Abstract
Oxidative stress results in apoptosis of neuronal cells, leading to neurodegenerative disorders. However, the underlying molecular mechanism remains to be elucidated. Here we show that hydrogen peroxide (H2O2), a major oxidant generated when oxidative stress occurs, induced apoptosis of neuronal cells (PC12 cells and primary murine neurons), by inhibiting the mammalian target of rapamycin (mTOR)-mediated phosphorylation of ribosomal p70 S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1). N-acetyl-L-cysteine (NAC), a scavenger of reactive oxygen species (ROS), blocked H2O2 inhibition of mTOR signaling. Ectopic expression of wild type (wt) mTOR, constitutively active S6K1 or downregulation of 4E-BP1 partially prevented H2O2 induction of apoptosis. Furthermore, we identified that H2O2 induction of ROS inhibited the upstream kinases, Akt and phosphoinositide-dependent kinase 1 (PDK1), but not the type I insulin-like growth factor receptor (IGFR), and activated the negative regulator, AMP-activated protein kinase α (AMPKα), but not the phosphatase and tensin homolog (PTEN) in the cells. Expression of a dominant negative AMPKα or downregulation of AMPKα1 conferred partial resistance to H2O2 inhibition of phosphorylation of S6K1 and 4E-BP1, as well as cell viability, indicating that H2O2 inhibition of mTOR signaling is at least in part through activation of AMPK. Our findings suggest that AMPK inhibitors may be exploited for prevention of H2O2-induced neurodegenerative diseases.
Keywords: Hydrogen peroxide; Apoptosis; Akt, Phosphoinositide-dependent kinase 1; Mammalian target of rapamycin; Ribosomal p70 S6 kinase; Eukaryotic initiation factor 4E-binding protein 1; AMP-activated kinase
Introduction
Oxidative stress elicits elevated levels of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), superoxide (O2−) and hydroxyl radical (HO−), which cause increased permeability of the blood-brain barrier, tubulin alterations, and perturbation in synaptic transmission, 1 and are known to play an important role in neuronal cell death. 2 Therefore, ROS induction becomes a prominent feature of many neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Increasing evidence has further shown that under pathological conditions, excessive amounts of ROS can readily react with thiol groups of proteins, thereby disrupting the structure of cellular proteins and altering their functions, and also activate related signaling pathways, leading to neuronal apoptosis or neurodegeneration. 3-9
The mammalian target of rapamycin (mTOR), a 289 kDa serine/threonine (Ser/Thr) protein kinase, lies downstream of insulin-like growth factor 1 (IGF-1) receptor (IGFR). 10 In response to ligand binding, IGFR, a transmembrane tyrosine kinase, is activated via auto-phosphorylation of multiple tyrosine residues. Activated IGFR phosphorylates the insulin receptor substrates, which in turn triggers activation of phosphatidylinositol 3’ kinase (PI3K). Activation of PI3K results in activation of PDK1, Akt and mTOR, which is negatively regulated by phosphatase and tensin homolog (PTEN), a dual-specificity protein and lipid phosphatase. Activated mTOR further phosphorylates ribosomal p70 S6 kinase (S6K1), eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1), and Akt, controlling cell proliferation, growth, and survival. 10,11 Activated PDK1 also positively regulates S6K1. 10 Studies have placed tuberous sclerosis complex (TSC) 1/2 as a modulator between PI3K/Akt and mTOR. 12-14 The TSC1/2 complex acts as a repressor of mTOR function. 12-14 TSC2 has GTPase-activating protein (GAP) activity towards the Ras family small GTPase Rheb (Ras homolog enriched in brain), and TSC1/2 antagonizes the mTOR signaling pathway via stimulation of GTP hydrolysis of Rheb. 15-20 Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38, 21 though this remains controversial. 22 The TSC can also be activated by energy depletion through the activation of AMP-activated kinase (AMPK). 23 AMPK responds both to changes in AMP concentration and to changes in the ratio of AMP to ATP. 24 An increased AMP to ATP ratio allows AMPK phosphorylation and activation. 24 This, in turn, activates the TSC, which catalyzes the conversion of Rheb-GTP to Rheb-GDP and thus inhibits mTOR. 23 AMPK can also be phosphorylated and activated by the calcium- and calmodulin-dependent protein kinase kinase-β (CaMKK-β) in response to alterations in intracellular calcium homeostasis. 25 In addition, AMPK may be activated by oxidative stress. 25
Recently we have demonstrated that H2O2 induces neuronal apoptosis, which is associated with inhibition of protein phosphatase 2A and protein phosphatase 5, leading to activation of MAPK pathway. 9 Here we further show that H2O2-induced ROS inhibited mTOR-mediated phosphorylation of S6K1 and 4E-BP1 in PC12 cells and primary murine neurons. Furthermore, we identified that H2O2 induction of ROS inhibited the upstream kinases, Akt and PDK1, but not IGFR, and activated the negative regulator, AMPKα, but not PTEN in the cells. Expression of a dominant negative AMPKα or downregulation of AMPKα1 conferred partial resistance to H2O2 inhibition of phosphorylation of S6K1 and 4E-BP1, as well as cell viability, indicating that H2O2 inhibition of mTOR signaling is at least in part through activation of AMPK.
Materials and Methods
Materials
Hydrogen peroxide (H2O2, 30%) was purchased from Sigma (St. Louis, MO). Dulbecco's Modified Eagle Medium (DMEM) was provided by Mediatech (Herndon, VA). Horse serum and fetal bovine serum (FBS) were from Hyclone (Logan, UT), whereas 0.05% Trypsin-EDTA, NEUROBASAL™ Media, and B27 Supplement were from Invitrogen (Carlsbad, CA). Enhanced chemiluminescence solution was from Pierce (Rockford, IL, USA). CellTiter 96 AQueous One Solution Cell Proliferation Assay kit was from Promega (Madison, WI). Caspase-3/7 Assay Kit (Cat.# 71118) was purchased from AnaSpec, Inc. (Fremont, CA). The following antibodies were used: phospho-AMPKα (Thr172), acetyl-CoA carboxylase (ACC), phospho-ACC (Ser79), phospho-mTOR (Ser2448), mTOR, phospho-Akt (Ser473), S6 ribosomal protein, phospho-S6 ribosomal protein (Ser235/236) (all from Cell Signaling Technology, Beverly, MA); hemagglutinin (HA), IGFRβ subunit (IGFRβ), Akt, AMPKα, phospho-S6K1 (Thr389), S6K1 (all from Santa Cruz Biotechnology, Santa Cruz, CA); 4E-BP1 (Zymed Laboratories, South San Francisco, CA); Phosphotyrosine (BD Biosciences, San Jose, CA); FLAG and β-tubulin (Sigma); goat anti-rabbit IgG-horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, and rabbit anti-goat IgG-HRP (Pierce). N-acetyl-L-cysteine (NAC), Poly-D-lysine (PDL), papain, DNase1 and all the other chemicals were purchased from Sigma.
Cell culture
Rat pheochromocytoma (PC12) and SH-SY5Y cell lines were purchased from American Type Culture Collection (ATCC) (Manassas, VA). PC12 cells were used for no more than 10 passages, and were in antibiotic-free DMEM supplemented with 10% horse serum and 5% FBS, whereas SH-SY5Y cells were grown in antibiotic-free DMEM supplemented with 10% FBS. Cells were maintained in a humid incubator (37°C, 5% CO2).
To isolate primary neurons, CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the Animal Resource Center at Louisiana State University Health Sciences Center-Shreveport under specific pathogen-free conditions. All procedures were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee and were in compliance with the guidelines set forth by the Guide for the Care and Use of Laboratory Animals. Fetal mouse cerebral cortexes of 14-18 days of gestation were used. Briefly, the pregnant mice were anaesthetized with pentobarbital and embryos were removed by a cesarean section under sterile conditions. Cerebral cortexes were isolated under dissection microscope and washed with ice-cold Ca2+/Mg2+-free Hank's buffered salt solution (HBSS) supplemented with 1% glucose and 50 μg/ml gentamicin. The isolated embryo cortexes were digested with papain solution [HBSS containing 500 μg/ml papain, 50 μg/ml DNase1, 1% glucose, 10 μM CaCl2, 5 mM EDTA] for 15 min at 37°C. Digested tissues were triturated into single cells using 1 ml pipette in NEUROBASAL™ Media supplemented with 2% B27 Supplement, 2 mM glutamine, 1 mM sodium pyruvate, 5 μg/ml insulin, and 40 μg/ml of gentamicin. Isolated cells were seeded at a density of 2 × 106 cells/well in a 6-well plate coated with 10 μg/ml PDL in the above culture medium, and grown in a humid incubator (37°C, 5% CO2). Fresh medium was replaced every 3 days. The cells were used for experiments after 6 days of culture.
Recombinant adenoviral constructs and infection of cells
To generate recombinant adenoviruses expressing FLAG-tagged wild type (wt) mTOR, wt-mTOR was excised from expression vector PIRESNeo-FLAG-mTOR 26 (gifts from Dr. Jie Chen, University of Illinois, Urbana, IL, USA), and sub-cloned to pShuttle-CMV vector. The recombinant adenoviruses were generated and amplified using “Ad-Easy system”. 27 Recombinant adenoviruses expressing HA-tagged constitutively active S6K1 (Ad-S6K1-ca) and the green fluorescence protein (GFP) (Ad-GFP) were generated and titrated, as described. 28 Recombinant adenovirus encoding HA-tagged dominant negative AMPKα1 (Ad-dn-AMPKα) 29 was a gift from Dr. Nicholas J. G. Webster (University of California at San Diego, La Jolla, CA). For experiments, PC12 cells were grown in the growth medium, and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5). Subsequently, cells were used for experiments. Cells infected with Ad-GFP alone served as a control. Expression of HA-tagged S6K1-ca or dn-AMPKα was confirmed by Western blot with antibodies to HA. Since S6 ribosomal protein and ACC are the substrates for S6K1 and AMPKα, the functions of S6K1-ca and dn-AMPKα in the cells were assessed by immunoblotting with antibodies to phospho-S6 ribosomal protein (Ser235/236) and phospho-ACC (Ser79), respectively.
Lentiviral shRNA cloning, production, and infection
To generate lentiviral shRNA to human AMPKα1, oligonucleotides containing the target sequences were synthesized, annealed and inserted into FSIPPW lentiviral vector via the EcoR1/BamH1 restriction enzyme site. Oligonucleiotides used were: sense: 5′-AATTCCCGTTGTGGCTCACCCAACTATGCAAGAGAtagttgggtgagccacaacTTTTTG-3′, anti-sense: 5′-GATCCAAAAAGTTGTGGCTCACCCAACTATCTCT TGCAtag ttgggtgagccacaacGGG-3′. Lentiviral shRNA construct targeting GFP (control) was described. 26 To produce lentiviral shRNAs, above constructs were co-transfected together with pMD2.G and psPAX2 (Addgene, Cambridge, MA) to 293TD cells using Lipfectamine™ 2000 reagent (Invitrogen). Each virus-containing medium was collected 36 and 60 h post-transfection, respectively. For use, monolayer cells, when grown to about 70% confluence, were infected with above lentivirus-containing supernatant in the presence of 8 μg/ml polybrene for 12 h twice at an interval of 6 h. Uninfected cells were eliminated by exposure to 2 □g/ml puromycin for 48 h before use.
Assays for cell vability, morphology and caspase-3/7 activity
PC12 cells, infected with Ad-mTOR, Ad-S6K1-ca, Ad-dn-AMPKα, or Ad-GFP (control), or infected with lentiviral shRNA to AMPKα1 or GFP, respectively, were seeded at a density of 1 × 104 cells/well in a flat-bottomed 96-well plate, pre-coated with PDL (0.2 μg/ml). Next day, cells were treated with/without H2O2 (0.5 and 0.7 mM) for 24 h with 6 replicates of each treatment. After incubation, each well was added 20 μl of one solution reagent (Promega, Madison, WI) and incubated for 3 h. Cell viability was determined by measuring the optical density (OD) at 490 nm using a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences, Wellesley, MA). Caspase-3/7 activity was determined using Caspase-3/7 Assay Kit (AnaSpec, Fremont, CA), following the instructions of the supplier. Additionally, the cells were also seeded at a density of 5 × 105 cells/well in a PDL-coated 6-well plate. Next day, cells were exposed to H2O2 (0.5 and 0.7 mM). After incubation for 24 h, images were taken with an Olympus inverted phase-contrast microscope (Olympus Optical Co., Melville, NY) (200 ×) equipped with the Quick Imaging system.
Western blot analysis
After treatment, cells were briefly washed with cold PBS. On ice, cells were lysed in RIPA buffer [50 mM Tris, pH 7.2; 150 mM NaCl; 1% sodium deoxycholate; 0.1% SDS; 1% Triton-X 100; 10 mM NaF; 1 mM Na3VO4; protease inhibitor cocktail (1:1000, Sigma). Lysates were sonicated for 10 s and centrifuged at 14,000 rpm for 10 min at 4°C. Protein concentration was determined by bicinchoninic acid assay with bovine serum albumin as standard (Pierce, Rockford, IL). Equivalent amounts of protein were separated on 7.5%-12% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were incubated with PBS containing 0.05% Tween 20 and 5% nonfat dry milk to block nonspecific binding and were incubated with primary antibodies, then with appropriate secondary antibodies conjugated to horseradish peroxidase. Immunoreactive bands were visualized by using enhanced chemiluminescence solution (Pierce). To check the amount of protein loaded, the immunoblots were treated with stripping solution (62.5 mM Tris buffer, pH 6.7, containing 2% SDS and 100 mM β-mercaptoethanol) for 30 min at 50°C and incubated with mouse monoclonal anti-β-tubulin antibody (Sigma) followed by horseradish peroxidase-coupled goat anti-mouse IgG (Pierce).
Detection of phosphorylation of IGFR
PC12 cells (5×105/ml) were seeded in 100-mm dishes, pre-coated with PDL (0.2 μg/ml). The next day, cells were treated with H2O2 (0-1 mM), or were treated with/without H2O2 (0.5 and 0.7 mM) following 1 h of NAC (5 mM) pre-incubation. After incubation for 2 h. Cells were washed once in ice-cold 1×PBS, followed by lysis in RIPA buffer. After clearing via centrifugation (14,000 rpm, 15 min, 4°C), 500 μl supernatants of cell lysates were collected and incubated overnight at 4°C with 1 μg of antibody to IGFRβ and 30 μl of protein A/G-agarose beads (Santa Cruz Biotechnology). Immunoprecipitates were washed with RIPA buffer two times and twice with ice-cold PBS, followed by immunoblotting with antibodies to p-Tyr and IGFRβ, respectively. IgG-heavy chain and light chain from each immunoprecipitated sample were used for loading control.
Statistical analysis
Results were expressed as mean values ± standard error (mean ± S.E.). Statistical analysis was performed by Student's t-test (STATISTICA, Statsoft Inc, Tulsa, OK). A level of P < 0.05 was considered to be significant.
Results
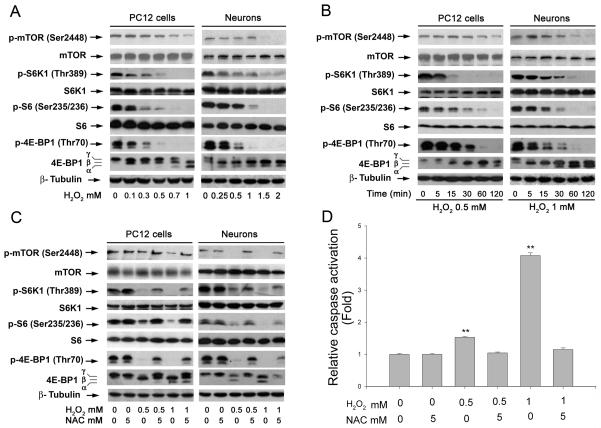
Hydrogen peroxide induction of ROS inhibits mTOR-mediated phosphorylation of S6K1 and 4E-BP1 in neuronal cells
We have shown H2O2 induces apoptosis of neuronal cells.9 mTOR is a central regulator of cell survival.10 To determine whether H2O2 induction of neuronal cell death is associated with inhibition of mTOR signaling, PC12 cells and primary murine neurons were used. By Western blotting analysis, we found that treatment of cells with different concentrations of H2O2 (0-2 mM) for 2 h decreased phosphorylation of mTOR, S6K1, and 4E-BP1 in a concentration-dependent manner, and at concentrations of >0.5 mM (for PC12) or >1 mM (for neurons), H2O2 dramatically reduced or completely blocked phosphorylation of S6K1 or the substrate of S6K1, S6, as well as 4E-BP1 (Fig. 1A). We also observed that H2O2 inhibited phosphorylation of S6K1 and 4E-BP1 in a time-dependent fashion. As shown in Fig.1B, at 15 min post experiment, H2O2 obviously reduced phosphorylation of S6K1 and 4E-BP1, and such effect was sustained for over 2 h. We also noticed that a complete abolishment occurred at 30 min (for PC12) or at 120 min (for neurons) post exposure to H2O2. H2O2 did not markedly alter total protein levels of those proteins (Fig. 1A and B). It should be mentioned that a considerable basal level of phosphorylation of 4E-BP1 was detected (Figs 1A, B) in PC12 cells and primary murine neurons. Phosphorylation state of 4E-BP1 was initially detected with an antibody to 4E-BP1. Phosphorylation of 4E-BP1 decreases its electrophoretic mobility during SDS-polyacrylamide gel electrophoresis.30 H2O2 also decreased phosphorylation of 4E-BP1 in a concentration-and time-dependent manner, as indicated by the decrease in the intensity of the uppermost band γ and by the increase in the higher mobility band α and β that corresponds to a less phosphorylated form of 4E-BP1 (Fig. 1A, B). We also verified the finding using the antibodies against to phospho-4E-BP1 (Thr70) (Fig. 1A, B). The findings indicate that H2O2 inhibits mTOR-mediated S6K1 and 4E-BP1 signaling pathways in the neuronal cells.
Fig. 1. Hydrogen peroxide induction of ROS inhibits phosphorylation of mTOR-mediated S6K1 and 4E-BP1 in neuronal cells.
(A) PC12 cells and primary neurons were treated with 0-2 mM H2O2 for 2 h, (B) treated with 0.5-1 mM H2O2 for indicated time (0-120 min), or (C) with 0.5 and 1 mM H2O2 for 2 h after pretreatment with NAC (5 mM) for 1 h. Total cell lysates were subjected to Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. Note: H2O2 markedly decreased phosphorylation of S6K1 and 4E-BP1 in a concentration-dependent (A) and time-dependent (B) manner, which was attenuated by NAC (C). (D) PC12 cells, seeded in 96-well plates (black wall), were exposed to 0.5 and 1 mM H2O2 for 24 h after pretreatment with NAC (5 mM) for 1 h. Subsequently, 50 μl of caspase-3/7 substrate solution was added into each well. After incubation on a plate shaker at 100 rpm at room temperature for 1 h (avoiding direct light), the fluorescence intensity was measured at Ex/Em = 354 nm/422 nm, using a Wallac 1420 Multilabel Counter. Results are presented as mean ± S.E. (n = 6). **P<0.01, difference vs. control group (H2O2 = 0 mM, and NAC = 0 mM).
To unveil whether H2O2 inhibition of mTOR signaling is due to induction of ROS, PC12 cells were exposed to H2O2 (0.5 and 1 mM) for 2 h after pretreatment with NAC, a ROS scavenger, for 1 h. As shown in Fig. 1C, H2O2 inhibited phosphorylation of mTOR, S6K1 and 4E-BP1, which was remarkably attenuated by NAC. Similar data were seen in primary neurons (Fig. 1C). In addition, consistent with our previous findings, 9we also found that NAC blocked H2O2 inhibition of cell viability, induction of cell roundup (data not shown), and activation of caspases 3/7 in PC12 cells (Fig. 1D), suggesting that NAC prevented H2O2 induced apoptosis of the cells (Fig. 1D). The findings support the notion that H2O2 induces ROS contributing to suppression of mTOR-mediated S6K1 and 4E-BP1 pathways, leading to neuronal cell death.
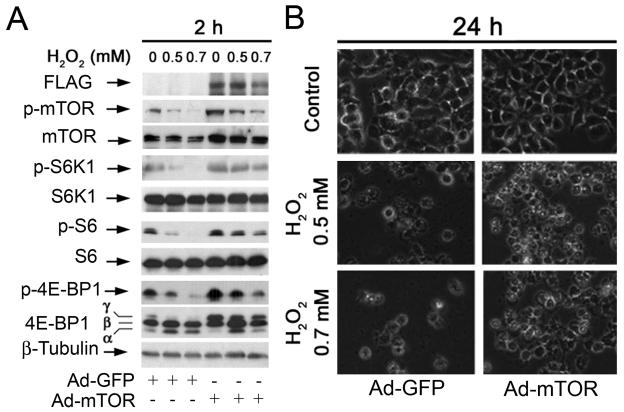
Ectopic expression of wild type (wt) mTOR or constitutively active S6K1, or downregulation of 4E-BP1 partially prevents hydrogen peroxide-induced neuronal cell death
To further assess the role of mTOR in H2O2 induced neuronal cell death, PC12 cells were infected with Ad-mTOR and Ad-GFP (control), respectively. Ectopic expression of FLAG-tagged wt-mTOR conferred considerable resistance to H2O2 inhibition of mTOR-mediated phosphorylation of S6K1 and 4E-BP1 (Fig. 2A), as well as H2O2 induced cell death (Fig. 2B), suggesting that H2O2 induced apoptosis is at least partially associated with inhibition of mTOR.
Fig. 2. Expression of wt-mTOR partially prevents hydrogen peroxide inhibition of mTOR signaling and cell death.
(A) PC12 cells, infected with replication replication-defective adenoviral recombinant expressing FLAG-tagged wt-mTOR (Ad-mTOR) or the control adenovirus (Ad-GFP), were exposed to H2O2 (0.5 and 0.7 mM) for 2 h, followed by Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B) PC12 cells, infected with Ad-GFP or Ad-mTOR, were exposed to H2O2 (0.5 and 0.7 mM) for 24 h, followed by morphological analysis using an Olympus inverted phase-contrast microscope (200 ×) equipped with Quick Imaging system.
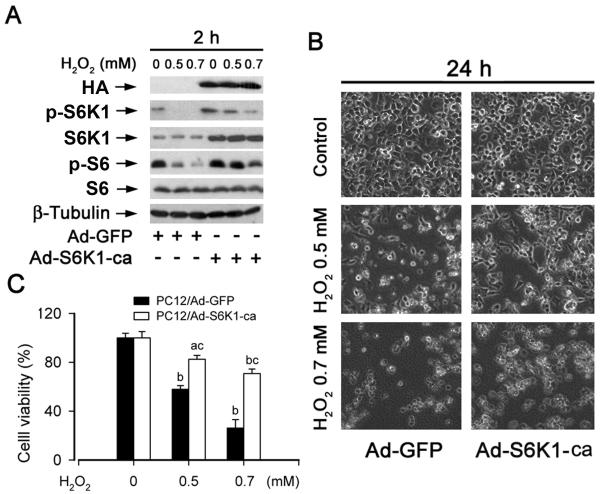
mTOR-mediated S6K1 and 4E-BP1 pathways are critical for cell survival.10 To evaluate the role of S6K1 pathway in H2O2 induction of neuronal cell death, PC12 cells were infected with recombinant adenoviruses expressing HA-tagged constitutively active S6K1 (Ad-S6K1-ca) or control virus encoding GFP alone. As shown in Fig. 3A, expression of high levels of recombinant S6K1-ca was observed in the cells infected with Ad-S6K1-ca, but not in those infected with Ad-GFP, as detected by immunoblotting with antibodies to HA and S6K1. The basal level of phosphorylation of S6 ribosomal protein, a substrate of S6K1, was considerably high in the cells, which was not enhanced by expression of S6K1-ca. However, when the cells were exposed to H2O2 (0.5 and 0.7 mM) for 2 h, expression of S6K1-ca obviously rendered resistance to H2O2 inhibition of phosphorylation of S6K1 and S6 ribosomal protein. Of interest, expression of S6K1-ca also significantly increased cell viability in the presence of H2O2 (Fig. 3B) or prevented H2O2-induced neuronal apoptosis (Fig. 3C).
Fig. 3. Expression of constitutively active S6K1 partially prevents hydrogen peroxide inhibition of S6K1 pathway and cell viability.
(A) PC12 cells, infected with replication-defective adenoviral recombinant expressing constitutively active S6K1 (Ad-S6K1-ca) or the control adenovirus (Ad-GFP), were exposed to H2O2 (0.5 and 0.7 mM) for 2 h, followed by Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B, C) PC12 cells, infected with Ad-GFP or Ad-S6K1-ca, were exposed to H2O2 (0.5 and 0.7 mM) for 24 h, followed by (B) morphological analysis using an Olympus inverted phase-contrast microscope (200 ×) equipped with Quick Imaging system, and (C) cell viability assay using one solution reagent, respectively. Results are presented as mean ± S.E. (n = 6). aP<0.05, bP<0.01, difference vs. control group; cP<0.01, Ad-S6K1-ca group vs. Ad-GFP group. Note: PC12 cells infected with an adenovirus expressing constitutively active S6K1 (Ad-S6K1-ca), but not a control virus (Ad-GFP), conferred partial resistance to H2O2 inhibition of phosphorylation of S6 ribosomal protein (A), as well as cell viability (B, C).
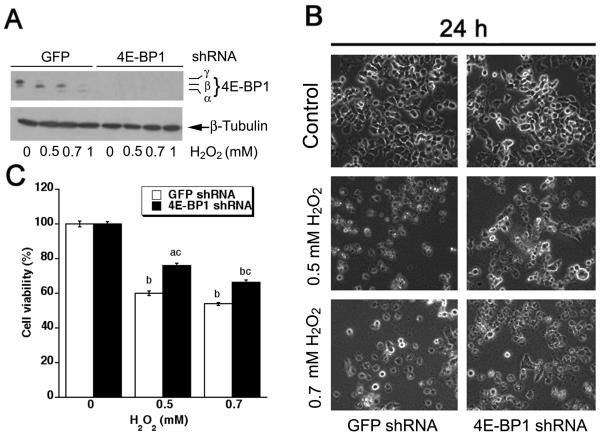
Next we assessed the role of 4E-BP1 pathway in H2O2 induction of neuronal cell death. Since 4E-BP1 functions as a suppressor of eIF4E,10 downregulation of 4E-BP1 would lead to loss of suppression of eIF4E. For this, 4E-BP1 was downregulated by >90% in PC12 cells using lentiviral shRNA to 4E-BP1 (Fig. 4A). Downregulation of 4E-BP1 significantly increased cell viability in the presence of H2O2 (0.5 and 0.7 mM) (Fig. 4B, C). Collectively, our results indicate that H2O2 induced neuronal cell death at least in part through inhibition of mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways.
Fig. 4. Downregulation of 4E-BP1 partially rescues hydrogen peroxide-induced neuronal cell death.
(A) PC12 cells, infected with lentiviral shRNAs to 4E-BP1 and GFP (as control), were exposed to H2O2 (0.5, 0.7 and 1 mM) for 2 h, followed by Western blot analysis with antibodies to 4E-BP1. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B and C) Morphology and cell viability of lentiviral shRNA-infected PC12 cells, treated with/without H2O2 (0.5 and 0.7 mM) for 24 h, were assessed using an Olympus inverted phase-contrast microscope (200 ×) equipped with Quick Imaging system, and using one solution assay, respectively. Results are presented as mean ± S.E. n=6. aP<0.05, bP<0.01, difference vs. control group; cP<0.01, 4E-BP1 shRNA group vs. GFP shRNA group.
Hydrogen peroxide inhibits phosphorylation of PDK1 and Akt, but not IGFR, in neuronal cells
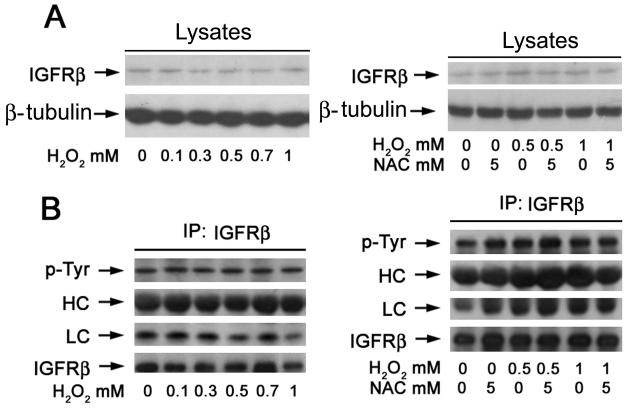
IGFR, a transmembrane protein tyrosine kinese, lies upstream of mTOR.10 To determine whether H2O2 induction of ROS inhibits mTOR signaling by suppressing the activity of IGFR, PC12 cells were treated with H2O2 (0-1 mM) for 2 h, or exposed to H2O2 (0.5 and 1 mM) for 2 h following 1 h of NAC (5 mM) pretreatment, followed by immnoprecipitation with antibodies to IGFRβ subunit (IGFRβ), and immunoblotting with antibodies to phosphotyrosine (p-Tyr) and IGFRβ, respectively. We found that treatment with H2O2 or NAC did not obviously impact protein expression of IGFRβ (Fig. 5A). Furthermore, H2O2 failed to alter the basal or NAC-pretreated tyrosine phosphorylaiton of IGFRβ, indicating that H2O2 did not affect IGFR activity (Fig. 5B).
Fig. 5. Hydrogen peroxide does not alter protein expression and phosphorylation of IGFR in neuronal cells.
PC12 cells were treated with 0-1 mM H2O2 for 2 h, or with 0.5 and 0.7 mM H2O2 for 2 h after pretreatment with NAC (5 mM) for 1 h, followed by (A) Western blot analysis with indicated antibodies, or (B) immnoprecipitation with antibodies to IGFRβ-subunit (IGFRβ) plus A/G-agarose, and immunoblotting with antibodies to phosphotyrosine (p-Tyr) and IGFRβ, respectively. Note: HC, heavy chain of IgG; LC, light chain of IgG.
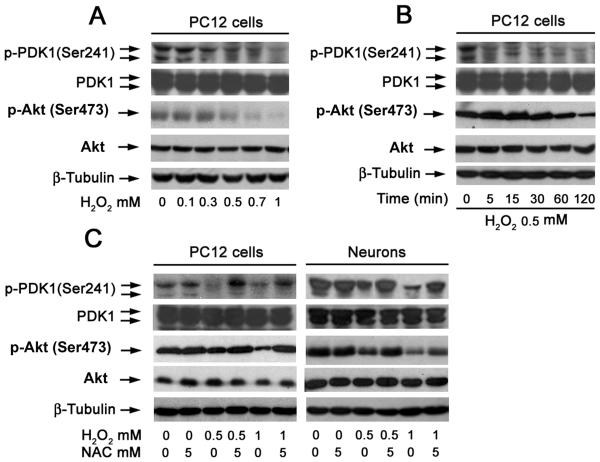
Since activated Akt and PDK1 may positively regulate mTOR and S6K1, respectively,10, we next investigated whether H2O2 induction of ROS inhibits mTOR-mediated phosphorylation of mTOR and S6K1 by suppressing the activity of Akt and PDK1, respectively. To this end, PC12 cells were treated with different concentrations of H2O2 (0-1 mM) for 2 h, or with 0.5 mM H2O2 for 0-2 h. We found that a concentration-dependent decrease of phosphorylation of Akt and PDK1 was detected by Western blot analysis, although H2O2 did not alter cellular protein levels of Akt and PDK1 (Fig. 6A). H2O2 inhibited phosphorylation of PDK1 in a time-dependent manner. However, H2O2 transiently activated Akt within 5-30 min, and then inhibited phosphorylation of Akt in 1-2 h. (Fig.6B). Treatment with NAC markedly prevented H2O2 inhibition of Akt and PDK1 phosphorylation in PC12 cells (Fig. 6C). Similar results were also observed in primary neurons (Fig. 6C), indicating that H2O2 induction of ROS inhibits Akt and PDK1 activity.
Fig. 6. Hydrogen peroxide inhibits Akt and PDK1 phosphorylation in neuronal cells.
(A) PC12 cells were treated with 0-1 mM H2O2 for 2 h, or (B) with 0.5 mM H2O2 for 0-120 min. Total lysates were subjected to Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. H2O2 inhibited phosphorylation of Akt and PDK1 in a concentration-dependent (A) and time-dependent (B) manner. (C) PC12 cells and primary neurons were pretreated with treated with/without NAC (5 mM) for 1 h, and then incubated with/without H2O2 at indicated concentrations for 2 h, followed by Western blot analysis using indicated antibodies. Note: NAC markedly reversed H2O2 inhibition of Akt or PDK1 activity.
Hydrogen peroxide induction of ROS activates AMPK, but not PTEN
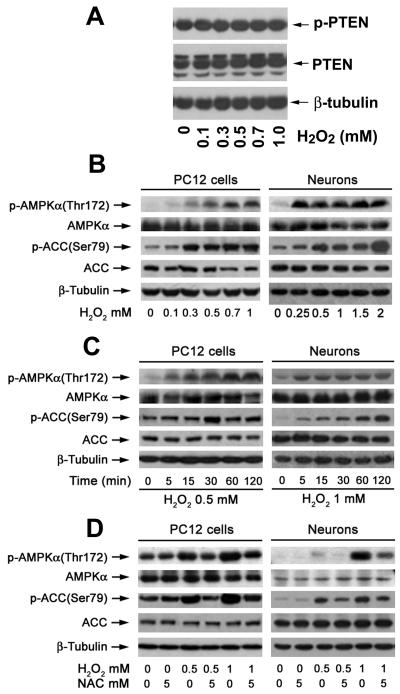
PTEN and AMPK are two major negative regulators of mTOR signaling.10,23 To determine whether H2O2 inhibition of mTOR signaling is associated with activation of PTEN and/or AMPK, PC12 cells were exposed to 0-1 mM H2O2 for 2 h or to 0.5 mM H2O2 for different time (0-120 min). Our Western blot analysis revealed that exposure to H2O2 did not obviously alter protein expression and phosphorylation of PTEN (Fig.7A). However, H2O2 increased phosphorylation of AMPKα and its substrate (ACC) in a concentration- and time-dependent manner, although there was no effect on cellular protein expression of AMPKα (Fig.7B, C). Similar data were observed in primary neurons (Fig. 7B, C).
Fig. 7. Hydrogen peroxide induction of ROS activates AMPK signaling in neuronal cells.
(A) PC12 cells and primary neurons were treated with 0-2 mM H2O2 for 2 h, (B) or treated with 0.5-1 mM H2O2 for indicated time (0-120 min), or (C) with 0.5 and 0.7 mM H2O2 for 2 h after pretreatment with NAC (5 mM) for 1 h. Total cell lysates were subjected to Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. Note: H2O2 obviously increased phosphorylation of AMPKα as well as its substrate (ACC) in a concentration-dependent (A) and time-dependent (B) manner, which was remarkably blocked by NAC (C).
Next we tested whether H2O2 activation of AMPKα is related to ROS induction. By Western blot analysis, we found that pre-treatment with NAC markedly attenuated H2O2 increase of phosphorylation of AMPKα and ACC in PC12 cells and primary neurons (Fig. 7D). Our findings suggest that H2O2-induced oxidative stress activates AMPKα signaling.
Expression of dominant negative AMPKα or downregulation of AMPKα1 partially prevents hydrogen peroxide inhibition of mTOR-mediated phosphorylation of S6K1 and 4E-BP1, as well as neuronal cell death
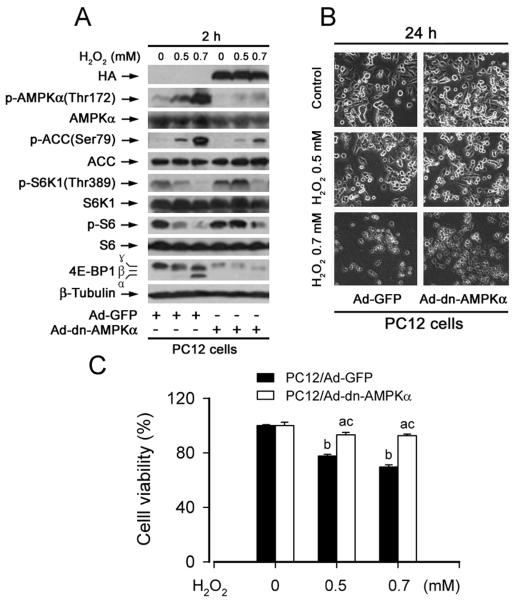
To further determine whether H2O2 activation of AMPKα is correlated with its inhibition of mTOR-mediated phosphorylation of S6K1 and 4E-BP1, PC12 cells, infected with Ad-dn-AMPKα and Ad-GFP (as control), were exposed to H2O2 (0.5 and 0.7 mM) for 2 h. Western blot analysis revealed that ectopic expression of dn-AMPKα obviously blocked H2O2-induced phosphorylation of AMPKα and its substrate, ACC (Fig. 8A). Consistently, H2O2 inhibition of phosphorylation of S6K1, S6 and 4E-BP1 was apparently attenuated by expression of dn-AMPKα (Fig. 8A). Furthermore, our morphological analysis and cell viability assay revealed that expression of dn-AMPKα also partially protected PC12 cells from apoptosis induced by H2O2 (Fig. 8B, C). Therefore, our results suggest that H2O2 induces apoptosis of the neuronal cells at least in part through activation of AMPKα, leading to inhibition of mTOR-mediated phosphorylation of S6K1 and 4E-BP1.
Fig. 8. Expression of a dominant negative AMPKα partially prevents hydrogen peroxide inhibition of phosphorylation of S6K1 and 4E-BP1, as well as cell viability.
(A) PC12 cells, infected with Ad-dn-AMPKα and Ad-GFP (as control), were exposed to H2O2 (0.5 and 0.7 mM) for 2 h, followed by Western blot analysis using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B, C) PC12 cells, infected with Ad-dn-AMPKα and Ad-GFP (as control), were exposed to H2O2 (0.5 and 0.7 mM) for 24 h, followed by (B) morphological analysis using an Olympus inverted phase-contrast microscope (200 ×) equipped with Quick Imaging system, and (C) cell viability assay using one solution reagent, respectively. Results are presented as mean ± S.E. (n = 6). aP<0.05, bP<0.01, difference vs. control group; cP<0.01, Ad-dn-AMPKα group vs. Ad-GFP group. Note: Overexpression of a dominant negative AMPKα partially rescued cells from death induced by H2O2, by inhibiting phosphorylation of AMPKα and ACC and increasing phosphorylations of mTOR-mediated S6K1, S6 and 4E-BP1.
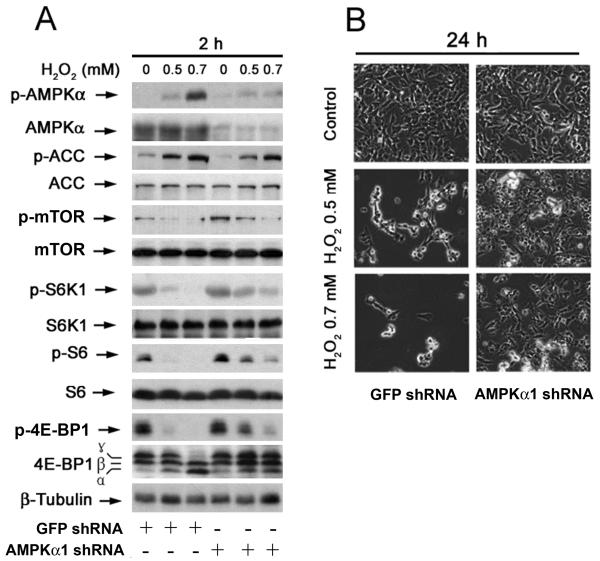
To further demonstrate the role of AMPK in H2O2 inhibition of mTOR signaling and induction of neuronal cell death, expression of AMPKα1 was silenced by RNA interference. As shown in Fig. 9A, lentiviral shRNA to AMPKα1, but not to GFP, downregulated AMPKα1 expression by ~85% in SH-SY5Y cells. Downregulation of AMPKα1 decreased the basal level of phosphorylation of ACC but increased the basal level of phosphorylation of mTOR. Of importance, silencing expression of AMPKα1 obviously attenuated H2O2 induced phosphorylation of AMPKα and ACC, particularly at 0.5 mM. Consistently, downregulation of AMPKα1 conferred partial resistance to H2O2 inhibition of mTOR signaling pathways (Fig. 9A), as well as H2O2 induced cell death in SH-SY5Y cells (Fig. 9B).
Fig. 9. Downregulation of AMPKα1 partially prevents hydrogen peroxide inhibition of mTOR signaling and induction of neuronal cell death.
(A) SH-SY5Y cells, infected with lentiviral shRNAs to human AMPKα1 and GFP (as control), were exposed to H2O2 (0.5, 0.7 and 1 mM) for 2 h, followed by Western blot analysis with indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (B) Morphology of lentiviral shRNA-infected SH-SY5Y cells, treated with/without H2O2 (0.5 and 0.7 mM) for 24 h, were assessed using an Olympus inverted phase-contrast microscope (200 ×) equipped with Quick Imaging system.
Discussion
Increasing evidence has implicated oxidative stress as one of critical factors leading to neuronal cell death in many neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, Huntington's disease and amyotrophic lateral sclerosis.2,31-39 However, how oxidative stress causes neuronal cell death is not well understood. mTOR has been widely recognized as a central controller for cell proliferation, growth, and survival. 10,23 Recently we have demonstrated that H2O2 induces apoptosis in PC12 cells and primary murine neurons.9 Here we show that H2O2 induction of neuronal cell death is in part associated with inhibition of mTOR signaling pathway. This is strongly supported by the findings (Fig. 1-4) that i) H2O2 treatment dramatically inhibited phosphorylation of mTOR, S6K1 and 4E-BP1 in a concentration- and time-dependent manner; ii) The above event was blocked by NAC in PC12 cells and primary neurons; iii) Expression of wt-mTOR or constitutively active S6K1, or downregulation of 4E-BP1 conferred partial resistance to H2O2 induction of neuronal cell death. In addition, we also noticed that inhibition of mTOR with rapamycin (100 ng/ml) alone did not significantly induce apoptosis of PC12 cells, though rapamycin slightly enhanced H2O2 induced cell death (data not shown). The results suggest that H2O2 induced neuronal cell death may involve more signaling pathways. At least JNK, Erk1/2 and p38 MAPK pathways have been described.9 Our results are in contrast to the findings in hepatoma (H4IIE) and epidermal (JB6) cells, 40-44 but in agreement with the data from PC12 cells.45 Treatment of H4IIE or JB6 cells with H2O2 or its inducers, such as arsenite and ultraviolet irradiation, increased phosphorylation of S6K1, which was blocked by NAC or catalase (a specific H2O2 scavenger).40-44 Ultraviolet irradiation-induced H2O2 contributes to tumorigenesis.41 However, treatment of PC12 cells with H2O2 inhibited phosphorylation of 4E-BP1 and initiation factor 4E (eIF4E), which was completely abolished by NAC.45 The findings in this study and others 40-45 imply that the effect of H2O2 on cell fate or mTOR signaling, to some degree, may be dependent on cell type.
In the studies, we observed that H2O2 inhibited phosphorylation of 4E-BP1 (Thr70), but increased expression of hypophosphorylated forms (α and β bands) of 4E-BP1 in PC12 and primary murine neurons. It has been reported that decreased phosphorylation of 4E-BP1 may increase the stability of 4E-BP1. 46 With a great possibility, H2O2 inhibition of phosphorylation of 4E-BP1 may increase stability of 4E-BP1, leading to increased expression of α and β bands of 4E-BP1.
mTOR signaling is positively regulated by IGFR/Akt, and negatively regulated by AMPK and PTEN.10,23 Besides, activated PDK1 may positively regulate mTOR-mediated S6K1.10 In order to identify the mechanism by which H2O2 inhibits mTOR signaling in neuronal cells, first of all, we focused on the upstream kinases, IGFR, Akt and PDK1. Our data show that H2O2 did not impact protein expression and tyrosine phosphorylation of IGFRβ (Fig. 5), but inhibited phosphorylation of Akt and PDK1 in PC12 cells and primary neurons in a concentration-dependent manner (Fig. 6). H2O2 inhibited PDK1 phosphorylation in a time-dependent manner, but activated Akt transiently within 10-30 min and then inhibited Akt in 1-2 h. The mechanism remains to be defined. Pretreatment with NAC, a ROS scavenger, dramatically prevented H2O2-reduced phosphorylation of Akt and PDK1 in the cells (Fig. 6), revealing that H2O2 induction of ROS inhibits mTOR signaling is concurrently related to inhibition of Akt and PDK1, but not IGFR, in neuronal cells. PI3K is an upstream kinase of Akt and PDK1. 23 Whether this is due to inhibition of PI3K remains to be determined.
In this study, we also observed that H2O2 failed to alter protein expression and phosphorylation of PTEN, but dramatically increased the activity of AMPK (Fig. 7). H2O2 treatment resulted in increase of phosphorylation of AMPKα and its substrate, ACC, in a dose- and time-dependent manner in PC12 cells and primary neurons. Pre-treatment with NAC inhibited H2O2 activation of AMPKα. Clearly, NAC inhibited phosphorylation of AMPKα and ACC induced by H2O2 at 0.5 mM than at 1 mM more potently. Probably, at 1 mM, H2O2 activates AMPK through ROS-independent mechanism. Of importance, expression of dominant negative AMPKα or downregulation of AMPKα1 partially prevented H2O2 inhibition of phosphorylation of mTOR, S6K1 and 4E-BP1, as well as induction of cell death (Fig. 8 and Fig. 9). In particular, in response to a higher concentration (1 mM) of H2O2, the protective effect of dominant negative AMPKα or AMPKα1 shRNA against H2O2 induced phosphorylation of ACC and apoptosis was marginal. We also noticed that overexpression of wild type AMPK alone, like treatment with rapamycin alone, inhibited phosphorylation of mTOR and its downstream effector molecules, including S6K1 and 4E-BP1, but failed to induce significant apoptosis in PC12 cells (data not shown). However, overexpression of wt-AMPK did sensitize PC12 cells to H2O2 induced cell death (data not shown). The results are in agreement with the findings that AMPK/mTOR-indepenndent pathways, such as JNK, Erk1/2 and p38 MAPK pathways, 9 also contribute to H2O2 induced cell death. The above data indicate that H2O2 induction of ROS causes apoptosis of the neuronal cells at least in part through activation of AMPKα signaling, leading to inhibition of mTOR-mediated phosphorylation of S6K1 and 4E-BP1.
In summary, we have identified that oxidative stress by H2O2 inhibits mTOR-mediated phosphorylation of S6K1 and 4E-BP1, leading to apoptosis of neuronal cells. H2O2 induction of ROS inhibits Akt and PDK1, but not IGFR; activates AMPK, but not PTEN. Expression of dominant negative AMPKα or downregulation of AMPKα1 conferred partial resistance to H2O2 inhibition of mTOR signaling and cell viability, indicating that H2O2 induction of ROS inhibits mTOR pathway at least in part by activating AMPKα. Our findings suggest that AMPK inhibitors may be exploited for prevention of oxidative stress (e.g. H2O2)-induced neurodegenerative diseases.
Acknowledgements
This work was supported in part by NIH CA115414 (S. H.), American Cancer Society Award #RSG-08-135-01-CNE (S.H.), Louisiana Board of Regents Award #NSF(2009)-PFUND-144 (S.H.), Feist-Weiller Cancer Research Award (S.H.), and a Start-up Fund (S.H.) jointly from Louisiana State University Health Sciences Center in Shreveport, LA.
References
- 1.Baxter LC, Sparks DL, Johnson SC, et al. Relationship of cognitive measures and gray and white matter in Alzheimer's disease. J Alzheimers Dis. 2006;9:253–260. doi: 10.3233/jad-2006-9304. [DOI] [PubMed] [Google Scholar]
- 2.Ruffels J, Griffin M, Dickenson JM. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol. 2004;483:163–173. doi: 10.1016/j.ejphar.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman E. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 4.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 5.Figueiredo-Pereira ME, Yakushin S, Cohen G. Disruption of the Intracellular Sulfhydryl Homeostasis by Cadmium-induced Oxidative Stress Leads to Protein Thiolation and Ubiquitination in Neuronal Cells. J Biol Chem. 1998;273:12703–12709. doi: 10.1074/jbc.273.21.12703. [DOI] [PubMed] [Google Scholar]
- 6.Green KN, Peers C. Divergent pathways account for two distinct effects of amyloid beta peptides on exocytosis and Ca2+ currents: involvement of ROS and NF-κB. J Neurochem. 2002;81:1043–1051. doi: 10.1046/j.1471-4159.2002.00907.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Arnaud L, Rockwell P, et al. A single amino acid substitution in a proteasome subunit triggers aggregation of ubiquitinated proteins in stressed neuronal cells. J Neurochem. 2004;90:19–28. doi: 10.1111/j.1471-4159.2004.02456.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Moon C, Eun S, et al. Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem Biophys Res Commun. 2005;328:326–334. doi: 10.1016/j.bbrc.2004.11.173. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Liu L, Yin J, et al. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol. 2009;41:1284–1295. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 11.Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Zhang Y, Arrazola P, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 13.Inoki K, Li Y, Zhu T, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 14.Tee AR, Fingar DC, Manning BD, et al. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stocker H, Radimerski T, Schindelholz B, et al. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Gao X, Saucedo LJ, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 17.Garami A, Zwartkruis FJ, Nobukuni T, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 18.Inoki K, Li Y, Xu T, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tee AR, Manning BD, Roux PP, et al. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 20.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Bai X, Ma D, Liu A, et al. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Fonseca BD, Tang H, et al. Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem. 2008;283:30482–30492. doi: 10.1074/jbc.M803348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91:1017–1028. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- 25.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 26.Vilella-Bach M, Nuzzi P, Fang Y, et al. The FKBP12-rapamycin-binding domain is required for FKBP12-rapamycin-associated protein kinase activity and G1 progression. J Biol Chem. 1999;274:4266–4272. doi: 10.1074/jbc.274.7.4266. [DOI] [PubMed] [Google Scholar]
- 27.He TC, Zhou S, da Costa LT, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Chen L, Chung J, Huang S. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene. 2008;27:4998–5010. doi: 10.1038/onc.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu M, Tang Q, Olefsky JM, et al. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–777. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunn GJ, Hudson CC, Sekulić A, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 31.Behl C, Davis JB, Lesley R, et al. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 32.Owen AD, Schapira AH, Jenner P, et al. Oxidative stress and Parkinson's disease. Ann NY Acad Sci. 1996;786:217–223. doi: 10.1111/j.1749-6632.1996.tb39064.x. [DOI] [PubMed] [Google Scholar]
- 33.Behl C. Alzheimer's disease and oxidative stress: implications for novel therapeutic approaches. Prog Neurobiol. 1999;57:301–323. doi: 10.1016/s0301-0082(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 34.Alexi T, Borlongan CV, Faull RL, et al. Neuroprotective strategies for basal ganglia degeneration: Parkinson's and Huntington's diseases. Prog Neurobiol. 2000;60:409–470. doi: 10.1016/s0301-0082(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 35.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 36.Jellinger KA. Cell death mechanisms in Parkinson's disease. J Neural Transm. 2000;107:1–29. doi: 10.1007/s007020050001. [DOI] [PubMed] [Google Scholar]
- 37.Rottkamp CA, Nunomura A, Raina AK, et al. Oxidative stress, antioxidants, and Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(Suppl 1):S62–S66. doi: 10.1097/00002093-200000001-00010. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson's disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 39.Wu DC, Re DB, Nagai M, et al. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bae G-U, Seo D-W, Kwon H-K, et al. Hydrogen peroxide activates p70S6k signaling pathway. J Biol Chem. 1999;274:32596–32602. doi: 10.1074/jbc.274.46.32596. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, Li J, Ke Q, et al. Ultraviolet-induced phosphorylation of p70(S6K) at Thr(389) and Thr(421)/Ser(424) involves hydrogen peroxide and mammalian target of rapamycin but not Akt and atypical protein kinase C. Cancer Res. 2002;62:5689–5697. [PubMed] [Google Scholar]
- 42.Jung DK, Bae GU, Kim YK, et al. Hydrogen peroxide mediates arsenite activation of p70(s6k) and extracellular signal-regulated kinase. Exp Cell Res. 2003;290:144–154. doi: 10.1016/s0014-4827(03)00320-3. [DOI] [PubMed] [Google Scholar]
- 43.Radisavljevic ZM, Gonzalez-Flecha B. TOR kinase and Ran are downstream from PI3K/Akt in H2O2-induced mitosis. J Cell Biochem. 2004;91:1293–1300. doi: 10.1002/jcb.20037. [DOI] [PubMed] [Google Scholar]
- 44.Kimball SR, Abbas A, Jefferson LS. Melatonin represses oxidative stress-induced activation of the MAP kinase and mTOR signaling pathways in H4IIE hepatoma cells through inhibition of Ras. J Pineal Res. 2008;44:379–386. doi: 10.1111/j.1600-079X.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Loghlen A, Perez-Morgado MI, Salinas M, et al. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cell Signal. 2006;18:21–31. doi: 10.1016/j.cellsig.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Elia A, Constantinou C, Clemens MJ. Effects of protein phosphorylation on ubiquitination and stability of the translational inhibitor protein 4E-BP1. Oncogene. 2008;27:811–822. doi: 10.1038/sj.onc.1210678. [DOI] [PubMed] [Google Scholar]