Abstract
Long-term adherence to training programmes is difficult to attain. Yet, the benefits of exercise to general health and well-being are undeniable. Any measure to demonstrate the minimum required exercise for maximal benefit to a person is a promising avenue towards increasing the uptake and adherence to physical activity for the general public. The purpose of this study was to compare the effects of two different intensities of resistance training in healthy older adults. We hypothesised that compared to high-intensity resistance exercise, relatively low training intensity could also improve in vivo markers of healthy physiologic and endocrine functions in previously sedentary older individuals. Thirty (out of a possible 34 recruited) older adults were randomly assigned to low (LowR, i.e. ∼40% one repetition maximum (1RM)) versus high-resistance training (HighR, i.e. ∼80% 1RM) for 12 weeks. Neither intervention significantly impacted upon body composition markers including: body mass index (BMI), waist/hip ratio and bioelectric impedance. Muscle strength data showed an advantage for the HighR protocol with 51 ± 4% and 22.4 ± 10.2% (P < 0.05) improvements in 1RM strength and bilateral knee extension torque, respectively, compared with 17 ± 1% and 10.3 ± 4.7% (P < 0.05) increments in 1RM strength and bilateral torque in the LowR group. Unilateral torque did not change significantly in either group. Quadriceps muscle thickness data also showed a significantly greater benefit of the HighR protocol (5.8 ± 2.6% increase) compared with the LowR protocol (no change). Functional ability tests, including Get-up-and-go (GUG), Standing from lying and the 6-min walk, showed changes of −11.6 ± 4.8%, −15.6% and 8.5 ± 1.7% (P < 0.05), respectively, in HighR compared with only one significant improvement in the LowR, namely a −10.8 ± 3% (P < 0.05) improvement in the GUG test. Overnight fasting serum levels of IGFBP-3 increased, NPY decreased and TNF-α decreased significantly in the LowR group. Serum levels of glucose increased and NPY decreased significantly in HighR. Circulating levels of I, IL-6 and IGF-1 did not change with either intervention. In vivo physiologic changes show functional advantages for older persons carrying out high-resistance training. At the endocrine level, such an advantage is not clear. In fact, in terms of changes in sera levels of fasting glucose, IGFBP-3 and TNF-α, there appears to be an advantage to carrying out the lower intensity exercises for the aged populations where endocrine adaptations are key.
Keywords: Ageing, Endocrinology, Cytokines, Physiology, Resistance exercise intensity
Introduction
Increased dependence in old age occurs through a number of circumstances, with decreased mobility being one of the most acutely debilitating (Doherty 2003). In hand with decreased mobility comes a notable reduction in skeletal muscle mass (sarcopenia), which occurs at ∼5% per decade after the 4th decade (Roubenoff et al. 2000) and increases by a further ∼1% every year after the 65th birthday (Greenlund and Nair 2003; Klitgaard et al. 1990; Lexell et al. 1988; Narici et al. 2003; Young et al. 1985). Sarcopenia is also associated with asthenia or decreased muscle strength whereby in the knee extensors for instance, strength losses are in the order of 20–50% between the ages of 25 to 73 years (Poulin et al. 1992; Vandervoort et al. 1990; Young et al. 1985).
That lifelong (Pearson et al. 2002) and/or newly adopted (Onambele et al. 2008) high levels of physical activity slow down and/or reverse age-related declines in strength is undoubtedly demonstrable. What is clear also is that whilst endurance (Klitgaard et al. 1990) and aerobic (Sipila et al. 1997) work have their cardiovascular benefits, resistance work more specifically can be associated with improved functional ability in daily tasks requiring strength (Coggan et al. 1992; Tracy et al. 1999). In their seminal paper, Frontera and co-workers (1988) used high-intensity strength training in healthy older men aged 60–72. The striking increments in strength (∼150%) and mid-thigh muscle mass (11.4%) after 12 weeks of resistance training have since established the training programme used as ‘normal practice’. This protocol involved three sets of eight repetitions (at 80% of the one repetition maximum (1RM)), three times a week, for each muscle group being trained. In the same vein, in recent work, it was concluded that 70–90% 1RM training in healthy older individuals was the most effective protocol for obtaining training-induced increments in muscle mass, with strength improvements being comparable to those seen in young individuals (Chin et al. 2008; Fielding 1995).
Another important aspect of ageing, which may in fact interfere with responsiveness to exercise, is the background of increased levels of inflammatory cytokines, a phenomenon referred to as ‘inflamed ageing’ (Butcher and Lord 2004), coupled with worsened metabolic profile, particularly in terms of hormones associated with both appetite and glucose metabolism (Adamczak et al. 2005; Baranowska et al. 2006; Schutte et al. 2007). In a context where (a) muscle hypertrophy can be achieved through either the decreased breakdown or the increased production of proteins (Short et al. 2005), (b) ageing-related sarcopaenia is related to decreased myosin heavy chain (crucial in muscle contractile function) synthesis rate and (c) hypo-activity is linked to a greater degree with decreased synthesis than any changes in breakdown activity (Rennie et al. 2009), the synthesis rate of myosin heavy chain has been shown to correlate to circulating levels of insulin-like growth factor-I (IGF-I), dehydroepiandrosterone sulphate (DHEAS) and testosterone (Balagopal et al. 1997). Thus, whilst a causal relationship has not necessarily been shown, and in fact increased levels of GH and/or IGF-I are beneficial in deficient but not healthy individuals in terms of hypertophic response (for a review, read (Velloso 2008)), these compounds may yet offer an insight into protein synthesis (and thus muscle anabolism) through implied modulated ability to synthesise myosin heavy chain. Together, the above phenomena suggest that the strategy to prescribing exercise in an older population ought to be manipulated in order to also take the altered hormonal environment into account, thus potentially maximising all of the potential positive effects of an exercise intervention.
Given the worldwide age demographics, coupled with the fact that older people have the greatest barriers (mostly in terms of accessibility and social context) to participating in exercise, simple advice on the minimum requirement for maximal benefits are likely to be the key to empowering older persons in terms of their personal lifestyle strategies. There are currently no systematic studies geared towards minimising the level of exercise intensity whilst maximising muscle strength and size as well as improving the endocrine profile of older adults.
The current study, therefore, aimed to determine whether, in an otherwise healthy older population, (a) high resistance activity would have positive muscle volume, functional and endocrine effects and (b) low-resistance activity could in fact elicit similar degrees of improvement (as high resistance activity) in hormonal, metabolic and functional parameters associated with physical well-being. Based on the principle that a previously reduced physical activity background entails a relatively greater potential for positive response to increased activity particularly in the older individual (Mroszczyk-McDonald et al. 2007), we hypothesised that compared to high-intensity resistance exercise, relatively low training intensity could also improve markers of healthy physiologic and endocrine functions in previously sedentary older individuals. In other words, in view of the fact that the literature has already reported a link with several endocrine factors and resistance exercise (for reviews, see (Crewther et al. 2006) and (Velloso 2008)), the purpose of the current study was in fact to (a) determine the effectiveness of relatively low resistance exercise and then (b) establish any link of changes in endocrine factors with exercise intensity in older people.
Methods
Participants
Thirty-nine older adults volunteered to participate in the current study having responded to advertisements posted locally. All applicants gave written informed consent to take part in the study though five of these prospective volunteers were excluded as they had a known history of cardiovascular, neurological, inflammatory or myopathic disease. Each participant’s general practitioner gave medical consent for the exercise regime. Thus, the current study participants were healthy, community dwelling and habitually active individuals, with no recent history of structured resistance training. The local Human Ethics Committee approved all experimental procedures.
Of the 34 healthy older adults who started the study, 30 completed the 12-week exercise intervention. Participants were randomly assigned to either a low (LowR) or a high (HighR) resistance-training group: the gender repartition, as well as age and physical characteristics of the completing participants are presented in Table 1.
Table 1.
Baseline characteristics of completing participant
| Group | Gender | Number | Age (years) | Activity (min/week) | Height (m) | Weight (Kg) |
|---|---|---|---|---|---|---|
| LowR | Male | 10 | 76 ± 2 | 245 ± 214 | 1.7 ± 0.05 | 79.1 ± 9.4 |
| Female | 8 | 76 ± 4 | 396 ± 457 | 1.6 ± 0.05 | 68.3 ± 11.7 | |
| All | 18 | 76 ± 3 | 321 ± 336 | 1.6 ± 0.05 | 73.7 ± 10.6 | |
| HighR | Male | 6 (4a) | 68 ± 7 | 273 ± 175 | 1.7 ± 0.03 | 81.7 ± 8.9 |
| Female | 6 | 66 ± 5 | 313 ± 183 | 1.6 ± 0.04 | 70.6 ± 13.2 | |
| All | 12 | 69 ± 6 | 293 ± 179 | 1.7 ± 0.04 | 76.2 ± 11.1 |
aThe number of participants who dropped out of the study before completion. Means ± SD
Muscle strength measurements
One repeated maximum measurement
During a familiarisation session no more than 7 days prior to the 12-week intervention, participants’ 1RM was determined for all exercises employed in the training programme. Participants first performed a standardised warm-up on the leg press (6 × 50% perceived 1RM; 4 × 70% perceived 1RM with 3-min recovery). Note here that we used ‘perceived sub-maximal efforts’ so as to not induce fatigue in the participants and/or injury by asking them to perform a maximal contraction on a ‘cold’ muscle. After warming up, the load was set at 90 % of the initially estimated 1RM and increased after each successful lift by 5 kg until failure. Each participant was given six lifting attempts in order to achieve their 1RM and a maximum of two attempts to lift the weight, once it had been established. The greatest amount of weight lifted successfully was recorded to determine the training load. Between successive attempts, 3-min rest periods were allowed. A repetition was valid if the participant used correct form and was able to complete the entire lift in a controlled manner without assistance. Participants’ 1RM for each exercise was reviewed every 2 weeks during training and if 1RM had increased the training load was adjusted accordingly. Additionally, if any participants felt that in-between 1RM assessments the training load was not providing adequate resistance, the load was increased so they were always lifting at the desired percentage of their maximum. Participants were familiarised with the resistance exercise training protocol on a subsequent visit to the laboratory.
Isometric knee extensors muscle strength measurements
Participants were familiarised with the experimental procedures on a separate occasion no more than 7 days prior to the baseline test sessions. In one single testing session, quadriceps isometric strength measures were taken on the right leg and isokinetic measures on the left leg, using a Cybex Dynamometer (Cybex Norm, Cybex International Inc., NY, USA). The centre of rotation of the lever arm of the dynamometer was aligned with the axis of rotation of the knee. Participants were positioned with the hip joint at 85° (supine = 0°). In order to minimise any extraneous movement of the hip joint or the trunk, participants were strapped over the shoulders, pelvis and thighs. Settings of chair height and positioning relative to the dynamometer were adjusted individually with all settings recorded and replicated at the post-intervention testing phase. Gravity corrections were then made following the manufacturers’ own procedure, having adjusted the attachment of the lever arm cuff relative to the length of the participant’s shank. Previous work from within our laboratory has utilised similar methods of measurement, finding them to be both valid and reliable (Pearson and Onambele 2005, 2006).
Maximal unilateral isometric torque Maximal isometric knee-extension torque was measured with the knee at 70° angle (full knee extension = 0°) on the right leg of all participants. After a series of warm-up trials consisting of 10 isokinetic contractions at 60°s−1 at 50–75% maximal effort, participants were instructed to rapidly exert maximal isometric force against the Cybex lever arm over a 3- to 4-s period. Participants were given both verbal and visual encouragement/feedback throughout their effort. Joint torque data were displayed on the screen of a computer (Macintosh G4; Apple Computer, Cupertino, CA), which was interfaced with an A/D system (Acknowledge, Biopac Systems, Santa Barbara, CA) with a sample frequency of 500 Hz. Isometric contractions were held for ∼2 s at the plateau, with a 90-s rest period between contractions. Peak torque was averaged over a 500-ms period at the plateau phase. The mean peak torque (MVCext) of three extensions was used as the measure of strength in each participant.
Maximal bilateral isometric torque Maximal bilateral isometric knee-extension torque was measured with both knees at 70° angle (full knee extension = 0°) pushing against a customised lever arm. Similar precautions to record time of day were taken. After a series of warm-up trials consisting of three isokinetic contractions at 60°s−1 at 50% Maximal effort, participants were instructed to rapidly exert maximal isometric force against the Cybex lever arm over a 3- to 4-s period. Participants were given both verbal and visual encouragement/feedback throughout their effort. Joint torque data were acquired and processed as during the unilateral efforts, with the only difference that, here, the best of the three efforts was used as the measure of strength for each participant.
Mid-thigh muscle thickness
Real-time B mode ultrasonography with a 7.5-MHz linear-array probe (AU5, Esaote, Genoa, Italy) was used to study mid-thigh muscle thickness in the area of the vastus intermedius (VI) and the vastus lateralis (VL) muscles. Muscle thickness was taken at rest with the knee at 70° angle. Scans were acquired in the mid-sagittal plane, at approximately 50% length of the VL muscle as measured from the origin at the linea aspera and lateral femur to insertion at the tibial tuberosity via patella tendon on the anterior surface. Medio-lateral width of the VL was determined over the skin surface, and the position of one half of the width was used as the measurement site. The ultrasound probe was coated with water-soluble transmission gel to provide acoustic contact and was held in place without depressing the dermal surface by the assessor. Three separate recordings of mid-thigh muscle thickness were made with 90-s rests between each recording. Ultrasound images were acquired using a digital recorder and frames exported to capture software (iMovie HD6, Apple computer Inc, USA). The VL thickness was measured as the distance from the top of the peripheral muscle aponeurosis to the deep aponeurosis. VI thickness was from the deep aponeuroses to the surface of the femur. The three points of interest were measured at three standardised points on each ultrasound frame to obtain an average tissue thickness using ImageJ analysis software (ImageJ 1.37, NIH, Bethesda, MD). Total muscle thickness was computed as the sum of VI+VL muscle thickness measured.
Body composition
Waist–hip ratio
A measuring tape was used to measure the circumference of the hips at the widest part of the buttocks and the waist at the smaller circumference of the individual’s natural waist (just above the navel). The waist–hip ratio was used as the individual’s score of central adiposity.
Bioelectrical impedance analysis
Bioelectrical impedance analysis (BIA; BODYSTAT, Isle of Man, British Isles) was used to estimate body composition based on the difference in electric conductive properties of various tissues. The BODYSTAT applied 500 µA at a single frequency of 50 kHz through self-adhesive electrodes placed on the right hand and foot, of a participant lying flat on their back with their arms away from the trunk, thighs not touching and ankles at least 20 cm apart. Although BIA has acknowledged limitations when applied to non-standard or elderly populations due to the built in equation utilised to calculate body composition, it is important to state that the BIA in this study was used to assess within participant adaptations as a consequence of the intervention and as such carries more validity. What is more, to lend greater external validity to data from this instrument, raw values were corrected for the inherent ∼15% overestimation in body fat content (when comparing pilot participants BIA versus DEXA outputs—others have drawn similar conclusions regarding the differences in body fat content values depending on the methodology used (Jorgensen et al. 1996; Lintsi et al. 2004)) and have tended to consider DEXA readings as gold standard since it yields values similar to those obtained from the hydrostatic weighing method (Prior et al. 1997).
Body mass index
BMI was defined as the individual’s body mass (kilograms) divided by the square of their height (square meters).
Functional abilities measures
The tests of functional ability were all performed on 1 day at the outset and completion of the study and were similar to a battery of functional tests performed by participants in previous research (Chandler et al. 1998; Skelton et al. 1995).
Get-up-and-go
Three cones were placed 1 m apart on the floor in front of a rigid chair of adjustable height. The participant’s knee-to-floor height (i.e. the distance from the knee joint axis to the floor) was recorded prior to testing to determine the appropriate chair height for each test. Once knee height was determined, the chair height was set at 100%, 80% and 60% of each individual knee-to-floor height for the tests. The test started with the participant seated on the chair (at the appropriate height), with feet flat on the floor and arms folded across the chest. They were then asked to rise unaided as quickly as possible, walk around the furthest cone (3 m away) and back to the initial seated position on the chair. The elapsed time between ‘chair rise’ to ‘sitting back down’ was recorded. The quickest of three trials was used as the participant’s score.
Standing from lying
Participants were asked to lie flat on the floor on a gym mat and on their preferred side, with their arm on the floor outstretched and their head flat on the outstretched arm. Participants were then instructed to rise as quickly as possible using their preferred technique. The time elapsed between the instruction to ‘Go’ and the participant standing upright and steadily with both feet firmly on the ground was recorded. The fastest of three trials was utilised as their score.
Six-minute walk
A 10-m course was set with cones 1 m apart in a straight line. Participants were instructed to walk around this course using their fastest, non-running, walking pace, with the aim of completing as many revolutions of the circuit as possible in 6 min. The score was calculated as the total distance covered in the allocated time.
Metabolic and endocrine profiling
At the onset and end of the intervention and following an overnight fasting period, participants reported to the laboratory. A 21-gauge 1-in. ultra-thin wall needle (Terumo medical corporation, New Jersey, USA) was inserted into the anticubital vein of the forearm. Using a vacutainer assembly and serum separator tubes (Monovette, Sarstedt, Numbrecht, Germany), 10 mL blood samples were collected. Blood glucose was analysed immediately using a single drop of freshly sampled blood using the AccuChek Advantage System (Roche Diagnostics Ltd, Lewes, UK; Sensitivity of <10 mg/dL (i.e. minimum detectable concentration); Intra-assay variability of 2% (i.e. coefficient of variation)). The remainder of the sample was centrifuged at 2–5°C for 5 min at 4,000 rpm, with the supernatant being removed and stored in eppendorfs at −70°C for later analyses. Insulin (Biosource, Nivelles, Belgium; Sensitivity of 0.15 µU/ml; Intra-assay variability of 4.2%), IGF-I (Biocode-Hycel, Liege, Belgium; Sensitivity of 4.9 ng/ml; Intra-assay variability of 8.0%), tumour necrosis factor-α (TNF-α; Diaclone, Besancon Cedex, France; Sensitivity <8 pg/ml; Intra-assay variability of 3.3%), NeuroPeptide Y (NPY; Phoenix Europe GmbH, Karlsruhe, Germany; Sensitivity of 0.13 ng/ml; Intra-assay variability <5%), insulin-like growth factor binding protein-3 (IGFBP-3; Biocode-Hycel, Liege, Belgium; Sensitivity of 10.5 ng/ml; Intra-assay variability of 6.5%) and interleukin-6 (IL-6; Diaclone, Besancon Cedex, France; Sensitivity <0.8 pg/ml; Intra-assay variability of 3.3%) were analysed using standard enzyme-linked immuno-sorbent assay procedures.
Training programme
The training programme was 12 weeks in duration and consisted of one supervised gym-based class and two home-based sessions per week in LowR. In HighR, the programme was for two supervised gym-based classes and one home-based session per week. All exercise sessions were 1 h in duration.
Briefly, the supervised exercise classes consisted of a warm-up (stretching, aerobic and coordination work), resistance exercises (using therabands for all major muscle groups, Leg Press, Leg Extension, Calf Rotator and Glute conditioner (Technogym, Gambettola, Italy) as partly illustrated on Fig. 1 below, with a progression from 8–11 reps in 2–4 sets at 40% or 80% 1RM) and a cool-down (i.e. stretches, Pilates, Tai Chi). The unsupervised home-based exercises were similar in design to the supervised classes with the exception that all the resistance work was carried out using therabands, and a 20-min brisk walk was also included. An exercise booklet illustrated, using photographic and/or cartoons, all the exercises in detail. Home-based exercise was not to be performed the day preceding or following the supervised class exercise.
Fig. 1.
a–c Supervised exercise classes
Statistical analyses
T tests were carried out to compare data at baseline. Two-way factorial ANOVAs were carried out with group as one factor (two levels: HighR vs. LowR) and phase as the second factor (two levels: baseline vs. post-intervention) to determine any main effects of the group or interventions. An ANCOVA was run on the get-up-and-go (GUG) IGFBP-3 datasets since the LowR and HighR populations differed in these parameters at the onset of the study. Data are expressed as mean ± SEM unless otherwise stated. Significance was set at P ≤ 0.05.
Results
The two intervention groups did not significantly differ in age, habitual physical activity levels, height or weight at the onset of the current programme. Interestingly, however, the HighR group showed a tendency towards a greater overall body mass increase (∼3%; from 76.2 ± 3.5 to 78.3 ± 4.2 Kg) after the 12-week intervention compared to the changes seen in the LowR group (∼1%; from 73.7 ± 2.50 to 74.5 ± 2.1 Kg). This effect, however, was not significant.
Body composition changes
No significant difference in BMI was observed in the two groups after the 12-week interventions. What is more, there was no change in waist–hip ratio in either the LowR population (0.90 ± 0.02 to 0.91 ± 0.02) or the HighR population (0.88 ± 0.03 to 0.87 ± 0.02). Nonetheless, interestingly, LowR exhibited a marked trend for increased body-fat percentage of 18.5 ± 9.0% (from a value of 26.3 ± 2.1% to 29.8 ± 2.2% post-intervention, P = NS), whereas such a trend for a rise in body fat percentage was attenuated in the HighR population (value of 9.2 ± 7.9%, i.e. from 29.1 ± 2.7% to 30.8 ± 2.6%, P = NS).
Knee extensors muscle strength changes
One repetition maximum
Prior to training, there was no significant difference in the mean 1RM strength measured by leg press, leg extension, calf rotation or gluteus conditioner between groups (P = NS). At 4, 8 and 12 weeks (i.e. the post-intervention phase), the average strength increase for each exercise was significant for both intervention groups (P < 0.05). However, the increase for LowR was significantly less pronounced in all exercises compared to HighR (see Table 2). The average 12-week increase in strength was 17 ± 1% for LowR (8.9 ± 1.7 kg) and 51 ± 4% for HighR (23.6 ± 3.4 kg).
Table 2.
Change in 1RM load lifted (Kg) and maximum isometric torque (Nm) from pre- to 12-week post-intervention
| LowR | HighR | |||||
|---|---|---|---|---|---|---|
| PRE | POST | Δ (%) | PRE | POST | Δ (%) | |
| Leg Press (Kg) | 90.4 ± 6.2 | 105.5 ± 7.1 | 17a | 89.7 ± 7.7 | 125.4 ± 7.5 | 40 |
| Leg Extensor (Kg) | 30.5 ± 2.7 | 35.5 ± 3 | 16a | 28.3 ± 1.8 | 42.8 ± 1.9 | 51 |
| Calf Rotator (Kg) | 34.7 ± 2 | 42.1 ± 2.4 | 21a | 33.4 ± 3.2 | 55.8 ± 3.8 | 67 |
| Glute Conditioner (Kg) | 55 ± 4.1 | 63.1 ± 4 | 15a | 45.7 ± 4.1 | 67.4 ± 5 | 47 |
| Unilateral MVC (Nm) | 122.2 ± 9.5 | 122.8 ± 9.3 | 1.3 ± 3.0 | 122.6 ± 12.3 | 133.0 ± 13.6 | 9.3 ± 5.1 |
| Bilateral MVC (Nm) | 152.0 ± 13.0 | 165.7 ± 14.0 | 10.3 ± 4.7 | 137.5 ± 19.2 | 160.3 ± 18.3 | 22.4 ± 10.2 |
aSignificantly lower increment in LowR compared to HighR (P < 0.05). Values are Mean ± SEM
Isometric: unilateral and bilateral torque
Prior to training, there was no significant difference in either the mean isometric unilateral or bilateral knee extensor strength (MVCext) between the two intervention groups (P = NS). Table 2 shows the mean changes in MVCext after the 12-week intervention in each group. Briefly, trends towards increased unilateral isometric force were seen post-interventions, though these effects were not significant. The HighR group exhibited a non-significant change in MVCext of 9.3 ± 5.1% (122.6 ± 13.6 to 133 ± 13.6 Nm, P = NS). LowR MVCext changed non-significantly by 1.3 ± 3% (122.2 ± 9.5 to 122.8 ± 9.3 Nm, P = NS). However, the 12-week intervention resulted in a significant increase in bilateral torque in both HighR (22.4 ± 10.2%, P = 0.024) and LowR (10.3 ± 4.7 %, P = 0.024) groups.
Mid-thigh muscle thickness
Total muscle thickness (VL+VI) was significantly increased in the HighR participants by 5.8 ± 2.6% (34.4 ± 1.8 to 36.4 ± 2.0 mm; P = 0.038). LowR was without effect on total muscle thickness (32.6 ± 2.0 to 34.6 ± 2.2 mm; 6.8 ± 5.4% change, P = NS).
Functional measures
Get-up-and-go
At baseline, there was a significant difference in reaction time (as determined by the GUG test) between the two groups, whereby LowR participants were taking significantly longer to complete the task (P < 0.04). Both groups completed the GUG test significantly quicker post-intervention. GUG time was significantly improved by 10.8 ± 3% (6.8 ± 0.4 to 6.0 ± 0.3 s) in the LowR population. Similarly, HighR was also significantly improved by 11.6 ± 4.8% (5.5 ± 0.3 to 4.9 ± 0.3 s). Even after accounting for the between-groups differing baseline values through the ANCOVA test, the difference in response to treatments remained significant (P < 0.05).
Standing from lying
Functional power determined by standing from lying (SFL) time showed significant improvement in the HighR population (2.73 ± 0.19 to 2.36 ± 0.2, i.e −15.6% change, P = 0.025 ) whilst the LowR population in fact showed a trend towards increased SFL (3.69 ± 0.33 to 3.75 ± 0.42, i.e 5.2% change, P = NS).
Six-minute walk
The distance covered during the 6-min walk test was increased by 8.5 ± 1.7% (25.7 ± 1.3 to 28 ± 1.7 m) in the HighR participants (P < 0.003). The LowR participants showed no change in distance covered (24.2 ± 1 to 24.5 ± 0.9 m; 1.9 ± 2.3% change, P = NS).
Metabolic and endocrine characteristic changes
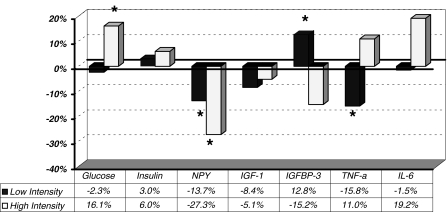
Data of the endocrine changes (i.e. post-intervention values normalised for baseline values) are summarised in Fig. 2.
Fig. 2.
Summary of endocrine characteristics changes with the two levels of exercise (high versus low intensity); *P < 0.05, post-intervention data were significantly different to baseline values
Metabolic profile—plasma levels of glucose and insulin
At baseline, there was no significant difference in the mean plasma glucose levels between intervention groups. The 12-week intervention resulted in a significant elevation in fasting plasma glucose of 16.1 ± 4.1% (P = 0.001) in the HighR group (4.80 ± 0.19 to 5.51 ± 0.08 mmol/L). The LowR group exhibited a (non-significant) trend for lower post-intervention plasma glucose of −2.3 ± 4.9% (5.34 ± 0.20 to 5.11 ± 0.19 mmol/L).
Prior to training, there was no significant difference in the mean plasma insulin levels between the two groups. The 12-week intervention resulted in no change in plasma insulin levels for both LowR (3 ± 7.5% change (i.e. 10 ± 1 to 10.4 ± 1.4 µU/ml)) and HighR (6 ± 6.6% change (i.e. 8.06 ± 0.69 to 8.51 ± 0.92 µU/ml)) groups.
Control of energy homeostasis in relation to metabolic status—plasma levels of NPY
Prior to training, there was no significant difference in the mean plasma NPY levels between the two intervention groups. The 12-week interventions resulted in significant decreases in plasma NPY levels for both LowR (−13.7% change (i.e. 23.4 ± 1.66 to 20.2 ± 1.67 pg/ml), P = 0.026) and HighR groups (−27.3% change (i.e. 28.4 ± 4.03 to 20.7 ± 3.53 pg/ml), P = 0.044).
Plasma levels of IGF-I and IGFBP-3
Serum levels of IGF-I did not differ significantly between the two groups at baseline. Post-intervention, in the LowR group, values changed from 342.6 ± 23.1 ng/ml at baseline to 313.8 ± 20.8 ng/ml post-training, a −8.4% change with the intervention, which was not, however, significant. Similarly in the HighR group, values decreased from 404.2 ± 31.2 ng/ml at baseline to 383.5 ± 19.0 ng/ml post-training, also a non-significant −5.1% change in serum IGF-I levels with the intervention.
Serum levels of IGFBP-3 differed significantly between the two groups at baseline (P = 0.035). Post-intervention, in the LowR group, values changed significantly from 3,827.2 ± 214.9 ng/ml at baseline to 4,317.8 ± 282.0 ng/ml post-training, a 12.8% (P = 0.005) increase with the intervention. However, in the HighR group, values did not change significantly with the intervention whilst decreasing from 3,050.9 ± 259.4 ng/ml at baseline to 2,587.8 ± 121.1 ng/ml post-training, a −15.2% non-significant change. Even after accounting for the between-groups differing baseline values through the ANCOVA test, the difference in response to treatments by group remained significant (P = 0.012).
Cellular degradation/inflammation signal—plasma levels of TNF-α and IL-6
Serum levels of TNF-α did not differ significantly between the two groups at baseline. Post-intervention, values decreased in both groups. Thus, in the LowR group, values changed significantly from 35.2 ± 8.7 pg/ml at baseline to 29.6 ± 7.7 pg/ml post-training, a −15.8% (P = 0.030) change with the intervention. In the HighR group, values did not change significantly (29.0 ± 18.6 pg/ml at baseline vs. 31.0 ± 20.9 pg/ml post-training; an 11% change, P = NS).
Serum levels of IL-6 did not differ significantly between the two groups at baseline and were unaltered by training (LowR = 3.00 ± 0.58 pg/ml at baseline to 2.95 ± 0.31 pg/ml post-training; HighR group = 2.14 ± 0.45 pg/ml at baseline to 2.56 ± 0.71 pg/ml post-training).
Discussion
Summary of findings in view of the study hypothesis
The current study sought to compare in vivo changes in skeletal muscle function, body composition and endocrine parameters in older people using two intensities of exercise, where one group of older persons performed resistance exercise once a week under supervision at 40% 1RM using resistance machines plus two unsupervised home-based training sessions using mainly therabands, and the second group performed resistance exercise twice a week under supervision at 80% 1RM using resistance machines and one unsupervised training session at home, using mainly therabands. We aimed to determine whether (a) high-resistance activity had positive muscle volume, functional and endocrine effects in older persons and (b) low resistance activity could in fact elicit a similar degree of improvement (as high-resistance activity) in several factors (e.g. hormonal and functional) associated with physical well-being. We hypothesised that, owing to the impact of baseline function on responsiveness to training, compared to high-intensity resistance exercise, relatively low training intensity could also improve in vivo markers of healthy physiologic and endocrine functions in previously sedentary older individuals.
Our current findings have partially supported our hypotheses. Indeed, whilst none of the interventions significantly impacted upon body composition markers, including BMI, waist–hip ratio and bioelectric impedance, muscle strength data showed an advantage for the HighR protocol with 51 ± 4% and 22.4 ± 10.2% (P < 0.05) improvements in 1RM strength and bilateral knee extension torque, respectively, compared with 17 ± 1% and 10.3 ± 4.7% 1% (P < 0.05) increments in the LowR group. Muscle thickness data also showed a greater benefit of a HighR protocol (5.8 ± 2.6% increase, P < 0.05) compared with the LowR protocol (no significant change). Functional ability tests including GUG, standing from lying and the 6-min walk all showed significant changes of −11.6 ± 4.8%, −15.6% and 8.5 ± 1.7% (P < 0.05), respectively, in HighR. In comparison, only the GUG test showed a significant decrease of −10.8 ± 3% (P < 0.05) in LowR. Finally, overnight fasting serum levels of IGFBP-3, NPY and TNF-α changed significantly in LowR whereas only serum levels of glucose and NPY changed significantly in HighR groups. Circulating levels of IL-6, insulin and IGF-I did not alter significantly with either intervention.
Muscle size and strength changes
Sixteen weeks of resistance exercise training has been shown to increase not only the strength but also type II muscle fibre size in old adults (Kosek et al. 2006). Indeed, numerous studies have demonstrated that resistance training is an effective measure to counteract sarcopenia in both healthy and frail older adults (Carmeli et al. 2000; Charette et al. 1991; Ferri et al. 2003; Frontera et al. 1988, 2000, 2003). It would appear that with an appropriate and effective exercise regimen, older adults may expect gains of 5–10% muscle CSA, accompanied by increases of 20–100% or more in muscle strength, depending on the muscle group under investigation. Our data thus agree with previous studies of similar duration (Charette et al. 1991; Frontera et al. 1988; Hakkinen et al. 2001; Lexell 1995) in terms of the magnitude of muscle strength and size benefits to be had from high-intensity resistance exercise. Studies other than the current one, however, have not systematically compared lower intensity resistance exercise with high-intensity programmes to try to quantify physiologic and endocrine adaptations, making our data novel in the current exercise intervention literature, as far as we are aware.
In agreement also with our current data, the majority of previous studies investigating the effects of strength training on muscle strength and mass in older adults have observed greater increases in muscle strength than in muscle CSA. Similar to the events seen in younger adults, the discrepancies between gains in muscle strength and gains in muscle size have mainly been attributed to neural adaptations (Fiatarone et al. 1990). Findings indicate that fibre activation and recruitment are more rapidly modulated than muscle hypertrophy response. This is especially true of the low-intensity-trained participants in the current study who exhibited significant strength increments post-intervention without, however, showing any muscle size increments.
Molecular background of the observed in vivo muscle characteristics
Repeated bouts of resistance exercise increase protein synthesis (Hartman et al. 2006; Yarasheski 2003), the metabolic determinant of gains in skeletal muscle mass (Kosek et al. 2006) in young and old adults, even if this increase in muscle protein synthesis with resistance exercise training in the old is lower than that seen in the young (Welle et al. 1996). Our current findings suggest that there is no direct link between physical exercise and presence of chronically elevated, systemic IGF-I. In fact, there were no discernable changes in the levels of IGF-I at the end of the two training interventions. Interestingly, levels of IGFBP-3 increased significantly but only in the lower-intensity-trained population, whilst the high-intensity-trained participants showed no change in the levels of this IGF-modulatory protein. Most IGF-I (∼80%) circulates in a 150-kDa, high-affinity complex, which also contains IGFBP-3 and an acid-labile subunit. Analogous to the present findings, studies have found no change in IGF-I and IGFBP-3 concentrations following 8 (Bermon et al. 1999) and 12 weeks (Kraemer et al. 1999) of resistance training in a similarly aged population. In contrast, a significant increase in plasma IGF-I concentrations following an acute exercise bout was reported in one of these studies (Bermon et al. 1999). In endurance training studies, IGFBP-3 has been shown to be independent of IGF-I responses and potentially to have its own biological activity at the level of the cell (Jones and Clemmons 1995). Eliakim et al. (Eliakim et al. 1997) showed that in rat, muscle IGF-I concentrations can increase with relatively short-duration endurance training, despite lack of change in muscle IGF-I mRNA or serum IGF-I. These authors proposed that the mechanisms through which muscle IGF-I concentrations post-training could alter with no concurrent changes in muscle IGF-I mRNA nor indeed serum IGF-I levels may either involve (a) translational and/or post-translational-mediated events, (b) exercise-induced increase in total blood volume or (c) modulated characteristics of the IGF binding proteins in the muscle tissue. Then again, a lack of change in serum IGF-I with training may suggest that IGF-I in the circulation may not be a meaningful marker of the activity of the GH-IGF-I system. Alternatively, increased IGF-I secretion from the liver may be quickly sequestered by the tissue in order to maintain a homeostatic balance of IGF-I in the systemic circulation (Kraemer et al. 1999), which may explain why we observed little change in these endocrine measures with either intervention but pronounced gains in muscle strength. Finally, it is also possible that the issue of timing of the sera sampling might have played a role in our observations and that hormonal adaptations may have occurred in a more acute fashion, which we would have missed (Kraemer and Ratamess 2005). In addition, it is notable that IGF-I basal levels in our two populations tended to show high values at baseline in the HighR group and for IGFBP-3 levels to be in fact lower at baseline in this group compared with LowR. In the case of IGF-I, this was possibly owing to the gender repartition in that the HighR group had proportionally (relative to the gender repartition in the LowR group) more females, and these tend to exhibit higher levels of the ligand (Brabant and Wallaschofski 2007). In the case of IGFBP-3, the between-groups difference might be owing to the overall higher absolute number (8 vs 6) of female participants in LowR compared with HighR (Berrigan et al. 2009). Either way, the above two statements simply highlight the need for future single gender studies to elucidate an answer.
The increments in muscle strength found in the present study do not appear to be due to an increase in muscle mass resulting from an increase in the myofibrillar protein synthesis rate. Of course, an increased synthetic rate is insufficient to stimulate muscle hypertrophy. Notably, ageing is also associated with increases in the protein breakdown mechanism (Trappe et al. 2004), and the impairment of this signalling may be an alternative pathway for maintained and/or improved skeletal muscle mass and, hence, performance. Our data illustrated significantly decreased TNF-α levels in the lower intensity exercise group only, with the high-intensity-trained counterparts exhibiting no significant IL-6 or TNF-α responses.
Physiological age-dependant hormonal changes may partly explain the impaired muscle protein synthesis in older adults. A subsequent increase in levels of catabolic cytokines, particularly TNF-α may suppress protein synthesis (Sakurai et al. 1996) and exacerbate protein degradation in the muscle (Bales and Ritchie 2002; Moulias et al. 1999). Resistance exercise may attenuate this process by suppressing skeletal muscle TNF-α expression (Greiwe et al. 2001), an effect seen in the LowR group only in this study. However, in the case of high-intensity exercise, there may be a potential for greater ‘micro-muscle-damage’ (Nikolaidis et al. 2008) resulting in a changed inflammatory response with this exercise intensity. The strength gains observed in the present study for HighR strengthen claims that these gains may be attributed to neural factors; however, acute adaptations of anabolic hormones cannot be ruled out. An inverse relationship between local levels of TNF-α and skeletal muscle protein synthesis in frail older adults has been hypothesised (Greiwe et al. 2001), and this is in agreement with the effect shown through the LowR intervention which stimulated a reduction in plasma concentrations of TNF-α. If increased myofibrillar protein synthesis was responsible for strength gains in the present study, one would postulate that circulating levels of TNF-α would decrease following 12 weeks of resistance training, as Greiwe et al. (2001) reported in an octogenarian population. The preference for decreased catabolic signalling for LowR in the present study was accompanied by modest strength gains compared to the strength gains in HighR. This suggests that low-intensity exercise in older populations may be sufficient to help alleviate one important modulator of inflamed ageing, while not impacting on the phenotypic expression of skeletal muscle mass. What is clear, however, is that the hormonal pathways used for LowR appear different to those seen with HighR exercise.
Finaly NPY, the sympathetic co-transmitter which causes vasoconstriction, decreases coronary blood flow and decreases cardiac output (Hauser et al. 1996) was seen to diminish significantly with the two levels of exercise, hence, denoting positive circulatory adaptations with exercise. The fact that the chronic circulating levels of the immunoreactive NPY decreased to a similar extent with both exercise intensities reinforces the effectiveness of lower exercise intensity in the older age group.
Body composition and metabolism at the whole body as well as endocrine level
Our findings are that body fat content did not significantly change whilst the body weight of participants was maintained from baseline to the post-intervention phase. These data on body composition changes appear to be at odds with a previous study that demonstrated a decrease in body fat content following a programme of resistance exercise in older populations (Kitamura et al. 2003). It should, however, be noted that more than resistance training, aerobic exercise is generally linked to decreased adiposity, and even then, there are aerobic studies showing no body composition changes post-intervention (Tonino 1989). This fact, combined with the fact that the current programme was limited in terms of aerobic work (participants did not carry out the aerobic phase of their programme at 85% of maximum heart rate, nor was this phase more than 15–20 min per session altogether), may explain the lack of changes in this parameter in the current study. What is more, ageing is linked to a decline in resting metabolic rate (RMR), presumably owing to the ageing-associated loss of muscle mass (Welle and Nair 1990), decreased physical activity (Vaughan et al. 1991) and decreased rate of protein metabolism (Welle and Nair 1990). The mechanism by which RMR is increased with resistance training is presumed to be linked to an increase in protein turnover and associated increased (a) muscle protein synthesis, (b) muscle-tissue damage and (c) muscle-tissue repair. Thus, the fact that these functions were generally not significantly changed with the training interventions may explain why any impact on body composition was largely limited. What is more, our current findings are aligned with the suggestions that maintenance of fat mass may be an important coping mechanism in the elderly, even in the presence of increased physical activity. Indeed, loss of adipose tissue predisposes individuals, especially the elderly, to chronic skin ulcers, disturbances of body temperature and decreased energy reserves in the face of chronic illness. Thus, there is no evidence to date to suggest that reduction of adipogenesis and/or excessive fat loss per se have any beneficial effects (Zhu et al. 2007) in older persons. In contrast, impaired adipogenesis has been reported to be associated with insulin resistance i.e. increased glucose intolerance (Heilbronn et al. 2004; Yang et al. 2004), thus potentially contributing to the paradoxical development of type II diabetes in very old lean patients.
Moreover, in the current study, whilst there were no significant changes or in fact any noticeable trends in the levels of insulin in the two groups, the high-intensity-trained participants in fact concluded the programme with significantly higher levels of glucose, whereas glucose levels in the low-intensity-trained participants remained unchanged. A study by others proposes that exercise alone may not be sufficient to induce changes in fasting plasma glucose and insulin levels (Matthews et al. 1985). In the face of no changes in body composition in our high-intensity-trained study population, it is, therefore, not surprising that the glucose profiles also bucked the trend for expected (Ryan 2000) reductions in glucose levels as well as fat content and/or central adiposity. It cannot be ruled out, however, whether this study group in fact altered their habitual nutritional intake, hence altering glucose metabolism through a different mechanism. It should also be pointed out here that although the glucose level changes were significant in HighR (∼16.1% increment), they were not clinically relevant as the status of the participants remained healthy (absolute glucose levels increased from ∼4.80 ± 0.19 to 5.51 ± 0.08 mmol/L, thus remaining within the expected healthy norm, post-intervention). Whatever the case, the effect of resistance training as a single intervention for impacting on plasma glucose warrants further investigations.
Functional abilities changes with exercise training
Finally, the two training programmes employed in the present study appear to have enhanced habitual function, more successfully than previous studies’ attempts to do so, particularly where the get-up-and-go test is concerned (Earles et al. 2001; Fiatarone et al. 1990; Schlicht et al. 2001; Skelton et al. 1995). Nevertheless, our findings of improved function with training do corroborate with the findings of some earlier studies (Skelton et al. 1997; Taaffe et al. 1999), even where the lower intensity group is concerned.
Conclusion
The two training intensities yielded a number of positive adaptations, including for both, improvements in strength and functional abilities. It is notable, however, that whilst in vivo physiologic function changes showed a clear advantage for older persons to carrying out high resistance training, at the endocrine level, such an advantage (at least chronically) is not seen. In fact, in terms of changes in sera levels of overnight fasted resting levels of glucose, IGFBP-3 and TNF-α, there appears to be an advantage to carrying out the lower intensity, long-term exercises for the aged populations where only endocrine factors are taken into account. Together, these studies suggest that mixed exercise intensity interventions should be advocated.
References
- Adamczak M, Rzepka E, Chudek J, Wiecek A. Ageing and plasma adiponectin concentration in apparently healthy males and females. Clin Endocrinol (Oxf) 2005;62(1):114–118. doi: 10.1111/j.1365-2265.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Proctor D, Nair KS. Sarcopenia and hormonal changes. Endocrine. 1997;7(1):57–60. doi: 10.1007/BF02778064. [DOI] [PubMed] [Google Scholar]
- Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–323. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- Baranowska B, Bik W, Baranowska-Bik A, Wolinska-Witort E, Szybinska A, Martynska L, et al. Neuroendocrine control of metabolic homeostasis in Polish centenarians. J Physiol Pharmacol. 2006;57(Suppl 6):55–61. [PubMed] [Google Scholar]
- Bermon S, Ferrari P, Bernard P, Altare S, Dolisi C. Responses of total and free insulin-like growth factor-I and insulin-like growth factor binding protein-3 after resistance exercise and training in elderly subjects. Acta Physiol Scand. 1999;165(1):51–56. doi: 10.1046/j.1365-201x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Berrigan D, Potischman N, Dodd KW, Hursting SD, Lavigne J, Barrett JC, et al. Race/ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm IGF Res. 2009;19:146–155. doi: 10.1016/j.ghir.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant G, Wallaschofski H. Normal levels of serum IGF-I: determinants and validity of current reference ranges. Pituitary. 2007;10(2):129–133. doi: 10.1007/s11102-007-0035-9. [DOI] [PubMed] [Google Scholar]
- Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3(4):151–160. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Reznick AZ, Coleman R, Carmeli V. Muscle strength and mass of lower extremities in relation to functional abilities in elderly adults. Gerontology. 2000;46(5):249–257. doi: 10.1159/000022168. [DOI] [PubMed] [Google Scholar]
- Chandler JM, Duncan PW, Kochersberger G, Studenski S. Is lower extremity strength gain associated with improvement in physical performance and disability in frail, community-dwelling elders? Arch Phys Med Rehabil. 1998;79(1):24–30. doi: 10.1016/S0003-9993(98)90202-7. [DOI] [PubMed] [Google Scholar]
- Charette SL, McEvoy L, Pyka G, Snow-Harter C, Guido D, Wiswell RA, et al. Muscle hypertrophy response to resistance training in older women. J Appl Physiol. 1991;70(5):1912–1916. doi: 10.1152/jappl.1991.70.5.1912. [DOI] [PubMed] [Google Scholar]
- Chin APMJ, Uffelen JG, Riphagen I, Mechelen W. The functional effects of physical exercise training in frail older people : a systematic review. Sports Med. 2008;38(9):781–793. doi: 10.2165/00007256-200838090-00006. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72(5):1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- Crewther B, Keogh J, Cronin J, Cook C. Possible stimuli for strength and power adaptation: acute hormonal responses. Sports Med. 2006;36(3):215–238. doi: 10.2165/00007256-200636030-00004. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Earles DR, Judge JO, Gunnarsson OT. Velocity training induces power-specific adaptations in highly functioning older adults. Arch Phys Med Rehabil. 2001;82(7):872–878. doi: 10.1053/apmr.2001.23838. [DOI] [PubMed] [Google Scholar]
- Eliakim A, Moromisato M, Moromisato D, Brasel JA, Roberts C, Jr, Cooper DM. Increase in muscle IGF-I protein but not IGF-I mRNA after 5 days of endurance training in young rats. Am J Physiol. 1997;273(4 Pt 2):R1557–R1561. doi: 10.1152/ajpregu.1997.273.4.R1557. [DOI] [PubMed] [Google Scholar]
- Ferri A, Scaglioni G, Pousson M, Capodaglio P, Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003;177(1):69–78. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263(22):3029–3034. doi: 10.1001/jama.263.22.3029. [DOI] [PubMed] [Google Scholar]
- Fielding RA. The role of progressive resistance training and nutrition in the preservation of lean body mass in the elderly. J Am Coll Nutr. 1995;14(6):587–594. doi: 10.1080/07315724.1995.10718547. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64(3):1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Krivickas LS, Kim SK, Foldvari M, Roubenoff R. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve. 2003;28(5):601–608. doi: 10.1002/mus.10480. [DOI] [PubMed] [Google Scholar]
- Greenlund LJ, Nair KS. Sarcopenia–consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124(3):287–299. doi: 10.1016/S0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15(2):475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- Hakkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand. 2001;171(1):51–62. doi: 10.1046/j.1365-201x.2001.171001051.x. [DOI] [PubMed] [Google Scholar]
- Hartman JW, Moore DR, Phillips SM. Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males. Appl Physiol Nutr Metab. 2006;31(5):557–564. doi: 10.1139/H06-031. [DOI] [PubMed] [Google Scholar]
- Hauser GJ, Danchak MR, Colvin MP, Hopkins RA, Wocial B, Myers AK, et al. Circulating neuropeptide Y in humans: relation to changes in catecholamine levels and changes in hemodynamics. Neuropeptides. 1996;30(2):159–165. doi: 10.1016/S0143-4179(96)90083-9. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Rood J, Janderova L, Albu JB, Kelley DE, Ravussin E, et al. Relationship between serum resistin concentrations and insulin resistance in nonobese, obese, and obese diabetic subjects. J Clin Endocrinol Metab. 2004;89(4):1844–1848. doi: 10.1210/jc.2003-031410. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen JO, Vahl N, Hansen TB, Thuesen L, Hagen C, Christiansen JS. Growth hormone versus placebo treatment for one year in growth hormone deficient adults: increase in exercise capacity and normalization of body composition. Clin Endocrinol (Oxf) 1996;45(6):681–688. doi: 10.1046/j.1365-2265.1996.8720883.x. [DOI] [PubMed] [Google Scholar]
- Kitamura IT, Tokudome N, Yamanouchi M, Oshida K, Sato Y. Effects of aerobic and resistance training on insulin action in the elderly. Geriatr Gerontol Int. 2003;3:50–55. doi: 10.1046/j.1444-1586.2003.00054.x. [DOI] [Google Scholar]
- Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, et al. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140(1):41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101(2):531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Hakkinen K, Newton RU, Nindl BC, Volek JS, McCormick M, et al. Effects of heavy-resistance training on hormonal response patterns in younger vs. older men. J Appl Physiol. 1999;87(3):982–992. doi: 10.1152/jappl.1999.87.3.982. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lintsi M, Kaarma H, Kull I. Comparison of hand-to-hand bioimpedance and anthropometry equations versus dual-energy X-ray absorptiometry for the assessment of body fat percentage in 17-18-year-old conscripts. Clin Physiol Funct Imaging. 2004;24(2):85–90. doi: 10.1111/j.1475-097X.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Moulias R, Meaume S, Raynaud-Simon A. Sarcopenia, hypermetabolism, and aging. Z Gerontol Geriatr. 1999;32(6):425–432. doi: 10.1007/s003910050140. [DOI] [PubMed] [Google Scholar]
- Mroszczyk-McDonald A, Savage PD, Ades PA. Handgrip strength in cardiac rehabilitation: normative values, interaction with physical function, and response to training. J Cardiopulm Rehabil Prev. 2007;27(5):298–302. doi: 10.1097/01.HCR.0000291297.70517.9a. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol. 2003;95(6):2229–2234. doi: 10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- Nikolaidis MG, Jamurtas AZ, Paschalis V, Fatouros IG, Koutedakis Y, Kouretas D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: magnitude and time-course considerations. Sports Med. 2008;38(7):579–606. doi: 10.2165/00007256-200838070-00005. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Maganaris CN, Mian OS, Tam E, Rejc E, McEwan IM, et al. Neuromuscular and balance responses to flywheel inertial versus weight training in older persons. J Biomech. 2008;41(15):3133–3138. doi: 10.1016/j.jbiomech.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Onambele GN. Acute changes in knee-extensors torque, fiber pennation, and tendon characteristics. Chronobiol Int. 2005;22(6):1013–1027. doi: 10.1080/07420520500397900. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Onambele GN. Influence of time of day on tendon compliance and estimations of voluntary activation levels. Muscle Nerve. 2006;33(6):792–800. doi: 10.1002/mus.20529. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M, et al. Muscle function in elite master weightlifters. Med Sci Sports Exerc. 2002;34(7):1199–1206. doi: 10.1097/00005768-200207000-00023. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Vandervoort AA, Paterson DH, Kramer JF, Cunningham DA. Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci. 1992;17(1):3–7. [PubMed] [Google Scholar]
- Prior BM, Cureton KJ, Modlesky CM, Evans EM, Sloniger MA, Saunders M, et al. In vivo validation of whole body composition estimates from dual-energy X-ray absorptiometry. J Appl Physiol. 1997;83(2):623–630. doi: 10.1152/jappl.1997.83.2.623. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Selby A, Atherton P, Smith K, Kumar V, Glover EL, et al. Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scand J Med Sci Sports. 2009;20:5–9. doi: 10.1111/j.1600-0838.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Hughes VA, Dallal GE, Nelson ME, Morganti C, Kehayias JJ, et al. The effect of gender and body composition method on the apparent decline in lean mass-adjusted resting metabolic rate with age. J Gerontol A Biol Sci Med Sci. 2000;55(12):M757–M760. doi: 10.1093/gerona/55.12.m757. [DOI] [PubMed] [Google Scholar]
- Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30(5):327–346. doi: 10.2165/00007256-200030050-00002. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Zhang XJ, Wolfe RR. TNF directly stimulates glucose uptake and leucine oxidation and inhibits FFA flux in conscious dogs. Am J Physiol. 1996;270(5 Pt 1):E864–E872. doi: 10.1152/ajpendo.1996.270.5.E864. [DOI] [PubMed] [Google Scholar]
- Schlicht J, Camaione DN, Owen SV. Effect of intense strength training on standing balance, walking speed, and sit-to-stand performance in older adults. J Gerontol A Biol Sci Med Sci. 2001;56(5):M281–M286. doi: 10.1093/gerona/56.5.m281. [DOI] [PubMed] [Google Scholar]
- Schutte AE, Huisman HW, Schutte R, Rooyen JM, Malan L, Malan NT. Aging influences the level and functions of fasting plasma ghrelin levels: the POWIRS-Study. Regul Pept. 2007;139(1–3):65–71. doi: 10.1016/j.regpep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Coenen-Schimke JM, Rys P, Nair KS. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99(1):95–102. doi: 10.1152/japplphysiol.00129.2005. [DOI] [PubMed] [Google Scholar]
- Sipila S, Elorinne M, Alen M, Suominen H, Kovanen V. Effects of strength and endurance training on muscle fibre characteristics in elderly women. Clin Physiol. 1997;17(5):459–474. doi: 10.1046/j.1365-2281.1997.05050.x. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Young A, Greig CA, Malbut KE. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc. 1995;43(10):1081–1087. doi: 10.1111/j.1532-5415.1995.tb07004.x. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Young A, Greig CA. Muscle function of women aged 65–89 years meeting two sets of health criteria. Aging (Milano) 1997;9(1–2):106–111. doi: 10.1007/BF03340135. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–1214. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- Tonino RP. Effect of physical training on the insulin resistance of aging. Am J Physiol. 1989;256(3 Pt 1):E352–E356. doi: 10.1152/ajpendo.1989.256.3.E352. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Ivey FM, Hurlbut D, Martel GF, Lemmer JT, Siegel EL, et al. Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J Appl Physiol. 1999;86(1):195–201. doi: 10.1152/jappl.1999.86.1.195. [DOI] [PubMed] [Google Scholar]
- Trappe T, Williams R, Carrithers J, Raue U, Esmarck B, Kjaer M, et al. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. J Physiol. 2004;554(Pt 3):803–813. doi: 10.1113/jphysiol.2003.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort AA, Kramer JF, Wharram ER. Eccentric knee strength of elderly females. J Gerontol. 1990;45(4):B125–B128. doi: 10.1093/geronj/45.4.b125. [DOI] [PubMed] [Google Scholar]
- Vaughan L, Zurlo F, Ravussin E. Aging and energy expenditure. Am J Clin Nutr. 1991;53(4):821–825. doi: 10.1093/ajcn/53.4.821. [DOI] [PubMed] [Google Scholar]
- Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154(3):557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Nair KS. Relationship of resting metabolic rate to body composition and protein turnover. Am J Physiol. 1990;258(6 Pt 1):E990–E998. doi: 10.1152/ajpendo.1990.258.6.E990. [DOI] [PubMed] [Google Scholar]
- Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci. 1996;51(6):M270–M275. doi: 10.1093/gerona/51a.6.m270. [DOI] [PubMed] [Google Scholar]
- Yang X, Jansson PA, Nagaev I, Jack MM, Carvalho E, Sunnerhagen KS, et al. Evidence of impaired adipogenesis in insulin resistance. Biochem Biophys Res Commun. 2004;317(4):1045–1051. doi: 10.1016/j.bbrc.2004.03.152. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci. 2003;58(10):M918–M922. doi: 10.1093/gerona/58.10.m918. [DOI] [PubMed] [Google Scholar]
- Young A, Stokes M, Crowe M. The size and strength of the quadriceps muscles of old and young men. Clin Physiol. 1985;5(2):145–154. doi: 10.1111/j.1475-097X.1985.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Zhu M, Lee GD, Ding L, Hu J, Qiu G, Cabo R, et al. Adipogenic signaling in rat white adipose tissue: modulation by aging and calorie restriction. Exp Gerontol. 2007;42(8):733–744. doi: 10.1016/j.exger.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]