M, Santos CS, Gaspar C, Barros J, Rouleau GA, Coutinho P, Sequeiros J. Improvement in the molecular diagnosis of Machado-Joseph disease. Arch Neurol. 2001;58:1821–1827. doi: 10.1001/archneur.58.11.1821. [DOI] [PubMed] [Google Scholar]
- Mittal U, Srivastava AK, Jain S, Jain S, Mukerji M. Founder haplotype for Machado-Joseph disease in the Indian population: novel insights from history and polymorphism studies. Arch Neurol. 2005;62:637–640. doi: 10.1001/archneur.62.4.637. [DOI] [PubMed] [Google Scholar]
- Takano H, Cancel G, Ikeuchi T, Lorenzetti D, Mawad R, Stevanin G, Didierjean O, Dürr A, Oyake M, Shimohata T, Sasaki R, Koide R, Igarashi S, Hayashi S, Takiyama Y, Nishizawa M, Tanaka H, Zoghbi H, Brice A, Tsuji S. Close associations between prevalences of dominantly inherited spinocerebellar ataxias with CAG-repeat expansions and frequencies of large normal CAG alleles in Japanese and Caucasian populations. Am J Hum Genet. 1998;63:1060–1066. doi: 10.1086/302067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay B, Basu P, Gangopadhyay PK, Mukherjee SC, Sinha KK, Chakraborty A, Roy T, Roychoudhury S, Majumder PP, Bhattacharyya NP. Variation of CAG repeats and two intragenic polymorphisms at SCA3 locus among Machado-Joseph disease/SCA3 patients and diverse normal populations from eastern India. Acta Neurol Scand. 2003;108:407–414. doi: 10.1034/j.1600-0404.2003.00167.x. [DOI] [PubMed] [Google Scholar]
- Bauer PO, Zumrova A, Matoska V, Marikova T, Krilova S, Boday A, Singh B, Goetz P. Absence of spinocerebellar ataxia type 3/Machado-Joseph disease within ataxic patients in the Czech population. Eur J Neurol. 2005;12:851–857. doi: 10.1111/j.1468-1331.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- Saleem Q, Choudhry S, Mukerji M, Bashyam L, Padma MV, Chakravarthy A, Maheshwari MC, Jain S, Brahmachari SK. Molecular analysis of autosomal dominant hereditary ataxias in the Indian population: high frequency of SCA2 and evidence for a common founder mutation. Hum Genet. 2000;106:179–187. doi: 10.1007/s004390051026. [DOI] [PubMed] [Google Scholar]
- Storey E, du Sart D, Shaw JH, Lorentzos P, Kelly L, McKinley Gardner RJ, Forrest SM, Biros I, Nicholson GA. Frequency of Spinocerebellar Ataxia Types 1, 2, 3, 6, and 7 in Australian Patients with Spinocerebellar Ataxia. Am J Med Genet. 2000;95:351–357. doi: 10.1002/1096-8628(20001211)95:4<351::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Stevanin G, Lebre AS, Mathieux C, Cancel G, Abbas N, Didierjean O, Dürr A, Trottier Y, Agid Y, Brice A. Linkage disequilibrium between the spinocerebellar ataxia 3/Machado-Joseph disease mutation and two intrageneic polymorphisms, one of which, X359Y, affects the stop codon. Am J Hum Genet. 1997;60:1548–1552. doi: 10.1016/S0002-9297(07)64251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YR, Lin HY, Chen CM, Gwinn-Hardy K, Ro LS, Wang YC, Li SH, Hwang JC, Fang K, Hsieh-Li HM, Li ML, Tung LC, Su MT, Lu KT, Lee-Chen GJ. Genetic testing in spinocerebellar ataxia in Taiwan: expansions of trinucleotide repeats in SCA8 and SCA17 are associated with typical Parkinson's disease. Clin Genet. 2004;65:209–214. doi: 10.1111/j.0009-9163.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- Silveira I, Lopes-Cendes I, Kish S, Maciel P, Gaspar C, Coutinho P, Botez MI, Teive H, Arruda W, Steiner CE, Pinto-Júnior W, Maciel JA, Jerin S, Sack G, Andermann E, Sudarsky L, Rosenberg R, MacLeod P, Chitayat D, Babul R, Sequeiros J, Rouleau GA. Frequency of spinocerebellar ataxia type1, dentatorubropallidoluysian atrophy, and Machado-Joseph disease mutations in a large group of spinocerebellar ataxia patients. Neurology. 1996;46:214–218. doi: 10.1212/wnl.46.1.214. [DOI] [PubMed] [Google Scholar]
- Trott A, Jardim LB, Ludwig HT, Saute JAM, Artigalas O, Kieling C, Wanderley HYC, Rieder CRM, Monte TL, Socal M, Alonso I, Ferro A, Carvalho T, do Ceu Moreira M, Mendonca P, Ferreirinha F, Silveira I, Sequeiros J, Giugliani R, Saraiva-Pereira ML. Spinocerebellar ataxias in 114 Brazilian families: clinical and molecular findings. Clin Genet. 2006;70:173–176. doi: 10.1111/j.1399-0004.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- Harding AE. Clinical features and classification of inherited ataxias. Adv Neurol. 1994;61:1–14. [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZY, Zhao GX, Chen WJ, Wang N, Wan B, Lin MT, Murong SX, Yu L. Mutation analysis of 218 Chinese patients with Wilson disease revealed no correlation between the canine copper toxicosis gene MURR1 and Wilson disease. J Mol Med. 2006;84:438–442. doi: 10.1007/s00109-005-0036-y. [DOI] [PubMed] [Google Scholar]
- Limprasert P, Nouri N, Heyman RA, Nopparatana C, Kamonsilp M, Deininger PL, Keats BJ. Analysis of CAG repeat of the Machado-Joseph gene in human, chimpanzee and monkey populations: a variant nucleotide is associated with the number of CAG repeats. Hum Mol Genet. 1996;5:207–213. doi: 10.1093/hmg/5.2.207. [DOI] [PubMed] [Google Scholar]
- Jiang M, Jin CL, Lin CK, Qiu GR, Liu ZL, Wang CX, Sun KL. Analysis and application of SCA1 and SCA3/MJD gene CAG repeats in Han population in Northeastern China. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:83–85. in Chinese. [PubMed] [Google Scholar]
- Jiang H, Tang BS, Xu B, Zhao GH, Shen L, Tang JG, Li QH, Xia K. Frequency analysis of autosomal dominant spinocerebellar ataxias in Mainland Chinese patients and clinical and molecular characterization of spinocerebellar ataxia type 6. Chin Med J (Engl) 2005;118:837–843. [PubMed] [Google Scholar]
- Tang B, Liu C, Shen L, Dai H, Pan Q, Jing L, Ouyang S, Xia J. Frequency of SCA1, SCA2, SCA3/MJD, SCA6, SCA7, and DRPLA CAG trinucleotide repeat expansion in patients with hereditary spinocerebellar ataxia from Chinese kindreds. Arch Neurol. 2000;57:540–544. doi: 10.1001/archneur.57.4.540. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Qiao WH, Gu WH, Xie H, Tang BS, Zhou LS, Yang BX, Takiyama Y, Tsuji S, He HY, Deng CX, Goldfarb LG, Wang GX. Spinocerebellar ataxia type 1 in China:molecular analysis and genotypephenotype correlation in 5 families. Arch Neurol. 2001;58:789–794. doi: 10.1001/archneur.58.5.789. [DOI] [PubMed] [Google Scholar]
- Soong BW, Lu YC, Choo KB, Lee HY. Frequency analysis of autosomal dominant cerebellar ataxias in Taiwanese patients and clinical and molecular characterization of spinocerebellar ataxia type 6. Arch Neurol. 2001;58:1105–1109. doi: 10.1001/archneur.58.7.1105. [DOI] [PubMed] [Google Scholar]
- Tsai HF, Liu CS, Leu TM, Wen FC, Lin SJ, Liu CC, Yang DK, Li C, Hsieh M. Analysis of trinucleotide repeats in different SCA loci in spinocerebellar ataxia patients and in normal population of Taiwan. Acta Neurol Scand. 2004;109:355–360. doi: 10.1046/j.1600-0404.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tan EK, Law HY, Yoon CS, Wong MC, Ng I. Prevalence and ethnic differences of autosomal-dominant cerebellar ataxia in Singapore. Clin Genet. 2002;62:478–481. doi: 10.1034/j.1399-0004.2002.620610.x. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Sugiura Y, Shimoji S, Kumagai T, Tochikubo S, Yamamoto T. Incidence of genetic subgroups of hereditary spinocerebellar ataxia in Fukushima Prefecture. Tohoku J Exp Med. 2001;195:85–91. doi: 10.1620/tjem.195.85. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Izumi Y, Morino H, Oda M, Toji H, Nakamura S, Kawakami H. Difference in disease-free survival curve and regional distribution according to subtype of spinocerebellar ataxia: a study of 1286 Japanese patients. Am J Med Genet. 2002;114:578–583. doi: 10.1002/ajmg.10514. [DOI] [PubMed] [Google Scholar]
- Basri R, Yabe I, Soma H, Sasaki H. Spectrum and prevalence of autosomal dominant spinocerebellar ataxia in Hokkaido, the northern island of Japan: a study of 113 Japanese families. J Hum Genet. 2007;52:848–855. doi: 10.1007/s10038-007-0182-x. [DOI] [PubMed] [Google Scholar]
- Matsumura R, Futamura N, Ando N, Ueno S. Frequency of spinocerebellar ataxia mutation in the Kinki district of Japan. Acta Neurol Scand. 2003;107:38–41. doi: 10.1034/j.1600-0404.2003.01347.x. [DOI] [PubMed] [Google Scholar]
- Onodera Y, Aoki M, Tsuda T, Kato H, Nagata T, Kameya T, Abe K, Itoyama Y. High prevalence of spinocerebellar ataxia type 1 (SCA1) in an isolated region of Japan. J Neurol Sci. 2000;178:153–158. doi: 10.1016/S0022-510X(00)00390-7. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Adachi Y, Mori M, Nakano T, Nakashima K. Clinical and genetic epidemiological study of 16q22.1-linked autosomal dominant cerebel lar ataxia in western Japan. Acta Neurol Scand. 2007;116:123–127. doi: 10.1111/j.1600-0404.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Yoshida K, Okano T, Ohara S, Hashimoto T, Fukushima Y, Ikeda S. Regional features of autosomal-dominant cerebellar ataxia in Nagano: clinical and molecular genetic analysis of 86 families. J Hum Genet. 2004;49:610–616. doi: 10.1007/s10038-004-0196-6. [DOI] [PubMed] [Google Scholar]
- Sura T, Eu-Ahsunthornwattana J, Youngcharoen S, Busabaratana M, Dejsuphong D, Trachoo O, Theerasasawat S, Tunteeratum A, Noparutchanodom C, Tunlayadechanont S. Frequencies of spinocerebellar ataxia subtypes in Thailand: window to the population history? J Hum Genet. 2009;54:284–288. doi: 10.1038/jhg.2009.27. [DOI] [PubMed] [Google Scholar]
- Jin DK, Oh MR, Song SM, Koh SW, Lee M, Kim GM, Lee WY, Chung CS, Lee KH, Im JH, Lee MJ, Kim JW, Lee MS. Frequency of spinocerebellar ataxia types 1,2,3,6,7 and dentatorubral pallidoluysian atrophy mutations in Korean patients with spinocerebellar ataxia. J Neurol. 1999;246:207–210. doi: 10.1007/s004150050335. [DOI] [PubMed] [Google Scholar]
- Lee WY, Jin DK, Oh MR, Lee JE, Song SM, Lee EA, Kim GM, Chung JS, Lee KH. Frequency analysis and clinical characterization of spinocerebellar ataxia types 1, 2, 3, 6, and 7 in Korean patients. Arch Neurol. 2003;60:858–863. doi: 10.1001/archneur.60.6.858. [DOI] [PubMed] [Google Scholar]
- Chakravarty A, Mukherjee SC. Autosomal dominant cerebellar ataxias in ethnic Bengalees in West Bengal-an Eastern Indian state. Acta Neurol Scand. 2002;105:202–208. doi: 10.1034/j.1600-0404.2002.1o054.x. [DOI] [PubMed] [Google Scholar]
- Krishna N, Mohan S, Yashavantha BS, Rammurthy A, Kiran Kumar HB, Mittal U, Tyagi S, Mukerji M, Jain S, Pal PK, Purushottam M. SCA 1, SCA 2 & SCA 3/MJD mutations in ataxia syndromes in southern India. Indian J Med Res. 2007;126:465–470. [PubMed] [Google Scholar]
- Alluri RV, Komandur S, Wagheray A, Chaudhuri JR, Sitajayalakshmi, Meena AK, Jabeen A, Chawda K, Subhash K, Krishnaveni A, Hasan Q. Molecular analysis of CAG repeats at five different spinocerebellar ataxia loci: correlation and alternative explanations for disease pathogenesis. Mol Cells. 2007;24:338–342. [PubMed] [Google Scholar]
- Srivastava AK, Choudhry S, Gopinath MS, Roy S, Tripathi M, Brahmachari SK, Jain S. Molecular and clinical correlation in five Indian families with spinocerebellar ataxia 12. Ann Neurol. 2001;50:796–800. doi: 10.1002/ana.10048. [DOI] [PubMed] [Google Scholar]
- Sinha KK, Worth PF, Jha DK, Sinha S, Stinton VJ, Davis MB, Wood NW, Sweeney MG, Bhatia KP. Autosomal dominant cerebellar ataxia: SCA2 is the most frequent mutation in eastern India. J Neurol Neurosurg Psychiatr. 2004;75:448–452. doi: 10.1136/jnnp.2002.004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira I, Miranda C, Guimarães L, Moreira MC, Alonso I, Mendonça P, Ferro A, Pinto-Basto J, Coelho J, Ferreirinha F, Poirier J, Parreira E, Vale J, Januário C, Barbot C, Tuna A, Barros J, Koide R, Tsuji S, Holmes SE, Margolis RL, Jardim L, Pandolfo M, Coutinho P, Sequeiros J. Trinucleotide repeats in 202 families with ataxia: a small expanded (CAG)n allele at the SCA17 locus. Arch Neurol. 2002;59:623–629. doi: 10.1001/archneur.59.4.623. [DOI] [PubMed] [Google Scholar]
- Schöls L, Amoiridis G, Büttner T, Przuntek H, Epplen JT, Riess O. Autosomal dominant cerebellar ataxia: phenotypic differences in genetically defined subtypes? Ann Neurol. 1997;42:924–932. doi: 10.1002/ana.410420615. [DOI] [PubMed] [Google Scholar]
- Dürr A, Stevanin G, Cancel G, Duyckaerts C, Abbas N, Didierjean O, Chneiweiss H, Benomar A, Lyon-Caen O, Julien J, Serdaru M, Penet C, Agid Y, Brice A. Spinocerebellar ataxia 3 and Machado- Joseph disease: clinical, molecular, and neuropathological features. Ann Neurol. 1996;39:490–499. doi: 10.1002/ana.410390411. [DOI] [PubMed] [Google Scholar]
- Warrenburg BP van de, Sinke RJ, Verschuuren-Bemelmans CC, Scheffer H, Brunt ER, Ippel PF, Maat-Kievit JA, Dooijes D, Notermans NC, Lindhout D, Knoers NV, Kremer HP. Spinocerebellar ataxias in the Netherlands: prevalence and age at onset variance analysis. Neurology. 2002;58:702–708. doi: 10.1212/wnl.58.5.702. [DOI] [PubMed] [Google Scholar]
- Ranum LP, Lundgren JK, Schut LJ, Ahrens MJ, Perlman S, Aita J, Bird TD, Gomez C, Orr HT. Spinocerebellar ataxia type 1 and Machado-Joseph disease: incidence of CAG expansions among adult-onset ataxia patients from 311 families with dominant, recessive, or sporadic ataxia. Am J Hum Genet. 1995;57:603–608. [PMC free article] [PubMed] [Google Scholar]
- Pujana MA, Corral J, Gratacòs M, Combarros O, Berciano J, Genís D, Banchs I, Estivill X, Volpini V. Spinocerebellar ataxias in Spanish patients: genetic analysis of familial and sporadic cases. The Ataxia Study Group. Hum Genet. 1999;104:516–522. doi: 10.1007/s004390050997. [DOI] [PubMed] [Google Scholar]
- Alonso E, Martínez-Ruano L, De Biase I, Mader C, Ochoa A, Yescas P, Gutiérrez R, White M, Ruano L, Fragoso-Benítez M, Ashizawa T, Bidichandani SI, Rasmussen A. Distinct distribution of autosomal dominant spinocerebellar ataxia in the Mexican population. Mov Disord. 2007;22:1050–1053. doi: 10.1002/mds.21470. [DOI] [PubMed] [Google Scholar]
- Bryer A, Krause A, Bill P, Davids V, Bryant D, Butler J, Heckmann J, Ramesar R, Greenberg J. The hereditary adult-onset ataxias in South Africa. J Neurol Sci. 2003;216:47–54. doi: 10.1016/S0022-510X(03)00209-0. [DOI] [PubMed] [Google Scholar]
- Brusco A, Gellera C, Cagnoli C, Saluto A, Castucci A, Michielotto C, Fetoni V, Mariotti C, Migone N, Di Donato S, Taroni F. Molecular genetics of hereditary spinocerebellar ataxia: mutation analysis of spinocerebellar ataxia genes and CAG/CTG repeat expansion detection in 225 Italian families. Arch Neurol. 2004;61:727–733. doi: 10.1001/archneur.61.5.727. [DOI] [PubMed] [Google Scholar]
- Leggo J, Dalton A, Morrison PJ, Dodge A, Connarty M, Kotze MJ, Rubinsztein DC. Analysis of spinocerebellar ataxia types 1, 2, 3, and 6, dentatorubral-pallidoluysian atrophy, and Friedreich's ataxia genes in spinocerebellar ataxia patients in the UK. J Med Genet. 1997;34:982–985. doi: 10.1136/jmg.34.12.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragasević NT, Culjković B, Klein C, Ristić A, Keckarević M, Topisirović I, Vukosavić S, Svetel M, Kock N, Stefanova E, Romac S, Kostić VS. Frequency analysis and clinical characterization of different types of spinocerebellar ataxia in Serbian patients. Mov Disord. 2006;21:187–191. doi: 10.1002/mds.20687. [DOI] [PubMed] [Google Scholar]
- Juvonen V, Hietala M, Kairisto V, Savontaus ML. The occurrence of dominant spinocerebellar ataxias among 251 Finnish ataxia patients and the role of predisposing large normal alleles in a genetically isolated population. Acta Neurol Scand. 2005;111:154–162. doi: 10.1111/j.1600-0404.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- Koht J, Tallaksen CM. Cerebellar ataxia in the eastern and southern parts of Norway. Acta Neurol Scand Suppl. 2007;187:76–79. doi: 10.1111/j.1600-0404.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- Maciel P, Gaspar C, Guimarães L, Goto J, Lopes-Cendes I, Hayes S, Arvidsson K, Dias A, Sequeiros J, Sousa A, Rouleau GA. Study of three intragenic polymorphisms in the Machado-Joseph disease gene (MJD1) in relation to genetic instability of the (CAG)n tract. Eur J Hum Genet. 1999;7:147–156. doi: 10.1038/sj.ejhg.5200264. [DOI] [PubMed] [Google Scholar]
- Lima M, Costa MC, Montiel R, Ferro A, Santos C, Silva C, Bettencourt C, Sousa A, Sequeiros J, Coutinho P, Maciel P. Population genetics of wild-type CAG repeats in the Machado-Joseph disease gene in Portugal. Hum Hered. 2005;60:156–163. doi: 10.1159/000090035. [DOI] [PubMed] [Google Scholar]
- Martins S, Calafell F, Gaspar C, Wong VC, Silveira I, Nicholson GA, Brunt ER, Tranebjaerg L, Stevanin G, Hsieh M, Soong BW, Loureiro L, Dürr A, Tsuji S, Watanabe M, Jardim LB, Giunti P, Riess O, Ranum LP, Brice A, Rouleau GA, Coutinho P, Amorim A, Sequeiros J. Asian origin for the worldwidespread mutational event in Machado-Joseph disease. Arch Neurol. 2007;64:1502–1508. doi: 10.1001/archneur.64.10.1502. [DOI] [PubMed] [Google Scholar]
- Shibata-Hamaguchi A, Ishida C, Iwasa K, Yamada M. Prevalence of spinocerebellar degenerations in the Hokuriku district in Japan. Neuroepidemiology. 2009;32:176–183. doi: 10.1159/000195686. [DOI] [PubMed] [Google Scholar]