Abstract
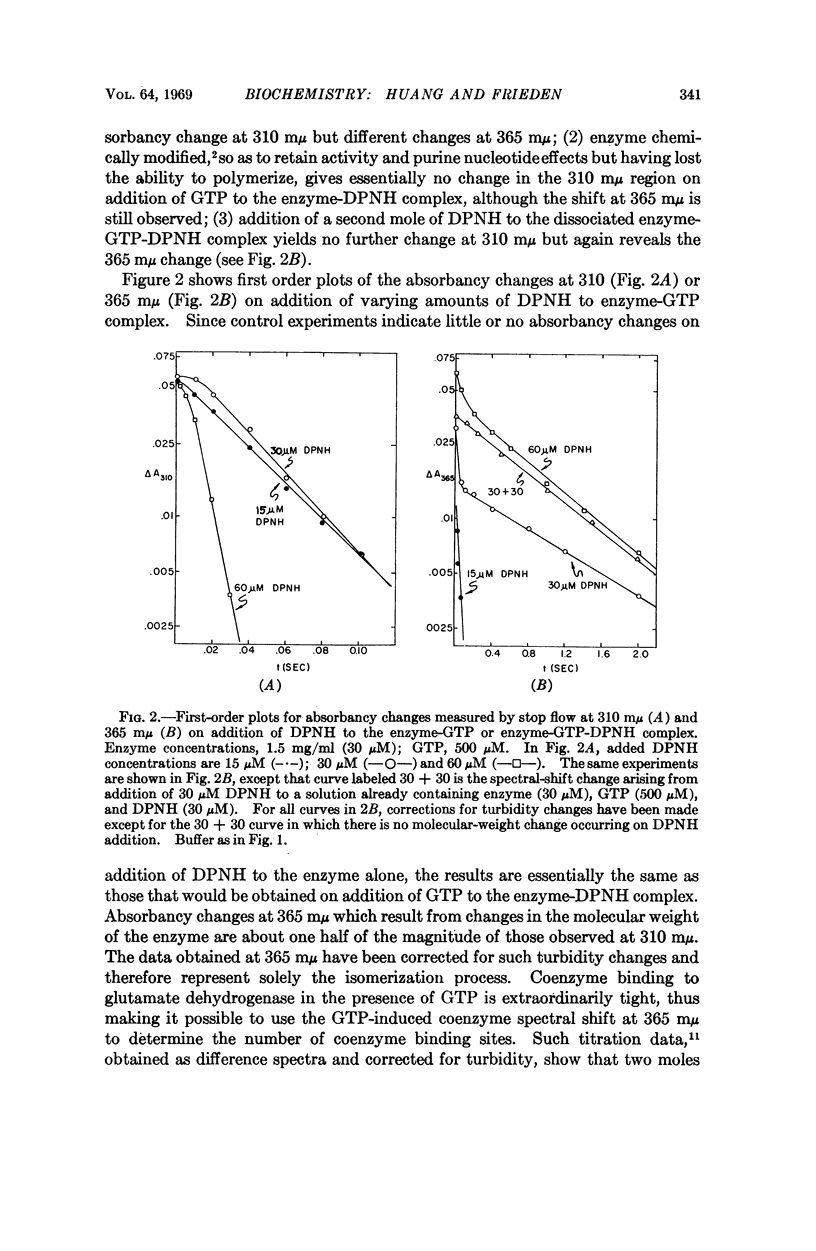
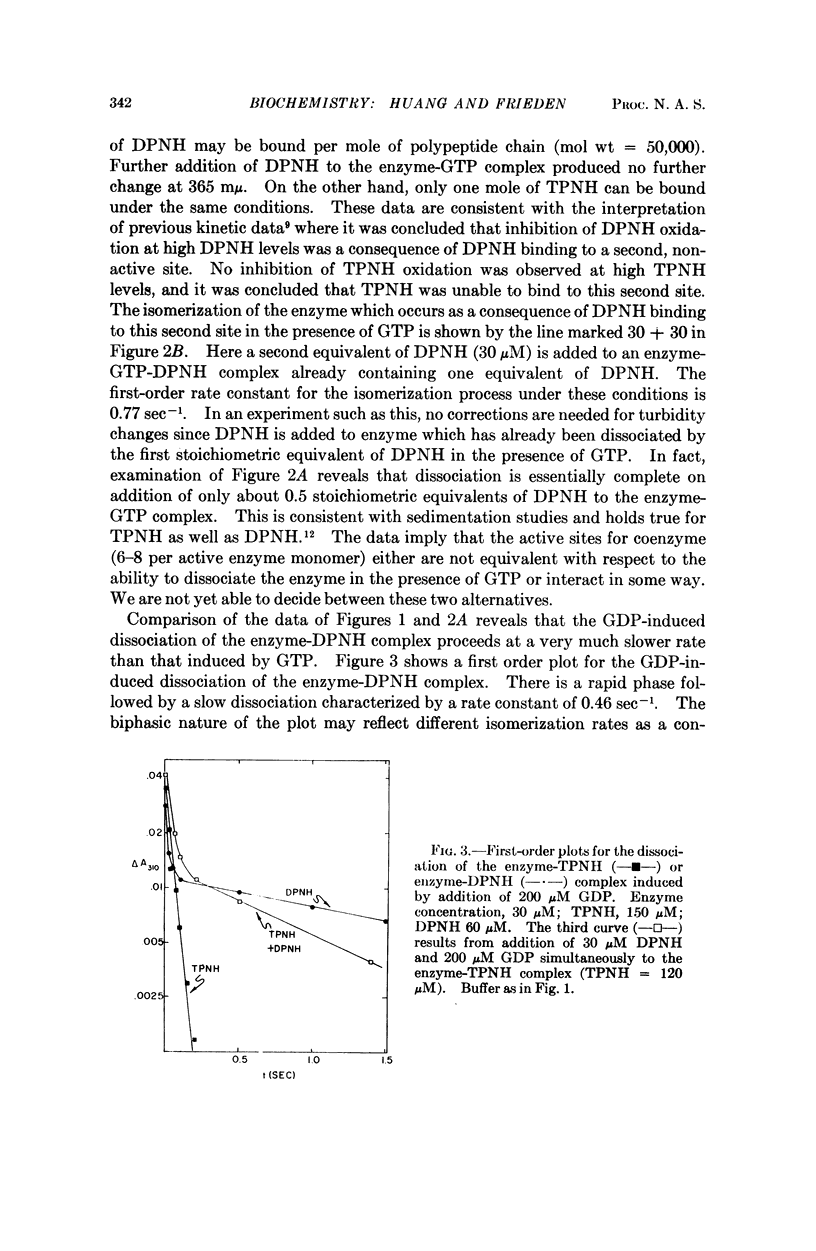
The rate of the depolymerization of beef liver glutamate dehydrogenase induced by coenzyme and the purine nucleotides guanosine 5′-diphosphate and guanosine 5′-triphosphate, which are potent inhibitors of enzymatic activity, has been measured by rapid light scattering techniques and by absorbancy changes with stop flow. It is shown that the rate constant for this process may vary from several milliseconds to several seconds depending upon the nucleotides used. The widely varying rate constants for the nucleotide-induced depolymerization may serve a role in determining the nature of the regulation of enzyme activity by nucleotides. Depolymerization induced by guanosine 5′-diphosphate in the presence of diphosphopyridine nucleotide is slower than in the presence of triphosphopyridine nucleotide as coenzyme, and this difference is apparently due to the isomerization of the enzyme as a result of diphosphopyridine nucleotide binding to a second, nonactive site. This binding, as well as binding of the coenzyme to the active site, may be conveniently measured by a purine nucleotide-induced spectral shift in the coenzyme absorption spectrum. It is also shown that complete depolymerization of the enzyme in the presence of guanosine 5′-triphosphate is accomplished by about half saturation of the coenzyme active sites (6-8 active “monomer”).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber E. D., Bright H. J. The rate of an allosteric process: inhibition of homoserine dehydrogenase I from E. coli by threonine. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1363–1370. doi: 10.1073/pnas.60.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun P. W., Kim S. J. Determination of the equilibrium constants of associating protein systems. IV. The application of the weight-average partition coefficient to analysis of BM1 nonideality term (as applied to bovine liver L-glutamate dehydrogenase). Biochemistry. 1969 Apr;8(4):1633–1643. doi: 10.1021/bi00832a045. [DOI] [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. I. Effect of acetylation on catalytic and regulatory properties. J Biol Chem. 1966 Aug 25;241(16):3652–3660. [PubMed] [Google Scholar]
- Eisenberg H., Tomkins G. M. Molecular weight of the subunits, oligomeric and associated forms of bovine liver glutamate dehydrogenase. J Mol Biol. 1968 Jan 14;31(1):37–49. doi: 10.1016/0022-2836(68)90052-1. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. I. The effect of coenzyme on the sedimentation velocity and kinetic behavior. J Biol Chem. 1959 Apr;234(4):809–814. [PubMed] [Google Scholar]
- Frieden C., Colman R. F. Glutamate dehydrogenase concentration as a determinant in the effect of purine nucleotides on enzymatic activity. J Biol Chem. 1967 Apr 25;242(8):1705–1715. [PubMed] [Google Scholar]
- MARLER E., TANFORD C. THE MOLECULAR WEIGHT OF THE POLYPEPTIDE CHAINS OF L-GLUTAMATE DEHYDROGENASE. J Biol Chem. 1964 Dec;239:4217–4218. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nichol L. W., Jackson W. J., Winzor D. J. A theoretical study of the binding of small molecules to a polymerizing protein system. A model for allosteric effects. Biochemistry. 1967 Aug;6(8):2449–2456. doi: 10.1021/bi00860a022. [DOI] [PubMed] [Google Scholar]


